Dual Roles of miR-10a-5p and miR-10b-5p as Tumor Suppressors and Oncogenes in Diverse Cancers
Abstract
1. Introduction
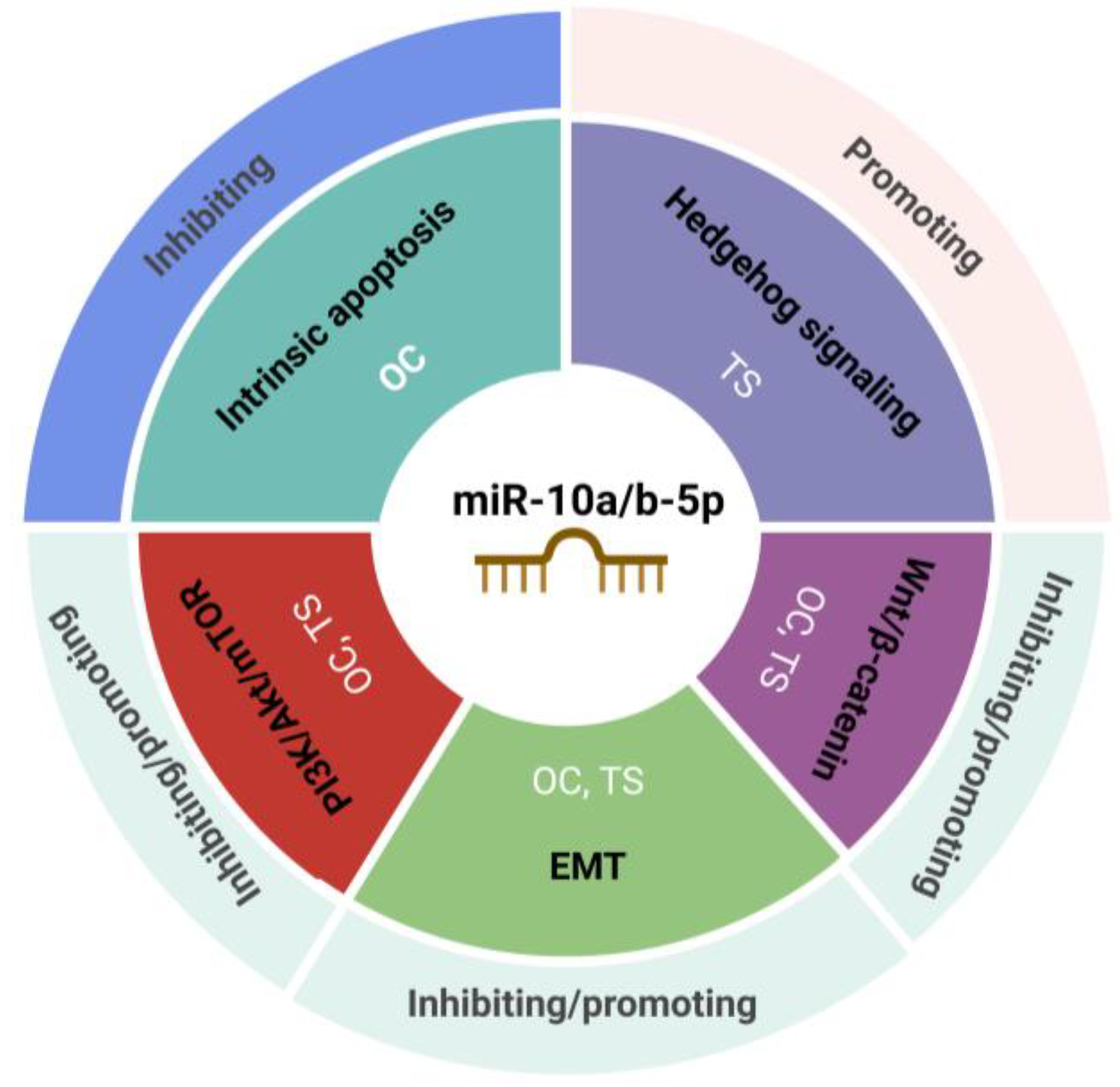
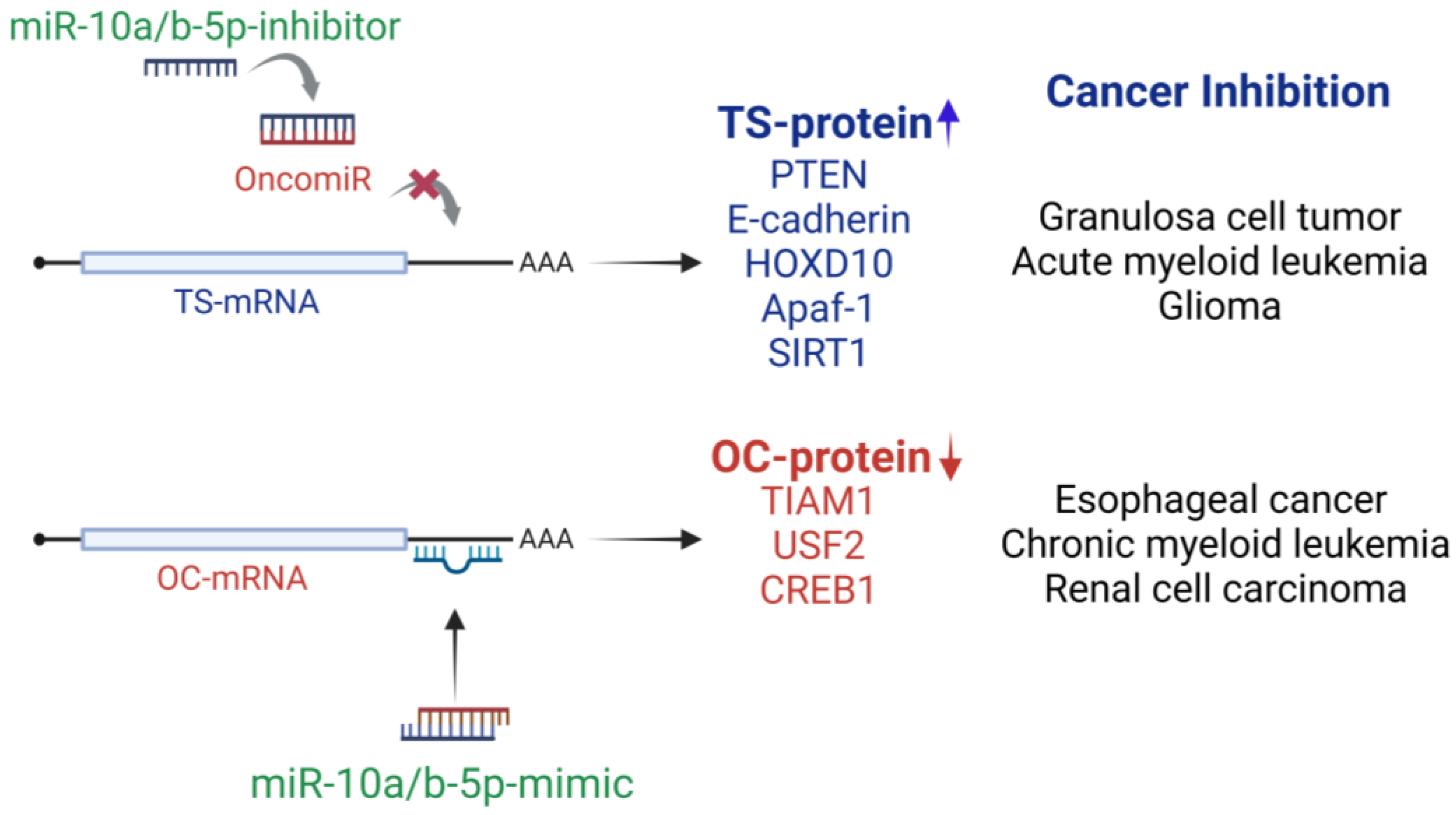
2. Dual Roles of miR-10a/b-5p as Tumor Suppressors and Oncogenes
3. miR-10a/b-5p as Tumor-Suppressive miRNAs (TSmiRs)
3.1. Chronic Myeloid Leukemia
3.2. Esophageal Squamous Cell Carcinoma
3.3. Renal Cell Carcinoma
4. miR-10a/b-5p as Oncogenic miRNAs (oncomiRs)
4.1. Cholangiocarcinoma
4.2. Granulosa Cell Tumors
4.3. Acute Myeloid Leukemia
4.4. Prostate Cancer
4.5. Glioma and Glioblastoma
5. miR-10a/b-5p as Both TSmiRs and oncomiRs
5.1. Breast Cancer
5.2. Bladder Cancer
5.3. Endometrial Cancer
5.4. Cervical Cancer
5.5. Ovarian Cancer
5.6. Gastric Cancer
5.7. Colorectal Cancer
5.8. Hepatocellular Carcinoma
6. miR10a/b-5p in the Regulation of Key Cancer Pathways
7. miR-10a/b-5p and Tumor Microenvironment
8. miRNA Therapeutic Targets in Cancer
9. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swanton, C.; Bernard, E.; Abbosh, C.; Andre, F.; Auwerx, J.; Balmain, A.; Bar-Sagi, D.; Bernards, R.; Bullman, S.; DeGregori, J.; et al. Embracing cancer complexity: Hallmarks of systemic disease. Cell 2024, 187, 1589–1616. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Offord, C. Duo honored for tiny RNAs key to development and disease. Science 2024, 386, 134. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Kim, V.N. Small and long non-coding RNAs: Past, present, and future. Cell 2024, 187, 6451–6485. [Google Scholar] [CrossRef]
- Hussen, B.M.; Hidayat, H.J.; Salihi, A.; Sabir, D.K.; Taheri, M.; Ghafouri-Fard, S. MicroRNA: A signature for cancer progression. Biomed. Pharmacother. 2021, 138, 111528. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Hausser, J.; Zavolan, M. Identification and consequences of miRNA-target interactions--beyond repression of gene expression. Nat. Rev. Genet. 2014, 15, 599–612. [Google Scholar] [CrossRef]
- Gurtan, A.M.; Sharp, P.A. The role of miRNAs in regulating gene expression networks. J. Mol. Biol. 2013, 425, 3582–3600. [Google Scholar] [CrossRef]
- Singh, R.; Ha, S.E.; Wei, L.; Jin, B.; Zogg, H.; Poudrier, S.M.; Jorgensen, B.G.; Park, C.; Ronkon, C.F.; Bartlett, A.; et al. miR-10b-5p Rescues Diabetes and Gastrointestinal Dysmotility. Gastroenterology 2021, 160, 1662–1678.e18. [Google Scholar] [CrossRef]
- Zogg, H.; Singh, R.; Ha, S.E.; Wang, Z.; Jin, B.; Ha, M.; Dafinone, M.; Batalon, T.; Hoberg, N.; Poudrier, S.; et al. miR-10b-5p rescues leaky gut linked with gastrointestinal dysmotility and diabetes. United Eur. Gastroenterol. J. 2023, 11, 750–766. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.E.; Singh, R.; Jin, B.; Baek, G.; Jorgensen, B.G.; Zogg, H.; Debnath, S.; Park, H.S.; Cho, H.; Watkins, C.M.; et al. miR-10a/b-5p-NCOR2 Regulates Insulin-Resistant Diabetes in Female Mice. Int. J. Mol. Sci. 2024, 25, 10147. [Google Scholar] [CrossRef]
- Singh, R.; Zogg, H.; Ro, S. Role of microRNAs in Disorders of Gut-Brain Interactions: Clinical Insights and Therapeutic Alternatives. J. Pers. Med. 2021, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Zogg, H.; Singh, R.; Ro, S. Current Advances in RNA Therapeutics for Human Diseases. Int. J. Mol. Sci. 2022, 23, 2736. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.H. miR-10 in development and cancer. Cell Death Differ. 2010, 17, 209–214. [Google Scholar] [CrossRef]
- Li, C.; Zhu, X.; Lv, X.; Han, X.; Xu, Y.; Huang, J.; Chen, X.; Yu, Z. Recent Updates on the Role of the MicroRNA-10 Family in Gynecological Malignancies. J. Oncol. 2022, 2022, 1544648. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shi, Y.; Liu, Z.; Li, Z.; Xu, W. The emerging role of miR-10 family in gastric cancer. Cell Cycle 2021, 20, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, P.; Medarova, Z. The fundamental role of miR-10b in metastatic cancer. Am. J. Cancer Res. 2018, 8, 1674–1688. [Google Scholar] [PubMed]
- Singh, R.; Ha, S.E.; Park, H.S.; Debnath, S.; Cho, H.; Baek, G.; Yu, T.Y.; Ro, S. Sustained Effectiveness and Safety of Therapeutic miR-10a/b in Alleviating Diabetes and Gastrointestinal Dysmotility without Inducing Cancer or Inflammation in Murine Liver and Colon. Int. J. Mol. Sci. 2024, 25, 2266. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yang, X.; Zhang, H.; Yu, M.; Long, J.; Yang, T. Inhibition of miR-10a-5p suppresses cholangiocarcinoma cell growth through downregulation of Akt pathway. Onco Targets Ther. 2018, 11, 6981–6994. [Google Scholar] [CrossRef]
- Shen, D.; Zhao, H.Y.; Gu, A.D.; Wu, Y.W.; Weng, Y.H.; Li, S.J.; Song, J.Y.; Gu, X.F.; Qiu, J.; Zhao, W. miRNA-10a-5p inhibits cell metastasis in hepatocellular carcinoma via targeting SKA1. Kaohsiung J. Med. Sci. 2021, 37, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Wang, D.; Lu, J. MicroRNA-10b inhibits proliferation, migration and invasion in cervical cancer cells via direct targeting of insulin-like growth factor-1 receptor. Oncol. Lett. 2017, 13, 5009–5015. [Google Scholar] [CrossRef] [PubMed]
- Guessous, F.; Alvarado-Velez, M.; Marcinkiewicz, L.; Zhang, Y.; Kim, J.; Heister, S.; Kefas, B.; Godlewski, J.; Schiff, D.; Purow, B.; et al. Oncogenic effects of miR-10b in glioblastoma stem cells. J. Neuro-Oncol. 2013, 112, 153–163. [Google Scholar] [CrossRef]
- Tu, J.; Cheung, H.H.; Lu, G.; Chen, Z.; Chan, W.Y. MicroRNA-10a promotes granulosa cells tumor development via PTEN-AKT/Wnt regulatory axis. Cell Death Dis. 2018, 9, 1076. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wu, H.; Li, Y.; Zhang, Y.; Liu, M.; Li, X.; Tang, H. miR-10a suppresses colorectal cancer metastasis by modulating the epithelial-to-mesenchymal transition and anoikis. Cell Death Dis. 2017, 8, e2739. [Google Scholar] [CrossRef]
- Ke, K.; Lou, T. MicroRNA-10a suppresses breast cancer progression via PI3K/Akt/mTOR pathway. Oncol. Lett. 2017, 14, 5994–6000. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.; Hunter, K.; Pandha, H.S. Downstream of the HOX genes: Explaining conflicting tumour suppressor and oncogenic functions in cancer. Int. J. Cancer 2022, 150, 1919–1932. [Google Scholar] [CrossRef]
- Lin, L.; Mahner, S.; Jeschke, U.; Hester, A. The Distinct Roles of Transcriptional Factor KLF11 in Normal Cell Growth Regulation and Cancer as a Mediator of TGF-beta Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 2928. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Li, Y.; Lan, X.; Ai, L. MicroRNA-10a-5p suppresses cancer proliferation and division in human cervical cancer by targeting BDNF. Exp. Ther. Med. 2017, 14, 6147–6151. [Google Scholar] [CrossRef] [PubMed]
- Agirre, X.; Jimenez-Velasco, A.; San Jose-Eneriz, E.; Garate, L.; Bandres, E.; Cordeu, L.; Aparicio, O.; Saez, B.; Navarro, G.; Vilas-Zornoza, A.; et al. Down-regulation of hsa-miR-10a in chronic myeloid leukemia CD34+ cells increases USF2-mediated cell growth. Mol. Cancer Res. 2008, 6, 1830–1840. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Jiang, X.; Yan, P.; Zhan, L.; Zhu, H.; Wang, T.; Wen, J. Tumor-suppressive microRNA-10a inhibits cell proliferation and metastasis by targeting Tiam1 in esophageal squamous cell carcinoma. J. Cell. Biochem. 2019, 120, 7845–7857. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Sun, X.Y.; Yang, C.X.; Zou, X.Y. MiR-10a-5p restrains the aggressive phenotypes of ovarian cancer cells by inhibiting HOXA1. Kaohsiung J. Med. Sci. 2021, 37, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wu, Q.; Lu, D. MicroRNA-10a-5p-mediated downregulation of GATA6 inhibits tumor progression in ovarian cancer. Hum. Cell 2024, 37, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.X.; Sun, G.; Shangguan, M.Y.; Gui, Z.; Bao, Y.; Li, Y.F.; Jia, Z.H. Novel role of lncRNA CHRF in cisplatin resistance of ovarian cancer is mediated by miR-10b induced EMT and STAT3 signaling. Sci. Rep. 2020, 10, 14768. [Google Scholar] [CrossRef]
- Zaravinos, A.; Radojicic, J.; Lambrou, G.I.; Volanis, D.; Delakas, D.; Stathopoulos, E.N.; Spandidos, D.A. Expression of miRNAs involved in angiogenesis, tumor cell proliferation, tumor suppressor inhibition, epithelial-mesenchymal transition and activation of metastasis in bladder cancer. J. Urol. 2012, 188, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, C.H.; Pursell, B.; Mercurio, A.M. miR-10b targets Tiam1: Implications for Rac activation and carcinoma migration. J. Biol. Chem. 2010, 285, 20541–20546. [Google Scholar] [CrossRef]
- Zou, D.; Zhou, Q.; Wang, D.; Guan, L.; Yuan, L.; Li, S. The Downregulation of MicroRNA-10b and its Role in Cervical Cancer. Oncol. Res. 2016, 24, 99–108. [Google Scholar] [CrossRef]
- Yu, M.; Xu, Y.; Pan, L.; Feng, Y.; Luo, K.; Mu, Q.; Luo, G. miR-10b Downregulated by DNA Methylation Acts as a Tumor Suppressor in HPV-Positive Cervical Cancer via Targeting Tiam1. Cell. Physiol. Biochem. 2018, 51, 1763–1777. [Google Scholar] [CrossRef]
- Khella, H.W.Z.; Daniel, N.; Youssef, L.; Scorilas, A.; Nofech-Mozes, R.; Mirham, L.; Krylov, S.N.; Liandeau, E.; Krizova, A.; Finelli, A.; et al. miR-10b is a prognostic marker in clear cell renal cell carcinoma. J. Clin. Pathol. 2017, 70, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Hiroki, E.; Akahira, J.; Suzuki, F.; Nagase, S.; Ito, K.; Suzuki, T.; Sasano, H.; Yaegashi, N. Changes in microRNA expression levels correlate with clinicopathological features and prognoses in endometrial serous adenocarcinomas. Cancer Sci. 2010, 101, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, O.; Miura, K.; Mishima, H.; Abe, S.; Kaneuchi, M.; Higashijima, A.; Miura, S.; Kinoshita, A.; Yoshiura, K.; Masuzaki, H. Identification of endometrioid endometrial carcinoma-associated microRNAs in tissue and plasma. Gynecol. Oncol. 2014, 132, 715–721. [Google Scholar] [CrossRef]
- Song, J.J.; Li, W. miR-10b suppresses the growth and metastasis of colorectal cancer cell by targeting FGF13. Eur Rev Med Pharmacol Sci. 2019, 23, 576–587. [Google Scholar]
- Liu, F.; An, X.; Zhao, X.; Zhang, N.; Chen, B.; Li, Z.; Xu, W. MiR-10b-5p inhibits tumorigenesis in gastric cancer xenograft mice model through down-regulating Tiam1. Exp. Cell Res. 2021, 407, 112810. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Shi, Y.; Zhao, X.; Gao, D.; Qu, L.; Chen, L.; Zhao, K.; Du, J.; Xu, W. CBFbeta/RUNX3-miR10b-TIAM1 molecular axis inhibits proliferation, migration, and invasion of gastric cancer cells. Int. J. Clin. Exp. Pathol. 2019, 12, 3185–3196. [Google Scholar] [PubMed]
- Li, Z.; Lei, H.; Luo, M.; Wang, Y.; Dong, L.; Ma, Y.; Liu, C.; Song, W.; Wang, F.; Zhang, J.; et al. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer 2015, 18, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, H.C.; Park, J.L.; Kim, M.; Kim, S.Y.; Noh, S.M.; Song, K.S.; Kim, J.C.; Kim, Y.S. Epigenetic regulation of microRNA-10b and targeting of oncogenic MAPRE1 in gastric cancer. Epigenetics 2011, 6, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, D.; Li, Y.; Jin, L.; Liu, J.; Su, Z.; Qi, Z.; Shi, M.; Jiang, Z.; Ni, L.; et al. Oncogenic cAMP responsive element binding protein 1 is overexpressed upon loss of tumor suppressive miR-10b-5p and miR-363-3p in renal cancer. Oncol. Rep. 2016, 35, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lin, J.X.; Yu, Y.H.; Zhang, M.Y.; Wang, H.Y.; Zheng, M. Downregulation of six microRNAs is associated with advanced stage, lymph node metastasis and poor prognosis in small cell carcinoma of the cervix. PLoS ONE 2012, 7, e33762. [Google Scholar] [CrossRef] [PubMed]
- Sommerova, L.; Anton, M.; Bouchalova, P.; Jasickova, H.; Rak, V.; Jandakova, E.; Selingerova, I.; Bartosik, M.; Vojtesek, B.; Hrstka, R. The role of miR-409-3p in regulation of HPV16/18-E6 mRNA in human cervical high-grade squamous intraepithelial lesions. Antivir. Res. 2019, 163, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Lv, Y.; Zhang, C.; Guo, S. LncRNA meg3 suppresses hepatocellular carcinoma in vitro and vivo studies. Am. J. Transl. Res. 2019, 11, 4089–4099. [Google Scholar]
- Nakayama, I.; Shibazaki, M.; Yashima-Abo, A.; Miura, F.; Sugiyama, T.; Masuda, T.; Maesawa, C. Loss of HOXD10 expression induced by upregulation of miR-10b accelerates the migration and invasion activities of ovarian cancer cells. Int. J. Oncol. 2013, 43, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wang, W. LncRNA SNHG4 regulates miR-10a/PTEN to inhibit the proliferation of acute myeloid leukemia cells. Hematology 2020, 25, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Wang, X.; Zou, B.; Mei, J.; Peng, X.; Wu, Z. Extracellular vesicles-encapsulated microRNA-10a-5p shed from cancer-associated fibroblast facilitates cervical squamous cell carcinoma cell angiogenesis and tumorigenicity via Hedgehog signaling pathway. Cancer Gene Ther. 2021, 28, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Li, G. MicroRNA-10a enhances the metastatic potential of cervical cancer cells by targeting phosphatase and tensin homologue. Mol. Med. Rep. 2014, 10, 1377–1382. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Zhang, H.; Lin, F.; Tan, Q.; Qin, Q.; Bao, W.; Liu, Y.; Xie, J.; Zeng, Q. Long intergenic non-protein coding RNA 324 prevents breast cancer progression by modulating miR-10b-5p. Aging 2020, 12, 6680–6699. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Z.; Zhang, L.; Sun, S. LncRNA PDCD4-AS1 alleviates triple negative breast cancer by increasing expression of IQGAP2 via miR-10b-5p. Transl. Oncol. 2021, 14, 100958. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Zhang, P.Y.; Zhang, Y.; Sun, S.Y.; Yu, S.Y.; Xi, Q.S. MicroRNA-10b targets E-cadherin and modulates breast cancer metastasis. Med. Sci. Monit. 2012, 18, BR299–BR308. [Google Scholar] [CrossRef]
- Ma, L.; Teruya-Feldstein, J.; Weinberg, R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 2007, 449, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Li, L.; Ye, Z.Y.; Zhao, Z.S.; Yan, Z.L. MicroRNA-10b promotes migration and invasion through Hoxd10 in human gastric cancer. World J. Surg. Oncol. 2015, 13, 259. [Google Scholar] [CrossRef] [PubMed]
- Stadthagen, G.; Tehler, D.; Hoyland-Kroghsbo, N.M.; Wen, J.; Krogh, A.; Jensen, K.T.; Santoni-Rugiu, E.; Engelholm, L.H.; Lund, A.H. Loss of miR-10a activates lpo and collaborates with activated Wnt signaling in inducing intestinal neoplasia in female mice. PLoS Genet. 2013, 9, e1003913. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Jiang, W.; Hui, B.; Rong, D.; Fu, K.; Dong, C.; Tang, W.; Cao, H. The circ_0021977/miR-10b-5p/P21 and P53 regulatory axis suppresses proliferation, migration, and invasion in colorectal cancer. J. Cell. Physiol. 2020, 235, 2273–2285. [Google Scholar] [CrossRef]
- Wang, Y.F.; Li, Z.; Zhao, X.H.; Zuo, X.M.; Zhang, Y.; Xiao, Y.H.; Li, J.; Peng, Z.H. MicroRNA-10b is upregulated and has an invasive role in colorectal cancer through enhanced Rhoc expression. Oncol. Rep. 2015, 33, 1275–1283. [Google Scholar] [CrossRef]
- Abdelmaksoud-Dammak, R.; Chamtouri, N.; Triki, M.; Saadallah-Kallel, A.; Ayadi, W.; Charfi, S.; Khabir, A.; Ayadi, L.; Sallemi-Boudawara, T.; Mokdad-Gargouri, R. Overexpression of miR-10b in colorectal cancer patients: Correlation with TWIST-1 and E-cadherin expression. Tumour Biol. 2017, 39, 1010428317695916. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, J.; Chen, Y.; Ma, C.; Li, B.; Hao, T. Up-regulation of mir-10b predicate advanced clinicopathological features and liver metastasis in colorectal cancer. Cancer Med. 2016, 5, 2932–2941. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, L.; Li, Y.; Cui, Y.; Wang, Y.; Liu, C. Long non-coding RNA CHRF promotes proliferation and mesenchymal transition (EMT) in prostate cancer cell line PC3 requiring up-regulating microRNA-10b. Biol. Chem. 2019, 400, 1035–1045. [Google Scholar] [CrossRef]
- Ma, C.; Wei, F.; Xia, H.; Liu, H.; Dong, X.; Zhang, Y.; Luo, Q.; Liu, Y.; Li, Y. MicroRNA-10b mediates TGF-beta1-regulated glioblastoma proliferation, migration and epithelial-mesenchymal transition. Int. J. Oncol. 2017, 50, 1739–1748. [Google Scholar] [CrossRef]
- Sun, L.; Yan, W.; Wang, Y.; Sun, G.; Luo, H.; Zhang, J.; Wang, X.; You, Y.; Yang, Z.; Liu, N. MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10. Brain Res. 2011, 1389, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, J.; Zhang, H.; Li, H. Long noncoding RNA-GAS5 attenuates progression of glioma by eliminating microRNA-10b and Sirtuin 1 in U251 and A172 cells. Biofactors 2020, 46, 487–496. [Google Scholar] [CrossRef]
- Chen, H.; Fan, Y.; Xu, W.; Chen, J.; Xu, C.; Wei, X.; Fang, D.; Feng, Y. miR-10b Inhibits Apoptosis and Promotes Proliferation and Invasion of Endometrial Cancer Cells via Targeting HOXB3. Cancer Biother. Radiopharm. 2016, 31, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Li, H.; Yu, G.; Xiao, W.; Hu, J.; Tang, K.; Zeng, J.; He, W.; Zeng, G.; Ye, Z.; et al. MicroRNA-10b promotes migration and invasion through KLF4 and HOXD10 in human bladder cancer. Oncol. Rep. 2014, 31, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Chen, Z.Y.; Li, Y.; Yang, Z.Q.; Zeng, F.; Cui, Y.; He, Y.; Chen, J.B.; Chen, H.Q. miR-10b suppresses cell invasion and metastasis through targeting HOXA3 regulated by FAK/YAP signaling pathway in clear-cell renal cell carcinoma. BMC Nephrol. 2019, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shirvani-Farsani, Z.; Branicki, W.; Taheri, M. MicroRNA Signature in Renal Cell Carcinoma. Front. Oncol. 2020, 10, 596359. [Google Scholar] [CrossRef]
- Vu, T.T.; Stolzel, F.; Wang, K.W.; Rollig, C.; Tursky, M.L.; Molloy, T.J.; Ma, D.D. miR-10a as a therapeutic target and predictive biomarker for MDM2 inhibition in acute myeloid leukemia. Leukemia 2021, 35, 1933–1948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, J.; Zhang, X.; Xu, J.; Sun, Y.; Zhang, P. MicroRNA-10b expression in breast cancer and its clinical association. PLoS ONE 2018, 13, e0192509. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Fan, T.C.; Yu, J.C.; Liao, G.S.; Lin, Y.C.; Shih, A.C.; Li, W.H.; Yu, A.L. The prognostic significance of RUNX2 and miR-10a/10b and their inter-relationship in breast cancer. J. Transl. Med. 2014, 12, 257. [Google Scholar] [CrossRef]
- Yoo, B.; Kavishwar, A.; Ross, A.; Wang, P.; Tabassum, D.P.; Polyak, K.; Barteneva, N.; Petkova, V.; Pantazopoulos, P.; Tena, A.; et al. Combining miR-10b-Targeted Nanotherapy with Low-Dose Doxorubicin Elicits Durable Regressions of Metastatic Breast Cancer. Cancer Res. 2015, 75, 4407–4415. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yan, S.; Weijie, Z.; Feng, W.; Liuxing, W.; Mengquan, L.; Qingxia, F. Critical role of miR-10b in transforming growth factor-beta1-induced epithelial-mesenchymal transition in breast cancer. Cancer Gene Ther. 2014, 21, 60–67. [Google Scholar] [CrossRef]
- Yang, L.; Sun, H.F.; Guo, L.Q.; Cao, H.B. MiR-10a-5p: A Promising Biomarker for Early Diagnosis and Prognosis Evaluation of Bladder Cancer. Cancer Manag. Res. 2021, 13, 7841–7850. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.G. miR-34—A microRNA replacement therapy is headed to the clinic. Front Genet. 2012, 3, 120. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qin, H.; Cui, Y. MiR-34a targets GAS1 to promote cell proliferation and inhibit apoptosis in papillary thyroid carcinoma via PI3K/Akt/Bad pathway. Biochem Biophys Res Commun. 2013, 441, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Kalfert, D.; Pesta, M.; Kulda, V.; Topolcan, O.; Ryska, A.; Celakovsky, P.; Laco, J.; Ludvikova, M. MicroRNA profile in site-specific head and neck squamous cell cancer. Anticancer Res. 2015, 35, 2455–2463. [Google Scholar] [PubMed]
- Ling, H.; Fabbri, M.; Calin, G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013, 12, 847–865. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer. 2020, 122, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Makowska, M.; Smolarz, B.; Romanowicz, H. microRNAs (miRNAs) in Glioblastoma Multiforme (GBM)-Recent Literature Review. Int. J. Mol. Sci. 2023, 24, 3521. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/beta-catenin signaling in cancers and targeted therapies. Signal Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/beta-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Gotze, S.; Coersmeyer, M.; Muller, O.; Sievers, S. Histone deacetylase inhibitors induce attenuation of Wnt signaling and TCF7L2 depletion in colorectal carcinoma cells. Int. J. Oncol. 2014, 45, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yan, H.; Wang, Q.; Zhang, L.; Liu, Y.; Yu, H. MicroRNA 10a induces glioma tumorigenesis by targeting myotubularin-related protein 3 and regulating the Wnt/beta-catenin signaling pathway. FEBS J. 2019, 286, 2577–2592. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Jing, J.; Wu, Z.; Wang, J.; Luo, G.; Lin, H.; Fan, Y.; Zhou, C. Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies. Signal Transduct. Target. Ther. 2023, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Padua, D.; Massague, J. Roles of TGFbeta in metastasis. Cell Res. 2009, 19, 89–102. [Google Scholar] [CrossRef]
- Seoane, J.; Gomis, R.R. TGF-beta Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb. Perspect. Biol. 2017, 9, a022277. [Google Scholar] [CrossRef]
- Suzuki, H.I.; Katsura, A.; Matsuyama, H.; Miyazono, K. MicroRNA regulons in tumor microenvironment. Oncogene 2015, 34, 3085–3094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, P.; Wang, X.F. Microenvironmental regulation of cancer metastasis by miRNAs. Trends Cell Biol. 2014, 24, 153–160. [Google Scholar] [CrossRef]
- Buck, A.H. Cells choose their words wisely. Cell 2022, 185, 1114–1116. [Google Scholar] [CrossRef]
- Nedaeinia, R.; Najafgholian, S.; Salehi, R.; Goli, M.; Ranjbar, M.; Nickho, H.; Haghjooy Javanmard, S.; Ferns, G.A.; Manian, M. The role of cancer-associated fibroblasts and exosomal miRNAs-mediated intercellular communication in the tumor microenvironment and the biology of carcinogenesis: A systematic review. Cell Death Discov. 2024, 10, 380. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, M.; Wu, L.; Yang, H.; Yao, Y.; Yang, Q.; Du, J.; Liu, L.; Li, Y.; Bai, Y. Stromal cells in the tumor microenvironment: Accomplices of tumor progression? Cell Death Dis. 2023, 14, 587. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. MicroRNAs won the Nobel-will they ever be useful as medicines? Nature 2024. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. miRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 11502. [Google Scholar] [CrossRef]
- Cuciniello, R.; Filosa, S.; Crispi, S. Novel approaches in cancer treatment: Preclinical and clinical development of small non-coding RNA therapeutics. J. Exp. Clin. Cancer Res. 2021, 40, 383. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics-challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef]
- Shah, M.Y.; Ferrajoli, A.; Sood, A.K.; Lopez-Berestein, G.; Calin, G.A. microRNA Therapeutics in Cancer—An Emerging Concept. EBioMedicine 2016, 12, 34–42. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Al-Qadi, N.; Kenyon, E.; Conner, K.N.; Mondal, S.K.; Medarova, Z.; Moore, A. Inhibition of miR-10b treats metastatic breast cancer by targeting stem cell-like properties. Oncotarget 2024, 15, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Diener, C.; Keller, A.; Meese, E. The miRNA-target interactions: An underestimated intricacy. Nucleic Acids Res. 2024, 52, 1544–1557. [Google Scholar] [CrossRef]
- Shu, Y.; Wu, K.; Zeng, Z.; Huang, S.; Ji, X.; Yuan, C.; Zhang, L.; Liu, W.; Huang, B.; Feng, Y.; et al. A Simplified System to Express Circularized Inhibitors of miRNA for Stable and Potent Suppression of miRNA Functions. Mol. Ther. Nucleic Acids 2018, 13, 556–567. [Google Scholar] [CrossRef]
- Belter, A.; Rolle, K.; Piwecka, M.; Fedoruk-Wyszomirska, A.; Naskret-Barciszewska, M.Z.; Barciszewski, J. Inhibition of miR-21 in glioma cells using catalytic nucleic acids. Sci. Rep. 2016, 6, 24516. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Deng, J.; Zhang, B.; He, X.; Meng, Z.; Li, G.; Ye, H.; Zheng, S.; Wei, L.; Deng, X.; et al. LncRNA HOTAIR epigenetically suppresses miR-122 expression in hepatocellular carcinoma via DNA methylation. EBioMedicine 2018, 36, 159–170. [Google Scholar] [CrossRef]
- Jorgensen, B.G.; Ro, S. MicroRNAs and ’Sponging’ Competitive Endogenous RNAs Dysregulated in Colorectal Cancer: Potential as Noninvasive Biomarkers and Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 2166. [Google Scholar] [CrossRef] [PubMed]

| miRNA | Target Gene (Oncogene) | Cancer | Human | Mouse | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Name | Role | Tissue (n) | Cell (n) | Blood (n) | ||||
| miR-10a-5p | TSmiR | PI3K/Akt/mTOR | Breast cancer (BC) | BC (2) | [25] | |||
| miR-10a-5p | TSmiR | AKT | Cholangiocarcinoma (CCA) | CCA (3) | CCA xenograft mice | [19] | ||
| miR-10a-5p | TSmiR | BDNF | Cervical cancer (CC) | CC (5) | [28] | |||
| miR-10a-5p | TSmiR | USF2 | Chronic myeloid leukemia (CML) | CML (6) Bone marrow (85) | CML (5) | [29] | ||
| miR-10a-5p | TSmiR | MMP14/ACTG1 | Colorectal cancer (CRC) | CRC (26) | CRC (2) | [24] | ||
| miR-10a-5p | TSmiR | TIAM1 | Esophageal squamous cell carcinoma (ESCC) | ESCC (54) | ESCC (2) | ESCC xenograft mice Pulmonary metastasis mice | [30] | |
| miR-10a-5p | TSmiR | SKA1 | Hepatocellular carcinoma (HCC) | HCC (30) | HCC (4) | Plasma (32) | [20] | |
| miR-10a-5p | TSmiR | HOXA1 | Ovarian cancer (OC) | OC (56) | OC (4) | [31] | ||
| miR-10a-5p | TSmiR | GATA6 | Ovarian cancer (OC) | OC (376) | OC (2) | OC xenograft mice | [32] | |
| miR-10b-5p | TSmiR | STAT3 | Ovarian cancer (OC) | OC (6) | OC (3) | [33] | ||
| miR-10b-5p | TSmiR | Bladder cancer (BLC) | BLC (77) | [34] | ||||
| miR-10b-5p | TSmiR | TIAM1 | Breast cancer (BC) | BC (4) | [35] | |||
| miR-10b-5p | TSmiR | HOXA1 | Cervical cancer (CC) | CC (40) | CC (2) | [36] | ||
| miR-10b-5p | TSmiR | IGF-1R | Cervical cancer (CC) | CC (46) | CC (5) | [21] | ||
| miR-10b-5p | TSmiR | TIAM1 | Cervical cancer (CC) | CC (70) | CC (3) | [37] | ||
| miR-10b-5p | TSmiR | Clear cell renal cell carcinoma (ccRCC) | ccRCC (250) | [38] | ||||
| miR-10b-5p | TSmiR | Endometrial serous adenocarcinoma (ESA) | ESA (21) | ESA (1) | [39] | |||
| miR-10b-5p | TSmiR | Endometrioid endometrial carcinoma (EEC) | EEC (28) | Plasma (12) | [40] | |||
| miR-10b-5p | TSmiR | FGF13 | Colorectal cancer (CRC) | CRC (3) | CRC xenograft mice | [41] | ||
| miR-10b-5p | TSmiR | TIAM1 | Gastric cancer (GC) | GC (12) | GC (3) | GC xenograft mice | [42] | |
| miR-10b-5p | TSmiR | TIAM1 | Gastric cancer (GC) | GC (19) | GC (4) | [43] | ||
| miR-10b-5p | TSmiR | TIAM1 | Gastric cancer (GC) | GC (100) | GC (4) | [44] | ||
| miR-10b-5p | TSmiR | MAPRE1 | Gastric cancer (GC) | GC (32) | GC (11) | [45] | ||
| miR-10b-5p | TSmiR | CREB1 | Renal cancer (RC) | RC (35) | RC (4) | [46] | ||
| miR-10b-5p | TSmiR | Small cell cervical carcinoma (SCCC) | SCCC (44) | [47] | ||||
| miR-10b-5p | TSmiR | Cervical cancer (CC) | CC (44) | [48] | ||||
| miRNA | Target Gene (Tumor Suppressor) | Cancer | Human | Mouse | Reference | ||
|---|---|---|---|---|---|---|---|
| Name | Role | Tissue (n) | Cell (n) | ||||
| miR-10a-5p | oncomiR | PTEN | Granulosa cell tumor (GCT) | Granulosa cells (2) | mir-10a KO mice GCT xenograft mice | [23] | |
| miR-10a-5p | oncomiR | PTEN | Hepatocellular carcinoma (HCC) | HCC (30) | HCC (1) | [49] | |
| miR-10b-5p | oncomiR | HOXD10 | Ovarian cancer (OC) | OC (68) | OC (3) | [50] | |
| miR-10a-5p | oncomiR | PTEN | Acute myeloid leukemia (AML) | AML (60) | AML (1) | [51] | |
| miR-10a-5p | oncomiR | TBX5 | Cervical squamous cell carcinoma (CSCC) | CSCC (60) | CSCC (2) | CSCC xenograft mice | [52] |
| miR-10a-5p | oncomiR | PTEN | Cervical cancer (CC) | CC (40) | CC (2) | [53] | |
| miR-10b-5p | oncomiR | E-cadherin | Breast cancer (BC) | BC (45) | BC (2) | BC xenograft mice | [54] |
| miR-10b-5p | oncomiR | IQGAP2 | Triple-negative breast cancer (TNBC) | TNBC (42) | TNBC (3) | [55] | |
| miR-10b-5p | oncomiR | E-cadherin | Breast cancer (BC) | BC (44) | BC (1) | [56] | |
| miR-10b-5p | oncomiR | HOXD10 | Breast cancer (BC) | BC (18) | BC (6) | [57] | |
| miR-10b-5p | oncomiR | HOXD10 | Gastric cancer (GC) | GC (436) | GC (7) | [58] | |
| miR-10a-5p | oncomiR | LPO/KLF4 | Colorectal cancer (CRC) | CRC (16) | CRC (Apc) mice mir-10a KO mice | [59] | |
| miR-10b-5p | oncomiR | P21 and P53 | Colorectal cancer (CRC) | CRC (63) | CRC (5) | CRC xenograft mice | [60] |
| miR-10b-5p | oncomiR | HOXD10 | Colorectal cancer (CRC) | CRC (70) | [61] | ||
| miR-10b-5p | oncomiR | E-cadherin | Colorectal cancer (CRC) | CRC (50) | CRC (1) | [62] | |
| miR-10b-5p | oncomiR | Colorectal cancer (CRC) | CRC (246) | [63] | |||
| miR-10b-5p | oncomiR | GSK3β | Prostate cancer (PC) | PC (2) | [64] | ||
| miR-10b-5p | oncomiR | Apaf-1, E-cadherin | GBM | GBM (15) | GBM (2) | [65] | |
| miR-10b-5p | oncomiR | HOXD10 | Glioma | Glioma (22) | Glioma (4) | [66] | |
| miR-10b-5p | oncomiR | SIRT1 | Glioma | Glioma (2) | [67] | ||
| miR-10b-5p | oncomiR | HOXB3 | Endometrial cancer (EC) | EC (20) | [68] | ||
| miR-10b-5p | oncomiR | KLF4/HOXD10 | Bladder cancer (BLC) | BLC (20) | BLC cancer (6) | [69] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.; Ha, S.E.; Yu, T.Y.; Ro, S. Dual Roles of miR-10a-5p and miR-10b-5p as Tumor Suppressors and Oncogenes in Diverse Cancers. Int. J. Mol. Sci. 2025, 26, 415. https://doi.org/10.3390/ijms26010415
Singh R, Ha SE, Yu TY, Ro S. Dual Roles of miR-10a-5p and miR-10b-5p as Tumor Suppressors and Oncogenes in Diverse Cancers. International Journal of Molecular Sciences. 2025; 26(1):415. https://doi.org/10.3390/ijms26010415
Chicago/Turabian StyleSingh, Rajan, Se Eun Ha, Tae Yang Yu, and Seungil Ro. 2025. "Dual Roles of miR-10a-5p and miR-10b-5p as Tumor Suppressors and Oncogenes in Diverse Cancers" International Journal of Molecular Sciences 26, no. 1: 415. https://doi.org/10.3390/ijms26010415
APA StyleSingh, R., Ha, S. E., Yu, T. Y., & Ro, S. (2025). Dual Roles of miR-10a-5p and miR-10b-5p as Tumor Suppressors and Oncogenes in Diverse Cancers. International Journal of Molecular Sciences, 26(1), 415. https://doi.org/10.3390/ijms26010415







