The Pentose Phosphate Pathway: From Mechanisms to Implications for Gastrointestinal Cancers
Abstract
1. Introduction
2. Regulatory Mechanisms of the PPP in GI Cancers
2.1. Esophageal Cancer
2.2. Gastric Cancer
2.3. Colorectal Cancer
2.4. Liver Cancer
2.5. Pancreatic Cancer
2.6. Other Cancers
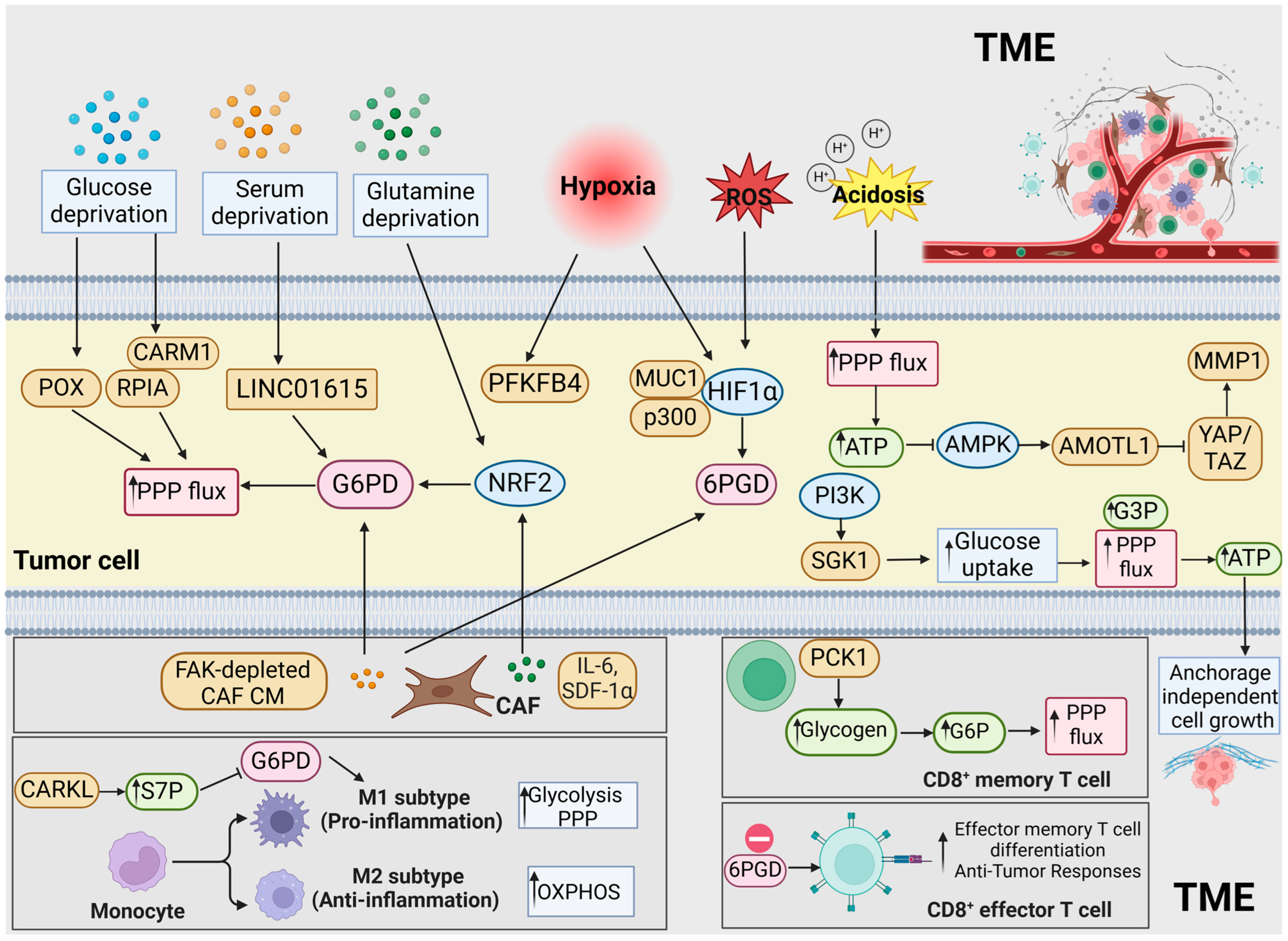
3. The PPP and the TME in GI Cancers
3.1. The PPP and Nutrient Deprivation
3.2. The PPP and Hypoxia
3.3. The PPP and Acidosis
3.4. The PPP and Tumor-Infiltrating Immunocytes
3.5. The PPP and the Mechanical Microenvironment
4. The Role of the PPP in Therapeutic Strategies for GI Cancers
4.1. Chemoradiotherapy
| GI Cancer Type | NcRNA | Role | Functions | Mechanisms | Reference |
|---|---|---|---|---|---|
| CRC | LINC01615 | Oncogene | ↑Survival, ↑nucleotide and lipid synthesis, ↓ROS production, ↑oxaliplatin resistance, and ↑PPP flux | Serum starvation/↓METTL3/↑LINC01615/competitive binding with hnRNPA1/↑G6PD | [87] |
| CircNOLC1 | Oncogene | ↑Proliferation, ↑migration, ↑liver metastasis, and ↑PPP flux | YY1/↑CircNOLC1/AZGP1/↑mTOR/SREBP1 signaling/↑G6PD; YY1/↑CircNOLC1/↓miR-212-5p/↑c-Met/↑G6PD | [34] | |
| Circ_0003215 | Tumor suppressor gene | ↓Proliferation, ↓migration, ↓invasion, ↓metastasis, and ↓PPP flux | Circ_0003215/↓miR-663b/↑DLG4/↓G6PD | [115] | |
| ELFN1-AS1 | Oncogene | ↑Proliferation, ↑migration, ↑invasion, ↓apoptosis, and↑PPP flux | YY1/↑ELFN1-AS1/↓TP53/↑G6PD | [35] | |
| Lnc-AP | Tumor suppressor gene | ↓Oxaliplatin resistance, ↑ROS accumulation, ↑apoptosis, and ↓PPP flux | Lnc-AP encoded pep-AP/↓TAL | [116] | |
| miR-124 | Tumor suppressor gene | ↓Growth, ↓nucleotide synthesis, and ↓PPP flux | miR-124/↓PRPS1 and RPIA. | [117] | |
| HCC | miR-206 | Tumor suppressor gene | ↓Proliferation, ↓lipid accumulation, and ↓PPP flux | miR-206/↓G6PD | [118] |
| miR-206 | Tumor suppressor gene | ↓Growth, ↓cholesterol synthesis, and ↓PPP flux | miR-206/↓G6PD and HMGCR | [57] | |
| miR-122, miR-1 | Tumor suppressor gene | ↓Viability and ↓PPP flux | miR-122 and miR-1/↓G6PD | [119] | |
| PDAC | GAS5 | Oncogene | ↓Proliferation,↑quiescence, ↑metastasis, ↑invasion, and ↑PPP flux | Sox2/↑GAS5/↓glucocorticoid receptor transcriptional activity | [71] |
| miR-4763-3p, miR-3663-5p | Tumor suppressor gene | ↓Nucleotide synthesis, ↑gemcitabine sensitivity, and ↓PPP flux | PRLR/↑miR-4763-3p/↓G6PD PRLR/↑miR-3663-5p/↓TKT | [114] | |
| GC | LINC00242 | Oncogene | ↑Aerobic glycolysis, ↑proliferation, ↓apoptosis, and ↓PPP flux | LINC00242/↓miR-1-3p/↑G6PD | [22] |
4.2. Targeted Therapy
| GI Cancer Type | Agent | Target | Characteristics | Mechanisms | Reference |
|---|---|---|---|---|---|
| CRC | M4IDP | G6PD | Zoledronic acid derivative | ↑Unprenylated Rap1A, RhoA and CDC42,↓G6PD,↑ROS, ↓NADPH and GSH, ↓mevalonate pathway, and ↓PPP flux | [127] |
| Ankaferd hemostat | 6PGD | Plant extracts of Thymus vulgaris, Glycyrrhiza glabra, Vitis vinifera, Alpinia officinarum, and Urtica dioica | ↓6PGD,↑oxidative stress, and ↓PPP flux | [128] | |
| GO-203 | MUC1 C-terminal subunit | D-amino acid cell-penetrating peptide | ↓AKT-S6K1-elF4A pathway, ↓TIGAR,↓GSH/mitochondrial transmembrane potential, ↑ROS, and ↓PPP flux | [129] | |
| Piperlongumine/auranofin | Glutathione S-transferase π/Thioredoxin reductase | Natural alkaloid from piper longum L/trialkylphosphine gold complex | ↑NRF2 target genes (G6PD), ↓CD44v9-positive fraction, ↓tumor formation and growth, and ↓PPP flux | [130] | |
| INK128/Avemar | mTOR | mTOR1/2 inhibitor/fermented wheat germ extract | ↓PPP enzymes (G6PD, PGD, TKT), ↓NADPH/NADP+ and GSH/GSSG ratios, and ↓PPP flux | [131] | |
| Halofuginone | Akt/mTORC1 signaling | Derivative of the febrifugine | ↓G6PD, ↑ROS, ↓NADPH, ↓glycolysis and lipid biosynthesis, and ↓PPP flux | [132] | |
| Epicatechin gallate | G6PD, TKT | Catechins in green tea and grape | ↓Enzymatic activity of G6PD and TKT, ↓de novo synthesis of RNA ribose, and ↓PPP flux | [133] | |
| Aspirin | G6PD, TKT | Salicylate | ↑Acetylation of G6PD and TKT,↓activity of G6PD, and ↓PPP flux | [134] | |
| Cu2O@Au nanocomposites | Ferroptosis pathway | Nanoparticles | ↓GSH, ↑H2O2, ↑ferroptosis, ↑immune therapy response, and ↓PPP flux | [135] | |
| Phy@PLGdH nanosheets | 6PGD | Nanoparticles | ↓NADPH and nucleotide synthesis, ↑radiation-therapy-mediated oxidative stress and DNA damage, ↑immunogenic cell death, and ↓PPP flux | [136] | |
| HCC | Oroxylin A | TKT | Herbal extracts | ↓TKT activity, ↑non-oxPPP substrates, ↑p53 signaling, and ↓PPP flux | [126] |
| CP-91149 | Glycogen phosphorylase | Indole carboxamide | ↑Effect of 6AN, ↑phosphorylation of AMPK, and ↓PPP flux | [137] | |
| Zerumbone | PI3K/AKT/mTOR and STAT3 signaling pathways | Sesquiterpene derived from the ginger plant zingiber zerumbet | ↓Enzymes in PPP (G6PD, RPIA, RPE, TKT, and TAL), and ↓PPP flux | [138] | |
| PDAC | Hypericin | G6PD | Naphthodianthrone, anthraquinone derivative, and active constituents of Hypericum | ↓G6PD, ↓GSH, ↑ROS, ↑effect of gemcitabine, and ↓PPP flux | [139] |
| Cholangiocarcinoma | Chloroquine | Autophagy lysosome pathway | Antimalarials drug | ↓G6PD, ↑mitochondrial ROS, ↑cisplatin-induced apoptosis, and ↓PPP flux | [73] |
4.3. Immunotherapy
4.4. Combination Therapy
4.5. Emerging Therapy
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Jensen, E.T.; Kim, H.P.; Egberg, M.D.; Lund, J.L.; Moon, A.M.; Pate, V.; Barnes, E.L.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology 2022, 162, 621–644. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Chandel, N.S. We need to talk about the Warburg effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.P.; Sabatini, D.M. Cancer cell metabolism: Warburg and beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, Y. Crucial role of the pentose phosphate pathway in malignant tumors. Oncol. Lett. 2019, 17, 4213–4221. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Du, W.; Wu, M. Regulation of the pentose phosphate pathway in cancer. Protein Cell 2014, 5, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, N.; El-Baba, C.; Araji, K.; El-Khoury, R.; Usta, J.; Darwiche, N. The Pentose Phosphate Pathway in Cancer: Regulation and Therapeutic Opportunities. Chemotherapy 2021, 66, 179–191. [Google Scholar] [CrossRef]
- Patra, K.C.; Hay, N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 2014, 39, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wamelink, M.M.; Struys, E.A.; Jakobs, C. The biochemistry, metabolism and inherited defects of the pentose phosphate pathway: A review. J. Inherit. Metab. Dis. 2008, 31, 703–717. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.M.; Krüger, A.; Tauqeer Alam, M.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- TeSlaa, T.; Ralser, M.; Fan, J.; Rabinowitz, J.D. The pentose phosphate pathway in health and disease. Nat. Metab. 2023, 5, 1275–1289. [Google Scholar] [CrossRef] [PubMed]
- Munemoto, M.; Mukaisho, K.I.; Miyashita, T.; Oyama, K.; Haba, Y.; Okamoto, K.; Kinoshita, J.; Ninomiya, I.; Fushida, S.; Taniura, N.; et al. Roles of the hexosamine biosynthetic pathway and pentose phosphate pathway in bile acid-induced cancer development. Cancer Sci. 2019, 110, 2408–2420. [Google Scholar] [CrossRef]
- Perl, A.; Hanczko, R.; Telarico, T.; Oaks, Z.; Landas, S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends Mol. Med. 2011, 17, 395–403. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhang, X.; Fan, R.; Gu, H.; Shi, Y.; Liu, H. Glucose-6-phosphate dehydrogenase expression is correlated with poor clinical prognosis in esophageal squamous cell carcinoma. Eur. J. Surg. Oncol. 2015, 41, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, L.; Huang, D.; Li, Y.; Yang, D.; Li, T.; Li, F.; Sun, L.; Wei, H.; He, K.; et al. Polo-like kinase 1 coordinates biosynthesis during cell cycle progression by directly activating pentose phosphate pathway. Nat. Commun. 2017, 8, 1506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lu, T.; Bi, G.; Hu, Z.; Liang, J.; Bian, Y.; Feng, M.; Zhan, C. PLK1 regulating chemoradiotherapy sensitivity of esophageal squamous cell carcinoma through pentose phosphate pathway/ferroptosis. Biomed. Pharmacother. 2023, 168, 115711. [Google Scholar] [CrossRef]
- Su, Z.; Gao, A.; Li, X.; Zou, S.; He, C.; Wu, J.; Ding, W.Q.; Zhou, J. DNA Polymerase Iota Promotes Esophageal Squamous Cell Carcinoma Proliferation Through Erk-OGT-Induced G6PD Overactivation. Front. Oncol. 2021, 11, 706337. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Meng, Q.; Sun, H.; Li, Y.; Li, Y.; Gu, L.; Liu, B.; Zhang, Y.; Zhou, H.; Xu, Z.; et al. The role of transketolase in human cancer progression and therapy. Biomed. Pharmacother. 2022, 154, 113607. [Google Scholar] [CrossRef]
- Li, J.; Zhu, S.C.; Li, S.G.; Zhao, Y.; Xu, J.R.; Song, C.Y. TKTL1 promotes cell proliferation and metastasis in esophageal squamous cell carcinoma. Biomed. Pharmacother. 2015, 74, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Zhao, Y.; Li, Q.T.; Lei, X.Y.; He, K.Y.; Guo, J.R.; Yang, J.Y.; Yan, Z.H.; Wu, D.H.; Zhang, L.; et al. HMGA1 promotes the progression of esophageal squamous cell carcinoma by elevating TKT-mediated upregulation of pentose phosphate pathway. Cell Death Dis. 2024, 15, 541. [Google Scholar] [CrossRef]
- Deng, P.; Li, K.; Gu, F.; Zhang, T.; Zhao, W.; Sun, M.; Hou, B. LINC00242/miR-1-3p/G6PD axis regulates Warburg effect and affects gastric cancer proliferation and apoptosis. Mol. Med. 2021, 27, 9. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Yu, H.; Liang, R.; Jia, R.; Wang, J.; Jiang, K.; Wang, Z. Rev-erbα inhibits proliferation by reducing glycolytic flux and pentose phosphate pathway in human gastric cancer cells. Oncogenesis 2019, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Staiger, W.I.; Coy, J.F.; Grobholz, R.; Hofheinz, R.D.; Lukan, N.; Post, S.; Schwarzbach, M.H.; Willeke, F. Expression of the mutated transketolase TKTL1, a molecular marker in gastric cancer. Oncol. Rep. 2006, 16, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, D.; He, G. TKTL1 and p63 are biomarkers for the poor prognosis of gastric cancer patients. Cancer Biomark. 2015, 15, 591–597. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Wang, B.; Xu, F.; Ma, F.; Qu, Y.; Jiang, D.; Li, K.; Feng, J.; Tian, S.; et al. Proteomic characterization of gastric cancer response to chemotherapy and targeted therapy reveals new therapeutic strategies. Nat. Commun. 2022, 13, 5723. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Wu, S.; Guo, J.; Chen, Z.; Ge, J.; Yang, P.; Hu, B.; Chen, Z. Silencing of TKTL1 by siRNA inhibits proliferation of human gastric cancer cells in vitro and in vivo. Cancer Biol. Ther. 2010, 9, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.Q.; Lu, Y.X.; Wu, Q.N.; Liu, J.; Zeng, Z.L.; Mo, H.Y.; Chen, Y.; Tian, T.; Wang, Y.; Kang, T.B.; et al. Disrupting G6PD-mediated Redox homeostasis enhances chemosensitivity in colorectal cancer. Oncogene 2017, 36, 6282–6292. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Li, Y.; Shao, Y.; Xiao, J.; Zhu, G.; Li, F. PAK4 regulates G6PD activity by p53 degradation involving colon cancer cell growth. Cell Death Dis. 2017, 8, e2820. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Li, Y.; Li, J.; Zhao, H.; Song, G.; Miyagishi, M.; Wu, S.; Kasim, V. NeuroD1 promotes tumor cell proliferation and tumorigenesis by directly activating the pentose phosphate pathway in colorectal carcinoma. Oncogene 2021, 40, 6736–6747. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, F.; Zhou, L.; Cao, T.; Sun, D.; Wen, S.; Zhu, J.; Xiong, Z.; Tsau, M.T.; Cheng, M.L.; et al. c-Src facilitates tumorigenesis by phosphorylating and activating G6PD. Oncogene 2021, 40, 2567–2580. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Q.; Xia, L.; Lin, J.; Oyang, L.; Tan, S.; Peng, Q.; Jiang, X.; Xu, X.; Wu, N.; et al. Rac1 promotes the reprogramming of glucose metabolism and the growth of colon cancer cells through upregulating SOX9. Cancer Sci. 2023, 114, 822–836. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wei, M.; Li, W.; Zhao, H.; Kasim, V.; Wu, S. PBX3 promotes pentose phosphate pathway and colorectal cancer progression by enhancing G6PD expression. Int. J. Biol. Sci. 2023, 19, 4525–4538. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Zhang, X.; Yue, F.; Zhang, F.; Jiang, S.; Zhou, X.; Lv, J.; Zhang, Z.; Sun, Y.; Chen, Z.; et al. CircNOLC1 Promotes Colorectal Cancer Liver Metastasis by Interacting with AZGP1 and Sponging miR-212-5p to Regulate Reprogramming of the Oxidative Pentose Phosphate Pathway. Adv. Sci. 2023, 10, e2205229. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, W.; Wei, H.; Ma, H.; Leng, G.; Zhang, Y. lncRNA ELFN1-AS1 promotes proliferation, migration and invasion and suppresses apoptosis in colorectal cancer cells by enhancing G6PD activity. Acta Biochim. Biophys. Sin. 2023, 55, 649–660. [Google Scholar] [CrossRef]
- Wu, S.; Wang, H.; Li, Y.; Xie, Y.; Huang, C.; Zhao, H.; Miyagishi, M.; Kasim, V. Transcription Factor YY1 Promotes Cell Proliferation by Directly Activating the Pentose Phosphate Pathway. Cancer Res. 2018, 78, 4549–4562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, X.; Yan, B.; Yu, W.; Shan, L. CircAGFG1 drives metastasis and stemness in colorectal cancer by modulating YY1/CTNNB1. Cell Death Dis. 2020, 11, 542. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, Z.; Yu, B.; Ning, Z.; Lu, Z.; Li, L.; Long, F.; Hu, Q.; Zhong, C.; Zhang, Y.; et al. ATP13A2 activates the pentose phosphate pathway to promote colorectal cancer growth though TFEB-PGD axis. Clin. Transl. Med. 2023, 13, e1272. [Google Scholar] [CrossRef]
- Li, M.; Zhao, X.; Yong, H.; Xu, J.; Qu, P.; Qiao, S.; Hou, P.; Li, Z.; Chu, S.; Zheng, J.; et al. Transketolase promotes colorectal cancer metastasis through regulating AKT phosphorylation. Cell Death Dis. 2022, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Chen, Y.; Wang, Y.Q.; Tao, E.W.; Tan, J.; Liu, Q.Q.; Li, C.M.; Tong, X.M.; Gao, Q.Y.; Hong, J.; et al. Sirtuin5 protects colorectal cancer from DNA damage by keeping nucleotide availability. Nat. Commun. 2022, 13, 6121. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Z.; Cai, Q.; Zhao, C.; Xiao, Y.; Quan, X.; Tang, C.; Gao, J. Inhibition of Transketolase Improves the Prognosis of Colorectal Cancer. Front. Med. 2022, 9, 837143. [Google Scholar] [CrossRef]
- Langbein, S.; Zerilli, M.; Zur Hausen, A.; Staiger, W.; Rensch-Boschert, K.; Lukan, N.; Popa, J.; Ternullo, M.P.; Steidler, A.; Weiss, C.; et al. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br. J. Cancer 2006, 94, 578–585. [Google Scholar] [CrossRef]
- Buj, R.; Chen, C.W.; Dahl, E.S.; Leon, K.E.; Kuskovsky, R.; Maglakelidze, N.; Navaratnarajah, M.; Zhang, G.; Doan, M.T.; Jiang, H.; et al. Suppression of p16 Induces mTORC1-Mediated Nucleotide Metabolic Reprogramming. Cell Rep. 2019, 28, 1971–1980.e78. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.T.; Jiang, J.K.; Yang, M.H.; Lu, J.W.; Lin, H.K.; Wang, H.D.; Yuh, C.H. Identification of a noncanonical function for ribose-5-phosphate isomerase A promotes colorectal cancer formation by stabilizing and activating β-catenin via a novel C-terminal domain. PLoS Biol. 2018, 16, e2003714. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xing, J.; Zhou, X.; Song, X.; Gao, S. Wnt/β-catenin signalling, epithelial-mesenchymal transition and crosslink signalling in colorectal cancer cells. Biomed. Pharmacother. 2024, 175, 116685. [Google Scholar] [CrossRef]
- Tang, J.; Chen, L.; Qin, Z.H.; Sheng, R. Structure, regulation, and biological functions of TIGAR and its role in diseases. Acta Pharmacol. Sin. 2021, 42, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; Athineos, D.; Lee, P.; Ridgway, R.A.; Lambie, W.; Nixon, C.; Strathdee, D.; Blyth, K.; Sansom, O.J.; Vousden, K.H. TIGAR is required for efficient intestinal regeneration and tumorigenesis. Dev. Cell 2013, 25, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Lu, L.; Dong, Q.; Yu, G.; Chen, J.; Qin, L.; Wang, L.; Zhu, W.; Jia, H. Elevated G6PD expression contributes to migration and invasion of hepatocellular carcinoma cells by inducing epithelial-mesenchymal transition. Acta Biochim. Biophys. Sin. 2018, 50, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, M.A.; Guzzo, G.; Morandi, A.; Perra, A.; Menegon, S.; Masgras, I.; Trevisan, E.; Angioni, M.M.; Fornari, F.; Quagliata, L.; et al. Metabolic reprogramming identifies the most aggressive lesions at early phases of hepatic carcinogenesis. Oncotarget 2016, 7, 32375–32393. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fang, M.; He, Z.; Cui, D.; Jia, S.; Lin, X.; Xu, X.; Zhou, T.; Liu, W. Hepatitis B virus stimulates G6PD expression through HBx-mediated Nrf2 activation. Cell Death Dis. 2015, 6, e1980. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Ding, X.; Yang, Y.; Zhang, H.; Li, H.; Tong, S.; An, X.; Zhong, Q.; Liu, X.; Ma, L.; et al. Changes in glucose-6-phosphate dehydrogenase expression results in altered behavior of HBV-associated liver cancer cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G611–G622. [Google Scholar] [CrossRef]
- Hong, X.; Song, R.; Song, H.; Zheng, T.; Wang, J.; Liang, Y.; Qi, S.; Lu, Z.; Song, X.; Jiang, H.; et al. PTEN antagonises Tcl1/hnRNPK-mediated G6PD pre-mRNA splicing which contributes to hepatocarcinogenesis. Gut 2014, 63, 1635–1647. [Google Scholar] [CrossRef]
- Li, M.; He, X.; Guo, W.; Yu, H.; Zhang, S.; Wang, N.; Liu, G.; Sa, R.; Shen, X.; Jiang, Y.; et al. Aldolase B suppresses hepatocellular carcinogenesis by inhibiting G6PD and pentose phosphate pathways. Nat. Cancer 2020, 1, 735–747. [Google Scholar] [CrossRef]
- Kong, D.H.; Li, S.; Du, Z.X.; Liu, C.; Liu, B.Q.; Li, C.; Zong, Z.H.; Wang, H.Q. BAG3 elevation inhibits cell proliferation via direct interaction with G6PD in hepatocellular carcinomas. Oncotarget 2016, 7, 700–711. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rao, K.N.; Elm, M.S.; Kelly, R.H.; Chandar, N.; Brady, E.P.; Rao, B.; Shinozuka, H.; Eagon, P.K. Hepatic hyperplasia and cancer in rats: Metabolic alterations associated with cell growth. Gastroenterology 1997, 113, 238–248. [Google Scholar] [CrossRef]
- Ally, A.; Balasundaram, M.; Carlsen, R.; Chuah, E.; Clarke, A.; Dhalla, N.; Ferguson, M.L. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017, 169, 1327–1341.e23. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, N.; Song, D.; Steer, C.J.; Zheng, G.; Song, G. A positive feedback between cholesterol synthesis and the pentose phosphate pathway rather than glycolysis promotes hepatocellular carcinoma. Oncogene 2023, 42, 2892–2904. [Google Scholar] [CrossRef]
- Liu, T.; Qi, J.; Wu, H.; Wang, L.; Zhu, L.; Qin, C.; Zhang, J.; Zhu, Q. Phosphogluconate dehydrogenase is a predictive biomarker for immunotherapy in hepatocellular carcinoma. Front. Oncol. 2022, 12, 993503. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.J.; Saeidi, S.; Chi, N.H.K.; Kim, S.J.; Kim, D.H.; Kim, S.H.; Park, S.A.; Cha, Y.N.; Na, H.K.; Surh, Y.J. The positive feedback loop between Nrf2 and phosphogluconate dehydrogenase stimulates proliferation and clonogenicity of human hepatoma cells. Free Radic. Res. 2020, 54, 906–917. [Google Scholar] [CrossRef]
- Xu, I.M.; Lai, R.K.; Lin, S.H.; Tse, A.P.; Chiu, D.K.; Koh, H.Y.; Law, C.T.; Wong, C.M.; Cai, Z.; Wong, C.C.; et al. Transketolase counteracts oxidative stress to drive cancer development. Proc. Natl. Acad. Sci. USA 2016, 113, E725–E734. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Xiang, C.; Zhong, F.; Liu, Y.; Dong, Q.; Li, K.; Shi, W.; Ding, C.; Qin, L.; He, F. Transketolase (TKT) activity and nuclear localization promote hepatocellular carcinoma in a metabolic and a non-metabolic manner. J. Exp. Clin. Cancer Res. 2019, 38, 154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, X.T.; Yu, H.Q.; Fang, L.; Wu, D.; Luo, Y.D.; Zhang, Y.J.; Xie, C.M. Elevated FBXL6 expression in hepatocytes activates VRK2-transketolase-ROS-mTOR-mediated immune evasion and liver cancer metastasis in mice. Exp. Mol. Med. 2023, 55, 2162–2176. [Google Scholar] [CrossRef]
- Zheng, Y.; Ming, P.; Zhu, C.; Si, Y.; Xu, S.; Chen, A.; Wang, J.; Zhang, B. Hepatitis B virus X protein-induced SH2 domain-containing 5 (SH2D5) expression promotes hepatoma cell growth via an SH2D5-transketolase interaction. J. Biol. Chem. 2019, 294, 4815–4827. [Google Scholar] [CrossRef]
- Chou, Y.T.; Chen, L.Y.; Tsai, S.L.; Tu, H.C.; Lu, J.W.; Ciou, S.C.; Wang, H.D.; Yuh, C.H. Ribose-5-phosphate isomerase A overexpression promotes liver cancer development in transgenic zebrafish via activation of ERK and β-catenin pathways. Carcinogenesis 2019, 40, 461–473. [Google Scholar] [CrossRef]
- Li, X.; Qian, X.; Peng, L.X.; Jiang, Y.; Hawke, D.H.; Zheng, Y.; Xia, Y.; Lee, J.H.; Cote, G.; Wang, H.; et al. A splicing switch from ketohexokinase-C to ketohexokinase-A drives hepatocellular carcinoma formation. Nat. Cell Biol. 2016, 18, 561–571. [Google Scholar] [CrossRef]
- Li, Y.; Tang, S.; Shi, X.; Lv, J.; Wu, X.; Zhang, Y.; Wang, H.; He, J.; Zhu, Y.; Ju, Y.; et al. Metabolic classification suggests the GLUT1/ALDOB/G6PD axis as a therapeutic target in chemotherapy-resistant pancreatic cancer. Cell Rep. Med. 2023, 4, 101162. [Google Scholar] [CrossRef] [PubMed]
- Bechard, M.E.; Word, A.E.; Tran, A.V.; Liu, X.; Locasale, J.W.; McDonald, O.G. Pentose conversions support the tumorigenesis of pancreatic cancer distant metastases. Oncogene 2018, 37, 5248–5256. [Google Scholar] [CrossRef]
- Zeng, X.; Guo, H.; Liu, Z.; Qin, Z.; Cong, Y.; Ren, N.; Zhang, Y.; Zhang, N. S100A11 activates the pentose phosphate pathway to induce malignant biological behaviour of pancreatic ductal adenocarcinoma. Cell Death Dis. 2022, 13, 568. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Huang, P.Q.; Jiang, S.H.; Yang, Q.; Hu, L.P.; Yang, X.M.; Li, J.; Wang, Y.H.; Li, Q.; Zhang, Y.F.; et al. The short isoform of PRLR suppresses the pentose phosphate pathway and nucleotide synthesis through the NEK9-Hippo axis in pancreatic cancer. Theranostics 2021, 11, 3898–3915. [Google Scholar] [CrossRef] [PubMed]
- Santana-Codina, N.; Roeth, A.A.; Zhang, Y.; Yang, A.; Mashadova, O.; Asara, J.M.; Wang, X.; Bronson, R.T.; Lyssiotis, C.A.; Ying, H.; et al. Oncogenic KRAS supports pancreatic cancer through regulation of nucleotide synthesis. Nat. Commun. 2018, 9, 4945. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.S.; Gnamlin, P.; Durden, B.; Gupta, V.K.; Kesh, K.; Garrido, V.T.; Dudeja, V.; Saluja, A.; Banerjee, S. Long non-coding RNA GAS5 acts as proliferation “brakes” in CD133+ cells responsible for tumor recurrence. Oncogenesis 2019, 8, 68. [Google Scholar] [CrossRef]
- Xu, K.; He, Z.; Chen, M.; Wang, N.; Zhang, D.; Yang, L.; Xu, Z.; Xu, H. HIF-1α regulates cellular metabolism, and Imatinib resistance by targeting phosphogluconate dehydrogenase in gastrointestinal stromal tumors. Cell Death Dis. 2020, 11, 586. [Google Scholar] [CrossRef]
- Qu, X.; Sheng, J.; Shen, L.; Su, J.; Xu, Y.; Xie, Q.; Wu, Y.; Zhang, X.; Sun, L. Autophagy inhibitor chloroquine increases sensitivity to cisplatin in QBC939 cholangiocarcinoma cells by mitochondrial ROS. PLoS ONE 2017, 12, e0173712. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, Q.; Su, Y.; Lu, X.; Wang, Y.; Yin, M.; Hu, W.; Wen, W.; Lei, Q.Y. Arginine methylation of ribose-5-phosphate isomerase A senses glucose to promote human colorectal cancer cell survival. Sci. China Life Sci. 2020, 63, 1394–1405. [Google Scholar] [CrossRef]
- Yin, X.; Tang, B.; Li, J.H.; Wang, Y.; Zhang, L.; Xie, X.Y.; Zhang, B.H.; Qiu, S.J.; Wu, W.Z.; Ren, Z.G. ID1 promotes hepatocellular carcinoma proliferation and confers chemoresistance to oxaliplatin by activating pentose phosphate pathway. J. Exp. Clin. Cancer Res. 2017, 36, 166. [Google Scholar] [CrossRef] [PubMed]
- Ciou, S.C.; Chou, Y.T.; Liu, Y.L.; Nieh, Y.C.; Lu, J.W.; Huang, S.F.; Chou, Y.T.; Cheng, L.H.; Lo, J.F.; Chen, M.J.; et al. Ribose-5-phosphate isomerase A regulates hepatocarcinogenesis via PP2A and ERK signaling. Int. J. Cancer 2015, 137, 104–115. [Google Scholar] [CrossRef]
- Wu, Y.S.; Looi, C.Y.; Subramaniam, K.S.; Masamune, A.; Chung, I. Soluble factors from stellate cells induce pancreatic cancer cell proliferation via Nrf2-activated metabolic reprogramming and ROS detoxification. Oncotarget 2016, 7, 36719–36732. [Google Scholar] [CrossRef]
- Sharma, N.; Bhushan, A.; He, J.; Kaushal, G.; Bhardwaj, V. Metabolic plasticity imparts erlotinib-resistance in pancreatic cancer by upregulating glucose-6-phosphate dehydrogenase. Cancer Metab. 2020, 8, 19. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Parlani, M.; Jorgez, C.; Friedl, P. Plasticity of cancer invasion and energy metabolism. Trends Cell Biol. 2023, 33, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ward, K.M.; Zhang, D.; Dayanandam, E.; Denittis, A.S.; Prendergast, G.C.; Ayene, I.S. A bioactive probe of the oxidative pentose phosphate cycle: Novel strategy to reverse radioresistance in glucose deprived human colon cancer cells. Toxicol. In Vitro 2013, 27, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Pandhare, J.; Donald, S.P.; Cooper, S.K.; Phang, J.M. Regulation and function of proline oxidase under nutrient stress. J. Cell Biochem. 2009, 107, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef]
- Polat, I.H.; Tarrado-Castellarnau, M.; Benito, A.; Hernandez-Carro, C.; Centelles, J.; Marin, S.; Cascante, M. Glutamine Modulates Expression and Function of Glucose 6-Phosphate Dehydrogenase via NRF2 in Colon Cancer Cells. Antioxidants 2021, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- De Falco, P.; Lazzarino, G.; Felice, F.; Desideri, E.; Castelli, S.; Salvatori, I.; Ciccarone, F.; Ciriolo, M.R. Hindering NAT8L expression in hepatocellular carcinoma increases cytosolic aspartate delivery that fosters pentose phosphate pathway and purine biosynthesis promoting cell proliferation. Redox Biol. 2023, 59, 102585. [Google Scholar] [CrossRef]
- Lo Re, O.; Douet, J.; Buschbeck, M.; Fusilli, C.; Pazienza, V.; Panebianco, C.; Castracani, C.C.; Mazza, T.; Li Volti, G.; Vinciguerra, M. Histone variant macroH2A1 rewires carbohydrate and lipid metabolism of hepatocellular carcinoma cells towards cancer stem cells. Epigenetics 2018, 13, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, L.; Ren, Z.; Liu, X.; Song, J.; Zhang, P.; Zhang, C.; Gong, S.; Wu, N.; Zhang, X.; et al. LINC01615 maintains cell survival in adaptation to nutrient starvation through the pentose phosphate pathway and modulates chemosensitivity in colorectal cancer. Cell Mol. Life Sci. 2022, 80, 20. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.Z.; Jin, M.Z.; Dai, J.X.; Sun, W.; Feng, J.H.; Jin, W.L. Reprogramming of the Tumor in the Hypoxic Niche: The Emerging Concept and Associated Therapeutic Strategies. Trends Pharmacol. Sci. 2017, 38, 669–686. [Google Scholar] [CrossRef] [PubMed]
- Schito, L.; Semenza, G.L. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef]
- Chaika, N.V.; Gebregiworgis, T.; Lewallen, M.E.; Purohit, V.; Radhakrishnan, P.; Liu, X.; Zhang, B.; Mehla, K.; Brown, R.B.; Caffrey, T.; et al. MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 13787–13792. [Google Scholar] [CrossRef]
- Gunda, V.; Souchek, J.; Abrego, J.; Shukla, S.K.; Goode, G.D.; Vernucci, E.; Dasgupta, A.; Chaika, N.V.; King, R.J.; Li, S.; et al. MUC1-Mediated Metabolic Alterations Regulate Response to Radiotherapy in Pancreatic Cancer. Clin. Cancer Res. 2017, 23, 5881–5891. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Purohit, V.; Mehla, K.; Gunda, V.; Chaika, N.V.; Vernucci, E.; King, R.J.; Abrego, J.; Goode, G.D.; Dasgupta, A.; et al. MUC1 and HIF-1alpha Signaling Crosstalk Induces Anabolic Glucose Metabolism to Impart Gemcitabine Resistance to Pancreatic Cancer. Cancer Cell 2017, 32, 71–87.e77. [Google Scholar] [CrossRef]
- Kam, C.S.; Ho, D.W.; Ming, V.S.; Tian, L.; Sze, K.M.; Zhang, V.X.; Tsui, Y.M.; Husain, A.; Lee, J.M.; Wong, C.C.; et al. PFKFB4 Drives the Oncogenicity in TP53-Mutated Hepatocellular Carcinoma in a Phosphatase-Dependent Manner. Cell Mol. Gastroenterol. Hepatol. 2023, 15, 1325–1350. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.P.; Xie, J.M.; Li, B.; Sun, Y.H.; Gao, Q.G.; Ding, Z.H.; Wu, H.R.; Qin, Z.H. TIGAR regulates DNA damage and repair through pentosephosphate pathway and Cdk5-ATM pathway. Sci. Rep. 2015, 5, 9853. [Google Scholar] [CrossRef]
- Tamada, M.; Nagano, O.; Tateyama, S.; Ohmura, M.; Yae, T.; Ishimoto, T.; Sugihara, E.; Onishi, N.; Yamamoto, T.; Yanagawa, H.; et al. Modulation of glucose metabolism by CD44 contributes to antioxidant status and drug resistance in cancer cells. Cancer Res. 2012, 72, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef]
- Lamonte, G.; Tang, X.; Chen, J.L.; Wu, J.; Ding, C.K.; Keenan, M.M.; Sangokoya, C.; Kung, H.N.; Ilkayeva, O.; Boros, L.G.; et al. Acidosis induces reprogramming of cellular metabolism to mitigate oxidative stress. Cancer Metab. 2013, 1, 23. [Google Scholar] [CrossRef] [PubMed]
- Schoonjans, C.A.; Joudiou, N.; Brusa, D.; Corbet, C.; Feron, O.; Gallez, B. Acidosis-induced metabolic reprogramming in tumor cells enhances the anti-proliferative activity of the PDK inhibitor dichloroacetate. Cancer Lett. 2020, 470, 18–28. [Google Scholar] [CrossRef]
- Chen, S.; Ning, B.; Song, J.; Yang, Z.; Zhou, L.; Chen, Z.; Mao, L.; Liu, H.; Wang, Q.; He, S.; et al. Enhanced pentose phosphate pathway activity promotes pancreatic ductal adenocarcinoma progression via activating YAP/MMP1 axis under chronic acidosis. Int. J. Biol. Sci. 2022, 18, 2304–2316. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Hardie, D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013, 493, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Haschemi, A.; Kosma, P.; Gille, L.; Evans, C.R.; Burant, C.F.; Starkl, P.; Knapp, B.; Haas, R.; Schmid, J.A.; Jandl, C.; et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012, 15, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Yang, D.; Klement, J.D.; Colson, Y.L.; Oberlies, N.H.; Pearce, C.J.; Colby, A.H.; Grinstaff, M.W.; Ding, H.F.; Shi, H.; et al. G6PD functions as a metabolic checkpoint to regulate granzyme B expression in tumor-specific cytotoxic T lymphocytes. J. Immunother. Cancer 2022, 10, e003543. [Google Scholar] [CrossRef] [PubMed]
- Daneshmandi, S.; Cassel, T.; Lin, P.; Higashi, R.M.; Wulf, G.M.; Boussiotis, V.A.; Fan, T.W.; Seth, P. Blockade of 6-phosphogluconate dehydrogenase generates CD8(+) effector T cells with enhanced anti-tumor function. Cell Rep. 2021, 34, 108831. [Google Scholar] [CrossRef]
- Ma, R.; Ji, T.; Zhang, H.; Dong, W.; Chen, X.; Xu, P.; Chen, D.; Liang, X.; Yin, X.; Liu, Y.; et al. A Pck1-directed glycogen metabolic program regulates formation and maintenance of memory CD8(+) T cells. Nat. Cell Biol. 2018, 20, 21–27. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, F.; Liu, X.; Lu, Y.; Yao, K.; Tian, N.; Tong, L.; Figge, D.A.; Wang, X.; Han, Y.; et al. Non-oxidative pentose phosphate pathway controls regulatory T cell function by integrating metabolism and epigenetics. Nat. Metab. 2022, 4, 559–574. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Cai, T.; Huang, C.; Zang, X.; Sun, L.; Guo, S.; Wang, Q.; Chen, Z.; Zhao, Y.; Han, Z.; et al. G6PD-NF-κB-HGF Signal in Gastric Cancer-Associated Mesenchymal Stem Cells Promotes the Proliferation and Metastasis of Gastric Cancer Cells by Upregulating the Expression of HK2. Front. Oncol. 2021, 11, 648706. [Google Scholar] [CrossRef] [PubMed]
- Demircioglu, F.; Wang, J.; Candido, J.; Costa, A.S.H.; Casado, P.; de Luxan Delgado, B.; Reynolds, L.E.; Gomez-Escudero, J.; Newport, E.; Rajeeve, V.; et al. Cancer associated fibroblast FAK regulates malignant cell metabolism. Nat. Commun. 2020, 11, 1290. [Google Scholar] [CrossRef]
- Schafer, Z.T.; Grassian, A.R.; Song, L.; Jiang, Z.; Gerhart-Hines, Z.; Irie, H.Y.; Gao, S.; Puigserver, P.; Brugge, J.S. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 2009, 461, 109–113. [Google Scholar] [CrossRef]
- Mason, J.A.; Cockfield, J.A.; Pape, D.J.; Meissner, H.; Sokolowski, M.T.; White, T.C.; Valentín López, J.C.; Liu, J.; Liu, X.; Martínez-Reyes, I.; et al. SGK1 signaling promotes glucose metabolism and survival in extracellular matrix detached cells. Cell Rep. 2021, 34, 108821. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Miyata, H.; Miyazaki, Y.; Makino, T.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; Nakajima, K.; Takiguchi, S.; Mori, M.; et al. Pyruvate Kinase M2 Modulates Esophageal Squamous Cell Carcinoma Chemotherapy Response by Regulating the Pentose Phosphate Pathway. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), S1461–S1468. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Chen, Y.; Liu, M.; Shi, J.; Tang, Y.; Yang, X.; Xu, D.; Yao, H.; Lu, P.; Sun, Y.; et al. Glycolysis addiction compensating for a defective pentose phosphate pathway confers gemcitabine sensitivity in SETD2-deficient pancreatic cancer. Biochem. Biophys. Res. Commun. 2022, 615, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.N.; Huang, P.Q.; Pan, H.; Gai, Y.Z.; Zhan, Y.F.; Li, S.X.; Nie, H.Z. Prolactin receptor potentiates chemotherapy through miRNAs-induced G6PD/TKT inhibition in pancreatic cancer. Faseb J. 2024, 38, e23705. [Google Scholar] [CrossRef]
- Chen, B.; Hong, Y.; Gui, R.; Zheng, H.; Tian, S.; Zhai, X.; Xie, X.; Chen, Q.; Qian, Q.; Ren, X.; et al. N6-methyladenosine modification of circ_0003215 suppresses the pentose phosphate pathway and malignancy of colorectal cancer through the miR-663b/DLG4/G6PD axis. Cell Death Dis. 2022, 13, 804. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Yin, S.; Yang, Y.; Yang, H.; Yang, J.; Zhou, Z.; Li, S.; Ying, G.; Ba, Y. lncRNA-encoded pep-AP attenuates the pentose phosphate pathway and sensitizes colorectal cancer cells to Oxaliplatin. EMBO Rep. 2022, 23, e53140. [Google Scholar] [CrossRef]
- Qiu, Z.; Guo, W.; Wang, Q.; Chen, Z.; Huang, S.; Zhao, F.; Yao, M.; Zhao, Y.; He, X. MicroRNA-124 reduces the pentose phosphate pathway and proliferation by targeting PRPS1 and RPIA mRNAs in human colorectal cancer cells. Gastroenterology 2015, 149, 1587–1598.e1511. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Chen, B.; Jian, S.; Cai, W.; Xiao, M.; Du, G. miR-206-G6PD axis regulates lipogenesis and cell growth in hepatocellular carcinoma cell. Anticancer. Drugs 2021, 32, 508–516. [Google Scholar] [CrossRef]
- Barajas, J.M.; Reyes, R.; Guerrero, M.J.; Jacob, S.T.; Motiwala, T.; Ghoshal, K. The role of miR-122 in the dysregulation of glucose-6-phosphate dehydrogenase (G6PD) expression in hepatocellular cancer. Sci. Rep. 2018, 8, 9105. [Google Scholar] [CrossRef]
- Lin, J.; Xia, L.; Oyang, L.; Liang, J.; Tan, S.; Wu, N.; Yi, P.; Pan, Q.; Rao, S.; Han, Y.; et al. The POU2F1-ALDOA axis promotes the proliferation and chemoresistance of colon cancer cells by enhancing glycolysis and the pentose phosphate pathway activity. Oncogene 2022, 41, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Lin, J.; Peng, M.; Jiang, X.; Peng, Q.; Cui, S.; Zhang, W.; Li, S.; Wang, J.; Oyang, L.; et al. Diallyl disulfide induces DNA damage and growth inhibition in colorectal cancer cells by promoting POU2F1 ubiquitination. Int. J. Biol. Sci. 2024, 20, 1125–1141. [Google Scholar] [CrossRef]
- Geng, L.; Zhu, M.; Luo, D.; Chen, H.; Li, B.; Lao, Y.; An, H.; Wu, Y.; Li, Y.; Xia, A.; et al. TKT-PARP1 axis induces radioresistance by promoting DNA double-strand break repair in hepatocellular carcinoma. Oncogene 2024, 43, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Hammel, P.; Huguet, F.; van Laethem, J.L.; Goldstein, D.; Glimelius, B.; Artru, P.; Borbath, I.; Bouché, O.; Shannon, J.; André, T.; et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016, 315, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.Y.; Kang, Y.K.; Nishida, T.; von Mehren, M. Gastrointestinal stromal tumours. Nat. Rev. Dis. Primers 2021, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, D.; Wu, Y.; Zhou, H.; Diao, W.; Liu, G.; Li, Q. A feedback loop of PPP and PI3K/AKT signal pathway drives regorafenib-resistance in HCC. Cancer Metab. 2023, 11, 27. [Google Scholar] [CrossRef]
- Jia, D.; Liu, C.; Zhu, Z.; Cao, Y.; Wen, W.; Hong, Z.; Liu, Y.; Liu, E.; Chen, L.; Chen, C.; et al. Novel transketolase inhibitor oroxylin A suppresses the non-oxidative pentose phosphate pathway and hepatocellular carcinoma tumour growth in mice and patient-derived organoids. Clin. Transl. Med. 2022, 12, e1095. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, Q.Z.; Xu, D.; Fu, J.Y.; Zhang, L.X.; Qiu, L.; Lin, J.G. M(4)IDP stimulates ROS elevation through inhibition of mevalonate pathway and pentose phosphate pathway to inhibit colon cancer cells. Biochem. Pharmacol. 2023, 217, 115856. [Google Scholar] [CrossRef] [PubMed]
- Koçak, E.; Çelebier, M.; Haznedaroglu, I.C.; Altınöz, S. Analysis of the Antiproliferative Effect of Ankaferd Hemostat on Caco-2 Colon Cancer Cells via LC/MS Shotgun Proteomics Approach. Biomed. Res. Int. 2019, 2019, 5268031. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Alam, M.; Hasegawa, M.; Uchida, Y.; Al-Obaid, O.; Kharbanda, S.; Kufe, D. Targeting MUC1-C inhibits the AKT-S6K1-elF4A pathway regulating TIGAR translation in colorectal cancer. Mol. Cancer 2017, 16, 33. [Google Scholar] [CrossRef]
- Tanaka, G.; Inoue, K.; Shimizu, T.; Akimoto, K.; Kubota, K. Dual pharmacological inhibition of glutathione and thioredoxin systems synergizes to kill colorectal carcinoma stem cells. Cancer Med. 2016, 5, 2544–2557. [Google Scholar] [CrossRef]
- Shibuya, N.; Inoue, K.; Tanaka, G.; Akimoto, K.; Kubota, K. Augmented pentose phosphate pathway plays critical roles in colorectal carcinomas. Oncology 2015, 88, 309–319. [Google Scholar] [CrossRef]
- Chen, G.Q.; Tang, C.F.; Shi, X.K.; Lin, C.Y.; Fatima, S.; Pan, X.H.; Yang, D.J.; Zhang, G.; Lu, A.P.; Lin, S.H.; et al. Halofuginone inhibits colorectal cancer growth through suppression of Akt/mTORC1 signaling and glucose metabolism. Oncotarget 2015, 6, 24148–24162. [Google Scholar] [CrossRef]
- Sánchez-Tena, S.; Alcarraz-Vizán, G.; Marín, S.; Torres, J.L.; Cascante, M. Epicatechin gallate impairs colon cancer cell metabolic productivity. J. Agric. Food Chem. 2013, 61, 4310–4317. [Google Scholar] [CrossRef]
- Marimuthu, S.; Chivukula, R.S.; Alfonso, L.F.; Moridani, M.; Hagen, F.K.; Bhat, G.J. Aspirin acetylates multiple cellular proteins in HCT-116 colon cancer cells: Identification of novel targets. Int. J. Oncol. 2011, 39, 1273–1283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, X.; Chen, H.; Deng, T.; Cai, B.; Xia, Y.; Xie, L.; Wang, H.; Huang, C. Improved Immune Response for Colorectal Cancer Therapy Triggered by Multifunctional Nanocomposites with Self-Amplifying Antitumor Ferroptosis. ACS Appl. Mater. Interfaces 2024, 16, 13481–13495. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Duan, R.; Gu, R.; Wang, W.; Wu, J.; Lian, H.; Hu, Y.; Yuan, A. High-Z-Sensitized Radiotherapy Synergizes with the Intervention of the Pentose Phosphate Pathway for In Situ Tumor Vaccination. Adv. Mater. 2022, 34, e2109726. [Google Scholar] [CrossRef] [PubMed]
- Barot, S.; Stephenson, O.J.; Priya Vemana, H.; Yadav, A.; Bhutkar, S.; Trombetta, L.D.; Dukhande, V.V. Metabolic alterations and mitochondrial dysfunction underlie hepatocellular carcinoma cell death induced by a glycogen metabolic inhibitor. Biochem. Pharmacol. 2022, 203, 115201. [Google Scholar] [CrossRef] [PubMed]
- Wani, N.A.; Zhang, B.; Teng, K.Y.; Barajas, J.M.; Motiwala, T.; Hu, P.; Yu, L.; Brüschweiler, R.; Ghoshal, K.; Jacob, S.T. Reprograming of Glucose Metabolism by Zerumbone Suppresses Hepatocarcinogenesis. Mol. Cancer Res. 2018, 16, 256–268. [Google Scholar] [CrossRef]
- Sun, L.; Shang, H.; Wu, Y.; Xin, X. Hypericin-mediated photodynamic therapy enhances gemcitabine induced Capan-2 cell apoptosis via inhibiting NADPH level. J. Pharm. Pharmacol. 2022, 74, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Daneshmandi, S.; Cassel, T.; Higashi, R.M.; Fan, T.W.; Seth, P. 6-Phosphogluconate dehydrogenase (6PGD), a key checkpoint in reprogramming of regulatory T cells metabolism and function. eLife 2021, 10, e67476. [Google Scholar] [CrossRef]
- Gu, M.; Zhou, X.; Sohn, J.H.; Zhu, L.; Jie, Z.; Yang, J.Y.; Zheng, X.; Xie, X.; Yang, J.; Shi, Y.; et al. NF-κB-inducing kinase maintains T cell metabolic fitness in antitumor immunity. Nat. Immunol. 2021, 22, 193–204. [Google Scholar] [CrossRef]
- Lei, J.; Yang, Y.; Lu, Z.; Pan, H.; Fang, J.; Jing, B.; Chen, Y.; Yin, L. Taming metabolic competition via glycolysis inhibition for safe and potent tumor immunotherapy. Biochem. Pharmacol. 2022, 202, 115153. [Google Scholar] [CrossRef] [PubMed]
- Povo-Retana, A.; Fariñas, M.; Landauro-Vera, R.; Mojena, M.; Alvarez-Lucena, C.; Fernández-Moreno, M.A.; Castrillo, A.; de la Rosa Medina, J.V.; Sánchez-García, S.; Foguet, C.; et al. Immunometabolic actions of trabectedin and lurbinectedin on human macrophages: Relevance for their anti-tumor activity. Front. Immunol. 2023, 14, 1211068. [Google Scholar] [CrossRef]
- Lao, Y.; Cui, X.; Xu, Z.; Yan, H.; Zhang, Z.; Zhang, Z.; Geng, L.; Li, B.; Lu, Y.; Guan, Q.; et al. Glutaryl-CoA dehydrogenase suppresses tumor progression and shapes an anti-tumor microenvironment in hepatocellular carcinoma. J. Hepatol. 2024, 81, 847–861. [Google Scholar] [CrossRef]
- Chen, H.; Wu, D.; Bao, L.; Yin, T.; Lei, D.; Yu, J.; Tong, X. 6PGD inhibition sensitizes hepatocellular carcinoma to chemotherapy via AMPK activation and metabolic reprogramming. Biomed. Pharmacother. 2019, 111, 1353–1358. [Google Scholar] [CrossRef]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene therapy comes of age. Science 2018, 359, eaan4672. [Google Scholar] [CrossRef]
- Jiang, P.; Du, W.; Wang, X.; Mancuso, A.; Gao, X.; Wu, M.; Yang, X. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat. Cell Biol. 2011, 13, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lu, Y.; Li, H.; Zhang, J.; Ju, Y.; Ouyang, M. Role of long non-coding RNAs in metabolic reprogramming of gastrointestinal cancer cells. Cancer Cell Int. 2024, 24, 15. [Google Scholar] [CrossRef] [PubMed]
- Vincent, R.L.; Gurbatri, C.R.; Li, F.; Vardoshvili, A.; Coker, C.; Im, J.; Ballister, E.R.; Rouanne, M.; Savage, T.; de Los Santos-Alexis, K.; et al. Probiotic-guided CAR-T cells for solid tumor targeting. Science 2023, 382, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Shao, M.; Teng, X.; Huang, Y.; Meng, Y.; Wu, L.; Wei, J.; Liu, L.; Gu, T.; Song, J.; et al. Mitochondrial isocitrate dehydrogenase impedes CAR T cell function by restraining antioxidant metabolism and histone acetylation. Cell Metab. 2024, 36, 176–192.e110. [Google Scholar] [CrossRef] [PubMed]
- Horecker, B.L. The pentose phosphate pathway. J. Biol. Chem. 2002, 277, 47965–47971. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e713. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Hu, L.; Lu, Y.; Tong, L.; Feng, M.; Liu, Q.; Li, Y.; Zhu, Y.; Wu, L.; Ji, Y.; et al. TKT maintains intestinal ATP production and inhibits apoptosis-induced colitis. Cell Death Dis. 2021, 12, 853. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Shao, F.; Bian, X.; Meng, Y.; Liang, T.; Lu, Z. The Evolving Landscape of Noncanonical Functions of Metabolic Enzymes in Cancer and Other Pathologies. Cell Metab. 2021, 33, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Meskers, C.J.W.; Franczak, M.; Smolenski, R.T.; Giovannetti, E.; Peters, G.J. Are we still on the right path(way)?: The altered expression of the pentose phosphate pathway in solid tumors and the potential of its inhibition in combination therapy. Expert. Opin. Drug Metab. Toxicol. 2022, 18, 61–83. [Google Scholar] [CrossRef] [PubMed]
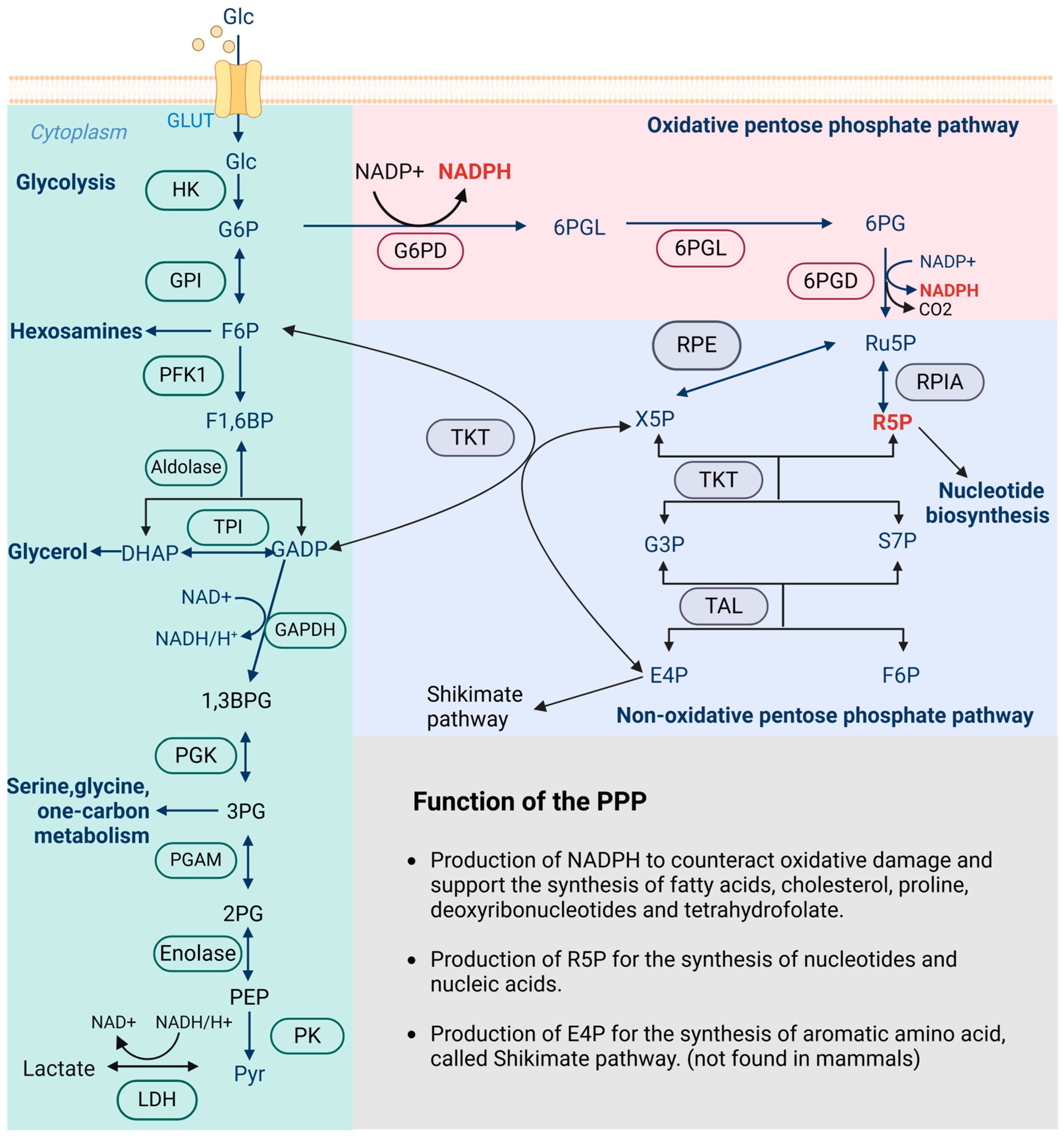

| GI Cancer Type | Enzyme | Branch of PPP | Regulatory Axis | Functions | Reference |
|---|---|---|---|---|---|
| ESCC | G6PD | OxPPP | YY1/↑PLK1/↑G6PD | ↓Ferroptosis ↓Chemoradiotherapy sensitivity | [17] |
| Pol ι/↑ERK/↑OGT/↑O-GlcNAc of G6PD | ↑Proliferation | [18] | |||
| TKT | Non-oxPPP | HMGA1/↑Sp1/↑TKT | ↑Proliferation ↑PPP flux ↑NADPH and GSH | [21] | |
| GC | G6PD | OxPPP | Rev-erbα/↓G6PD | ↑Proliferation ↑Glycolysis | [23] |
| CRC | G6PD | OxPPP | PAK4/↑MDM2-mediated p53 ubiquitination/↑G6PD | ↑Glucose consumption ↑NADPH production | [29] |
| NeuroD1/↑G6PD | ↑Proliferation ↓Apoptosis ↑NADPH production ↓ROS level | [30] | |||
| c-Src/↑G6PD Tyr 112 phosphorylation | ↑Tumor growth ↑NADPH production ↑Nucleotides synthesis ↑Lipid biosynthesis | [31] | |||
| Rac1/↑PI3K-AKT/↑SOX9/↑G6PD | ↑Proliferation ↑Migration ↑Invasion ↑Tumor growth | [32] | |||
| PBX3/↑G6PD | ↑Viability ↑Proliferation ↓Apoptosis ↑NADPH production ↓ROS level ↑Lipid biosynthesis ↑Tumor growth | [33] | |||
| YY1/↑G6PD | ↑Proliferation ↑Nucleotides synthesis ↑Lipid biosynthesis ↑NADPH production | [36] | |||
| 6PGD | ATP13A2/↑TFEB nuclear localization/↑6PGD | ↑Proliferation ↑PPP activity ↑Tumor growth | [38] | ||
| TKT | Non-oxPPP | TKT/↑GRP78/↑AKT phosphorylation | ↑Proliferation ↑Metastasis ↑Aerobic glycolysis | [39] | |
| RPIA | Nuclear localization of RPIA/↑β-catenin | ↑Proliferation ↑Tumor growth | [44] | ||
| p16/↓mTORC1/↓RPIA | ↑Proliferation ↓Senescence ↑Nucleotide synthesis | [43] | |||
| Glucose deprivation/↑CARM1-RPIA interaction/↑RPIA R42 methylation | ↑PPP flux ↑ROS clearance ↑Cell growth | [74] | |||
| HCC | G6PD | OxPPP | G6PD/↑STAT3 phosphorylation | ↑Proliferation ↑Migration ↑Invasion ↑Tumor growth ↑EMT | [48] |
| PTEN/↑GSK3β/↓Tcl1/↑hnRNPK/↓G6PD pre-mRNA splicing | ↓G6PD dimer formation ↓Proliferation ↑Senescence ↑Sensitivity of HCC to sorafenib | [52] | |||
| Aldob–G6PD–p53 protein complex/↓G6PD activity | ↓Tumorigenesis | [53] | |||
| BAG3/↓G6PD | ↓Proliferation | [54] | |||
| HBx–p62–Keap1 complex/↑Nrf2/↑G6PD | ↑Proliferation | [50] | |||
| ID1/↑Wnt/β-catenin pathway/↑c-MYC/↑G6PD | ↑Proliferation ↓Apoptosis ↑Oxaliplatin resistance | [75] | |||
| 6PGD | Nrf2/↑6PGD, 6PGD/↓Keap1/↑Nrf2 | ↑Proliferation ↑Migration | [59] | ||
| TKT | Non-oxPPP | TKT nuclear localization/↑EGFR pathway | ↑Proliferation ↑Viability ↑Migration ↑Invasion ↑Metastasis | [61] | |
| HBx/↑SH2D5/↑interaction of SH2D5 and TKT/↑STAT3 pathway | ↑Proliferation ↑Migration ↑Invasion | [63] | |||
| VRK2/↑TKT phosphorylation/↑FBXL6/↑TKT ubiquitination and activation/↑ROS-mTOR axis/↑PD-L1 | ↑Tumorigenesis ↑Immune evasion ↑Metastasis | [62] | |||
| RPIA | RPIA/↓PP2A activity/↑ERK signaling | ↑Proliferation ↑Tumor growth | [76] | ||
| PDAC | G6PD | OxPPP | ↑GLUT1/↓Aldob/↑G6PD activity | ↑Chemoresistance | [66] |
| PSC-CM/↑SDF-1α, IL-6/↑Nrf2/↑G6PD | ↑Proliferation ↑Glucose metabolism ↑Glutaminolysis ↑Glutathione biosynthesis ↓ROS level | [77] | |||
| ID1/↑c-MYC/↑G6PD | ↓Glycolysis ↑PPP flux ↑Erlotinib resistance | [78] | |||
| TKT | Non-oxPPP | ↑S100A11 expression/↑H3K4me3 on TKT promoter/↑TKT expression | ↑Proliferation ↑Tumor growth | [68] | |
| GIST | 6PGD | OxPPP | Long-term imatinib exposure/↑HIF-1α/↑6PGD | ↑Proliferation ↓Apoptosis ↑Imatinib-resistant | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, J.; Yu, Z.; Zhou, H.; Wang, W.; Wu, H.; Ye, J. The Pentose Phosphate Pathway: From Mechanisms to Implications for Gastrointestinal Cancers. Int. J. Mol. Sci. 2025, 26, 610. https://doi.org/10.3390/ijms26020610
Qiao J, Yu Z, Zhou H, Wang W, Wu H, Ye J. The Pentose Phosphate Pathway: From Mechanisms to Implications for Gastrointestinal Cancers. International Journal of Molecular Sciences. 2025; 26(2):610. https://doi.org/10.3390/ijms26020610
Chicago/Turabian StyleQiao, Jincheng, Zhengchen Yu, Han Zhou, Wankun Wang, Hao Wu, and Jun Ye. 2025. "The Pentose Phosphate Pathway: From Mechanisms to Implications for Gastrointestinal Cancers" International Journal of Molecular Sciences 26, no. 2: 610. https://doi.org/10.3390/ijms26020610
APA StyleQiao, J., Yu, Z., Zhou, H., Wang, W., Wu, H., & Ye, J. (2025). The Pentose Phosphate Pathway: From Mechanisms to Implications for Gastrointestinal Cancers. International Journal of Molecular Sciences, 26(2), 610. https://doi.org/10.3390/ijms26020610






