Various Options for Covalent Immobilization of Cysteine Proteases—Ficin, Papain, Bromelain
Abstract
1. Introduction
2. Results
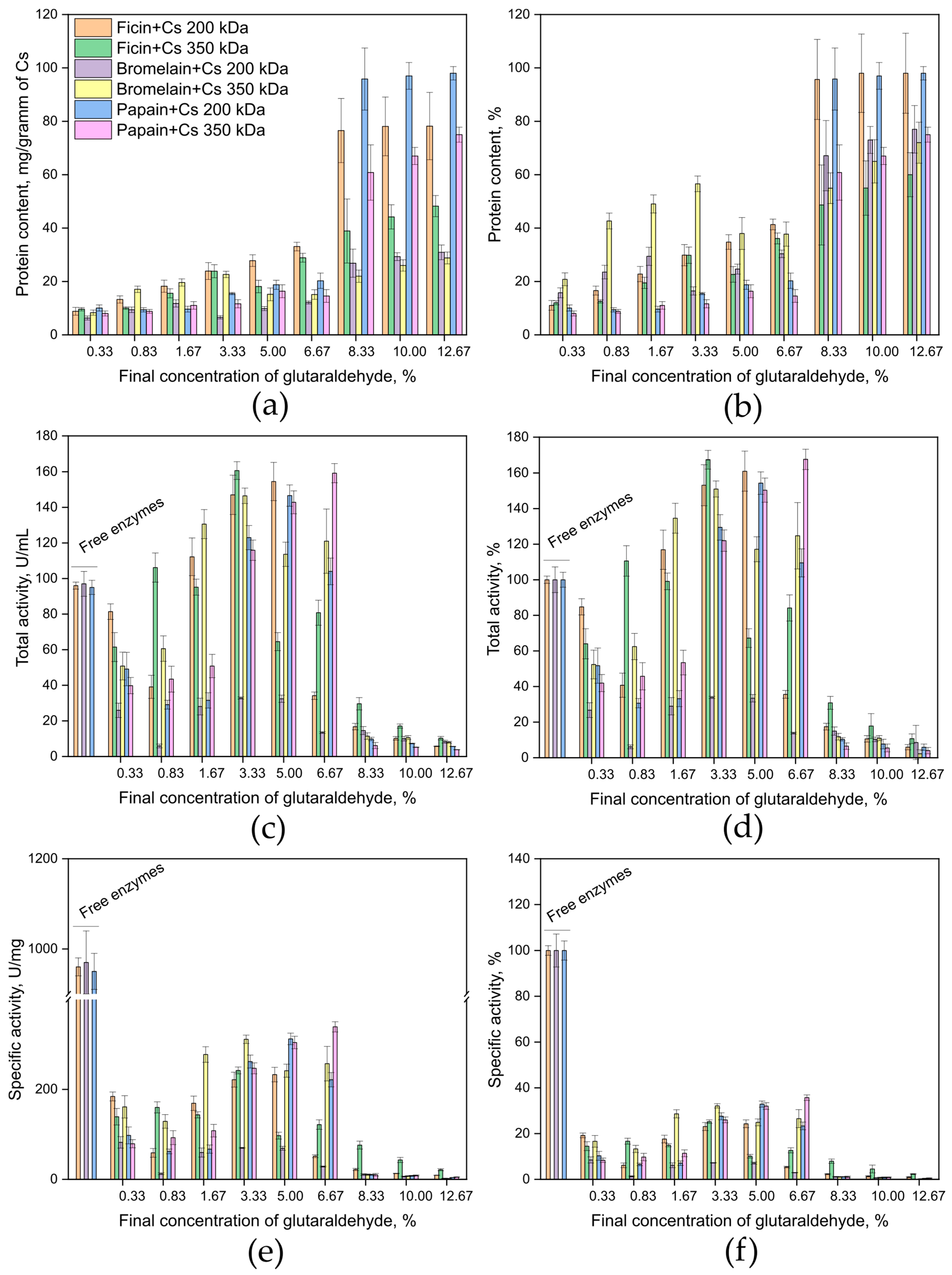
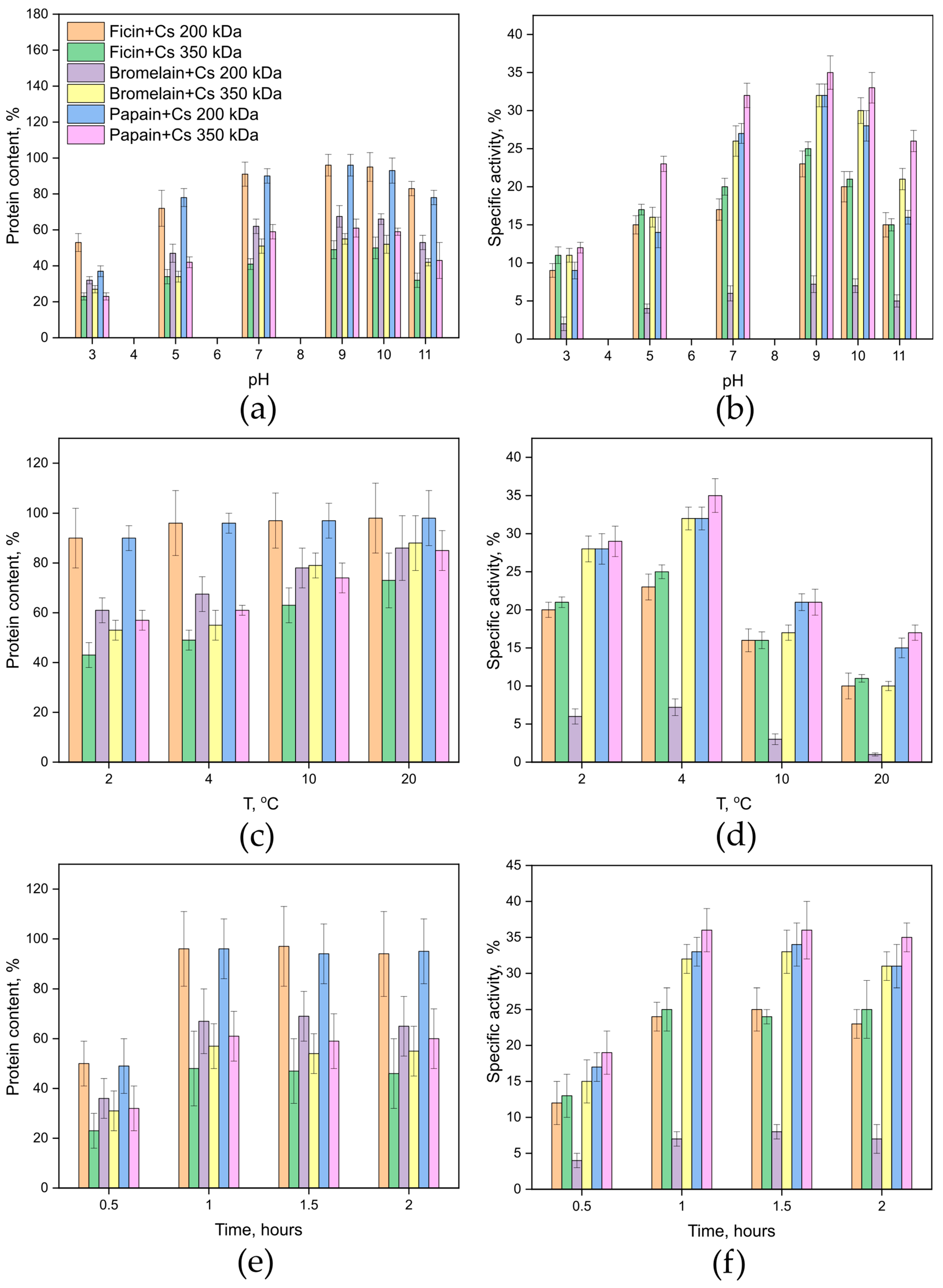
2.1. Developing a Method for Covalent Immobilization of Cysteine Proteases on a Chitosan Matrix
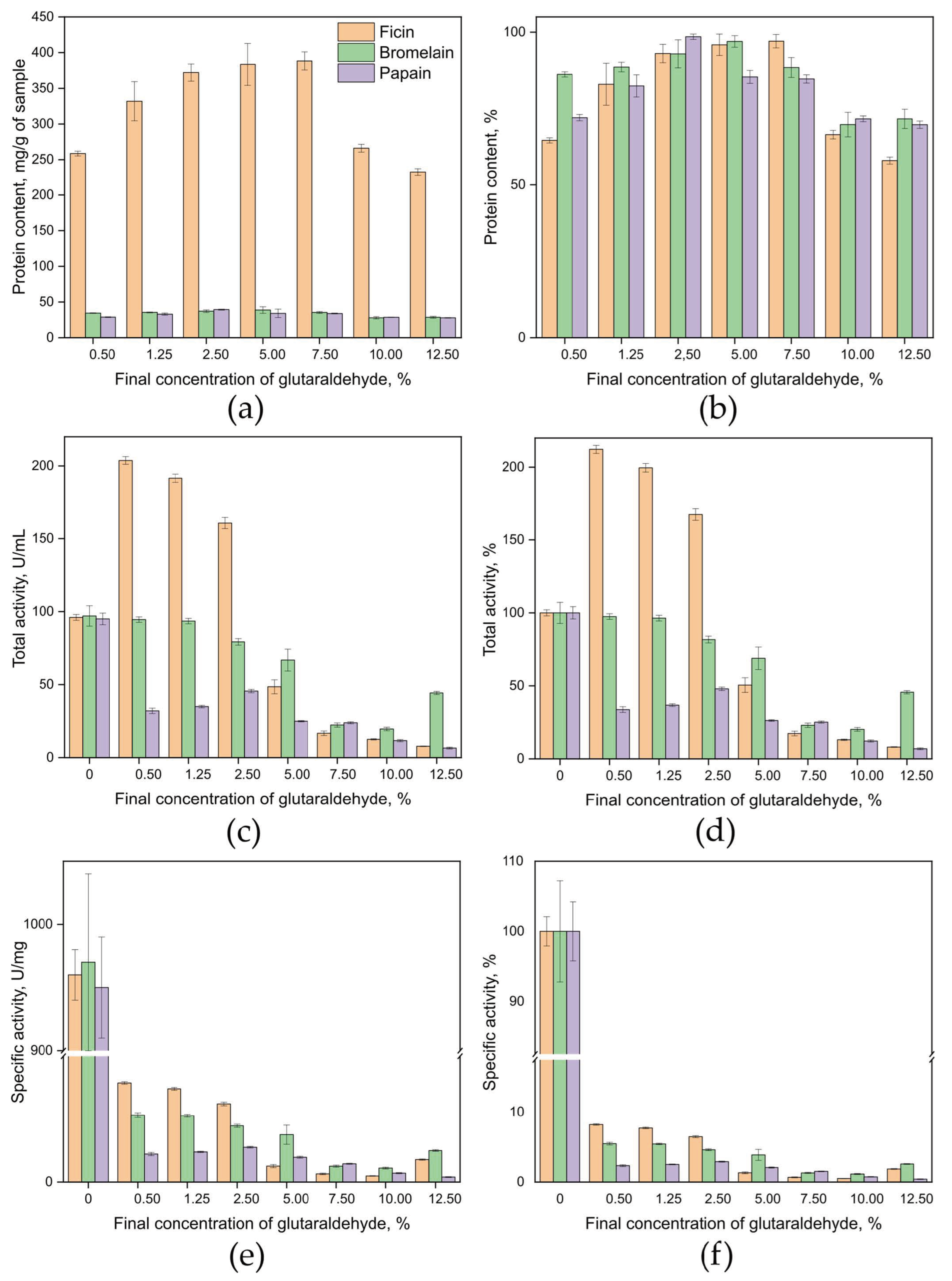
2.2. Developing a Method for Covalent Immobilization of Cysteine Proteases Without a Carrier Matrix
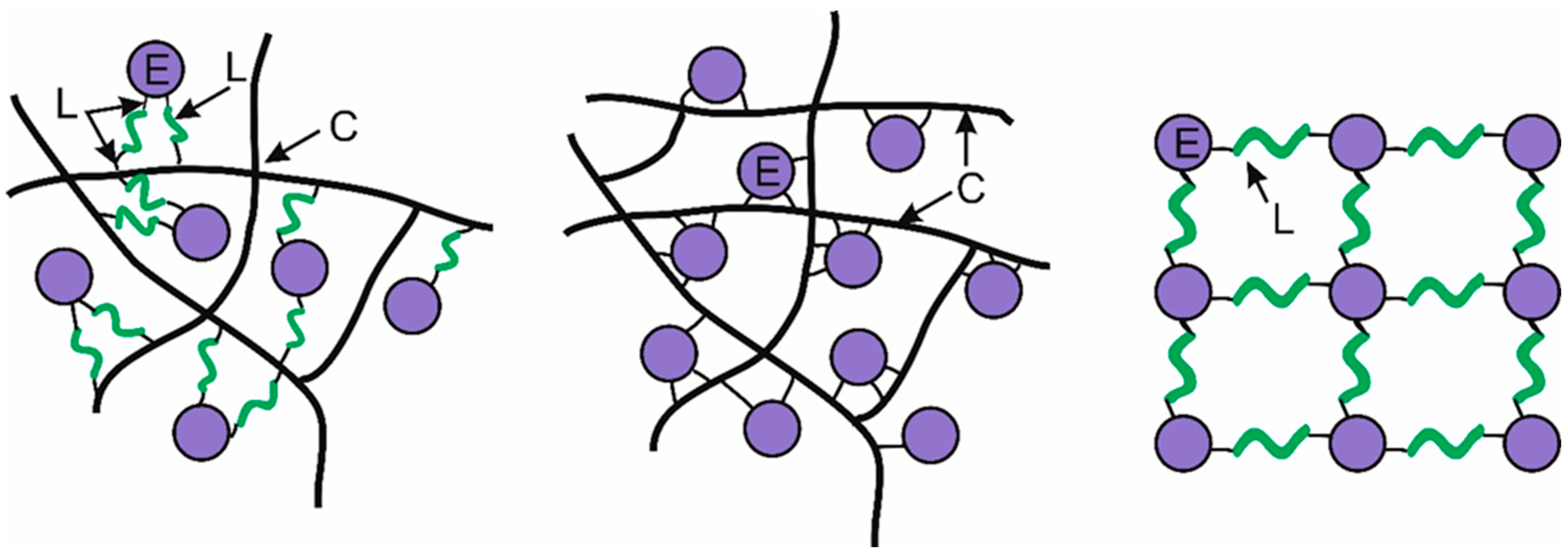
3. Discussion
- E–C–L, where the enzyme is linked to both the carrier and the crosslinking reagent;
- E–C, where the enzyme is directly attached to the carrier;
- E–L, where the enzyme is linked to the crosslinking reagent.
4. Materials and Methods
4.1. Materials
4.2. Method for Covalent Immobilization on a Chitosan Matrix
4.3. Method for Covalent Immobilization of Enzymes Without a Carrier Matrix
4.4. Method for Determining Proteolytic Activity of Enzymes
4.5. Fourier-Transform Infrared Spectroscopy
4.6. SEM
4.7. Statistical Processing of Research Results
5. Conclusions
- The optimal enzyme-to-chitosan ratios are 8:100 for ficin, 10:100 for papain, and 4:100 for bromelain.
- Since glutaraldehyde reacts reversibly with amino groups across a broad pH range (≥pH 3.0), with minimal reversibility between pH 7.0 and 9.0, it is advisable to use buffers with a pH of 9–10 for covalent immobilization. Lysine ε-amino groups have a pKa > 9.5, but the small percentage of unprotonated amines at lower pH levels is sufficient for reacting with glutaraldehyde. Excessive crosslinking may increase steric hindrance for large protein substrates.
- Final concentrations of glutaraldehyde in the immobilization system should be in the range of 3.33–6.67%.
- To protect the active site from oxidation, immobilization of cysteine proteases should be carried out in a 0.04 M cysteine solution.
- The immobilization process should be conducted at a temperature of 4 °C.
- To ensure that no unbound enzyme remains, the final step in sample processing should involve dialysis against a 0.05 M tris-HCl buffer, pH 7.5, using a cellophane membrane with a pore size of 25 kDa.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Domsalla, A.; Melzig, M.F. Occurrence and properties of proteases in plant latices. Planta Med. 2008, 74, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Golovkin, B.N. Latex and proteolytic enzymes in plants. Byulleten Gl. Bot. Sada. 2006, 191, 157–160. [Google Scholar]
- Radauer, C.; Breiteneder, H. Evolutionary biology of plant food allergens. J. Allergy Clin. Immunol. 2007, 120, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.W.; Coghlan, V.M.; Dihel, L.C. Cloning and sequencing of Papain-encoding cDNA. Gene 1986, 48, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Vernet, T.; Tessier, D.C.; Richardson, C.; Laliberté, F.; Khouri, H.E.; Bell, A.W.; Storer, A.C.; Thomas, D.Y. Secretion of functional Papain precursor from insect cells. Requirement for N-glycosylation of the pro-region. J. Biol Chem. 1990, 265, 16661–16666. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.J.; Rawlings, N.D.; Woessner, J.F. Handbook of Proteolytic Enzymes; Academic Press: New York, NY, USA, 1998. [Google Scholar]
- Starley, I.F.; Mohammed, P.; Schneider, G.; Bickler, S.W. The treatment of paediatric burns using topical papaya. Burns 1999, 25, 636–639. [Google Scholar] [CrossRef]
- Berger, J.; Asenjo, C.F. Anthelmintic activity of crystalline Papain. Science 1940, 91, 387–388. [Google Scholar] [CrossRef]
- Yaakobi, T.; Cohen-Hadar, N.; Yaron, H.; Hirszowicz, E.; Simantov, Y.; Bass, A.; Freeman, A. Wound debridement by continuous streaming of proteolytic enzyme solution: Effects on experimental chronic wound model in porcin. Wounds–A Compend. Clin. Res. Pract. 2007, 19, 192–200. [Google Scholar]
- Baidamshina, D.R.; Koroleva, V.A.; Olshannikova, S.S.; Trizna, E.Y.; Bogachev, M.I.; Artyukhov, V.G.; Holyavka, M.G.; Kayumov, A.R. Biochemical properties and anti-biofilm activity of chitosan-immobilized Papain. Mar. Drugs 2021, 19, 197. [Google Scholar] [CrossRef] [PubMed]
- Buttle, D.J. Affinity chromatography of cysteine peptidases. Methods Enzymol. 1994, 244, 639–648. [Google Scholar] [PubMed]
- Chetia, D.; Nath, L.K.; Dutta, S.K. Extraction, purification and physicochemical properties of a proteolytic enzyme from the latex of Ficus hispida Linn. Indian J. Pharm. Sci. 1999, 61, 29–33. [Google Scholar]
- Baidamshina, D.R.; Trizna, E.Y.; Holyavka, M.G.; Bogachev, M.I.; Artyukhov, V.G.; Akhatova, F.S.; Rozhina, E.V.; Fakhrullin, R.F.; Kayumov, A.R. Targeting microbial biofilms using Ficin, a nonspecific plant protease. Sci. Rep. 2017, 7, 46068. [Google Scholar] [CrossRef]
- Baidamshina, D.R.; Trizna, E.Y.; Goncharova, S.S.; Sorokin, A.V.; Lavlinskaya, M.S.; Melnik, A.P.; Gafarova, L.F.; Kharitonova, M.A.; Ostolopovskaya, O.V.; Artyukhov, V.G.; et al. The Effect of Ficin Immobilized on Carboxymethyl Chitosan on Biofilms of Oral Pathogens. Int. J. Mol. Sci. 2023, 24, 16090. [Google Scholar] [CrossRef] [PubMed]
- Baidamshina, D.R.; Koroleva, V.A.; Trizna, E.Y.; Pankova, S.M.; Agafonova, M.N.; Chirkova, M.N.; Vasileva, O.S.; Akhmetov, N.; Shubina, V.V.; Porfiryev, A.G.; et al. Anti-biofilm and wound-healing activity of chitosan-immobilized Ficin. Int. J. Biol. Macromol. 2020, 164, 4205–4217. [Google Scholar] [CrossRef]
- Rosenberg, L.; Krieger, Y.; Bogdanov-Berezovski, A.; Silberstein, E.; Shoham, Y.; Singer, A.J. A novel rapid and selective enzymatic debridement agent for burn wound management: A multi-center RCT. Burns 2014, 40, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Rowan, A.D. Stem Bromelain. In Handbook of Proteolytic Enzymes; Academic Press: Cambridge, MA, USA, 2013; Volume 2, pp. 1871–1873. [Google Scholar]
- Sharaf, A.; Muthayya, P. Microbial profile of burn wounds managed with enzymatic debridement using bromelain-based agent, NexoBrid. Burns 2021, 48, 1618–1625. [Google Scholar] [CrossRef]
- Feijoo-Siota, L.; Villa, T.G. Native and biotechnologically engineered plant proteases with industrial applications. Food Bioprocess Technol. 2011, 4, 1066–1088. [Google Scholar] [CrossRef]
- Alpay, P.; Uygun, D.A. Usage of immobilized papain for enzymatic hydrolysis of proteins. J. Mol. Catal. B Enzym. 2015, 111, 56–63. [Google Scholar] [CrossRef]
- Abbas, S.; Shanbhag, T.; Kothare, A. Applications of bromelain from pineapple waste towards acne. Saudi J. Biol. Sci. 2020, 28, 1001–1009. [Google Scholar] [CrossRef]
- Sampietro de Luis, J.M. Experience with NexoBrid® in enzymatic debridement of facial burns. Burns 2018, 44, 1013–1014. [Google Scholar] [CrossRef]
- Holyavka, M.G.; Goncharova, S.S.; Sorokin, A.V.; Lavlinskaya, M.S.; Redko, Y.A.; Faizullin, D.A.; Baidamshina, D.R.; Zuev, Y.F.; Kondratyev, M.S.; Kayumov, A.R. Novel biocatalysts based on bromelain immobilized on functionalized chitosans and research on their structural features. Polymers 2022, 14, 5110. [Google Scholar] [CrossRef] [PubMed]
- Holyavka, M.; Pankova, S.; Koroleva, V.; Vyshkvorkina, Y.; Lukin, A.; Kondratyev, M.; Artyukhov, V. Influence of UV radiation on molecular structure and catalytic activity of free and immobilized bromelain, ficin and papain. J. Photochem. Photobiol. B 2019, 201, 111681. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Grazu, V.; Palomo, J.M.; Lopez-Gallego, F.; Fernandez-Lafuente, R.; Guisan, J.M. Immobilization of enzymes on heterofunctional epoxy supports. Nat. Protoc. 2007, 2, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez- Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernandez-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef]
- Santos-Moriano, P.; Monsalve-Ledesma, L.; Ortega-Munoz, M.; Fernandez-Arrojo, L.; Ballesteros, A.O.; Santoyo-Gonzalez, F.; Plou, F.J. Vinyl sulfone-activated silica for efficient covalent immobilization of alkaline unstable enzymes: Application to levansucrase for fructooligosaccharide synthesis. RSC Adv. 2016, 6, 64175–64181. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Recent advances in engineered chitosan-based nanogels for biomedical applications. J. Mater. Chem. B 2017, 5, 6986–7007. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.R.; Singh, U.S.; Momin, M.; Bhavsar, C. Chitosan: Application in tissue engineering and skin grafting. J. Polym. Res. 2017, 24, 125. [Google Scholar] [CrossRef]
- Desbrières, J.; Guibal, E. Chitosan for wastewater treatment. Polym. Int. 2018, 67, 7–14. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Recent advances of chitosan applications in plants. Polymers. 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, B. Application of chitin-and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Wang, K. Enzyme immobilization on chitosan-based supports. Chitosan-Based Hydrogels 2011, 18, 339–406. [Google Scholar]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.S.; Chamundeeswari, M. Enzyme immobilization on chitin and chitosan-based supports for biotechnological applications. Sustain. Agric. Rev. 2019, 35, 147–173. [Google Scholar]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Sulej, J.; Osińska-Jaroszuk, M.; Jaszek, M.; Olszewska, A.; Belcarz, A.; Piątek-Gołda, W. Chitosan as a Promising Support of a CDH Activity Preservation System for Biomedical and Industrial Applications. Int. J. Mol. Sci. 2023, 24, 4535. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.S.; de Farias, B.S.; Junior, T.R.S.A.C.; de Almeida Pinto, L.A.; Diaz, P.S. Chitosan–based nanofibers for enzyme immobilization. Int. J. Biol. Macromol. 2021, 183, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef]
- Santos, J.C.S.; Rueda, N.; Barbosa, O.; Millan-Linares, M.D.C.; Pedroche, J.; del Mar Yuste, M.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Bovine trypsin immobilization on agarose activated with divinylsulfone: Improved activity and stability via multipoint covalent attachment. J. Mol. Catal. B Enzym. 2015, 117, 38–44. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Lopez-Gallego, F.; Bolivar, J.M.; Rocha-Martin, J.; Moreno-Perez, S.; Guisán, J.M. Immobilization of proteins on highly activated glyoxyl supports: Dramatic increase of the enzyme stability via multipoint immobilization on pre-existing carriers. Curr. Org. Chem. 2015, 19, 1719–1731. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, A.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of enzymes via immobilization: Multipoint covalent attachment and other stabilization strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Mondal, K.; Mondal, K.C.; Thakur, N. Hunt for α-amylase from metagenome and strategies to improve its thermostability: A systematic review. World J. Microbiol. Biotechnol. 2022, 38, 203. [Google Scholar] [CrossRef] [PubMed]
- Montoya, N.A.; Roth, R.E.; Funk, E.K.; Gao, P.; Corbin, D.R.; Shiflett, M.B. Review on porous materials for the thermal stabilization of proteins. Microporous Mesoporous Mater. 2022, 333, 111750. [Google Scholar] [CrossRef]
- Anastas, P.T.; Rodriguez, A.; de Winter, T.M.; Coish, P.; Zimmerman, J.B. A review of immobilization techniques to improve the stability and bioactivity of lysozyme, Green. Chem. Lett. Rev. 2021, 14, 302–338. [Google Scholar]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Pedroche, J.; del Mar Yust, M.; Mateo, C.; Fernandez-Lafuente, R.; Giron-Calle, J.; Alaiz, M.; Vioque, J.; Guisan, J.M.; Millan, F. Effect of the support and experimental conditions in the intensity of the multipoint covalent attachment of proteins on glyoxyl-agarose supports: Correlation between enzyme–support linkages and thermal stability. Enzym. Microb. Technol. 2007, 40, 1160–1166. [Google Scholar] [CrossRef]
- Braham, S.A.; Morellon-Sterling, R.; de Andrades, D.; Rodrigues, R.C.; Siar, E.-H.; Aksas, A.; Pedroche, J.; Millan, M.d.C.; Fernandez-Lafuente, R. Effect of tris buffer in the intensity of the multipoint covalent immobilization of enzymes in glyoxyl- agarose beads. Appl. Biochem. Biotechnol. 2021, 193, 2843–2857. [Google Scholar] [CrossRef] [PubMed]
- Morellon-Sterling, R.; Siar, E.-H.; Braham, S.A.; de Andrades, D.; Pedroche, J.; Millán, M.D.C.; Fernandez-Lafuente, R. Effect of amine length in the interference of the multipoint covalent immobilization of enzymes on glyoxyl agarose beads. J. Biotechnol. 2021, 329, 128–142. [Google Scholar] [CrossRef]
- Lopez-Gallego, F.; Montes, T.; Fuentes, M.; Alonso, N.; Grazu, V.; Betancor, L.; Guis’an, J.M.; Fern’andez-Lafuente, R. Improved stabilization of chemically aminated enzymes via multipoint covalent attachment on glyoxyl supports. J. Biotechnol. 2005, 116, 1–10. [Google Scholar] [CrossRef]
- Cui, J.D.; Zhang, S.; Sun, L.-M. Cross-linked enzyme aggregates of phenylalanine ammonia lyase: Novel biocatalysts for synthesis of l-phenylalanine. Appl. Biochem. Biotechnol. 2012, 167, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.D.; Sun, L.M.; Li, L.L. A Simple Technique of Preparing Stable CLEAs of Phenylalanine Ammonia Lyase Using Coaggregation with Starch and Bovine Serum Albumin. Appl. Biochem. Biotechnol. 2013, 170, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Q.; Wang, S.S.; Li, L.N.; Gao, J.; Zhang, Y.W. Combined cross-linked enzyme aggregates as biocatalysts. Catalysts 2018, 8, 460. [Google Scholar] [CrossRef]
- Sheldon, R.A. CLEAs, combi-cleas and ‘smart’ magnetic cleas: Biocatalysis in a bio-based economy. Catalysts 2019, 9, 261. [Google Scholar] [CrossRef]
- Rios, N.S.; Pinheiro, B.B.; Pinheiro, M.P.; Bezerra, R.M.; dos Santos, J.C.S.; Barros Gonçalves, L.R. Biotechnological potential of lipases from Pseudomonas: Sources, properties and applications. Process Biochem. 2018, 75, 99–120. [Google Scholar] [CrossRef]
- Reis, C.L.B.; de Sousa, E.Y.A.; Serpa, J.d.F.; Oliveira, R.C.; dos Santos, J.C.S. Design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Quim. Nova 2019, 42, 768–783. [Google Scholar] [CrossRef]
- Costa, I.O.; Morais, J.R.F.; de Medeiros Dantas, J.M.; Gonçalves, L.R.B.; Santos, E.S.D.; Rios, N.S. Enzyme immobilization technology as a tool to innovate in the production of biofuels: A special review of the Cross-Linked Enzyme Aggregates (CLEAs) strategy. Enzym. Microb. Technol. 2023, 170, 110300. [Google Scholar] [CrossRef]
- Chen, N.; Chang, B.; Shi, N.; Yan, W.; Lu, F.; Liu, F. Cross-linked enzyme aggregates immobilization: Preparation, characterization, and applications. Crit. Rev. Biotechnol. 2023, 43, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Bouguerra, O.M.; Wahab, R.A.; Huyop, F.; Al-Fakih, A.M.; Mahmood, W.M.A.W.; Mahat, N.A.; Sabullah, M.K. An overview of crosslinked enzyme aggregates: Concept of development and trends of applications. Appl. Biochem. Biotechnol. 2024, 196, 5711–5739. [Google Scholar] [CrossRef] [PubMed]
- Arco, J.D.; Alc’antara, A.R.; Fern’andez-Lafuente, R.; Fern’andez-Lucas, J. Magnetic micro-macro biocatalysts applied to industrial bioprocesses. Bioresour. Technol. 2021, 322, 124547. [Google Scholar]
- Velasco-Lozano, S.; L’opez-Gallego, F.; Mateos-Díaz, J.C.; Favela-Torres, E. Cross- linked enzyme aggregates (CLEA) in enzyme improvement—A review. Biocatalysis 2016, 1, 166–177. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R.; Rosell, C.M.; Rodriguez, V.; Guisan, J.M. Strategies for enzyme stabilization by intramolecular crosslinking with bifunctional reagents. Enzym. Microb. Technol. 1995, 17, 517–523. [Google Scholar] [CrossRef]
- Aissaoui, N.; Landoulsi, J.; Bergaoui, L.; Boujday, S.; Lambert, J.F. Catalytic activity and thermostability of enzymes immobilized on silanized surface: Influence of the crosslinking agent. Enzym. Microb. Technol. 2013, 52, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Betancor, L.; López-Gallego, F.; Hidalgo, A.; Alonso-Morales, N.; Mateo, D.O.C.; Fernández-Lafuente, R.; Guisán, J.M. Different mechanisms of protein immobilization on glutaraldehyde activated supports: Effect of support activation and immobilization conditions. Enzym. Microb. Technol. 2006, 39, 877–882. [Google Scholar] [CrossRef]
- Wahba, M.I. Porous chitosan beads of superior mechanical properties for the covalent immobilization of enzymes. Int. J. Biol. Macromol. 2017, 105, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Shahedi, M.; Ahrari, F.; Mostafavi, M.; Habibi, Z.; Yousefi, M. Isocyanide-based multi-component reactions for carrier-free and carrier-bound covalent immobilization of enzymes. Nat. Protoc. 2023, 18, 1641–1657. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Faar, A.L.; Randall, R.J. Protein measurement with Folin-phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Sabirova, A.R.; Rudakova, N.L.; Balaban, N.P.; Ilyinskaya, O.N.; Demidyuk, I.V.; Kostrov, S.V.; Rudenskaya, G.N.; Sharipova, M.R. A novel secreted metzincin metalloproteinase from Bacillus intermedius. FEBS Lett. 2010, 584, 4419–4425. [Google Scholar] [CrossRef]
- Andreas, L.; Hilterhaus, L. Evaluation of Immobilized Enzymes for Industrial Applications. Chem. Soc. Rev. 2013, 42, 6236. [Google Scholar] [CrossRef]
- Artyukhov, V.G.; Kovaleva, T.A.; Holyavka, M.G.; Bityutskaya, L.A.; Grechkina, M.V. Thermal inactivation of free and immobilized inulinase. Appl. Biochem. Microbial. 2010, 46, 422–427. [Google Scholar] [CrossRef]
- Morellon-Sterling, R.; Carballares, D.; Arana-Pena, S.; Siar, E.-H.; Braham, S.A.; Fernandez-Lafuente, R. Advantages of supports activated with divinyl sulfone in enzyme coimmobilization: Possibility of multipoint covalent immobilization of the most stable enzyme and immobilization via ion exchange of the least stable enzyme. ACS Sustain. Chem. Eng. 2021, 9, 7508–7518. [Google Scholar] [CrossRef]
- Morellon-Sterling, R.; Bolivar, J.M.; Fernandez-Lafuente, R. Switch off/switch on of a cysteinyl protease as a way to preserve the active catalytic group by modification with a reversible covalent thiol modifier: Immobilization of ficin on vinyl-sulfone activated supports. Int. J. Biol. Macromol. 2022, 220, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Siar, E.H.; Morellon-Sterling, R.; Zidoune, M.N.; Fernandez-Lafuente, R. Amination of ficin extract to improve its immobilization on glyoxyl-agarose: Improved stability and activity versus casein. Int. J. Biol. Macromol. 2019, 133, 412–419. [Google Scholar] [CrossRef]
- Siar, E.H.; Arana-Pena, S.; Barbosa, O.; Zidoune, M.N.; Fernandez-Lafuente, R. Solid phase chemical modification of agarose glyoxyl-ficin: Improving activity and stability properties by amination and modification with glutaraldehyde. Process Biochem. 2018, 73, 109–116. [Google Scholar] [CrossRef]
- Siar, E.H.; Zaak, H.; Kornecki, J.F.; Zidoune, M.N.; Barbosa, O.; Fernandez- Lafuente, R. Stabilization of ficin extract by immobilization on glyoxyl agarose. Preliminary characterization of the biocatalyst performance in hydrolysis of proteins. Process Biochem. 2017, 58, 98–104. [Google Scholar] [CrossRef]
- Walt, D.R.; Agayn, V. The chemistry of enzyme and protein immobilization with glutaraldehyde. Trends Anal. Chem. 1994, 13, 425–430. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 798–802. [Google Scholar] [CrossRef]
- Asgher, M.; Noreen, S.; Bilal, M. Enhancing catalytic functionality of Trametes versicolor IBL-04 laccase by immobilization on chitosan microspheres. Chem. Eng. Res. Des. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Jarzabek, B.; Kaczmarczyk, B.; Sek, D. Characteristic and spectroscopic properties of the Schiff-base model compounds. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 74, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Malykhina, N.V.; Olshannikova, S.S.; Holyavka, M.G.; Sorokin, A.V.; Lavlinskaya, M.S.; Artyukhov, V.G.; Faizullin, D.A.; Zuev, Y.F. Preparation of Ficin complexes with carboxymethylchitosan and N-(2-hydroxy)propyl-3-trimethylammoniumchitosan and studies of their structural features. Russ. J. Bioorganic Chem. 2022, 48 (Suppl. 1), S50–S60. [Google Scholar] [CrossRef]
- Bowes, J.H.; Cater, C.W. The interaction of aldehydes with collagen. Bio-chim. Biophys. Acta 1968, 168, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, D.; Callen, C.R.; McCabe, M. The reactions between glutaraldehyde and various proteins. An investigation of their kinetics. Histochem. J. 1970, 2, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Alexa, G.; Chisalita, D.; Chirita, G. Reaction of dialdehyde with functional groups in collagen. Rev. Technol. Ind. Cuir. 1971, 63, 5–14. [Google Scholar]
- Avrameas, S.; Ternynck, T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoad-sorbents. Immunochemistry 1969, 6, 53–66. [Google Scholar] [CrossRef]
- Okuda, K.; Urabe, I.; Yamada, Y.; Okada, H. Reaction of glutaraldehyde with amino and thiol compounds. J. Ferment. Bioeng. 1991, 71, 100–105. [Google Scholar] [CrossRef]
- Weetall, H.H. Immobilized enzymes: Analytical applications. Anal. Chem. 1974, 46, 602A–604A. [Google Scholar]
- Guisán, J.M. Aldehyde-agarose gels as activated supports for immobilization—Stabilization of enzymes. Enzym. Microb. Technol. 1988, 10, 375–382. [Google Scholar] [CrossRef]
- Quiocho, F.A.; Richards, F.M. Intermolecular cross-linking of a protein in the crystalline state: Carboxypeptidase A. Proc. Natl. Acad. Sci. USA 1964, 52, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Avrameas, S. Coupling of enzymes to proteins with glutaraldehyde. Use of the con-jugates for the detection of antigens and anti-bodies. Immunochemistry 1969, 6, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Billah, R.E.K.; Islam, M.A.; Nazal, M.K.; Bahsis, L.; Soufiane, A.; Abdellaoui, Y.; Achak, M. A novel glutaraldehyde cross-linked chitosan@acid-activated bentonite composite for effective Pb (II) and Cr (VI) adsorption: Experimental and theoretical studies. Sep. Purif. Technol. 2024, 334, 126094. [Google Scholar] [CrossRef]
- Broun, G.B. Chemically aggregated enzymes. Methods Enzymol. 1976, 44, 263–280. [Google Scholar] [PubMed]
- Kennedy, J.F.; Cabral, J.M.S. Immobilized enzymes. Chem. Anal. 1983, 66, 253–391. [Google Scholar]
- Zaborsky, O.R. Immobilized Enzymes; CRC Press: Cleveland, OH, USA, 1973; 175p. [Google Scholar]
- Jansen, E.F.; Tomimatsu, Y.; Olson, A.C. Cross-linking of α-chymotrypsin and other proteins by reaction with glutaraldehyde. Arch. Biochem. Biophys. 1971, 144, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Tomimatsu, Y.; Jansen, E.F.; Gaffield, W.; Olson, A.C. Physical chemical observations on the α-chymotrypsin glutaralde-hyde system during formation of an insoluble derivative. J. Colloid Interface Sci. 1971, 36, 51–64. [Google Scholar] [CrossRef]
- Ottesen, M.; Svensson, B. Modification of papain by treatment with glutaraldehyde under reducing and non-reducing conditions. Comptes-Rendus Trav. Lab. Carlsberg. 1971, 38, 171–185. [Google Scholar]
- Jansen, E.F.; Olson, A.C. Properties and enzymatic activities of papain insolubilized with glutaraldehyde. Arch. Biochem. Biophys. 1969, 129, 221–227. [Google Scholar] [CrossRef]
- Bano, B.; Saleemuddin, M. Studies on chemically aggregated trypsin using glutaraldehyde. Ind. J. Biochem. Biophys. 1980, 17, 12–17. [Google Scholar]
- Gupta, M.N. Cross-linking techniques: Application to enzyme and protein stabilization and bioconjugate preparation. Biocatal. Des. Stab. Specif. 1993, 307–324. [Google Scholar] [CrossRef]
- Chui, W.K.; Wan, L.S. Prolonged retention of cross-linked trypsin in calcium alginate microspheres. J. Microencapsul. 1997, 14, 51–61. [Google Scholar] [CrossRef]
- Habeeb, A.F.S.A.; Hiramoto, R. Reaction of proteins with glutaraldehyde. Arch. Biochem. Biophys. 1968, 126, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, A.F. Preparation of enzymically active, water-insoluble derivatives of trypsin. Arch. Biochem. Biophys. 1967, 119, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Herzog, V.; Fahimi, H.D. Effect of glutaraldehyde on catalase. Biochemical and cytochemical studies with beef liver cata-lase and rat liver peroxisomes. J. Cell Biol. 1974, 60, 303–311. [Google Scholar] [CrossRef]
- Holyavka, M.; Faizullin, D.; Koroleva, V.; Olshannikova, S.; Zakhartchenko, N.; Zuev, Y.; Kondratyev, M.; Zakharova, E.; Artyukhov, V. Novel Biotechnological Formulations of Cysteine Proteases, Immobilized on Chitosan. Structure, Stability and Activity. Int. J. Biol. Macromol. 2021, 180, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Ol’shannikova, S.S.; Holyavka, M.G.; Artyukhov, V.G. Method Development for Ficin Entrapment into Gels Based on Food-Grade Chitosan and Chitosan Succinate. Pharm. Chem. J. 2021, 54, 1067–1070. [Google Scholar] [CrossRef]
- Holyavka, M.G.; Goncharova, S.S.; Redko, Y.A.; Lavlinskaya, M.S.; Sorokin, A.V.; Artyukhov, V.G. Novel biocatalysts based on enzymes in complexes with nano and micromaterials. Biophys. Rev. 2023, 15, 1127–1158. [Google Scholar] [CrossRef] [PubMed]
- Holyavka, M.; Redko Yu Goncharova, S.; Lavlinskaya, M.; Sorokin, A.; Kondratyev, M.; Artyukhov, V. Novel Hybrid Catalysts of Cysteine Proteases Enhanced by Chitosan and Carboxymethyl Chitosan Micro- and Nanoparticles. Polymers 2024, 16, 3111. [Google Scholar] [CrossRef]
- Jafari, N.; Najavand, S.; Pazhang, M.; Matin, A. Entrapment of Papain in Chitosan–Polyethylene Glycol Hybrid Nanohydrogels: Presenting a Model for Protein Delivery Systems. Mol Biotechnol. 2024. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Castañeda-Valbuena, D.; Berenguer-Murcia, Á.; Kamli, M.R.; Tavano, O.; Fernandez-Lafuente, R. Immobilization of papain: A review. Int. J. Biol. Macromol. 2021, 118, 94–113. [Google Scholar] [CrossRef] [PubMed]
- Benucci, I.; Lombardelli, C.; Cacciotti, I.; Esti, M. Papain Covalently Immobilized on Chitosan–Clay Nanocomposite Films: Application in Synthetic and Real White Wine. Nanomaterials 2020, 10, 1622. [Google Scholar] [CrossRef] [PubMed]
- Olshannikova, S.; Koroleva, V.; Goncharova, M.; Pashkov, A.; Artyukhov, V. Covalent immobilization of thiol proteinases on chitosan. In Proceedings of the 1st International Electronic Conference on Catalysis Sciences, Virtual, 10–30 November 2020; p. 7. [Google Scholar] [CrossRef]




| Adsorption [10,23,29,85,109] | + |
|
| - |
| |
| Gel entrapment [110,113] | + |
|
| - |
| |
| Complexation with nanomaterials [111,112] | + |
|
| - |
| |
| Chemical immobilization [114,115] | + |
|
| - |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holyavka, M.G.; Goncharova, S.S.; Artyukhov, V.G. Various Options for Covalent Immobilization of Cysteine Proteases—Ficin, Papain, Bromelain. Int. J. Mol. Sci. 2025, 26, 547. https://doi.org/10.3390/ijms26020547
Holyavka MG, Goncharova SS, Artyukhov VG. Various Options for Covalent Immobilization of Cysteine Proteases—Ficin, Papain, Bromelain. International Journal of Molecular Sciences. 2025; 26(2):547. https://doi.org/10.3390/ijms26020547
Chicago/Turabian StyleHolyavka, Marina G., Svetlana S. Goncharova, and Valeriy G. Artyukhov. 2025. "Various Options for Covalent Immobilization of Cysteine Proteases—Ficin, Papain, Bromelain" International Journal of Molecular Sciences 26, no. 2: 547. https://doi.org/10.3390/ijms26020547
APA StyleHolyavka, M. G., Goncharova, S. S., & Artyukhov, V. G. (2025). Various Options for Covalent Immobilization of Cysteine Proteases—Ficin, Papain, Bromelain. International Journal of Molecular Sciences, 26(2), 547. https://doi.org/10.3390/ijms26020547







