Strategies for Survival of Staphylococcus aureus in Host Cells
Abstract
1. Introduction
2. Internalization of S. aureus
3. Survival Strategies for S. aureus
3.1. Alpha-Hemolysin (α-Toxin)
3.2. Phenol Soluble Modulins
3.3. Two-Component Leukotoxins
3.4. S. aureus Extracellular Vesicles
3.5. Formation of S. aureus Biofilms
3.6. Small Colony Variants (SCVs)
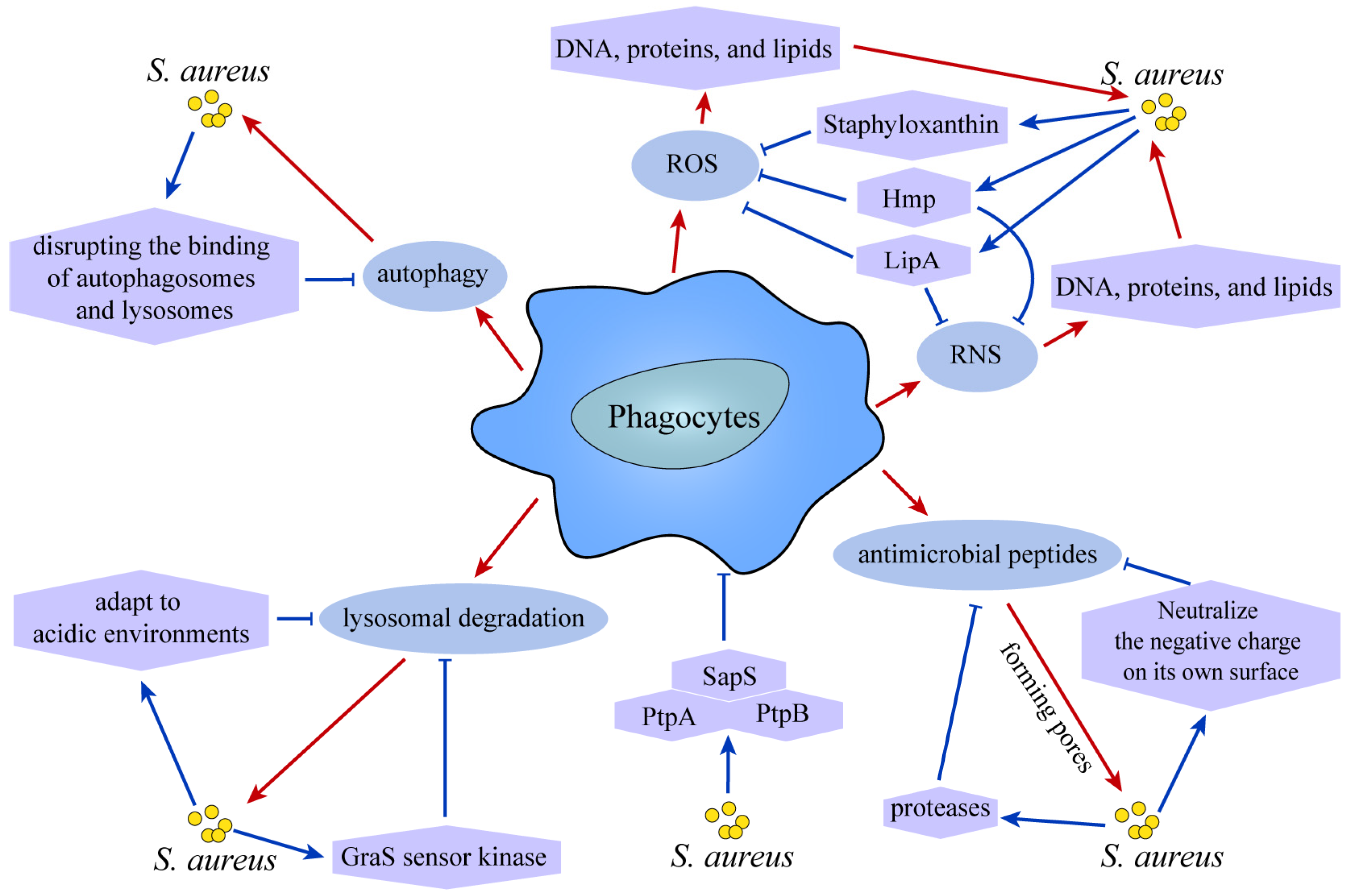
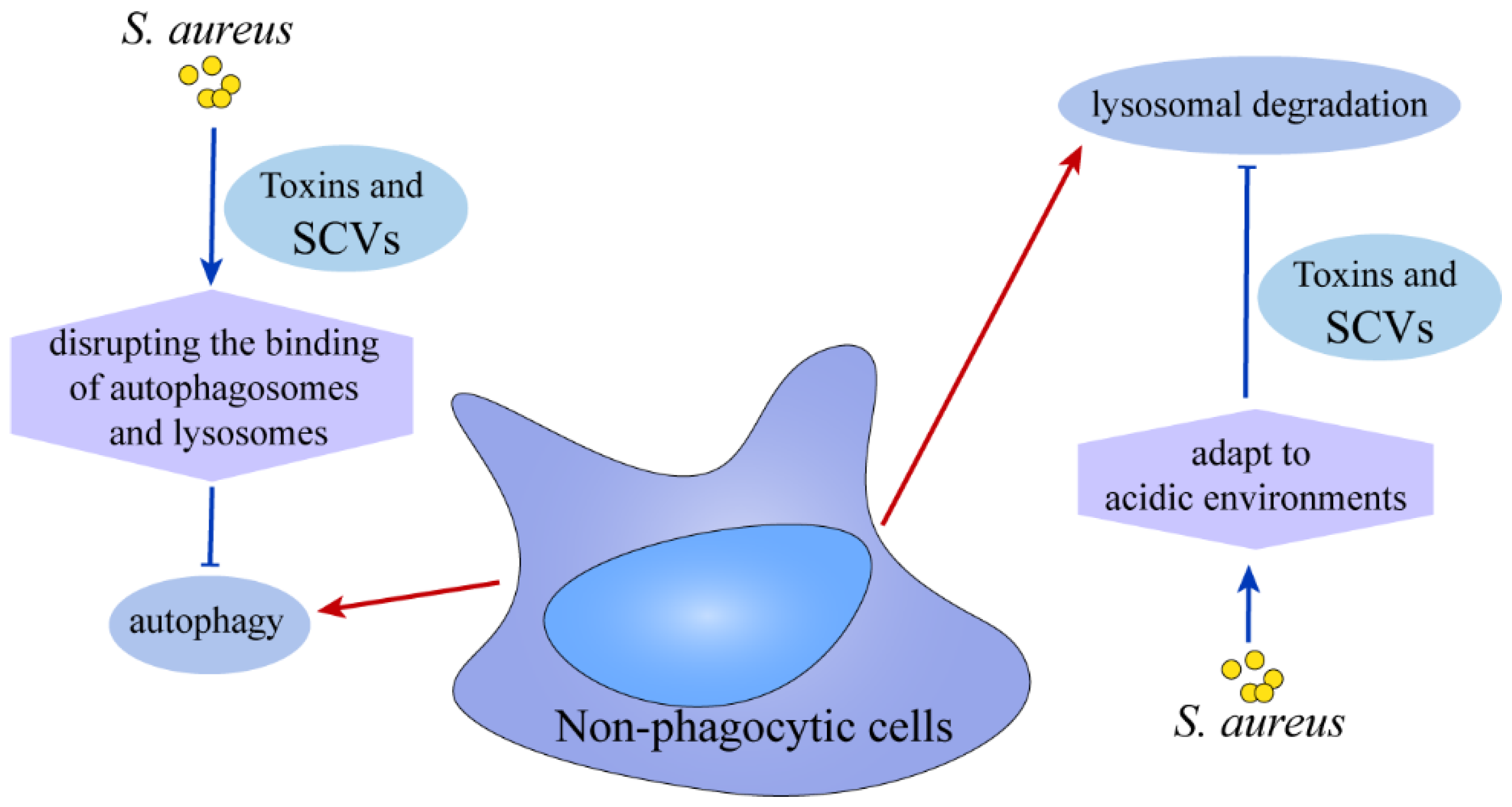
4. The Ultimate Fate of S. aureus
5. Summary
6. Complementary
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Oliveira, D.; Borges, A.; Simões, M. Staphylococcus aureus Toxins and Their Molecular Activity in Infectious Diseases. Toxins 2018, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.; Kucklick, M.; Marbach, H.; Ehling-Schulz, M.; Engelmann, S.; Grunert, T. Within-Host Adaptation of Staphylococcus aureus in a Bovine Mastitis Infection Is Associated with Increased Cytotoxicity. Int. J. Mol. Sci. 2021, 22, 8840. [Google Scholar] [CrossRef] [PubMed]
- Zaatout, N.; Ayachi, A.; Kecha, M. Staphylococcus aureus persistence properties associated with bovine mastitis and alternative therapeutic modalities. J. Appl. Microbiol. 2020, 129, 1102–1119. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef]
- Tuchscherr, L.; Löffler, B.; Proctor, R.A. Persistence of Staphylococcus aureus: Multiple Metabolic Pathways Impact the Expression of Virulence Factors in Small-Colony Variants (SCVs). Front. Microbiol. 2020, 11, 1028. [Google Scholar] [CrossRef]
- Laabei, M.; Uhlemann, A.C.; Lowy, F.D.; Austin, E.D.; Yokoyama, M.; Ouadi, K.; Feil, E.; Thorpe, H.A.; Williams, B.; Perkins, M.; et al. Evolutionary Trade-Offs Underlie the Multi-faceted Virulence of Staphylococcus aureus. PLoS Biol. 2015, 13, e1002229. [Google Scholar] [CrossRef] [PubMed]
- Frutis-Murillo, M.; Sandoval-Carrillo, M.A.; Alva-Murillo, N.; Ochoa-Zarzosa, A.; López-Meza, J.E. Immunomodulatory molecules regulate adhesin gene expression in Staphylococcus aureus: Effect on bacterial internalization into bovine mammary epithelial cells. Microb. Pathog. 2019, 131, 15–21. [Google Scholar] [CrossRef]
- Niemann, S.; Nguyen, M.T.; Eble, J.A.; Chasan, A.I.; Mrakovcic, M.; Böttcher, R.T.; Preissner, K.T.; Roßlenbroich, S.; Peters, G.; Herrmann, M. More Is Not Always Better-the Double-Headed Role of Fibronectin in Staphylococcus aureus Host Cell Invasion. mBio 2021, 12, e0106221. [Google Scholar] [CrossRef]
- Meng, M.; Wang, J.; Li, H.; Wang, J.; Wang, X.; Li, M.; Gao, X.; Li, W.; Ma, C.; Wei, L. Eliminating the invading extracellular and intracellular FnBp(+) bacteria from respiratory epithelial cells by autophagy mediated through FnBp-Fn-Integrin α5β1 axis. Front. Cell. Infect. Microbiol. 2023, 13, 1324727. [Google Scholar] [CrossRef]
- Schlesier, T.; Siegmund, A.; Rescher, U.; Heilmann, C. Characterization of the Atl-mediated staphylococcal internalization mechanism. Int. J. Med. Microbiol. IJMM 2020, 310, 151463. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.T.; Ren, W.J.; Tan, X.; Yang, J.; Liu, R.; Du, A.F. Annexin A2-Mediated Internalization of Staphylococcus aureus into Bovine Mammary Epithelial Cells Requires Its Interaction with Clumping Factor B. Microorganisms 2021, 9, 2090. [Google Scholar] [CrossRef] [PubMed]
- Tribelli, P.M.; Luqman, A.; Nguyen, M.T.; Madlung, J.; Fan, S.H.; Macek, B.; Sass, P.; Bitschar, K.; Schittek, B.; Kretschmer, D.; et al. Staphylococcus aureus Lpl protein triggers human host cell invasion via activation of Hsp90 receptor. Cell. Microbiol. 2020, 22, e13111. [Google Scholar] [CrossRef]
- Truong-Bolduc, Q.C.; Wang, Y.; Hooper, D.C. Tet38 of Staphylococcus aureus Binds to Host Cell Receptor Complex CD36-Toll-Like Receptor 2 and Protects from Teichoic Acid Synthesis Inhibitors Tunicamycin and Congo Red. Infect. Immun. 2019, 87, e00194-19. [Google Scholar] [CrossRef] [PubMed]
- Inoshima, I.; Inoshima, N.; Wilke, G.A.; Powers, M.E.; Frank, K.M.; Wang, Y.; Bubeck Wardenburg, J. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat. Med. 2011, 17, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Seilie, E.S.; Bubeck Wardenburg, J. Staphylococcus aureus pore-forming toxins: The interface of pathogen and host complexity. Semin. Cell Dev. Biol. 2017, 72, 101–116. [Google Scholar] [CrossRef]
- Liang, X.; Ji, Y. Involvement of alpha5beta1-integrin and TNF-alpha in Staphylococcus aureus alpha-toxin-induced death of epithelial cells. Cell. Microbiol. 2007, 9, 1809–1821. [Google Scholar] [CrossRef]
- Virreira Winter, S.; Zychlinsky, A.; Bardoel, B.W. Genome-wide CRISPR screen reveals novel host factors required for Staphylococcus aureus α-hemolysin-mediated toxicity. Sci. Rep. 2016, 6, 24242. [Google Scholar] [CrossRef] [PubMed]
- Popov, L.M.; Marceau, C.D.; Starkl, P.M.; Lumb, J.H.; Shah, J.; Guerrera, D.; Cooper, R.L.; Merakou, C.; Bouley, D.M.; Meng, W.; et al. The adherens junctions control susceptibility to Staphylococcus aureus α-toxin. Proc. Natl. Acad. Sci. USA 2015, 112, 14337–14342. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Rouaud, F.; Guerrera, D.; Vasileva, E.; Popov, L.M.; Kelley, W.L.; Rubinstein, E.; Carette, J.E.; Amieva, M.R.; Citi, S. A Dock-and-Lock Mechanism Clusters ADAM10 at Cell-Cell Junctions to Promote α-Toxin Cytotoxicity. Cell Rep. 2018, 25, 2132–2147.e2137. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Zhou, M.; Jiang, C.; Chen, X.; Guo, L.; Zhang, M.; Chu, Z.; Wang, Y. Staphylococcus aureus Phenol-Soluble Modulins α1-α3 Act as Novel Toll-Like Receptor (TLR) 4 Antagonists to Inhibit HMGB1/TLR4/NF-κB Signaling Pathway. Front. Immunol. 2018, 9, 862. [Google Scholar] [CrossRef]
- Lebtig, M.; Scheurer, J.; Muenkel, M.; Becker, J.; Bastounis, E.; Peschel, A.; Kretschmer, D. Keratinocytes use FPR2 to detect Staphylococcus aureus and initiate antimicrobial skin defense. Front. Immunol. 2023, 14, 1188555. [Google Scholar] [CrossRef]
- Richardson, J.R.; Armbruster, N.S.; Günter, M.; Henes, J.; Autenrieth, S.E. Staphylococcus aureus PSM Peptides Modulate Human Monocyte-Derived Dendritic Cells to Prime Regulatory T Cells. Front. Immunol. 2018, 9, 2603. [Google Scholar] [CrossRef]
- Spaan, A.N.; Vrieling, M.; Wallet, P.; Badiou, C.; Reyes-Robles, T.; Ohneck, E.A.; Benito, Y.; de Haas, C.J.; Day, C.J.; Jennings, M.P.; et al. The staphylococcal toxins γ-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat. Commun. 2014, 5, 5438. [Google Scholar] [CrossRef]
- Alonzo, F., 3rd; Kozhaya, L.; Rawlings, S.A.; Reyes-Robles, T.; DuMont, A.L.; Myszka, D.G.; Landau, N.R.; Unutmaz, D.; Torres, V.J. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 2013, 493, 51–55. [Google Scholar] [CrossRef]
- Reyes-Robles, T.; Alonzo, F., 3rd; Kozhaya, L.; Lacy, D.B.; Unutmaz, D.; Torres, V.J. Staphylococcus aureus leukotoxin ED targets the chemokine receptors CXCR1 and CXCR2 to kill leukocytes and promote infection. Cell Host Microbe 2013, 14, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Vrieling, M.; Koymans, K.J.; Heesterbeek, D.A.; Aerts, P.C.; Rutten, V.P.; de Haas, C.J.; van Kessel, K.P.; Koets, A.P.; Nijland, R.; van Strijp, J.A. Bovine Staphylococcus aureus Secretes the Leukocidin LukMF’ To Kill Migrating Neutrophils through CCR1. mBio 2015, 6, e00335. [Google Scholar] [CrossRef] [PubMed]
- Spaan, A.N.; Henry, T.; van Rooijen, W.J.M.; Perret, M.; Badiou, C.; Aerts, P.C.; Kemmink, J.; de Haas, C.J.C.; van Kessel, K.P.M.; Vandenesch, F.; et al. The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell Host Microbe 2013, 13, 584–594. [Google Scholar] [CrossRef]
- DuMont, A.L.; Yoong, P.; Day, C.J.; Alonzo, F., 3rd; McDonald, W.H.; Jennings, M.P.; Torres, V.J. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc. Natl. Acad. Sci. USA 2013, 110, 10794–10799. [Google Scholar] [CrossRef] [PubMed]
- Agerer, F.; Michel, A.; Ohlsen, K.; Hauck, C.R. Integrin-mediated invasion of Staphylococcus aureus into human cells requires Src family protein-tyrosine kinases. J. Biol. Chem. 2003, 278, 42524–42531. [Google Scholar] [CrossRef]
- Latomanski, E.A.; Newton, H.J. Taming the Triskelion: Bacterial Manipulation of Clathrin. Microbiol. Mol. Biol. Rev. MMBR 2019, 83, e00058-18. [Google Scholar] [CrossRef]
- Miyake, R.; Iwamoto, K.; Sakai, N.; Matsunae, K.; Aziz, F.; Sugai, M.; Takahagi, S.; Tanaka, A.; Hide, M. Uptake of Staphylococcus aureus by keratinocytes is reduced by interferon-fibronectin pathway and filaggrin expression. J. Dermatol. 2022, 49, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- García-Betancur, J.C.; Goñi-Moreno, A.; Horger, T.; Schott, M.; Sharan, M.; Eikmeier, J.; Wohlmuth, B.; Zernecke, A.; Ohlsen, K.; Kuttler, C.; et al. Cell differentiation defines acute and chronic infection cell types in Staphylococcus aureus. eLife 2017, 6, e28023. [Google Scholar] [CrossRef]
- Grumann, D.; Nübel, U.; Bröker, B.M. Staphylococcus aureus toxins--their functions and genetics. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2014, 21, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Kubica, M.; Guzik, K.; Koziel, J.; Zarebski, M.; Richter, W.; Gajkowska, B.; Golda, A.; Maciag-Gudowska, A.; Brix, K.; Shaw, L.; et al. A potential new pathway for Staphylococcus aureus dissemination: The silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS ONE 2008, 3, e1409. [Google Scholar] [CrossRef]
- Cohen, T.S.; Hilliard, J.J.; Jones-Nelson, O.; Keller, A.E.; O’Day, T.; Tkaczyk, C.; DiGiandomenico, A.; Hamilton, M.; Pelletier, M.; Wang, Q.; et al. Staphylococcus aureus α toxin potentiates opportunistic bacterial lung infections. Sci. Transl. Med. 2016, 8, 329ra331. [Google Scholar] [CrossRef]
- Fernandez, J.S.; Tuttobene, M.R.; Montaña, S.; Subils, T.; Cantera, V.; Iriarte, A.; Tuchscherr, L.; Ramirez, M.S. Staphylococcus aureus α-Toxin Effect on Acinetobacter baumannii Behavior. Biology 2022, 11, 570. [Google Scholar] [CrossRef]
- Hong, S.W.; Choi, E.B.; Min, T.K.; Kim, J.H.; Kim, M.H.; Jeon, S.G.; Lee, B.J.; Gho, Y.S.; Jee, Y.K.; Pyun, B.Y.; et al. An important role of α-hemolysin in extracellular vesicles on the development of atopic dermatitis induced by Staphylococcus aureus. PLoS ONE 2014, 9, e100499. [Google Scholar] [CrossRef]
- Pivard, M.; Moreau, K.; Vandenesch, F. Staphylococcus aureus Arsenal To Conquer the Lower Respiratory Tract. mSphere 2021, 6, e00059-21. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.A.; Fahsel, B.; Kemper, H.; Mayeres, J.; Li, C.; Wilker, B.; Keitsch, S.; Soddemann, M.; Sehl, C.; Kohnen, M.; et al. Staphylococcus aureus Alpha-Toxin Disrupts Endothelial-Cell Tight Junctions via Acid Sphingomyelinase and Ceramide. Infect. Immun. 2018, 86, e00606-17. [Google Scholar] [CrossRef]
- Keitsch, S.; Riethmüller, J.; Soddemann, M.; Sehl, C.; Wilker, B.; Edwards, M.J.; Caldwell, C.C.; Fraunholz, M.; Gulbins, E.; Becker, K.A. Pulmonary infection of cystic fibrosis mice with Staphylococcus aureus requires expression of α-toxin. Biol. Chem. 2018, 399, 1203–1213. [Google Scholar] [CrossRef]
- Monecke, S.; Müller, E.; Büchler, J.; Stieber, B.; Ehricht, R. Staphylococcus aureus in vitro secretion of alpha toxin (hla) correlates with the affiliation to clonal complexes. PLoS ONE 2014, 9, e100427. [Google Scholar] [CrossRef] [PubMed]
- Bonifacius, A.; Goldmann, O.; Floess, S.; Holtfreter, S.; Robert, P.A.; Nordengrün, M.; Kruse, F.; Lochner, M.; Falk, C.S.; Schmitz, I.; et al. Staphylococcus aureus Alpha-Toxin Limits Type 1 While Fostering Type 3 Immune Responses. Front. Immunol. 2020, 11, 1579. [Google Scholar] [CrossRef] [PubMed]
- Teymournejad, O.; Li, Z.; Beesetty, P.; Yang, C.; Montgomery, C.P. Toxin expression during Staphylococcus aureus infection imprints host immunity to inhibit vaccine efficacy. NPJ Vaccines 2023, 8, 3. [Google Scholar] [CrossRef]
- Kim, N.H.; Choi, Y.; Kwon, K.; Park, J.S.; Park, K.U.; Moon, S.M.; Song, K.H.; Kim, E.S.; Park, W.B.; Kim, H.B. Anti-Alpha-Toxin Antibody Responses and Clinical Outcomes of Staphylococcus aureus Bacteremia. J. Korean Med. Sci. 2023, 38, e129. [Google Scholar] [CrossRef] [PubMed]
- Lade, H.; Chung, S.H.; Lee, Y.; Joo, H.S.; Kim, J.S. Genotypes of Staphylococcus aureus Clinical Isolates Are Associated with Phenol-Soluble Modulin (PSM) Production. Toxins 2022, 14, 556. [Google Scholar] [CrossRef]
- Kristoffersen, K.; Hansen, K.H.; Andreasen, M. Differential Effects of Lipid Bilayers on αPSM Peptide Functional Amyloid Formation. Int. J. Mol. Sci. 2023, 25, 102. [Google Scholar] [CrossRef]
- Otto, M. Phenol-soluble modulins. Int. J. Med. Microbiol. IJMM 2014, 304, 164–169. [Google Scholar] [CrossRef]
- Siegmund, A.; Afzal, M.A.; Tetzlaff, F.; Keinhörster, D.; Gratani, F.; Paprotka, K.; Westermann, M.; Nietzsche, S.; Wolz, C.; Fraunholz, M.; et al. Intracellular persistence of Staphylococcus aureus in endothelial cells is promoted by the absence of phenol-soluble modulins. Virulence 2021, 12, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Hanzelmann, D.; Joo, H.S.; Franz-Wachtel, M.; Hertlein, T.; Stevanovic, S.; Macek, B.; Wolz, C.; Götz, F.; Otto, M.; Kretschmer, D.; et al. Toll-like receptor 2 activation depends on lipopeptide shedding by bacterial surfactants. Nat. Commun. 2016, 7, 12304. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Archer, N.K.; Dillen, C.A.; Wang, Y.; Ashbaugh, A.G.; Ortines, R.V.; Kao, T.; Lee, S.K.; Cai, S.S.; Miller, R.J.; et al. Staphylococcus aureus Epicutaneous Exposure Drives Skin Inflammation via IL-36-Mediated T Cell Responses. Cell Host Microbe 2017, 22, 653–666.e655. [Google Scholar] [CrossRef]
- Nakajima, I.; Fukuda, K.; Ishida, W.; Kishimoto, T.; Kuwana, A.; Suzuki, T.; Kaito, C.; Yamashiro, K. Staphylococcus aureus-derived virulent phenol-soluble modulin α triggers alarmin release to drive IL-36-dependent corneal inflammation. Microbes Infect. 2024, 26, 105237. [Google Scholar] [CrossRef]
- Damour, A.; Robin, B.; Deroche, L.; Broutin, L.; Bellin, N.; Verdon, J.; Lina, G.; Leclère, F.M.; Garcia, M.; Cremniter, J.; et al. Phenol-soluble modulins α are major virulence factors of Staphylococcus aureus secretome promoting inflammatory response in human epidermis. Virulence 2021, 12, 2474–2492. [Google Scholar] [CrossRef]
- Ventura, C.L.; Malachowa, N.; Hammer, C.H.; Nardone, G.A.; Robinson, M.A.; Kobayashi, S.D.; DeLeo, F.R. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS ONE 2010, 5, e11634. [Google Scholar] [CrossRef] [PubMed]
- Münzenmayer, L.; Geiger, T.; Daiber, E.; Schulte, B.; Autenrieth, S.E.; Fraunholz, M.; Wolz, C. Influence of Sae-regulated and Agr-regulated factors on the escape of Staphylococcus aureus from human macrophages. Cell. Microbiol. 2016, 18, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.Y.; Lin, C.C.; Liao, I.C.; Yao, Y.C.; Shen, F.C.; Liu, C.C.; Lin, C.F. Panton-Valentine leukocidin facilitates the escape of Staphylococcus aureus from human keratinocyte endosomes and induces apoptosis. J. Infect. Dis. 2014, 209, 224–235. [Google Scholar] [CrossRef]
- Genestier, A.L.; Michallet, M.C.; Prévost, G.; Bellot, G.; Chalabreysse, L.; Peyrol, S.; Thivolet, F.; Etienne, J.; Lina, G.; Vallette, F.M.; et al. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Investig. 2005, 115, 3117–3127. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.; Bandyopadhyay, A.; Roberts, N.; Mughal, N.; Moore, L.S.P.; Fuller, L.C. Panton-Valentine leucocidin-producing Staphylococcus aureus: A clinical review. Clin. Exp. Dermatol. 2022, 47, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Leistner, R.; Hanitsch, L.G.; Krüger, R.; Lindner, A.K.; Stegemann, M.S.; Nurjadi, D. Skin Infections Due to Panton-Valentine Leukocidin-Producing S. Aureus. Dtsch. Arztebl. Int. 2022, 119, 775–784. [Google Scholar] [CrossRef]
- Duployez, C.; Le Guern, R.; Tinez, C.; Lejeune, A.L.; Robriquet, L.; Six, S.; Loïez, C.; Wallet, F. Panton-Valentine Leukocidin-Secreting Staphylococcus aureus Pneumonia Complicating COVID-19. Emerg. Infect. Dis. 2020, 26, 1939–1941. [Google Scholar] [CrossRef]
- Liu, Z.H.; Wu, Q.Y.; Xu, F.; Zhang, X.; Liao, X.B. Biofunction and clinical potential of extracellular vesicles from methicillin-resistant Staphylococcus aureus. Microbiol. Res. 2023, 266, 127238. [Google Scholar] [CrossRef]
- Wang, X.; Koffi, P.F.; English, O.F.; Lee, J.C. Staphylococcus aureus Extracellular Vesicles: A Story of Toxicity and the Stress of 2020. Toxins 2021, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Uppu, D.S.; Dickey, S.W.; Burgin, D.J.; Otto, M.; Lee, J.C. Staphylococcus aureus delta toxin modulates both extracellular membrane vesicle biogenesis and amyloid formation. mBio 2023, 14, e0174823. [Google Scholar] [CrossRef]
- Asano, K.; Hirose, S.; Narita, K.; Subsomwong, P.; Kawai, N.; Sukchawalit, R.; Nakane, A. Extracellular vesicles from methicillin resistant Staphylococcus aureus stimulate proinflammatory cytokine production and trigger IgE-mediated hypersensitivity. Emerg. Microbes Infect. 2021, 10, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Bitto, N.J.; Cheng, L.; Johnston, E.L.; Pathirana, R.; Phan, T.K.; Poon, I.K.H.; O’Brien-Simpson, N.M.; Hill, A.F.; Stinear, T.P.; Kaparakis-Liaskos, M. Staphylococcus aureus membrane vesicles contain immunostimulatory DNA, RNA and peptidoglycan that activate innate immune receptors and induce autophagy. J. Extracell. Vesicles 2021, 10, e12080. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Wang, J.; Xu, H.; Xue, K.; Liu, X.; Zhang, Z.; Liu, J.; Liu, Y. Staphylococcus aureus extracellular vesicles induce apoptosis and restrain mitophagy-mediated degradation of damaged mitochondria. Microbiol. Res. 2023, 273, 127421. [Google Scholar] [CrossRef]
- Lister, J.L.; Horswill, A.R. Staphylococcus aureus biofilms: Recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 2014, 4, 178. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, K.; Horswill, A.R. Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies. Microbiol. Mol. Biol. Rev. MMBR 2020, 84, e00026-19. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal infections: Mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu. Rev. Med. 2013, 64, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Grando, K.; Nicastro, L.K.; Tursi, S.A.; De Anda, J.; Lee, E.Y.; Wong, G.C.L.; Tükel, Ç. Phenol-Soluble Modulins From Staphylococcus aureus Biofilms Form Complexes With DNA to Drive Autoimmunity. Front. Cell. Infect. Microbiol. 2022, 12, 884065. [Google Scholar] [CrossRef] [PubMed]
- Scherr, T.D.; Hanke, M.L.; Huang, O.; James, D.B.; Horswill, A.R.; Bayles, K.W.; Fey, P.D.; Torres, V.J.; Kielian, T. Staphylococcus aureus Biofilms Induce Macrophage Dysfunction Through Leukocidin AB and Alpha-Toxin. mBio 2015, 6, e01021-15. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Lee, S.; Soper, S.A.; Mitchell, R.J. Staphylococcus aureus extracellular vesicles (EVs): Surface-binding antagonists of biofilm formation. Mol. Biosyst. 2017, 13, 2704–2714. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Reviews. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef]
- Sabino, Y.N.V.; Araújo Domingues, K.C.; Mathur, H.; Gómez-Mascaraque, L.G.; Drouin, G.; Martínez-Abad, A.; Tótola, M.R.; Abreu, L.M.; Cotter, P.D.; Mantovani, H.C. Exopolysaccharides produced by Bacillus spp. inhibit biofilm formation by Staphylococcus aureus strains associated with bovine mastitis. Int. J. Biol. Macromol. 2023, 253, 126689. [Google Scholar] [CrossRef]
- Guo, H.; Tong, Y.; Cheng, J.; Abbas, Z.; Li, Z.; Wang, J.; Zhou, Y.; Si, D.; Zhang, R. Biofilm and Small Colony Variants-An Update on Staphylococcus aureus Strategies toward Drug Resistance. Int. J. Mol. Sci. 2022, 23, 1241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Rao, Y.; Li, J.; Huang, Q.; Rao, X. Staphylococcus aureus small-colony variants: Formation, infection, and treatment. Microbiol. Res. 2022, 260, 127040. [Google Scholar] [CrossRef] [PubMed]
- Tuchscherr, L.; Löffler, B. Staphylococcus aureus dynamically adapts global regulators and virulence factor expression in the course from acute to chronic infection. Curr. Genet. 2016, 62, 15–17. [Google Scholar] [CrossRef]
- Tuchscherr, L.; Geraci, J.; Löffler, B. Staphylococcus aureus Regulator Sigma B is Important to Develop Chronic Infections in Hematogenous Murine Osteomyelitis Model. Pathogens 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Huang, C.; Chen, X.; Chen, Y.; Huang, Z.; Zhang, C.; Zhang, W.; Fang, X. The role of Staphylococcus aureus small colony variants in intraosseous invasion and colonization in periprosthetic joint infection. Bone Jt. Res. 2022, 11, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Löffler, B.; Tuchscherr, L.; Niemann, S.; Peters, G. Staphylococcus aureus persistence in non-professional phagocytes. Int. J. Med. Microbiol. IJMM 2014, 304, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Raineri, E.J.M.; Yedavally, H.; Salvati, A.; van Dijl, J.M. Time-resolved analysis of Staphylococcus aureus invading the endothelial barrier. Virulence 2020, 11, 1623–1639. [Google Scholar] [CrossRef]
- Pidwill, G.R.; Gibson, J.F.; Cole, J.; Renshaw, S.A.; Foster, S.J. The Role of Macrophages in Staphylococcus aureus Infection. Front. Immunol. 2020, 11, 620339. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.P.; Clarke, R.S.; Kim, G.L.; Brittan, J.L.; Rowley, J.E.; Mavridou, D.A.I.; Parker, D.; Clarke, T.B.; Nobbs, A.H.; Edwards, A.M. Staphylococcal DNA Repair Is Required for Infection. mBio 2020, 11, e02288-20. [Google Scholar] [CrossRef]
- Cordero, M.; García-Fernández, J.; Acosta, I.C.; Yepes, A.; Avendano-Ortiz, J.; Lisowski, C.; Oesterreicht, B.; Ohlsen, K.; Lopez-Collazo, E.; Förstner, K.U.; et al. The induction of natural competence adapts staphylococcal metabolism to infection. Nat. Commun. 2022, 13, 1525. [Google Scholar] [CrossRef]
- Gao, P.; Davies, J.; Kao, R.Y.T. Dehydrosqualene Desaturase as a Novel Target for Anti-Virulence Therapy against Staphylococcus aureus. mBio 2017, 8, e01224-17. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I.; Lushchak, O. Interplay between reactive oxygen and nitrogen species in living organisms. Chem. -Biol. Interact. 2021, 349, 109680. [Google Scholar] [CrossRef]
- Grayczyk, J.P.; Alonzo, F., 3rd. Staphylococcus aureus Lipoic Acid Synthesis Limits Macrophage Reactive Oxygen and Nitrogen Species Production To Promote Survival during Infection. Infect. Immun. 2019, 87, e00344-19. [Google Scholar] [CrossRef]
- Nobre, L.S.; Gonçalves, V.L.; Saraiva, L.M. Flavohemoglobin of Staphylococcus aureus. Methods Enzymol. 2008, 436, 203–216. [Google Scholar] [CrossRef]
- Ryu, S.; Song, P.I.; Seo, C.H.; Cheong, H.; Park, Y. Colonization and infection of the skin by S. aureus: Immune system evasion and the response to cationic antimicrobial peptides. Int. J. Mol. Sci. 2014, 15, 8753–8772. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Faulhaber, A.; Sieber, C.; Pfeifer, D.; Hochberg, T.; Gansz, M.; Deshmukh, S.D.; Dauth, S.; Brix, K.; Saftig, P.; et al. The endolysosomal cysteine cathepsins L and K are involved in macrophage-mediated clearance of Staphylococcus aureus and the concomitant cytokine induction. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2014, 28, 162–175. [Google Scholar] [CrossRef]
- Bayer, J.; Becker, J.; Liu, X.; Gritsch, L.; Daiber, E.; Korn, N.; Oesterhelt, F.; Fraunholz, M.; Weber, A.; Wolz, C. Differential survival of Staphylococcal species in macrophages. Mol. Microbiol. 2024, 121, 470–480. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Kuiack, R.C.; McGavin, M.J.; Heinrichs, D.E. Staphylococcus aureus Uses the GraXRS Regulatory System To Sense and Adapt to the Acidified Phagolysosome in Macrophages. mBio 2018, 9, e01143-18. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Li, J.; Zhou, Y.; Wang, J.; Li, J.; Cui, L.; Meng, X.; Zhu, G.; Wang, H. Staphylococcus aureus facilitates its survival in bovine macrophages by blocking autophagic flux. J. Cell. Mol. Med. 2020, 24, 3460–3468. [Google Scholar] [CrossRef] [PubMed]
- Vozza, E.G.; Mulcahy, M.E.; McLoughlin, R.M. Making the Most of the Host; Targeting the Autophagy Pathway Facilitates Staphylococcus aureus Intracellular Survival in Neutrophils. Front. Immunol. 2021, 12, 667387. [Google Scholar] [CrossRef]
- Gannoun-Zaki, L.; Pätzold, L.; Huc-Brandt, S.; Baronian, G.; Elhawy, M.I.; Gaupp, R.; Martin, M.; Blanc-Potard, A.B.; Letourneur, F.; Bischoff, M.; et al. PtpA, a secreted tyrosine phosphatase from Staphylococcus aureus, contributes to virulence and interacts with coronin-1A during infection. J. Biol. Chem. 2018, 293, 15569–15580. [Google Scholar] [CrossRef] [PubMed]
- Youssouf, N.; Martin, M.; Bischoff, M.; Soubeyran, P.; Gannoun-Zaki, L.; Molle, V. The secreted tyrosine phosphatase PtpA promotes Staphylococcus aureus survival in RAW 264.7 macrophages through decrease of the SUMOylation host response. Microbiol. Spectr. 2023, 11, e0281323. [Google Scholar] [CrossRef]
- Elhawy, M.I.; Huc-Brandt, S.; Pätzold, L.; Gannoun-Zaki, L.; Abdrabou, A.M.M.; Bischoff, M.; Molle, V. The Phosphoarginine Phosphatase PtpB from Staphylococcus aureus Is Involved in Bacterial Stress Adaptation during Infection. Cells 2021, 10, 645. [Google Scholar] [CrossRef]
- Elhawy, M.I.; Molle, V.; Becker, S.L.; Bischoff, M. The Low-Molecular Weight Protein Arginine Phosphatase PtpB Affects Nuclease Production, Cell Wall Integrity, and Uptake Rates of Staphylococcus aureus by Polymorphonuclear Leukocytes. Int. J. Mol. Sci. 2021, 22, 5342. [Google Scholar] [CrossRef]
- Ahmad-Mansour, N.; Elhawy, M.I.; Huc-Brandt, S.; Youssouf, N.; Pätzold, L.; Martin, M.; Abdel-Wadood, N.; Aljohmani, A.; Morsli, M.; Krasteva-Christ, G.; et al. Characterization of the Secreted Acid Phosphatase SapS Reveals a Novel Virulence Factor of Staphylococcus aureus That Contributes to Survival and Virulence in Mice. Int. J. Mol. Sci. 2022, 23, 4031. [Google Scholar] [CrossRef]
- Caire, R.; Audoux, E.; Thomas, M.; Dalix, E.; Peyron, A.; Rodriguez, K.; Pordone, N.; Guillemot, J.; Dickerscheit, Y.; Marotte, H.; et al. YAP promotes cell-autonomous immune responses to tackle intracellular Staphylococcus aureus in vitro. Nat. Commun. 2022, 13, 6995. [Google Scholar] [CrossRef] [PubMed]
- Geng, N.; Wang, X.; Yu, X.; Wang, R.; Zhu, Y.; Zhang, M.; Liu, J.; Liu, Y. Staphylococcus aureus Avoids Autophagy Clearance of Bovine Mammary Epithelial Cells by Impairing Lysosomal Function. Front. Immunol. 2020, 11, 746. [Google Scholar] [CrossRef]
- Gauron, M.C.; Newton, A.C.; Colombo, M.I. PKCα Is Recruited to Staphylococcus aureus-Containing Phagosomes and Impairs Bacterial Replication by Inhibition of Autophagy. Front. Immunol. 2021, 12, 662987. [Google Scholar] [CrossRef] [PubMed]
- Abad, L.; Chauvelot, P.; Audoux, E.; Andre, C.; Josse, J.; Dupieux, C.; Lustig, S.; Ferry, T.; Verhoeven, P.O.; Diot, A.; et al. Lysosomal alkalization to potentiate eradication of intra-osteoblastic Staphylococcus aureus in the bone and joint infection setting. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2022, 28, e131–e135. [Google Scholar] [CrossRef]
- Schröder, A.; Kland, R.; Peschel, A.; von Eiff, C.; Aepfelbacher, M. Live cell imaging of phagosome maturation in Staphylococcus aureus infected human endothelial cells: Small colony variants are able to survive in lysosomes. Med. Microbiol. Immunol. 2006, 195, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Homma, F.; Huang, J.; van der Hoorn, R.A.L. AlphaFold-Multimer predicts cross-kingdom interactions at the plant-pathogen interface. Nat. Commun. 2023, 14, 6040. [Google Scholar] [CrossRef] [PubMed]
| Toxicogenic Proteins (Effectors) | Target Host Factors | Reference | |
|---|---|---|---|
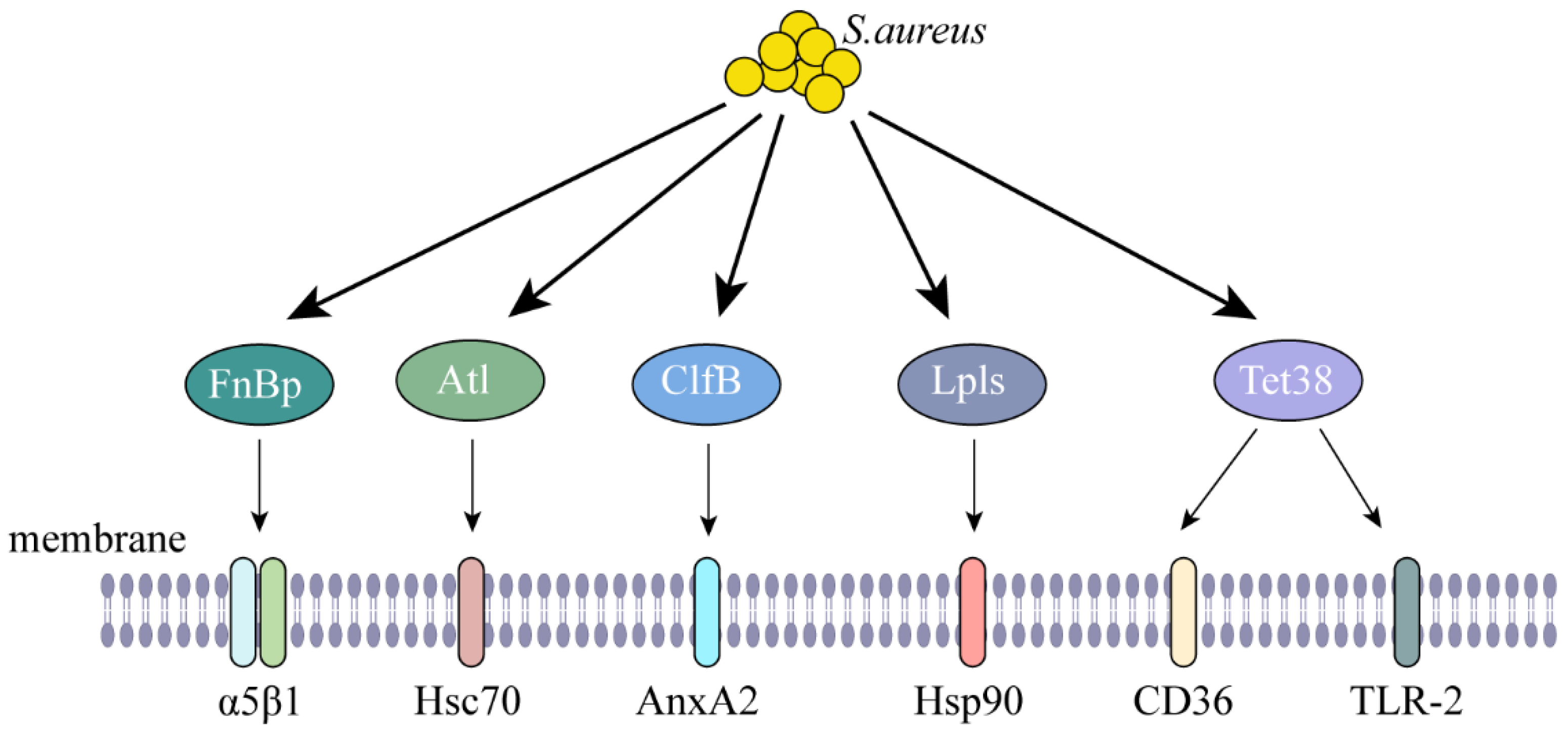
| Internalization | FnBp | α5β1 | [9] |
| Atl | Hsc70 | [10] | |
| ClfB | AnxA2 | [11] | |
| Lpls | Hsp90 | [12] | |
| Tet38 | CD36, TLR2 | [13] | |
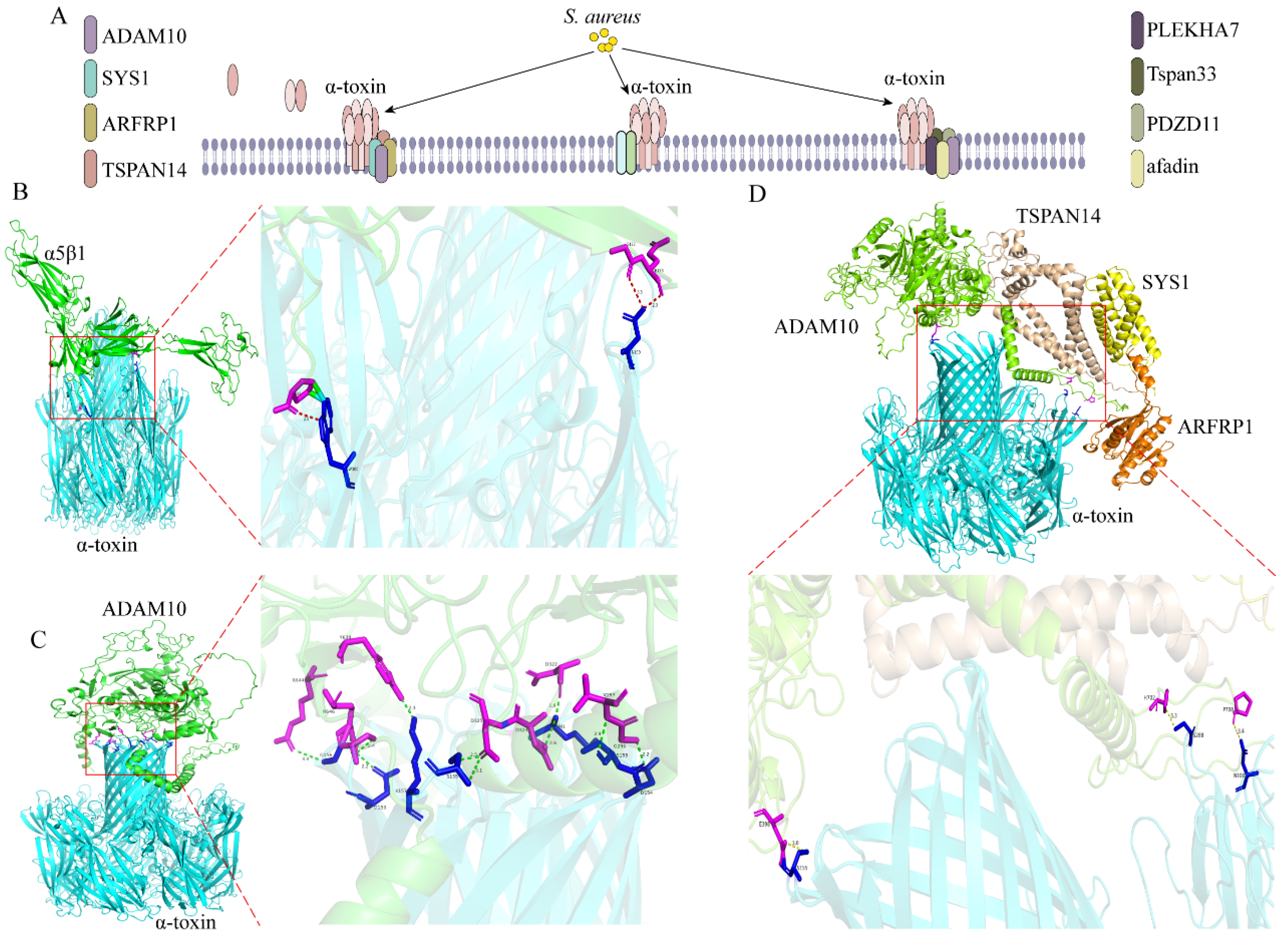
| alpha-hemolysin (α-toxin) | α-toxin | ADAM10 | [14,15] |
| α5β1 | [16] | ||
| SYS1, ARFRP1, TSPAN14 | [17] | ||
| Tspan33, PDZD11, PLEKHA7, afadin | [18,19] | ||
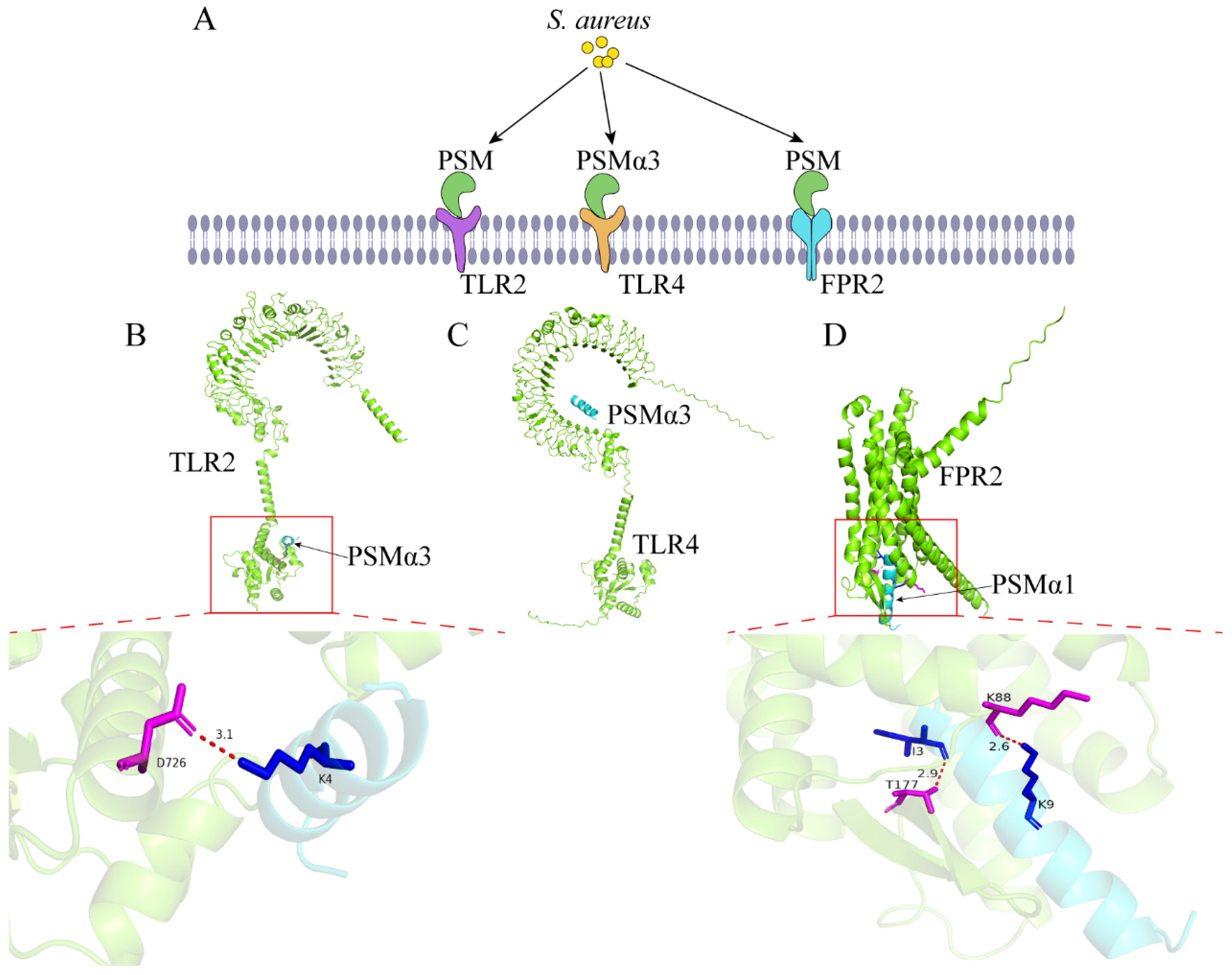
| Phenol soluble modulins | PSM | TLR4 | [20] |
| FPR2 | [21] | ||
| PSMα3 | TLR2, TLR4 | [22] | |
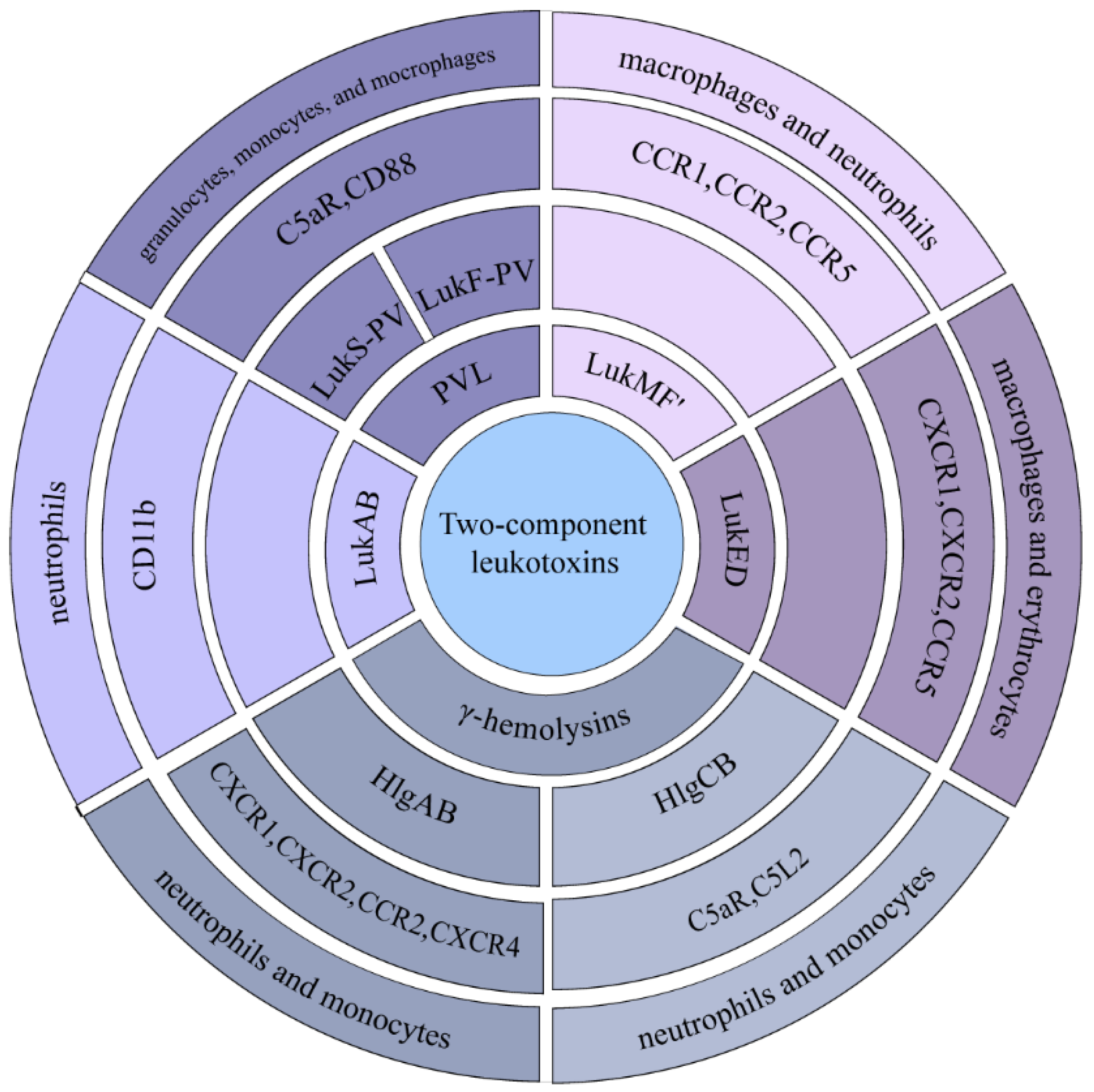
| Two-component leukotoxins | HlgAB | CXCR1, CXCR2, CCR2, CXCR4 | [23] |
| HlgCB | C5aR, C5L2 | [23] | |
| LukED | CCR5 | [15,24] | |
| CXCR1, CXCR2 | [25] | ||
| LukMF’ | CCR1, CCR2, CCR5 | [26] | |
| PVL | C5aR, CD88 | [27] | |
| LukAB | CD11b | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Wang, S.; Liu, X.; Li, M.; Wang, X.; Chen, H.; Qu, C.; Liu, Y.; Liu, J. Strategies for Survival of Staphylococcus aureus in Host Cells. Int. J. Mol. Sci. 2025, 26, 720. https://doi.org/10.3390/ijms26020720
Xu H, Wang S, Liu X, Li M, Wang X, Chen H, Qu C, Liu Y, Liu J. Strategies for Survival of Staphylococcus aureus in Host Cells. International Journal of Molecular Sciences. 2025; 26(2):720. https://doi.org/10.3390/ijms26020720
Chicago/Turabian StyleXu, Huiling, Shengnan Wang, Xiaoting Liu, Muzi Li, Xiaozhou Wang, Huahua Chen, Chaonan Qu, Yongxia Liu, and Jianzhu Liu. 2025. "Strategies for Survival of Staphylococcus aureus in Host Cells" International Journal of Molecular Sciences 26, no. 2: 720. https://doi.org/10.3390/ijms26020720
APA StyleXu, H., Wang, S., Liu, X., Li, M., Wang, X., Chen, H., Qu, C., Liu, Y., & Liu, J. (2025). Strategies for Survival of Staphylococcus aureus in Host Cells. International Journal of Molecular Sciences, 26(2), 720. https://doi.org/10.3390/ijms26020720







