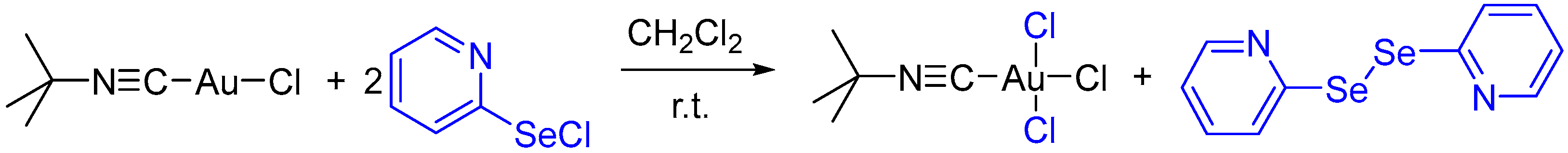Abstract
In this study, we report the first example of acyclic (amino)(N-pyridinium)carbenoid gold(III) complexes synthesized via a coupling reaction between 2-pyridylselenyl chloride and Au(I)-bound isonitriles. The reaction involves an initial oxidative addition of the Se–Cl moiety to Au(I), followed by the nucleophilic addition of the pyridine fragment to the isonitrile’s C≡N bond, furnishing a metallacycle. Importantly, this is the first example of the pyridine acting as a nucleophile towards metal-bound isonitriles. Arguably, such an addition is due to the chelate effect. The structures of the gold(III) carbenoid complexes were unambiguously established using X-ray diffraction and NMR spectroscopy. Theoretical calculations, including DFT, Natural Resonance Theory (NRT), and Meyer bond order (MBO) analyses, were used to analyze the different resonance forms. The reaction mechanism was further elucidated using DFT calculations, which identified the oxidative addition as the rate-determining step with a barrier of 29.7 kcal/mol. The nucleophilic addition proceeds with a minimal barrier, making the reaction highly favorable. The antiproliferative activity of new compounds 2a–2e was tested against two human cancer cell lines: A2780 ovarian adenocarcinoma and the A278Cis cisplatin-resistant variant.
1. Introduction
Nitrogen-stabilized carbene complexes are of paramount importance in organometallic chemistry, with applications in catalysis, the development of novel antitumor agents, etc. [1,2,3]. N-heterocyclic carbenes (NHCs) currently represent one of the most applicable classes of ancillary ligands in catalysis [4,5]. These carbenes are stable in the free state and were first isolated by Arduengo in 1991 (Scheme 1) [6].
Scheme 1.
Synthesis of isolable NHC by Arduengo.
These ligands are particularly significant due to their prominent role in organometallic chemistry and catalysis, where they have emerged as strong competitors to the widely employed phosphine ligands. Many of their complexes serve as catalysts of choice in various organic transformations. Additionally, their electronic and geometric properties can be modified more easily than those of phosphines, offering greater versatility and tunability in catalytic applications.
The most popular NHCs are typically derived from imidazolium salts and, to a lesser extent, imidazolidinium salts. These carbenes are generated through the action of strong bases on the respective salts. Numerous methods have been developed for synthesizing various precursors of NHCs [7]. The significance of this class of ligands has driven extensive research on diverse approaches to the synthesis of their precursors. While these methods are not detailed in this work, they are comprehensively summarized in several review papers [7,8,9,10,11,12,13].
In contrast, acyclic diaminocarbenes (ADCs) have received less attention. Importantly, ADCs have demonstrated their ability to act as strong σ-donor ligands. In 2002, Herrmann showed that ADC ligands induce greater electron density at Rh(I) compared to both saturated and unsaturated five-membered NHCs, as evidenced by the ν(CO) values of the square planar Rh(I) carbonyl complexes [14].
However, ADC complexes have one significant advantage over NHC complexes, which is their synthesis via nucleophilic addition to metal-activated isonitriles [15,16,17,18,19,20,21,22,23,24,25]. This route unlocks structures with easily variable steric and electronic properties.
Gold(I) and (III) have shown remarkable efficacy in promoting nucleophilic addition to isonitriles producing ADC complexes [26,27,28,29,30]. The latter has shown promise in catalysis and antiproliferative activity, which is widely being explored currently [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46].
Here, we describe the addition of 2-pyridylselenyl chloride to gold(I)-bound isonitriles producing acyclic (amino)(N-pyridinium)carbenoid gold(III) complexes. This is the first example of pyridine acting as a nucleophile in metal-mediated addition to isonitriles. Moreover, novel structures were tested against two human cancer cell lines.
2. Results and Discussion
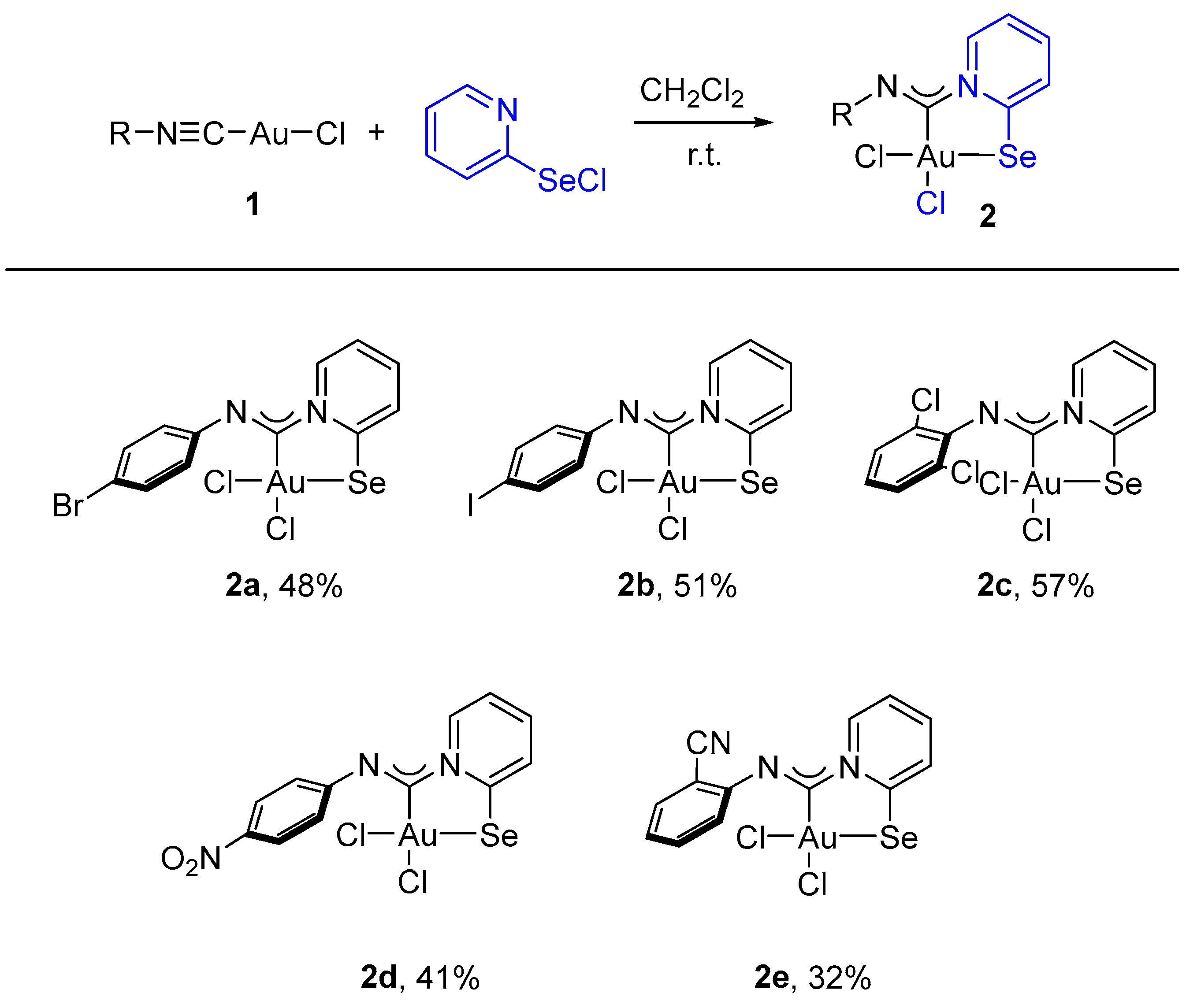
For this study, we used Au(I) isocyanide complexes, which were obtained in situ by reacting the corresponding isocyanides with [(Me2S)AuCl] in dichloromethane. The addition of 2-pyridylselenyl chloride to solutions of Au(I) isocyanide complexes in CH2Cl2 resulted in a gradual formation of orange precipitates (Scheme 2). The isolation and analysis of the solids suggested the formation of the adducts of 2-pyridylselenyl chloride with isocyanide-gold(I) chloride in 32–57% yields (Scheme 2).
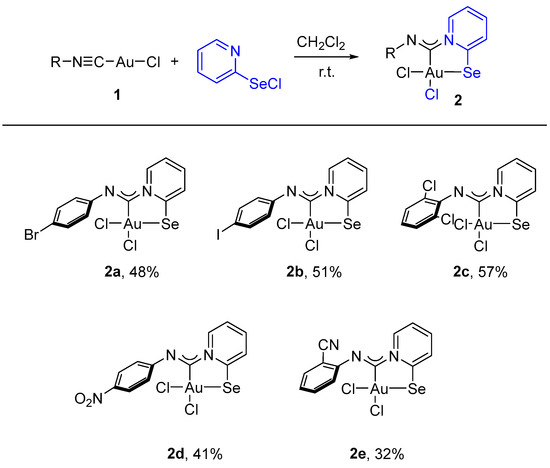
Scheme 2.
Synthesis of 2a–2e.
Complexes 2a–2e are stable at room temperature and exhibit very poor solubility in most organic solvents. However, they are moderately soluble in highly polar solvents such as DMF or DMSO, which allows their characterization by the NMR technique. The 13C NMR spectra of 2a–2e (see Section 3) exhibited new characteristic peaks in the range of 163–153 ppm, indicating carbenoid species formation.
Interestingly, aliphatic isocyanide gold(I) complexes did not produce carbenoid species like 2a–2e upon reaction with 2-PySeCl. The addition of 2-PySeCl to tert-butyl isocyanide gold(I) chloride resulted in the formation of isocyanide Au(III) trichloride and 2,2′-dipyridyl diselenide as products (Scheme 3). In this case, 2-PySeCl acted as a chlorinating agent.

Scheme 3.
Oxidation of tert-butyl isocyanide gold(I) chloride to gold(III) trichloride by 2-PySeCl.
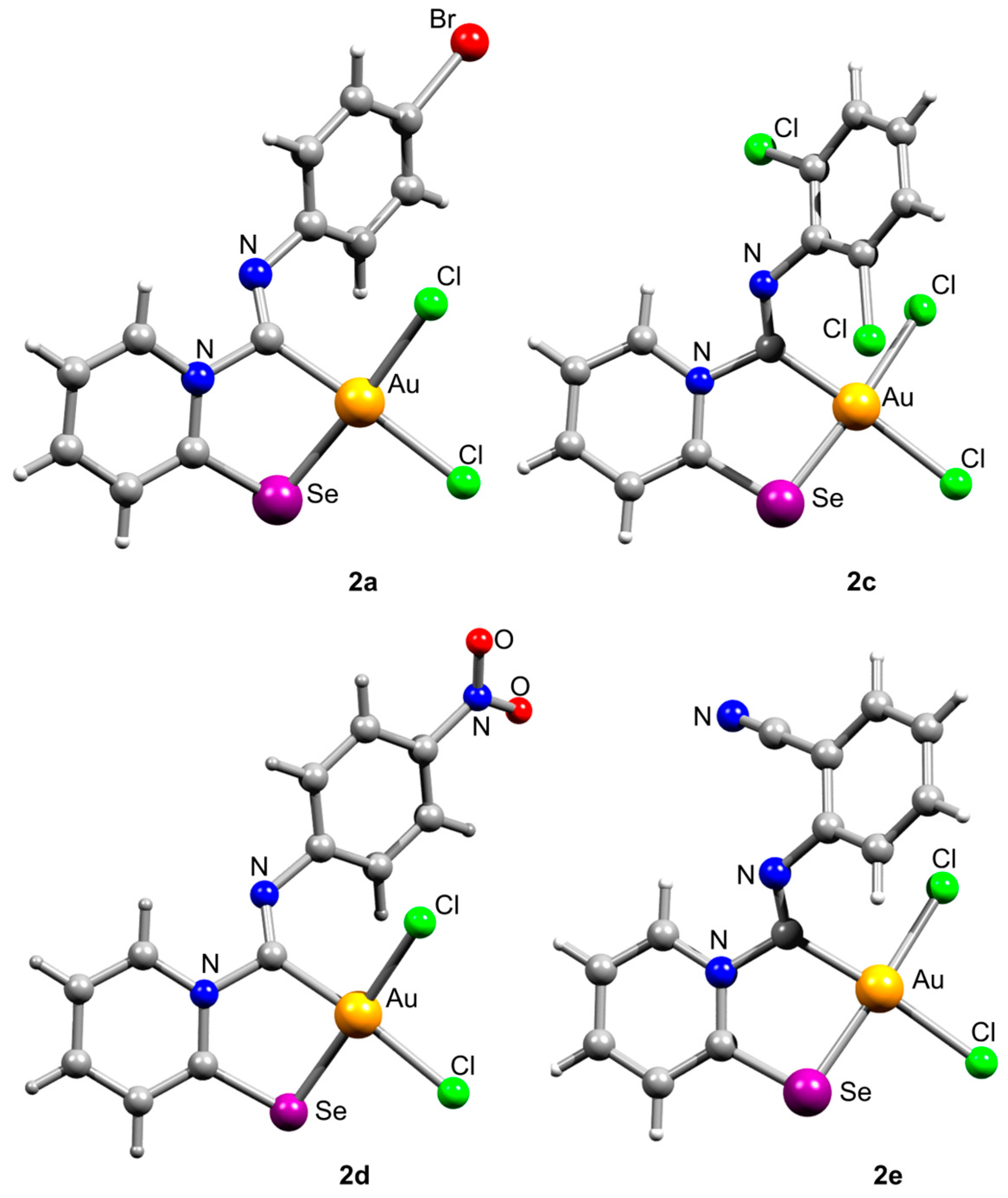
The carbenoid complexes 2a and 2c–2e could be recrystallized from dichloromethane to produce crystals suitable for X-ray single-crystal analysis, which confirmed the formation of Au(III) carbenoid complexes (Figure 1, Tables S1–S13 and Figures S1–S4).
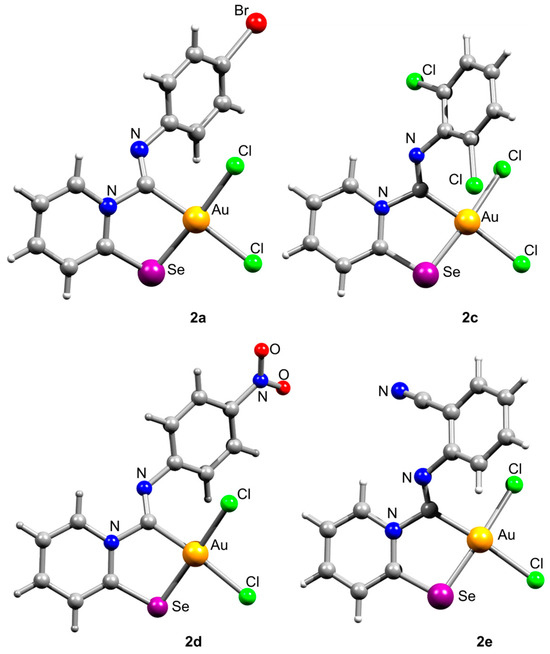
Figure 1.
Ball-and-stick representations of crystal structures of 2a and 2c–2e. Gray and light-gray spheres represent carbon and hydrogen, respectively.
Single crystals of 2e contain dichloromethane molecules, while the other complexes crystallize without a solvent. The asymmetric unit of 2c contains two independent molecules. For all complexes, Au(III) achieves a distorted square geometry with a twisted disposition of two chlorine and C, Se atoms (Figure 1, Table 1). The Au–C distance is the shortest, and the Au–Se distance is the longest.

Table 1.
Selected interatomic distances and angles (Å, °) in 2a and 2c–2e.
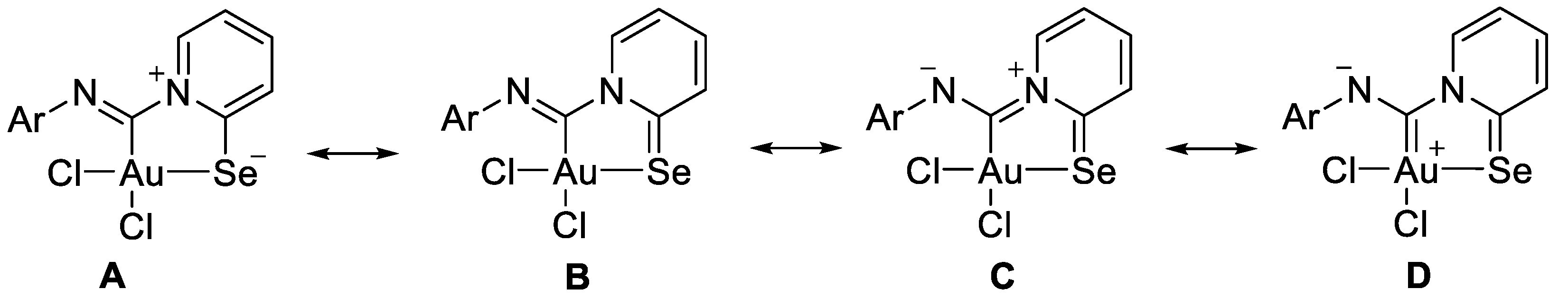
The structures of 2a–2e could be described by the mesomeric forms A–D (Scheme 4).
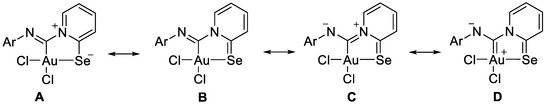
Scheme 4.
Mesomeric forms for AuIII carbenoid complexes 2a–2e.
The bond distances in the ligand demonstrate that the main mesomeric form for 2-pyridine-selenyl carbene complexes is mesomeric form A with single C–Se and double N=C(carbenoid) bonds. The length of the C–NPh bond is affected by electronic effects from substituents at different positions in the phenyl ring. The ligand chelates the gold(III) atom with the formation of a rigid five-membered cycle; however, the angle between the pyridine ring and the mean plane formed by the AuCl2 fragment varies from 20.1(2) to 36.50(13). The angle between an aryl ring and the pyridine cycle is equal to 15.3(3)–60.4(10) due to rotation around a single N–C bond.
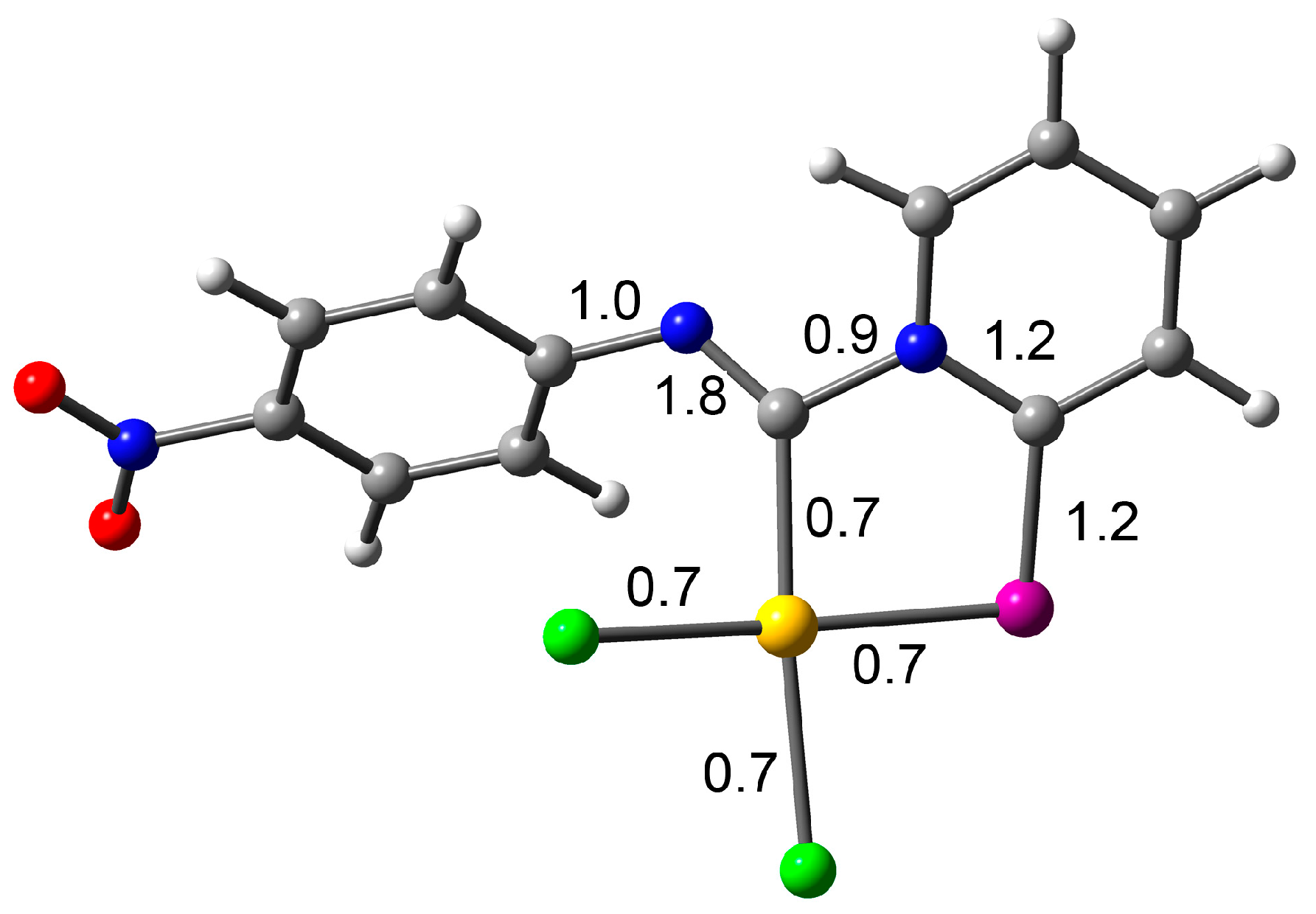
To gain a deeper insight into which mesomeric forms (Scheme 4) contribute most to the final structure, we performed additional theoretical calculations. Specifically, the percentage of each form was computed using Natural Resonance Theory (NRT) within the Natural Bond Orbital (NBO) framework, along with Meyer bond order (MBO) values. The NRT results indicate negligible contributions from forms C and D, while form A dominates, with a 70% contribution. This aligns well with the MBO analysis (see Figure 2), which shows that the Npy–C(Au) bond has a bond index below 1, effectively ruling out any significant contribution from resonance form C, which involves a Npy=C(Au) double bond. The MBO values for all coordination bonds are approximately 0.7, typical of coordination bonds with bond orders less than 1, further excluding any double bond character between the gold and carbon atoms, thereby dismissing resonance form D. Additionally, the MBO value of nearly 2 for the Nph–C(Au) bond is consistent with forms A and B, confirming no contribution from forms C or D. Finally, the MBO value of 1.2 for both the C–Se and pyridine C–N bonds suggests some contribution from resonance form B, corroborated by NRT calculations.
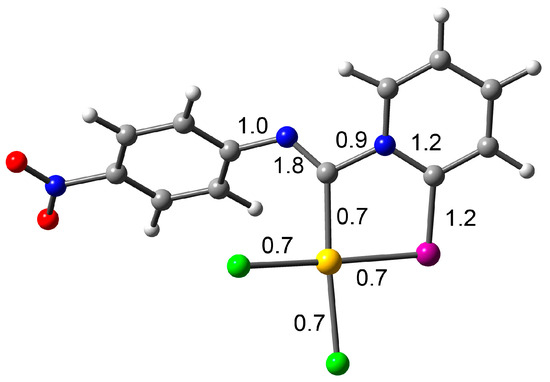
Figure 2.
RI-BP86-D4/def2-TZVP optimized geometry of compound 2d, indicating the Meyer bond order.
Although the nucleophilic addition to the C≡N triple bond of metal-bound isonitriles is well documented and widely studied in the context of the synthesis of metal carbene complexes and their applications, this is the first example of pyridine functioning as a nucleophile in a metal-mediated coupling with isonitriles. As a result, this represents the first example of acyclic (amino)(N-pyridinium)carbenoid metal complexes.
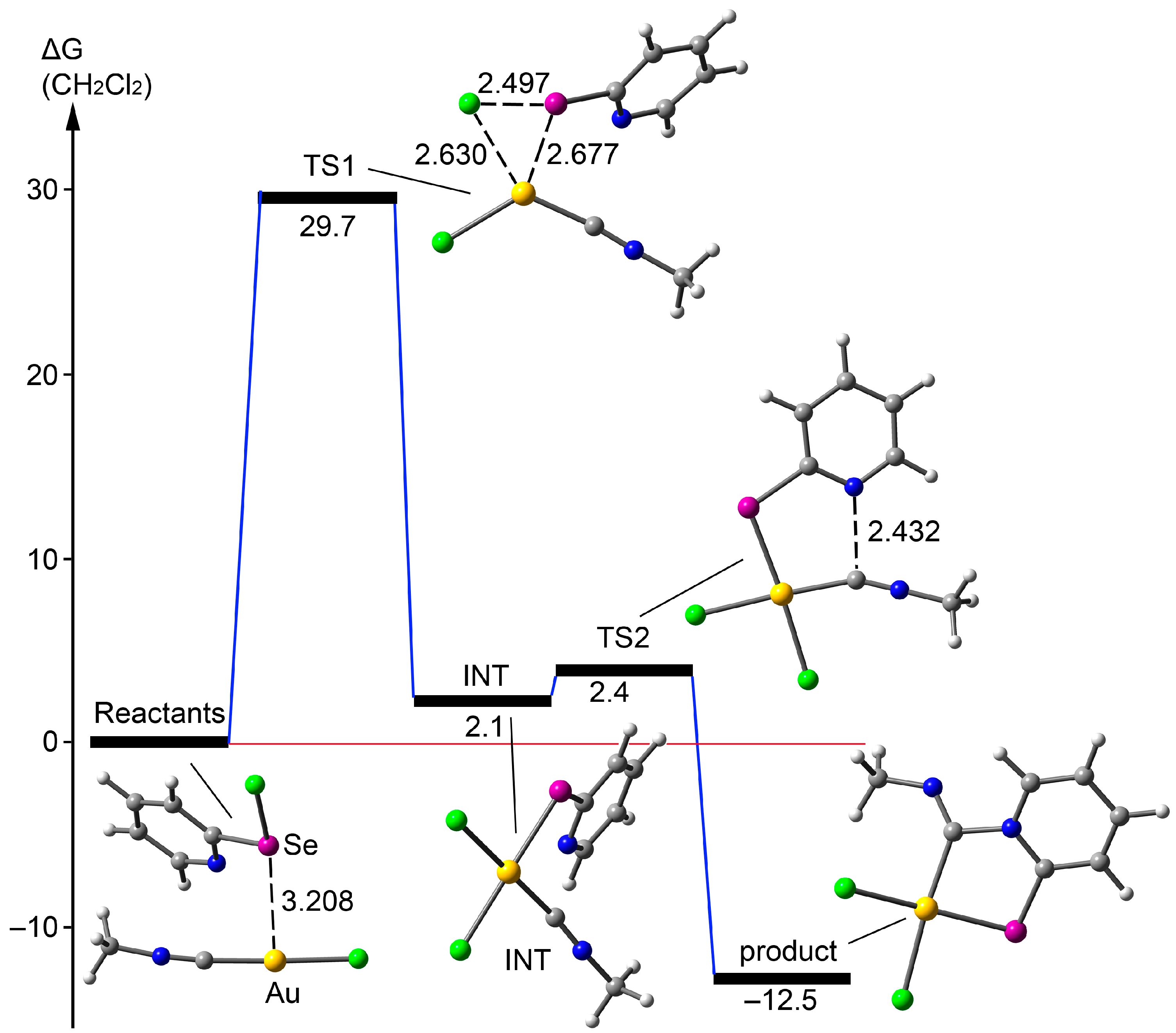
The mechanism of the new coupling reaction between 2-pyridylselenyl chloride and Au(I) isocyanide complexes formally involves two key steps: the oxidative addition of the Se–Cl moiety to Au(I) and the nucleophilic addition of the pyridine fragment to the isonitrile’s CN triple bond. These steps may occur either sequentially or simultaneously. To elucidate the mechanism, we performed additional DFT calculations, which ruled out the simultaneous mechanism. For computational efficiency, we simplified the isocyanide complex to CH3-N≡C–Au–Cl. We explored the potential energy surface connecting the [(Me2S)AuCl] and CH3-N≡C–Au–Cl reactants to the final adduct using the NEB-TS (Nudged Elastic Band with TS optimization) method, which locates the transition state (TS) by using the geometries of the reactants and products. The NEB-TS analysis identified an intermediate corresponding to the oxidative addition of the Se–Cl moiety to Au(I) (Figure 3). Starting from this intermediate, we computed two transition states, linking it to both the reactants and the product. The ΔG values in CH2Cl2 reveal that the oxidative addition is the rate-determining step, with a barrier of 29.7 kcal/mol. Interestingly, the reactants form a supramolecular complex prior to the oxidative addition, where the Se atom is preorganized 3.028 Å from the Au atom. The second step, the nucleophilic addition of the pyridine fragment to the CN bond, occurs with a minimal barrier of 0.3 kcal/mol, leading to the final product, which is 12.5 kcal/mol more stable than the starting material.
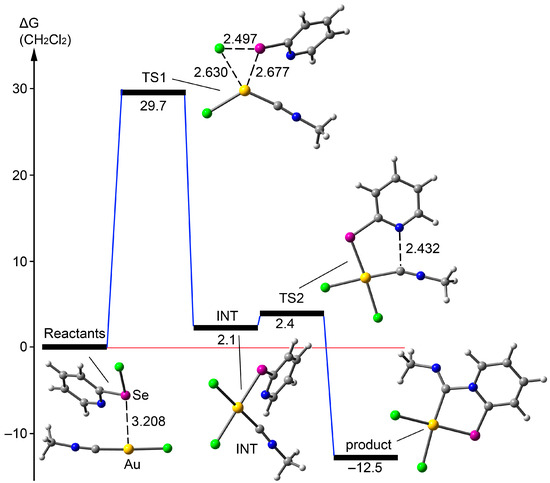
Figure 3.
Energetic profile for the reaction of [(Me2S)AuCl] with CH3-N≡C–Au–Cl at the RI-BP86-D4(CH2Cl2)/def2-TZVP level of theory. Distances are shown in Å.
Gold carbene complexes are known for their anticancer activity. The antiproliferative activity of new compounds 2a–2e was tested against two human cancer cell lines, A2780 ovarian adenocarcinoma and the A278Cis cisplatin-resistant variant, using the standard MTT colorimetric assay. Cisplatin was used as a control in the study. The new compounds 2a–2e demonstrated high antiproliferative activity in the low micromolar range. They were about 3–5 times more potent than cisplatin against the cisplatin-sensitive cell line and about 5–6 times more potent against the cisplatin-resistant variant (Table 2).

Table 2.
Antiproliferative activity of 2-pyridine-selenyl gold (III) carbene complexes 2a–e and cisplatin against human cancer cells. Rf (resistance factors) showed the resistance coefficient. The results are the mean values ± SD of three independent experiments, each of which was performed in triplicate.
Among the new compounds, the one with the nitro group 2d was found to be the most active against both cancer cell lines. When comparing the antiproliferative activity of the new compounds against the A2780 and A2780 cis cell lines, it was found that the complexes were less potent against cisplatin-resistant cells (Table 2, Rf); however, the resistant index was lower compared to the cisplatin. These results suggest that the new compounds may have a different mode of action compared to cisplatin, which will be studied in more details in the future.
3. Methods
3.1. Experimental Details
General remarks. No uncommon hazards were noted as stemming from the experimental work carried out. All manipulations were carried out in air. All the reagents used in this study were obtained from commercial sources (Aldrich, TCI-Europe, Strem, ABCR). Commercially available solvents were purified by conventional methods and distilled immediately prior to use. Mass spectra were recorded on a Bruker micrOTOF spectrometer equipped with an electrospray ionization (ESI) source; MeOH or MeCN was used as a solvent. NMR spectra were recorded on a Bruker Avance neo 700; chemical shifts (δ) are given in ppm, and coupling constants (J) are given in Hz. IR spectra were recorded on a Shimadzu Inspirit IR–Fourier spectrometer equipped with the QATAR-Singapore attenuated total reflectance sample holder.
Synthetic part.
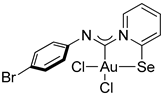
Compound 2a. 1 [(Me2S)AuCl] (15 mg, 0.05 mmol) was added to a solution of p-bromophenyl isocyanide (9 mg, 0.05 mmol) in CH2Cl2 (2 mL), and the solution was stirred for 15 min. After that, a solution of 2-PySeCl (10 mg, 0.05 mmol) in CH2Cl2 (3 mL) was added to the mixture, which was stirred for 4 h. An orange precipitate formed, which was filtered off and washed with MeOH (3 mL) and Et2O (3 mL) and dried under a vacuum. Yield: 15 mg, 48%. 1H NMR (400 MHz, DMSO-d6) δ 9.33 (d, J = 6.6 Hz, 1H), 8.34 (d, J = 8.2 Hz, 1H), 8.26 (t, J = 7.7 Hz, 1H), 7.74 (t, J = 6.9 Hz, 1H), 7.62 (d, J = 8.5 Hz, 2H), 7.14 (d, J = 8.5 Hz, 2H). 13C NMR (101 MHz, DMSO) δ 154.4, 146.5, 146.1, 144.4, 142.3, 131.7, 127.6, 123.3, 122.9, 119.0. MS (ESI+): found, 606.8143 [M]+; calcd for C12H8AuBrCl2N2Se, 606.8151. Crystals, suitable for X-ray analysis, were obtained directly from the reaction mixture.
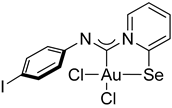
Compound 2b [(Me2S)AuCl] (15 mg, 0.05 mmol) was added to a solution of p-iodophenyl isocyanide (12 mg, 0.05 mmol) in CH2Cl2 (2 mL), and the solution was stirred for 15 min. A white precipitate formed. After that, a solution of 2-PySeCl (10 mg, 0.05 mmol) in CH2Cl2 (3 mL) was added to the mixture, which caused the white precipitate to dissolve, and the mixture was stirred for 4 h. An orange precipitate formed, which was filtered off and washed with MeOH (3 mL) and Et2O (3 mL) and dried under a vacuum. Yield: 20 mg, 51%. 1H NMR (400 MHz, DMSO-d6) δ 9.32 (d, J = 6.3 Hz, 1H), 8.34 (d, J = 8.2 Hz, 1H), 8.25 (t, J = 7.7 Hz, 1H), 7.81–7.68 (m, 3H), 7.00 (d, J = 8.5 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 154.3, 146.5, 145.9, 144.7, 142.3, 138.6, 137.5, 127.6, 123.4, 122.9. MS (ESI+): found, 654.8017 [M]+; calcd for C12H8AuICl2N2Se, 654.8013.
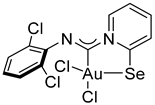
Compound 2c [(Me2S)AuCl] (15 mg, 0.05 mmol) was added to a solution of 2,6-dichlorophenyl isocyanide (9 mg, 0.05 mmol) in CH2Cl2 (2 mL), and the solution was stirred for 15 min. After that, a solution of 2-PySeCl (10 mg, 0.05 mmol) in CH2Cl2 (3 mL) was added to the mixture, which was stirred for 4 h. The reaction mixture was left overnight, and the residue was washed with MeOH (3 mL) and Et2O (3 mL) and dried under a vacuum. Yield: 17 mg, 57%. 1H NMR (700 MHz, DMSO-d6) δ 9.10 (d, J = 6.7, 1H), 8.41 (d, J = 8.0 Hz, 1H), 8.33–8.29 (m, 1H), 7.79 (td, J = 7.1, 1.2 Hz, 1H), 7.53 (d, J = 8.2 Hz, 2H), 7.25 (t, J = 8.1 Hz, 1H). 13C NMR (176 MHz, DMSO-d6) δ 155.79, 153.30, 147.16, 141.32, 140.17, 128.44, 128.01, 126.94, 124.61, 123.40. MS (ESI+): found, 560.8614 [M-Cl]+; calcd for C12H7AuCl3N2Se, 560.8506. Crystals, suitable for X-ray analysis, were obtained from the reaction mixture.
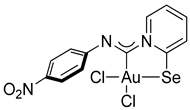
Compound 2d [(Me2S)AuCl] (15 mg, 0.05 mmol) was added to a solution of p-nitrophenyl isocyanide (8 mg, 0.05 mmol) in CH2Cl2 (2 mL) and then stirred for 15 min. A white precipitate formed. After that, a solution of 2-PySeCl (10 mg, 0.05 mmol) in CH2Cl2 (3 mL) was added to the mixture, which caused the white precipitate to dissolve, and the mixture was stirred for 4 h. The reaction mixture was left overnight, and the residue was washed with MeOH (3 mL) and Et2O (3 mL) and dried under a vacuum. Yield: 12 mg, 41%. 1H NMR (400 MHz, DMSO-d6) δ 9.36 (d, J = 6.5 Hz, 1H), 8.38 (d, J = 8.2 Hz, 1H), 8.33–8.23 (m, 3H), 7.76 (t, J = 6.9 Hz, 1H), 7.39 (d, J = 8.9 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 155.0, 150.6, 148.0, 146.8, 144.9, 142.5, 127.60, 124.6, 122.9, 121.8. MS (ESI+): found, 573.8892 [M]+; calcd for C12H9AuCl2N3O2Se, 573.8897. Crystals, suitable for X-ray analysis, were obtained from the reaction mixture.
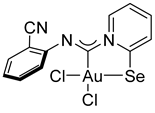
Compound 2e [(Me2S)AuCl] (15 mg, 0.05 mmol) was added to a solution of 2-cyanophenyl isocyanide (7 mg, 0.05 mmol) in CH2Cl2 (2 mL) and then stirred for 15 min. After that, a solution of 2-PySeCl (10 mg, 0.05 mmol) in CH2Cl2 (3 mL) was added to the mixture, which was stirred for 4 h. The reaction mixture was left overnight, and the residue was washed with MeOH (3 mL) and Et2O (3 mL) and dried under a vacuum. Yield: 9 mg, 32%. 1H NMR (400 MHz, DMSO-d6) δ 9.11 (d, J = 6.4 Hz, 1H), 8.39 (d, J = 8.2 Hz, 1H), 8.34–8.24 (m, 1H), 7.87 (d, J = 8.8 Hz, 1H), 7.82 (t, J = 6.9 Hz, 1H), 7.75 (t, J = 8.5 Hz, 1H), 7.43 (t, J = 7.7 Hz, 1H), 7.36 (d, J = 7.9 Hz, 1H).13C NMR (101 MHz, DMSO-d6) δ 155.6, 150.5, 147.7, 146.9, 141.5, 134.0, 132.7, 127.8, 126.6, 123.3, 121.5, 117.1, 103.9. MS (ESI+): found, 553.8992 [M]+; calcd for C13H8AuCl2N3Se, 553.8999. Crystals, suitable for X-ray analysis, were obtained from the reaction mixture.
Reaction of [(tBuNC)AuCl] with 2-PySeCl
[(Me2S)AuCl] (15 mg, 0.05 mmol) was added to a solution of tert-butyl isocyanide (4 mg, 0.05 mmol) in CH2Cl2 (2 mL) and the solution was stirred for 15 min. After that, a solution of 2-PySeCl (20 mg, 0.1 mmol) in CH2Cl2 (3 mL) was added to the mixture and stirred overnight. Then, the reaction mixture was concentrated under a vacuum and separated by column chromatography (eluent: DCM) to give white crystals of [(tBuNC)AuCl3] and yellow crystals of Py2Se2. Yield: 15 mg (74%) and 15 mg (94%), respectively.
3.2. Theoretical Methods
The structures and relative energies of all systems considered in the mechanistic analysis were optimized at the PBE0-D4/def2-TZVP level of theory using ORCA 5.1 [47]. The hybrid functional PBE0 [48], corrected for dispersion effects with the D4 method [49], was employed alongside a triple-ζ quality basis set [50]. Frequency calculations confirmed the stationary points, ensuring they correspond to minima or transition states. To locate the transition states, the Nudged Elastic Band (NEB) method available in ORCA was applied. This approach identifies a minimum energy pathway by constructing a series of atomic configurations, known as “images” that link the reactant and product states. A detailed explanation of the NEB implementation can be found in the study by Ásgeirsson et al. [51].
4. Conclusions
The reaction between the isonitrile gold(I) complex and 2-pyridylselenyl chloride resulted in the formation of unprecedented (amino)(N-pyridinium)carbenoid gold(III) derivatives. The addition proceeds via two key steps: the oxidative addition of Se–Cl moiety to the Au(I) and the nucleophilic addition of the pyridine group to the CN triple bond of metal-bound isonitrile. Importantly, this is a first example of pyridine acting as a nucleophile in the reaction with the CN bond of isonitrile. Arguably, such an addition is due to the chelate effect. The reaction mechanism was elucidated using DFT calculations, which identified the oxidative addition as the rate-determining step with a barrier of 29.7 kcal/mol.
Moreover, we tested the new compounds 2a–2e against two human cancer cell lines, A2780 ovarian adenocarcinoma and the A278Cis cisplatin-resistant variant, and the new compounds were about 3–5 times more potent than cisplatin against the cisplatin-sensitive cell line and about 5–6 times more potent against the cisplatin-resistant variant.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/ijms26020483/s1.
Author Contributions
Conceptualization, A.G.T.; investigation, K.M.V., D.M.S., R.M.G., A.F., A.S.K. (Andreii S. Kritchenkov), O.V.R., A.V.V., A.V.B., M.V.G., A.S.K. (Alexey S. Kubasov) and I.S.K.; methodology, A.A.N.; visualization, V.G.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was performed with the support of the Russian Science Foundation (award No. 22-73-10007).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The single crystal X-ray diffraction data for 2a, 2c, and 2e were obtained using the equipment of the Shared Facility Center at the Kurnakov Institute, operating with financial support from the Ministry of Science and Higher Education of the Russian Federation. The XRD for 2d was supported by the Ministry of Science and Higher Education of the Russian Federation (Contract No. 075-00277-24-00, project № 021341-2-000). Theoretical investigations were funded by the “Ministerio de Ciencia, Investigacion y Universidades/Agencia Estatal de Investigación” (MICIU/AEI/10.13039/501100011033) of Spain (project PID2020-115637GB-I00. FEDER funds). The authors acknowledge support from the M.V. Lomonosov Moscow State University Program of Development (the “Feyond-A400” microplate reader (Allsheng, China) for the MTT assay).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Cardin, D.J.; Cetinkaya, B.; Lappert, M.F.; Manojlović-Muir, L.; Muir, K.W. An electron-rich olefin as a source of co-ordinated carbene; synthesis of trans-PtCl2[C(NPhCH2)2]PEt3. J. Chem. Soc. D Chem. Commun. 1971, 400–401. [Google Scholar] [CrossRef]
- Cardin, D.J.; Cetinkaya, B.; Lappert, M.F. Transition metal-carbene complexes. Chem. Rev. 1972, 72, 545–574. [Google Scholar] [CrossRef]
- Slaughter, L.M. Acyclic aminocarbenes in catalysis. ACS Catal. 2012, 2, 1802–1816. [Google Scholar] [CrossRef]
- Nelson, D.J.; Nolan, S.P. N-Heterocyclic Carbenes. In N-Heterocyclic Carbenes; Wiley: Hoboken, NJ, USA, 2014; pp. 1–24. ISBN 9783527671229. [Google Scholar]
- Arduengo, A.J.; Goerlich, J.R.; Marshall, W.J. A stable diaminocarbene. J. Am. Chem. Soc. 2002, 117, 11027–11028. [Google Scholar] [CrossRef]
- Benhamou, L.; Chardon, E.; Lavigne, G.; Bellemin-Laponnaz, S.; César, V. Synthetic Routes to N-Heterocyclic Carbene Precursors. Chem. Rev. 2011, 111, 2705–2733. [Google Scholar] [CrossRef] [PubMed]
- Riener, K.; Haslinger, S.; Raba, A.; Högerl, M.P.; Cokoja, M.; Herrmann, W.A.; Kühn, F.E. Chemistry of Iron N-Heterocyclic Carbene Complexes: Syntheses, Structures, Reactivities, and Catalytic Applications. Chem. Rev. 2014, 114, 5215–5272. [Google Scholar] [CrossRef]
- Jayaraj, A.; Raveedran, A.V.; Latha, A.T.; Priyadarshini, D.; Swamy, P.C.A. Coordination Versatility of NHC-metal Topologies in Asymmetric Catalysis: Synthetic Insights and Recent Trends. Coord. Chem. Rev. 2023, 478, 214922. [Google Scholar] [CrossRef]
- Bellotti, P.; Koy, M.; Hopkinson, M.N.; Glorius, F. Recent advances in the chemistry and applications of N-heterocyclic carbenes. Nat. Rev. Chem. 2021, 5, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Tyagi, R. Acyclic diaminocarbenes (ADCs) and their catalytic activity in metal catalysed organic transformation reactions. Rev. Inorg. Chem. 2024, 44, 255–270. [Google Scholar] [CrossRef]
- Zhou, T.; Utecht-Jarzyńska, G.; Szostak, M. Ring-expanded N-heterocyclic carbene (reNHC) complexes: Applications in transition metal catalysis. Coord. Chem. Rev. 2024, 512, 215867. [Google Scholar] [CrossRef]
- Zhao, Q.; Han, B.; Peng, C.; Zhang, N.; Huang, W.; He, G.; Li, J.-L. A promising future of metal-N-heterocyclic carbene complexes in medicinal chemistry: The emerging bioorganometallic antitumor agents. Med. Res. Rev. 2024, 44, 2194–2235. [Google Scholar] [CrossRef]
- Denk, K.; Sirsch, P.; Herrmann, W.A. The first metal complexes of bis(diisopropylamino)carbene: Synthesis, structure and ligand properties. J. Organomet. Chem. 2002, 649, 219–224. [Google Scholar] [CrossRef]
- Boyarskiy, V.P.; Bokach, N.A.; Luzyanin, K.V.; Kukushkin, V.Y. Metal-Mediated and Metal-Catalyzed Reactions of Isocyanides. Chem. Rev. 2015, 115, 2698–2779. [Google Scholar] [CrossRef]
- Bolotin, D.S.; Bokach, N.A.; Kritchenkov, A.S.; Haukka, M.; Kukushkin, V.Y. Amidrazone Complexes from a Cascade Platinum(II)-Mediated Reaction between Amidoximes and Dialkylcyanamides. Inorg. Chem. 2013, 52, 6378–6389. [Google Scholar] [CrossRef] [PubMed]
- Tskhovrebov, A.G.; Goddard, R.; Fürstner, A. Two Amphoteric Silver Carbene Clusters. Angew. Chemie Int. Ed. 2018, 57, 8089–8094. [Google Scholar] [CrossRef] [PubMed]
- Kritchenkov, A.S.; Luzyanin, K.V.; Bokach, N.A.; Kuznetsov, M.L.; Gurzhiy, V.V.; Kukushkin, V.Y. Selective Nucleophilic Oxygenation of Palladium-Bound Isocyanide Ligands: Route to Imine Complexes That Serve as Efficient Catalysts for Copper-/Phosphine-Free Sonogashira Reactions. Organometallics 2013, 32, 1979–1987. [Google Scholar] [CrossRef]
- Luzyanin, K.V.; Tskhovrebov, A.G.; Carolina Carias, M.; da Silva, M.F.C.G.; Pombeiro, A.J.L.; Kukushkin, V.Y. Novel Metal-mediated (M = Pd, Pt) coupling between isonitriles and benzophenone hydrazone as a route to aminocarbene complexes exhibiting high catalytic activity (M = Pd) in the Suzuki-Miyaura reaction. Organometallics 2009, 28, 6559–6566. [Google Scholar] [CrossRef]
- Luzyanin, K.V.; Tskhovrebov, A.G.; Guedes da Silva, M.F.C.; Haukka, M.; Pombeiro, A.J.L.; Kukushkin, V.Y. Metal-mediated [2+3] cycloaddition of nitrones to palladium-bound isonitriles. Chem. A Eur. J. 2009, 15, 5969–5978. [Google Scholar] [CrossRef] [PubMed]
- Luzyanin, K.V.; Pombeiro, A.J.L. Carbene Complexes Derived from Metal-Bound Isocyanides: Recent Advances. In Isocyanide Chemistry; Wiley: Hoboken, NJ, USA, 2012; pp. 531–550. ISBN 9783527652532. [Google Scholar]
- Tskhovrebov, A.G.; Luzyanin, K.V.; Dolgushin, F.M.; Guedes Da Silva, M.F.C.; Pombeiro, A.J.L.; Kukushkin, V.Y. Novel reactivity mode of metal diaminocarbenes: Palladium(II)-mediated coupling between acyclic diaminocarbenes and isonitriles leading to dinuclear species. Organometallics 2011, 30, 3362–3370. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Luzyanin, K.V.; Kuznetsov, M.L.; Sorokoumov, V.N.; Balova, I.A.; Haukka, M.; Kukushkin, V.Y. Substituent R-dependent regioselectivity switch in nucleophilic addition of N-phenylbenzamidine to PdII-and PtII-complexed isonitrile RN-C giving aminocarbene-like species. Organometallics 2011, 30, 863–874. [Google Scholar] [CrossRef]
- Mikhaylov, V.N.; Sorokoumov, V.N.; Liakhov, D.M.; Tskhovrebov, A.G.; Balova, I.A. Polystyrene-supported acyclic diaminocarbene palladium complexes in Sonogashira cross-coupling: Stability vs. catalytic activity. Catalysts 2018, 8, 141. [Google Scholar] [CrossRef]
- Mikhaylov, V.N.; Sorokoumov, V.N.; Novikov, A.S.; Melnik, M.V.; Tskhovrebov, A.G.; Balova, I.A. Intramolecular hydrogen bonding stabilizes trans-configuration in a mixed carbene/isocyanide PdII complexes. J. Organomet. Chem. 2020, 912, 121174. [Google Scholar] [CrossRef]
- Usón, R.; Laguna, A.; Vicente, J.; García, J.; Bergareche, B.; Brun, P. Neutral isocyanide and carbene pentafluorophenyl complexes of gold(I) and gold(III). Inorganica Chim. Acta 1978, 28, 237–243. [Google Scholar] [CrossRef]
- Usón, R.; Laguna, A.; Vicente, J.; García, J.; Bergareche, B. Preparation of pentahalophenyl p-tolylisocyanide complexes of gold(I) and their reactions with amines, ammonia and alcohols. J. Organomet. Chem. 1979, 173, 349–355. [Google Scholar] [CrossRef]
- Parks, J.E.; Balch, A.L. Gold carbene complexes: Preparation, oxidation, and ligand displacement. J. Organomet. Chem. 1974, 71, 453–463. [Google Scholar] [CrossRef]
- Minghetti, G.; Bonati, F. Bis(carbene) complexes of gold(I) and gold (III). J. Organomet. Chem. 1973, 54, C62–C63. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Luzyanin, K.V.; Haukka, M.; Kukushkin, V.Y. Synthesis and characterization of cis-(RNC)2PtII species useful as synthons for generation of various (aminocarbene)Pt II complexes. J. Chem. Crystallogr. 2012, 42, 1170–1175. [Google Scholar] [CrossRef]
- Ruch, A.A.; Ellison, M.C.; Nguyen, J.K.; Kong, F.; Handa, S.; Nesterov, V.N.; Slaughter, L.M. Highly Sterically Encumbered Gold Acyclic Diaminocarbene Complexes: Overriding Electronic Control in Regiodivergent Gold Catalysis. Organometallics 2021, 40, 1416–1433. [Google Scholar] [CrossRef]
- Khrakovsky, D.A.; Tao, C.; Johnson, M.W.; Thornbury, R.T.; Shevick, S.L.; Toste, F.D. Enantioselective, Stereodivergent Hydroazidation and Hydroamination of Allenes Catalyzed by Acyclic Diaminocarbene (ADC) Gold(I) Complexes. Angew. Chemie Int. Ed. 2016, 55, 6079–6083. [Google Scholar] [CrossRef] [PubMed]
- Zuccarello, G.; Nannini, L.J.; Arroyo-Bondía, A.; Fincias, N.; Arranz, I.; Pérez-Jimeno, A.H.; Peeters, M.; Martín-Torres, I.; Sadurní, A.; García-Vázquez, V.; et al. Enantioselective Catalysis with Pyrrolidinyl Gold(I) Complexes: DFT and NEST Analysis of the Chiral Binding Pocket. JACS Au 2023, 3, 1742–1754. [Google Scholar] [CrossRef] [PubMed]
- Montanel-Pérez, S.; Elizalde, R.; Laguna, A.; Villacampa, M.D.; Gimeno, M.C. Synthesis of Bioactive N-Acyclic Gold(I) and Gold(III) Diamino Carbenes with Different Ancillary Ligands. Eur. J. Inorg. Chem. 2019, 2019, 4273–4281. [Google Scholar] [CrossRef]
- Iacopetta, D.; Rosano, C.; Sirignano, M.; Mariconda, A.; Ceramella, J.; Ponassi, M.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Is the Way to Fight Cancer Paved with Gold? Metal-Based Carbene Complexes with Multiple and Fascinating Biological Features. Pharmaceuticals 2020, 13, 91. [Google Scholar] [CrossRef]
- Weaver, J.; Gaillard, S.; Toye, C.; Macpherson, S.; Nolan, S.P.; Riches, A. Cytotoxicity of Gold(I) N-Heterocyclic Carbene Complexes Assessed by Using Human Tumor Cell Lines. Chem. A Eur. J. 2011, 17, 6620–6624. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Romanov, A.S.; Brooks, M.; Davis, J.; Schmidt, C.; Ott, I.; O’Connell, M.; Bochmann, M. Synthesis, structure and cytotoxicity of cyclic (alkyl)(amino) carbene and acyclic carbene complexes of group 11 metals. Dalt. Trans. 2017, 46, 15875–15887. [Google Scholar] [CrossRef]
- Chan, S.-L.; Chun, Y.-K.; Ko, C.-C. Transition-metal acyclic carbene complexes: Building blocks for luminescent, stimuli-responsive, bioactive materials and catalysts. Mater. Chem. Front. 2023, 7, 2958–2972. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, L.; Xie, B.; Wang, Y.; Ren, L.; Chen, X.; Cen, B.; Lv, H.; Wang, H. Novel fast-acting pyrazole/pyridine-functionalized N-heterocyclic carbene silver complexes assembled with nanoparticles show enhanced safety and efficacy as anticancer therapeutics. Dalt. Trans. 2020, 49, 2505–2516. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Junquera, M.; Lalinde, E.; Moreno, M.T.; Alfaro-Arnedo, E.; López, I.P.; Larráyoz, I.M.; Pichel, J.G. Luminescent cyclometalated platinum(ii) complexes with acyclic diaminocarbene ligands: Structural, photophysical and biological properties. Dalt. Trans. 2021, 50, 4539–4554. [Google Scholar] [CrossRef] [PubMed]
- Jazzar, R.; Soleilhavoup, M.; Bertrand, G. Cyclic (Alkyl)- and (Aryl)-(amino)carbene Coinage Metal Complexes and Their Applications. Chem. Rev. 2020, 120, 4141–4168. [Google Scholar] [CrossRef] [PubMed]
- Beerhues, J.; Neubrand, M.; Sobottka, S.; Neuman, N.I.; Aberhan, H.; Chandra, S.; Sarkar, B. Directed Design of a AuI Complex with a Reduced Mesoionic Carbene Radical Ligand: Insights from 1,2,3-Triazolylidene Selenium Adducts and Extensive Electrochemical Investigations. Chem. A Eur. J. 2021, 27, 6557–6568. [Google Scholar] [CrossRef] [PubMed]
- Mikherdov, A.S.; Baikov, S.V.; Proskurina, I.K.; Shetnev, A.A.; Kotov, A.D. Synthesis and Properties of C,N-Chelated Carbene Complexes of Palladium(II) with 2-Aminobenzo[d]thiazole Fragment. Russ. J. Gen. Chem. 2019, 89, 2062–2068. [Google Scholar] [CrossRef]
- Mikherdov, A.S.; Orekhova, Y.A.; Boyarskii, V.P. Formation of Homo- and Heteronuclear Platinum(II) and Palladium(II) Carbene Complexes in the Reactions of Coordinated Isocyanides with Aminothiazaheterocycles. Russ. J. Gen. Chem. 2018, 88, 2119–2124. [Google Scholar] [CrossRef]
- Zhang, C.; Maddelein, M.-L.; Wai-Yin Sun, R.; Gornitzka, H.; Cuvillier, O.; Hemmert, C. Pharmacomodulation on Gold-NHC complexes for anticancer applications—Is lipophilicity the key point? Eur. J. Med. Chem. 2018, 157, 320–332. [Google Scholar] [CrossRef] [PubMed]
- La Manna, S.; Marasco, D. Insights into molecular mechanisms of metallodrugs using metallomic studies. Inorganica Chim. Acta 2024, 560, 121816. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Caldeweyher, E.; Ehlert, S.; Hansen, A.; Neugebauer, H.; Spicher, S.; Bannwarth, C.; Grimme, S. A generally applicable atomic-charge dependent London dispersion correction. J. Chem. Phys. 2019, 150, 154122. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Ásgeirsson, V.; Birgisson, B.O.; Bjornsson, R.; Becker, U.; Neese, F.; Riplinger, C.; Jónsson, H. Nudged Elastic Band Method for Molecular Reactions Using Energy-Weighted Springs Combined with Eigenvector Following. J. Chem. Theory Comput. 2021, 17, 4929–4945. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).