Bioactive Compounds from Vegetal Organs of Taraxacum Species (Dandelion) with Biomedical Applications: A Review
Abstract
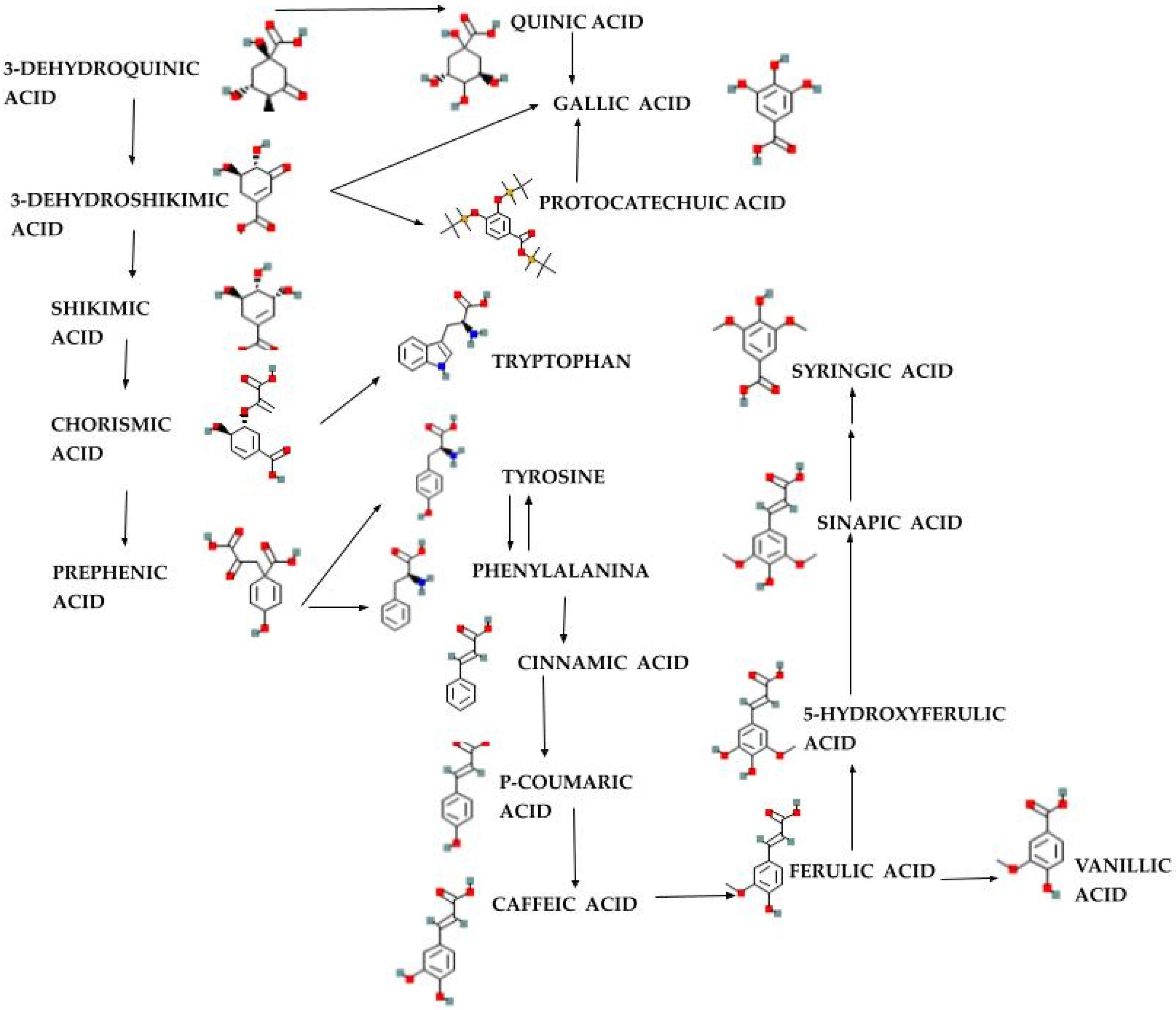
1. Introduction
2. General Description of the Species
2.1. Taraxacum Plant Characterization
2.2. Phytochemistry
| Vegetable Organs | Taraxacum (T.) Species | Bioactive Compounds | Chemical Formula | Diseases Involved | References |
|---|---|---|---|---|---|
| Root | Taraxacum officinale | ainsloside | C37H62O16 | antitumor, antioxidant | [49] |
| beta-carotene | C40H56 | antitumor, antioxidant, immunostimulant | [32] | ||
| caffeic acid | C9H8O4 | anti-inflammatory, antitumor, antioxidant | [50,51] | ||
| caffeoylquinic acid | C16H18O9 | anti-influenza | [52] | ||
| chicoric acid | C22H18O12 | antioxidant, anti-inflammatory | [53] | ||
| cyclo-artenol | C30H50O | anti-inflammatory, antitumor, antioxidant | [54] | ||
| faradiol | C30H50O2 | anti-inflammatory | [55] | ||
| ferulic acid | C10H10O4 | anticarcinogenic, antioxidant, antimicrobial, hepatoprotective | [56] | ||
| inulin | C228H382O191 | kidney diseases, antibacterial | [57] | ||
| isterine | C21H30O9 | anti-inflammatory | [58] | ||
| lupeol | C30H50O | anti-inflammatory, antitumor, antidiabetic, heart diseases | [59] | ||
| hydroxybenzoic acid | C7H6O3 | antioxidant | [60] | ||
| monocaffeoyltartaric acid | C13H12O9 | antioxidant, anti-inflammatory | [53] | ||
| p-coumaric acid | C9H8O3 | anti-inflammatory, antitumor, antioxidant | [54] | ||
| protocatechuic acid | C7H6O4 | anti-inflammatory | [55] | ||
| stigmasterol | C29H48O | antioxidant, antitumor, antimicrobial, hepatoprotective | [56] | ||
| syringin | C9H10O5 | kidney diseases, anti-bacterial | [57] | ||
| taraxalisin | 67-kD glycoprotein | anti-inflammatory | [58] | ||
| taraxasterol | C30H50O | anti-inflammatory, antitumor, antidiabetic, for heart diseases | [59] | ||
| taraxerol | C30H50O | antioxidant | [60] | ||
| taraxicinic acid | C15H18O4 | anti-inflammatory | [58] | ||
| tetrahydroridentin B | C15H24O | antimicrobial, anti-inflammatory | [55] | ||
| umbelliferone | C9H6O3 | anti-inflammatory, antihyperglycemic, antitumor, antibacterial, antifungal | [60] | ||
| vanillic acid | C8H8O4 | anti-inflammatory, antioxidant, cytoprotective, neurological disorders | [26,61] | ||
| 3-formyl indole | C9H7NO | anti-inflammatory, antitumor, antioxidant, antidiabetic | [29,62] | ||
| T. fomosanum | beta-carotene | C40H56 | antitumor, antioxidant, immunostimulant | [63] | |
| caffeic acid | C9H8O4 | anti-inflammatory, antitumor, antioxidant | [64] | ||
| caffeoylquinic acid | C16H18O9 | anti-influenza | [64] | ||
| caftaric acid | C13H12O9 | antioxidant, antidiabetic | [64] | ||
| chicoric acid | C22H18O12 | antioxidant, anti-inflammatory, antidiabetic | |||
| Root | T. fomosanum | chlorogenic acid | C16H18O9 | anti-inflammatory | [64] |
| luteolin-7-O-glucoside | C21H20O11 | anti-inflammatory, anticancer, antidiabetic | [64] | ||
| nicotinamide | C6H6N2O | anticancer, skin diseases | [29] | ||
| protocatechuic acid | C7H6O4 | anti-inflammatory | [55] | ||
| stigmasterol | C29H48O | antitumor, antioxidant, antimicrobial | [29] | ||
| syringic acid | C9H10O5 | antimicrobial, antidiabetic, antitumor | [29,65] | ||
| taraxafolide, taraxafolin-B | Substance SID: 275594471 | antibacterial, anti-inflammatory | [29,66] | ||
| vanillic acid | C8H8O4 | antioxidant, cytoprotective | [29,67] | ||
| T. campylodes | apigenin | C15H10O5 | antioxidant, antitumor, antiviral, antibacterial, nervous, kidney diseases | [68,69] | |
| chicoric acid | C22H18O12 | antidiabetic, antioxidant, anti-inflammatory | [70] | ||
| chlorogenic acid | C16H18O9 | anti-inflammatory | [70] | ||
| isoquercitrin | C21H20O12 | antitumor, antioxidant, antidiabetic, cardiovascular disorders | [68] | ||
| luteolin | C15H10O6 | antiviral, antidiabetic, anti-asthmatic, antitumor | [68] | ||
| taraxacin | C15H14O3 | liver and kidney disorders, antitumor | [71] | ||
| T. mongolicum | ainsloside | C37H62O16 | antioxidant, antitumor | [72] | |
| apigenin | C15H10O5 | antitumor, antioxidant, anti-inflammatory | [72] | ||
| baicalein | C15H10O5 | anti-inflammatory, cardiovascular, respiratory and gastrointestinal disorders | [73] | ||
| caffeic acid | C9H8O4 | anti-inflammatory, antitumor, antioxidant | [72] | ||
| chicoric acid | C22H18O12 | antidiabetic, antioxidant | [72] | ||
| chlorogenic acid | C16H18O | anti-inflammatory | [72] | ||
| galacturonic acid | C6H10O9 | anti-inflammatory, gastrointestinal disorders | [74] | ||
| glucose | C6H12O6 | ubiquitous energy source | [74] | ||
| heperetin-5′-O-β-rhamno-glucoside | C22H24O11 | antioxidant, antitumor | [73] | ||
| kaempferol-3-glucoside | C21H20O11 | anti-inflammatory, antioxidant, antiviral, antiallergic | [73] | ||
| lutein | C40H56O2 | anticarcinogenic, photo-protector, antioxidant, anti-inflammatory | [72] | ||
| p-coumaric acid | C9H8O3 | anticancer, antiulcer, antioxidant, anti-inflammatory, anti-mutagenic | [72] | ||
| quercetin | C15H10O7 | anti-inflammatory, antiallergic, antitumoral, antioxidant | [73] | ||
| T. coreanum | caffeic acid | C9H8O4 | anti-inflammatory, antitumor, antioxidant | [75] | |
| decursinol | C14H14O4 | anti-inflammatory, analgesic, antineoplastic agent | [30] | ||
| inositol | C6H12O6 | nervous and metabolic disorders, antidiabetic | [30] | ||
| Root | T. coreanum | isoferulic acid | C10H10O4 | antidiabetic | [30] |
| pinoresinol | C20H22O6 | hypoglycaemic agent, increase apoptosis | [30] | ||
| syringaldehyde | C9H10O4 | antioxidant | [30] | ||
| taraxathin | C40H56O3 | membrane stabilizer, flavoring agent | [75] | ||
| taraxinositols A (1), B (2) | 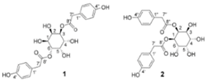 Not found in the literature | [30,76] | |||
| taraxinol | 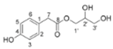 Not found in the literature | [30] | |||
| vanillic acid | C8H8O4 | antioxidant, cytoprotector | [30] | ||
| Leaves | Taraxacum officinale | anthraquinones | C14H8O2 | antiviral, immunostimulatory, diuretic, laxative, antifungal | [77] |
| apigenin-7-glucoside | C21H20O10 | anticancer, antifungal | [78] | ||
| β-branched glucomannan | C24H42O21 | antidiabetic, laxative, hypocholesterolaemiant | [79] | ||
| beta-sitosterol | C29H50O | reduction of benign prostatic hyperplasia and blood cholesterol levels | [54,80,81] | ||
| betulin | C30H50O2 | anticancer, antiobesity, antidiabetic | [82] | ||
| caffeoyl glucoside | C15H18O9 | antiviral, antitumor, antidiabetic, antifungal, antioxidant | [83] | ||
| caffeoylmalic acid | C13H12O8 | antidiabetic, antioxidant, antiproliferative, apoptotic effect | [84,85] | ||
| chicoric acid | C22H18O12 | antidiabetic, antioxidant | [51] | ||
| cichorin | C18H20O3 | antiparasitic activity | [55,86] | ||
| dodecane | C15H24O | antimicrobial | [77] | ||
| stafiatin | C15H18O3 | anti-inflammatory, antitumor | [82] | ||
| ferulic acid | C10H10O4 | antioxidant, anti-inflammatory, neuroprotective, skin disease | [87] | ||
| hesperidin | C28H34O15 | anti-inflammatory, antitumoral, antihypertensive, antihyperlipidemic | [88] | ||
| hydroxycinnamic acid | C9H8O3 | antioxidant, skin protector | [51] | ||
| kaempferol | C15H10O6 | anti-tumoral, anti-inflammatory, antioxidant, respiratory diseases | [89] | ||
| lettucenin A | C15H12O3 | antifungal, antimicrobial | [90,91] | ||
| lupeol acetate | C32H52O | anticancer, antidiabetic, anti-inflammatory and antiprotozoal | [54] | ||
| lutein | C40H56O2 | antitumoral, antioxidant, anti-inflammatory, photoprotector | [32,92] | ||
| luteolin diglycoside | C27H30O16 | antidiabetic | [83] | ||
| Leaves | T. officinale | monocaffeoyltartaric acid | C13H12O9 | antioxidant, anti-inflammatory | [38] |
| phytol | C20H40O | antioxidant, antimicrobial activity | [54] | ||
| protocatechuic acid | C7H6O4 | anti-inflammatory, antihyperglycemic, antioxidant, neuroprotector | [93] | ||
| quercetine glycosides | C21H19O12 | antioxidant, anti-inflammatory, cardiovascular disorders | [83,94] | ||
| scopoletin | C10H8O4 | antifungal, antitumoral | [95] | ||
| sesquiterpene lactones | C22H30O7 | anticancer, anti-inflammatory, antitumoral, antiviral, antibacterial, antifungal | [38] | ||
| sinapic acid | C11H12O5 | antioxidant, antimicrobial, anticancer, anti-inflammatory and antianxiety activity | [44] | ||
| stigmasterol | C29H48O | antitumor, antioxidant, antimicrobial | [96,97] | ||
| violaxanthin | C40H56O4 | anti-inflammatory, antitumoral, antioxidant | [39] | ||
| T.coreanum | chrysoeriol | C16H12O6 | anti-inflammatory, antioxidant, antitumor effects | [98,99,100,101] | |
| daucosterol | C35H60O | antitumor, anti-inflammatory, antioxidant | [99,102,103] | ||
| diosmetin | C16H12O6 | antioxidant, antineoplastic, antitumoral, antimicrobial, antidepressive | [99] | ||
| esculetin | C9H6O4 | antioxidant, antidiabetic, antitumor | [99,104] | ||
| luteolin | C15H10O6 | antiviral, antidiabetic, antiasthmatic, antitumoral | [99,105] | ||
| luteolin-7-O-glucoside | C21H20O11 | anti-inflammatory, anticancer, antidiabetic | [99,105] | ||
| sitosterol | C29H50O10 | anti-inflammatory, antioxidant, antidiabetic, antianxiety, liver-protector | [99] | ||
| taraxasteryl acetate | C32H52O2 | anticancer, anti-inflammatory, antidiabetic, anticaught and lung diseases | [99,106] | ||
| vanilinic acid | C8H8O4 | antioxidant, cytoprotector | [30] | ||
| T. campylodes | chlorogenic acid | C16H18O9 | anti-inflammatory | [70] | |
| chicoric acid | C22H18O12 | antidiabetic, antioxidant | [70] | ||
| T. mongolicum | caffeic acid | C9H8O4 | anti-inflammatory, antitumor, antioxidant | [107] | |
| esculetin | C9H6O4 | antioxidant, antidiabetic, antitumor | [108] | ||
| isoetin | C15H10O7 | antioxidant, anti-inflammatory | [109] | ||
| inositol | C6H12O6 | nervous and metabolic disorders, antidiabetic | [21] | ||
| luteolin 7-O-β-D-glucopyranoside | C21H20O11 | anti-inflammatory, anticancer, antidiabetic | [107] | ||
| stigmasterol | C29H48O | anti-inflammatory | [110] | ||
| quercetin | C15H10O7 | anti-inflammatory, antiallergic, antitumor, antioxidant | [111] | ||
| taraxasterol | C30H50O | anti-inflammatory, antitumor, antioxidant, lung disease | [110] | ||
| Flowers | T. officinale | arnidiol | C30H50O2 | anti-inflammatory | [55,94] |
| beta-carotene | C40H56 | antitumor, antioxidant, immunostimulant | [32] | ||
| chlorogenic acid | C16H18O | anti-inflammatory | [44,51,94] | ||
| chrysoeriol | C16H12O6 | antineoplastic agent, antioxidant, antimicrobial | [83] | ||
| heneicosane | CH3(CH2)19CH3 | antimicrobial, antioxidant, analgesic, antipyretic | [60] | ||
| luteoline 7-O-glucoside | C21H20O11 | anticancer, antidiabetic antioxidant | [112,113] | ||
| hydroxycinnamic acid | C9H8O3 | antioxidant, skin protector | [53,94] | ||
| monocaffeoyltartaric acid | C13H12O9 | antioxidant, anti-inflammatory | [114] | ||
| pectin | C6H10O7 | anti-inflammatory, antibacterial, antioxidant, antitumor activities | [115] | ||
| routine | C27H30O16 | antimicrobial | [83] | ||
| quercetin | C15H10O7 | anti-inflammatory, antihypertensive, anti-obesity | [83] | ||
| stigmasterol | C29H48O | anti-inflammatory | [96] | ||
| tricosane | C23H48 | antimicrobial | [37] | ||
| T.formosanum | not found in literature | ||||
| T. mongolicum | caffeic acid caffeic acid | C9H8O4 | anti-inflammatory, antitumor, antioxidant | [52] | |
| caffeoylquinic acid | C16H18O9 | anti-influenza | [52] | ||
| caftaric acid | C13H12O9 | antioxidant, antidiabetic | [52,116] | ||
| chicoric acid | C22H18O12 | antioxidant, anti-inflammatory, antidiabetic | [52] | ||
| chlorogenic acid | C16H18O9 | anti-inflammatory | [52,117] | ||
| delphinidin 3-O-glucoside | C21H21O12 | antitumor, hypolipidemic, endothelial protective | [116] | ||
| luteolin | C15H10O6 | antiviral, antidiabetic, antiasthmatic, antitumor | [52,117] | ||
| T. coreanum | adenosine | C10H13N5O4 | neuromodulator, reduce tissue injury and promote repair | [118] | |
| astragalin | C21H20O11 | anti-inflammatory, antitumor, antioxidant, neuroprotective | [118] | ||
| chicoric acid | C22H18O12 | antioxidant, anti-inflammatory, antidiabetic | [75] | ||
| isoquercitine | C21H20O12 | antiviral, anti-inflammatory, antioxidant | [118] | ||
| luteolin | C15H10O6 | antiviral, antidiabetic, anti-asthmatic, antitumor | [75] | ||
| Entire vegetal product | T. officinale | alfa and beta-amyrin | C30H50O | anti-inflammatory, analgesic, gastroprotective, hepatoprotective, antihyperglycemic, anti-obesity effects | [58,59] |
| chicoric acid | C22H18O12 | anti-inflammatory, antioxidant | [83] | ||
| monocaffeoyltartaric acid | C13H12O9 | antioxidant, anti-inflammatory | [114] | ||
| chlorogenic acid | C16H18O9 | anti-inflammatory, antioxidant, antiviral, antibacterial, antihypertensive | [83] | ||
| ferulic acid | C10H10O4 | anticarcinogenic, antioxidant, antimicrobial, hepatoprotective | [51] | ||
| taraxacin | C15H14O3 | anti-inflammatory and anticancer | [16] | ||
| vanillic acid | C8H8O4 | antioxidant, cytoprotector | [30] | ||
| T. coreanum | adenosine | C10H13N5O4 | neuromodulator, reduce tissue injury and promote repair | [118] | |
| guanosine | C10H13N5O5 | neuroprotective, reduce neuroinflammation and oxidative stress | [118] | ||
| luteolin | C15H10O6 | antiviral, antidiabetic, anti-asthmatic, antitumor | [75] | ||
| 5′-methyl-thioadenosine | C11H15N5O3S | anti-inflammatory, liver damage | [118] | ||
| quercetin | C15H10O7 | anti-inflammatory, antihypertensive, anti-obesity | [75] | ||
| taraxinic acid | C15H18O4 | anti-inflammatory | [75] | ||
| T. campylodes | austricin 8-O-β-D-glucopyranoside | C21H28O9 | anti-inflammatory, anticancer | [119,120] | |
| caftaric acid | C13H12O9 | antioxidant, antidiabetic | [119] | ||
| 3,5-di-O-caffeoyl-quinic acid | C25H24O12 | antimutagenic | [119] | ||
| chichoric acid | C22H18O12 | antidiabetic, antioxidant | [119] | ||
| chlorogenic acid | C16H18O9 | antioxidant, anti-inflammatory, antiviral, antibacterial, antihypertensive | [119] | ||
| inositol | C30H30O12 | nervous and metabolic disorders, antidiabetic | [119] | ||
| luteoline 7-O-glucoside | C21H20O11 | anticancer, antidiabetic antioxidant | [119] | ||
| taraxinic acid | C15H18O4 | anti-inflammatory | [119] | ||
| T. mongolicum | caffeic acid | C9H8O4 | anti-inflammatory, antitumor, antioxidant | [52] | |
| caffeoylquinic acid | C16H18O9 | anti-influenza | [52] | ||
| caftaric acid | C13H12O9 | antioxidant, antidiabetic | [52,116] | ||
| chicoric acid | C22H18O12 | antioxidant, anti-inflammatory, antidiabetic | [52] | ||
| chlorogenic acid | C16H18O9 | anti-inflammatory | [52,117] | ||
3. Biomedical Effects
3.1. Anti-Inflammatory Activity
3.2. Antiviral Activity
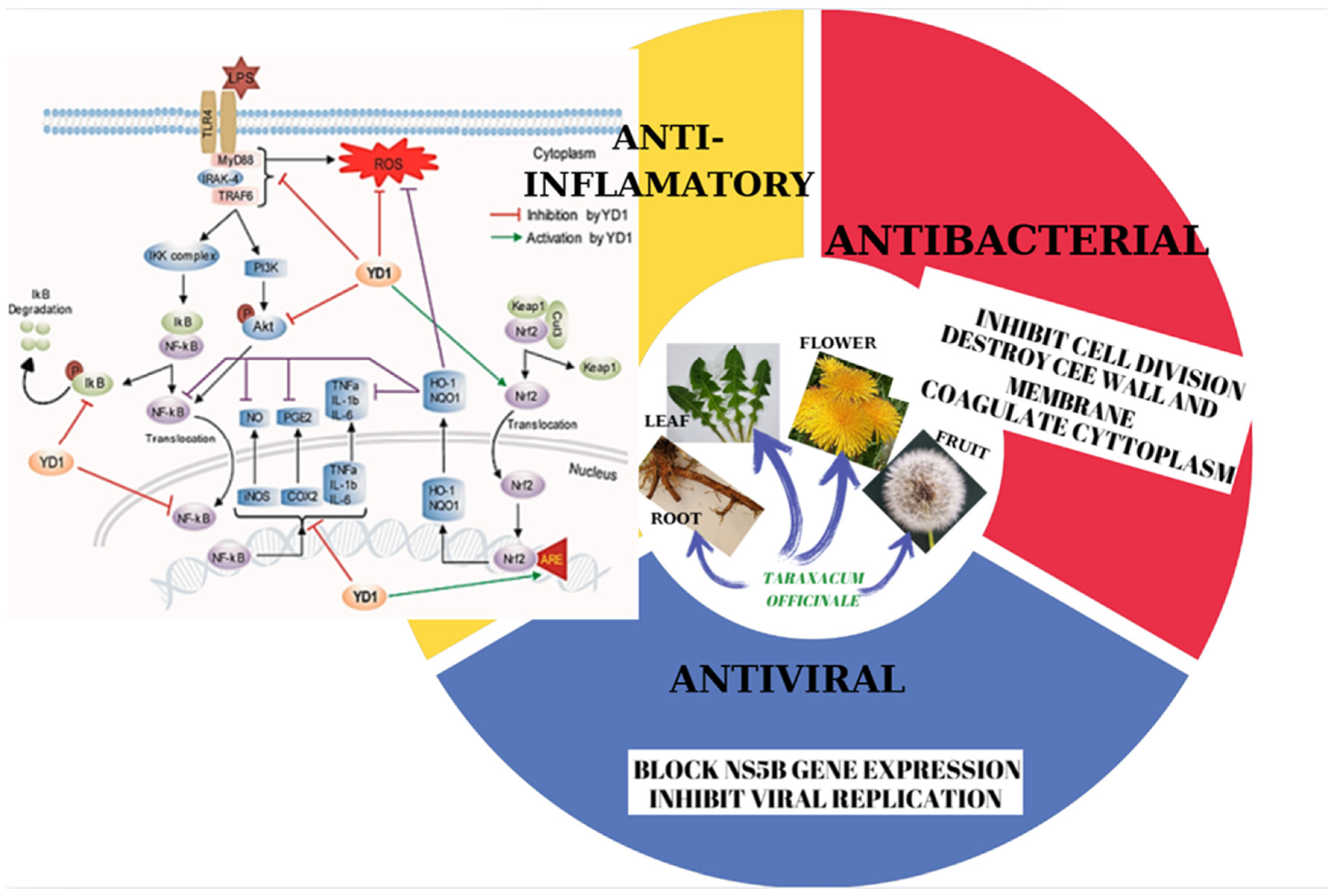
3.3. Antimicrobial Activity
| Vegetal Organ/Phytocompounds | Type of Experiment | Results | Reference |
|---|---|---|---|
| T. o. ethanolic and aqueous leaf extracts | E. coli, K. pneumoniae, P. aeruginosa, S. aureus | ethanolic leaf extract at concentrations of 200 mg/mL and 100 mg/mL inhibited only E. coli and S. aureus, while the 50 mg/mL ethanolic leaf extract inhibited only E. coli. The aqueous extract showed inhibition against E. coli at concentrations of both 200 mg/mL and 100 mg/mL | [197] |
| T. m. leaves water extract | silver nanoparticles (AgNPs) were synthesized with biological material tested on Xanthomonas oryzae pv. oryzae (Xoo) | strong bacteriostatic against the Xoo strain at 20 µg/mL with an inhibition zone of 12.4 mm, while bacterial numbers in a liquid broth (measured by OD600) decreased by 65.60% | [198] |
| T. o. ethanol extract of leaves from 3 cytotypes | B. subtilis, S. aureus, E. coli, K. pneumonia, P. aeruginosa | diploid cytotype, with the best cichoric acid concentration, exhibited the highest antibacterial activities | [199] |
| T. o. flowers and seeds polypeptides | pathogenic fungi: A. niger, A. fumigatus, P. chrysogenum, F. oxysporum, C. albicans | three mg/mL of dandelion flower concentrate increased the survival rate of the yeast test culture CFU by more than 1.5 times, while the dandelion seed extract was inactive. None of the peptides had activity against A. niger, ToAMP3 had moderate antifungal action against P. chryzogenum and a weak against A. fumigatus | [201] |
| T. o. Ethanol extract of leaves | bacterial strains—S. aureus, E. coli, S. abony enterica | exhibited antimicrobial activity against E. coli and S. abony enterica, but no antimicrobial activity against S. aureus | [202] |
| T. o. root extract | P. aeruginosa, S. aureus, E. coli, K. pneumoniae, S. pneumoniae | efficient capping or reducing agent for the synthesis of nanoparticles, which can be developed as an antibacterial agent that is highly specific for a broad range of microorganisms in order to prevent bacterial contamination | [204] |
| T. o. root extract | E. coli, P. aeruginosa, S. aureus, S. typhi, B. subtilis, L. rhamnosum | maximum values of the antibacterial properties were related to polysaccharides isolated from T. o. against four studied bacteria, and polysaccharides isolated, cyanidin-3-O-β-glucoside, N-acetylcysteine, and glutamic acid may play a major role | [206] |
3.4. Hepatoprotective Activity
| Vegetal Organ/Phytocompounds | Type of Experiment | Results | Reference |
|---|---|---|---|
| T. o. leaves water extract | carbon tetrachloride-induced liver damage in male Wister albino rats | increased enzyme activities (AST, ALT, and LDH) at the end of the 2nd, 4th, and 6th weeks of the study. By the 6th week of treatment, significant improvement and repair of genomic DNA were observed compared to the genomic DNA of untreated animals | [209] |
| T. o. root water extract | CCl4-induced hepatic fibrosis in mice | reduced the accumulation of hepatic fibrinous deposits, restored the histological structure, and regulated the expression of GFAP and α-SMA by inactivating hepatic stellate cells while also enhancing the liver’s regenerative capacity | [216] |
| T. o. extract | human hepatoma cell line, Hep G2 | decreased the cell viability by 26% and significantly increased the (TNF)-α and IL-1α | [218] |
| T. s. Ethanol extract of root | male Wistar rats with hepatocellular injuries induced by acetaminophen (APAP) | decrease in serum levels of glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), and ALPwas observed, accompanied by the prevention of histopathological alterations in the liver | [219] |
| T. o. leaves extract | APAP-induced hepatotoxicity | reduce thiobarbituric acid-reactive substance levels, prevent the reduction of sulfhydryl levels, and increase serum aspartate and alanine aminotransferase levels | [220] |
| T. o. ethanolic and n-hexane extract of leaves | CCl4-induced liver toxicity in rats | both leaf extracts reduced the concentrations of TBARS, H2O2, and nitrite, with the ethanolic leaf extract demonstrating a more effective protective effect. | [224] |
| Taraxasterol | APAP-induced acute liver injury in mice | protected liver cells from APAP-induced damage by reducing oxidative stress and inflammation and increasing Nrf2 expression. | [226] |
| Chicoric acid | High-fat diet-induced obese mice treated with chicoric acid | reduced obesity in high-fat diet-fed mice by alleviating insulin resistance, liver injury, and inflammation while boosting the antioxidant defense system | [230] |
3.5. Anti-Diabetic Activity
| Vegetal Organ/Phytocompounds | Type of Experiment | Results | Reference |
|---|---|---|---|
| T. o. methanol extract of stem, flowers, and roots | alpha-amylase and alpha-glucoside inhibition | stem exhibited the strongest antidiabetic activity, followed by the roots, while the flowers were the least effective | [242] |
| T. o. root water, methanol, Ethanol, n-hexane, ethyl acetate, and chloroform extract | IR-HepG2 cells were grown in complete medium DMEM | water extract of dandelion, rich in polysaccharides, total flavonoids, phenolic compounds, and saponins, showed significant inhibitory effects on α-glucosidase and α-amylase while enhancing hexokinase and pyruvate kinase activity | [243] |
| T. o. roots ethanol extract | chemical compounds in dandelion and burdock roots | liquid chromatography-mass spectrometry was employed to tentatively identify chemical components. Qualitative analysis confirmed the presence of inulin in the root, with higher tannin content and α-amylase activity observed in burdock compared to dandelion | [244] |
| T. o. root water extract | normoglycemic and diabetic mice evaluated at two dosages (200 mg/kg and 400 mg/kg) using antidiabetic tests and subcutaneous glucose tolerance assessments | 400 mg/kg extract effectively lowered blood glucose levels, while the aqueous extract significantly enhanced glucose uptake | [245] |
| T. o. root Ethanol extract | hypoglycemic properties of the extracts based on α-amylase activity | plant extract did not match the efficacy of acarbose, and its suitability as an antidiabetic agent remains uncertain without further in vivo studies | [246] |
| T. o. ethanolic and aqueous extract of leaves and roots | normal and streptozotocin-induced diabetic Wistar albino rats (Rattus rattus) were studied | extracts at 6% and 10% concentrations reduced fasting blood glucose levels, with the ethanolic root extract showing relatively higher potency | [247] |
| Chicoric acid | high-fat diet-induced obese C57BL/6J mice treated with chicoric acid | extract alleviated insulin resistance, liver damage, and inflammation in mice | [230] |
| T. o. and M. c. ethanol extracts | streptozotocin-nicotinamide induced diabetic rats—male Wistar albino rats and male Sprague Dawley rats | polyherbal combination demonstrated enhanced antidiabetic effects, including enzyme inhibition and blood glucose reduction, comparable to standard treatments like Glibenclamide and Metformin. | [252] |
3.6. Immunomodulatory Action
3.7. Antitumoral Activity
| Vegetal Organ/Phytocompounds | Type of Experiment | Results | Reference |
|---|---|---|---|
| T. o. taraxasterol | concanavalin A-induced acute hepatic injury in mice | inhibiting T toll-like receptors/NF-κB (-) inflammatory signaling pathway and promoting Bax/Bc1-2 anti-apoptotic signaling pathway | [171] |
| T. o. water root extract | BxPC-3 and PANC-1 pancreatic cells | induce apoptosis and autophagy in human pancreatic cancer cells with no significant effect on noncancerous cells | [217] |
| T. c. aerial part chloroform fraction | mouse peritoneal macrophages stimulated in vitro with interferon-γ and lipopolysaccharide in a mouse model of lethal septic shock | inhibited the production of (TNF)-αIL-1β, and IL-6, and increased survival by 83% | [266] |
| T. o. methanolic extract of roots | HepG2, MCF7, HCT116, and normal Hs27 cell lines | 500 µg/mL decreased the growth of the HepG2 cell line, while the effect on MCF7 and HCT116 cell lines was less pronounced, and no effect has been observed in Hs27 cell lines; enhanced the phosphorylation level of AMPK of HepG2 cells, which is considered crucial in cancer treatment | [268] |
| T. o. water extract of roots | prostate cancer cell line DU-145 cultured in Eagle’s Minimum Essential Medium | exhibit selective anticancer activity, and in addition to the chemotherapeutics, taxol, and mitoxantrone were determined to enhance the induction of apoptosis, significantly reduce the tumor burden in prostate cancer xenograft models | [269] |
| T. o. water extract of roots | female albino rats with breast cancer | regulated PI3K and Akt pathways involved in the suppression of breast cancer growth and proliferation | [270] |
| T. o. water extract of roots, leaves and flowers | MCF-7/AZ breast cancer cells and LNCaP prostate cancer cells | T. o. root extract blocks the invasion of MCF-7/AZ cells, but leaf extract blocked the invasion of LNCaP cells into collagen type I and diminishes the expansion of MCF-7/AZ cells | [271] |
| T. o. water extract of roots | gastric cancer (GS) cell lines (SGC7901, BGC823), and a normal gastric epithelium cell line (GES-1) | decrease the expression of the long non-coding RNA colon cancer-associated transcript-1 in gastric cancer cells, which is associated with reduced cell proliferation and migration | [272] |
| T. o. water extract of plant | neuroblastoma cell lines, SH-SY5Y and Kelly | caused apoptosis and loss of mitochondrial integrity, as well as an inhibition of invasion and migration | [273] |
| T. m., T. f. water extract | MDA-MB-231 human triple-negative breast cancer cells, as well as ZR75-1 and MCF-7 non-triple-negative breast cancer cells | cytotoxic effects against all three breast cancer cell lines were observed, particularly in MDA-MB-231 cells. The cytotoxicity was mediated through apoptosis, reduced cell proliferation, and disruption of the mitochondrial membrane potential | [277] |
| T. o. taraxerol | MDA-MB-231 human breast cancer cell | inhibited the migration and invasion of cells via the ERK/Slug axis | [281] |
| T. o. ethanol and dimethyl sulfoxide extract of leaves | human glioblastoma cell lines U-138 MG DMEM medium | T. o. extracts were compared with doxorubicin and were close to that. The best antitumor activity was shown by T. o. extracts prepared with DMSO (110,000 µg/L—17.3 ± 8%) | [282] |
| T. o. ethanol extract root | PANC-1 (human epithelioid carcinoma) cell in DMEM medium | 10 mg/mL of DRE produced the maximum inhibition rate, and the lowest IC50 value was reached during the 72-h treatment | [285] |
| T. o. flavone | human MM = Multiple myeloma cell line U266 | reduced the expression of matrix metalloproteinases (MMP-2 and MMP-9) while increasing the expression of tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2). Additionally, it promoted the expression of pro-apoptotic proteins and inhibited the polarization of macrophages towards the M2 phenotype by suppressing the PI3K/AKT signaling pathway | [286] |
3.8. Antioxidant Activity
4. Potential Toxicity of Taraxacum Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nascimento, C.C.H.C.; Vasconcelos, S.D.D.D.; Camacho, A.C.L.F.; Nascimento, S.F.; Oliveira, J.F.F.M.; Nogueira, R.I.; Barreto, A.S.; Diré, G.F. A Literature review on the Medicinal Properties and toxicological Profile of Costus spicatus plant. Res. J. Life Sci. Bioinform. Pharm. Chem. 2016, 2, 56–68. [Google Scholar]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Martillanes, S.; Rocha-Pimienta, J.; Cabrera-Bañegil, M.; Martín-Vertedor, D.; Delgado-Adámez, J. Application of Phenolic Compounds for Food Preservation: Food Additive and Active Packaging. In Phenolic Compounds-Biological Activity; InTech: London, UK, 2017. [Google Scholar]
- Stewart-Wade, S.M.; Neumann, S.; Collins, L.L.; Boland, G.J. The biology of Canadian weeds. 117. Taraxacum officinale GH Weber ex Wiggers. Can. J. Plant Sci. 2002, 82, 825–853. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Batsatsashvili, K.; Kikvidze, Z.; Paniagua-Zambrana, N.Y.; Khutsishvili, M.; Maisaia, I.; Tchelidze, D. Taraxacum confusum Schischk. Taraxacum officinale FH Wigg Asteraceae. In Ethnobotany of the Mountain Regions of Far Eastern Europe: Ural, Northern Caucasus; Springer: Cham, Switzerland, 2020; pp. 1–10. [Google Scholar]
- Jalili, C.; Taghadosi, M.; Pazhouhi, M.; Bahrehmand, F.; Miraghaee, S.S.; Pourmand, D.; Rashidi, I. An overview of therapeutic potentials of Taraxacum officinale (dandelion): A traditionally valuable herb with a reach historical background. World Cancer Res. J. 2020, 7, e1679. [Google Scholar]
- Wirngo, F.E.; Lambert, M.N.; Jeppesen, P.B. The Physiological Effects of Dandelion (Taraxacum officinale) in Type 2 Diabetes. Rev. Diabet. Stud. 2016, 13, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, M.; Goldberg, A.; Brinckman, J. Herbal Medicine; Expanded Commision E Monograph; Integrative Medicine Communication: Newton, MA, USA, 2000; pp. 96–97. [Google Scholar]
- Pearn, J. On “officinalis” the names of plants as one enduring history of therapeutic medicine. Vesalius 2010, 24–28. [Google Scholar]
- Odontuya, G.; Murata, T.; Sasaki, K.; Yoshizaki, F. Non-polar constituents from Taraxacum officinale Weber ex Wigg. Mong. Pharm. Pharmacol. 2015, 65–71. [Google Scholar]
- Yang, H.J.; Kim, M.J.; Kwon, D.Y.; Kang, E.S.; Kang, S.; Park, S. Gastroprotective actions of Taraxacum coreanum Nakai water extracts in ethanol-induced rat models of acute and chronic gastritis. J. Ethnopharmacol. 2017, 208, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wan, D.; Yi, F.; Luan, L. Purification, Preliminary Characterization and Hepatoprotective Effects of Polysaccharides from Dandelion Root. Molecules 2017, 22, 1409. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M.; Mahboubi, M. Hepatoprotection by dandelion (Taraxacum officinale) and mechanisms. Asian Pac. J. Trop. Biomed. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Roberts, T.H.; Matthews, K.R.; Bezerra, C.F.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; Sharopov, F.; Salehi, B.; Yousaf, Z.; Sharifi-Rad, M.; et al. Ethnobotany of the genus Taraxacum—Phytochemicals and antimicrobial activity. Phytother. Res. 2018, 32, 2131–2145. [Google Scholar] [CrossRef]
- Ramirez-Cadavid, D.A.; Valles-Ramirez, S.; Cornish, K.; Michel, F.C., Jr. Simultaneous quantification of rubber, inulin, and resins in Taraxacum kok-saghyz (TK) roots by sequential solvent extraction. Ind. Crops Prod. 2018, 122, 647–656. [Google Scholar] [CrossRef]
- Qureshi, M.N.; Stecher, G.; Bonn, G.K. Quality control of herbs: Determination of amino acids in Althaea officinalis, Matricaria chamomilla and Taraxacum officinale. Pak. J. Pharm. Sci. 2014, 27, 459–462. [Google Scholar] [PubMed]
- Lee, S.; Han, S.; Kim, H.M.; Lee, J.M.; Mok, S.Y.; Lee, S. Isolation and identification of phytochemical constituents from Taraxacum coreanum. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 73–78. [Google Scholar] [CrossRef]
- Berganayeva, G.; Kudaibergenova, B.; Litvinenko, Y.; Nazarova, I.; Sydykbayeva, S.; Vassilina, G.; Izdik, N.; Dyusebaeva, M. Medicinal plants of the flora of Kazakhstan used in the treatment of skin diseases. Molecules 2023, 28, 4192. [Google Scholar] [CrossRef] [PubMed]
- MacAdam, J.W. Structure and Function of Plants; John Wiley & Sons: Ames, IA, USA, 2011; p. 56. [Google Scholar]
- Wang, L.; Li, S.; Zhang, L. Separating mechanism of dandelion (Taraxacum officinale) seed: Results from model analysis and separation force measurement. Wear 2021, 477, 203846. [Google Scholar] [CrossRef]
- Jedrejek, D.; Pawelec, S. Comprehensive Qualitative and Quantitative Analysis of Flavonoids in Dandelion (Taraxacum officinale) Flowers and Food Products. J. Agric. Food Chem. 2024, 72, 17368–17376. [Google Scholar] [CrossRef]
- Corleto, K.A.; Singh, J.; Jayaprakasha, G.K.; Patil, B.S. Storage stability of dietary nitrate and phenolic compounds in beetroot (Beta vulgaris) and arugula (Eruca sativa) juices. J. Food Sci. 2018, 83, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Jayaprakasha, G.K.; Patil, B.S. An optimised solvent extraction and characterization of unidentified flavonoid glucuronide derivatives from spinach by UHPLC-HR-QTOF-MS. Talanta 2018, 188, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Patil, B.S. A metabolomics approach to identify and quantify the phytochemicals in watermelons by quantitative (1) H NMR. Talanta 2016, 153, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Kenny, O.; Smyth, T.J.; Hewage, C.M.; Brunton, N.P. Antioxidant properties and quantitative UPLC-MS/MS analysis of phenolic compounds in dandelion (Taraxacum officinale) root extracts. Free Radic. Antioxid. 2014, 4, 55–61. [Google Scholar] [CrossRef]
- Hu, C.; Kitts, D.D. Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells. Mol. Cell. Biochem. 2004, 265, 107–113. [Google Scholar] [CrossRef]
- Michalska, K.; Kisiel, W. Sesquiterpene lactones from Taraxacum obovatum. Planta Med. 2003, 69, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Leu, Y.L.; Wang, Y.L.; Huang, S.C.; Shi, L.S. Chemical constituents from roots of Taraxacum formosanum. Chem. Pharm. Bull. 2005, 53, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Mo, E.J.; Ahn, J.H.; Jo, Y.H.; Kim, S.B.; Hwang, B.Y.; Lee, M.K. Inositol derivatives and phenolic compounds from the roots of Taraxacum coreanum. Molecules 2017, 22, 1349. [Google Scholar] [CrossRef]
- Tanasa (Acretei), M.V.; Negreanu-Pirjol, T.; Popoviciu, D.R.; Radu (Drăgoi), S.-I.; Negreanu-Pirjol, B.S.; Rosoiu, N. Comparative flavonoids content of Taraxacum officinale vegetal organs extracts from different Romanian regions. Afr. J. Biol. Sci. 2024, 6, 2799–2810. [Google Scholar] [CrossRef]
- Tanasa (Acretei), M.V.; Negreanu-Pirjol, T.; Chifiriuc, C.; Popoviciu, D.R.; Petcu, A.; Anghel (Cireasa), L.; Rosoiu, N. Carotenoids content in plant organs of Taraxacum officinale (L.) species from two Romanian regions. Acad. Rom. Sci. Ann. Ser. Biol. Sci. 2023, 12, 71–81. [Google Scholar] [CrossRef]
- Dedić, S.; Džaferović, A.; Jukić, H. Chemical composition and antioxidant activity of water-ethanol extracts of dandelion (Taraxacum officinale). Food Health Dis. Sci. Prof. J. Nutr. Diet. 2022, 11, 8–14. [Google Scholar]
- Tanasa (Acretei), M.V.; Negreanu-Pirjol, T.; Chifiriuc, C.; Popoviciu, D.R.; Anghel (Cireasa), L.; Rosoiu, N. Preliminary Data Regarding Polyphenols, Carotenoids and Flavonoids Content Correlated with Antioxidant Activity of Some Taraxacum sp. Fluid Extracts. Acad. Rom. Sci. Ann. Ser. Biol. Sci. 2022, 11, 31–44. [Google Scholar]
- Grauso, L.; Emrick, S.; Bonanomi, G.; Lanzotti, V. Metabolomics of the alimurgic plants Taraxacum officinale, Papaver rhoeas and Urtica dioica by combined NMR and GC—MS analysis. Phytochem. Anal. 2019, 30, 535–546. [Google Scholar] [CrossRef]
- Guo, Y.; Busta, L.; Jetter, R. Cuticular wax coverage and composition differ among organs of Taraxacum officinale. Plant Physiol. Biochem. 2017, 115, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Obermair, B.; Dorn, T.; Siems, K.; Rimbach, G.; Birringer, M. Sesquiterpene lactone composition and cellular Nrf2 induction of Taraxacum officinale leaves and roots and taraxinic acid ß-d-glucopyranosyl ester. J. Med. Food 2017, 20, 71–78. [Google Scholar] [CrossRef]
- Gomez, M.K.; Singh, J.; Acharya, P.; Jayaprakasha, G.K.; Patil, B.S. Identification and quantification of phytochemicals, antioxidant activity, and bile acid-binding capacity of Garnet Stem dandelion (Taraxacum officinale). J. Food Sci. 2018, 83, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Yoon, K.D.; Kim, J. Chemical constituents from Taraxacum officinale and their α-glucosidase inhibitory activities. Bioorg. Med. Chem. Lett. 2018, 28, 476–481. [Google Scholar] [CrossRef]
- Yarnell, E.; Abascal, K. Dandelion (Taraxacum officinale and Taraxacum mongolicum). Integr. Med. 2009, 8, 34–38. [Google Scholar]
- Mir, M.A.; Sawhney, S.S.; Jassal, M.M.S. Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. Wudpecker. J. Pharm. Pharmacol. 2013, 2, 1–5. [Google Scholar]
- Ghaima, K.K.; Hashim, N.M.; Ali, S.A. Antibacterial and antioxidant activities of ethyl acetate extract of nettle (Urtica dioica) and dandelion (Taraxacum officinale). J. Appl. Pharm. Sci. 2013, 3, 96–99. [Google Scholar]
- Ivanov, I. Polyphenols content and antioxidant activities of Taraxacum officinale F.H. Wigg (Dandelion) leaves. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 889–893. [Google Scholar]
- Forson, K.A.A.M. Evaluation of organoleptic and phytochemical properties of bread fortified with Taraxacum officinale leaf powder. J. Chem. Pharm. Res. 2022, 13, 1–9. [Google Scholar]
- Gallaher, R.N.; Gallaher, K.; Marshall, A.J. Mineral analysis of ten types of commercially available tea. J. Food Compos. Anal. 2006, 19, 53–57. [Google Scholar] [CrossRef]
- Popescu, M.L.; Dinu, M.; Ursache, D.D. Contributions to the pharmacognostical and phytobiological study on Taraxacum officinale (L.) Weber. Farmacia 2010, 58, 646–653. [Google Scholar]
- Meléndez-Martfnez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. HPLC analysis of geometrical isomers of lutein epoxide isolated from dandelion (Taraxacum officinale F. Weber ex Wiggers). Phytochemistry 2006, 67, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lee, C.; Kim, Y.H.; Ma, J.Y.; Shim, S.H. Chemical constituents of the aerial part of Taraxacum mongolicum and their chemotaxonomic significance. Nat. Prod. Res. 2017, 31, 2303–2307. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Rho, J.H.; Kim, K.T.; Cho, C.W.; Rhee, Y.K.; Choi, U.K. Phenolic acid contents and ROS scavenging activity of dandelion (Taraxacum officinale). Korean J. Food Preserv. 2008, 15, 325–331. [Google Scholar]
- Duan, L.; Zhang, C.; Zhao, Y.; Chang, Y.; Guo, L. Comparison of bioactive phenolic compounds and antioxidant activities of different parts of Taraxacum mongolicum. Molecules 2020, 25, 3260. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.I.; Barros, L.; Alves, R.C.; Oliveira, M.B.P.; Santos-Buelga, C.; Ferreira, I.C. Nutritional composition, antioxidant activity and phenolic compounds of wild Taraxacum sect. Ruderalia. Food Res. Int. 2014, 56, 266–271. [Google Scholar] [CrossRef]
- Ivanov, I.; Petkova, N.; Tumbarski, J.; Dincheva, I.; Badjakov, I.; Denev, P.; Pavlov, A. GC-MS characterization of n-hexane soluble fraction from dandelion (Taraxacum officinale Weber ex FH Wigg.) aerial parts and its antioxidant and antimicrobial properties. Z. Naturforsch. C 2018, 73, 41–47. [Google Scholar] [CrossRef]
- Ajmire, P.V.; Chavhan, S.A.; Thete, P.V.; Bakal, R.L. Pharmacognosy, phytochemistry, pharmacology and clinical applications of Taraxacum officinale. J. Pharmacogn. Phytochem. 2021, 10, 165–171. [Google Scholar]
- Xu, P.; Xu, X.B.; Khan, A.; Fotina, T.; Wang, S.H. Antibiofilm activity against Staphylococcus aureus and content analysis of Taraxacum officinale phenolic extract. Pol. J. Vet. Sci. 2021, 24, 243–251. [Google Scholar] [CrossRef]
- Savych, A.; Bilyk, O.; Vaschuk, V.; Humeniuk, I. Analysis of inulin and fructans in Taraxacum officinale L. roots as the main inulin-containing component of antidiabetic herbal mixture. Pharmacia 2021, 68, 527–532. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Takanaka, K.; Tsukada, H.; Miwa, Y.; Taga, T.; Tanaka, S. Sesquiterpene glucosides from anti leukotriene B4 release fraction of Taraxacum officinale. J. Asian Nat. Prod. Res. 2001, 3, 191–197. [Google Scholar] [CrossRef]
- Ovadje, P.; Ammar, S.; Guerrero, J.A.; Arnason, J.T.; Pandey, S. Dandelion root extract affects colorectal cancer proliferation and survival through the activation of multiple death signalling pathways. Oncotarget 2016, 7, 73080–73100. [Google Scholar] [CrossRef]
- Bylka, W.; Matlawska, I.; Frański, R. Essential oil composition of Taraxacum officinale. Acta Physiol. Plant 2010, 32, 231–234. [Google Scholar] [CrossRef]
- Sharma, N.; Tiwari, N.; Vyas, M.; Khurana, N.; Muthuraman, A.; Utreja, P. An overview of therapeutic effects of vanillic acid. Plant Arch. 2020, 20, 3053–3059. [Google Scholar]
- Tajner-Czopek, A.; Gertchen, M.; Rytel, E.; Kita, A.; Kucharska, A.Z.; Sokół-Łętowska, A. Study of antioxidant activity of some medicinal plants having high content of caffeic acid derivatives. Antioxidants 2020, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.H.; Loh, C.H.; Inbaraj, B.S.; Chen, B. Determination of carotenoids in Taraxacum formosanum by HPLC-DAD-APCI-MS and preparation by column chromatography. J. Pharmaceut. Biomed. 2012, 66, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Inbaraj, B.S.; Chen, B.H. Determination of Phenolic Acids and Flavonoids in Taraxacum formosanum Kitam by Liquid Chromatography-Tandem Mass Spectrometry Coupled with a Post-Column Derivatization Technique. Int. J. Mol. Sci. 2012, 13, 260–285. [Google Scholar] [CrossRef]
- Zielińska, K.; Kisiel, W. Sesquiterpenoids from roots of Taraxacum laevigatum and Taraxacum disseminatum. Phytochem. 2000, 54, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Zhang, X.; Song, H.; Zhang, Y. Dandelion (Taraxacum Genus): A review of chemical constituents and pharmacological effects. Molecules 2023, 28, 5022. [Google Scholar] [CrossRef]
- Zhou, J.; Xie, G.; Yan, X. Volume 5: Isolated Compounds (TZ) References TCM Plants and Congeners. In Encyclopedia of Traditional Chinese Medicines-Molecular Structures, Pharmacological Activities, Natural Sources and Applications; Springer: Berlin/Heidelberg, Germany, 2011; Volume 5, pp. 1–601. [Google Scholar]
- Banjari, I.; Misir, A.; Pavlić, M.; Herath, P.N.; Waisundara, V.Y. Traditional herbal medicines for diabetes used in Europe and Asia: Remedies from Croatia and Sri Lanka. Altern. Ther. Health Med. 2019, 25, 40–52. [Google Scholar]
- Wu, J.; Dai, W.; Li, K.; Ding, X.; Gao, H.; Wang, W.; Xiao, W. Apigenin’s Health Benefits in Kidney Disease: Pharmacological Insights and Future Perspectives. In Food Reviews International; Taylor & Francis Group, LLC: Philadelphia, PA, USA, 2024; pp. 1–29. [Google Scholar]
- Kronberga, A.; Nakurte, I.; Kaļāne, L.; Vecvanags, A.; Jakovels, D.; Fiļipovs, J.; Mežaka, I. Cultivation potential of Taraxacum campylodes G.E.Haglund wild populations: Morphological and biochemical variation. Plant Genet. Resour. Charact. Util. 2023, 21, 71–80. [Google Scholar] [CrossRef]
- Shikov, A.N.; Narkevich, I.A.; Akamova, A.; Nemyatykh, O.D.; Flisyuk, E.; Luzhanin, V.G.; Povydysh, M.N.; Mikhailova, I.; Pozharitskaya, O.N. Medical Species Used in Russia for the Management of Diabetes and Related Disorders. Front. Pharmacol. 2021, 12, 697411. [Google Scholar] [CrossRef]
- Li, C.; Tian, Y.; Zhao, C.; Li, S.; Wang, T.; Qiao, B.; Fu, Y. Application of fingerprint combined with quantitative analysis and multivariate chemometric methods in quality evaluation of dandelion (Taraxacum mongolicum). R. Soc. Open Sci. 2021, 8, 210614. [Google Scholar] [CrossRef]
- Wang, R.; Li, W.; Fang, C.; Zheng, X.; Liu, C.; Huang, Q. Extraction and identification of new flavonoid compounds in dandelion Taraxacum mongolicum Hand. -Mazz. with evaluation of antioxidant activities. Sci. Rep. 2023, 13, 2166. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Liu, Z.; Zhang, N.; Bao, C.; Li, X.; Liu, M.; Yuan, W.; Wu, H.; Shang, H. Effects of dietary dandelion (Taraxacum mongolicum Hand. -Mazz.) polysaccharides on the performance and gut microbiota of laying hens. Int. J. Biol. Macromol. 2023, 240, 124422. [Google Scholar] [CrossRef] [PubMed]
- Yoo, I.S.; Kim, A.J. A Systematic Review of the Functional Ingredients and Physiological Activities of Taraxacum coreanum Nakai. Asian J. Beauty Cosmetol. 2023, 21, 719–732. [Google Scholar] [CrossRef]
- Jung, Y.; Ahn, Y.G.; Kim, H.K.; Moon, B.C.; Lee, A.Y.; Hwang, G.S. Characterization of dandelion species using 1H NMR-and GC-MS-based metabolite profiling. Analyst 2011, 136, 4222–4231. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, P.; Ganesan, S.; Jayaseelan, T.; Azhagumadhavan, S.; Padma, M.; Senthilkumar, S.; Mani, P. Phytochemicals and GC–MS analysis of bioactive compounds present in ethanolic leaves extract of Taraxacum officinale (L). J. Drug Deliv. Ther. 2019, 9, 90–94. [Google Scholar] [CrossRef]
- Davaatseren, M.; Hur, H.J.; Yang, H.J.; Hwang, J.T.; Park, J.H.; Kim, H.J.; Kim, M.J.; Kwon, D.Y.; Sung, M.J. Taraxacum officinale (Dandelion) Leaf Extract Alleviates High—Fat Diet—Induced Nonalcoholic Fatty Liver. Food Chem. Toxicol. 2013, 58, 30–36. [Google Scholar] [CrossRef]
- El-Emam, S.Z.; El-Ella, D.M.A.; Fayez, S.M.; Asker, M.; Nazeam, J.A. Novel dandelion mannan-lipid nanoparticle: Exploring the molecular mechanism underlying the potent anticancer effect against non-small lung carcinoma. J. Funct. Foods 2021, 87, 104781. [Google Scholar] [CrossRef]
- Kristó, T.S.; Szoke, E.; Kery, A.; Terdy, P.P.; Selmeczi, L.K.; Simándi, B. Production and characterisation of Taraxacum officinale extracts prepared by supercritical fluid and solvent extractions. Acta Hortic. 2004, 597, 57–61. [Google Scholar] [CrossRef]
- Simandi, B.; Kristo, S.T.; Kery, A.; Selmeczi, L.K.; Kmecz, I.; Kemeny, S. Supercritical fluid extraction of dandelion leaves. J. Supercrit. Fluids 2002, 23, 135–142. [Google Scholar] [CrossRef]
- Díaz, K.; Espinoza, L.; Madrid, A.; Pizarro, L.; Chamy, R. Isolation and identification of compounds from bioactive extracts of Taraxacum officinale Weber ex FH Wigg. (Dandelion) as a potential source of antibacterial agents. Evid. Based Complement. Altern. Med. 2018, 1, 2706417. [Google Scholar] [CrossRef]
- Schütz, K.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale Web. ex-Wigg.) root and herb by high-performance liquid chromatography/electro spray ionization mass spectrometry. Radip Commn Mass. Spect. 2005, 19, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, G.; Xie, J.; Wu, M.; Wang, W.; Xiao, L.; Qian, Z. Screening Antioxidant Components in Different Parts of Dandelion Using Online Gradient Pressure Liquid Extraction Coupled with High-Performance Liquid Chromatography Antioxidant Analysis System and Molecular Simulations. Molecules 2024, 29, 2315. [Google Scholar] [CrossRef] [PubMed]
- Rehman, G.; Khan, I.; Rauf, A.; Rashid, U.; Siddique, A.; Shah, S.M.M.; Akram, Z.; AlMasoud, N.; Alomar, T.S.; Shah, Z.A.; et al. Antidiabetic Properties of Caffeoylmalic Acid, a Bioactive Natural Compound Isolated from Urtica dioica. Fitoterapia 2024, 176, 106024. [Google Scholar] [CrossRef] [PubMed]
- Miłek, M.; Marcinčáková, D.; Legáth, J. Polyphenols content, antioxidant activity, and cytotoxicity assessment of Taraxacum officinale extracts prepared through the micelle-mediated extraction method. Molecules 2019, 24, 1025. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, S.; Du, M.; Zhu, M.J. Dandelion extract suppresses reactive oxidative species and inflammasome in intestinal epithelial cells. J. Funct. Foods 2017, 29, 10–18. [Google Scholar] [CrossRef]
- García-Carrasco, B.; Fernandez-Dacosta, R.; Dávalos, A.; Ordovás, J.M.; Rodriguez-Casado, A. In vitro hypolipidemic and antioxidant effects of leaf and root extracts of Taraxacum officinale. Med. Sci. 2015, 3, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Jassim, A.K.M.; Farhan, S.A.; Noori, O.M. Identification of dandelion Taraxacum officinale leaves components and study its extracts effect on different microorganisms. Al-Nahrain J. Sci. 2012, 15, 7–14. [Google Scholar] [CrossRef]
- Lim, T.K. Taraxacum officinale. In Edible Medicinal and Non-Medicinal Plants: Vol. 7, Flowers; Springer: Dordrecht, The Netherlands, 2013; pp. 516–536. [Google Scholar]
- Merchán-Gaitán, J.B.; Mendes, J.H.; Nunes, L.E.; Buss, D.S.; Rodrigues, S.P.; Fernandes, P.M. The Role of Plant Latex in Virus Biology. Viruses 2023, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Antika, L.; Tasfiyati, A.; Hikmat, H.; Septama, A. Scopoletin: A review of its source, biosynthesis, methods of extraction, and pharmacological activities. Z. Naturforschung C 2022, 77, 303–316. [Google Scholar] [CrossRef]
- Xie, P.J.; Huang, L.X.; Zhang, C.H.; Ding, S.S.; Deng, Y.J.; Wang, X.J. Skin-care effects of dandelion leaf extract and stem extract: Antioxidant properties, tyrosinase inhibitory and molecular docking simulations. Ind. Crop Prod. 2018, 111, 238–246. [Google Scholar] [CrossRef]
- Molinu, M.G.; Piluzza, G.; Campesi, G.; Sulas, L.; Re, G.A. Antioxidant sources from leaves of Russian dandelion. Chem. Biodivers. 2019, 16, e1900250. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Muks, E.; Carle, R.; Schieber, A. Separation and quantification of inulin in selected artichoke (Cynara scolymus L.) cultivars and dandelion (Taraxacum officinale Web. ex-Wigg.) roots by high-performance anion exchange chromatography with pulsed amperometric detection. Biomed. Chromatogr. 2006, 20, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.; Todorova, M.; Petkova, N.; Dincheva, I. Non-polar phytochemical compounds from dandelion (Taraxacum officinale Weber ex FH Wigg.) flowers. Bulg. Chem. Commun. 2024, 56, 96–99. [Google Scholar] [CrossRef]
- Kong, J.; Chen, J.; Yue, Y.; Ma, Q.; Dong, Y.; Zhang, J. Ultrasonic/microwave–assisted extraction and rapid quantitative determination of active ingredients in Taraxacum koksaghyz Rodin by ultra-high-performance liquid chromatography tandem mass spectrometry. Int. J. Mass. Spectrom. 2021, 470, 116700. [Google Scholar] [CrossRef]
- Kim, M.H.; Kwon, S.Y.; Woo, S.Y.; Seo, W.D.; Kim, D.Y. Antioxidative effects of chrysoeriol via activation of the Nrf2 signaling pathway and modulation of mitochondrial function. Molecules 2021, 26, 313. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Jo, Y.J.; Jeong, S.H.; Kim, Y.H.; Han, J.H. An investigation of antioxidative and anti-inflammatory effects of Taraxacum coreanum (white dandelion) in lactating Holstein dairy cows. J. Adv. Vet. Anim. Res. 2024, 11, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Nickavar, B.; Rezaee, J.; Nickavar, A. Effect-directed analysis for the antioxidant compound in Salvia verticillata. Iran. J. Pharm. Res. 2016, 15, 241. [Google Scholar] [PubMed]
- Zhang, Y.; Li, Z.; Min, Q.; Palida, A.; Zhang, Y.; Tang, R.; Li, H. 8-Chrysoeriol, as a potential BCL-2 inhibitor triggers apoptosis of SW1990 pancreatic cancer cells. Bioorg Chem. 2018, 77, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Jiang, P.; Liu, W.; Xu, H.; Li, Y.; Li, Z.; Ma, H.; Yu, Y.; Li, X.; Ye, X. Role of Daucosterol Linoleate on Breast Cancer: Studies on Apoptosis and Metastasis. J. Agric. Food Chem. 2018, 66, 6031–6041. [Google Scholar] [CrossRef] [PubMed]
- Omari, N.; Jaouadi, I.; Lahyaoui, M.; Benali, T.; Taha, D.; Bakrim, S.; Menyiy, N.; Kamari, F.; Zengin, G.; Bangar, S.P.; et al. Natural Sources, Pharmacological Properties, and Health Benefits of Daucosterol: Versatility of Actions. Appl. Sci. 2022, 12, 5779. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J.; Sahu, D.; Liu, C.J. Pharmacological and Therapeutic Applications of Esculetin. Int. J. Mol. Sci. 2022, 23, 12643. [Google Scholar] [CrossRef]
- Lee, S.; Choi, M.J.; Choi, J.M.; Lee, S.; Kim, H.Y.; Cho, E.J. Flavonoids from Taraxacum coreanum protect from radical-induced oxidative damage. J. Med. Plant Res. 2012, 6, 5377–5384. [Google Scholar]
- Jiao, F.; Tan, Z.; Yu, Z.; Zhou, B.; Meng, L.; Shi, X. The phytochemical and pharmacological profile of taraxasterol. Front. Pharmacol. 2022, 13, 1663. [Google Scholar] [CrossRef]
- Jia, Y.-Y.; Guan, R.-F.; Wu, Y.-H.; Yu, X.-P.; Lin, W.-Y.; Zhang, Y.-Y.; Liu, T.; Zhao, J.; Shi, S.-Y.; Zhao, Y. Taraxacum mongolicum extract exhibits a protective effect on hepatocytes and an antiviral effect against hepatitis B virus in animal and human cells. Mol. Med. Rep. 2014, 9, 1381–1387. [Google Scholar] [CrossRef]
- Peng, S.; Dai, W.; Yu, H.; Wang, Y.; Wang, X.; Wang, X.; Peng, S. Anti-bacterial activity of aqueous and ethanolic extracts of Portulaca oleracea L. and Taraxacum mongolicum against pathogenic bacteria of cow mastitis. Int. J. Appl. Res. Vet. Med. 2014, 12, 210–213. [Google Scholar]
- Shi, S.; Zhao, Y.; Zhou, H.; Zhang, Y.; Jiang, X.; Huang, K. Identification of antioxidants from Taraxacum mongolicum by high-performance liquid chromatography-diode array detection-radical-scavenging detection-electrospray ionization mass spectrometry and nuclear magnetic resonance experiments. J. Chromatogr. A 2008, 1209, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, Y.; Zhang, X. Taraxacum mongolicum extract exhibits antimicrobial activity against respiratory tract bacterial strains in vitro and in neonatal rats by enhancing systemic Th1 immunity. Trop. J. Pharm. Res. 2018, 17, 1833–1838. [Google Scholar] [CrossRef]
- Oh, S.M.; Kim, H.R.; Park, Y.J.; Lee, Y.H.; Chung, K.H. Ethanolic extract of dandelion (Taraxacum mongolicum) induces estrogenic activity in MCF-7 cells and immature rats. Chin. J. Nat. Med. 2015, 13, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Igarashi, K.; Li, Y. Anti-diabetic effects of luteolin and luteolin-7-O-glucoside on KK-A(y) mice. Biosci. Biotechnol. Biochem. 2016, 80, 1580–1586. [Google Scholar] [CrossRef]
- Lee, A.Y.; Lee, S.; Kim, H.Y.; Lee, S.; Cho, E.J. Anti-inflammatory effects of luteolin and luteoloside from Taraxacum coreanum in RAW264. 7 macrophage cells. Appl. Biol. Chem. 2016, 59, 747–754. [Google Scholar] [CrossRef]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Świątek, P.; Stolarczyk, P.; Kocki, J. Immunodetection of pectic epitopes, arabinogalactan proteins, and extensins in mucilage cells from the ovules of Pilosella officinarum Vaill. and Taraxacum officinale Agg. (Asteraceae). Int. J. Mol. Sci. 2020, 21, 9642. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, K.; Yan, C.; Wu, M.; Wang, Y. Revealing the differences in phenolics in different parts of Taraxacum mongolicum using UPLC-MS/MS. Phytochem. Lett. 2023, 56, 13–18. [Google Scholar] [CrossRef]
- Qiao, H.; Sun, T. Antibacterial activity of ethanol extract and fractions obtained from Taraxacum mongolicum flower. Res. J. Pharmacogn. 2014, 1, 35–39. [Google Scholar]
- Jang, H.; Choi, M.; Lee, E.; Jang, K.S. Comparative Phytochemical Profiling of Methanolic Extracts of Different Parts of White Dandelion (Taraxacum coreanum) using Hybrid Ion-mobility Q-TOF MS. Mass. Spectrom. Lett. 2024, 15, 95–106. [Google Scholar]
- Lee, Y.S.; Kim, J.; Woo, S.; Park, J.Y.; Park, H.S.; Shim, H.; Choi, H.; Kang, J.H.; Lee, T.J.; Sung, S.H.; et al. Assessing the genetic and chemical diversity of Taraxacum species in the Korean Peninsula. Phytochemistry 2021, 181, 112576. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Rasul, A.; Hussain, G.; Shah, M.A.; Sarfraz, I.; Nageen, B.; Riaz, A.; Khalid, R.; Asrar, M.; Selamoglu, Z.; et al. Physcion and Physcion 8-O-β-D-glucopyranoside: Natural anthraquinones with potential anticancer activities. Curr. Drug Targets 2021, 22, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.; Mir, T.A.; Khare, R.K. Traditional use of medicinal plants among the indigenous communities in Baramulla district, Jammu and Kashmir, India. Nord. J. Bot. 2022, 6, e03387. [Google Scholar] [CrossRef]
- Biel, W.; Jaroszewska, A.; Łysoń, E.; Telesiński, A. The chemical composition and antioxidant properties of common dandelion leaves compared with sea buckthorn. Can. J. Plant Sci. 2017, 97, 1165–1174. [Google Scholar] [CrossRef]
- Rasool, S.; Sharma, B. Taraxacum officinale: A high value less known medicinal plant. Ann. Plant Sci. 2014, 3, 908–915. [Google Scholar]
- Pfingstgraf, I.O.; Taulescu, M.; Pop, R.M.; Orăsan, R.; Vlase, L.; Uifalean, A.; Pârvu, A.E. Protective effects of Taraxacum officinale L. (dandelion) root extract in experimental acute on chronic liver failure. Antioxidants 2021, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Bensky, D.; Clavey, S.; Stöger, E.; Gamble, A. Chinese Herbal Medicine Materia Medica, 3rd ed.; Eastland Press: Seattle, WA, USA, 2004. [Google Scholar]
- Malik, A.H.; Khuroo, A.A.; Dar, G.H.; Khan, Z.S. Ethnomedicinal uses of some plants in the Kashmir Himalaya. Indian J. Tradit. Knowl. 2011, 10, 362–366. [Google Scholar]
- Martinez, M.; Poirrier, P.; Chamy, R.; Prüfer, D.; Schulze-Gronover, C.; Jorquera, L.; Ruiz, G. Taraxacum officinale and related species—An ethnopharmacological review and its potential as a commercial medicinal plant. J. Ethnopharmacol. 2015, 69, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Aremu, O.O.; Oyedeji, A.O.; Oyedeji, O.O.; Nkeh-Chungag, B.N.; Rusike, C.R.S. In vitro and in vivo antioxidant properties of Taraxacum officinale in N ω-Nitro-l-Arginine Methyl ester (L-NAME)-induced hypertensive rats. Antioxidants 2019, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, E. Hepatoprotective properties of Dandelion: Recent update. J. Appl. Pharm. Sci. 2016, 6, 202–205. [Google Scholar] [CrossRef]
- Sweeney, B.; Vora, M.; Ulbricht, C.; Basch, E. Evidence-based systematic review of dandelion (Taraxacum officinale) by natural standard research collaboration. J. Herb. Pharmacother. 2005, 5, 79–93. [Google Scholar] [CrossRef]
- Choi, U.K.; Lee, O.H.; Yim, J.H.; Cho, C.W.; Rhee, Y.K.; Lim, S.I.; Kim, Y.C. Hypolipidemic and Antioxidant Effects of Dandelion (Taraxacum officinale) Root and Leaf on Cholesterol-Fed Rabbits. Int. J. Mol. Sci. 2010, 11, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, A.; Jeyachandran, R.; Cindrella, L.; Thangadurai, D.; Veerapur, V.P.; Muralidhara Rao, D. Hepatocurative Potential of Sesquiterpene Lactones of Taraxacum officinale on Carbon Tetrachloride Induced Liver Toxicity in Mice. Acta Biol. Hung. 2010, 61, 175–190. [Google Scholar] [CrossRef]
- You, Y.; Yoo, S.; Yoon, H.G.; Park, J.; Lee, Y.H.; Kim, S.; Oh, K.T.; Lee, J.; Cho, H.Y.; Jun, W. In vitro and in vivo hepatopro- tective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem. Toxicol. 2010, 48, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Liu, X.; Guan, L.; Yu, L.; Zhang, X. Anti-inflammatory and anti-arthritic effects of taraxasterol on adjuvant-induced arthritis in rats. J. Ethnopharmacol. 2016, 187, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F. Dandelion. In Nonvitamin and Nonmineral Nutritional Supplements; Academic Press: San Diego, CA, USA, 2019; pp. 203–204. [Google Scholar]
- Lis, B.; Olas, B. Pro-health activity of dandelion (Taraxacum officinale L.) and its food products—History and present. J. Funct. Foods 2019, 59, 40–48. [Google Scholar] [CrossRef]
- Neamtu, G.; Tabacaru, C.; Socaciu, C. Phytochemical research on higher plants. V. The content of carotenoids, chlorophylls, substances used as food and mineral elements in Taraxacum officinale L. (dandelion). Bul. Univ. Științe Cluj-Napoca Ser. Agric. Hortic. 1992, 46, 93–99. [Google Scholar]
- Al-Eisawi, Z.; Abderrahman, S.M.; Al-Khalaf, I.F.; Al-Abbassi, R.; Bustanji, Y.K. Taraxacum officinale extracts exhibit safe and selective anticancer activity. Nat. Prod. J. 2022, 12, 69–77. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The Effects of Plant Flavonoids on Mammalian Cells: Implications for Inflammation, Heart Disease, and Cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [CrossRef]
- Urquiaga, J.; Leighton, F. Plant Polyphenol Antioxidants and Oxidative Stress. Biol. Res. 2000, 33, 55–64. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; Di Benedetto, R.; Filesi, C.; Masella, R. Polyphenols, Intracellular Signalling and Inflammation. Ann. Ist. Super. Sanita 2007, 43, 394–405. [Google Scholar] [PubMed]
- Malireddy, S.; Kotha, S.R.; Secor, J.D.; Gurney, T.O.; Abbott, J.L.; Maulik, G.; Maddipati, K.R.; Parinandi, N.L. Phytochemical Antioxidants Modulate Mammalian Cellular Epigenome: Implications in Health and Disease. Antioxid. Redox Signal. 2012, 17, 327–339. [Google Scholar] [CrossRef]
- Wilkinson-Berka, J.L.; Rana, I.; Armani, R.; Agrotis, A. Reactive Oxygen Species, Noxand Angiotensin II in Angiogenesis: Implications for Retinopathy. Clin. Sci. 2013, 124, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Clare, B.A.; Conroy, R.S.; Spelman, K. The diuretic effect in human subjects of an extract of Taraxacum officinale folium over a single day. J. Altern. Complement. Med. 2009, 15, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Metrani, R.; Shivanagoudra, S.R.; Jayaprakasha, G.K.; Patil, B.S. Review on bile acids: Effects of the gut microbiome, interactions with dietary fiber, and alterations in the bioaccessibility of bioactive compounds. J. Agric. Food Chem. 2019, 67, 9124–9138. [Google Scholar] [CrossRef]
- Salmond, S. The Hepatobiliary System. In Clinical Naturopathic Medicine-Ebook; Churchill Livingstone: London, UK, 2013; p. 210. [Google Scholar]
- Zhou, S.; Wang, Z.; Hao, Y.; An, P.; Luo, J.; Luo, Y. Dandelion Polysaccharides Ameliorate High-Fat-Diet-Induced Atherosclerosis in Mice through Antioxidant and Anti-Inflammatory Capabilities. Nutrients 2023, 15, 4120. [Google Scholar] [CrossRef]
- Majewski, M.; Lis, B.; Juśkiewicz, J.; Ognik, K.; Borkowska-Sztachańska, M.; Jędrejek, D.; Stochmal, A.; Olas, B. Phenolic fractions from dandelion leaves and petals as modulators of the antioxidant status and lipid profile in an in vivo study. Antioxidants 2020, 9, 131. [Google Scholar] [CrossRef]
- Park, C.M.; Youn, H.J.; Chang, H.K.; Song, Y.S. TOP1 and 2, Polysaccharides from Taraxacum officinale, Attenuate CCl4 -Induced Hepatic Damage through the Modulation of NF-kappa B and Its Regulatory Mediators. Food Chem. Toxicol. 2010, 48, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.J.; Cha, D.S.; Ko, J.S.; Park, H.J.; Choi, H.D. Anti-Inflammatory Effect of Taraxacum officinale Leaves on Lipopoly-saccharide—Induced Inflammatory Responses RAW264.7. Cells. J. Med. Food 2010, 13, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Deng, X.; Yu, X.; Wang, W.; Yan, W.; Zhao, X.; Wang, X.; Bai, C.; Wang, Z.; Han, L. Taraxacum: A Review of Ethnopharmacology, Phytochemistry and Pharmacological Activity. Am. J. Chin. Med. 2024, 52, 183–215. [Google Scholar] [CrossRef] [PubMed]
- Chovatiya, R.; Medzhitov, R. Stress, inflammation, and defense of homeostasis. Mol. Cell 2014, 54, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive oxygen species in macrophages: Sources and targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Hamidzadeh, K.; Goncalves, R. Macrophages and the maintenance of homeostasis. J. Cell Mol. Immunol. 2021, 18, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Leick, M.; Azcutia, V.; Newton, G.; Luscinskas, F.W. Leukocyte recruitment in inflammation: Basic concepts and new mechanistic insights based on new models and microscopic imaging technologies. Cell Tissue Res. 2014, 355, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Shin, H.Y.; Lim, K.H.; Ryu, S.T.; Shin, T.Y.; Chae, H.J.; Kim, H.R.; Lyu, Y.S.; An, N.H.; Lim, K.S. Taraxacum officinale inhibits tumor necrosis factor-a production from rat astrocytes. Immunopharmacol. Immunotoxicol. 2000, 22, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Wang, J.; Hong, D.; Zhang, T.; Duan, H.; Mu, X.; Yang, Z. Effects of aqueous extracts of Taraxacum officinale on expression of tumor necrosis factor-alpha and intracellular adhesion molecule 1 in LPS-stimulated RMMVECs. BMC Complement. Altern. Med. 2017, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.W.; Koo, H.N.; An, H.J.; Kwon, K.B.; Lim, B.C.; Seo, E.A.; Ryu, D.G.; Moon, G.; Kim, H.Y.; Kim, H.M.; et al. Taraxacum officinale protects against cholecystokinin-induced acute pancreatitis in rats. World J. Gastroenterol. 2005, 11, 597–599. [Google Scholar] [CrossRef]
- Hudec, J.; Burdovå, M.; Kobida, L.U.; Komora, L.; Macho, V.; Kogan, G.; Turianica, I.; Kochanova, R.; Lozek, O.; Haban, M.; et al. Antioxidant capacity changes and phenolic profile of Echinacea purpurea, nettle (Urtica dioica L.), and dandelion (Taraxacum officinale) after application of polyamine and phenolic biosynthesis regulators. J. Agric. Food Chem. 2007, 55, 5689–5696. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.J.; Kang, H.J.; Jung, H.J.; Kang, Y.S.; Lim, C.J.; Kim, Y.M.; Park, E.H. Anti-inflam Hagymasi matory activity of Taraxacum officinale. J. Ethnopharmacol. 2008, 115, 82–88. [Google Scholar] [CrossRef]
- Liu, B.; He, Z.; Wang, J.; Xin, Z.; Wang, J.; Li, F.; Fu, Y. Taraxasterol Inhibits LPS-Induced Inflammatory Response in BV2 Microglia Cells by Activating LXRα. Front. Pharmacol. 2018, 9, 278. [Google Scholar] [CrossRef]
- Liu, L.; Xiong, H.; Ping, J.; Ju, Y.; Zhang, X. Taraxacum officinale protects against lipopolysaccharide induced acute lung injury in mice. J. Ethnopharmacol. 2010, 130, 392–397. [Google Scholar] [CrossRef]
- Lee, J.P.; Li, Y.C.; Chen, H.Y.; Lin, R.H.; Huang, S.S.; Chen, H.L.; Kuan, P.C.; Liao, M.F.; Chen, C.J.; Kuan, Y.H. Protective effects of luteolin against lipopolysaccharide-induced acute lung injury involve inhibition of MEK/ERK and PI3K/Akt pathways in neutrophils. Acta Pharmacol. Sin. 2010, 31, 831–838. [Google Scholar] [CrossRef]
- Park, C.M.; Jin, K.S.; Lee, Y.W.; Song, Y.S. Luteolin and chicoric acid synergistically inhibited inflammatory responses via inactivation of PI3K-Akt pathway and impairment of NF-kB translocation in LPS stimulated RAW264.7 cells. Eur. J. Pharmacol. 2011, 660, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Awortwe, C.; Sackeyfio, A.; Osei-Safo, D.; Bugyei, K.A.; Asiedu-Gyekye, I.J. Dual effect of Taraxacum officinale leaves: Anticholinergic and inhibitory effect on inflammatory cells in ovalbumin-sensitized guineapigs. Afr. J. Pharm. Pharmacol. 2011, 5, 2613–2619. [Google Scholar] [CrossRef]
- Awortwe, C.; Osei-Safo, D.; Asiedu-Gyekye, I.J.; Sackeyfio, A. Anti-inflammatory activity of Taraxacum officinale leaves in ovalbumin-sensitized guineapigs. Int. J. Pharm. Pharma Sci. 2013, 5, 628–633. [Google Scholar]
- Xiong, H.; Cheng, Y.; Zhang, X.; Zhang, X. Effects of taraxasterol on iNOS and COX-2 expression in LPS-induced RAW 264.7 macrophages. J. Ethnopharmacol. 2014, 155, 753–757. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, H.; Li, H.; Cheng, Y. Protective effect of taraxasterol against LPS-induced endotoxic shock by modulating inflammatory responses in mice. Immunopharmacol. Immunotoxicol. 2014, 36, 11–16. [Google Scholar] [CrossRef]
- Park, C.M.; Cho, C.W.; Song, Y.S. TOP 1 and 2, polysaccharides from Taraxacum officinale, inhibit NFκB-mediated inammation and accelerate Nrf2-induced antioxidative potential through the modulation of PI3K-Akt signaling pathway in RAW 264.7 cells. Food Chem. Toxicol. 2014, 66, 56–64. [Google Scholar] [CrossRef]
- Sang, R.; Yu, Y.; Ge, B.; Xu, L.; Wang, Z.; Zhang, X. Taraxasterol from Taraxacum prevents concanavalin A-induced acute hepatic injury in mice via modulating TLRs/NF-κB and Bax/Bc1-2 signalling pathways. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3929–3937. [Google Scholar] [CrossRef] [PubMed]
- Epure, A.; Pârvu, A.E.; Vlase, L.; Benedec, D.; Hanganu, D.; Oniga, O.; Vlase, A.-M.; Ielciu, I.; Toiu, A.; Oniga, I. New Approaches on the Anti-Inflammatory and Cardioprotective Properties of Taraxacum officinale Tincture. Pharmaceuticals 2023, 16, 358. [Google Scholar] [CrossRef] [PubMed]
- Hee, H.S.; HakDong, L.; Sanghyun, L.; Young, L.A. Taraxacum coreanum Nakai extract attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier dysfunction in Caco-2 cells. J. Ethnopharmacol. 2023, 319, 117105. [Google Scholar]
- Bingjie, G.; Rui, S.; Wei, W.; Kexin, Y.; Yifan, Y.; Lin, K.; Minghong, Y.; Xinman, L.; Xuemei, Z. Protection of taraxasterol against acetaminophen-induced liver injury elucidated through network pharmacology and in vitro and in vivo experiments. Phytomedicine 2023, 116, 154872. [Google Scholar]
- Yi, L.; Jin, X.; Chen, C.Y.; Fu, Y.J.; Zhang, T.; Chang, H.; Zhou, Y.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Chemical structures of 4-oxo-flavonoids in relation to inhibition of oxidized low-density lipoprotein (LDL)-induced vascular endothelial dysfunction. Int. J. Mol. Sci. 2011, 12, 5471–5489. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Jia, Q.; Guo, X.; Fan, X. Clinical application of dandelion external application in acute mastitis. Guizhou Med. J. 2014, 38, 360–361. [Google Scholar]
- Chaoyong, X.; Yu, Z.; Yuliang, W. Extraction, Purification and in vitro Anti-inflammatory Activity Analysis of Total Polysaccharides from Taraxacum mongolicum. Chin. J. Exp. Tradit. Med. Formulae 2016, 22, 25–28. [Google Scholar]
- Qiao, N.; Ding, X.; Ni, S. Clinical observation on treatment of acute lactation mastitis complicated with abscess formation with combination of traditional Chinese and western medicine. China, J. Tradit. Chin. Med. Pharm. 2020, 35, 1580–1582. [Google Scholar]
- An, L. Clinical study on dandelion granules combined with cefradine in the treatment of acute mastitis. Drugs Clin. 2021, 36, 796–798. [Google Scholar]
- Han, S.H.; Lee, S.; Kim, H.Y.; Lee, A.Y. Taraxacum coreanum (Korean Dandelion) Extract Protects against Lipopolysaccharide-Induced Blood-Brain Barrier Destruction via Regulation of Tight Junctions and Inflammatory Responses in bEnd.3 Cells. J. Med. Food 2024, 27, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.; Ozer, E.; Tugay, O.; Dik, I.; Kus, C. Evaluation of In Vitro Antiviral Activity of Taraxacum farinosum and Taraxacum mirabile Extracts against Herpes Simplex Virus Type 1 (HSV-1). Int. J. Sci. Technol. Res. 2016, 2, 29–35. [Google Scholar]
- Sengul, M.; Yildiz, H.; Gungor, N. Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pak. J. Pharm. Sci. 2009, 22, 102–106. [Google Scholar] [PubMed]
- Renantha, R.R.; Liga, A.R.; Tanugroho, C.B.; Denovian, L.X.; Az, S.L.; Budiyanto, Z.; Parikesit, A.A. Flavonoids as potential inhibitors of dengue virus 2 (DENV2) envelope protein. J. Pharm. Pharmacogn. Res. 2022, 10, 660–675. [Google Scholar] [CrossRef]
- Riwu, A.G.; Nugraha, J.; Purwanto, D.A.; Triyono, E.A. In silico analysis of anti-dengue activity of faloak (Sterculia quadrifida R. Br) stem bark compounds. J. Pharm. Pharmacogn. Res. 2022, 10, 1006–1014. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, A.R.; Chia, S.L.; Looi, Q.H.; Omar, A.R.; Noordin, M.M.; Ideris, A. Herbal Extracts as Antiviral Agents—Chapter 7 in Feed Additives; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Academic Press: Oxford, UK, 2020; pp. 115–132. [Google Scholar]
- Kulkarni, K.; Jagtap, G.; Magdum, S.A. Comprehensive review on herbal drug standardization. Am. J. PharmTech Res. 2019, 9, 97–122. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Rodríguez-Ortega, M.; Chumpitaz, Z.; Santiago, R.Í.O.S.; Méndez, M.; Méndez, J.; Cabrera, G. Actividad antiviral contra el virus de la fiebre amarilla, cepa vacunal 17D, de extractos de hojas de Taraxacum officinale G.H. Weber ex Wiggers. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2013, 12, 346–355. [Google Scholar]
- Rehman, S.; Ijaz, B.; Fatima, N.; Muhammad, S.A.; Riazuddin, S. Therapeutic potential of Taraxacum officinale against HCV NS5B polymerase: In vitro and in silico study. Biomed. Pharmacother. 2016, 83, 881–891. [Google Scholar] [CrossRef]
- Flores-Ocelotl, M.R.; Rosas-Murrieta, N.H.; Moreno, D.A.; Vallejo-Ruiz, V.; Reyes-Leyva, J.; Domínguez, F.; Santos-López, G. Taraxacum officinale and Urtica dioica extracts inhibit dengue virus serotype 2 replication in vitro. BMC Complement. Altern. Med. 2018, 18, 2163. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martinez, E. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8 (Suppl. S12), 7–16. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.T.; Le, N.P.K.; Gigl, M.; Dawid, C.; Lamy, E. Common dandelion (Taraxacum officinale) efficiently blocks the interaction between ACE2 cell surface receptor and SARS-CoV-2 spike protein D614, mutants D614G, N501Y, K417N and E484K in vitro. BioRxiv 2021. [Google Scholar] [CrossRef]
- Chittasupho, C.; Umsumarng, S.; Srisawad, K.; Arjsri, P.; Phongpradist, R.; Samee, W.; Tingya, W.; Ampasavate, C.; Dejkriengkraikul, P. Inhibition of SARS-CoV-2-Induced NLRP3 Inflammasome-Mediated Lung Cell Inflammation by Triphala-Loaded Nanoparticle Targeting Spike Glycoprotein S1. Pharmaceutics 2024, 16, 751. [Google Scholar] [CrossRef] [PubMed]
- Valdiviezo-Campos, J.E.; Rodriguez-Aredo, C.D.; Ruiz-Reyes, S.G.; Venegas-Casanova, E.A.; Bussmann, R.W.; Ganoza-Yupanqui, M.L. Identification of polyphenols by UPLC-MS/MS and their potential in silico antiviral activity from medicinal plants in Trujillo, Peru. J. Pharm. Pharmacogn. Res. 2024, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Parisi, M.G.; Ozón, B.; Vera González, S.M.; García-Pardo, J.; Obregón, W.D. Plant Protease Inhibitors as Emerging Antimicrobial Peptide Agents: A Comprehensive Review. Pharmaceutics 2024, 16, 582. [Google Scholar] [CrossRef] [PubMed]
- Oseni, A.; Yussif, I. Screening ethanolic and aqueous leaf extracts of T. officinale for in vitro bacteria growth inhibition. J. Pharm. Biomed. Sci. 2012, 20, 1–4. [Google Scholar]
- Tian, Y.; Luo, J.; Wang, H.; Zaki, H.E.M.; Yu, S.; Wang, X.; Ahmed, T.; Shahid, M.S.; Yan, C.; Chen, J.; et al. Bioinspired Green Synthesis of Silver Nanoparticles Using Three Plant Extracts and Their Antibacterial Activity against Rice Bacterial Leaf Blight Pathogen Xanthomonas oryzae pv. oryzae. Plants 2022, 11, 2892. [Google Scholar] [CrossRef] [PubMed]
- Goyal, H.; Gupta, R.C.; Pradhan, S.K.; Goel, R. Antimicrobial and Hepatoprotective Activity of Different Cytotypes of Taraxacum officinale from the Indian Himalayas. J. Herbs Spices Med. Plants 2023, 30, 12–25. [Google Scholar] [CrossRef]
- Rahman, M.S.; Alam, M.B.; Kim, Y.K.; Madina, M.H.; Fliss, I.; Lee, S.H.; Yoo, J.C. Activation of Nrf2/HO-1 by Peptide YD1 Attenuates Inflammatory Symptoms through Suppression of TLR4/MYyD88/NF-κB Signaling Cascade. Int. J. Mol. Sci. 2021, 22, 5161. [Google Scholar] [CrossRef] [PubMed]
- Astafieva, A.A.; Rogozhin, E.A.; Odintsova, T.I.; Khadeeva, N.V.; Grishin, E.V.; Egorov, T.A. Discovery of novel antimicrobial peptides with unusual cysteine motifs in dandelion Taraxacum officinale Wigg. flowers. Peptides 2012, 36, 266–271. [Google Scholar] [CrossRef]
- Ionescu, D.; Predan, G.; Rizea, G.D.; Mihele, D.; Dune, A.; Ivopol, G.; Ionita, C. Antimicrobial Activity of some Hydroalcoholic Extracts of Artichoke (“Cynara scolymus”), Burdock (“Arctium lappa”) and Dandelion (“Taraxacum officinale”). Bull. Transilv. Univ. Brasov. Ser. II Forestry. Wood Industry. Agric. Food Eng. 2013, 6, 113–120. [Google Scholar]
- Răducanu, A.E.; Tihauan, B.M.; Marinas, I.C.; Ciuperca, O.T.; Tebrencu, C.E.; Ionescu, E.; Onisei, T. The biological effects of novel nutraceuticals with curcuminoids and other plant-derived immunomodulators and pre-probiotics. Pharmaceutics 2021, 13, 666. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.; Gangwar, R.; Kumar Vishwakarma, R.; Singh Negi, D. Antibacterial, antioxidant and photodegradation potential of ZnO nanoparticles mediated via roots of Taraxacum officinale radix. Mater. Today Proc. 2022, 57, 2435–2443. [Google Scholar] [CrossRef]
- Negreanu-Pirjol, B.; Negreanu-Pirjol, T.; Tofan, L.; Sirbu, R.; Sava, C.; Meghea, A. Physicochemical and microbiological characterisation of two biological wastes of the Romanian Black Sea coast. J. Environ. Prot. Ecol. 2011, 4A, 2205–2217. [Google Scholar]
- Enteshari Najafabadi, M.; Roozbeh Nasiraie, L.; Ghasemi Pirblouti, A.; Noori, H.R. Biological and prebiotic activities of polysaccharides from Taraxacum officinale F.H. Wigg., Cichorium intybus L., and Gundelia tournefortii L. J. Food Meas. Charact. 2024, 18, 1412–1421. [Google Scholar] [CrossRef]
- Hassan, H.A.; Patel, V.B.; Rajendran, R.; Preedy, V.R. Oxidative stress as a crucial factor in liver associated disorders: Potential therapeutic effect of antioxidants. In The Liver; Academic Press: Boston, MA, USA, 2018; pp. 121–130. [Google Scholar]
- Kim, D.; Alshuwaykh, O.; Dennis, B.B.; Cholankeril, G.; Knowles, J.W.; Ahmed, A. Chronic liver disease-related mortality in diabetes before and during the COVID-19 in the United States. Dig. Liver Dis. 2023, 55, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Al-Malki, A.L.; Abo-Golayel, M.K.; Abo-Elnaga, G.; Al-Beshri, H. Hepatoprotective effect of dandelion (Taraxacum officinale) against induced chronic liver cirrhosis. J. Med. Plants Res. 2013, 7, 1494–1505. [Google Scholar]
- Kour, J.; Sharma, R.; Nayik, G.A.; Ramaiyan, B.; Sofi, S.A.; Alam, M.S.; Anand, N. Dandelion. In Antioxidants in Vegetables and Nuts-Properties and Health Benefits, 1st ed.; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 237–248. [Google Scholar]
- Sharma, P.K.; Lal, B. Ethnobotanical notes on some medicinal and aromatic plants of Himachal Pradesh Indian. J. Tradit. Know. 2005, 4, 424–428. [Google Scholar]
- Kshirsagar, A.D.; Mohite, R.; Aggrawal, A.S.; Suralkar, U.R. Hepatoprotective medicinal plants of Ayurveda—A review. Asian J. Pharm. Clin. Res. 2011, 4, 1–8. [Google Scholar]
- Erdem, M.; Özaslan, M. Investigation of the antioxidant capacity of Taraxacum officinale L. leaf extract. Eurasia Proc. Sci. Technol. Eng. Math. 2024, 28, 575–580. [Google Scholar] [CrossRef]
- Hu, C. Taraxacum: Phytochemistry and health benefits. Chin. Herb. Med. 2018, 10, 353–361. [Google Scholar] [CrossRef]
- Figurski, A.C.; Rakel, D. Cholelithiasis Integrative Medicine, 4th ed.; Elsevier: London, UK, 2018; pp. 450–456. [Google Scholar]
- Domitrović, R.; Jakovac, H.; Romić, Z.; Rahelić, D.; Tadić, Z. Antifibrotic activity of Taraxacum officinale root in carbon tetrachloride-induced liver damage in mice. J. Ethnopharmacol. 2010, 130, 569–577. [Google Scholar] [CrossRef]
- Ovadje, P.; Chochkeh, M.; Akbari-Asl, P.; Hamm, C.; Pandey, S. Selective induction of apoptosis and autophagy through treatment with dandelion root extract in human pancreatic cancer cells. Pancreas 2012, 41, 1039–1047. [Google Scholar] [CrossRef]
- Koo, H.N.; Hong, S.H.; Song, B.K.; Kim, C.H.; Yoo, Y.H.; Kim, H.M. Taraxacum officinale induces cytotoxicity through TNF-alpha and IL 1 alpha secretion in HepG2 cells. Life Sci. 2004, 74, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.; Fanaei, H.; Dehpour, A.R.; Hassanzadeh, G.; Jafari, M.; Salehi, M.; Mohammadi, M. Chemical composition and hepatoprotective activity of ethanolic root extract of Taraxacum syriacum Boiss against acetaminophen intoxication in rats. Bratisl. Med. J. 2015, 116, 41–46. [Google Scholar] [CrossRef]
- Colle, D.; Arantes, L.P.; Gubert, P.; da Luz, S.C.; Athayde, M.L.; Teixeira Rocha, J.B.; Soares, F.A. Antioxidant properties of Taraxacum officinale leaf extract is involved in the protective effect against hepatoxicity induced by acetaminophen in mice. J. Med. Food 2012, 15, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycemic effects used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Macía, M.J.; García, E.; Vidaurre, P.J. An ethnobotanical survey of medicinal plants commercialized in the markets of La Paz and El Alto, Bolivia. J. Ethnopharmacol 2005, 97, 337–350. [Google Scholar] [CrossRef]
- Yu, J.K.; La Rota, M.; Kantety, R.V.; Sorrells, M.E. EST derived SSR markers for comparative mapping in wheat and rice. Mol. Genet. Genom. 2004, 271, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Gulfraz, M.; Ahamd, D.; Ahmad, M.S.; Qureshi, R.; Mahmood, R.T.; Jabeen, N.; Abbasi, K.S. Effect of leaf extracts of Taraxacum officinale on CCl4 induced hepatotoxicity in rats, in vivo study. Pak. J. Pharm. Sci. 2014, 27, 825–829. [Google Scholar]
- Hamzawy, M.A.; El-Denshary, E.S.; Abdel-Wahhab, M.A. Effects of natural compounds in treatment and prevention of hepatotoxicity and hepatocellular carcinoma. Hepatoma Res. 2015, 1, 111–118. [Google Scholar] [CrossRef]
- Lin, W.; Gu, B.; Gu, Y.; Zhao, R.; Huang, Y.; Fan, R.; Rong, W.; Liu, Z. Taraxasterol protects against acetaminophen-induced hepatotoxicity by reducing liver inflammatory response and ameliorating oxidative stress in mice. Int. Immunopharmacol. 2024, 138, 112580. [Google Scholar] [CrossRef]
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Alshuwaykh, O.; Dennis, B.B.; Cholankeril, G.; Ahmed, A. Trends in etiology-based mortality from chronic liver disease before and during COVID-19 pandemic in the United States. Clin. Gastroenterol. Hepatol. 2022, 20, 2307–2316. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Xie, G.; Wang, J.; Hou, X.; Wang, X.; Wu, W.; Liu, X. Chicoric acid prevents obesity by attenuating hepatic steatosis, inflammation and oxidative stress in high-fat diet-fed mice. Food Res. Int. 2013, 54, 345–353. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 28 October 2024).
- Lewis, G.F.; Carpentier, A.; Adeli, K.; Giacca, A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr. Rev. 2014, 23, 201–229. [Google Scholar] [CrossRef]
- Hamden, K.; Carreau, S.; Boujbiha, M.A.; Lajmi, S.; Aloulou, D.; Kchaou, D.; Elfeki, A. Hyperglycaemia, stress oxidant, liver dysfunction and histological changes in diabetic male rat pancreas and liver: Protective effect of 17 beta-estradiol. Steroids 2008, 73, 495–501. [Google Scholar] [CrossRef] [PubMed]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and oxidative stress: An integral, updated and critical overview of their metabolic interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef] [PubMed]
- González-Castejón, M.; Visioli, F.; Rodriguez-Casado, A. Diverse biological activities of dandelions. Nutr. Rev. 2012, 70, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Ježek, P.; Jabůrek, M.; Plecitá-Hlavatá, L. Contribution of oxidative stress and impaired biogenesis of pancreatic β-cells to type 2 diabetes. Antioxid. Redox Signal 2019, 31, 722–751. [Google Scholar] [CrossRef]
- Cheng, D.M.; Pogrebnyak, N.; Kuhn, P.; Krueger, C.G.; Johnson, W.D.; Raskin, I. Development and phytochemical characterization of high polyphenol red lettuce with anti-diabetic properties. PLoS ONE 2014, 9, e91571. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Waheed, A.; Qureshi, R.A.; Burdi, D.K.; Verspohl, E.J.; Khan, N.; Hasan, M. The effect of medicinal plants of Islamabad and Murree region of Pakistan on insulin secretion from INS-1 cells. Phytother. Res. 2004, 18, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Ikram, A.; Khan, R.; Kauser, S.; Khan, A.A.; Arshad, M.T.; Ahmad, M. Taraxacum officinale (Dandelion). In Edible Flowers; Gupta, A.K., Kumar, V., Naik, B., Mishra, P., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Uttarakhand, India, 2024; pp. 281–300. [Google Scholar]
- Villiger, A.; Sala, F.; Suter, A.; Butterweck, V. In vitro inhibitory potential of Cynara scolymus, Silybum marianum, Taraxacum officinale, and Peumus boldus on key enzymes relevant to metabolic syndrome. Phytomedicine 2015, 22, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Pisonero-Vaquero, S.; González-Gallego, J.; Sánchez-Campos, S.; García-Mediavilla, M.V. Flavonoids and Related Compounds in Non-Alcoholic Fatty Liver Disease Therapy. Curr. Med. Chem. 2015, 22, 2991–3012. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.A.; Sawhney, S.S.; Jassal, M.M.S. In-vitro antidiabetic studies of various extracts of Taraxacum officinale. Pharma Innov. 2015, 4, 61–66. [Google Scholar]
- Li, J.; Luo, J.; Chai, Y.; Guo, Y.; Tianzhi, Y.; Bao, Y. Hypoglycemic effect of Taraxacum officinale root extract and its synergism with Radix Astragali extract. Food Sci. Nutr. 2021, 9, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Frolova, A.S.; Fokina, A.D.; Milentyeva, I.S.; Asyakina, L.K.; Proskuryakova, L.A.; Prosekov, A.Y. The Biological Active Substances of Taraxacum officinale and Arctium lappa from the Siberian Federal District. Int. J. Mol. Sci. 2024, 25, 3263. [Google Scholar] [CrossRef]
- Juee, L.Y.M.; Naqishbandi, A.M. In vivo and in vitro antidiabetic potential of Taraxacum officinale root extracts. Curr. Issues Pharm. Med. Sci. 2020, 33, 168–175. [Google Scholar] [CrossRef]
- Zolotova, D.; Teterovska, R.; Bandere, D.; Lauberte, L.; Niedra, S. Antidiabetic Properties of the Root Extracts of Dandelion (Taraxacum officinale) and Burdock (Arctium lappa). Plants 2024, 13, 1021. [Google Scholar] [CrossRef] [PubMed]
- Nnamdi, C.C.; Uwakwe, A.A.; Chuku, L.C. Hypoglycemic effects of aqueous and ethanolic extracts of dandelion (Taraxacum officinale FH Wigg.) leaves and roots on streptozotocin-induced albino rats. Glob. J. Res. Med. Plants Indig. Med. 2012, 1, 211. [Google Scholar]
- Kim, M.Y.; Kim, M.H.; Son, C.; Yook, H.; Kim, J.H.; Cho, Y.; Chun, H.; Kim, M.R. Leafy vegetable mix supplementation improves lipid profiles and antioxidant status in C57BL/6J mice fed a high fat and high cholesterol diet. J. Med. Food. 2009, 12, 877–884. [Google Scholar] [CrossRef]
- Hu, C.; Kitts, D.D. Dandelion (Taraxacum officinale) flower extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro. Phytomedicine 2005, 12, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Tfayli, H.; Bacha, F.; Gungor, N.; Arslanian, S. Phenotypic type 2 diabetes in obese youth: Insulin sensitivity and secretion in islet cell antibody-negative versus -positive patients. Diabetes 2009, 58, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P. Nutrients and oxidative stress: Friend or foe? Oxid. Med. Cell Longev. 2018, 1, 9719584. [Google Scholar] [CrossRef] [PubMed]
- Perumal, N.; Nallappan, M.; Shohaimi, S.; Kassim, N.K.; Tee, T.T.; Cheah, Y.H. Synergistic antidiabetic activity of Taraxacum officinale (L.) Weber ex FH Wigg and Momordica charantia L. polyherbal combination. Biomed. Pharmacother. 2022, 145, 112401. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Tousch, D.; Lajoix, A.D.; Hosy, E.; Azay-Milhau, J.; Ferrare, K.; Jahannault, C.; Cros, G.; Petit, P. Chicoric acid, a new compound able to enhance insulin release and glucose uptake. Biochem. Biophys. Res. Commun. 2008, 377, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef]
- Jarald, E.; Joshi, S.B.; Jain, D.C. Diabetes and herbal medicines. Iran. J. Pharmacol. Ther. 2008, 7, 97–106. [Google Scholar]
- Prabhakar, P.K.; Doble, M.A. A target-based therapeutic approach towards diabetes mellitus using medicinal plants. Curr. Diabetes Rev. 2008, 4, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Lis, B.; Jedrejek, D.; Rywaniak, J.; Soluch, A.; Stochmal, A.; Olas, B. Flavonoid Preparations from Taraxacum officinale L. Fruits—A Phytochemical, Antioxidant and Hemostasis Studies. Molecules 2020, 25, 5402. [Google Scholar] [CrossRef] [PubMed]
- Kania-Dobrowolska, M.; Baraniak, J. Dandelion (Taraxacum officinale L.) as a source of biologically active compounds supporting the therapy of co-existing diseases in metabolic syndrome. Foods 2022, 11, 2858. [Google Scholar] [CrossRef] [PubMed]
- Nworu, C.; Akah, P. Anti-inflammatory medicinal plants and the molecular mechanisms underlying their activities. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 52–61. [Google Scholar] [CrossRef]
- Nofal, A.E.; Shaaban, A.M.; Ibrahim, H.M.; Abouelmagd, F.; Mohamed, A.H. In Vivo Antischistosomicidal and Immunomodulatory Effects of Dietary Supplementation with Taraxacum officinale. J. Xenobiot. 2024, 14, 1003–1022. [Google Scholar] [CrossRef]
- Shekarabi, S.P.H.; Mostafavi, Z.S.; Mehrgan, M.S.; Islami, H.R. Dietary supplementation with dandelion (Taraxacum officinale) flower extract provides immunostimulation and resistance against Streptococcus iniae infection in rainbow trout (Oncorhynchus mykiss). Fish Shellfish. Immunol. 2021, 118, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Tanasa (Acretei), M.V.; Negreanu-Pirjol, T.; Chifiriuc, M.C.; Popoviciu, D.R.; Rosoiu, N. Bioactive Principles Content and Antioxidant Activity of Indigenous Dandelion (Taraxacum officinale L.) from Dobrudja Region of Romania. Int. J. Adv. Innov. Res. 2022, 9, 29–37. [Google Scholar]
- Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Yin, Y.; Kim, K.; Kumar Saha, S.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids. Int. J. Mol. Sci. 2016, 17, 569. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Dong, R. Integrated Microbiome-Metabolomics Analysis Reveals the Potential Mechanism of Dandelion Root Polysaccharides to Ameliorate Ulcerative Colitis. Metabolites 2024, 14, 351. [Google Scholar] [CrossRef]
- Lee, M.H.; Kang, H.; Lee, K.; Yang, G.; Ham, I.; Bu, Y.; Kim, H.; Choi, H.Y. The aerial part of Taraxacum coreanum extract has an anti-inflammatory effect on peritoneal macrophages in vitro and increases survival in a mouse model of septic shock. J. Ethnopharmacol. 2013, 146, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.J. Effect of water extracts from root of Taraxacum officinale on innate and adaptive immune responses in mice. Korean J. Food Nutr. 2008, 21, 275–282. [Google Scholar]
- Rehman, G.; Hamayun, M.; Iqbal, A.; Khan, S.A.; Khan, H.; Shehzad, A.; Lee, I.J. Effect of methanolic extract of dandelion roots on cancer cell lines and AMP-activated protein kinase pathway. Front. Pharmacol. 2017, 8, 875. [Google Scholar] [CrossRef]
- Nguyen, C.; Mehaidli, A.; Baskaran, K.; Grewal, S.; Pupulin, A.; Ruvinov, I.; Scaria, B.; Parashar, K.; Vegh, C.; Pandey, S. Dandelion root and lemongrass extracts induce apoptosis, enhance chemotherapeutic efficacy, and reduce tumour xenograft growth in vivo in prostate cancer. Evid. Based Complement. Alternat Med. 2019, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Nassan, M.A.; Soliman, M.M.; Ismail, S.A.; El-Shazly, S. Effect of Taraxacum officinale extract on PI3K/Akt pathway in DMBA-induced breast cancer in albino rats. Biosci. Rep. 2018, 38, 11–24. [Google Scholar] [CrossRef]
- Sigstedt, S.C.; Hooten, C.J.; Callewaert, M.C.; Jenkins, A.R.; Romero, A.E.; Pullin, M.J.; Kornienko, A.; Lowrey, T.K.; Slambrouck, S.V.; Steelant, W.F. Evaluation of aqueous extracts of Taraxacum officinale on growth and invasion of breast and prostate cancer cells. Int. J. Oncol. 2008, 32, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhao, H.; Zhang, L.; Xu, J.; Zhu, C.; Zhao, H.; Lv, G. Dandelion root extract suppressed gastric cancer cells proliferation and migration through targeting lncRNA-CCAT1. Biomed. Pharmacother. 2017, 93, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Menke, K.; Schwermer, M.; Felenda, J.; Beckmann, C.; Stintzing, F.; Schramm, A.; Zuzak, T.J. Taraxacum officinale extract shows antitumor effects on pediatric cancer cells and enhance mistletoe therapy. Complement. Ther. Med. 2018, 40, 158–164. [Google Scholar] [CrossRef]
- Cen, L.H.; Xiao, R.L.; Li, M.; Wang, Z.Z.; Chang, B.M. Antitumor and anti-inflammatory mechanisms of Taraxacum: Research advances, J. Int. Pharm. Res. 2020, 47, 954–961. [Google Scholar]
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 1–12. [Google Scholar] [PubMed]
- Schmitz, M.L.; Kracht, M. Cyclin-Dependent Kinases as Coregulators of Inflammatory Gene Expression. Trends Pharmacol. Sci. 2016, 37, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Chen, J.T.; Yeh, L.J.; Yang, R.C.; Huang, S.M.; Chen, T.W. Characteristics of the cytotoxicity of Taraxacum mongolicum and Taraxacum formosanum in human breast cancer cells. Int. J. Mol. Sci. 2022, 23, 11918. [Google Scholar] [CrossRef] [PubMed]
- Yucheng, S.; Xiaowei, L.; Guangzhe, P. Study on the effects of Taraxerol on proliferation and metastasis of gastric cancer cells through Hippo and Wnt pathway. J. Med. Sci. Univ. 2021, 44, 9–12. [Google Scholar]
- Yaoi, X.; Lu, B.; Lu, C.; Bai, Q.; Yan, D.; Xu, H. Taraxerol Induces Cell Apoptosis through Mitochondria-Mediated Pathway in HeLa Cells. Cell J. 2017, 19, 512–519. [Google Scholar]
- Zhang, Z.; Di Yan, C.; Yan, B.; Gao, Q.; Nan, X.; He, T. Effects of taraxerol on proliferation and apoptosis of bladder cancer T24 cells Chin. J. Clin. Pharmacol. 2020, 36, 2682–2685. [Google Scholar]
- Xia, Y.T.; Zhang, Y.Q.; Chen, L.; Min, L.; Huang, D.; Zhang, Y.; Li, C.; Li, Z.H. Suppression of migration and invasion by taraxerol in the triple-negative breast cancer cell line MDA-MB-231 via the ERK/Slug axis. PLoS ONE 2023, 18, e0291693. [Google Scholar] [CrossRef] [PubMed]
- Fulga, A.; Casian, A.; Casian, I.; Protopop, S.; Gudumac, V.; Tagadiuc, O. Effects of Taraxacum officinale on Glioblastoma Cell Culture and Their Correlation with Hydroxycinnamic Acids Content. Med. Sci. Forum 2023, 21, 18. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, K.; Jin, Y.; Fu, Y.; Wang, R.; Zhang, J. Protective Effects of Taraxasterol against Deoxynivalenol-Induced Damage to Bovine Mammary Epithelial Cells. Toxins 2022, 14, 211. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.; Kewlani, P.; Singh, L.; Rawat, S.; Bhatt, I.D.; Sundriyal, R.C.; Pande, V. A review on natural bioactive compounds of Taraxacum officinale Weber: A potential anticancer plant. Pharm. Pharmacol. Rep. 2024, 3, 9. [Google Scholar] [CrossRef]
- Aras, A.İ.; Arslan, E.; Özçay, B. In vitro Cytotoxicity Evaluation of Dandelion Root Ethanol Extract on PANC-1 Cell Line. Mutiara Med. J. Kedokt. Dan Kesehat. 2024, 24, 66–70. [Google Scholar] [CrossRef]
- Mao, B.; Wang, G. MicroRNAs involved with hepatocellular carcinoma (Review). Oncol. Rep. 2015, 34, 2811–2820. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.; Fan, X. Anti-tumor effect of dandelion flavone on multiple myeloma cells and its mechanism. Discov. Oncol. 2024, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Lis, B.; Juskiewicz, J.; Ognik, K.; Jedrejek, D.; Stochmal, A.; Olas, B. The composition and vascular/antioxidant properties of Taraxacum officinale flower water syrup in a normal-fat diet using an obese rat model. J. Ethnopharmacol. 2021, 265, 113393. [Google Scholar] [CrossRef] [PubMed]
- Ozougwu, J.C. The role of reactive oxygen species and antioxidants in oxidative stress. Int. J. Res. 2016, 1, 1–8. [Google Scholar]
- Salzano, S.; Checconi, P.; Hanschmann, E.M.; Lillig, C.H.; Bowler, L.D.; Chan, P.; Vaudry, D.; Mengozzi, M.; Coppo, L.; Sacre, S.; et al. Linkage of Inflammation and Oxidative Stress via Release of Glutathionylated Peroxiredoxin-2, Which Acts as a Danger Signal. Proc. Natl. Acad. Sci. USA 2014, 111, 12157–12162. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.; Ahswin, H.; Smart, N.; Bayon, Y.; Wohlert, S.; Hunt, J.A. Reactive Oxygen Species ROS-A Family of Fate Deciding Molecules Pivotal in Constructive Inflammation and Wound Healing. Eur. Cells Mater. 2012, 24, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Naik, E.; Dixit, V.M. Mitochondrial Reactive Oxygen Species Drive Proinflammatory Cytokine Production. J. Exp. Med. 2011, 208, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Valente, A.J. Nuclear Factor Kappa B Activation by NADPH Oxidases Mech. Ageing Dev. 2004, 125, 799–810. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Sharma, A.K.; Kumar, S.; Saxena, A.K.; Pandey, A.K. Bauhinia Variegata Leaf Extracts Exhibit Considerable Antibacterial, Antioxidant, and Anticancer Activities. BioMed Res. Int. 2013, 2013, 915436. [Google Scholar] [CrossRef] [PubMed]
- Hagymasi, K.; Blazovics, A.; Feher, J.; Lugasi, A.; Kristo, S.T.; Kery, A. The in vitro effect of dandelions antioxidants on micro- somal lipid peroxidation. Phytother. Res. 2000, 14, 43–44. [Google Scholar] [CrossRef]
- Wang, H.B. Effect of Dandelion Polysaccharides on the Retardation of the Quality Changes of White Shrimp. Int. J. Biol. Macromol. 2014, 68, 205–208. [Google Scholar] [CrossRef]
- Khan, A.S.; Arif, K.; Munir, B.; Kiran, S.; Jalal, F.; Qureshi, N.; Hassan, S.M.; Soomro, G.A.; Nazir, A.; Ghaffar, A.; et al. Estimating total phenolics in Taraxacum officinale (L.) extracts. Pol. J. Environ. Stud. 2019, 28, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Park, C.M. Alleviated oxidative damage by Taraxacum officinale through the induction of Nrf2-MAPK/PI3K mediated HO-1 activation in murine macrophages RAW 264.7 cell line. Biomolecules 2019, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Jedrejek, D.; Lis, B.; Rolnik, A.; Stochmal, A.; Olas, B. Comparative phytochemical, cytotoxicity, antioxidant, and haemostatic studies of Taraxacum officinale root preparations. Food Chem. Toxicol. 2019, 126, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.Y.; Zang, E.H.; Lv, L.J.; Li, Q.Y.; Zhang, C.H.; Xia, Y.; Zhang, L.; Dang, L.S.; Li, M.H. Flavonoids in myocardial ischemia-reperfusion injury: Therapeutic effects and mechanisms. Chin. Herb. Med. 2020, 13, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Cebova, M.; Pechanova, O. Protective Effects of Polyphenols against Ischemia/Reperfusion Injury. Molecules 2020, 25, 3469. [Google Scholar] [CrossRef] [PubMed]
- Radoman, K.; Zivkovic, V.; Zdravkovic, N.; Chichkova, N.V.; Bolevich, S.; Jakovljevic, V. Effects of dandelion root on rat heart function and oxidative status. BMC Complement. Med. Ther. 2023, 23, 78. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Nima, L.; Wang, S.; Tan, J. Ultrasound assisted hot water extraction of polysaccharides from Taraxacum mongolicum: Optimization, purification, structure characterization, and antioxidant activity. Int. J. Food Sci. 2024, 89, 2827–2842. [Google Scholar] [CrossRef] [PubMed]
- Wójciak, K.; Materska, M.; Pełka, A.; Michalska, A.; Małecka-Massalska, T.; Kačániová, M.; Čmiková, N.; Słowiński, M. Effect of the Addition of Dandelion (Taraxacum officinale) on the Protein Profile, Antiradical Activity, and Microbiological Status of Raw-Ripening Pork Sausage. Molecules 2024, 29, 2249. [Google Scholar] [CrossRef]
- Wu, X.; Li, N.; Dong, Z.; Yin, Q.; Zhou, T.; Zhu, L.; Yan, H.; Chen, Z.; Zhai, K. Extraction, Purification, Sulfated Modification, and Biological Activities of Dandelion Root Polysaccharides. Foods 2024, 13, 2393. [Google Scholar] [CrossRef]
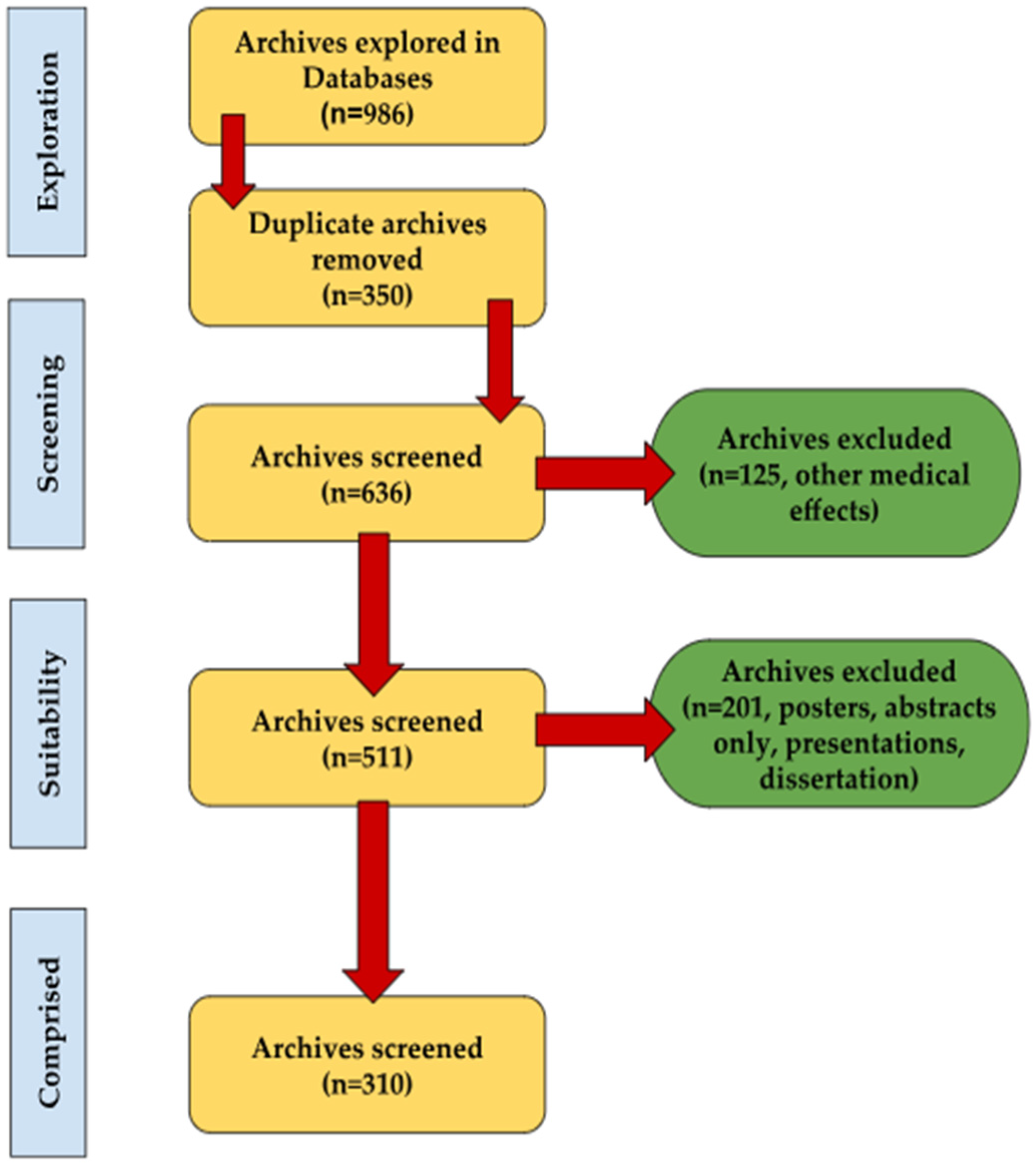

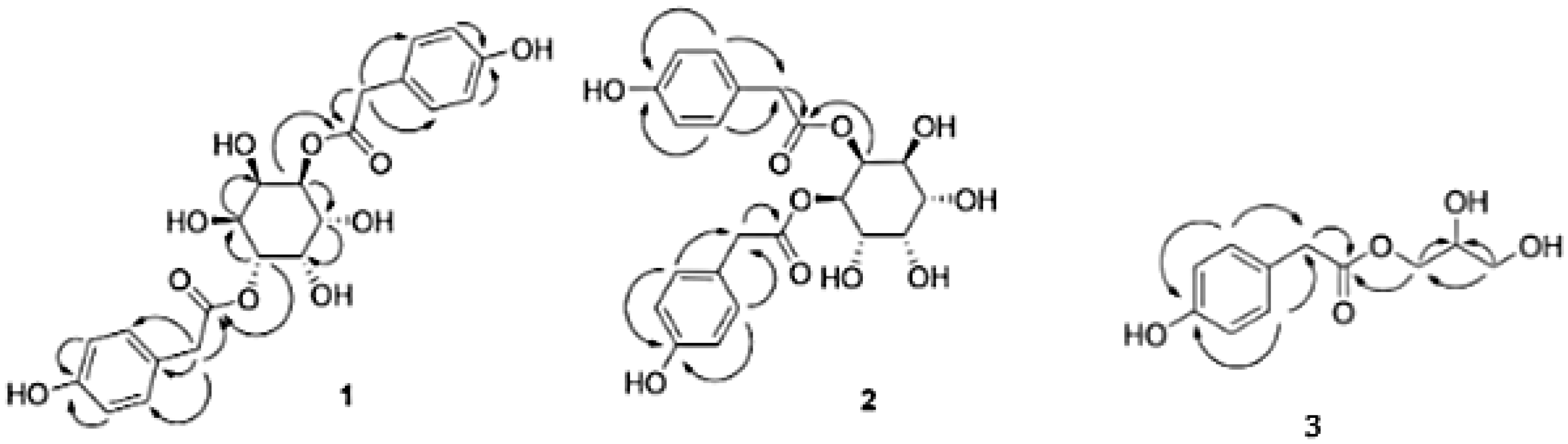

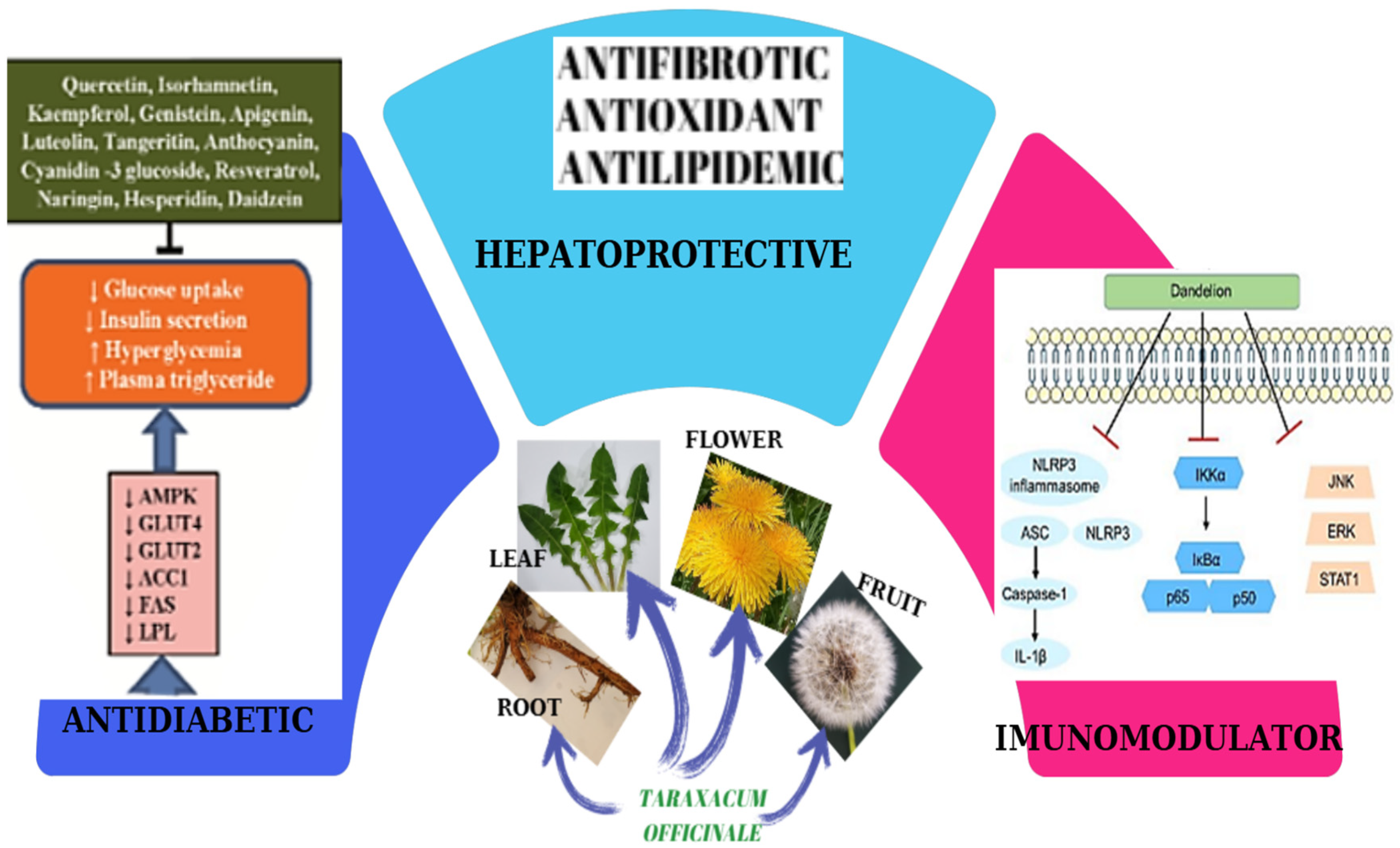

| Compounds | Name and Structure |
|---|---|
| Sesquiterpenoids |  Taraxafolide Taraxinic acid β-D-glucopyranosyl ester |
 Taraxinic acid β-glucopyranosyl ester 11β,13-Dihydrotaraxinic acid | |
| Phenolics | 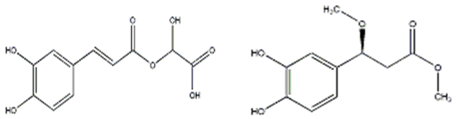 (+)-Taraxafolin B Taraxafolin |
| Triterpenoids | 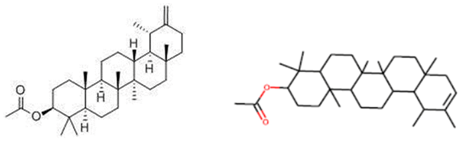 Taraxasteryl acetate Ψ-taraxasteryl acetate |
| Other types |  Taraxacine A Taraxacine B |
 Taraxinositol A Taraxinositol B |
| Vegetal Organ/Phytocompounds | Type of Experiment | Results | Reference |
|---|---|---|---|
| T. o. root extract | human neutrophils | MeOH extract inhibits the formation of leukotriene B4 | [57] |
| T. o. extract | primary cultures of rat astrocytes | treatment of TO (100 and 1000 µg/mL) to astrocytes inhibited the TNF-alpha production by inhibiting Interleukin-1 production | [157] |
| T. o. leaves aqueous extracts | rat mammary microvascular endothelial cells | inhibit both TNF-α and ICAM-1 expression | [158] |
| T. o. dried herbs from Pharmacy | in vivo, octapeptide-induced acute pancreatitis in rats | treatment of TO 10 mg/kg orally administered increased pancreatic levels of HSP60 and HSP72 and decreased the secretion of IL-6 and TNF-α | [159] |
| T. o. dried plant | chicken chorioallantoic assay | ethanol extracts diminished leukocyte levels | [161] |
| Taraxasterol | BV2 microglia cells culture | dose-dependently inhibited LPS-induced production of TNF-α and IL-1β, suppressed NF-κB activation, and activated the LXRα-ABCA1 signaling pathway | [162] |
| T.o. extract | acute lung injury induced by lipopolysaccharide in mice | oral administration of 2.5, 5, and 10 mg/kg significantly inhibited the inflammatory cytokines TNF-α and IL-6 in the bronchoalveolar lavage fluid | [163] |
| T.o. luteolin, chicoric acid | RAW 264.7 cells | luteolin activates the NF-κB and Akt pathways, while chicoric acid enhances luteolin’s anti-inflammatory activity by attenuating NF-κB activation. | [165] |
| T. o. leaves extract | ovalbumin-sensitized guinea-pig trachea | reduced monocytes, lymphocytes, neutrophils, eosinophils, and basophils | [166] |
| T. o. ethanolic leaf extract | ovalbumin-sensitized guinea-pigs | infiltration of eosinophils and basophils were reduced in the lungs | [167] |
| T. o. taraxasterol | RAW 264.7 macrophages | inhibit mRNA and protein expression levels of iNOS and COX-2 | [168] |
| T. o. taraxasterol | murine model of endotoxic shock on mice | reduced TNF-α, IFN-γ, IL-1β, IL-6, NO and PGE2 levels in sera | [169] |
| T. o. lipopolysaccharide | RAW 264.7 cells | inhibited NF-κB-mediated inflammation and enhanced Nrf2-mediated antioxidative activity by modulating the PI3K/Akt pathway | [170] |
| T. m. taraxasterol | concanavalin A-induced acute hepatic injury in mice | inhibited pro-inflammatory cytokines TNF-α, IL-6, IL-1β, interferon-γ (IFN-γ), and IL-4 | [171] |
| T. o. aerial parts, polyphenolic tincture | rat turpentine-induced inflammation model | Reduced serum oxidative markers: malondialdehyde (MDA), thiols (SH), and nitrites/nitrates (NOx), NF-κB levels | [172] |
| T. c. ethanol extract of aerial parts | lipopolysaccharide-stimulated Caco-2 cells | 100 μg/mL extract reduced inducible NO synthase, cyclooxygenase-2, TNF-α, IL-6, and IL-1β | [173] |
| T. o. taraxasterol | drug (APAP-induced liver injury AML12 cells and mice) | promoted Nrf2 and HO-1 expression, suppressed JNK phosphorylation, and decreased the Bax/Bcl-2 ratio and caspase-3 expression | [174] |
| T. o. tincture | rat isoprenaline-induced myocardial infarction models | administration of the tincture led to a decrease in aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine kinase-MB (CK-MB), and nuclear factor kappa B (NF-κB) levels | [175] |
| Vegetal Organ/Phytocompounds | Type of Experiment | Results | Reference |
|---|---|---|---|
| T. f. and T. m. methanol and aqueous extract | in vitro HSV-1/Vero cells system | 15.63 μg/mL concentrations of acyclovir showed 100% protection against HSV-1, and 195.31 μg/mL methanol extract of T. mirabile only showed 7.08% protection | [181] |
| T. o. chloroform and exane leaf extracts | lavivirus, using the 17D vaccine strain of yellow fever virus as a model | Extract from leaves of 5 months of growth is 8 times more effective than extract from leaves of 2 months of growth | [189] |
| T. o. methanol extract of leaves | human hepatoma (Huh-7) and CHO cell lines transfected with pCR3.1/Flagtag/HCV NS5B gene cloned vector (genotype 1a) | 65% inhibition of NS5B expression was documented at nontoxic dose concentration (200 μg/mL) | [190] |
| T. o. methanol extract of leaves | replication of dengue virus serotype 2 in baby hamster kidney BHK-21 cells | extracts at 60 °C showed higher inhibitory effects and present bioactive compounds: luteolin, caffeoylquinic acids, quercetin diclycosides | [191] |
| T. o. water extract of leaves | wild type and mutant forms of SARS-CoV-2 in human HEK293 -hACE2 kidney and A549-hACE2 TMPRSS2 lung cells | Infection of the lung cells was efficiently prevented, and so was virus-triggered pro-inflammatory interleukin 6 secretion | [193] |
| T. c. water extract | viral proteins NS2B/NS3 (DENV-2), NS5B (HCV), and ICP27 (HSV-1) | inhibitory effects of chlorogenic acid (for DENV-2 and HCV), rutin (for HCV and HSV-1), and rosmarinic acid (for DENV-2 and HCV) | [195] |
| Vegetal Organ/Phyto-Compounds | Type of Experiment | Results | Reference |
|---|---|---|---|
| T. o. root powder (capsule contained 500 mg) | male albino mice of the CD1 strain and laboratory-raised snails of the species Biomphalaria alexandrina were infected with Schistosoma mansoni miracidia. | combined treatment with praziquantel and Taraxacum root resulted in the most significant improvements—morphological changes in adult worms, reduced worm burden and egg count, decreased granuloma size, altered immune cell distribution, and modulated cytokine levels compared to the infected untreated group | [261] |
| T. o. flower extract | immune response and disease resistance in rainbow trout fed with the extract fed with 3 and 4 g/kg of the extract | dietary supplementation with the extract at 3 g/kg significantly increased total leukocyte and lymphocyte counts, immunoglobulin M, total protein, and lysozyme levels in fish. Additionally, the expression of interleukin-1β and interleukin-6 genes were upregulated in fish fed 3 and 4 g/kg of the extract. The recommended dietary dose is 2.49–2.74 g/kg. | [262] |
| T. c. aerial part chloroform fraction | isolated mouse peritoneal macrophages stimulated in vitro with interferon-γ (IFN-γ) and lipopolysaccharide in a mouse model of lethal septic shock | suppressed the production of nitric oxide (NO) and prostaglandin E2 (PGE2) as inflammatory mediators and repressed the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2); blocked the activation signaling pathways: NF-κB, MAPK, and STAT1 | [266] |
| T. o. hot-water and cold-water extract of roots | innate and adaptive immune responses in mice | thioglycollate-induced macrophages cultured with TO-100 and TO-4 produced a significantly higher quantity of various cytokines, such as IL-6 and IL-12 | [267] |
| T. o. methanolic extract of roots | HepG2, MCF7, HCT116, and normal Hs27 cell lines | 500 µg/mL inhibited the growth of HepG2 cells, and a less pronounced effect on MCF7 and HCT116 cells was registered. No significant effect was observed on Hs27 cells. Also, the treatment improved the phosphorylation of AMPK in HepG2 cells, a key issue in cancer therapy | [268] |
| Vegetal Organ/Phyto-Compounds | Type of Experiment | Results | Reference |
|---|---|---|---|
| Chicoric acid | high-fat diet mice treated with chicoric acid | inhibited the protein expressions of peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer-binding protein α (C/EBPα), increasing the activity of antioxidant enzymes and total antioxidant capacity in the liver, regulated the levels of leptin and adiponectin | [230] |
| T. o. methanol fruit extract | estimating antiradical, antiplatelet, and antioxidant properties related to hemostasis | significantly reduced plasma lipid peroxidation and protein thiol oxidation stimulated by H2O2/Fe at the highest tested concentrations of 10 and 50 µg/mL. | [258] |
| T. o. root | male CD1 strain albino mice and laboratory-bred snails (Biomphalaria alexandrina) infected with S. mansoni miracidia | potent antioxidant activity at a significant level, suggesting superior scavenging efficacy | [261] |
| T. o. flower water syrup | obese male albino-Wistar rats | increased plasma superoxide dismutase (SOD) activity (1.6-fold) and decreased lipid peroxidation (MDA, 0.81-fold), modulating ACh-induced relaxation but not carbon monoxide-releasing molecule-2 (CORM-2)-induced relaxation in isolated thoracic arteries | [287] |
| T. o. flower ethanol extract | bacterial-lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells | suppressed the stable DPPH radical in a concentration-dependent manner, also exhibited a significant synergistic effect with a-tocopherol in scavenging DPPH radical | [288] |
| T. o. water and ethanol extract | RAW 264.7 cells | both extracts dose-dependently induced HO-1 expression without any cytotoxicity. Water extract activated HO-1 via PI3K/Akt and JNK phosphorylation, while ethanol extract used PI3K/Akt. The antioxidant potential was confirmed by HO-1 inducer (CoPP) and inhibitor (SnPP). Both extracts dose-dependently induced HO-1 expression without cytotoxicity | [297] |
| T. o. water leaves extract | effect of adding T.O extract on the characteristics of raw-ripening pork sausages while reducing the nitrite addition from 150 to 80 mg/kg | dandelion addition (0.5–1%) significantly improved the oxidative stability of nitrite-reduced (80 mg/kg) raw-ripening sausages, as evidenced by ABTS+ and DPPH radical scavenging activity and TBARS assays | [304] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanasa, M.-V.; Negreanu-Pirjol, T.; Olariu, L.; Negreanu-Pirjol, B.-S.; Lepadatu, A.-C.; Anghel, L.; Rosoiu, N. Bioactive Compounds from Vegetal Organs of Taraxacum Species (Dandelion) with Biomedical Applications: A Review. Int. J. Mol. Sci. 2025, 26, 450. https://doi.org/10.3390/ijms26020450
Tanasa M-V, Negreanu-Pirjol T, Olariu L, Negreanu-Pirjol B-S, Lepadatu A-C, Anghel L, Rosoiu N. Bioactive Compounds from Vegetal Organs of Taraxacum Species (Dandelion) with Biomedical Applications: A Review. International Journal of Molecular Sciences. 2025; 26(2):450. https://doi.org/10.3390/ijms26020450
Chicago/Turabian StyleTanasa (Acretei), Maria-Virginia, Ticuta Negreanu-Pirjol, Laura Olariu, Bogdan-Stefan Negreanu-Pirjol, Anca-Cristina Lepadatu, Larisa Anghel (Cireasa), and Natalia Rosoiu. 2025. "Bioactive Compounds from Vegetal Organs of Taraxacum Species (Dandelion) with Biomedical Applications: A Review" International Journal of Molecular Sciences 26, no. 2: 450. https://doi.org/10.3390/ijms26020450
APA StyleTanasa, M.-V., Negreanu-Pirjol, T., Olariu, L., Negreanu-Pirjol, B.-S., Lepadatu, A.-C., Anghel, L., & Rosoiu, N. (2025). Bioactive Compounds from Vegetal Organs of Taraxacum Species (Dandelion) with Biomedical Applications: A Review. International Journal of Molecular Sciences, 26(2), 450. https://doi.org/10.3390/ijms26020450






