Bypass of Methoxyamine-Adducted Abasic Sites by Eukaryotic Translesion DNA Polymerases
Abstract
1. Introduction
2. Results
2.1. General Experimental Design
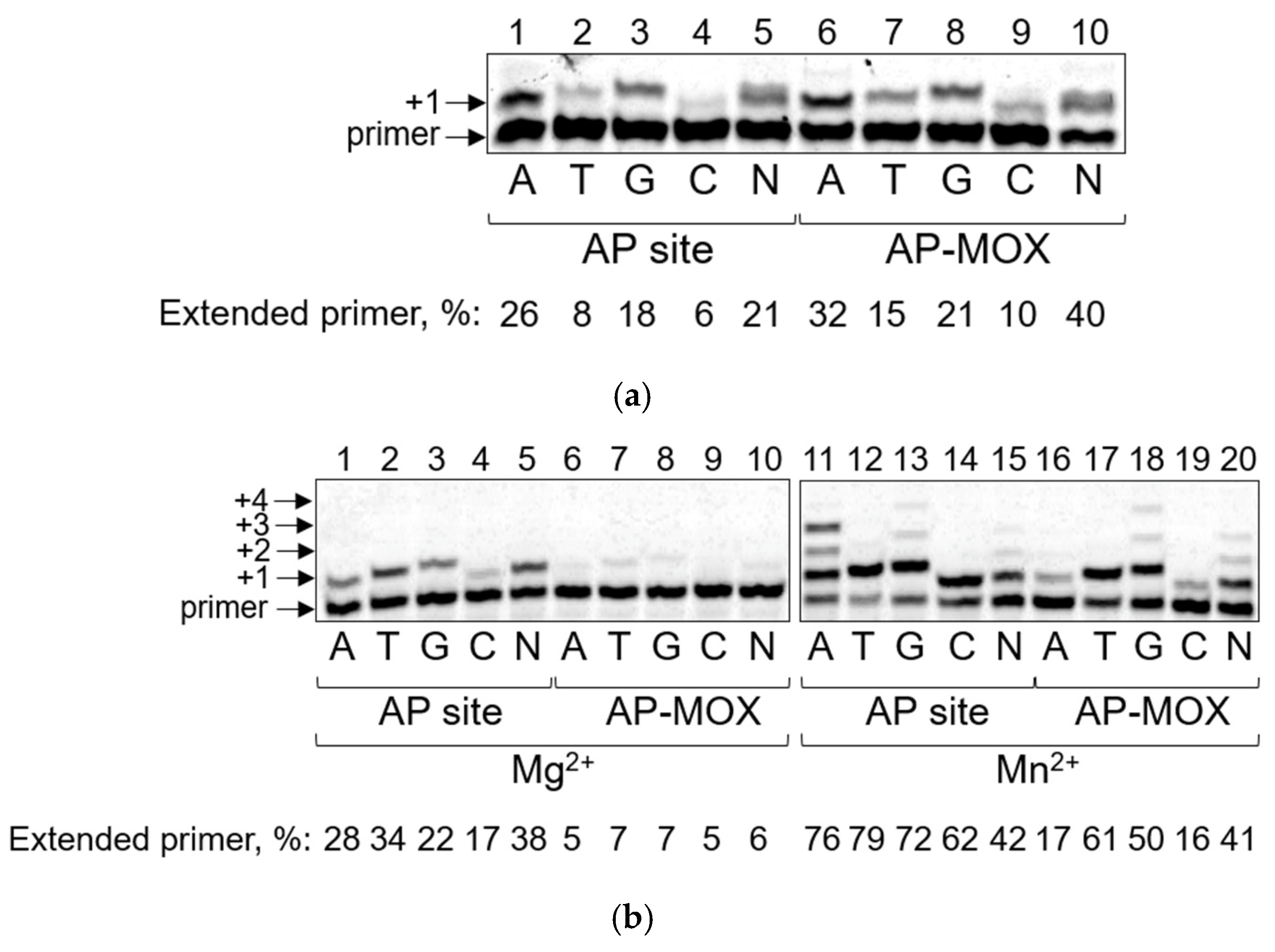
2.2. Pol η Bypasses AP-MOX and the AP Site with the Same Efficiency
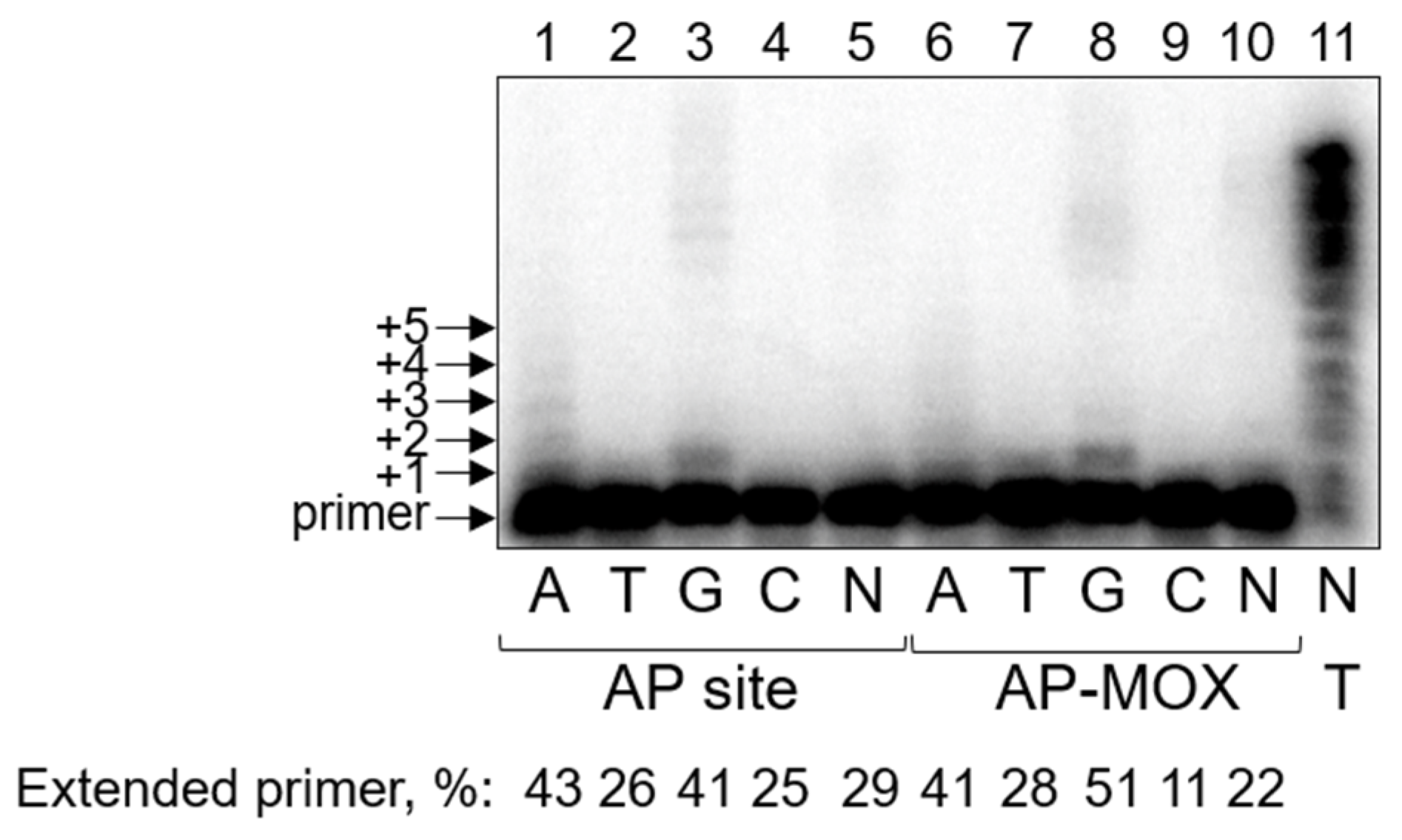
2.3. Pol ι Bypass of AP-MOX Is Significantly Decreased Compared to the AP Site
2.4. Pol ζ and Rev1 Bypass AP-MOX More Efficiently than the AP Site
2.5. PrimPol Bypass of the AP Site and AP-MOX Is Stimulated by Mn2+
3. Discussion
4. Materials and Methods
4.1. Enzymes and Oligonucleotides
4.2. Standing-Start Assay
4.3. Steady-State Kinetics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Bont, R.; van Larebeke, N. Endogenous DNA damage in humans: A review of quantitative data. Mutagenesis 2004, 19, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Rahimoff, R.; Kosmatchev, O.; Kirchner, A.; Pfaffeneder, T.; Spada, F.; Brantl, V.; Müller, M.; Carell, T. 5-Formyl- and 5-carboxydeoxycytidines do not cause accumulation of harmful repair intermediates in stem cells. J. Am. Chem. Soc. 2017, 139, 10359–10364. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yao, L.; Brown, C.; Rizzo, C.J.; Turesky, R.J. Quantitation of apurinic/apyrimidinic sites in isolated DNA and in mammalian tissue with a reduced level of artifacts. Anal. Chem. 2019, 91, 7403–7410. [Google Scholar] [CrossRef] [PubMed]
- Boiteux, S.; Guillet, M. Abasic sites in DNA: Repair and biological consequences in Saccharomyces cerevisiae. DNA Repair 2004, 3, 1–12. [Google Scholar] [CrossRef]
- Goodman, M.F.; Cai, H.; Bloom, L.B.; Eritja, R. Nucleotide insertion and primer extension at abasic template sites in different sequence contexts. Ann. N. Y. Acad. Sci. 1994, 726, 132–142. [Google Scholar] [CrossRef]
- Auerbach, P.A.; Demple, B. Roles of Rev1, Pol ζ, Pol32 and Pol η in the bypass of chromosomal abasic sites in Saccharomyces cerevisiae. Mutagenesis 2010, 25, 63–69. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Lim, S.; Kim, E.-J.; Jo, A.; Guengerich, F.P. Translesion synthesis across abasic lesions by human B-family and Y-family DNA polymerases α, δ, η, ι, κ, and REV1. J. Mol. Biol. 2010, 404, 34–44. [Google Scholar] [CrossRef]
- Nair, D.T.; Johnson, R.E.; Prakash, L.; Prakash, S.; Aggarwal, A.K. DNA synthesis across an abasic lesion by yeast Rev1 DNA polymerase. J. Mol. Biol. 2011, 406, 18–28. [Google Scholar] [CrossRef]
- Patra, A.; Zhang, Q.; Lei, L.; Su, Y.; Egli, M.; Guengerich, F.P. Structural and kinetic analysis of nucleoside triphosphate incorporation opposite an abasic site by human translesion DNA polymerase η. J. Biol. Chem. 2015, 290, 8028–8038. [Google Scholar] [CrossRef]
- Yang, J.; Wang, R.; Liu, B.; Xue, Q.; Zhong, M.; Zeng, H.; Zhang, H. Kinetic analysis of bypass of abasic site by the catalytic core of yeast DNA polymerase eta. Mutat. Res. 2015, 779, 134–143. [Google Scholar] [CrossRef]
- Efrati, E.; Tocco, G.; Eritja, R.; Wilson, S.H.; Goodman, M.F. Abasic translesion synthesis by DNA polymerase β violates the “A-rule”: Novel types of nucleotide incorporation by human DNA polymerase β at an abasic lesion in different sequence contexts. J. Biol. Chem. 1997, 272, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, K.M.; Kim, J.; Goodman, M.F.; Greenberg, M.M. Replication of an oxidized abasic site in Escherichia coli by a dNTP-stabilized misalignment mechanism that reads upstream and downstream nucleotides. Biochemistry 2006, 45, 5048–5056. [Google Scholar] [CrossRef] [PubMed]
- Steighner, R.J.; Povirk, L.F. Bleomycin-induced DNA lesions at mutational hot spots: Implications for the mechanism of double-strand cleavage. Proc. Natl. Acad. Sci. USA 1990, 87, 8350–8354. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Gold, B. Small-molecule inhibitors of DNA damage-repair pathways: An approach to overcome tumor resistance to alkylating anticancer drugs. Future Med. Chem. 2012, 4, 1093–1111. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.S.; Rosen, L.S.; Mendelson, D.; Ramanathan, R.K.; Goldman, J.; Liu, L.; Xu, Y.; Gerson, S.L.; Anthony, S.P.; Figg, W.D.; et al. A phase 1 study of TRC102, an inhibitor of base excision repair, and pemetrexed in patients with advanced solid tumors. Investig. New Drugs 2013, 31, 714–723. [Google Scholar] [CrossRef]
- Lawley, P.D.; Phillips, D.H. DNA adducts from chemotherapeutic agents. Mutat. Res. 1996, 355, 13–40. [Google Scholar] [CrossRef]
- Madhusudan, S.; Middleton, M.R. The emerging role of DNA repair proteins as predictive, prognostic and therapeutic targets in cancer. Cancer Treat. Rev. 2005, 31, 603–617. [Google Scholar] [CrossRef]
- Shrivastav, N.; Li, D.; Essigmann, J.M. Chemical biology of mutagenesis and DNA repair: Cellular responses to DNA alkylation. Carcinogenesis 2010, 31, 59–70. [Google Scholar] [CrossRef]
- Curtin, N.J. Inhibiting the DNA damage response as a therapeutic manoeuvre in cancer. Br. J. Pharmacol. 2013, 169, 1745–1765. [Google Scholar] [CrossRef]
- Hosoya, N.; Miyagawa, K. Targeting DNA damage response in cancer therapy. Cancer Sci. 2014, 105, 370–388. [Google Scholar] [CrossRef]
- Zharkov, D.O. Base excision DNA repair. Cell. Mol. Life Sci. 2008, 65, 1544–1565. [Google Scholar] [CrossRef] [PubMed]
- Mechetin, G.V.; Endutkin, A.V.; Diatlova, E.A.; Zharkov, D.O. Inhibitors of DNA glycosylases as prospective drugs. Int. J. Mol. Sci. 2020, 21, 3118. [Google Scholar] [CrossRef] [PubMed]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.J.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Lord, C.J.; Tutt, A.N.J.; Ashworth, A. Synthetic lethality and cancer therapy: Lessons learned from the development of PARP inhibitors. Annu. Rev. Med. 2015, 66, 455–470. [Google Scholar] [CrossRef]
- Liu, L.; Taverna, P.; Whitacre, C.M.; Chatterjee, S.; Gerson, S.L. Pharmacologic disruption of base excision repair sensitizes mismatch repair-deficient and -proficient colon cancer cells to methylating agents. Clin. Cancer Res. 1999, 5, 2908–2917. [Google Scholar]
- Taverna, P.; Liu, L.; Hwang, H.-S.; Hanson, A.J.; Kinsella, T.J.; Gerson, S.L. Methoxyamine potentiates DNA single strand breaks and double strand breaks induced by temozolomide in colon cancer cells. Mutat. Res. 2001, 485, 269–281. [Google Scholar] [CrossRef]
- Taverna, P.; Hwang, H.-s.; Schupp, J.E.; Radivoyevitch, T.; Session, N.N.; Reddy, G.; Zarling, D.A.; Kinsella, T.J. Inhibition of base excision repair potentiates iododeoxyuridine-induced cytotoxicity and radiosensitization. Cancer Res. 2003, 63, 838–846. [Google Scholar]
- Liu, L.; Nakatsuru, Y.; Gerson, S.L. Base excision repair as a therapeutic target in colon cancer. Clin. Cancer Res. 2002, 8, 2985–2991. [Google Scholar]
- She, M.; Pan, J.; Sun, L.; Yeung, S.-C.J. Enhancement of manumycin A-induced apoptosis by methoxyamine in myeloid leukemia cells. Leukemia 2005, 19, 595–602. [Google Scholar] [CrossRef]
- Yan, T.; Seo, Y.; Schupp, J.E.; Zeng, X.; Desai, A.B.; Kinsella, T.J. Methoxyamine potentiates iododeoxyuridine-induced radiosensitization by altering cell cycle kinetics and enhancing senescence. Mol. Cancer Ther. 2006, 5, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Fishel, M.L.; He, Y.; Smith, M.L.; Kelley, M.R. Manipulation of base excision repair to sensitize ovarian cancer cells to alkylating agent temozolomide. Clin. Cancer Res. 2007, 13, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Bulgar, A.; Miao, Y.; Mahajan, V.; Donze, J.R.; Gerson, S.L.; Liu, L. Combined treatment with temozolomide and methoxyamine: Blocking apurininc/pyrimidinic site repair coupled with targeting topoisomerase IIα. Clin. Cancer Res. 2007, 13, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Bulgar, A.D.; Weeks, L.D.; Miao, Y.; Yang, S.; Xu, Y.; Guo, C.; Markowitz, S.; Oleinick, N.; Gerson, S.L.; Liu, L. Removal of uracil by uracil DNA glycosylase limits pemetrexed cytotoxicity: Overriding the limit with methoxyamine to inhibit base excision repair. Cell Death Dis. 2012, 3, e252. [Google Scholar] [CrossRef]
- Guerreiro, P.S.; Fernandes, A.S.; Costa, J.G.; Castro, M.; Miranda, J.P.; Oliveira, N.G. Differential effects of methoxyamine on doxorubicin cytotoxicity and genotoxicity in MDA-MB-231 human breast cancer cells. Mutat. Res. 2013, 757, 140–147. [Google Scholar] [CrossRef]
- Montaldi, A.P.; Sakamoto-Hojo, E.T. Methoxyamine sensitizes the resistant glioblastoma T98G cell line to the alkylating agent temozolomide. Clin. Exp. Med. 2013, 13, 279–288. [Google Scholar] [CrossRef]
- Oleinick, N.L.; Biswas, T.; Patel, R.; Tao, M.; Patel, R.; Weeks, L.; Sharma, N.; Dowlati, A.; Gerson, S.L.; Fu, P.; et al. Radiosensitization of non-small-cell lung cancer cells and xenografts by the interactive effects of pemetrexed and methoxyamine. Radiother. Oncol. 2016, 121, 335–341. [Google Scholar] [CrossRef]
- Caimi, P.F.; Cooper, B.W.; William, B.M.; Dowlati, A.; Barr, P.M.; Fu, P.; Pink, J.; Xu, Y.; Lazarus, H.M.; de Lima, M.; et al. Phase I clinical trial of the base excision repair inhibitor methoxyamine in combination with fludarabine for patients with advanced hematologic malignancies. Oncotarget 2017, 8, 79864–79875. [Google Scholar] [CrossRef]
- Eads, J.R.; Krishnamurthi, S.S.; Saltzman, J.; Bokar, J.A.; Savvides, P.; Meropol, N.J.; Gibbons, J.; Koon, H.; Sharma, N.; Rogers, L.; et al. Phase I clinical trial of temozolomide and methoxyamine (TRC-102), an inhibitor of base excision repair, in patients with advanced solid tumors. Investig. New Drugs 2021, 39, 142–151. [Google Scholar] [CrossRef]
- Ahluwalia, M.S.; Ozair, A.; Drappatz, J.; Ye, X.; Peng, S.; Lee, M.; Rath, S.; Dhruv, H.; Hao, Y.; Berens, M.E.; et al. Evaluating the Base Excision Repair Inhibitor TRC102 and Temozolomide for Patients with Recurrent Glioblastoma in the Phase 2 Adult Brain Tumor Consortium Trial BERT. Clin. Cancer Res. 2024, 30, 3167–3178. [Google Scholar] [CrossRef]
- O’Sullivan Coyne, G.; Kummar, S.; Meehan, R.S.; Do, K.; Collins, J.M.; Anderson, L.; Ishii, K.; Takebe, N.; Zlott, J.; Juwara, L.; et al. Phase I trial of TRC102 (methoxyamine HCl) in combination with temozolomide in patients with relapsed solid tumors and lymphomas. Oncotarget 2020, 11, 3959–3971. [Google Scholar] [CrossRef] [PubMed]
- Talpaert-Borlé, M.; Liuzzi, M. Reaction of apurinic/apyrimidinic sites with [14C]methoxyamine: A method for the quantitative assay of AP sites in DNA. Biochim. Biophys. Acta 1983, 740, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, M.; Talpaert-Borlé, M. A new approach to the study of the base-excision repair pathway using methoxyamine. J. Biol. Chem. 1985, 260, 5252–5258. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, M.; Weinfeld, M.; Paterson, M.C. Selective inhibition by methoxyamine of the apurinic/apyrimidinic endonuclease activity associated with pyrimidine dimer-DNA glycosylases from Micrococcus luteus and bacteriophage T4. Biochemistry 1987, 26, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, H.; Friedberg, E.C.; Fuchs, R.P.; Goodman, M.F.; Hanaoka, F.; Hinkle, D.; Kunkel, T.A.; Lawrence, C.W.; Livneh, Z.; Nohmi, T.; et al. The Y-family of DNA polymerases. Mol. Cell 2001, 8, 7–8. [Google Scholar] [CrossRef]
- Livneh, Z.; Ziv, O.; Shachar, S. Multiple two-polymerase mechanisms in mammalian translesion DNA synthesis. Cell Cycle 2010, 9, 729–735. [Google Scholar] [CrossRef]
- Rizzo, A.A.; Korzhnev, D.M. The Rev1-Polζ translesion synthesis mutasome: Structure, interactions and inhibition. Enzymes 2019, 45, 139–181. [Google Scholar] [CrossRef]
- Kim, N.; Mudrak, S.V.; Jinks-Robertson, S. The dCMP transferase activity of yeast Rev1 is biologically relevant during the bypass of endogenously generated AP sites. DNA Repair 2011, 10, 1262–1271. [Google Scholar] [CrossRef]
- Otsuka, C.; Kunitomi, N.; Iwai, S.; Loakes, D.; Negishi, K. Roles of the polymerase and BRCT domains of Rev1 protein in translesion DNA synthesis in yeast in vivo. Mutat. Res. 2005, 578, 79–87. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Zhang, Y.; Wang, Z. The catalytic function of the Rev1 dCMP transferase is required in a lesion-specific manner for translesion synthesis and base damage-induced mutagenesis. Nucleic Acids Res. 2010, 38, 5036–5046. [Google Scholar] [CrossRef]
- Sarkies, P.; Reams, C.; Simpson, L.J.; Sale, J.E. Epigenetic instability due to defective replication of structured DNA. Mol. Cell 2010, 40, 703–713. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, S.; Reyes, A.; Martínez-Jiménez, M.I.; Chocrón, E.S.; Mourón, S.; Terrados, G.; Powell, C.; Salido, E.; Méndez, J.; Holt, I.J.; et al. PrimPol, an archaic primase/polymerase operating in human cells. Mol. Cell 2013, 52, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Yudkina, A.V.; Zharkov, D.O. Miscoding and DNA polymerase stalling by methoxyamine-adducted abasic sites. Chem. Res. Toxicol. 2022, 35, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Makarova, A.V.; Burgers, P.M. Eukaryotic DNA polymerase ζ. DNA Repair 2015, 29, 47–55. [Google Scholar] [CrossRef]
- Masutani, C.; Kusumoto, R.; Yamada, A.; Dohmae, N.; Yokoi, M.; Yuasa, M.; Araki, M.; Iwai, S.; Takio, K.; Hanaoka, F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase η. Nature 1999, 399, 700–704. [Google Scholar] [CrossRef]
- Sherrer, S.M.; Fiala, K.A.; Fowler, J.D.; Newmister, S.A.; Pryor, J.M.; Suo, Z. Quantitative analysis of the efficiency and mutagenic spectra of abasic lesion bypass catalyzed by human Y-family DNA polymerases. Nucleic Acids Res. 2011, 39, 609–622. [Google Scholar] [CrossRef]
- Zhao, B.; Xie, Z.; Shen, H.; Wang, Z. Role of DNA polymerase η in the bypass of abasic sites in yeast cells. Nucleic Acids Res. 2004, 32, 3984–3994. [Google Scholar] [CrossRef][Green Version]
- Yuan, F.; Zhang, Y.; Rajpal, D.K.; Wu, X.; Guo, D.; Wang, M.; Taylor, J.-S.; Wang, Z. Specificity of DNA lesion bypass by the yeast DNA polymerase η. J. Biol. Chem. 2000, 275, 8233–8239. [Google Scholar] [CrossRef]
- Haracska, L.; Washington, M.T.; Prakash, S.; Prakash, L. Inefficient bypass of an abasic site by DNA polymerase η. J. Biol. Chem. 2001, 276, 6861–6866. [Google Scholar] [CrossRef]
- McDonald, J.P.; Rapić-Otrin, V.; Epstein, J.A.; Broughton, B.C.; Wang, X.; Lehmann, A.R.; Wolgemuth, D.J.; Woodgate, R. Novel human and mouse homologs of Saccharomyces cerevisiae DNA polymerase eta. Genomics 1999, 60, 20–30. [Google Scholar] [CrossRef]
- McIntyre, J. Polymerase iota—An odd sibling among Y family polymerases. DNA Repair 2020, 86, 102753. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.E.; Washington, M.T.; Haracska, L.; Prakash, S.; Prakash, L. Eukaryotic polymerases ι and ζ act sequentially to bypass DNA lesions. Nature 2000, 406, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, F.; Wu, X.; Taylor, J.-S.; Wang, Z. Response of human DNA polymerase ι to DNA lesions. Nucleic Acids Res. 2001, 29, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Frank, E.G.; Woodgate, R. Increased Catalytic Activity and Altered Fidelity of Human DNA Polymerase ι in the Presence of manganese. J. Biol. Chem. 2007, 282, 24689–24696. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.T.; Johnson, R.E.; Prakash, L.; Prakash, S.; Aggarwal, A.K. DNA synthesis across an abasic lesion by human DNA polymerase ι. Structure 2009, 17, 530–537. [Google Scholar] [CrossRef]
- Nelson, J.R.; Lawrence, C.W.; Hinkle, D.C. Deoxycytidyl transferase activity of yeast REV1 protein. Nature 1996, 382, 729–731. [Google Scholar] [CrossRef]
- Stone, J.E.; Kumar, D.; Binz, S.K.; Inase, A.; Iwai, S.; Chabes, A.; Burgers, P.M.; Kunkel, T.A. Lesion bypass by S. cerevisiae Pol ζ alone. DNA Repair 2011, 10, 826–834. [Google Scholar] [CrossRef]
- Kow, Y.W.; Bao, G.; Minesinger, B.; Jinks-Robertson, S.; Siede, W.; Jiang, Y.L.; Greenberg, M.M. Mutagenic effects of abasic and oxidized abasic lesions in Saccharomyces cerevisiae. Nucleic Acids Res. 2005, 33, 6196–6202. [Google Scholar] [CrossRef]
- Stolyarenko, A.D.; Novikova, A.V.; Shilkin, E.S.; Poltorachenko, V.A.; Makarova, A.V. The Catalytic Activity of Human REV1 on Undamaged and Damaged DNA. Int. J. Mol. Sci. 2024, 25, 4107. [Google Scholar] [CrossRef]
- Haracska, L.; Prakash, S.; Prakash, L. Yeast Rev1 protein is a G template-specific DNA polymerase. J. Biol. Chem. 2002, 277, 15546–15551. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Rechkoblit, O.; Geacintov, N.E.; Taylor, J.-S.; Wang, Z. Response of human REV1 to different DNA damage: Preferential dCMP insertion opposite the lesion. Nucleic Acids Res. 2002, 30, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Makarova, A.V.; Boldinova, E.O.; Belousova, E.A.; Lavrik, O.I. In vitro lesion bypass by human PrimPol. DNA Repair 2018, 70, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, J.; Rudd, S.G.; Jozwiakowski, S.K.; Bailey, L.J.; Soura, V.; Taylor, E.; Stevanovic, I.; Green, A.J.; Stracker, T.H.; Lindsay, H.D.; et al. PrimPol bypasses UV photoproducts during eukaryotic chromosomal DNA replication. Mol. Cell 2013, 52, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Tokarsky, J.E.; Wallenmeyer, P.C.; Phi, K.K.; Suo, Z. Significant impact of divalent metal ions on the fidelity, sugar selectivity, and drug incorporation efficiency of human PrimPol. DNA Repair 2017, 49, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, W.; Morehouse, N.; Tree, M.O.; Zhao, L. Divalent cations alter the rate-limiting step of PrimPol catalyzed DNA elongation. J. Mol. Biol. 2019, 431, 673–686. [Google Scholar] [CrossRef]
- Martínez-Jiménez, M.I.; García-Gómez, S.; Bebenek, K.; Sastre-Moreno, G.; Calvo, P.A.; Díaz-Talavera, A.; Kunkel, T.A.; Blanco, L. Alternative solutions and new scenarios for translesion DNA synthesis by human PrimPol. DNA Repair 2015, 29, 127–138. [Google Scholar] [CrossRef]
- Liu, L.; Gerson, S.R. Therapeutic impact of methoxyamine: Blocking repair of abasic sites in the base excision repair pathway. Curr. Opin. Investig. Drugs 2004, 5, 623–627. [Google Scholar]
- Putu, E.P.G.N.; Cattiaux, L.; Lavergne, T.; Pommier, Y.; Bombard, S.; Granzhan, A. Unprecedented reactivity of polyamines with aldehydic DNA modifications: Structural determinants of reactivity, characterization and enzymatic stability of adducts. Nucleic Acids Res. 2023, 51, 10846–10866. [Google Scholar] [CrossRef]
- Bellamri, M.; Terrell, J.T.; Brandt, K.; Gruppi, F.; Turesky, R.J.; Rizzo, C.J. Anthracyclines react with apurinic/apyrimidinic sites in DNA. ACS Chem. Biol. 2023, 18, 1315–1323. [Google Scholar] [CrossRef]
- Minko, I.; Luzadder, M.; McCullough, A.; Lloys, R.S. Interactions of pixantrone with apurinic/apyrimidinic sites in DNA. MicroPubl. Biol. 2024. [Google Scholar] [CrossRef]
- Islam, T.; Shim, G.; Melton, D.; Lewis, C.D.; Lei, Z.; Gates, K.S. Ultrafast reaction of the drug hydralazine with apurinic/apyrimidinic sites in DNA gives rise to a stable triazolo[3,4-a]phthalazine adduct. Chem. Res. Toxicol. 2024, 17, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Teixeira Oliveira, T.; Lima Fontes-Dantas, F.; de Medeiros Oliveira, R.K.; Lopes Pinheiro, D.M.; Gomes Coutinho, L.; da Silva, V.L.; de Souza, S.J.; Fassarella Agnez-Lima, L. Chemical inhibition of apurinic-apyrimidinic endonuclease 1 redox and DNA repair functions affects the inflammatory response via different but overlapping mechanisms. Front. Cell Dev. Biol. 2021, 9, 731588. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, C.; Sanadai, S.; Hata, Y.; Okuto, H.; Noskov, V.N.; Loakes, D.; Negishi, K. Difference between deoxyribose- and tetrahydrofuran-type abasic sites in the in vivo mutagenic responses in yeast. Nucleic Acids Res. 2002, 30, 5129–5135. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, K.M.; Goodman, M.F.; Greenberg, M.M. A comprehensive comparison of DNA replication past 2-deoxyribose and its tetrahydrofuran analog in Escherichia coli. Nucleic Acids Res. 2004, 32, 5480–5485. [Google Scholar] [CrossRef]
- Biertümpfel, C.; Zhao, Y.; Kondo, Y.; Ramón-Maiques, S.; Gregory, M.; Lee, J.Y.; Masutani, C.; Lehmann, A.R.; Hanaoka, F.; Yang, W. Structure and mechanism of human DNA polymerase η. Nature 2010, 465, 1044–1048. [Google Scholar] [CrossRef]
- Boldinova, E.O.; Yudkina, A.V.; Shilkin, E.S.; Gagarinskaya, D.I.; Baranovskiy, A.G.; Tahirov, T.H.; Zharkov, D.O.; Makarova, A.V. Translesion activity of PrimPol on DNA with cisplatin and DNA–protein cross-links. Sci. Rep. 2021, 11, 17588. [Google Scholar] [CrossRef]
- Lindahl, T.; Nyberg, B. Rate of depurination of native deoxyribonucleic acid. Biochemistry 1972, 11, 3610–3618. [Google Scholar] [CrossRef]
- Acharya, N.; Haracska, L.; Prakash, S.; Prakash, L. Complex formation of yeast Rev1 with DNA polymerase eta. Mol. Cell. Biol. 2007, 23, 8401–8408. [Google Scholar] [CrossRef]
- Acharya, N.; Johnson, R.E.; Pagès, V.; Prakash, L.; Prakash, S. Yeast Rev1 protein promotes complex formation of DNA polymerase zeta with Pol32 subunit of DNA polymerase delta. Proc. Natl Acad. Sci. USA 2009, 106, 9631–9636. [Google Scholar] [CrossRef]
- Piao, J.; Masuda, Y.; Kamiya, K. Specific amino acid residues are involved in substrate discrimination and template binding of human REV1 protein. Biochem. Biophys. Res. Commun. 2010, 392, 140–144. [Google Scholar] [CrossRef]
- Suzuki, M.; Kino, K.; Kawada, T.; Morikawa, M.; Kobayashi, T.; Miyazawa, H. Analysis of nucleotide insertion opposite 2,2,4-triamino-5(2H)-oxazolone by eukaryotic B- and Y-family DNA polymerases. Chem. Res. Toxicol. 2015, 28, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Haracska, L.; Johnson, R.E.; Unk, I.; Phillips, B.B.; Hurwitz, J.; Prakash, L.; Prakash, S. Targeting of human DNA polymerase ι to the replication machinery via interaction with PCNA. Proc. Natl Acad. Sci. USA 2001, 98, 14256–14261. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, E.; Hanafusa, T.; Kamei, K.; Song, I.; Tomida, J.; Hashimoto, H.; Vaziri, C.; Ohmori, H. Identification of a novel REV1-interacting motif necessary for DNA polymerase kappa function. Genes Cells 2009, 14, 101–111. [Google Scholar] [CrossRef]
- Boehm, E.M.; Gildenberg, M.S.; Washington, M.T. The many roles of PCNA in eukaryotic DNA replication. Enzymes 2016, 39, 231–254. [Google Scholar] [CrossRef]
- Ramakrishnan, N.; Weaver, T.M.; Aubuchon, L.N.; Woldegerima, A.; Just, T.; Song, K.; Vindigni, A.; Freudenthal, B.D.; Verma, P. Nucleolytic processing of abasic sites underlies PARP inhibitor hypersensitivity in ALC1-deficient BRCA mutant cancer cells. Nat. Commun. 2024, 15, 6343. [Google Scholar] [CrossRef]
- Hanthi, Y.W.; Ramirez-Otero, M.A.; Appleby, R.; De Antoni, A.; Joudeh, L.; Sannino, V.; Waked, S.; Ardizzoia, A.; Barra, V.; Fachinetti, D.; et al. RAD51 protects abasic sites to prevent replication fork breakage. Mol. Cell 2024, 84, 3026–3043. [Google Scholar] [CrossRef]
- Meng, F.; Li, T.; Singh, A.K.; Wang, Y.; Attiyeh, M.; Kohram, F.; Feng, Q.; Li, Y.R.; Shen, B.; Williams, T.; et al. Base-excision repair pathway regulates transcription-replication conflicts in pancreatic ductal adenocarcinoma. Cell Rep. 2024, 43, 114820. [Google Scholar] [CrossRef]
- Boldinova, E.O.; Ignatov, A.; Kulbachinskiy, A.; Makarova, A.V. The active site residues Gln55 and Arg73 play a key role in DNA damage bypass by S. cerevisiae Pol η. Sci. Rep. 2018, 8, 10314. [Google Scholar] [CrossRef]
- Makarova, A.V.; Grabow, C.; Gening, L.V.; Tarantul, V.Z.; Tahirov, T.H.; Bessho, T.; Pavlov, Y.I. Inaccurate DNA synthesis in cell extracts of yeast producing active human DNA polymerase iota. PLoS ONE 2011, 6, e16612. [Google Scholar] [CrossRef]
- Makarova, A.V.; Stodola, J.L.; Burgers, P.M. A four-subunit DNA polymerase ζ complex containing Pol δ accessory subunits is essential for PCNA-mediated mutagenesis. Nucleic Acids Res. 2012, 40, 11618–11626. [Google Scholar] [CrossRef]


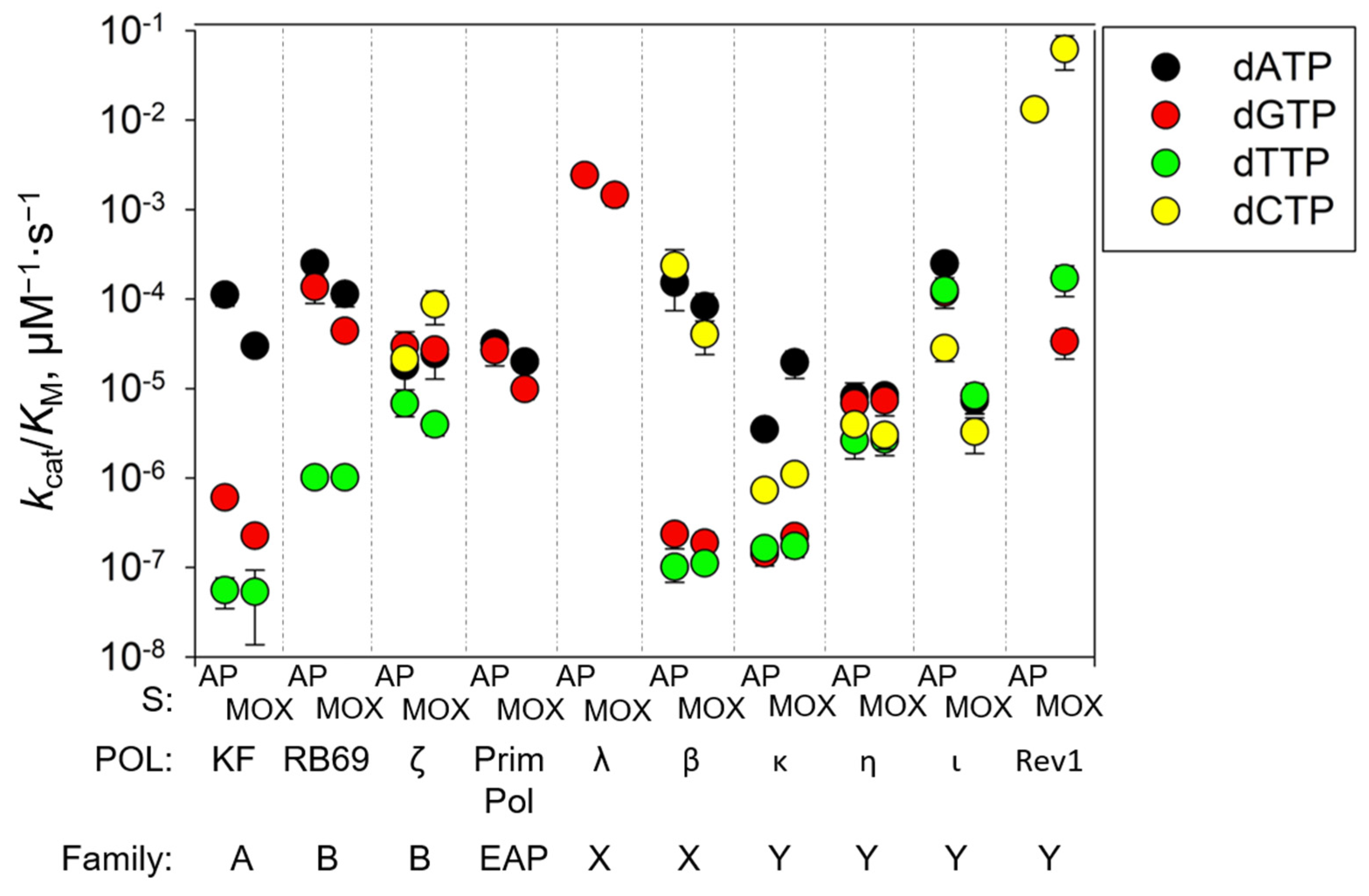
| Pol | Template | dNTP | KM, µM | kcat, s−1 (×105) | kcat/KM, µM−1s−1 (×106) |
|---|---|---|---|---|---|
| Pol η | AP site | dATP | 104 ± 40 | 84 ± 14 | 8.1 ± 3.4 |
| Pol η | AP site | dGTP | 116 ± 39 | 79 ± 9 | 6.8 ± 2.4 |
| Pol η | AP site | dTTP | 197 ± 69 | 52 ± 7 | 2.6 ± 1.0 |
| Pol η | AP site | dCTP | 127 ± 26 | 50 ± 5 | 4.0 ± 0.9 |
| Pol η | AP-MOX | dATP | 130 ± 27 | 108 ± 9 | 8.3 ± 1.8 |
| Pol η | AP-MOX | dGTP | 102 ± 32 | 75 ± 8 | 7.3 ± 2.4 |
| Pol η | AP-MOX | dTTP | 213 ± 67 | 57 ± 7 | 2.7 ± 0.9 |
| Pol η | AP-MOX | dCTP | 154 ± 45 | 47 ± 5 | 3.0 ± 0.9 |
| Pol | Template | dNTP | KM, µM | kcat, s−1 (×105) | kcat/KM, µM−1s−1 (×106) |
|---|---|---|---|---|---|
| Pol ι | AP site | dATP | 2.3 ± 0.7 | 57 ± 3 | 248 ± 75 |
| Pol ι | AP site | dGTP | 2.6 ± 0.8 | 30 ± 2 | 117 ± 39 |
| Pol ι | AP site | dTTP | 2.1 ± 0.5 | 27 ± 1 | 125 ± 33 |
| Pol ι | AP site | dCTP | 5.1 ± 1.5 | 14 ± 0.8 | 28 ± 8.2 |
| Pol ι | AP-MOX | dATP | 5.0 ± 1.4 | 3.7 ± 0.3 | 7.4 ± 2.1 |
| Pol ι | AP-MOX | dGTP | 5.4 ± 1.9 | 4.5 ± 0.4 | 8.2 ± 3.0 |
| Pol ι | AP-MOX | dTTP | 4.2 ± 1.5 | 3.5 ± 0.3 | 8.2 ± 3.1 |
| Pol ι | AP-MOX | dCTP | 3.7 ± 1.5 | 1.2 ± 0.1 | 3.3 ± 1.4 |
| Pol | Template | dNTP | KM, µM | kcat, s−1 (×105) | kcat/KM, µM−1s−1 (×106) |
|---|---|---|---|---|---|
| Pol ζ | AP site | dATP | 79 ± 35 | 141 ± 16 | 18 ± 8 |
| Pol ζ | AP site | dGTP | 32 ± 14 | 96 ± 9 | 30 ± 13 |
| Pol ζ | AP site | dTTP | 21 ± 6 | 15 ± 1 | 7 ± 2 |
| Pol ζ | AP site | dCTP | 14 ± 4 | 31 ± 2 | 21 ± 7 |
| Pol ζ | AP-MOX | dATP | 32 ± 15 | 78 ± 9 | 24 ± 11 |
| Pol ζ | AP-MOX | dGTP | 23 ± 7 | 63 ± 4 | 27 ± 8 |
| Pol ζ | AP-MOX | dTTP | 35 ± 9 | 14 ± 1 | 4 ± 1 |
| Pol ζ | AP-MOX | dCTP | 5 ± 2 | 41 ± 3 | 87 ± 35 |
| Pol | Template | dNTP | KM, µM | kcat, s−1 (×102) | kcat/KM, µM−1s−1 (×103) |
|---|---|---|---|---|---|
| Rev1 | AP site | dCTP | 21 ± 4 | 27 ± 1 | 13 ± 2 |
| Rev1 | AP site | dTTP | no incorporation | ||
| Rev1 | AP site | dGTP | no incorporation | ||
| Rev1 | AP-MOX | dCTP | 4.7 ± 1.9 | 29 ± 3 | 62 ± 26 |
| Rev1 | AP-MOX | dTTP | 108 ± 39 | 1.9 ± 0.2 | 0.17 ± 0.06 |
| Rev1 | AP-MOX | dGTP | 169 ± 57 | 0.6 ± 0.1 | 0.03 ± 0.01 |
| Rev1 | G | dCTP | 0.7 ± 0.1 | 25 ± 1 | 340 ± 50 |
| Rev1 | G | dTTP | 86 ± 24 | 3.0 ± 0.3 | 0.35 ± 0.10 |
| Rev1 | G | dGTP | 87 ± 26 | 3.1 ± 0.3 | 0.36 ± 0.11 |
| Pol | Template | dNTP | KM, µM | kcat, s−1 (×106) | kcat/KM, µM−1s−1 (×106) |
|---|---|---|---|---|---|
| PrimPol | AP site | dATP | 1.1 ± 0.3 | 35 ± 13 | 32 ± 9 |
| PrimPol | AP site | dGTP | 1.1 ± 0.4 | 30 ± 13 | 27 ± 19 |
| PrimPol | AP-MOX | dATP | 1.8 ± 0.4 | 35 ± 10 | 20 ± 4 |
| PrimPol | AP-MOX | dGTP | 3.7 ± 0.8 | 36 ± 13 | 10 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yudkina, A.V.; Novikova, A.A.; Stolyarenko, A.D.; Makarova, A.V.; Zharkov, D.O. Bypass of Methoxyamine-Adducted Abasic Sites by Eukaryotic Translesion DNA Polymerases. Int. J. Mol. Sci. 2025, 26, 642. https://doi.org/10.3390/ijms26020642
Yudkina AV, Novikova AA, Stolyarenko AD, Makarova AV, Zharkov DO. Bypass of Methoxyamine-Adducted Abasic Sites by Eukaryotic Translesion DNA Polymerases. International Journal of Molecular Sciences. 2025; 26(2):642. https://doi.org/10.3390/ijms26020642
Chicago/Turabian StyleYudkina, Anna V., Anna A. Novikova, Anastasia D. Stolyarenko, Alena V. Makarova, and Dmitry O. Zharkov. 2025. "Bypass of Methoxyamine-Adducted Abasic Sites by Eukaryotic Translesion DNA Polymerases" International Journal of Molecular Sciences 26, no. 2: 642. https://doi.org/10.3390/ijms26020642
APA StyleYudkina, A. V., Novikova, A. A., Stolyarenko, A. D., Makarova, A. V., & Zharkov, D. O. (2025). Bypass of Methoxyamine-Adducted Abasic Sites by Eukaryotic Translesion DNA Polymerases. International Journal of Molecular Sciences, 26(2), 642. https://doi.org/10.3390/ijms26020642






