The Nrf2 Inhibitor Brusatol Promotes Human Osteosarcoma (MG63) Growth and Blocks EB1089-Induced Differentiation
Abstract
1. Introduction
2. Results
2.1. Bru Promotes the Growth of MG63 Cells
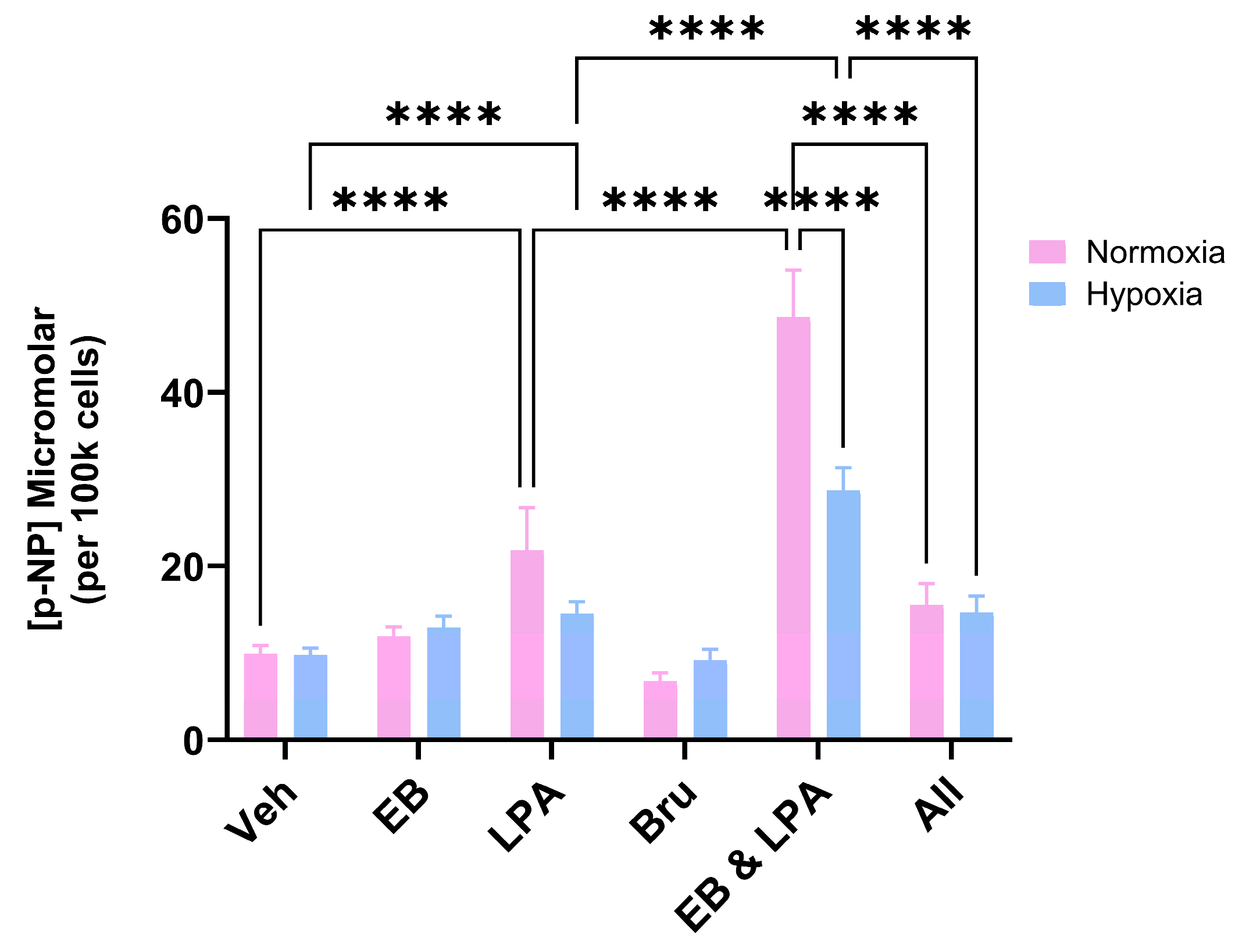
2.2. Bru Inhibits MG63 Differentiation
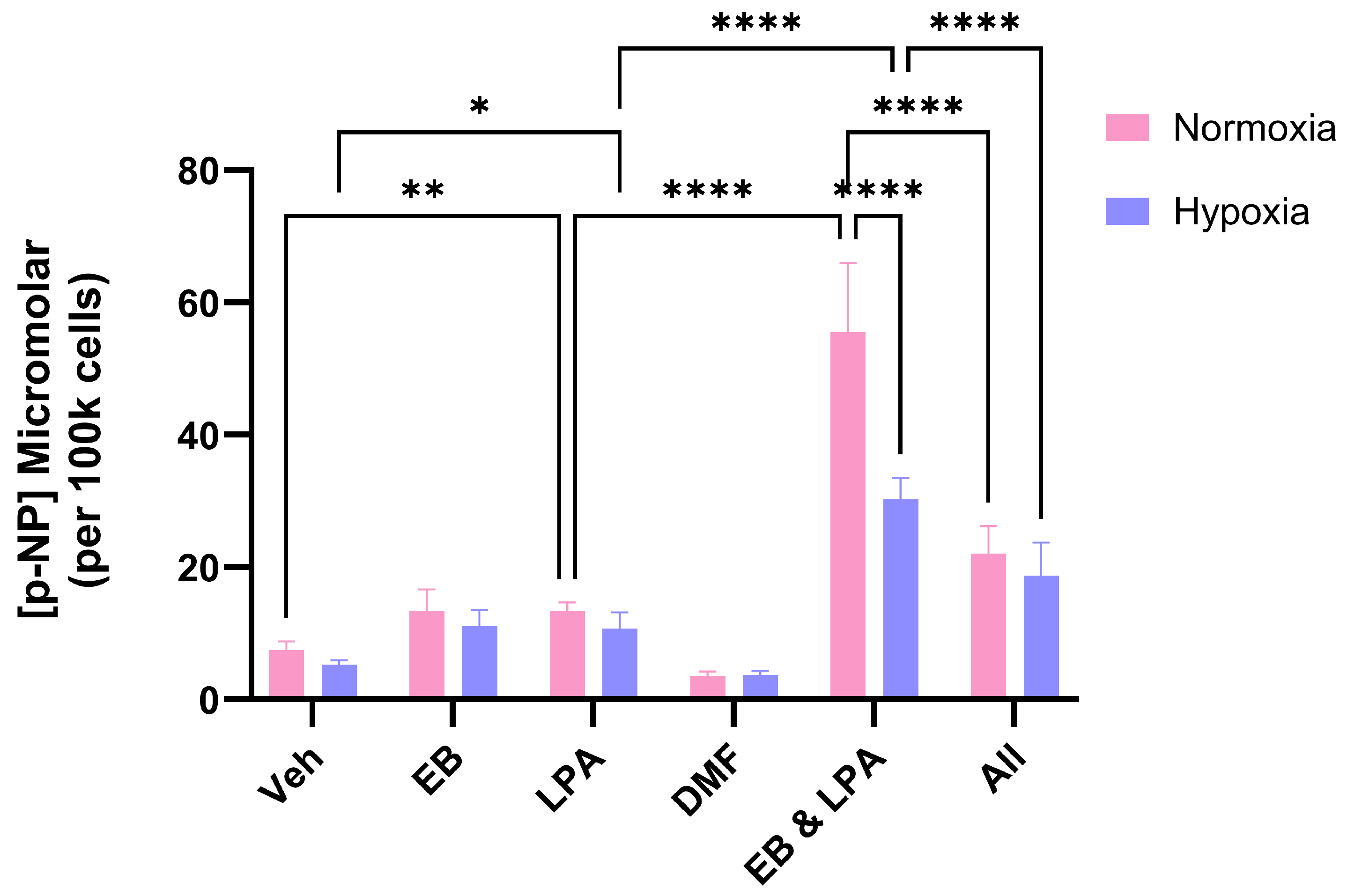
2.3. The Influence of the Nrf2 Activator Dimethyl Fumarate (DMF) on MG63 Growth and Differentiation
3. Discussion
4. Materials and Methods
4.1. General
4.2. Human Osteosarcoma (OS) Cells
4.3. Hypoxia Treatments
4.4. Cell Number
4.5. ALP Activity
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef]
- Coventry, M.B.; Dahlin, D.C. Osteogenic sarcoma; a critical analysis of 430 cases. J. Bone Jt. Surg. Am. 1957, 39, 741–758. [Google Scholar] [CrossRef]
- Gianferante, D.M.; Mirabello, L.; Savage, S.A. Germline and somatic genetics of osteosarcoma—Connecting aetiology, biology and therapy. Nat. Rev. Endocrinol. 2017, 13, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Abarrategi, A.; Tornin, J.; Martinez-Cruzado, L.; Hamilton, A.; Martinez-Campos, E.; Rodrigo, J.P.; Gonzalez, M.V.; Baldini, N.; Garcia-Castro, J.; Rodriguez, R. Osteosarcoma: Cells-of-Origin, Cancer Stem Cells, and Targeted Therapies. Stem Cells Int. 2016, 2016, 3631764. [Google Scholar] [CrossRef] [PubMed]
- Walia, M.K.; Castillo-Tandazo, W.; Mutsaers, A.J.; Martin, T.J.; Walkley, C.R. Murine models of osteosarcoma: A piece of the translational puzzle. J. Cell Biochem. 2018, 119, 4241–4250. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.A.; Hawkins, C.J. Recent and Ongoing Research into Metastatic Osteosarcoma Treatments. Int. J. Mol. Sci. 2022, 23, 3817. [Google Scholar] [CrossRef]
- Cai, S.J.; Liu, Y.; Han, S.; Yang, C. Brusatol, an NRF2 inhibitor for future cancer therapeutic. Cell Biosci. 2019, 9, 45. [Google Scholar] [CrossRef]
- Xiang, Y.; Ye, W.; Huang, C.; Lou, B.; Zhang, J.; Yu, D.; Huang, X.; Chen, B.; Zhou, M. Brusatol inhibits growth and induces apoptosis in pancreatic cancer cells via JNK/p38 MAPK/NF-kb/Stat3/Bcl-2 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 487, 820–826. [Google Scholar] [CrossRef]
- Xi, W.; Zhao, C.; Wu, Z.; Ye, T.; Zhao, R.; Jiang, X.; Ling, S. Brusatol’s anticancer activity and its molecular mechanism: A research update. J. Pharm. Pharacol. 2024, 76, 753–762. [Google Scholar] [CrossRef]
- Ren, D.; Villeneuve, N.F.; Jiang, T.; Wu, T.; Lau, A.; Toppin, H.A.; Zhang, D.D. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc. Natl. Acad. Sci. USA 2011, 108, 1433–1438. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Wu, W.; Dang, H.; Wang, B. Expression of the Nrf2 and Keap1 proteins and their clinical significance in osteosarcoma. Biochem. Biophys. Res. Commun. 2016, 473, 42–46. [Google Scholar] [CrossRef]
- Peng, X.; Feng, J.; Yang, H.; Xia, P.; Pu, F. Nrf2: A key regulator in chemoradiotherapy resistance of osteosarcoma. Genes Dis. 2025, 12, 101335. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, T.; Matsumoto, T.; Itoi, A.; Kawana, A.; Nishiyama, T.; Ogura, K.; Hiratsuka, A. Enhanced sensitivity of A549 cells to the cytotoxic action of anticancer drugs via suppression of Nrf2 by procyanidins from Cinnamomi Cortex extract. Biochem. Biophys. Res. Commun. 2011, 413, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wang, H.; Fan, L.; Wu, X.; Xin, A.; Ren, H.; Wang, X.J. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic. Biol. Med. 2011, 50, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Pierrevelcin, M.; Fuchs, Q.; Lhermitte, B.; Messé, M.; Guérin, E.; Weingertner, N.; Martin, S.; Lelong-Rebel, I.; Nazon, C.; Dontenwill, M.; et al. Focus on hypoxia-related pathways in pediatric osteosarcomas and their druggability. Cells 2020, 9, 1998. [Google Scholar] [CrossRef]
- Belisario, D.C.; Kopecka, J.; Pasino, M.; Akman, M.; De Smaele, E.; Donadelli, M.; Riganti, C. Hypoxia Dictates Metabolic Rewiring of Tumors: Implications for Chemoresistance. Cells 2020, 9, 2598. [Google Scholar] [CrossRef]
- Trump, D.L. Calcitriol and cancer therapy: A missed opportunity. Bone Rep. 2018, 9, 110–119, Erratum in: Bone Rep. 2021, 14, 101084. [Google Scholar] [CrossRef]
- Duffy, M.J.; Murray, A.; Synnott, N.C.; O’Donnovan, N.; Crown, J. Vitamin D analogues: Potential use in cancer treatment. Crit. Rev. Oncol. Hematol. 2017, 112, 190–197. [Google Scholar] [CrossRef]
- Elkhenany, H.; Elkodous, M.A.; Mansell, J.P. Ternary nanocomposite potentiates the lysophosphatidic acid effect on human osteoblast (MG63) maturation. Nanomedicine 2023, 18, 1459–1475. [Google Scholar] [CrossRef]
- Beresford, J.N.; Gallagher, J.A.; Russell, R.G.G. 1,25-dihydroxyvitamin D3 and human bone-derived cells in vitro: Effects on alkaline phosphatase, type I collagen and proliferation. Endocrinology 1986, 119, 1776–1785. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Romano, P.R.; Park, K.-Y. Regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D3 in human osteosarcoma cells. J. Biol. Chem. 1988, 263, 18938–18945. [Google Scholar] [CrossRef] [PubMed]
- Unni, S.; Deshmukh, P.; Krishnappa, G.; Kommu, P.; Padmanabhan, B. Structural insights into the multiple binding modes of Dimethyl Fumarate (DMF) and its analogs to the Kelch domain of Keap1. FEBS J. 2020, 288, 1599–1613. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.W. Osteosarcoma: Diagnosis, treatment, and emerging opportunities. Hematol. Oncol. Clin. N. Am. 2025, 39, 749–760. [Google Scholar] [CrossRef]
- Pouremamali, F.; Pouremamali, A.; Dadashpour, M.; Soozangar, N.; Jeddi, F. An update of Nrf2 activators and inhibitors in cancer prevention/promotion. Cell Commun. Signal. 2022, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Yu, S.; Lin, L.; Chen, Y.; Wu, Z.; Fang, X.; Zhang, W. Brusatol inhibits malignant phenotypes and lipid metabolism of osteosarcoma cells by regulating PI3K/AKT and MAPK pathways. Phytomedicine 2025, 139, 156464. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, H.; Jiang, X.; Huang, N.; Wei, Q. Hypoxia-Induced miR-378a-3p Inhibits Osteosarcoma Invasion and Epithelial-to-Mesenchymal Transition via BYSL Regulation. Front. Genet. 2022, 12, 804952. [Google Scholar] [CrossRef]
- Bae, T.; Hallis, S.P.; Kwak, M.-K. Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer. Exp. Mol. Med. 2024, 56, 501–514. [Google Scholar] [CrossRef]
- Evans, H.; Greenhough, A.; Perry, L.; Lasanta, G.; Gonzalez, C.M.; Mourino, A.; Mansell, J.P. Hypoxia Compromises the Differentiation of Human Osteosarcoma Cells to CAR-R, a Hydroxylated Derivative of Lithocholic Acid and Potent Agonist of the Vitamin D Receptor. Int. J. Mol. Sci. 2025, 26, 365. [Google Scholar] [CrossRef]
- Bresciani, G.; Manai, F.; Davinelli, S.; Tucci, P.; Saso, L.; Amadio, M. Novel potential pharmacological applications of dimethyl fumarate—An overview and update. Front. Pharmacol. 2023, 14, 1264842. [Google Scholar] [CrossRef]
- Nachliely, M.; Trachtenberg, A.; Khalfin, B.; Nalbandyan, K.; Cohen-Lahav, M.; Yasuda, K.; Sakaki, T.; Kutner, A.; Danilenko, M. Dimethyl fumarate and vitamin D derivatives cooperatively enhance VDR and Nrf2 signaling in differentiating AML cells in vitro and inhibit leukemia progression in a xenograft mouse model. J. Steroid Biochem. Mol. Biol. 2019, 188, 8–16. [Google Scholar] [CrossRef]
- Jramne-Saleem, Y.; Danilenko, M. Roles of Glutathione and AP-1 in the Enhancement of Vitamin D-Induced Differentiation by Activators of the Nrf2 Signaling Pathway in Acute Myeloid Leukemia Cells. Int. J. Mol. Sci. 2024, 25, 2284. [Google Scholar] [CrossRef]
- Masuda, H.; Miller, C.; Koeffler, H.P.; Battifora, H.; Cline, M.J. Rearrangement of the p53 gene in human osteogenic sarcomas. Proc. Natl. Acad. Sci. USA 1987, 84, 7716–7719. [Google Scholar] [CrossRef]
- Hamad, S.H.; Sellers, R.S.; Wamsley, N.; Zolkind, P.; Schrank, T.P.; Major, M.B.; Weissman, B.E. NRF2 Activation in Trp53;p16-deficient Mice Drives Oral Squamous Cell Carcinoma. Cancer Res. Commun. 2024, 4, 487–495. [Google Scholar] [CrossRef]
- Han, J.; Yang, K.; An, J.; Jiang, N.; Fu, S.; Tang, X. The Role of NRF2 in Bone Metabolism—Friend or Foe? Front. Endocrinol. 2022, 13, 813057. [Google Scholar] [CrossRef]



| Source of Brusatol | Experiment 1 | Experiment 2 | Experiment 3 |
|---|---|---|---|
| Merck | 449.5 ± 14.9 *** | 452.8 ± 11.5 *** | 526.5 ± 34.2 *** |
| Cayman Chemical | 463.8 ± 15.5 *** | 458.4 ± 29.1 *** | 536.9 ± 26.7 *** |
| TargetMol Chemicals Inc | 440.0 ± 3.5 *** | 429.6 ± 15.9 *** | 489.3 ± 16.2 *** |
| Vehicle control | 270.6 ± 9.3 | 277.4 ± 13.7 | 301.5 ± 5.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stephens, E.; Greenhough, A.; Mansell, J.P. The Nrf2 Inhibitor Brusatol Promotes Human Osteosarcoma (MG63) Growth and Blocks EB1089-Induced Differentiation. Int. J. Mol. Sci. 2025, 26, 9675. https://doi.org/10.3390/ijms26199675
Stephens E, Greenhough A, Mansell JP. The Nrf2 Inhibitor Brusatol Promotes Human Osteosarcoma (MG63) Growth and Blocks EB1089-Induced Differentiation. International Journal of Molecular Sciences. 2025; 26(19):9675. https://doi.org/10.3390/ijms26199675
Chicago/Turabian StyleStephens, Emily, Alexander Greenhough, and Jason P. Mansell. 2025. "The Nrf2 Inhibitor Brusatol Promotes Human Osteosarcoma (MG63) Growth and Blocks EB1089-Induced Differentiation" International Journal of Molecular Sciences 26, no. 19: 9675. https://doi.org/10.3390/ijms26199675
APA StyleStephens, E., Greenhough, A., & Mansell, J. P. (2025). The Nrf2 Inhibitor Brusatol Promotes Human Osteosarcoma (MG63) Growth and Blocks EB1089-Induced Differentiation. International Journal of Molecular Sciences, 26(19), 9675. https://doi.org/10.3390/ijms26199675






