Metal Ion Supplementation to Boost Melanin Production by Streptomyces nashvillensis
Abstract
1. Introduction
2. Results
2.1. Shake Flask Experiments
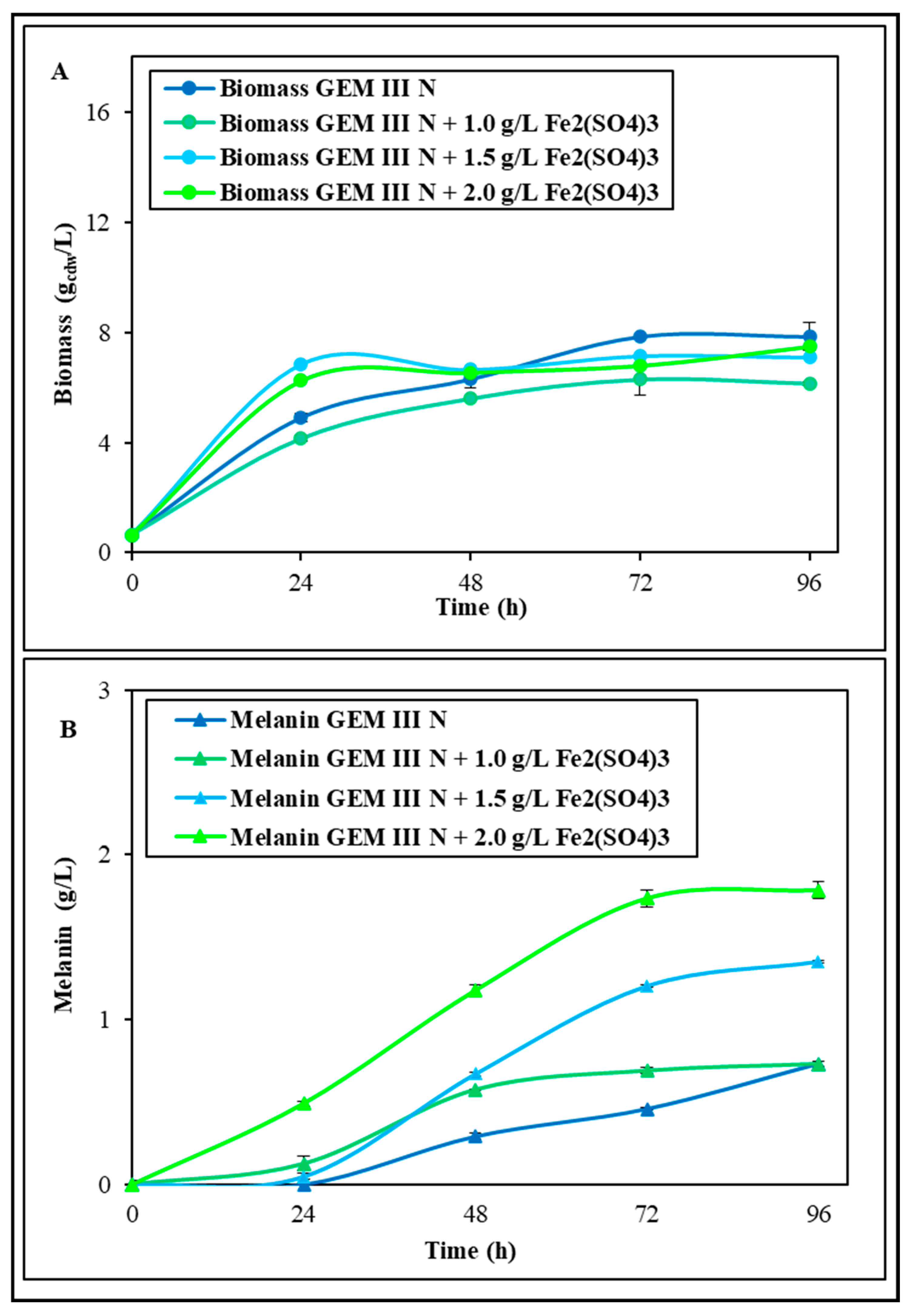
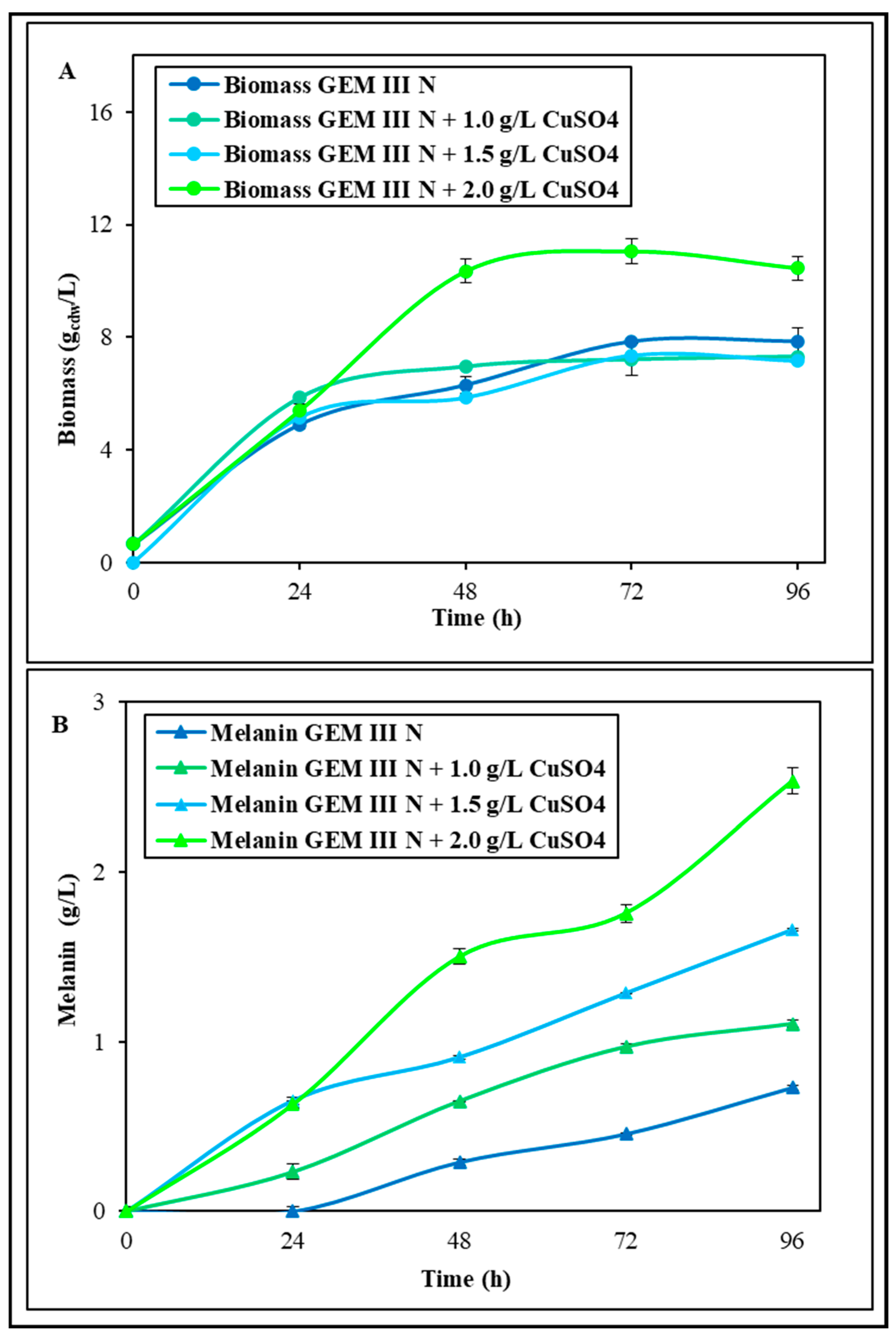
2.1.1. Shake Flask Experiments with Single Supplementations of Fe2(SO4)3 or CuSO4
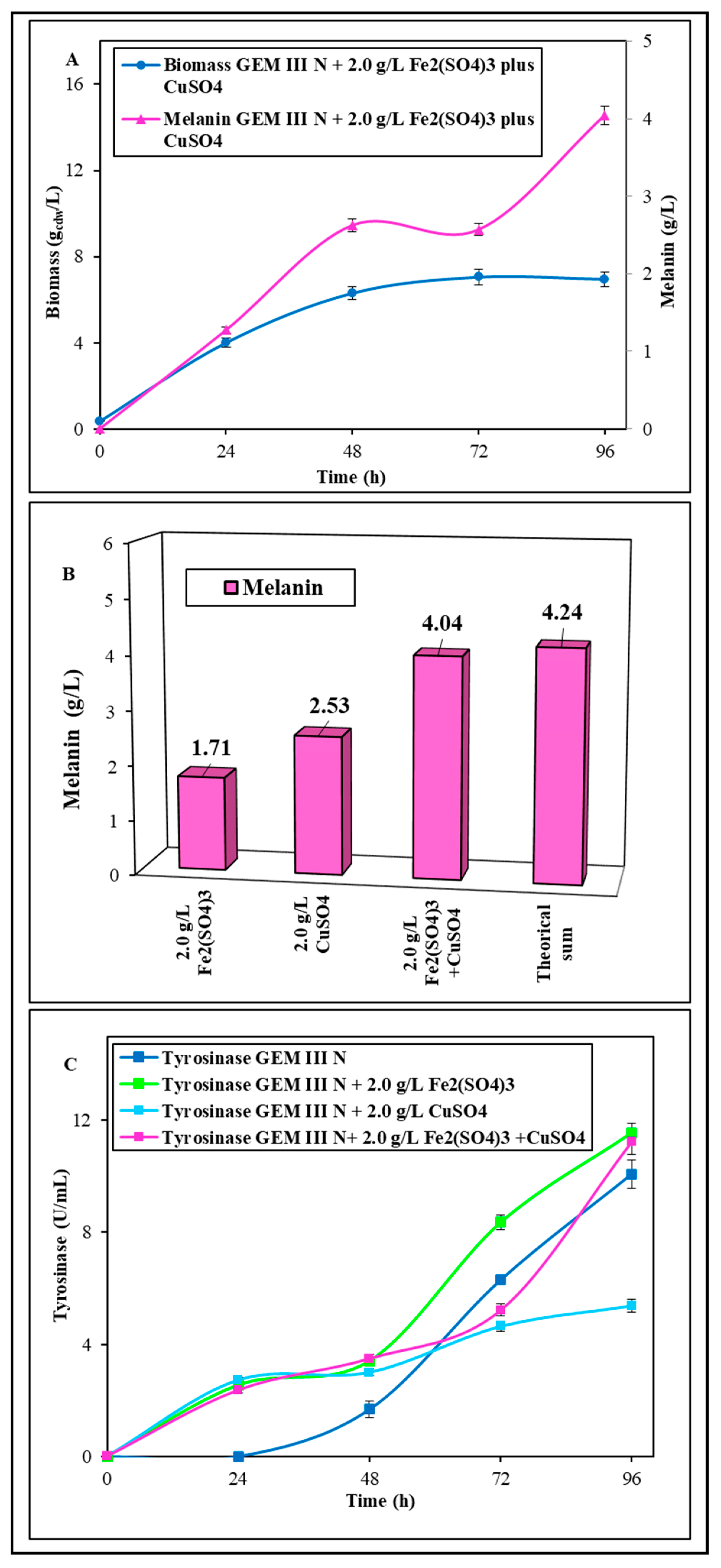
2.1.2. Shake Flask Experiments with Coupled Supplementation of Fe2(SO4)3 Plus CuSO4
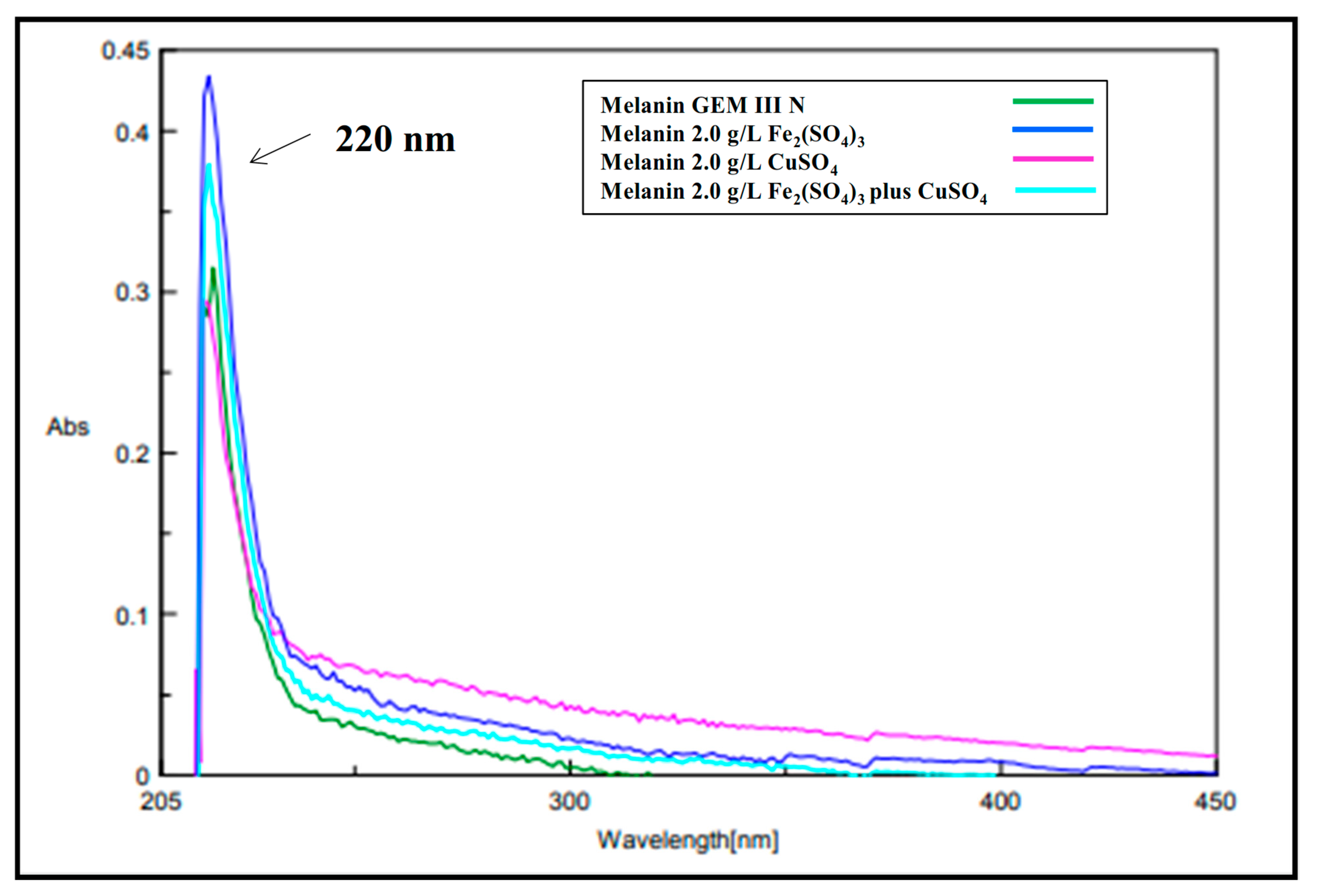
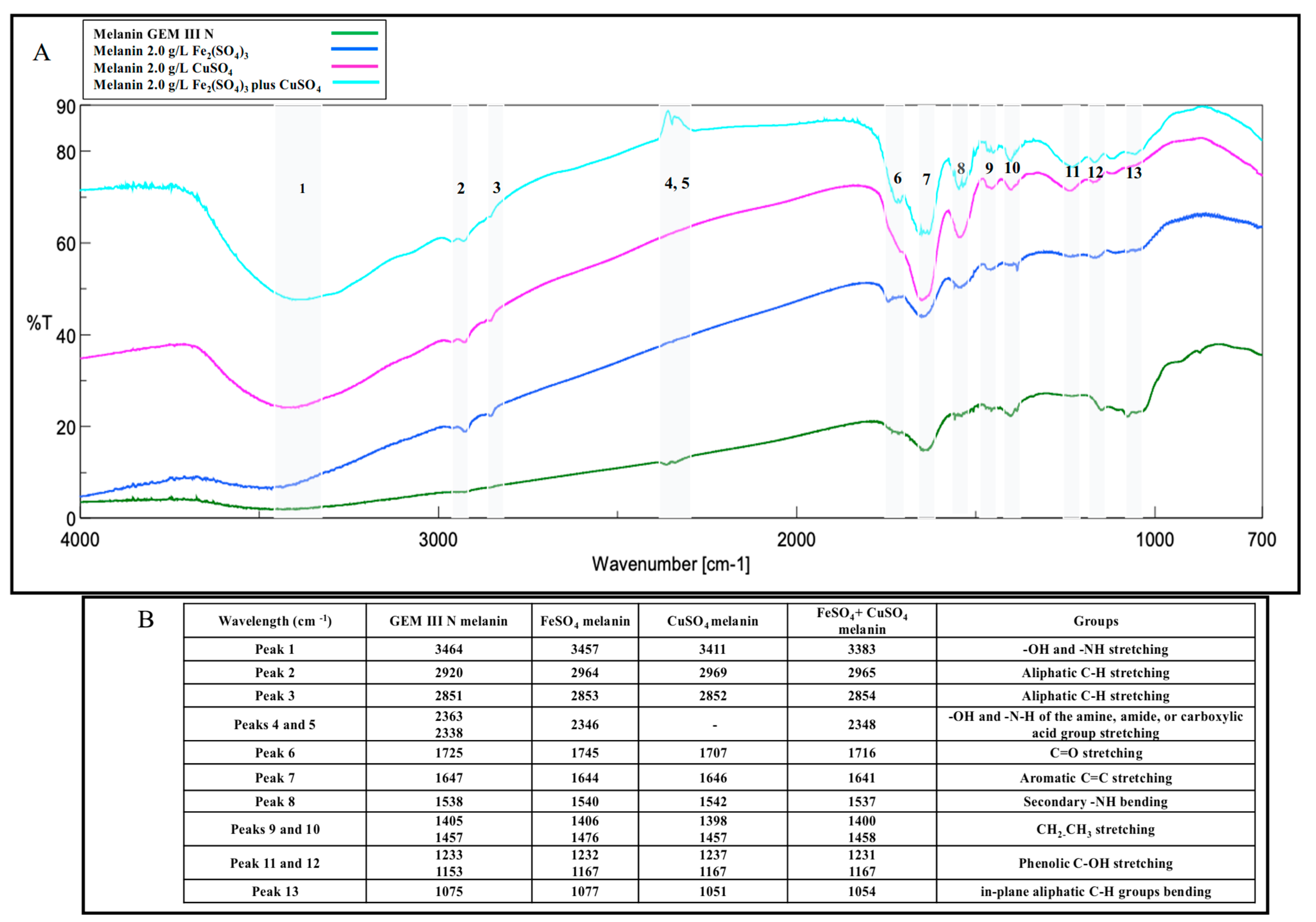
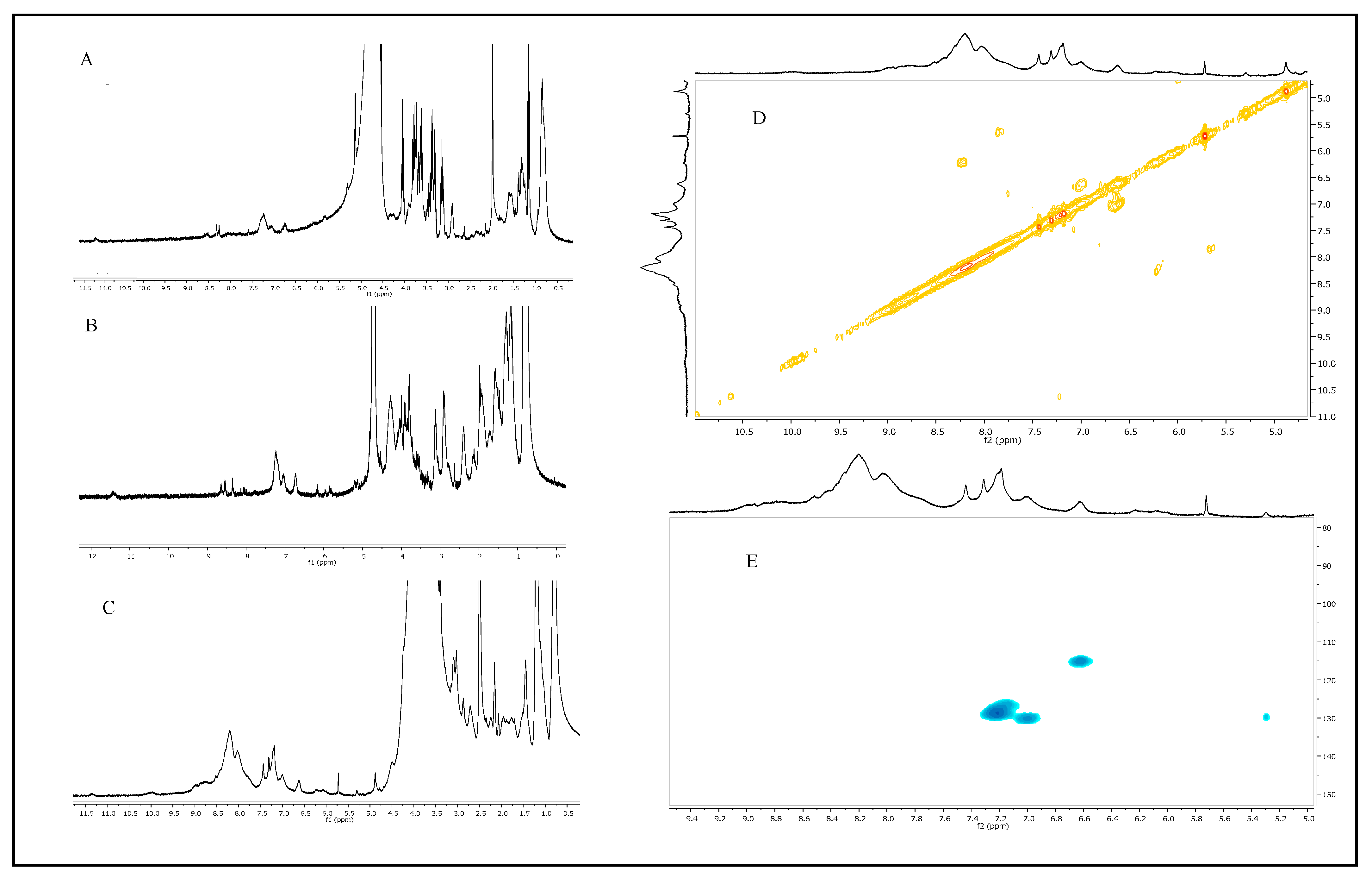
2.2. Melanin Purification and Characterization
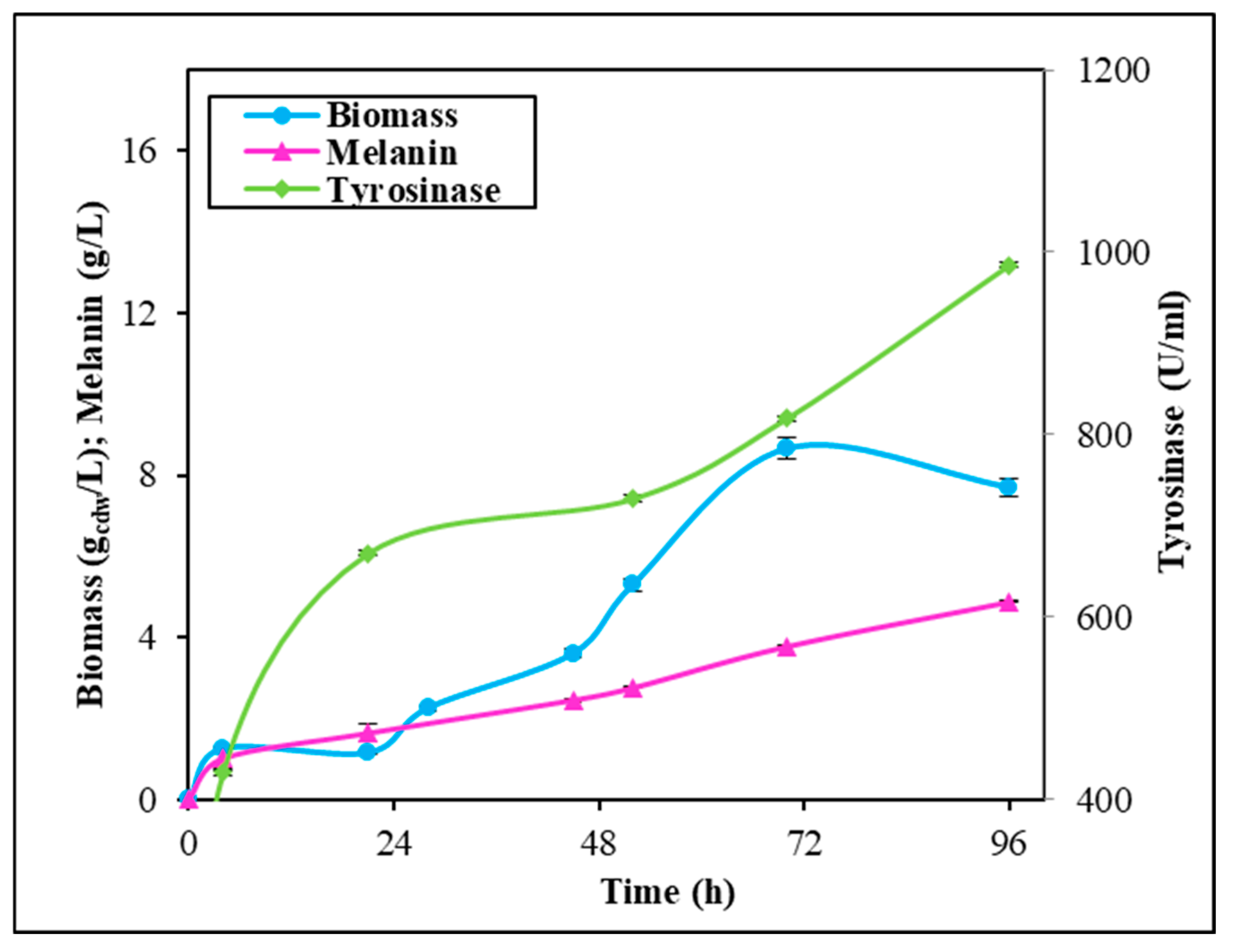
2.3. Fermentation Experiments in Stirred Tank Reactor
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Microorganism and Media
4.3. Shake Flask Experiments
4.4. Fermentation Experiments in Stirred Tank Reactor
4.5. Melanin Purification
4.6. Melanin Determination by UV-Visible Analyses
4.7. Tyrosinase Activity Assay
4.8. Melanin Structural Characterization
4.8.1. Melanin Analysis by Fourier-Transform Infrared (FT-IR) Spectroscopy
4.8.2. Melanin Analysis by Nuclear Magnetic Resonance (NMR) Spectroscopy
5. Data Analysis
6. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carletti, G.; Nervo, G.; Cattivelli, L. Flavonoids and melanins: A common strategy across two kingdoms. Int. J. Biol. Sci. 2014, 10, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanin and melanin-related polymers as materials with biomedical and biotechnological applications-cuttlefish ink and mussel foot proteins as inspired biomolecules. Int. J. Mol. Sci. 2017, 18, 1561. [Google Scholar] [CrossRef] [PubMed]
- Al Khatib, M.; Harir, M.; Costa, J.; Baratto, M.C.; Schiavo, I.; Trabalzini, L.; Pollini, S.; Rossolini, G.M.; Basosi, R.; Pogni, R. Spectroscopic characterization of natural melanin from Streptomyces cyaneofuscatus strain and comparison with melanin enzymetically synthesized by tyrosinase and laccase. Molecules 2018, 23, 916. [Google Scholar] [CrossRef] [PubMed]
- Kordjazi, T.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R.; Restaino, O.F. Streptomycetes as microbial cell factories for the bio-technological production of melanin. Int. J. Mol. Sci. 2024, 25, 3013. [Google Scholar] [CrossRef]
- Mbonyiryivuze, A.; Omollo, I.; Ngom, B.D.; Dhlamini, S.M.; Park, E.; Maaza, M. Natural dye sensitizer for Grätzel cells: Sepia melanin. Phys. Mater. Chem. 2015, 3, 1–6. [Google Scholar]
- Manini, P.; Lucci, V.; Lino, V.; Sartini, S.; Rossella, F.; Falco, G.; Chiappe, C.; d’Ischia, M. Synthetic mycomelanin thin films as emergent bio-inspired interfaces controlling the fate of embryonic stem cells. J. Mater. Chem. B 2020, 8, 4412–4418. [Google Scholar] [CrossRef]
- Tran-Ly, A.N.; Reyes, C.; Schwarze, F.W.M.R.; Ribera, J. Microbial production of melanin and its various applications. World J. Microbiol. Biotechnol. 2020, 36, 170. [Google Scholar] [CrossRef]
- Barbuto Ferraiuolo, S.; Cammarota, M.; Schiraldi, C.; Restaino, O.F. Streptomycetes as platform for biotechnological production processes of drugs. Appl. Microbiol. Biotechnol. 2021, 105, 551–568. [Google Scholar] [CrossRef]
- Pralea, I.-E.; Moldovan, R.-C.; Petrache, A.-M.; Ilies, M.; Heghes, S.-C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From extraction to advanced analytical methods: The challenges of melanin analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef]
- Claus, H.; Decker, H. Bacterial tyrosinases. Syst. Appl. Microbiol. 2006, 29, 3–14. [Google Scholar] [CrossRef]
- Funa, N.; Funabashi, M.; Ohnishi, Y.; Horinouchi, S. Biosynthesis of hexahydroxyperylenequinone melanin via oxidative aryl coupling by cytochrome P-450 in Streptomyces griseus. J. Bacteriol. 2005, 187, 8149–8155. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.-Y.; Jang, S.; Sudheer, P.D.V.N.; Choi, K.-Y. Microbial production of melanin pigments from caffeic acid and L-tyrosine using Streptomyces glaucescens and FCS-ECH-expressing Escherichia coli. Int. J. Mol. Sci. 2021, 22, 2413. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Chen, B.F.; Wu, S.Y.; Leu, W.M.; Lin, J.J.; Chen, C.W.; Lo, S.C. A trans-acting gene is required for the phenotypic expression of a tyrosinase gene in Streptomyces. Gene 1988, 65, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Leu, W.M.; Chen, L.Y.; Liaw, L.L.; Lee, Y.H. Secretion of the Streptomyces tyrosinase is mediated through its trans-activator protein, MelC1. J. Biol. Chem. 1992, 267, 20108–20113. [Google Scholar] [CrossRef] [PubMed]
- Liaw, L.L.; Lee, Y.H. Histidine residues 102 and 117 of MelC1 play different roles in the chaperone function for Streptomyces apotyrosinase. Biochem. Biophys. Res. Commun. 1995, 214, 447–453. [Google Scholar] [CrossRef]
- Tseng, H.C.; Lin, C.K.; Hsu, B.J.; Leu, W.M.; Lee, Y.H.; Chiou, S.J.; Hu, N.T.; Chen, C.W. The melanin operon of Streptomyces antibioticus: Expression and use as a marker in gram-negative bacteria. Gene 1990, 86, 123–128. [Google Scholar] [CrossRef]
- Chen, L.Y.; Leu, W.M.; Wang, K.T.; Lee, Y.H. Copper transfer and activation of the Streptomyces apotyrosinase are mediated through a complex formation between apotyrosinase and its trans-activator MelC1. J. Biol. Chem. 1992, 267, 20100–20107. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Lee, Y.H. Roles of copper ligands in the activation and secretion of Streptomyces tyrosinase. J. Biol. Chem. 1998, 273, 19243–19250. [Google Scholar] [CrossRef]
- Barbuto Ferraiuolo, S.; Restaino, O.F.; Gutiérrez-del-Río, I.; Ventriglia, R.; Cammarota, M.; Villar, C.; Lombó, F.; Schiraldi, C. Optimization of pre-inoculum, fermentation process parameters and precursor supplementation conditions to enhance apigenin production by a recombinant Streptomyces albus strain. Fermentation 2021, 7, 161. [Google Scholar] [CrossRef]
- Dholakiya, R.N.; Kumar, M.A.; Mody, K.H. Production and characterization of melanin from Streptomyces cavourensis strain RD8 using response surface optimization. Environ. Pollut. Prot. 2017, 2, 168–178. [Google Scholar]
- El-Ahmady El-Naggar, N.; El-Ewasy, S.M. Bioproduction, characterization, anticancer and antioxidant activities of extra-cellular melanin pigment produced by newly isolated microbial cell factories Streptomyces glaucescens NEAE-H. Sci. Rep. 2017, 7, 42129. [Google Scholar]
- Madhusudhan, D.; Zainab Mazhari, B.B.; Dastager, S.G.; Agsar, D. Production and cytotoxicity of extracellular insoluble and droplets of soluble melanin by Streptomyces lusitans DMZ-3. Biomed. Res. Int. 2014, 2014, 306895. [Google Scholar] [CrossRef] [PubMed]
- Restaino, O.F.; Scognamiglio, M.; Mirpoor, S.F.; Cammarota, M.; Ventriglia, R.; Giosafatto, C.V.L.; Fiorentino, A.; Porta, R.; Schiraldi, C. Enhanced Streptomyces roseochromogenes melanin production by using the marine renewable source Posidonia oceanica egagropili. Appl. Microbiol. Biotechnol. 2022, 106, 7265–7283. [Google Scholar] [CrossRef] [PubMed]
- Tarangini, K.; Mishra, S. Production of melanin by soil microbial isolate on fruit waste extract: Two step optimization of key parameters. Biotechnol. Rep. 2014, 4, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Rao, Z.; Yang, T.; Yang, T.; Man, Z.; Xu, M.; Zhang, X. High-level production of melanin by a novel isolate of Strep-tomyces kathirae. FEMS Microbiol. Lett. 2014, 357, 85–91. [Google Scholar] [CrossRef]
- Sivaperumal, P.; Kamala, K.; Rajaram, R.; Mishra, S.S. Melanin from marine Streptomyces sp. (MVCS13) with potential effect against ornamental fish pathogens of Carassius auratus (Linnaeus, 1758). Biocatal. Agric. Biotechnol. 2014, 3, 134–141. [Google Scholar] [CrossRef]
- Yousif, A.; Zhang, J.; Mulcahy, F.; Singh, O.V. Bio-economics of melanin biosynthesis using electromagnetic field resistant Streptomyces sp.-EF1 isolated from cave soil. Ann. Microbiol. 2015, 65, 1573–1582. [Google Scholar] [CrossRef]
- Srinivasan, M.; Merlyn Keziah, S.; Hemalatha, M.; Subathra Devi, C. Pigment from Streptomyces bellus MSA1 isolated from marine sediments. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 022049. [Google Scholar] [CrossRef]
- Kazi, Z.; Hungund, B.S.; Yaradoddi, J.S.; Banapurmath, R.; Yusuf, A.A.; Kishore, K.L.; Soudagar, M.E.M.; Khan, T.M.Y.; Elfasakhany, A.; Buyondo, K.A. Production, characterization, and antimicrobial activity of pigment from Streptomyces species. J. Biomater. Appl. 2022, 2022, 3962301. [Google Scholar] [CrossRef]
- Rudrappa, M.; Kumar, M.S.; Kumar, R.S.; Almansour, A.I.; Perumal, K.; Nayaka, S. Bioproduction, purification, and phy-s-icochemical characterization of melanin from Streptomyces sp. strain MR28. Microbiol. Res. 2022, 263, 12713. [Google Scholar] [CrossRef]
- El-Zawawy, N.A.; Kenawy, E.-R.; Ahmed, S.; El-Sapagh, S. Bioproduction and optimization of newly characterized melanin pigment from Streptomyces djakartensis NSS-3 with its anticancer, antimicrobial, and radioprotective properties. Microb. Cell Fact. 2024, 23, 23. [Google Scholar] [CrossRef] [PubMed]
- Polapally, R.; Mansani, M.; Rajkumar, K.; Burgula, S.; Hameeda, B.; Alhazmi, A.; Bantun, F.; Almalki, A.H.; Haque, S.; El Enshasy, H.A.; et al. Melanin pigment of Streptomyces puniceus RHPR9 exhibits antibacterial, antioxidant, and anticancer activities. PLoS ONE 2022, 17, e0197709. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Li, Y. Metal ions driven production, characterization and bioactivity of extracellular melanin from Streptomyces sp. ZL-24. Int. J. Biol. Macromol. 2019, 123, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Restaino, O.F.; Manini, P.; Kordjazi, T.; Alfieri, M.L.; Rippa, M.; Mariniello, L.; Porta, R. Biotechnological production and characterization of extracellular melanin by Streptomyces nashvillensis. Microorganisms 2024, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Krysenko, S. Impact of nitrogen-containing compounds on secondary metabolism in Streptomyces spp.—A source of metabolic engineering strategies. SynBio 2023, 1, 204–225. [Google Scholar] [CrossRef]
- Ghadge, V.; Kumar, P.; Maity, T.K.; Prasad, K.; Shinde, P.B. Facile alternative sustainable process for the selective extraction of microbial melanin. ACS Sustain. Chem. Eng. 2022, 10, 2681–2688. [Google Scholar] [CrossRef]
- Restaino, O.F.; Marseglia, M.; De Castro, C.; Diana, P.; Forni, P.; Parrilli, M.; De Rosa, M.; Schiraldi, C. Biotechnological transformation of hydrocortisone to 16α-hydroxy hydrocortisone by Streptomyces roseochromogenes. Appl. Microbiol. Biotechnol. 2014, 98, 1291–1299. [Google Scholar] [CrossRef]
- Li, C.; Ji, C.; Tang, B. Purification, characterization and biological activity of melanin from Streptomyces sp. FEMS Microbiol. Lett. 2018, 365, fny077. [Google Scholar] [CrossRef]
- Masterman, D.; Redding, K. Advanced Biology with Vernier: Experiments for AP and College General Biology; Vernier: Beaverton, OR, USA, 2010; pp. 130–136. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Restaino, O.F.; Kordjazi, T.; Tancredi, F.; Manini, P.; Lanzillo, F.; Raganati, F.; Marzocchella, A.; Porta, R.; Mariniello, L. Metal Ion Supplementation to Boost Melanin Production by Streptomyces nashvillensis. Int. J. Mol. Sci. 2025, 26, 416. https://doi.org/10.3390/ijms26010416
Restaino OF, Kordjazi T, Tancredi F, Manini P, Lanzillo F, Raganati F, Marzocchella A, Porta R, Mariniello L. Metal Ion Supplementation to Boost Melanin Production by Streptomyces nashvillensis. International Journal of Molecular Sciences. 2025; 26(1):416. https://doi.org/10.3390/ijms26010416
Chicago/Turabian StyleRestaino, Odile Francesca, Talayeh Kordjazi, Francesco Tancredi, Paola Manini, Fabiana Lanzillo, Francesca Raganati, Antonio Marzocchella, Raffaele Porta, and Loredana Mariniello. 2025. "Metal Ion Supplementation to Boost Melanin Production by Streptomyces nashvillensis" International Journal of Molecular Sciences 26, no. 1: 416. https://doi.org/10.3390/ijms26010416
APA StyleRestaino, O. F., Kordjazi, T., Tancredi, F., Manini, P., Lanzillo, F., Raganati, F., Marzocchella, A., Porta, R., & Mariniello, L. (2025). Metal Ion Supplementation to Boost Melanin Production by Streptomyces nashvillensis. International Journal of Molecular Sciences, 26(1), 416. https://doi.org/10.3390/ijms26010416










