Advancements and Perspectives in Biodegradable Polyester Elastomers: Toward Sustainable and High-Performance Materials
Abstract
1. Introduction
2. Bio-Based Polyester Elastomers
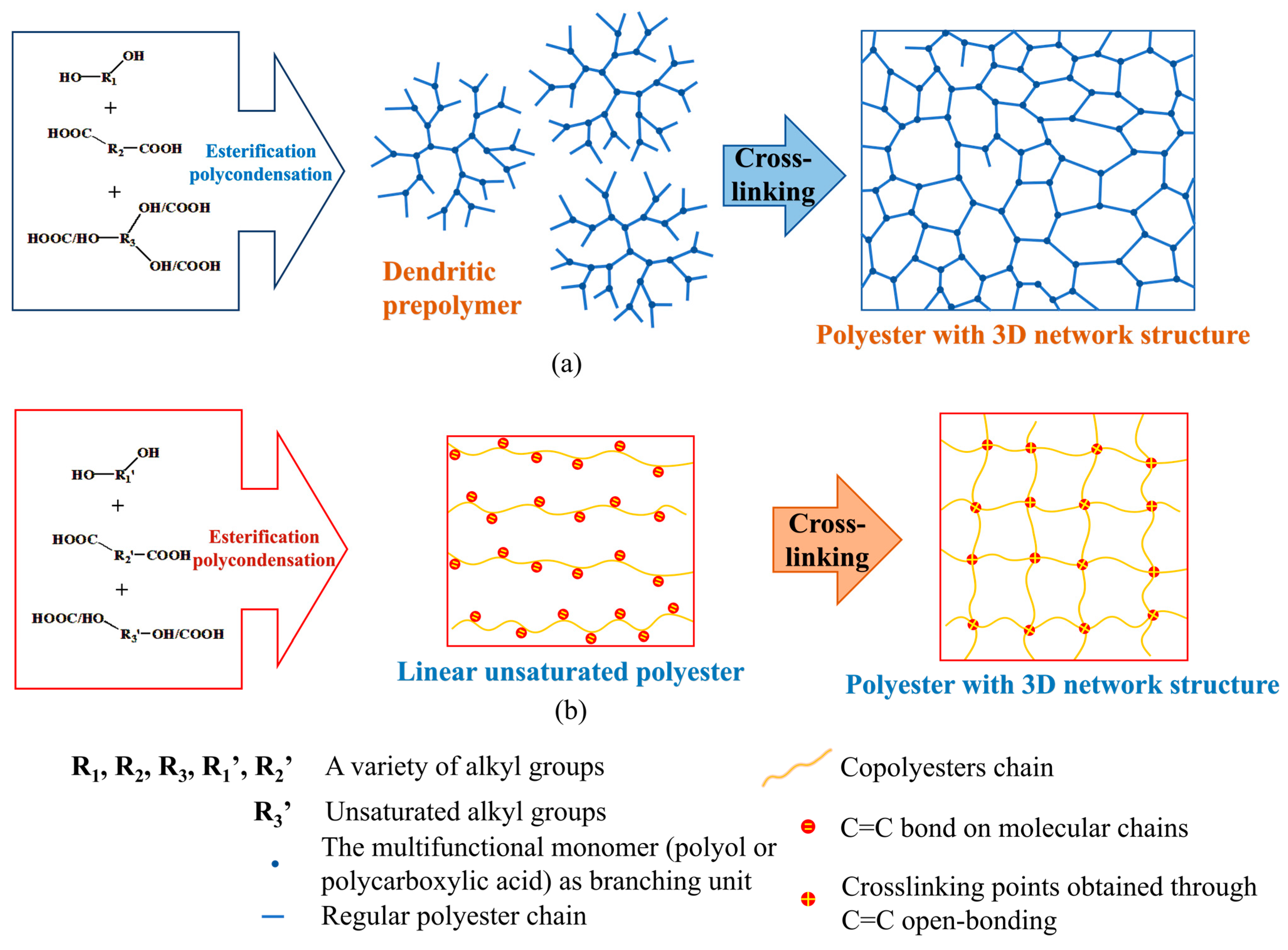
2.1. In Situ Cross-Linked Polyester Elastomer
2.1.1. Glycerol Based Polyester Elastomer
2.1.2. Citric-Acid-Based Polyester Elastomer
2.2. Polyolefin Cross-Linked Polyester Elastomers
3. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- L’Heveder, S.; Sportelli, F.; Isitman, N.A. Investigation of solubility in plasticised rubber systems for tire applications. Plast. Rubber Compos. 2016, 45, 319–325. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, J.; Han, L.; Ma, X.; Chen, W. Mechanism and Influence Factors of Abrasion Resistance of High-Flow Grade SEBS/PP Blended Thermoplastic Elastomer. Polymers 2022, 14, 1795. [Google Scholar] [CrossRef] [PubMed]
- Yoda, R. Elastomers for biomedical applications. J. Biomater. Sci. Polym. Ed. 1998, 9, 561–626. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Gao, X.; Chen, Z.; Feng, Y.; Liu, G.; Zhou, F.; Liu, W. Super-lubricating hybrid elastomer with rapid photothermal sterilization and strong anti-cell adhesion. Chem. Eng. J. 2022, 434, 134763. [Google Scholar] [CrossRef]
- Zhang, Y.; Ellingford, C.; Zhang, R.; Roscow, J.; Hopkins, M.; Keogh, P.; McNally, T.; Bowen, C.; Wan, C. Electrical and Mechanical Self-Healing in High-Performance Dielectric Elastomer Actuator Materials. Adv. Funct. Mater. 2019, 29, 1808431. [Google Scholar] [CrossRef]
- Gu, G.-Y.; Zhu, J.; Zhu, L.-M.; Zhu, X. A survey on dielectric elastomer actuators for soft robots. Bioinspir. Biomim. 2017, 12, 011003. [Google Scholar] [CrossRef] [PubMed]
- Benavides, P.T.; Lee, U.; Zarè-Mehrjerdi, O. Life cycle greenhouse gas emissions and energy use of polylactic acid, bio-derived polyethylene, and fossil-derived polyethylene. J. Clean. Prod. 2020, 277, 124010. [Google Scholar] [CrossRef]
- Benavides, P.T.; Dunn, J.B.; Han, J.; Biddy, M.; Markham, J. Exploring Comparative Energy and Environmental Benefits of Virgin, Recycled, and Bio-Derived PET Bottles. ACS Sustain. Chem. Eng. 2018, 6, 9725–9733. [Google Scholar] [CrossRef]
- Kim, T.; Bamford, J.; Gracida-Alvarez, U.R.; Benavides, P.T. Life Cycle Greenhouse Gas Emissions and Water and Fossil-Fuel Consumptions for Polyethylene Furanoate and Its Coproducts from Wheat Straw. ACS Sustain. Chem. Eng. 2022, 10, 2830–2843. [Google Scholar] [CrossRef]
- Kim, M.-N.; Kim, K.-H.; Jin, H.-J.; Park, J.-K.; Yoon, J.-S. Biodegradability of ethyl and n-octyl branched poly(ethylene adipate) and poly(butylene succinate). Eur. Polym. J. 2001, 37, 1843–1847. [Google Scholar] [CrossRef]
- Taib, N.-A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Qin, Q.; Yang, Y.; Yang, C.; Zhang, L.; Yin, H.; Yu, F.; Ma, J. Degradation and adsorption behavior of biodegradable plastic PLA under conventional weathering conditions. Sci. Total Environ. 2022, 842, 156775. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.V.; Petronyuk, Y.S.; Guseynov, N.A.; Tereshchuk, S.V.; Popov, A.A.; Volkov, A.V.; Gorshenev, V.N.; Olkhov, A.A.; Levin, V.M.; Dymnikov, A.B.; et al. Biocompatibility and Bioresorption of 3D-Printed Polylactide and Polyglycolide Tissue Membranes. Bull. Exp. Biol. Med. 2021, 170, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhao, Q.; Niu, Y.; Luo, C.; Zhang, Z.; He, B.; Hao, H. Synthesis, characterization, thermal stability, and in vitro and in vivo degradation study of polycaprolactone and polyglycolide block copolymers. J. Biomater. Sci. Polym. Ed. 2023, 34, 302–314. [Google Scholar] [CrossRef]
- Archer, E.; Torretti, M.; Madbouly, S. Biodegradable polycaprolactone (PCL) based polymer and composites. Phys. Sci. Rev. 2021, 8, 4391–4414. [Google Scholar] [CrossRef]
- Thakur, M.; Majid, I.; Hussain, S.; Nanda, V. Poly(ε-caprolactone): A potential polymer for biodegradable food packaging applications. Packag. Technol. Sci. 2021, 34, 449–461. [Google Scholar] [CrossRef]
- Rai, R.; Tallawi, M.; Grigore, A.; Boccaccini, A.R. Synthesis, properties and biomedical applications of poly(glycerol sebacate) (PGS): A review. Prog. Polym. Sci. 2012, 37, 1051–1078. [Google Scholar] [CrossRef]
- Chon, Y.J.; Koo, J.M.; Park, Y.J.; Hwang, S.Y.; Jung, Y.M.; Im, S.S. Synthesis of a high-performance citric acid-based polyester elastomer by a hot-pressing technique. Polymer 2017, 125, 283–291. [Google Scholar] [CrossRef]
- Godinho, B.; Gama, N.; Ferreira, A. Different methods of synthesizing poly(glycerol sebacate) (PGS): A review. Front. Bioeng. Biotechnol. 2022, 10, 1033827. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Energy. Report: Top Value Added Chemicals from Biomass, Volume I: Results of Screening for Potential Candidates from Sugars and Synthesis Gas. 2004. Available online: http://www.osti.gov/bridge (accessed on 4 December 2024).
- Vogt, L.; Ruther, F.; Salehi, S.; Boccaccini, A.R. Poly(glycerol sebacate) in Biomedical Applications—A Review of the Recent Literature. Adv. Healthc. Mater. 2021, 10, 2002026. [Google Scholar] [CrossRef] [PubMed]
- Piszko, P.; Kryszak, B.; Piszko, A.; Szustakiewicz, K. Brief review on poly(glycerol sebacate) as an emerging polyester in biomedical application: Structure, properties and modifications. Polim. W Med. 2021, 51, 43–50. [Google Scholar] [CrossRef]
- Lian, H.; Meng, Z. Fabrication, characterization and osteoblast responses of poly(octanediol citrate)/bioglass nanofiber composites. Mater. Sci. Eng. C 2018, 84, 123–129. [Google Scholar] [CrossRef]
- Wei, T.; Lei, L.; Kang, H.; Qiao, B.; Wang, Z.; Zhang, L.; Coates, P.; Hua, K.; Kulig, J. Tough Bio-Based Elastomer Nanocomposites with High Performance for Engineering Applications. Adv. Eng. Mater. 2012, 14, 112–118. [Google Scholar] [CrossRef]
- Zhang, H.; Grinstaff, M.W. Recent Advances in Glycerol Polymers: Chemistry and Biomedical Applications. Macromol. Rapid Commun. 2014, 35, 1906–19024. [Google Scholar] [CrossRef]
- Valerio, O.; Misra, M.; Mohanty, A.K. Poly(glycerol-co-diacids) Polyesters: From Glycerol Biorefinery to Sustainable Engineering Applications, A Review. ACS Sustain. Chem. Eng. 2018, 6, 5681–5693. [Google Scholar] [CrossRef]
- Migneco, F.; Huang, Y.-C.; Birla, R.K.; Hollister, S.J. Poly(glycerol-dodecanoate), a biodegradable polyester for medical devices and tissue engineering scaffolds. Biomaterials 2009, 30, 6479–6484. [Google Scholar] [CrossRef] [PubMed]
- Swainson, S.M.E.; Styliari, I.D.; Taresco, V.; Garnett, M.C. Poly (glycerol adipate) (PGA), an Enzymatically Synthesized Functionalizable Polyester and Versatile Drug Delivery Carrier: A Literature Update. Polymers 2019, 11, 1561. [Google Scholar] [CrossRef]
- Chongcharoenchaikul, T.; Thamyongkit, P.; Poompradub, S. Synthesis, characterization and properties of a bio-based poly(glycerol azelate) polyester. Mater. Chem. Phys. 2016, 177, 485–495. [Google Scholar] [CrossRef]
- Loh, X.J.; Abdul Karim, A.; Owh, C. Poly(glycerol sebacate) biomaterial: Synthesis and biomedical applications. J. Mater. Chem. B 2015, 3, 7641–7652. [Google Scholar] [CrossRef]
- Sha, D.; Wu, Z.; Zhang, J.; Ma, Y.; Yang, Z.; Yuan, Y. Development of modified and multifunctional poly(glycerol sebacate) (PGS)-based biomaterials for biomedical applications. Eur. Polym. J. 2021, 161, 110830. [Google Scholar] [CrossRef]
- Wang, Y.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.; Hu, H.; Geng, C.; Song, J.; Shen, Y.; Lei, D.; Guan, Q.; Zhao, S.; You, Z. Biofunctionalized chondrogenic shape-memory ternary scaffolds for efficient cell-free cartilage regeneration. Acta Biomater. 2020, 105, 97–110. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Q.; Wang, L.; Zhang, Y.; Liu, W.; Zhao, S.; Geng, X.; Fan, Y. A novel biomimetic nanofibrous cardiac tissue engineering scaffold with adjustable mechanical and electrical properties based on poly(glycerol sebacate) and polyaniline. Mater. Today Bio 2023, 23, 100798. [Google Scholar] [CrossRef]
- Piszko, P.; Kryszak, B.; Gazińska, M.; Słota, D.; Sobczak-Kupiec, A.; Włodarczyk, M.; Szwed-Georgiou, A.; Rudnicka, K.; Szustakiewicz, K. The effect of filler content on mechanical properties and cell response of elastomeric PGS/apatite foam scaffolds. Ceram. Int. 2023, 49, 25353–25363. [Google Scholar] [CrossRef]
- Godinho, B.; Gama, N.; Barros-Timmons, A.; Ferreira, A. Enzymatic synthesis of poly(glycerol sebacate) pre-polymer with crude glycerol, by-product from biodiesel prodution. AIP Conf. Proc. 2018, 1981, 020031. [Google Scholar]
- Lang, K.; Bhattacharya, S.; Ning, Z.; Sánchez-Leija, R.J.; Bramson, M.T.K.; Centore, R.; Corr, D.T.; Linhardt, R.J.; Gross, R.A. Enzymatic Polymerization of Poly(glycerol-1,8-octanediol-sebacate): Versatile Poly(glycerol sebacate) Analogues that Form Monocomponent Biodegradable Fiber Scaffolds. Biomacromolecules 2020, 21, 3197–3206. [Google Scholar] [CrossRef] [PubMed]
- Perin, G.B.; Felisberti, M.I. Enzymatic Synthesis of Poly(glycerol sebacate): Kinetics, Chain Growth, and Branching Behavior. Macromolecules 2020, 53, 7925–7935. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Lang, K.; Xia, K.; Linhardt, R.J.; Gross, R.A. Lipase-Catalyzed Synthesis and Characterization of Poly(glycerol sebacate). Biomacromolecules 2022, 23, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Aydin, H.M.; Salimi, K.; Rzayev, Z.M.O.; Pişkin, E. Microwave-assisted rapid synthesis of poly(glycerol-sebacate) elastomers. Biomater. Sci. 2013, 1, 503–509. [Google Scholar] [CrossRef]
- Lau, C.; Al Qaysi, M.; Owji, N.; Bayazit, M.; Xie, J.; Knowles, J.; Tang, J. Advanced biocomposites of poly(glycerol sebacate) and β-tricalcium phosphate by in situ microwave synthesis for bioapplication. Mater. Today Adv. 2020, 5, 100023. [Google Scholar] [CrossRef]
- Tevlek, A.; Agacik, D.T.; Aydin, H.M. Stretchable poly(glycerol-sebacate)/β-tricalcium phosphate composites with shape recovery feature by extrusion. J. Appl. Polym. Sci. 2020, 137, 48689. [Google Scholar] [CrossRef]
- Lau, C.C.; Bayazit, M.K.; Knowles, J.C.; Tang, J. Tailoring degree of esterification and branching of poly(glycerol sebacate) by energy efficient microwave irradiation. Polym. Chem. 2017, 8, 3937–3947. [Google Scholar] [CrossRef]
- Conejero-García, Á.; Gimeno, H.R.; Sáez, Y.M.; Vilariño-Feltrer, G.; Ortuño-Lizarán, I.; Vallés-Lluch, A. Correlating synthesis parameters with physicochemical properties of poly(glycerol sebacate). Eur. Polym. J. 2017, 87, 406–419. [Google Scholar] [CrossRef]
- Piszko, P.; Kryszak, B.; Szustakiewicz, K. Influence of cross-linking time on physico-chemical and mechanical properties of bulk poly(glycerol sebacate). Acta Bioeng. Biomech. 2022, 24, 85–93. [Google Scholar] [CrossRef]
- Golbaten-Mofrad, H.; Sahzabi, A.S.; Seyfikar, S.; Salehi, M.H.; Goodarzi, V.; Wurm, F.R.; Jafari, S.H. Facile template preparation of novel electroactive scaffold composed of polypyrrole-coated poly(glycerol-sebacate-urethane) for tissue engineering applications. Eur. Polym. J. 2021, 159, 110749. [Google Scholar] [CrossRef]
- Risley, B.B.; Ding, X.; Chen, Y.; Miller, P.G.; Wang, Y. Citrate Crosslinked Poly(glycerol sebacate) with Tunable Elastomeric Properties. Macromol. Biosci. 2021, 21, 2000301. [Google Scholar] [CrossRef]
- Pashneh-Tala, S.; Moorehead, R.; Claeyssens, F. Hybrid manufacturing strategies for tissue engineering scaffolds using methacrylate functionalised poly(glycerol sebacate). J. Biomater. Appl. 2020, 34, 1114–1130. [Google Scholar] [CrossRef] [PubMed]
- Becerril-Rodriguez, I.C.; Claeyssens, F. Low methacrylated poly(glycerol sebacate) for soft tissue engineering. Polym. Chem. 2022, 13, 3513–3528. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Ouyang, L.; Highley, C.B.; Burdick, J.A. Norbornene-modified poly(glycerol sebacate) as a photocurable and biodegradable elastomer. Polym. Chem. 2017, 8, 5091–5099. [Google Scholar] [CrossRef]
- Agach, M.; Delbaere, S.; Marinkovic, S.; Estrine, B.; Nardello-Rataj, V. Characterization, stability and ecotoxic properties of readily biodegradable branched oligoesters based on bio-sourced succinic acid and glycerol. Polym. Degrad. Stab. 2012, 97, 1956–1963. [Google Scholar] [CrossRef]
- Eirini, N.; Maria, L.; Georgia, P.; Anna, M.; Ioannis, T.; Liliana, L.; Marcela, A.; Anastasia, B.; Aldo, B.; Eleana, K.; et al. Poly(Glycerol Succinate) as Coating Material for 1393 Bioactive Glass Porous Scaffolds for Tissue Engineering Applications. Polymers 2022, 14, 5028. [Google Scholar] [CrossRef] [PubMed]
- Shukla, K.; Arunagiri, A.; Muthukumar, K. Synthesis and hydrolytic degradation of poly (glycerol succinate) based polyesters. J. Indian Chem. Soc. 2023, 100, 100841. [Google Scholar] [CrossRef]
- Mahtabi, R.; Benisi, S.; Goodarzi, V.; Shojaei, S. Application of Biodegradable Bone Scaffolds Based on Poly(Lactic Acid) / Poly(Glycerol Succinic Acid) Containing Nano-Hydroxyapatite. J. Polym. Environ. 2024, 32, 548–559. [Google Scholar] [CrossRef]
- Godinho, B.; Nogueira, R.; Gama, N.; Ferreira, A. Synthesis and Characterization of Poly(glycerol sebacate), Poly(glycerol succinate) and Poly(glycerol sebacate-co-succinate). J. Polym. Environ. 2024, 32, 4330–4347. [Google Scholar] [CrossRef]
- Amarasekara, A.; Razzaq, A.; Bonham, P. Synthesis and Characterization of All Renewable ResourcesBased Branched Polyester: Poly(2,5-furandicarboxylicacid-co-glycerol). Int. Sch. Res. Not. 2013, 2013, 645169. [Google Scholar]
- Medeiros, E.; Offeman, R.; Klamczynski, A.; Glenn, G.; Mattoso, L.; Orts, W. Synthesis, Characterization and Nanocomposite Formation of Poly(glycerol succinate-co-maleate) with Nanocrystalline Cellulose. J. Polym. Environ. 2014, 22, 219–226. [Google Scholar] [CrossRef]
- Yang, J.; Webb, A.R.; Ameer, G.A. Novel Citric Acid-Based Biodegradable Elastomers for Tissue Engineering. Adv. Mater. 2004, 16, 511–516. [Google Scholar] [CrossRef]
- Wang, Q.-Z.; Zhang, H.; Ding, T.; Yuan, N.-X.; Wang, Y.-S. Synthesis and characterization of a novel biodegradable elastomer based on citric-acid-crosslinked polyesters. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 475–482. [Google Scholar] [CrossRef]
- Yu, L.; He, W.; Peters, E.B.; Ledford, B.T.; Tsihlis, N.D.; Kibbe, M.R. Development of Poly(1,8-octanediol-co-citrate-co-ascorbate) Elastomers with Enhanced Ascorbate Performance for Use as a Graft Coating to Prevent Neointimal Hyperplasia. ACS Appl. Bio Mater. 2020, 3, 2150–2159. [Google Scholar] [CrossRef]
- Koper, F.; Świergosz, T.; Żaba, A.; Flis, A.; Trávníčková, M.; Bačáková, L.; Pamuła, E.; Bogdał, D.; Kasprzyk, W.P. Advancements in structure–property correlation studies of cross-linked citric acid-based elastomers from the perspective of medical application. J. Mater. Chem. B 2021, 9, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, Y.; Ji, Y. Fabricating poly(1,8-octanediol citrate) elastomer based fibrous mats via electrospinning for soft tissue engineering scaffold. J. Mater. Sci. Mater. Med. 2017, 28, 93. [Google Scholar] [CrossRef]
- Liang, K.; Zhou, Y.; Ji, Y. Full biodegradable elastomeric nanocomposites fabricated by chitin nanocrystal and poly(caprolactone-diol citrate) elastomer. J. Bioact. Compat. Polym. 2019, 34, 453–463. [Google Scholar] [CrossRef]
- Guo, J.; Xie, Z.; Tran, R.T.; Xie, D.; Jin, D.; Bai, X.; Yang, J. Click Chemistry Plays a Dual Role in Biodegradable Polymer Design. Adv. Mater. 2014, 26, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Zeimaran, E.; Pourshahrestani, S.; Pingguan-Murphy, B.; Kong, D.; Naveen, S.V.; Kamarul, T.; Kadri, N.A. Development of poly (1, 8-octanediol citrate)/chitosan blend films for tissue engineering applications. Carbohydr. Polym. 2017, 175, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.H.; Zhao, H.Y.; Cui, Y.; Ao, X.; Li, A.L.; Zhang, Z.M.; Qiu, D. Poly(1,8-octanediol citrate)/bioactive glass composite with improved mechanical performance and bioactivity for bone regeneration. Chin. Chem. Lett. 2017, 28, 2116–2120. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Ge, J.; Ma, P.X.; Lei, B. In situ silica nanoparticles-reinforced biodegradable poly(citrate-siloxane) hybrid elastomers with multifunctional properties for simultaneous bioimaging and bone tissue regeneration. Appl. Mater. Today 2018, 10, 153–163. [Google Scholar] [CrossRef]
- Piątek-Hnat, M.; Bomba, K.; Pęksiński, J. Structure and Properties of Biodegradable Poly (Xylitol Sebacate-Co-Butylene Sebacate) Copolyester. Molecules 2020, 25, 1541. [Google Scholar] [CrossRef]
- Firoozi, N.; Kang, Y. A Highly Elastic and Autofluorescent Poly(xylitol-dodecanedioic Acid) for Tissue Engineering. ACS Biomater. Sci. Eng. 2019, 5, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Geeti, D.K.; Niranjan, K. Environmentally benign bio-based waterborne polyesters: Synthesis, thermal-and bio-degradation studies. Prog. Org. Coat. 2019, 127, 419–428. [Google Scholar] [CrossRef]
- Piątek-Hnat, M.; Bomba, K.; Pęksiński, J. Synthesis and Selected Properties of Ester Elastomer Containing Sorbitol. Appl. Sci. 2020, 10, 1628. [Google Scholar] [CrossRef]
- Rahmani, M.; Khani, M.-M.; Rabbani, S.; Mashaghi, A.; Noorizadeh, F.; Faridi-Majidi, R.; Ghanbari, H. Development of poly (mannitol sebacate)/poly (lactic acid) nanofibrous scaffolds with potential applications in tissue engineering. Mater. Sci. Eng. C 2020, 110, 110626. [Google Scholar] [CrossRef]
- Kang, H.; Li, M.; Tang, Z.; Xue, J.; Hu, X.; Zhang, L.; Guo, B. Synthesis and characterization of Bio-based isosorbide-containing copolyesters as shape memory polymers for biomedical applications. J. Mater. Chem. B 2014, 2, 7877–7886. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zai, Y.; Yang, D.; Qiu, L.; Niu, C. Bio-based elastomer nanoparticles with controllable biodegradability. RSC Adv. 2016, 6, 102142–102148. [Google Scholar] [CrossRef]
- Hu, X.; Shen, X.; Huang, M.; Liu, C.; Geng, Y.; Wang, R.; Xu, R.; Qiao, H.; Zhang, L. Biodegradable unsaturated polyesters containing2,3-butanediol for engineering applications: Synthesis, characterization and performances. Polymer 2016, 84, 343–354. [Google Scholar] [CrossRef]
- Kang, H.; Qiao, B.; Wang, R.; Wang, Z.; Zhang, L.; Ma, J.; Coates, P. Employing a novel bioelastomer to toughen polylactide. Polymer 2013, 54, 2450–2458. [Google Scholar] [CrossRef]
- Hu, X.; Kang, H.; Li, Y.; Geng, Y.; Wang, R.; Zhang, L. Preparation, morphology and superior performances of Bio-based thermoplastic elastomer by in situ dynamical vulcanization for 3D-printed materials. Polymer 2017, 108, 11–20. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, H.; Shen, Z.; Guo, B.; Zhang, L.; Jia, D. Grafting of Polyester onto Graphene for Electrically and Thermally Conductive Composites. Macromolecules 2012, 45, 3444–3451. [Google Scholar] [CrossRef]
- Tang, L.; He, X.; Huang, R. Advanced Elasticity and Biodegradability of Bio-based Copolyester Elastomer achieved by the Crystallization Inhibition of Isosorbide and Flexibility of 1,6-Hexanediol. Polym. Bull. 2024, 1–12. [Google Scholar] [CrossRef]
- Tang, L.; Jin, Y.; He, X.; Huang, R. Biodegradable Poly(ethylene glycol-glycerol-itaconate-sebacate) Copolyester Elastomer with Significantly Reinforced Mechanical Properties by in-situ Construction of Bacterial Cellulose Interpenetrating Network. Sci. Rep. 2024, 14, 7172. [Google Scholar] [CrossRef] [PubMed]
| Study | Synthesis Method | Key Findings | Applications/Remarks |
|---|---|---|---|
| Wang et al. [32]; Xuan et al. [33]; Wu et al. [34]; Piszko et al. [35] | Polycondensation under reduced pressure | Early and promising method for synthesizing pre-PGS at high temperatures in an inert gas atmosphere. | Provides a strong foundation for producing PGS. |
| Godinho et al. [36]; Lang et al. [37] | Enzymatic synthesis | Mild conditions, high catalytic efficiency, and selectivity. | Suitable for environmentally friendly synthesis of PGS prepolymers. |
| Perin and Felisberti [38] | Enzymatic synthesis with CALB | Explored reaction kinetics, chain growth, and branching under various conditions. | Enhanced understanding of enzymatic polymerization parameters. |
| Ning et al. [39] | Enzymatic synthesis with N435 | Achieved higher-molecular-weight PGS compared to self-catalyzed polymerization. | Improved mechanical properties of PGS prepolymer. |
| Aydin et al. [40] | Microwave-assisted synthesis | Reduced pre-polymerization time for PGS. | Efficient and energy-saving synthesis technique. |
| Lau et al. [41] | Microwave synthesis of PGS/β-TCP composites | Successfully incorporated β-tricalcium phosphate nanoparticles into PGS. | Biomedical applications such as bone tissue engineering. |
| Tevlek et al. [42] | Microwave pre-polymerization and curing | Achieved PGS with good elasticity (elongation: 212.75 ± 37.25%, Young’s modulus: 0.09 ± 0.03 MPa). | Demonstrated the effectiveness of microwave synthesis for elastomer preparation. |
| Lau et al. [43] | Microwave-assisted synthesis | Produced highly branched pre-PGS, enabling faster cross-linking. | Enhances the production efficiency of crosslinked PGS. |
| Conejero-García et al. [44] | Thermal crosslinking | Correlated curing parameters with physicochemical properties of PGS. | Provides insights into controlling PGS mechanical properties through thermal treatment. |
| Golbaten-Mofrad et al. [46] | Chemical cross-linking with Sn(Oct)2 | Synthesized PGS-U with varied crosslinking densities using a mild and fast method. | Allows fine-tuning of PGS properties for specific applications. |
| Risley et al. [47] | Citric acid cross-linking | Introduced citric acid to accelerate cross-linking, reducing curing time. | Achieved similar properties to PGS in a fraction of the original time. |
| Pashneh-Tala et al. [48]; Becerril-Rodriguez et al. [49] | UV-curable pre-PGS | Functionalized PGS with double bonds (acrylation) for UV-curable crosslinking. | Quick and efficient elastomer formation under mild conditions. |
| Yeh et al. [50] | UV-curable pre-PGS | Functionalized PGS with double bonds (norbornenylation) for UV-curable crosslinking. | Quick and efficient elastomer formation under mild conditions. |
| Study | Synthesis Method | Key Findings | Applications/Remarks |
|---|---|---|---|
| Chon et al. [18] | Melt polycondensation | Citric acid and glycol monomers form a 3D network at 160 °C under nitrogen protection; good biodegradability and biocompatibility. | Versatile elastomer suitable for biomedical applications. |
| Yang et al. [58]; Wang et al. [59]; Yu et al. [60]; Koper et al. [61] | Direct melt polycondensation | Developed poly(citrate-1,8-octanediol) ester (POC) with excellent elasticity, biocompatibility, and flexibility. | Widely used in tissue engineering and biomedical applications. |
| Zhu et al. [62] | Electrospinning | Fabricated fibrous mats from poly(1,8-octanediol citrate) for tissue engineering. | Soft tissue scaffolds with biodegradable properties. |
| Liang et al. [63] | Biodegradable nanocomposite fabrication | Created nanocomposites using chitin nanocrystals and poly(caprolactone-diol citrate) elastomers. | Applications in biomedical engineering and biodegradable materials. |
| Guo et al. [64] | Thermal synchronous double cross-linking | Achieved POC elastomers with tensile strength exceeding 20 MPa by using azides and alkyne glycols. | Enhanced mechanical performance for biomedical and engineering applications. |
| Zeimaran et al. [65] | Composite synthesis with chitosan (CS) | Created POC/CS composites with adjustable mechanical properties; tensile strength reached 5.87 MPa. | Blend films for tissue engineering, mechanical properties dependent on CS concentration. |
| Ren et al. [66] | POC-based composite with bioactive glass | Enhanced crosslinking with calcium in bioactive glass, achieving a compressive strength of ~50 MPa. | Applications in bone regeneration and implants. |
| Li et al. [67] | In-situ nanoparticle reinforcement | Developed poly(citrate-siloxane) hybrid elastomers reinforced with silicon dioxide nanoparticles. | Multifunctional properties for bioimaging and bone tissue regeneration. |
| Piątek-Hnat et al. [68]; Firoozi et al. [69] | Melt polycondensation with xylitol | Synthesized biodegradable xylitol–sebacate copolyesters with tunable properties. | Potential for biomedical applications. |
| Geeti et al. [70]; Piątek-Hnat et al. [71] | Melt polycondensation with Sorbitol | Developed environmentally benign bio-based waterborne polyesters with good thermal and biodegradation properties. | Applications in coatings and environmentally friendly materials. |
| Rahmani et al. [72] | Development of poly(mannitol sebacate) nanofibers | Fabricated poly(mannitol sebacate)/poly(lactic acid) nanofibrous scaffolds for tissue engineering. | Suitable for soft tissue applications with biodegradable characteristics. |
| Study | Polyester Type | Synthesis Method | Key Monomers | Properties/Applications |
|---|---|---|---|---|
| Wei et al. [24] | PBPSIS Amorphous Polyester Elastomer | Condensation, vulcanization, and cross-linking with diisopropylene peroxide (DCP) | 1,4-butanediol, 1,3-propanediol, sebacic acid, itaconic acid, succinate | Initial development of cross-linked elastomers through classical vulcanization methods. Average molecular weight (Mn) ranging from 32,952 to 52,529 g/mol. |
| Kang et al. [73] | PBISI (Bio-based Shape Memory Polyester) | Copolymerization, and cross-linking | 1,4-butanediol, iso-sorbide, sebacic ac-id, itaconic acid | Outstanding shape recovery proper-ties, not elastomeric at room temper-ature due to lack of long-chain monomers. |
| Wang et al. [74] | Aliphatic Unsaturated Polyester | Melt polycondensation, emulsification, and radiation cross-linking | Succinic acid, sebacic acid, itaconic acid, 1,3-propanediol, 1,4-butanediol | Weight loss ratio of 52.3% within 5 days in the presence of lipase), low glass transition temperature of about −55 °C. |
| Hu et al. [75] | PBPSSI (Bio-based Elastomer) | Melt polycondensation and silica reinforcement | 1,3-propanediol, 2,3-butanediol, succinic acid, sebacic acid, itaconic acid | Excellent thermal stability, biocompatibility, improved tensile strength through silica reinforcement. |
| Kang et al. [76] | Bio-based Polyester Elastomer | Melt polycondensation and PLA reinforcement | Itaconic acid, succinic acid, 1,3-propanediol, 1,4-butanediol, sebacic acid | Enhanced notch impact strength (330% increase), potential for composite reinforcement. |
| Hu et al. [77] | PLBSI (Polyester Similar to PLA) | Condensation | Lactic acid, sebacic acid, itaconic acid, 1,4-butanediol | Compatible with PLA, stress-strain behavior shifted from plastic to elastic, potential in conductivity and shape memory. |
| Tang et al. [78] | Polyester/Graphene Composite | Condensation reaction with graphene grafting | Bio-based diols and diacids | Conductivity of 1.06 S/m at 0.33 vol.% graphene, applications in conductive materials. |
| Tang et al. [79] | PBHIIS (Bio-based Elastomer) | Copolymerization | 1,6-hexanediol, isosorbide, sebacic acid, itaconic acid | Constant elastomer behavior at room temperature with good tensile strength and elongation rate. |
| Tang et al. [80] | PEGIS-BC (Biodegradable Elastomer) | Combination of in-situ and polyolefin cross-linking | Glycerol, itaconic acid, sebacic acid | Dual cross-linking network, strengthened with bacterial cellulose. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; He, X.; Huang, R. Advancements and Perspectives in Biodegradable Polyester Elastomers: Toward Sustainable and High-Performance Materials. Int. J. Mol. Sci. 2025, 26, 727. https://doi.org/10.3390/ijms26020727
Tang L, He X, Huang R. Advancements and Perspectives in Biodegradable Polyester Elastomers: Toward Sustainable and High-Performance Materials. International Journal of Molecular Sciences. 2025; 26(2):727. https://doi.org/10.3390/ijms26020727
Chicago/Turabian StyleTang, Lisheng, Xiaoyan He, and Ran Huang. 2025. "Advancements and Perspectives in Biodegradable Polyester Elastomers: Toward Sustainable and High-Performance Materials" International Journal of Molecular Sciences 26, no. 2: 727. https://doi.org/10.3390/ijms26020727
APA StyleTang, L., He, X., & Huang, R. (2025). Advancements and Perspectives in Biodegradable Polyester Elastomers: Toward Sustainable and High-Performance Materials. International Journal of Molecular Sciences, 26(2), 727. https://doi.org/10.3390/ijms26020727






