Enhancing Soybean Salt Tolerance with GSNO and Silicon: A Comprehensive Physiological, Biochemical, and Genetic Study
Abstract
:1. Introduction
2. Results
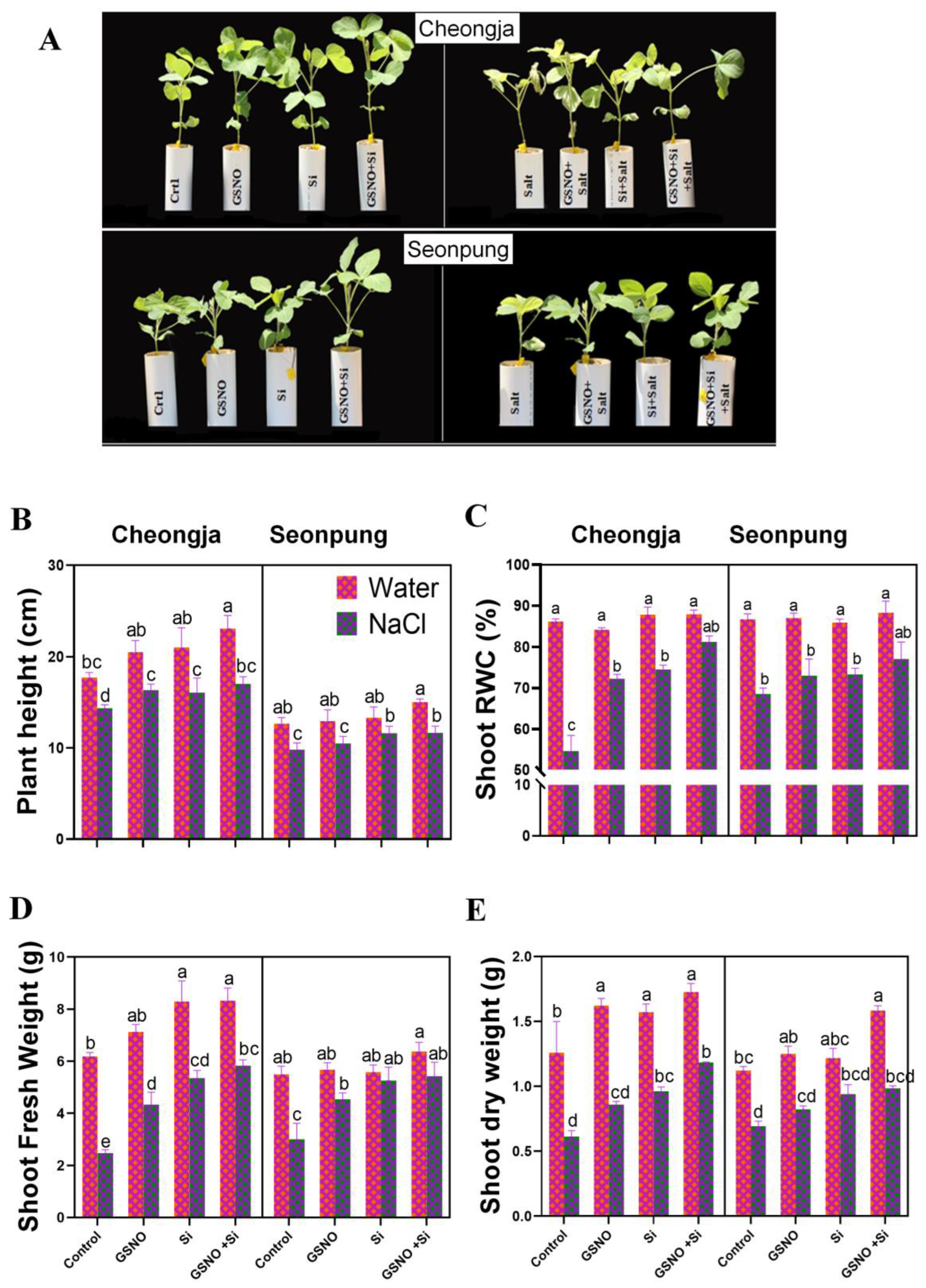
2.1. Combined GSNO and Si Treatment Promoted Plant Growth
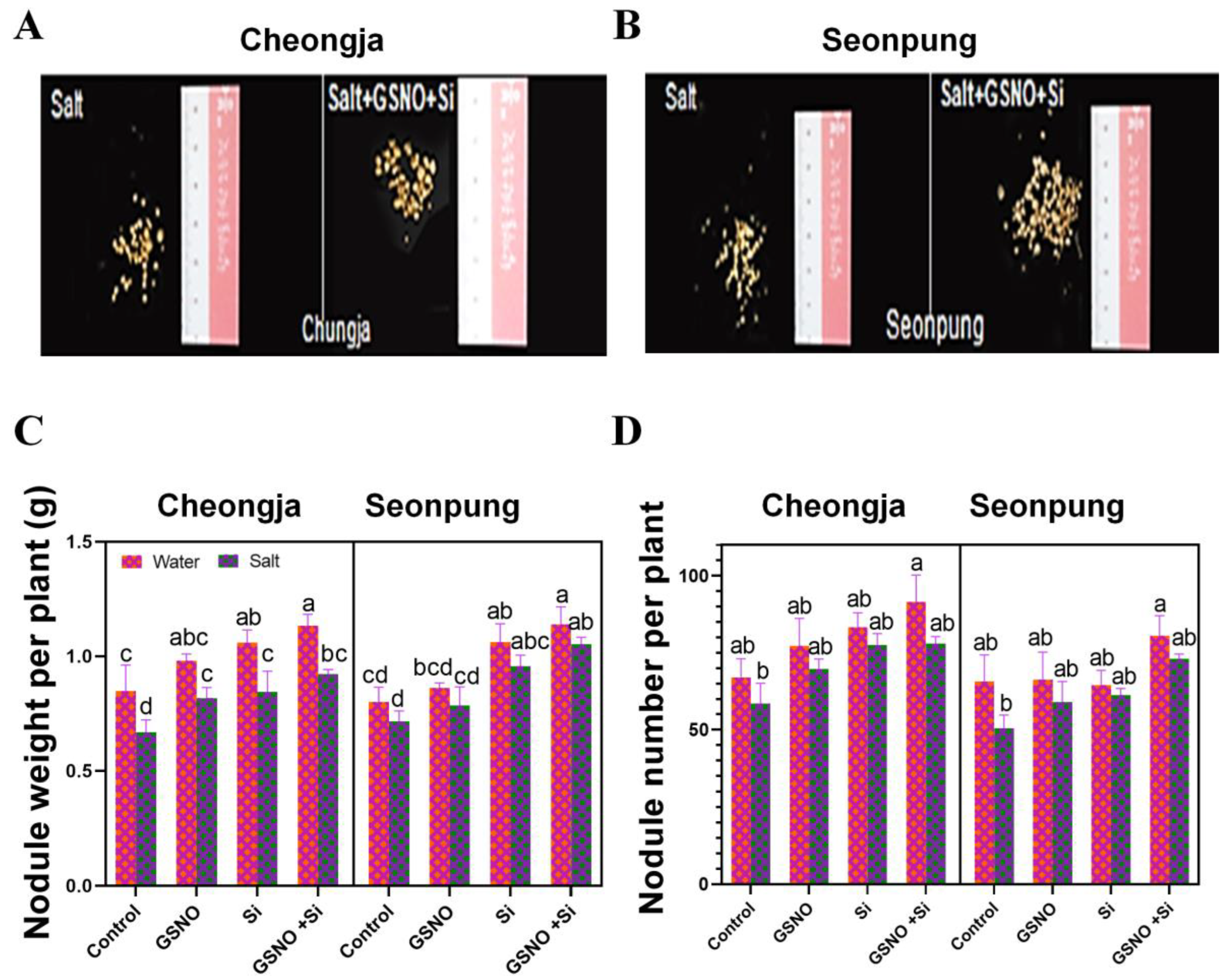
2.2. Combined GSNO and Si Treatment Enhanced Root Traits
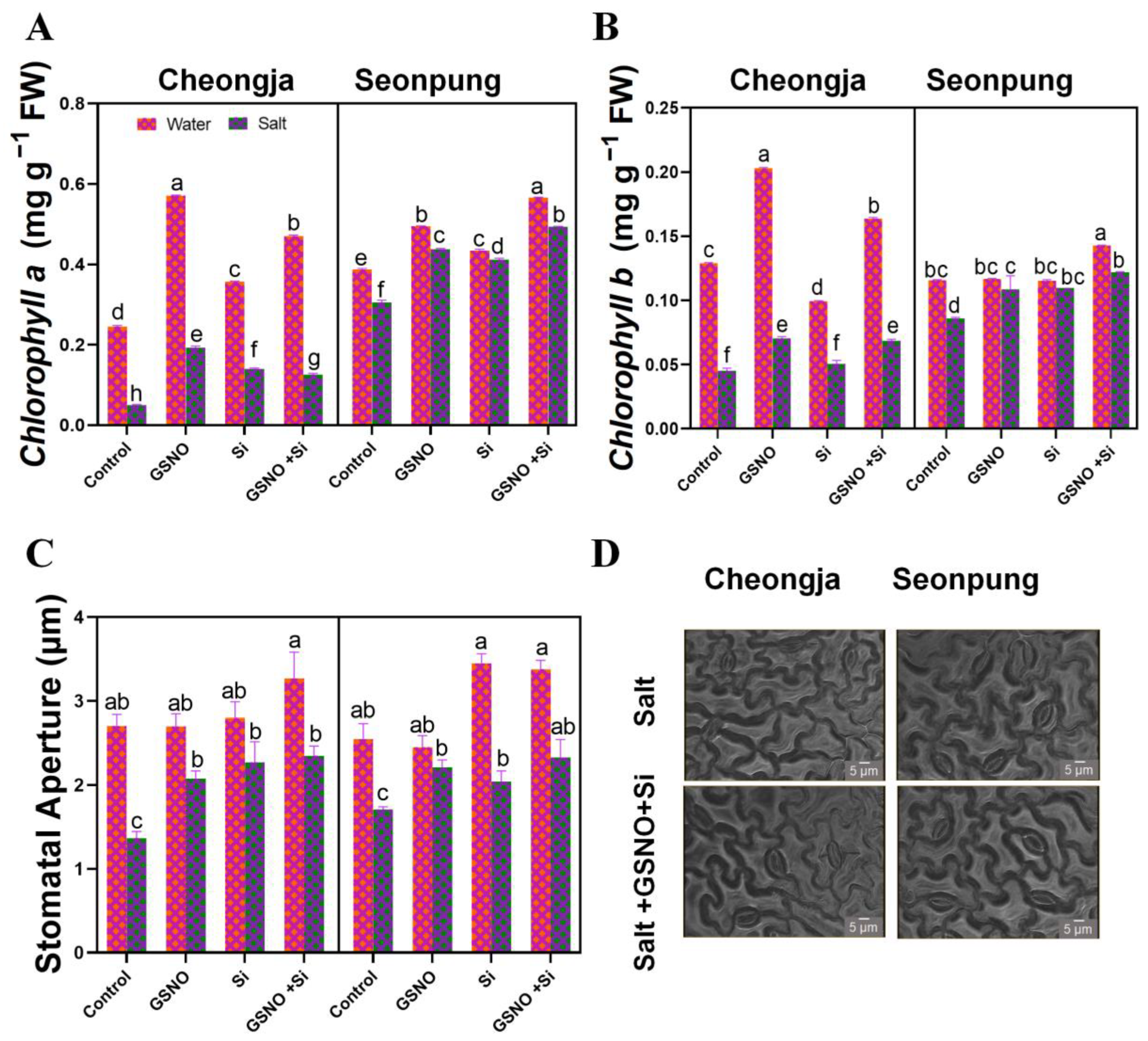
2.3. Combined GSNO and Si Treatment Improved Chlorophyll Content and Stomatal Aperture
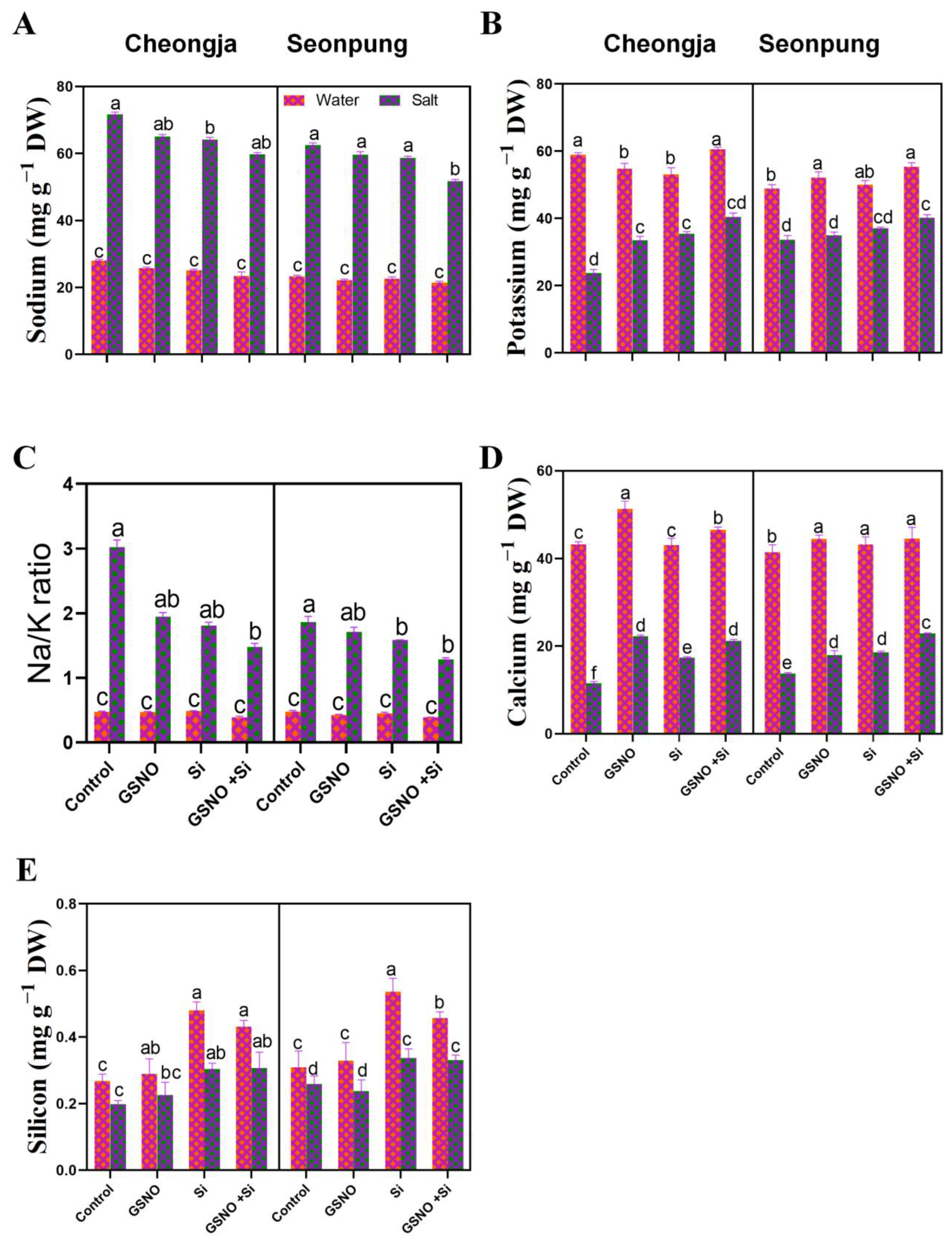
2.4. Combined GSNO and Si Treatment Optimized Ion Homeostasis
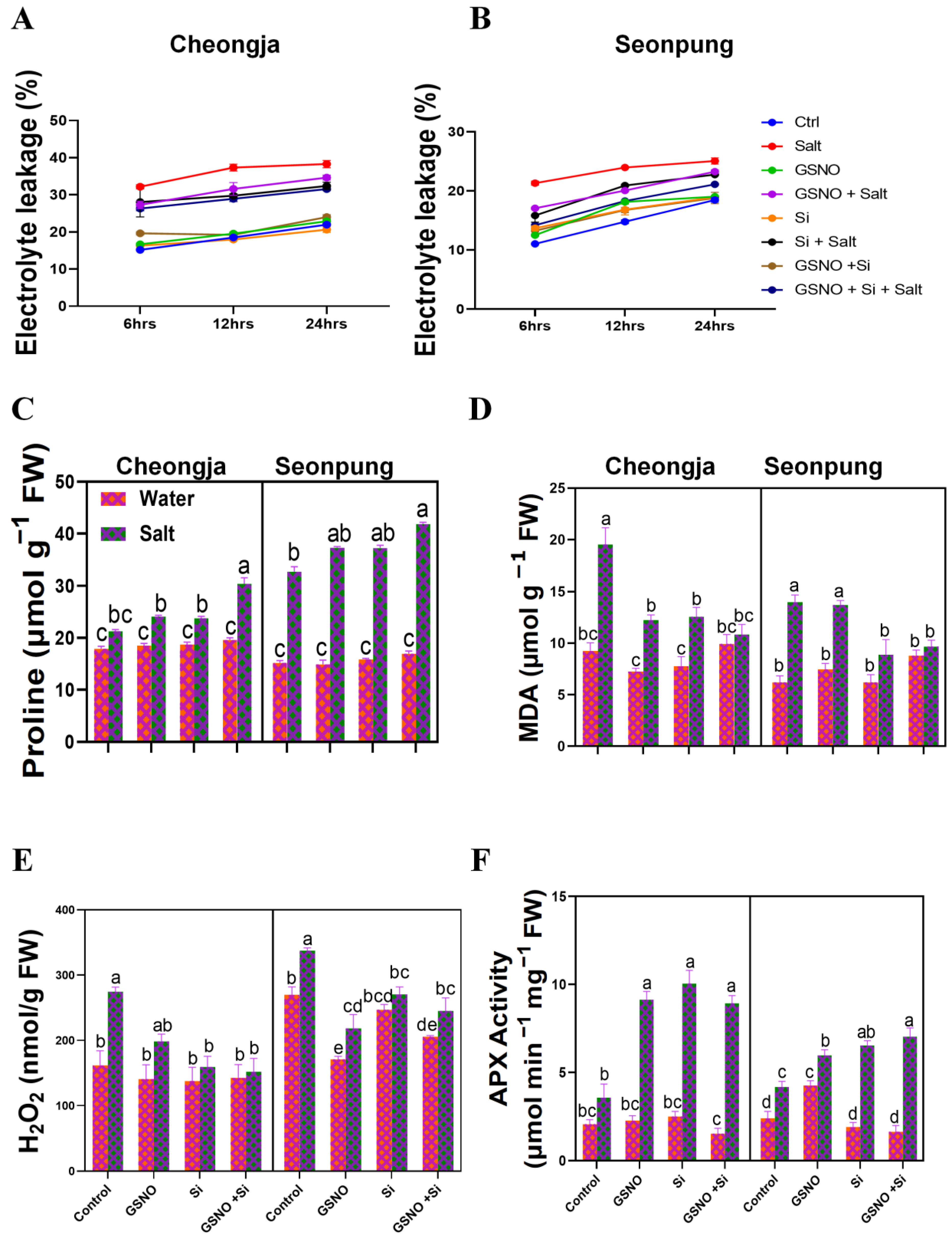
2.5. Combined GSNO and Si Treatment Reduced MDA and H2O2 Levels and Enhanced Cell Integrity, Proline, Water-Soluble Protein, and Antioxidant Activity
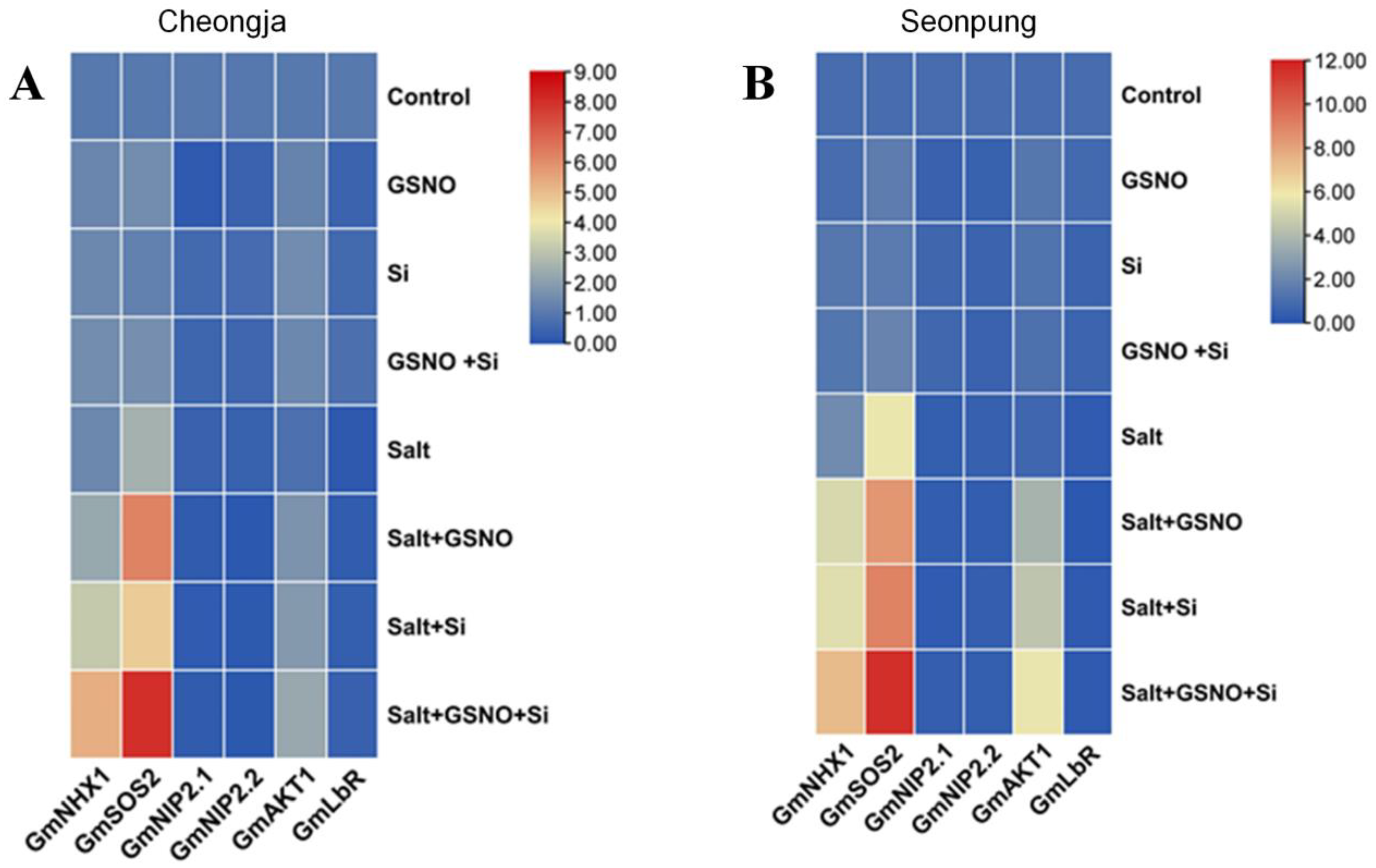
2.6. Combined GSNO and Si Treatment Modulates Gene Expression
3. Discussion
4. Materials and Methods
4.1. Synthesis of GSNO and Chemicals Used
4.2. Plant Material and Growth Condition
4.3. Plant Height and Relative Water Content
4.4. Analysis and Sampling of Roots
4.5. Chlorophyll Content and Stomatal Aperture
4.6. Ion Contents
4.7. Water-Soluble Protein, Electrolyte Leakage, Proline, Malondialdehyde (MDA), H2O2, and APX Content
4.8. RNA, cDNA Synthesis, and Relative Gene Expression Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Negacz, K.; Malek, Ž.; de Vos, A.; Vellinga, P. Saline soils worldwide: Identifying the most promising areas for saline agriculture. J. Arid Environ. 2022, 203, 104775. [Google Scholar] [CrossRef]
- FAO. Global Map of Salt-Affected Soils. 2021. Available online: https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/global-map-of-salt-affected-soils/en/ (accessed on 16 September 2024).
- Zhang, Y.; Ding, J.; Wang, H.; Su, L.; Zhao, C. Biochar addition alleviate the negative effects of drought and salinity stress on soybean productivity and water use efficiency. BMC Plant Biol. 2020, 20, 288. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mishra, R.; Rai, S.; Bano, A.; Pathak, N.; Fujita, M.; Kumar, M.; Hasanuzzaman, M. Mechanistic Insights of Plant Growth Promoting Bacteria Mediated Drought and Salt Stress Tolerance in Plants for Sustainable Agriculture. Int. J. Mol. Sci. 2022, 23, 3741. [Google Scholar] [CrossRef]
- Machado, R.; Serralheiro, R. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Tessema, N.; Yadeta, D.; Kebede, A.; Ayele, G.T. Soil and Irrigation Water Salinity, and Its Consequences for Agriculture in Ethiopia: A Systematic Review. Agriculture 2022, 13, 109. [Google Scholar] [CrossRef]
- Colletti, A.; Attrovio, A.; Boffa, L.; Mantegna, S.; Cravotto, G. Valorisation of by-products from soybean (Glycine max (L.) Merr.) processing. Molecules 2020, 25, 2129. [Google Scholar] [CrossRef]
- Medic, J.; Atkinson, C.; Hurburgh, C.R. Current Knowledge in Soybean Composition. J. Am. Oil Chem. Soc. 2014, 91, 363–384. [Google Scholar] [CrossRef]
- Malle, S.; Eskandari, M.; Morrison, M.; Belzile, F. Genome-wide association identifies several QTLs controlling cysteine and methionine content in soybean seed including some promising candidate genes. Sci. Rep. 2020, 10, 21812. [Google Scholar] [CrossRef]
- Anwar-Ul-Haq, M.; Iftikhar, I.; Akhtar, J.; Maqsood, M. Role of Exogenous Osmolyte Supplementation in Ameliorating Osmotic and Oxidative Stress and Promoting Growth in Salinity-Stressed Soybean Genotypes. J. Soil Sci. Plant Nutr. 2023, 23, 3682–3694. [Google Scholar] [CrossRef]
- Dolatabadian, A.; Modarres Sanavy, S.A.M.; Ghanati, F. Effect of Salinity on Growth, Xylem Structure and Anatomical Characteristics of Soybean. Not. Sci. Biol. 2011, 3, 41–45. [Google Scholar] [CrossRef]
- Feng, C.; Gao, H.; Zhou, Y.; Jing, Y.; Li, S.; Yan, Z.; Xu, K.; Zhou, F.; Zhang, W.; Yang, X. Unfolding molecular switches for salt stress resilience in soybean: Recent advances and prospects for salt-tolerant smart plant production. Front. Plant Sci. 2023, 14, 1162014. [Google Scholar] [CrossRef] [PubMed]
- Pande, A.; Mun, B.-G.; Methela, N.J.; Rahim, W.; Lee, D.-S.; Lee, G.-M.; Hong, J.K.; Hussain, A.; Loake, G.; Yun, B.-W. Heavy metal toxicity in plants and the potential NO-releasing novel techniques as the impending mitigation alternatives. Front. Plant Sci. 2022, 13, 1019647. [Google Scholar] [CrossRef]
- Wang, C.; Wei, L.; Zhang, J.; Hu, D.; Gao, R.; Liu, Y.; Feng, L.; Gong, W.; Liao, W. Nitric Oxide Enhances Salt Tolerance in Tomato Seedlings by Regulating Endogenous S-nitrosylation Levels. J. Plant Growth Regul. 2023, 42, 275–293. [Google Scholar] [CrossRef]
- Houmani, H.; Palma, J.M.; Corpas, F.J. High Salinity Stimulates the Adaptive Response to Potassium Deficiency Through the Antioxidant and the NADPH-Generating Systems in the Roots and Leaves of the Halophyte Cakile maritima. J. Plant Growth Regul. 2022, 42, 6286–6306. [Google Scholar] [CrossRef]
- Rahman, M.A.; Ahmed, K.R.; Haque, F.; Park, M.N.; Kim, B. Recent Advances in Cellular Signaling Interplay between Redox Metabolism and Autophagy Modulation in Cancer: An Overview of Molecular Mechanisms and Therapeutic Interventions. Antioxidants 2023, 12, 428. [Google Scholar] [CrossRef] [PubMed]
- Al Murad, M.; Khan, A.L.; Muneer, S. Silicon in Horticultural Crops: Cross-talk, Signaling, and Tolerance Mechanism under Salinity Stress. Plants 2020, 9, 460. [Google Scholar] [CrossRef]
- Rai, G.K.; Kumar, P.; Choudhary, S.M.; Singh, H.; Adab, K.; Kosser, R.; Magotra, I.; Kumar, R.R.; Singh, M.; Sharma, R.; et al. Antioxidant Potential of Glutathione and Crosstalk with Phytohormones in Enhancing Abiotic Stress Tolerance in Crop Plants. Plants 2023, 12, 1133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-X.; Gong, H.-J.; Yin, J.-L. Role of Silicon in Mediating Salt Tolerance in Plants: A Review. Plants 2019, 8, 147. [Google Scholar] [CrossRef]
- Kumari, S.; Chhillar, H.; Chopra, P.; Khanna, R.R.; Khan, M.I.R. Potassium: A track to develop salinity tolerant plants. Plant Physiol. Biochem. 2021, 167, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.-G.; Yun, B.-W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.M.; Farooq, M. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef]
- Osman, H.S.; Gowayed, S.M.; Elbagory, M.; Omara, A.E.-D.; El-Monem, A.M.A.; Abd El-Razek, U.A.; Hafez, E.M. Interactive Impacts of Beneficial Microbes and Si-Zn Nanocomposite on Growth and Productivity of Soybean Subjected to Water Deficit under Salt-Affected Soil Conditions. Plants 2021, 10, 1396. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Vishwakarma, K.; Singh, V.P.; Prakash, V.; Sharma, S.; Muneer, S.; Nikolic, M.; Deshmukh, R.; Vaculík, M.; Corpas, F.J. Silicon crosstalk with reactive oxygen species, phytohormones and other signaling molecules. J. Hazard. Mater. 2021, 408, 124820. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.S.; Vijayakumar, V.; Yadav, G.S.; Mitran, T. Response and interaction of Bradyrhizobium japonicum and arbuscular mycorrhizal fungi in the soybean rhizosphere. Plant Growth Regul. 2018, 84, 207–223. [Google Scholar] [CrossRef]
- Hussain, S.; Mumtaz, M.; Manzoor, S.; Shuxian, L.; Ahmed, I.; Skalicky, M.; Brestic, M.; Rastogi, A.; Ulhassan, Z.; Shafiq, I.; et al. Foliar application of silicon improves growth of soybean by enhancing carbon metabolism under shading conditions. Plant Physiol. Biochem. 2021, 159, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Farhangi-Abriz, S.; Torabian, S. Biochar improved nodulation and nitrogen metabolism of soybean under salt stress. Symbiosis 2017, 74, 215–223. [Google Scholar] [CrossRef]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Shahzad, R.; Al-Harrasi, A.; Al-Rawahi, A.; Al-Azhri, M.; Mohanta, T.K.; Lee, I.-J. Silicon and gibberellins: Synergistic function in harnessing ABA signaling and heat stress tolerance in date palm (Phoenix dactylifera L.). Plants 2020, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Husain, T.; Suhel, M.; Prasad, S.M.; Singh, V.P. Implication of Nitric Oxide Under Salinity Stress: The Possible Interaction with Other Signaling Molecules. J. Plant Growth Regul. 2021, 41, 163–177. [Google Scholar] [CrossRef]
- Shaffique, S.; Khan, M.A.; Wani, S.H.; Pande, A.; Imran, M.; Kang, S.-M.; Rahim, W.; Khan, S.A.; Bhatta, D.; Kwon, E.-H.; et al. A Review on the Role of Endophytes and Plant Growth Promoting Rhizobacteria in Mitigating Heat Stress in Plants. Microorganisms 2022, 10, 1286. [Google Scholar] [CrossRef] [PubMed]
- Veličković, D.; Liao, Y.-C.; Thibert, S.; Veličković, M.; Anderton, C.; Voglmeir, J.; Stacey, G.; Zhou, M. Spatial mapping of plant N-glycosylation cellular heterogeneity inside soybean root nodules provided insights into legume-rhizobia symbiosis. Front. Plant Sci. 2022, 13, 869281. [Google Scholar] [CrossRef]
- Etesami, H.; Adl, S.M. Can interaction between silicon and non–rhizobial bacteria help in improving nodulation and nitrogen fixation in salinity–stressed legumes? A review. Rhizosphere 2020, 15, 100229. [Google Scholar] [CrossRef]
- Schwember, A.R.; Schulze, J.; Del Pozo, A.; Cabeza, R.A. Regulation of Symbiotic Nitrogen Fixation in Legume Root Nodules. Plants 2019, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Danial, A.W.; Basset, R.A. Amelioration of NaCl stress on germination, growth, and nitrogen fixation of Vicia faba at isosmotic Na–Ca combinations and Rhizobium. Planta 2024, 259, 69. [Google Scholar] [CrossRef]
- Chung, Y.S.; Kim, S.-H.; Park, C.-W.; Na, C.I.; Kim, Y. Treatment with silicon fertilizer induces changes in root morphological traits in soybean (Glycine max L.) during early growth. J. Crop Sci. Biotechnol. 2020, 23, 445–451. [Google Scholar] [CrossRef]
- Sardar, H.; Khalid, Z.; Ahsan, M.; Naz, S.; Nawaz, A.; Ahmad, R.; Razzaq, K.; Wabaidur, S.M.; Jacquard, C.; Širić, I. Enhancement of salinity stress tolerance in lettuce (Lactuca sativa L.) via foliar application of nitric oxide. Plants 2023, 12, 1115. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.; Shah, A.N. An insight into salt stress tolerance mechanisms of Chenopodium album. Environ. Sci. Pollut. Res. 2017, 24, 16531–16535. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.-X.; Li, X.; Li, C.; Zhao, L. The Role of Nitric Oxide in Plant Responses to Salt Stress. Int. J. Mol. Sci. 2022, 23, 6167. [Google Scholar] [CrossRef] [PubMed]
- Peña Calzada, K.; Calero Hurtado, A.; Olivera Viciedo, D.; Habermann, E.; de Mello Prado, R.; de Oliveira, R.; Ajila, G.; Tenesaca, L.F.L.; Rodríguez, J.C.; Gratão, P.L. Regulatory Role of Silicon on Growth, Potassium Uptake, Ionic Homeostasis, Proline Accumulation, and Antioxidant Capacity of Soybean Plants Under Salt Stress. J. Plant Growth Regul. 2023, 42, 4528–4540. [Google Scholar] [CrossRef]
- Kausar, A.; Ashraf, M.Y.; Niaz, M. Some physiological and genetic determinants of salt tolerance in sorghum (Sorghum bicolor (L.) Moench): Biomass production and nitrogen metabolism. Pak. J. Bot 2014, 46, 515–519. [Google Scholar]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M. Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 2012, 27, 210–218. [Google Scholar] [CrossRef]
- Batelli, G.; Verslues, P.E.; Agius, F.; Qiu, Q.; Fujii, H.; Pan, S.; Schumaker, K.S.; Grillo, S.; Zhu, J.-K. SOS2 promotes salt tolerance in part by interacting with the vacuolar H+-ATPase and upregulating its transport activity. Mol. Cell. Biol. 2007, 27, 7781–7790. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.A.H.; Chaoxing, H. Does inoculation with Glomus mosseae improve salt tolerance in pepper plants? J. Plant Growth Regul. 2014, 33, 644–653. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Yuan, X.; Xuan, Y.; Gao, Y.; Yan, Y. Exogenous salicylic acid improves salinity tolerance of Nitraria tangutorum. Russ. J. Plant Physiol. 2016, 63, 132–142. [Google Scholar] [CrossRef]
- Reddy, P.S.; Jogeswar, G.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K.; Kishor, P.B.K. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef]
- Jogaiah, S.; Govind, S.R.; Tran, L.-S.P. Systems biology-based approaches toward understanding drought tolerance in food crops. Crit. Rev. Biotechnol. 2013, 33, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P. Growth and antioxidant responses in mustard (Brassica juncea L.) plants subjected to combined effect of gibberellic acid and salinity. Arch. Agron. Soil Sci. 2010, 56, 575–588. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd_Allah, E.F.; Gucel, S.; Tran, L.-S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef]
- Methela, N.J.; Pande, A.; Islam, M.S.; Rahim, W.; Hussain, A.; Lee, D.-S.; Mun, B.-G.; Maria Joseph Raj, N.P.; Kim, S.-J.; Kim, Y. Chitosan-GSNO nanoparticles: A positive modulator of drought stress tolerance in soybean. BMC Plant Biol. 2023, 23, 639. [Google Scholar] [CrossRef] [PubMed]
- Methela, N.J.; Islam, M.S.; Lee, D.-S.; Yun, B.-W.; Mun, B.-G. S-Nitrosoglutathione (GSNO)-Mediated Lead Detoxification in Soybean through the Regulation of ROS and Metal-Related Transcripts. Int. J. Mol. Sci. 2023, 24, 9901. [Google Scholar] [CrossRef]
- Bari, M.A.; Prity, S.A.; Das, U.; Akther, M.S.; Sajib, S.A.; Reza, M.A.; Kabir, A.H. Silicon induces phytochelatin and ROS scavengers facilitating cadmium detoxification in rice. Plant Biol. 2020, 22, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, W.U.; Ali Shah, A.; Yasin, N.A.; Naz, S.; Ali, A.; Tahir, A.; Iram Batool, A. Synergistic effects of nitric oxide and silicon on promoting plant growth, oxidative stress tolerance and reduction of arsenic uptake in Brassica juncea. Chemosphere 2021, 262, 128384. [Google Scholar] [CrossRef] [PubMed]
- Pirooz, P.; Amooaghaie, R.; Ahadi, A.; Sharififar, F. Silicon- induced nitric oxide burst modulates systemic defensive responses of Salvia officinalis under copper toxicity. Plant Physiol. Biochem. 2021, 162, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhu, W.; Zhang, H.; Ding, H.; Zhang, H.J. Exogenous nitric oxide protects against salt-induced oxidative stress in the leaves from two genotypes of tomato (Lycopersicom esculentum Mill.). Acta Physiol. Plant. 2011, 33, 1199–1209. [Google Scholar] [CrossRef]
- Biju, S.; Fuentes, S.; Gupta, D. Silicon modulates nitro-oxidative homeostasis along with the antioxidant metabolism to promote drought stress tolerance in lentil plants. Physiol. Plant. 2021, 172, 1382–1398. [Google Scholar] [CrossRef]
- Kuźniak, E.; Skłodowska, M. Compartment-specific role of the ascorbate–glutathione cycle in the response of tomato leaf cells to Botrytis cinerea infection. J. Exp. Bot. 2005, 56, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Meunier, J.D.; Davidian, J.C.; Pokrovsky, O.S.; Bovet, N.; Keller, C. Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ. Sci. Pollut. Res. 2016, 23, 1414–1427. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.F.; Wong, F.L.; Tsai, S.N.; Phang, T.H.; Shao, G.; Lam, H.M. Tonoplast-located GmCLC1 and GmNHX1 from soybean enhance NaCl tolerance in transgenic bright yellow (BY)-2 cells. Plant Cell Environ. 2006, 29, 1122–1137. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Hu, L.; Tang, G. The Application of Genome Editing Technologies in Soybean (Glycine max L.) for Abiotic Stress Tolerance. In Plant Genome Editing Technologies: Speed Breeding, Crop Improvement and Sustainable Agriculture; Chen, J.-T., Ahmar, S., Eds.; Springer Nature: Singapore, 2024; pp. 221–237. [Google Scholar]
- Khan, A.; Khan, A.L.; Muneer, S.; Kim, Y.-H.; Al-Rawahi, A.; Al-Harrasi, A. Silicon and salinity: Crosstalk in crop-mediated stress tolerance mechanisms. Front. Plant Sci. 2019, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.-J.; Fan, L.; Yang, J.; Cao, R.-Z.; Yang, C.-Y.; Zhang, J.; Wang, D.-M. A Glycine max sodium/hydrogen exchanger enhances salt tolerance through maintaining higher Na+ efflux rate and K+/Na+ ratio in Arabidopsis. BMC Plant Biol. 2019, 19, 469. [Google Scholar] [CrossRef]
- Al Murad, M.; Muneer, S. Physiological and molecular analysis revealed the role of silicon in modulating salinity stress in mung bean. Agriculture 2023, 13, 1493. [Google Scholar] [CrossRef]
- Lindberg, S.; Premkumar, A. Ion changes and signaling under salt stress in wheat and other important crops. Plants 2023, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, J.; Fang, Q.; Chang, X.; Sun, M.; Li, W.; Li, Y. GmAKT1 is involved in K+ uptake and Na+/K+ homeostasis in Arabidopsis and soybean plants. Plant Sci. 2021, 304, 110736. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Huang, H.; Du, W.; Jiang, Z.; Luo, Y.; Yi, D.; Yang, G.; Pang, Y. Overexpression of MsNIP2 improves salinity tolerance in Medicago sativa. J. Plant Physiol. 2024, 295, 154207. [Google Scholar] [CrossRef]
- Kumawat, S.; Aggarwal, B.; Rana, N.; Mandlik, R.; Mehra, A.; Shivaraj, S.M.; Sonah, H.; Deshmukh, R. Identification of aquaporins and deciphering their role under salinity stress in pomegranate (Punica granatum). J. Plant Biochem. Biotechnol. 2021, 30, 930–942. [Google Scholar] [CrossRef]
- Wang, S.; Liu, P.; Chen, D.; Yin, L.; Li, H.; Deng, X. Silicon enhanced salt tolerance by improving the root water uptake and decreasing the ion toxicity in cucumber. Front. Plant Sci. 2015, 6, 759. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.K.; Vivancos, J.; Guérin, V.; Sonah, H.; Labbé, C.; Belzile, F.; Bélanger, R.R. Identification and functional characterization of silicon transporters in soybean using comparative genomics of major intrinsic proteins in Arabidopsis and rice. Plant Mol. Biol. 2013, 83, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Patil, G.; Devkar, V.; D’Agostino, L.; Kshetry, A.; Yong-Villalobos, L.; Nadaf, A.; Thirumalaikumar, V.; Skirycz, A.; Ma, J.; Stupar, R. Cell-type-specific response to silicon treatment in soybean leaves revealed by single nucleus RNA-sequencing and targeted gene-editing. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Sultan, N.Z.; Jo, H.; Kim, T.; Kim, S.; Park, J.; Kang, G.M.; Lee, D.M.; Lee, Y.S.; Cho, H.; Kim, K. Reaction of Korean soybean cultivars to salt stress. 한국육종학회 학술발표회지 2024, 2024, 301. [Google Scholar]
- Pruthi, R.; Chaudhary, C.; Chapagain, S.; Abozaid, M.M.E.; Rana, P.; Kondi, R.K.R.; Fritsche-Neto, R.; Subudhi, P.K. Deciphering the genetic basis of salinity tolerance in a diverse panel of cultivated and wild soybean accessions by genome-wide association mapping. Theor. Appl. Genet. 2024, 137, 238. [Google Scholar] [CrossRef]
- Tripathi, P.; Tayade, R.; Mun, B.-G.; Yun, B.-W.; Kim, Y. Silicon application differentially modulates root morphology and expression of PIN and YUCCA family genes in soybean (Glycine max L.). Front. Plant Sci. 2022, 13, 842832. [Google Scholar] [CrossRef] [PubMed]
- Valentovic, P.; Luxova, M.; Kolarovic, L.; Gasparikova, O. Effect of osmotic stress on compatible solutes content, membrane stability and water relations in two maize cultivars. Plant Soil Environ. 2006, 52, 184. [Google Scholar] [CrossRef]
- Islam, M.S.; Ghimire, A.; Lay, L.; Khan, W.; Lee, J.-D.; Song, Q.; Jo, H.; Kim, Y. Identification of Quantitative Trait Loci Controlling Root Morphological Traits in an Interspecific Soybean Population Using 2D Imagery Data. Int. J. Mol. Sci. 2024, 25, 4687. [Google Scholar] [CrossRef] [PubMed]
- Hiscox, J.; Israelstam, G. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ana. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yun, B.-W.; Feechan, A.; Yin, M.; Saidi, N.B.B.; Le Bihan, T.; Yu, M.; Moore, J.W.; Kang, J.-G.; Kwon, E.; Spoel, S.H.; et al. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 2011, 478, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar]
- Steven, S.; Islam, M.S.; Ghimire, A.; Methela, N.J.; Kwon, E.-H.; Yun, B.-W.; Lee, I.-J.; Kim, S.-H.; Kim, Y. Chitosan-GSNO Nanoparticles and Silicon Priming Enhance the Germination and Seedling Growth of Soybean (Glycine max L.). Plants 2024, 13, 1290. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Msarie, M.W.; Methela, N.J.; Islam, M.S.; An, T.H.; Das, A.K.; Lee, D.-S.; Mun, B.-G.; Yun, B.-W. Enhancing Soybean Salt Tolerance with GSNO and Silicon: A Comprehensive Physiological, Biochemical, and Genetic Study. Int. J. Mol. Sci. 2025, 26, 609. https://doi.org/10.3390/ijms26020609
Msarie MW, Methela NJ, Islam MS, An TH, Das AK, Lee D-S, Mun B-G, Yun B-W. Enhancing Soybean Salt Tolerance with GSNO and Silicon: A Comprehensive Physiological, Biochemical, and Genetic Study. International Journal of Molecular Sciences. 2025; 26(2):609. https://doi.org/10.3390/ijms26020609
Chicago/Turabian StyleMsarie, Meshari Winledy, Nusrat Jahan Methela, Mohammad Shafiqul Islam, Tran Hoang An, Ashim Kumar Das, Da-Sol Lee, Bong-Gyu Mun, and Byung-Wook Yun. 2025. "Enhancing Soybean Salt Tolerance with GSNO and Silicon: A Comprehensive Physiological, Biochemical, and Genetic Study" International Journal of Molecular Sciences 26, no. 2: 609. https://doi.org/10.3390/ijms26020609
APA StyleMsarie, M. W., Methela, N. J., Islam, M. S., An, T. H., Das, A. K., Lee, D.-S., Mun, B.-G., & Yun, B.-W. (2025). Enhancing Soybean Salt Tolerance with GSNO and Silicon: A Comprehensive Physiological, Biochemical, and Genetic Study. International Journal of Molecular Sciences, 26(2), 609. https://doi.org/10.3390/ijms26020609







