Adhesion Mechanism, Applications, and Challenges of Ocular Tissue Adhesives
Abstract
1. Introduction
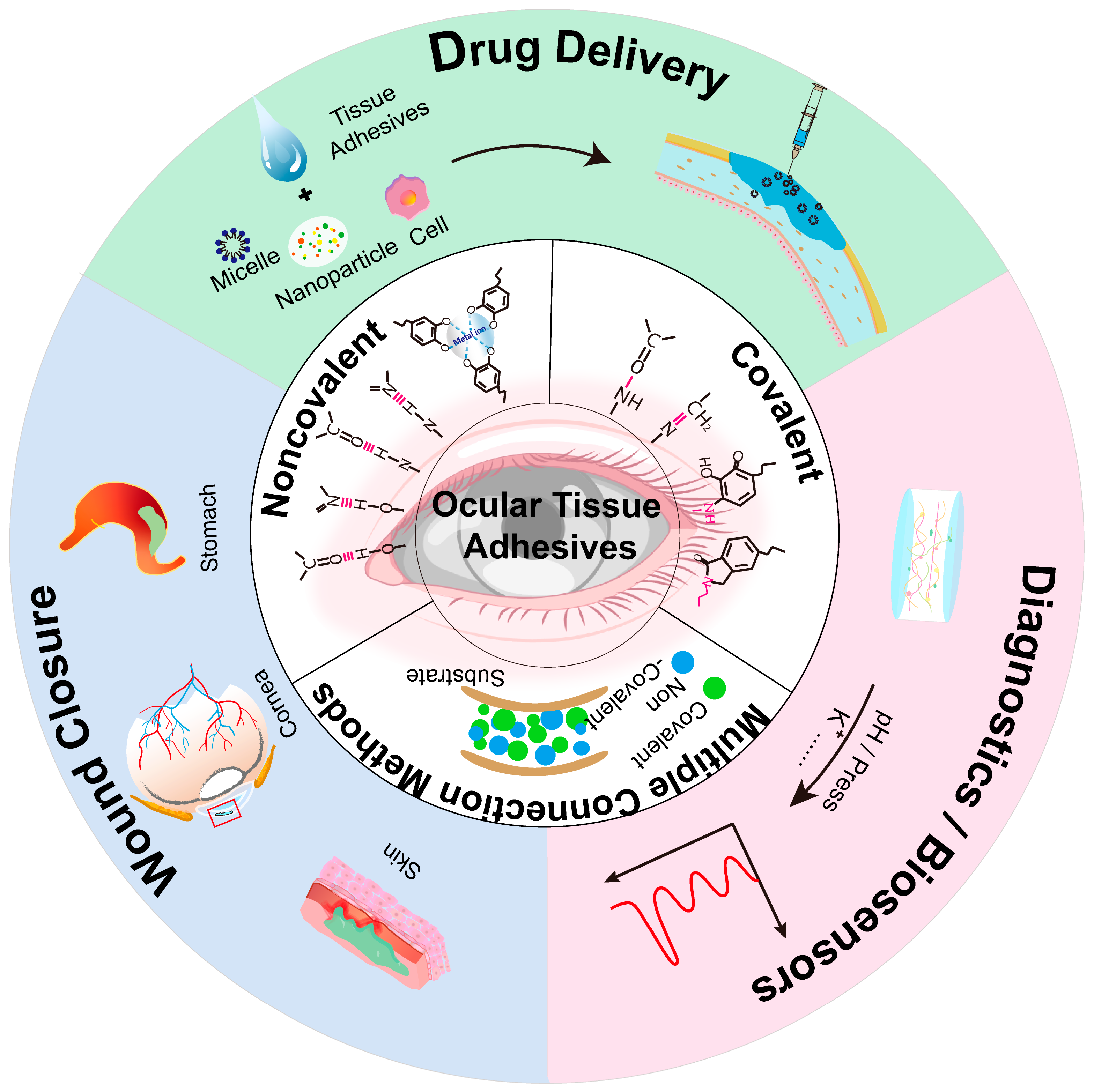
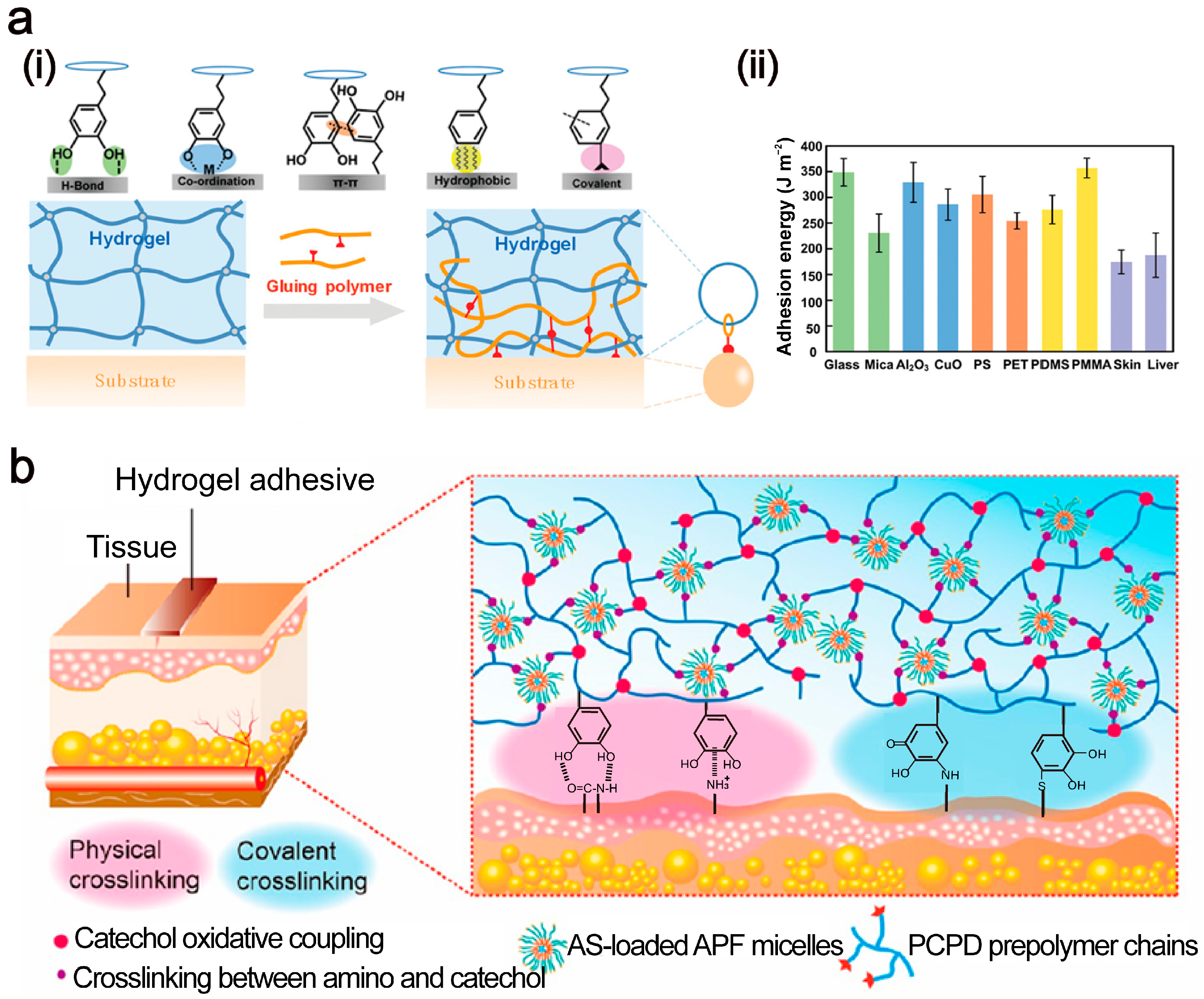
2. Adhesion Mechanisms Between Tissue Adhesives and Tissues
2.1. Van der Waals Force
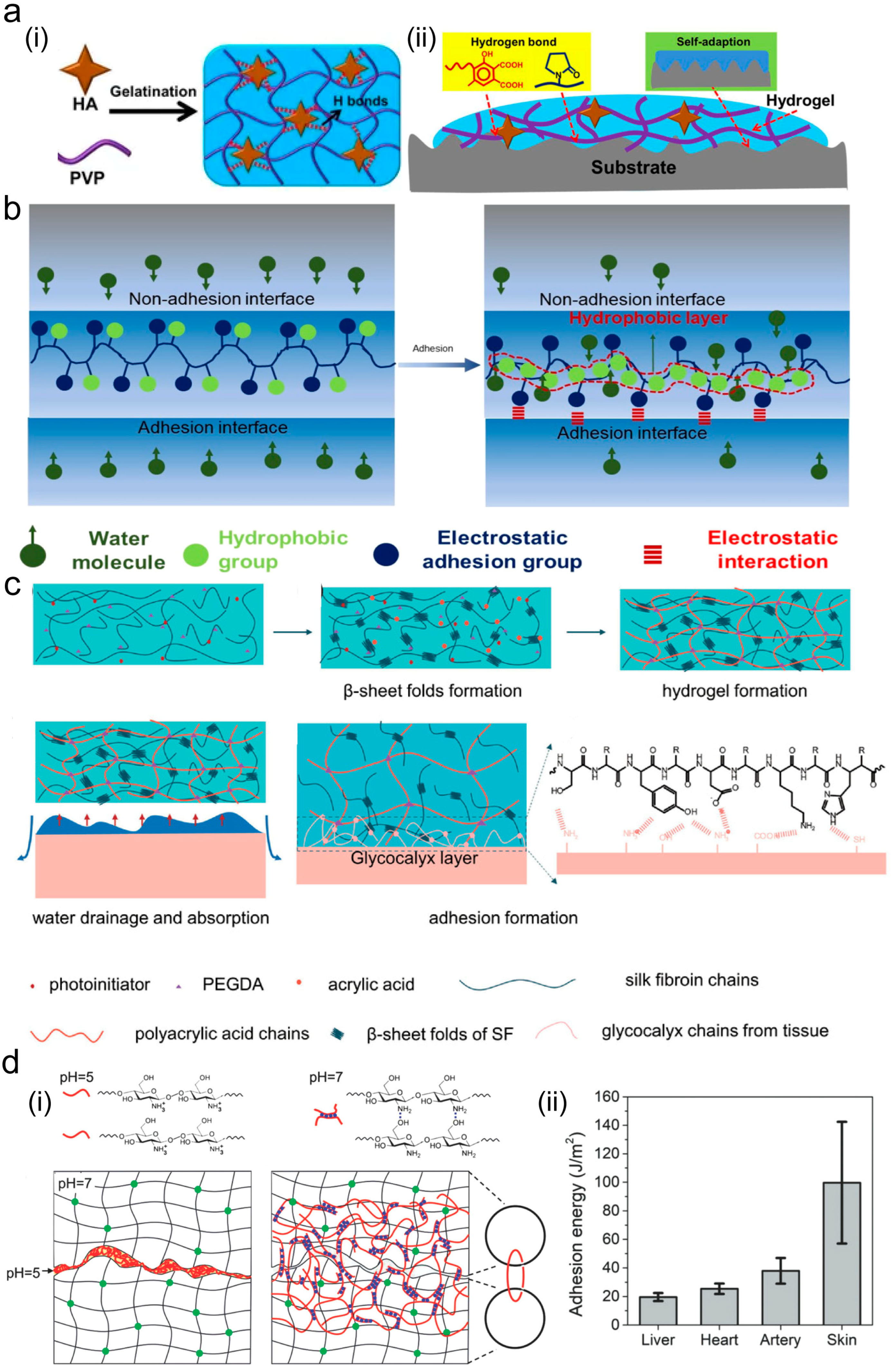
2.2. Hydrogen Bond
2.3. Electrostatic Interaction
2.4. Topological Structure
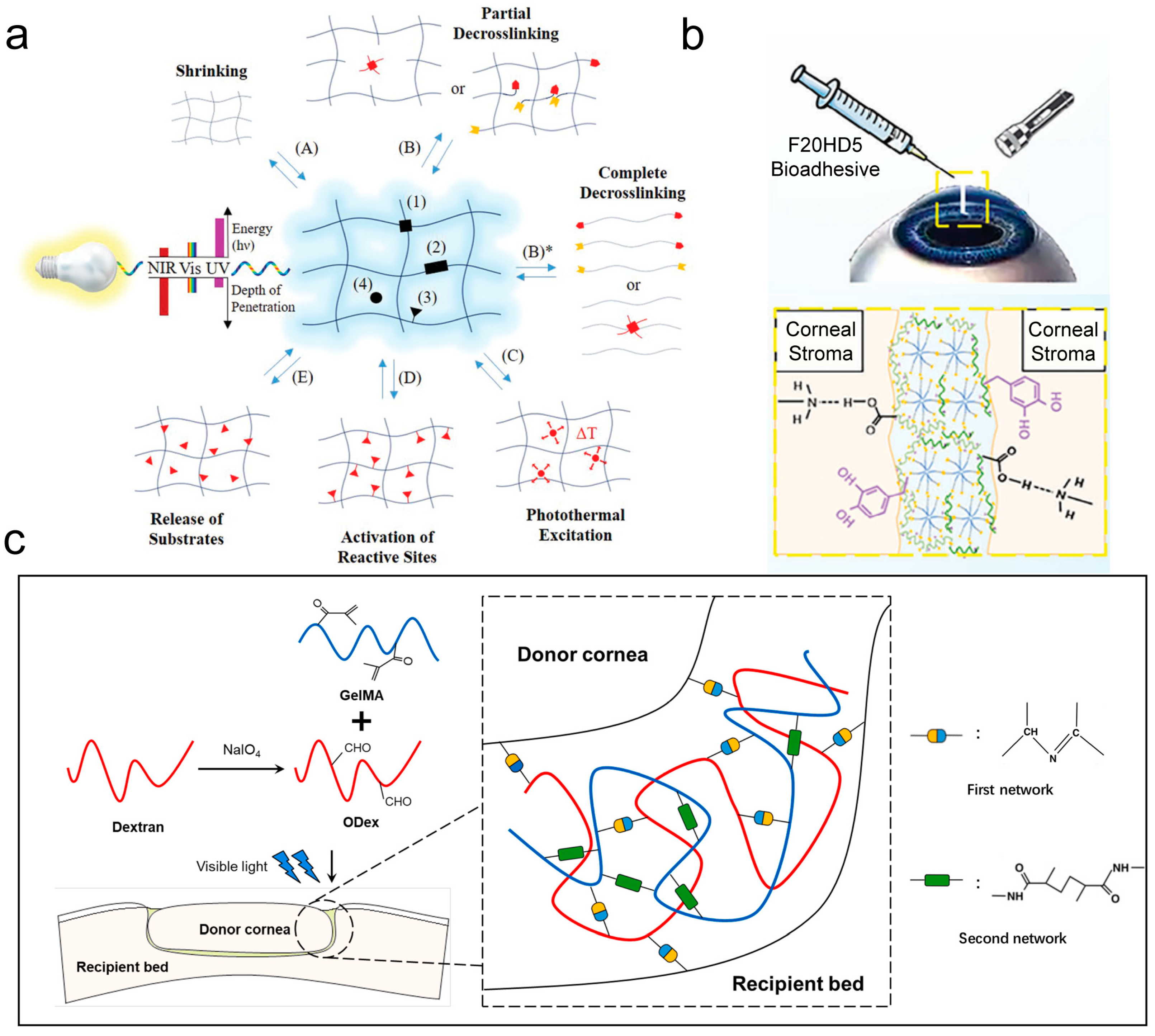
2.5. Covalent Bonding
2.6. Other Adhesion Mechanisms
| Components | Binding Mechanism | Adhesion Strength | Application | Ref. |
|---|---|---|---|---|
| Gelatin, HA | Schiff base reaction | 13 kPa | Corneal reconstruction | [47] |
| Fibrin | NA | 4 kPa | NA | [47] |
| Chitosan, dialdehyde starch | Schiff base reaction | NA | Eye drug delivery system | [48] |
| Sericin | Hydrogen bonding | 100 kPa | Wound closure | [49] |
| Fibrin | Coagulation reaction | 1.575 kPa | Corneal perforations | [50] |
| Poly(ethyleneglycol), peptide-based dendritic crosslinker | Pseudoproline/thiazolidine linkages | 40–700 kPa | Corneal adhesive | [51] |
| Polydextran-aldehydes | Schiff base reaction | 104.2 kPa | Wet adhesion, rapid hemostasis | [52] |
| Gelatin, tannic acid | Covalent bonding, hydrogen bonding, electrostatic interaction | 42 J/m2 | Wound closure | [46] |
| PVA, chitosan | Hydrogen bonding | 15.3 kPa | Wound closure, self-healing | [53] |
| PEG | Hydrogen bonding | 30 kPa | Prevent gas leakage | [54] |
| Pectin | Physical entanglement | 3500 kPa | Wound closure | [55] |
| Polyethylene glycol diacrylate, dopamine | Amido link | 21.6–656.7 kPa | Corneal perforations | [56] |
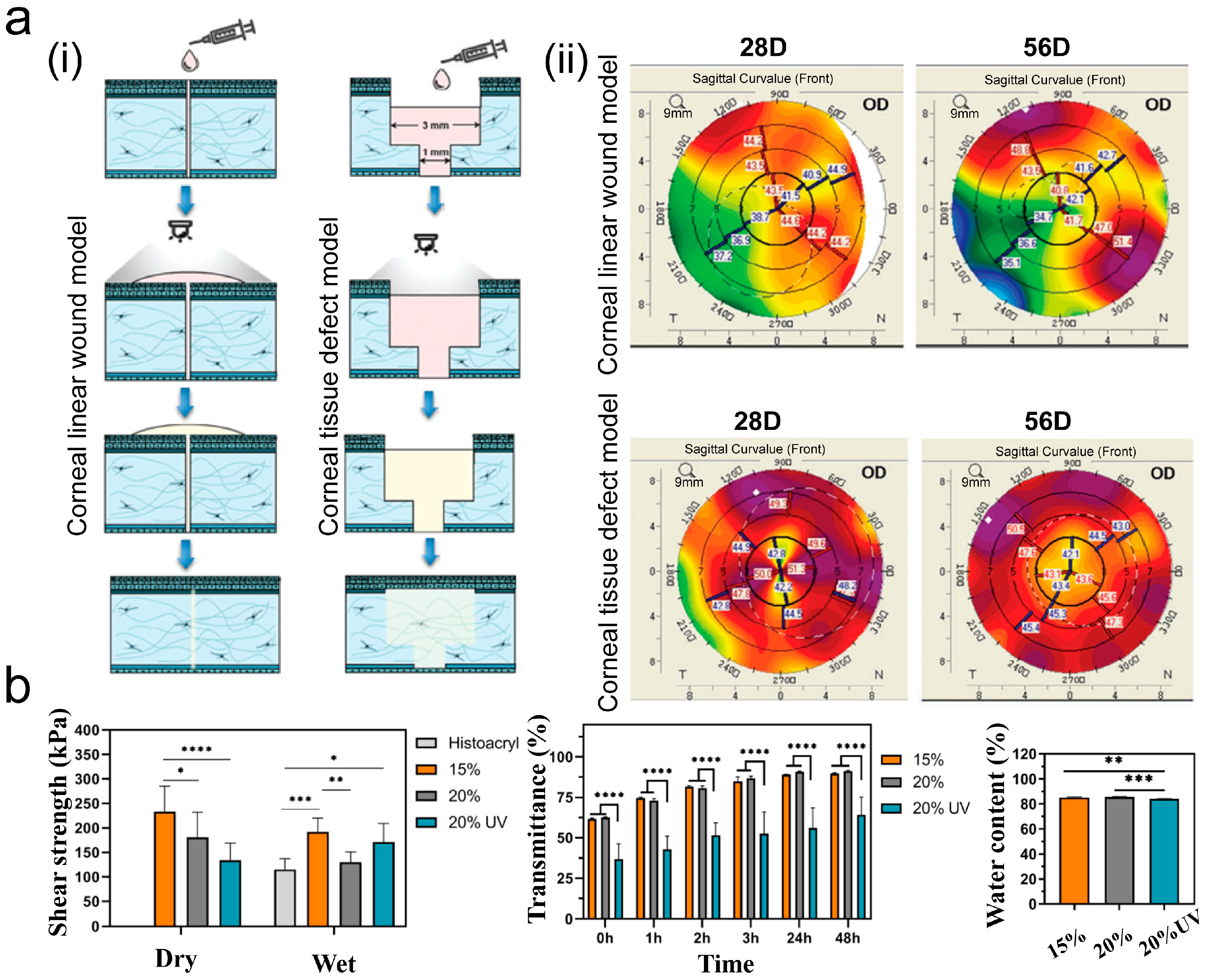
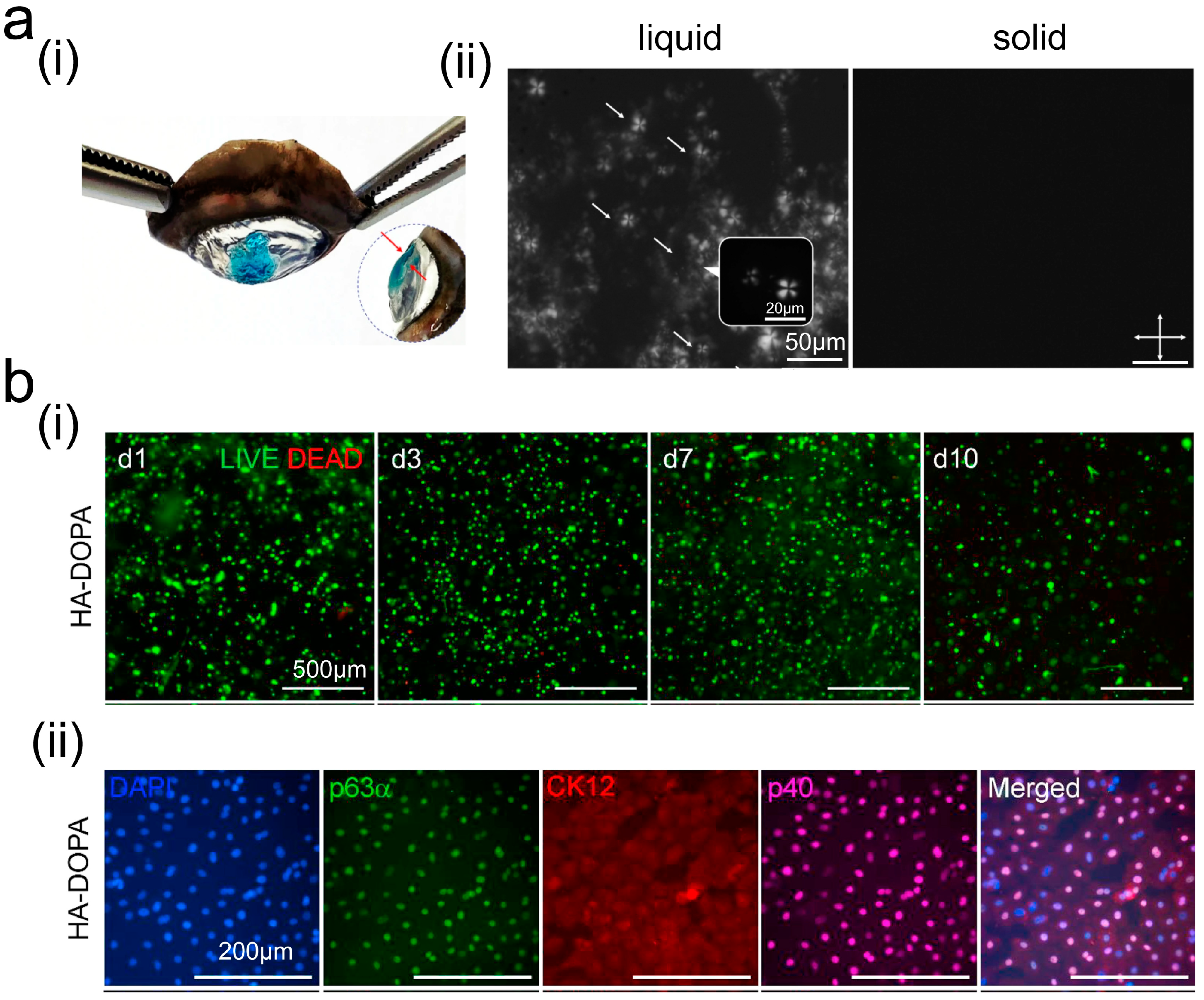
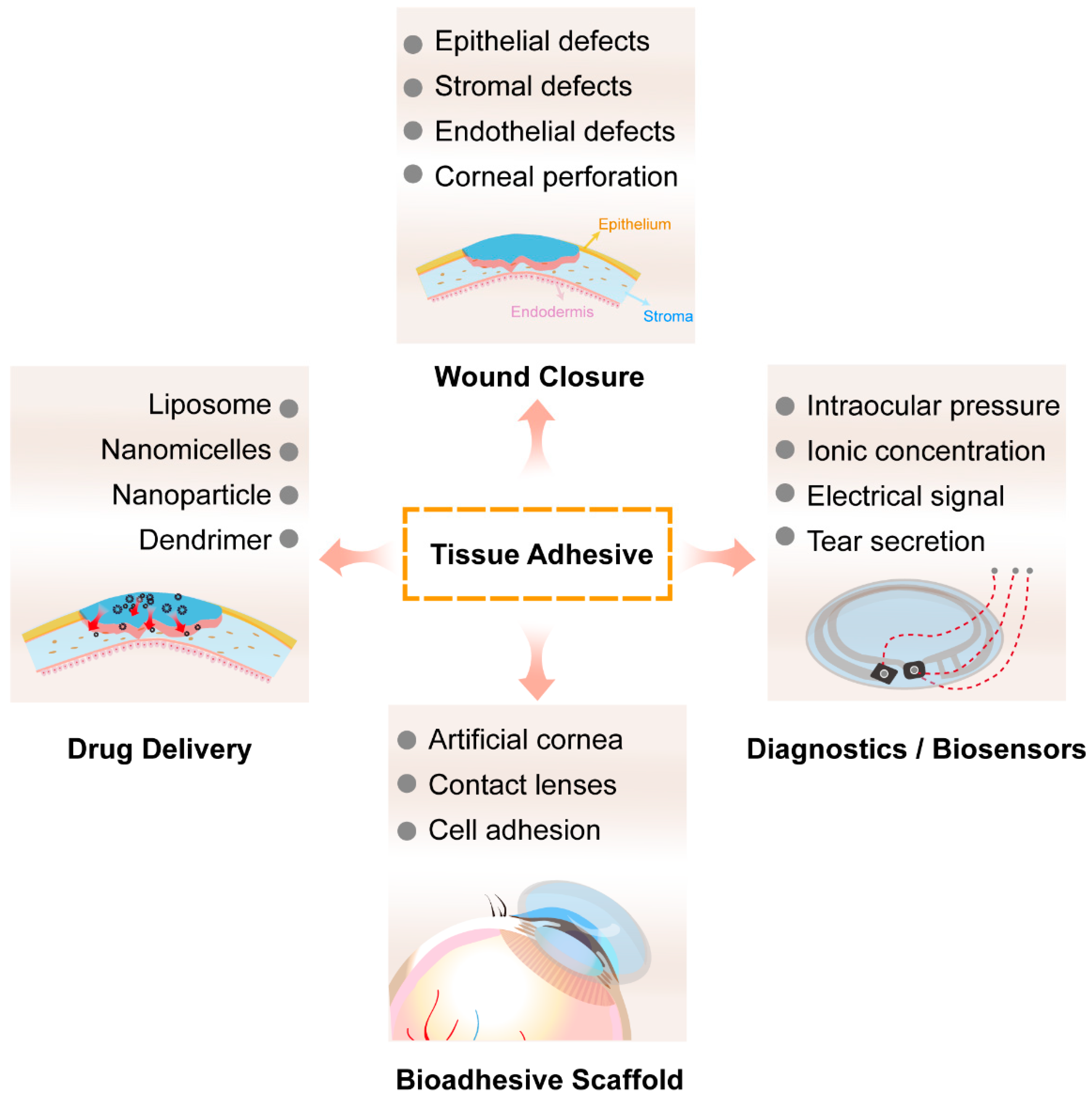
3. Application of Adhesives in Corneal Repair
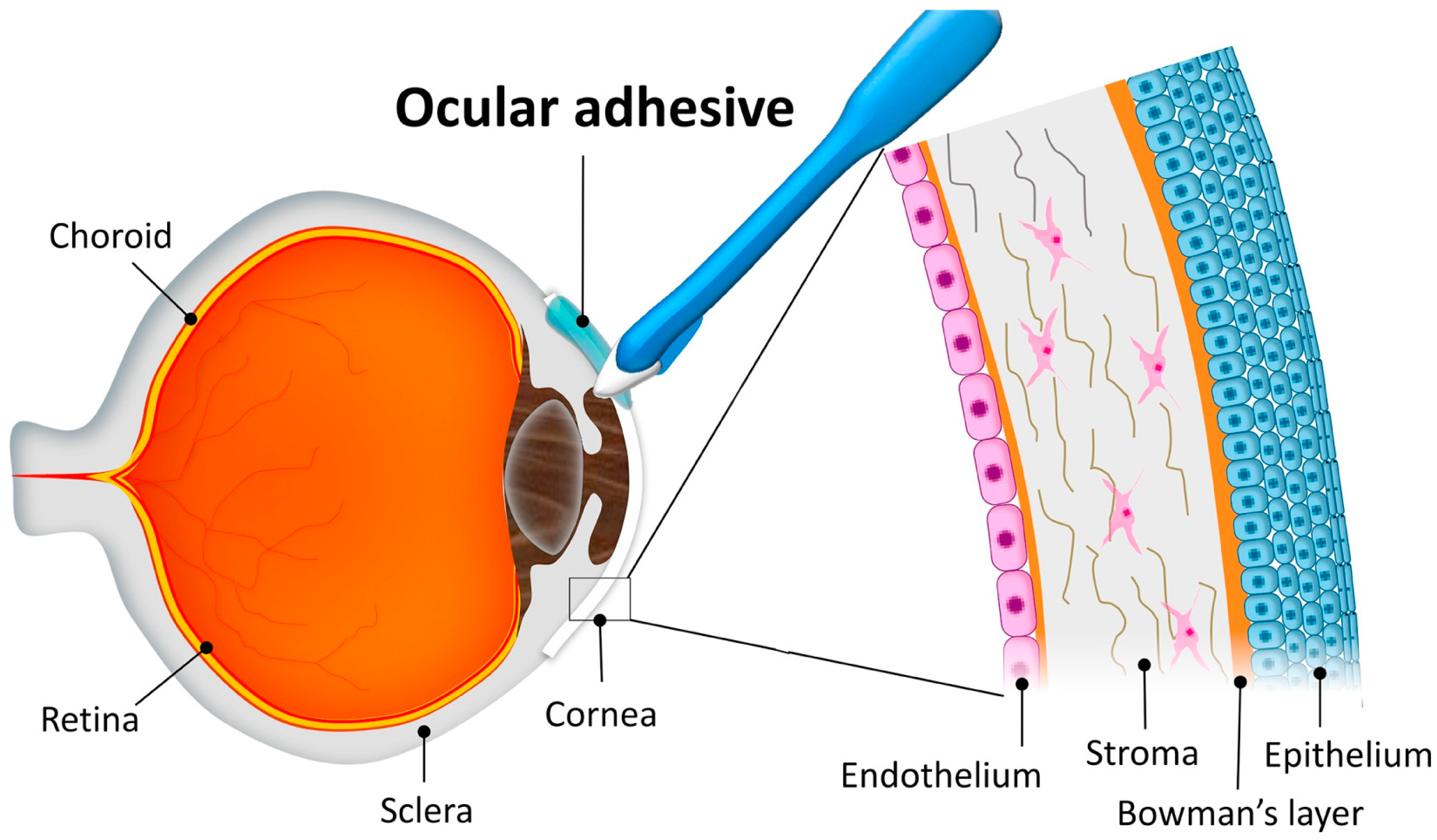
3.1. Wound Sealant
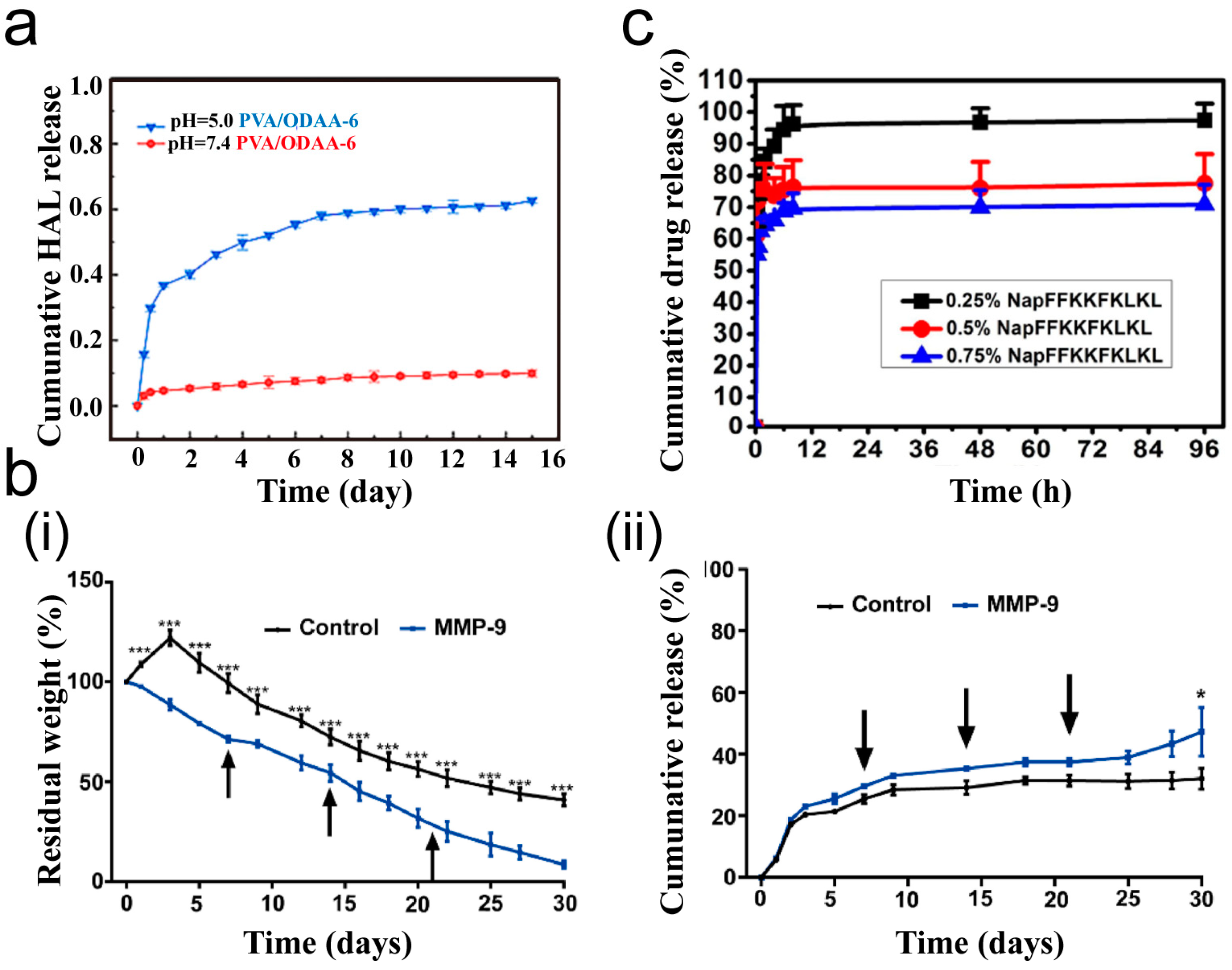
3.2. Drug Delivery
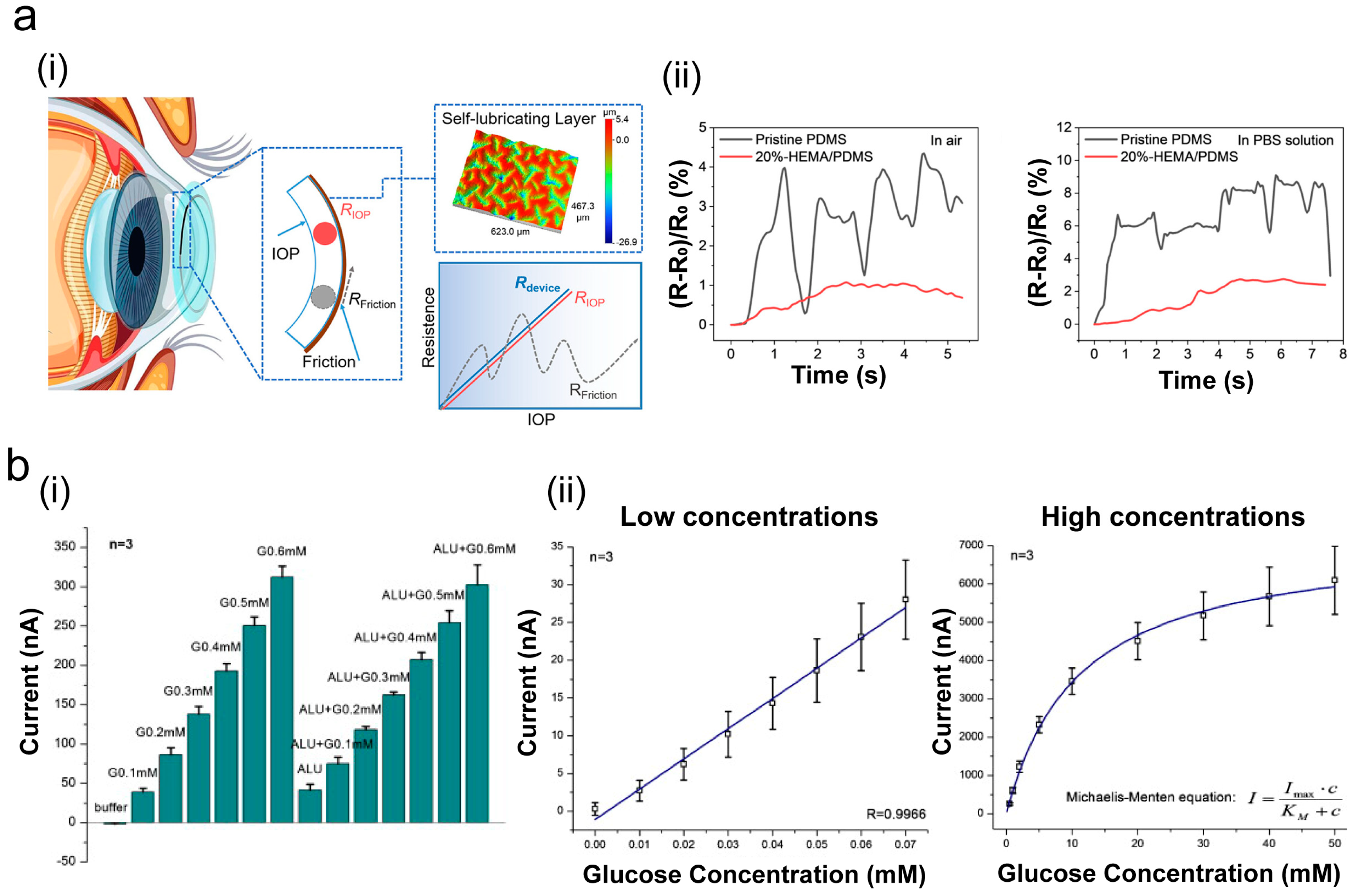
3.3. Eye Diagnostics and Biosensors
4. Summary and Outlook
Funding
Data Availability Statement
Conflicts of Interest
References
- Scognamiglio, F.; Travan, A.; Rustighi, I.; Tarchi, P.; Palmisano, S.; Marsich, E.; Borgogna, M.; Donati, I.; de Manzini, N.; Paoletti, S. Adhesive and Sealant Interfaces for General Surgery Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Wairkar, S. Recent Developments and Clinical Applications of Surgical Glues: An Overview. Int. J. Biol. Macromol. 2019, 137, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Matossian, C.; Makari, S.; Potvin, R. Cataract Surgery and Methods of Wound Closure: A Review. Clin. Ophthalmol. 2015, 9, 921–928. [Google Scholar]
- Du, D.; Chen, X.; Shi, C.; Zhang, Z.; Shi, D.; Kaneko, D.; Kaneko, T.; Hua, Z. Mussel-Inspired Epoxy Bioadhesive with Enhanced Interfacial Interactions for Wound Repair. Acta Biomater. 2021, 136, 223–232. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, Z.; Cheng, X.; Zou, Z.; Chen, X.; He, C. Injectable, Self-healing Hydrogel Adhesives with Firm Tissue Adhesion and On-demand Biodegradation for Sutureless Wound Closure. Sci. Adv. 2023, 9, eadh4327. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wu, X.; Zhang, X.; Xing, M. Bridging Wounds: Tissue Adhesives’ Essential Mechanisms, Synthesis and Characterization, Bioinspired Adhesives and Future Perspectives. Burn. Trauma 2022, 10, tkac033. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Zhou, W.; Liu, W.; Ou, Y.; Zheng, X.; Yang, H.; Wang, T. Injectable Carrier Hydrogel for Diabetic Foot Ulcer Wound Repair. J. Mater. Sci. 2023, 58, 11441–11468. [Google Scholar] [CrossRef]
- Zhu, X.; Yuan, W.; Li, Z.; Lin, Y.; Li, W.; Ji, L.; Wang, D.; Zhang, H.; Wang, Y. Progress of Research on Antioxidants and Carriers for Skin Wound Repair. Processes 2023, 11, 2069. [Google Scholar] [CrossRef]
- Zou, M.L.; Teng, Y.Y.; Wu, J.J.; Liu, S.Y.; Tang, X.Y.; Jia, Y.; Chen, Z.H.; Zhang, K.W.; Sun, Z.L.; Li, X.; et al. Fibroblasts: Heterogeneous Cells with Potential in Regenerative Therapy for Scarless Wound Healing. Front. Cell Dev. Biol. 2021, 9, 713605. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef] [PubMed]
- Autumn, K.; Sitti, M.; Liang, Y.A.; Peattie, A.M.; Hansen, W.R.; Sponberg, S.; Kenny, T.W.; Fearing, R.; Israelachvili, J.N.; Full, R.J. Evidence for Van Der Waals Adhesion in Gecko Setae. Proc. Natl. Acad. Sci. USA 2002, 99, 12252–12256. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Jain, D.; Zoltowski, C.M.; Voleti, S.; Stark, A.Y.; Niewiarowski, P.H.; Dhinojwala, A. Direct Evidence of Acid-Base Interactions in Gecko Adhesion. Sci. Adv. 2021, 7, eabd9410. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.; Jon, W.; Barnes, W.P.J. Adhesion and Detachment of the Toe Pads of Tree Frogs. J. Exp. Biol. 1991, 155, 103–125. [Google Scholar] [CrossRef]
- Drotlef, D.; Stepien, L.; Kappl, M.; Barnes, W.J.P.; Butt, H.; del Campo, A. Insights into the Adhesive Mechanisms of Tree Frogs using Artificial Mimics. Adv. Funct. Mater. 2013, 23, 1137–1146. [Google Scholar] [CrossRef]
- Ji, A.; Han, L.; Dai, Z. Adhesive Contact in Animal: Morphology, Mechanism and Bio-inspired Application. J. Bionic Eng. 2011, 8, 345–356. [Google Scholar] [CrossRef]
- Ashutosh, S. Surface Properties of Normal and Damaged Corneal Epithelia. J. Dispers. Sci. Technol. 1992, 13, 459–478. [Google Scholar]
- Zhao, Y.; Song, S.; Ren, X.; Zhang, J.; Lin, Q.; Zhao, Y. Supramolecular Adhesive Hydrogels for Tissue Engineering Applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef]
- Omar, J.; Ponsford, D.; Dreiss, C.A.; Lee, T.; Loh, X.J. Supramolecular Hydrogels: Design Strategies and Contemporary Biomedical Applications. Chem. Asian J. 2022, 17, e202200081. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xiao, Q.; Qi, G.; Chen, F.; Tu, B.; Zhang, S.; Li, Y.; Chen, Y.; Yu, H.; Duan, P. A Hydrogen Bonds-Crosslinked Hydrogels with Self-Healing and Adhesive Properties for Hemostatic. Front. Bioeng. Biotechnol. 2022, 10, 855013. [Google Scholar] [CrossRef]
- Tian, G.; Liu, Y.; Yu, M.; Liang, C.; Yang, D.; Huang, J.; Zhao, Q.; Zhang, W.; Chen, J.; Wang, Y.; et al. Electrostatic Interaction-Based High Tissue Adhesive, Stretchable Microelectrode Arrays for the Electrophysiological Interface. ACS Appl. Mater. Interfaces 2022, 14, 4852–4861. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Yu, H.; Wang, L.; Sheng, Y.; Huang, Z.; Yang, J.; Ni, Z.; Shen, D. Instant and Tough Adhesives for Rapid Gastric Perforation and Traumatic Pneumothorax Sealing. Adv. Healthc. Mater. 2022, 11, 2201798. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bai, R.; Suo, Z. Topological Adhesion of Wet Materials. Adv. Mater. 2018, 30, 1800671. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.K.; Lee, D.W.; Israelachvili, J.N.; Waite, J.H. Surface-initiated Self-healing of Polymers in Aqueous Media. Nat. Mater. 2014, 13, 867–872. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yuk, H.; Wu, J.; Nabzdyk, C.S.; Zhao, X. Instant Tough Bioadhesive with Triggerable Benign Detachment. Proc. Natl. Acad. Sci. USA 2020, 117, 15497–15503. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, J.; Gong, J.P. Barnacle Cement Proteins-Inspired Tough Hydrogels with Robust, Long-Lasting, and Repeatable Underwater Adhesion. Adv. Funct. Mater. 2021, 31, 2009334. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Wu, C.; Pei, X.; Cong, Y.; Zhang, R.; Fu, J. Antibacterial Zwitterionic Polyelectrolyte Hydrogel Adhesives with Adhesion Strength Mediated by Electrostatic Mismatch. ACS Appl. Mater. Interfaces 2020, 12, 46816–46826. [Google Scholar] [CrossRef]
- Roy, C.K.; Guo, H.L.; Sun, T.L.; Bin Ihsan, A.; Kurokawa, T.; Takahata, M.; Nonoyama, T.; Nakajima, T.; Gong, J.P. Self-Adjustable Adhesion of Polyampholyte Hydrogels. Adv. Mater. 2015, 27, 7344–7348. [Google Scholar] [CrossRef] [PubMed]
- Steck, J.; Kim, J.; Yang, J.; Hassan, S.; Suo, Z. Topological Adhesion. I. Rapid and Strong Topohesives. Extrem. Mech. Lett. 2020, 39, 100803. [Google Scholar] [CrossRef]
- Bhatia, S.K. Biomaterials for Clinical Applications; Springer Science & Business Media: New York, NY, USA, 2010. [Google Scholar]
- Tighe, B.J.; Mann, A. 11—Adhesives and Interfacial Phenomena in Wound Healing. In Advanced Wound Repair Therapies; Farrar, D., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 247–283. [Google Scholar]
- Wallace, D.G.; Cruise, G.M.; Rhee, W.M.; Schroeder, J.A.; Prior, J.J.; Ju, J.; Maroney, M.; Duronio, J.; Ngo, M.H.; Estridge, T.; et al. A Tissue Sealant Based on Reactive Multifunctional Polyethylene Glycol. J. Biomed. Mater. Res. 2001, 58, 545–555. [Google Scholar] [CrossRef]
- Wu, S.J.; Zhao, X. Bioadhesive Technology Platforms. Chem. Rev. 2023, 123, 14084–14118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, H.; Huang, C.; Sun, J.; Qu, X.; Lu, Y. A Novel Corneal Adhesive Based on Functionally Coupled PEG-lysozyme Hydrogel for Wound Closure After Surgical Eye Surgery. Chin. Chem. Lett. 2022, 33, 4321–4325. [Google Scholar] [CrossRef]
- Ge, L.; Chen, S. Recent Advances in Tissue Adhesives for Clinical Medicine. Polymers 2020, 12, 939. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Xia, Y.; Xu, C.; Meng, K.; Lian, J.; Zhang, X.; Xu, J.; Wang, C.; Zhao, B. Photocross-linked Silk Fibroin/Hyaluronic Acid Hydrogel Loaded with hDPSC for Pulp Regeneration. Int. J. Biol. Macromol. 2022, 215, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, X.; Yu, F.; Fang, P.; Liu, L.; Du, X.; Li, W.; He, D.; Bai, Y.; Li, S.; et al. Photocurable and Temperature-Sensitive Bioadhesive Hydrogels for Sutureless Sealing of Full-Thickness Corneal Wounds. Small Methods 2023, 8, 2300996. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, S.; Du, X.; Li, W.; Wang, Q.; He, D.; Yuan, J. Natural Polymer-derived Photocurable Bioadhesive Hydrogels for Sutureless Keratoplasty. Bioact. Mater. 2021, 8, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Shen, Y.; Adamcik, J.; Fischer, P.; Schneider, M.; Loessner, M.J.; Mezzenga, R. Polyphenol-Binding Amyloid Fibrils Self-Assemble into Reversible Hydrogels with Antibacterial Activity. ACS Nano 2018, 12, 3385–3396. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, J.; Han, X.; Pan, Y.; Wang, P.; Wang, T.; Lu, T. A Universal Strategy for Tough Adhesion of Wet Soft Material. Adv. Funct. Mater. 2020, 30, 2003207. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, J.; Huang, Y.; Mu, L.; Chen, J.; Liang, Z.; Yin, Z.; Chu, D.; Han, Y.; Guo, B. Injectable Antiswelling and High-Strength Bioactive Hydrogels with a Wet Adhesion and Rapid Gelling Process to Promote Sutureless Wound Closure and Scar-free Repair of Infectious Wounds. ACS Nano 2023, 17, 22015–22034. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Zhang, X.; Tang, P.; Zheng, T.; Ran, R.; Li, G. An Injectable, Self-healing, and Antioxidant Collagen- and Hyaluronic Acid-based Hydrogel Mediated with Gallic Acid and Dopamine for Wound Repair. Carbohydr. Polym. 2023, 320, 121231. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.M.G.; Herretes, S.; Pirouzmanesh, A.; Wang, D.-A.; Elisseeff, J.H.; Jun, A.; McDonnell, P.J.; Chuck, R.S.; Behrens, A. Modified Chondroitin Sulfate Aldehyde Adhesive for Sealing Corneal Incisions. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, X.; Deng, Z.; Chen, Y.; Yu, Q.; Tang, W.; Sun, T.L.; Zhang, Y.S.; Yue, K. Tough Bonding, On-Demand Debonding, and Facile Rebonding between Hydrogels and Diverse Metal Surfaces. Adv. Mater. 2019, 31, 1904732. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Whalen, J.J.; Humayun, M.S.; Thompson, M.E. Reversible Bioadhesives Using Tannic Acid Primed Thermally-Responsive Polymers. Adv. Funct. Mater. 2020, 30, 1907478. [Google Scholar] [CrossRef]
- Jia, L.; Yongrui, H.; Weiya, Y.; Xiaomin, S.; Yingni, X.; Yuehai, P.; Wenjing, S.; Jin, Y.; Li, R. Sutureless Transplantation Using a Semi-interpenetrating Polymer Network Bioadhesive for Ocular Surface Reconstruction. Acta Biomater. 2022, 153, 273–286. [Google Scholar]
- Aslzad, S.; Savadi, P.; Abdolahinia, E.D.; Omidi, Y.; Fathi, M.; Barar, J. Chitosan/dialdehyde Starch Hybrid In Situ Forming Hydrogel for Ocular Delivery of Betamethasone. Mater. Today Commun. 2022, 33, 104873. [Google Scholar] [CrossRef]
- Liu, H.; Qin, S.; Liu, J.; Zhou, C.; Zhu, Y.; Yuan, Y.; Fu, D.; Lv, Q.; Song, Y.; Zou, M.; et al. Bio-Inspired Self-Hydrophobized Sericin Adhesive with Tough Underwater Adhesion Enables Wound Healing and Fluid Leakage Sealing. Adv. Funct. Mater. 2022, 32, 2201108. [Google Scholar] [CrossRef]
- You, J.; Frazer, H.; Sayyar, S.; Chen, Z.; Liu, X.; Taylor, A.; Filippi, B.; Beirne, S.; Wise, I.; Petsoglou, C.; et al. Development of an In Situ Printing System with Human Platelet Lysate-Based Bio-Adhesive to Treat Corneal Perforations. Transl. Vis. Sci. Technol. 2022, 11, 26. [Google Scholar] [CrossRef]
- Oelker, A.M.; Berlin, J.A.; Wathier, M.; Grinstaff, M.W. Synthesis and Characterization of Dendron Cross-Linked PEG Hydrogels as Corneal Adhesives. Biomacromolecules 2011, 12, 1658–1665. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Liu, C.; Wang, N.; Chen, H.; Yao, W.; Sun, G.; Song, Q.; Qiao, W. A Highly Efficient, in situ Wet-Adhesive Dextran Derivative Sponge for Rapid Hemostasis. Biomaterials 2019, 205, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Fuyu, S.; Jiahui, Z.; Jie, L.; Yi, C.; Yehan, T.; Changyou, S.; Haisong, W. A Mussel-inspired Flexible Chitosan-based Bio-hydrogel as a Tailored Medical Adhesive. Int. J. Biol. Macromol. 2021, 189, 183–193. [Google Scholar]
- Yang, Z.; Yu, X.; Wei, P.; Huang, Y.; Zhou, S.; Jing, W.; Zhang, Y.; Sun, L.; Bao, G.; He, X.; et al. Tetra-armed PEG-based Rapid High-adhesion, Antibacterial and Biodegradable Pre-clinical Bioadhesives for Preventing Pancreas Leakage. Mater. Des. 2022, 224, 111281. [Google Scholar] [CrossRef]
- Liu, B.S.; Liao, M.; Wagner, W.L.; Khalil, H.A.; Chen, Z.; Ackermann, M.; Mentzer, S.J. Biomechanics of a Plant-Derived Sealant for Corneal Injuries. Transl. Vis. Sci. Technol. 2023, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Kabir, H.; Mahdavi, S.S.; Abdekhodaie, M.J.; Rafii, A.B.; Merati, M. Development of an In-situ Forming Collagen-based Hydrogel as a Regenerative Bioadhesive for Corneal Perforations. Int. J. Biol. Macromol. 2024, 278, 134761. [Google Scholar] [CrossRef]
- Chaurasia, S.S.; Lim, R.R.; Lakshminarayanan, R.; Mohan, R.R. Nanomedicine Approaches for Corneal Diseases. J. Funct. Biomater. 2015, 6, 277–298. [Google Scholar] [CrossRef]
- Tong, A.Y.; Gupta, P.K.; Kim, T. Wound Closure and Tissue Adhesives in Clear Corneal Incision Cataract Surgery. Curr. Opin. Ophthalmol. 2018, 29, 14–18. [Google Scholar] [CrossRef]
- Trujillo-de Santiago, G.; Sharifi, R.; Yue, K.; Sani, E.S.; Kashaf, S.S.; Alvarez, M.M.; Leijten, J.; Khademhosseini, A.; Dana, R.; Annabi, N. Ocular adhesives: Design, chemistry, crosslinking mechanisms, and applications. Biomaterials 2019, 197, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Dong, Z.; Tang, S.; Chu, W.; Zheng, X.; Zhen, L.; Chen, X.; Ding, C.; Luo, J.; Li, J. A Natural Polymer Based Bioadhesive with Self-healing Behavior and Improved Antibacterial Properties. Biomater. Sci. 2020, 8, 4346–4357. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, Y.; Fan, B.; Peng, W.; Qian, W.; Ji, X.; Gan, D.; Liu, P. Long-term Antibacterial, Antioxidative, and Bioadhesive Hydrogel Wound Dressing for Infected Wound Healing Applications. Biomater. Sci. 2023, 11, 2080–2090. [Google Scholar] [CrossRef]
- Hotta, K.; Hirakata, A.; Hida, T. The Management of Retinal Detachments Associated with Choroidal Colobomas by Vitrectomy with Cyanoacrylate Retinopexy. Jpn. J. Ophthalmol. 1998, 42, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Öhrström, A.; Stenkula, S.; Berglin, L.; Naeser, P. Scleral Reinforcement by a Teflon Graft and a Tissue Adhesive. Acta Ophthalmol. 2009, 66, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Leahey, A.B.; Gottsch, J.D.; Stark, W.J. Clinical Experience with N-butyl Cyanoacryiate (Nexacryl) Tissue Adhesive. Ophthalmology 1993, 100, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Nozari, N.; Biazar, E.; Kamalvand, M.; Keshel, S.H.; Shirinbakhsh, S. Photo Cross-linkable Biopolymers for Cornea Tissue Healing. Curr. Stem Cell Res. Ther. 2022, 17, 58–70. [Google Scholar] [CrossRef]
- Barroso, I.A.; Man, K.; Robinson, T.E.; Cox, S.C.; Ghag, A.K. Photocurable GelMA Adhesives for Corneal Perforations. Bioengineering 2022, 9, 53. [Google Scholar] [CrossRef]
- Tang, Q.; Lu, B.; He, J.; Chen, X.; Fu, Q.; Han, H.; Luo, C.; Yin, H.; Qin, Z.; Lyu, D.; et al. Exosomes-loaded Thermosensitive Hydrogels for Corneal Epithelium and Stroma Regeneration. Biomaterials 2022, 280, 121320. [Google Scholar] [CrossRef] [PubMed]
- Kassaee, S.N.; Nili-Ahmadabadi, A.; Mahboobian, M.M. Fabrication of Poloxamer Based Besifloxacin Thermosensitive in situ Gelling Nanoemulsions for Ophthalmic Delivery. J. Bioact. Compat. Polym. 2023, 38, 298–310. [Google Scholar] [CrossRef]
- McTiernan, C.D.; Simpson, F.C.; Haagdorens, M.; Samarawickrama, C.; Hunter, D.; Buznyk, O.; Fagerholm, P.; Ljunggren, M.K.; Lewis, P.; Pintelon, I.; et al. LiQD Cornea: Pro-regeneration Collagen Mimetics as Patches and Alternatives to Corneal Transplantation. Sci. Adv. 2020, 6, eaba2187. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, W.; Qiao, Y.; Shi, D.; Hu, L.; Cheng, J.; Wu, J.; Zhao, L.; Li, D.; Shi, W.; et al. ECM-Like Adhesive Hydrogel for the Regeneration of Large Corneal Stromal Defects. Adv. Healthc. Mater. 2023, 12, 2300192. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Imani, R.; Shokrollahi, P.; Jarchizadeh, R.; Keshel, S.H. Hydrogel-based Formulations for Drug Delivery to the Anterior Segment of the Eye. J. Drug Deliv. Sci. Technol. 2023, 81, 104250. [Google Scholar] [CrossRef]
- Ahmed, S.; Amin, M.M.; Sayed, S. Ocular Drug Delivery: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 66. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Y.; Hu, Q. Recent Advances in Overcoming Barriers to Cell-based Delivery Systems for Cancer Immunotherapy. Exploration 2022, 2, 20210106. [Google Scholar] [CrossRef] [PubMed]
- Naghib, S.M.; Ahmadi, B.; Kangarshahi, B.M.; Mozafari, M.R. Chitosan-based Smart Stimuli-responsive Nanoparticles for Gene Delivery and Gene Therapy: Recent Progresses on Cancer Therapy. Int. J. Biol. Macromol. 2024, 278, 134542. [Google Scholar] [CrossRef] [PubMed]
- Tarsitano, M.; Mancuso, A.; Cristiano, M.C.; Urbanek, K.; Torella, D.; Paolino, D.; Fresta, M. Perspective Use of Bio-adhesive Liquid Crystals as Ophthalmic Drug Delivery Systems. Sci. Rep. 2023, 13, 16188. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, L.; Kauppila, M.; Samanta, S.; Parihar, V.S.; Ilmarinen, T.; Miettinen, S.; Oommen, O.P.; Skottman, H. Tissue Adhesive Hyaluronic Acid Hydrogels for Sutureless Stem Cell Delivery and Regeneration of Corneal Epithelium and Stroma. Biomaterials 2019, 225, 119516. [Google Scholar] [CrossRef] [PubMed]
- Rakhmetova, A.; Yi, Z.; Sarmout, M.; Koole, L.H. Sustained Release of Voriconazole Using 3D-Crosslinked Hydrogel Rings and Rods for Use in Corneal Drug Delivery. Gels 2023, 9, 933. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, P.; Li, D.; Liu, M.; Zhang, J.; Yuan, K.; Zheng, H.; Liu, L. Preparation and Evaluation of Oxidized-dextran Based on Antibacterial Hydrogel for Synergistic Photodynamic Therapy. Int. J. Biol. Macromol. 2023, 253, 127648. [Google Scholar] [CrossRef]
- Chen, L.; Yan, D.; Wu, N.; Yao, Q.; Sun, H.; Pang, Y.; Fu, Y. Injectable Bio-responsive Hydrogel for Therapy of Inflammation Related Eyelid Diseases. Bioact. Mater. 2021, 6, 3062–3073. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Yu, A.; Lin, D.; Bao, Z.; Wang, Y.; Li, X. Bioinspired Self-assembly Supramolecular Hydrogel for Ocular Drug Delivery. Chin. Chem. Lett. 2021, 32, 3936–3939. [Google Scholar] [CrossRef]
- Jung, H.J.; Abou-Jaoude, M.; Carbia, B.E.; Plummer, C.; Chauhan, A. Glaucoma Therapy by Extended Release of Timolol from Nanoparticle Loaded Silicone-hydrogel Contact Lenses. J. Control. Release 2013, 165, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Burke, M.T.; Carbia, B.E.; Plummer, C.; Chauhan, A. Extended Drug Delivery by Contact Lenses for Glaucoma Therapy. J. Control. Release 2012, 162, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-H.; Gause, S.; Chauhan, A. Review of Ophthalmic Drug Delivery by Contact Lenses. J. Drug Deliv. Sci. Technol. 2014, 24, 123–135. [Google Scholar] [CrossRef]
- Mondal, H.; Kim, H.-J.; Mohanto, N.; Jee, J.-P. A Review on Dry Eye Disease Treatment: Recent Progress, Diagnostics, and Future Perspectives. Pharmaceutics 2023, 15, 990. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, B.; Kozioł, A.; Brzozowska, E.; Ferens-Sieczkowska, M. Application of Selected Biosensor Techniques in Clinical Diagnostics. Expert Rev. Mol. Diagn. 2021, 21, 925–937. [Google Scholar] [CrossRef]
- Farandos, N.M.; Yetisen, A.K.; Monteiro, M.J.; Lowe, C.R.; Yun, S.H. Contact Lens Sensors in Ocular Diagnostics. Adv. Healthc. Mater. 2014, 4, 792–810. [Google Scholar] [CrossRef]
- Han, K.J.; Moon, H.; Woo, J.M.; Min, J.K. Using RETeval System Flicker Electroretinography for Evaluation of Dense Vitreous Hemorrhage. Retina 2022, 42, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, J.; Marçal, H.; Watson, S.; Wakefield, D.; Sarris, M.; Foster, L.J.R. Sutureless Sealing of Penetrating Corneal Wounds Using a Laser-activated Thin Film Adhesive. Lasers Surg. Med. 2011, 43, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Chenault, H.K.; Bhatia, S.K.; DiMaio Jr, W.G.; Vincent, G.L.; Camacho, W.; Behrens, A. Sealing and Healing of Clear Corneal Incisions with an Improved Dextran Aldehyde-PEG Amine Tissue Adhesive. Curr. Eye Res. 2011, 36, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhou, Y.; Lu, F.; Zhai, L.; Wu, H.; Chen, Z.; Wang, C.; Zhu, X.; Xie, Y.; Cai, P.; et al. Contact Lens Sensor with Anti-jamming Capability and High Sensitivity for Intraocular Pressure Monitoring. ACS Sens. 2023, 8, 2691–2701. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Shum, A.J.; Cowan, M.; Lähdesmäki, I.; Parviz, B.A. A Contact Lens with Embedded Sensor for Monitoring Tear Glucose Level. Biosens. Bioelectron. 2011, 26, 3290–3296. [Google Scholar] [CrossRef]
- Willshire, C.; Buckley, R.J.; Bron, A.J. Estimating Basal Tear Osmolarity in Normal and Dry Eye Subjects. Contact Lens Anterior Eye 2018, 41, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Shinn, J.; Kwon, N.; Lee, S.A.; Lee, Y. Smart pH-responsive Nanomedicines for Disease Therapy. J. Pharm. Investig. 2022, 52, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, Y.; Deng, F.; Chen, Y.; Zhang, H. Eye-Movement-Controlled Wheelchair Based on Flexible Hydrogel Biosensor and WT-SVM. Biosensors 2021, 11, 198. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; He, X.; Teng, L.; Zeng, X.; Zhu, S.; Dong, Y.; Zeng, Z.; Zheng, Q.; Sun, X. Adhesion Mechanism, Applications, and Challenges of Ocular Tissue Adhesives. Int. J. Mol. Sci. 2025, 26, 486. https://doi.org/10.3390/ijms26020486
Hu Z, He X, Teng L, Zeng X, Zhu S, Dong Y, Zeng Z, Zheng Q, Sun X. Adhesion Mechanism, Applications, and Challenges of Ocular Tissue Adhesives. International Journal of Molecular Sciences. 2025; 26(2):486. https://doi.org/10.3390/ijms26020486
Chicago/Turabian StyleHu, Zuquan, Xinyuan He, Lijing Teng, Xiangyu Zeng, Simian Zhu, Yu Dong, Zhu Zeng, Qiang Zheng, and Xiaomin Sun. 2025. "Adhesion Mechanism, Applications, and Challenges of Ocular Tissue Adhesives" International Journal of Molecular Sciences 26, no. 2: 486. https://doi.org/10.3390/ijms26020486
APA StyleHu, Z., He, X., Teng, L., Zeng, X., Zhu, S., Dong, Y., Zeng, Z., Zheng, Q., & Sun, X. (2025). Adhesion Mechanism, Applications, and Challenges of Ocular Tissue Adhesives. International Journal of Molecular Sciences, 26(2), 486. https://doi.org/10.3390/ijms26020486








