Role of miRNAs in Regulating Ascending Aortic Dilation in Bicuspid Aortic Valve Patients Operated for Aortic Stenosis
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Population
2.2. Surgical Procedure
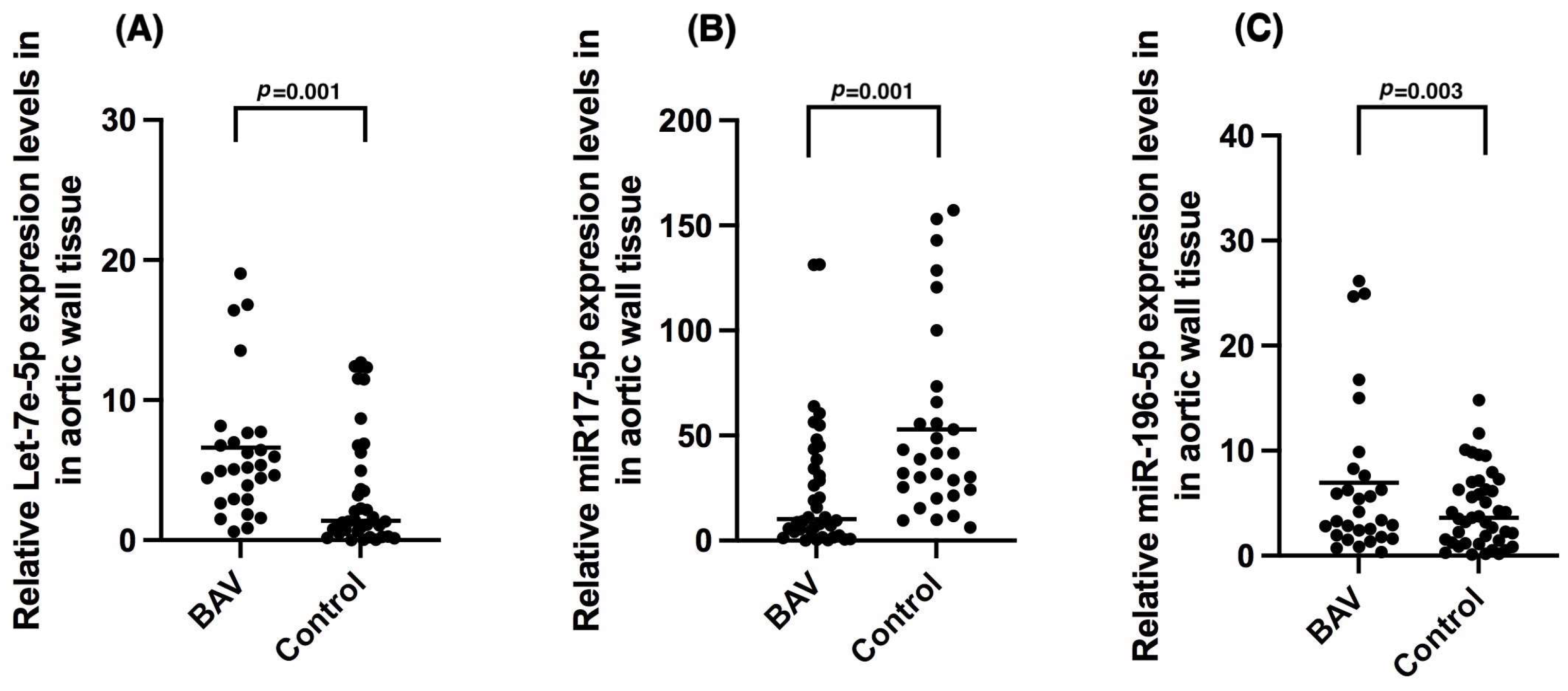
2.3. miR Expression in BAV
2.4. miRs Expression Profiles in Aortic Dilation in Bicuspid Aortic Valve
2.5. miRs Expression Profiles in Double Aortic Lesion and Stenosis in Patients with Bicuspid and Tricuspid Aortic Valves
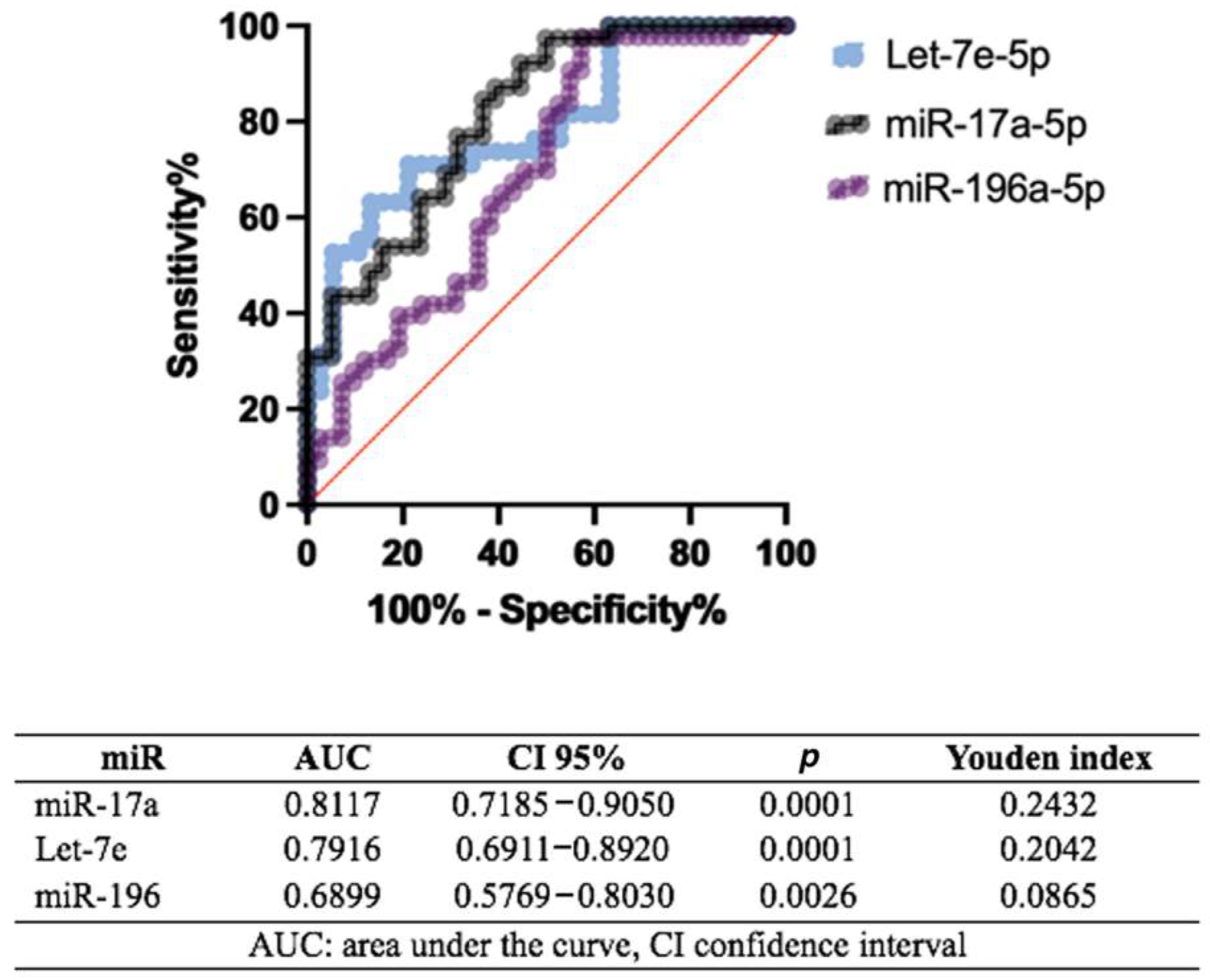
2.6. Correlations
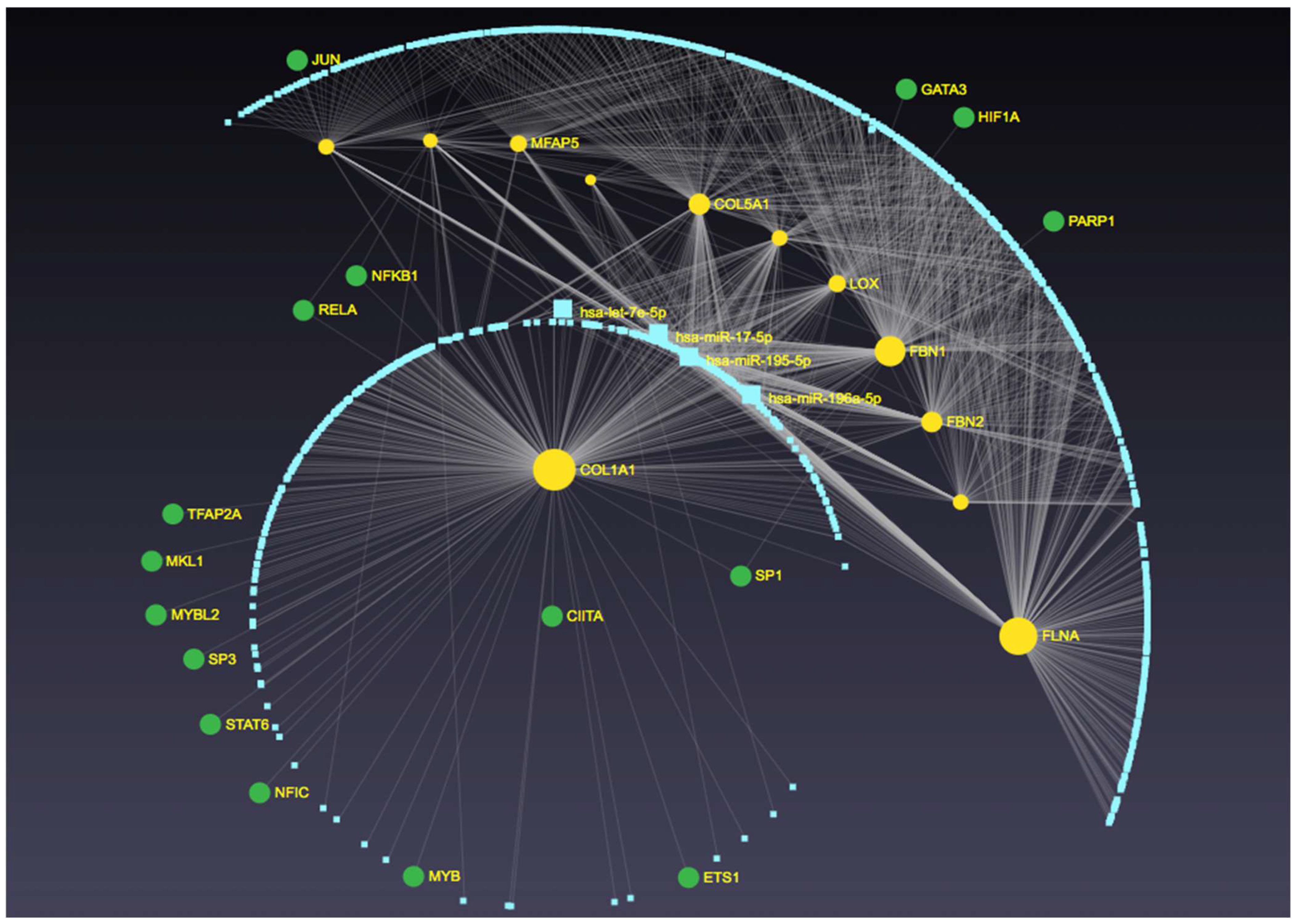
3. Discussion
4. Materials and Methods
4.1. Population
4.2. Measurement of Aortic Stenosis Echocardiographic Parameters
4.3. Measurement of Valvular Calcium
4.4. Measurement of Aortic Diameter
4.5. Ethical Considerations
4.6. Surgical Technique and Sample Collection
4.7. Sample Pulverization
4.8. miRNAs Extraction
4.9. Real-Time PCR
4.10. Statistical Analysis
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sillesen, A.-S.; Vøgg, O.; Pihl, C.; Raja, A.A.; Sundberg, K.; Vedel, C.; Zingenberg, H.; Jørgensen, F.S.; Vejlstrup, N.; Iversen, K.; et al. Prevalence of Bicuspid Aortic Valve and Associated Aortopathy in Newborns in Copenhagen, Denmark. JAMA 2021, 325, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Michelena, H.I.; Corte, A.; Evangelista, A.; Maleszewski, J.J.; Edwards, W.D.; Roman, M.J.; Devereux, R.B.; Fernández, B.; Asch, F.M.; Barker, A.J.; et al. International Consensus Statement on Nomenclature and Classification of the Congenital Bicuspid Aortic Valve and Its Aortopathy, for Clinical, Surgical, Interventional and Research Purposes. Radiol. Cardiothorac. Imaging 2021, 3, e200496. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, B.M.; Lewin, M.B.; Stout, K.K.; Gill, E.; Prueitt, A.; Byers, P.H.; Otto, C.M. The bicuspid aortic valve: An integrated phenotypic classification of leaflet morphology and aortic root shape. Heart 2008, 94, 1634–1638. [Google Scholar] [CrossRef]
- Sievers, H.-H.; Stierle, U.; Hachmann, R.M.S.; Charitos, E.I. New insights in the association between bicuspid aortic valve phenotype, aortic configuration and valve haemodynamics. Eur. J. Cardio-Thorac. Surgery Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2016, 49, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Sievers, H.-H.; Schmidtke, C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J. Thorac. Cardiovasc. Surg. 2007, 133, 1226–1233. [Google Scholar] [CrossRef]
- Michelena, H.I.; Della Corte, A.; Evangelista, A.; Maleszewski, J.J.; Edwards, W.D.; Roman, M.J.; Devereux, R.B.; Fernández, B.; Asch, F.M.; Barker, A.J.; et al. Summary: International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional, and research purposes. J. Thorac. Cardiovasc. Surg. 2021, 162, 781–797. [Google Scholar] [CrossRef]
- Bulut, H.I.; Arjomandi Rad, A.; Syrengela, A.-A.; Ttofi, I.; Djordjevic, J.; Kaur, R.; Keiralla, A.; Krasopoulos, G.A. Comprehensive Review of Management Strategies for Bicuspid Aortic Valve (BAV): Exploring Epidemiology, Aetiology, Aortopathy, and Interventions in Light of Recent Guidelines. J. Cardiovasc. Dev. Dis. 2023, 10, 398. [Google Scholar] [CrossRef]
- Coll, M.; Fernández-Falgueras, A.; Iglesias, A.; Brugada, R. Valvulopathies and Genetics: Where are We? Rev. Cardiovasc. Med. 2024, 25, 40. [Google Scholar] [CrossRef]
- Sabet, H.Y.; Edwards, W.D.; Tazelaar, H.D.; Daly, R.C. Congenitally bicuspid aortic valves: A surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2715 additional cases. Mayo Clin. Proc. 1999, 74, 14–26. [Google Scholar] [CrossRef]
- Muralles-Castillo, F.A. Characteristics of pediatric patients operated of aortic coartation in the years 2009 to 2018 at the National Institute of Cardiology Ignacio Chávez. Arch. Cardiol. Mex. 2020, 90, 436–441. [Google Scholar] [CrossRef]
- Ward, C. Clinical significance of the bicuspid aortic valve. Heart 2000, 83, 81–85. [Google Scholar] [CrossRef]
- Girdauskas, E.; Schulz, S.; Borger, M.A.; Mierzwa, M.; Kuntze, T. Transforming growth factor-beta receptor type II mutation in a patient with bicuspid aortic valve disease and intraoperative aortic dissection. Ann. Thorac. Surg. 2011, 91, e70–e71. [Google Scholar] [CrossRef]
- Wang, J.; Deng, W.; Lv, Q.; Li, Y.; Liu, T.; Xie, M. Aortic Dilatation in Patients with Bicuspid Aortic Valve. Front. Physiol. 2021, 12, 615175. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Cohen, G.; Colbert, J.; Fedak, P.W.M. Bicuspid aortic valve associated aortopathy: 2022 guideline update. Curr. Opin. Cardiol. 2023, 38, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, A. Phenotypic heterogeneity of bicuspid aortopathy: A potential key to decode the prognosis? Heart 2014, 100, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, S.; Sun, C.; Qi, H.; Qian, X.; Zheng, Z. Outcomes After Isolated Aortic Valve Replacement in Patients with Bicuspid vs Tricuspid Aortic Valve. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 854–865. [Google Scholar] [CrossRef]
- Girdauskas, E.; Borger, M.A. Bicuspid aortic valve and associated aortopathy: An update. Semin. Thorac. Cardiovasc. Surg. 2013, 25, 310–316. [Google Scholar] [CrossRef]
- Andreassi, M.G.; Della Corte, A. Genetics of bicuspid aortic valve aortopathy. Curr. Opin. Cardiol. 2016, 31, 585–592. [Google Scholar] [CrossRef]
- Yassine, N.M.; Shahram, J.T.; Body, S.C. Pathogenic mechanisms of bicuspid aortic valve aortopathy. Front. Physiol. 2017, 8, 687. [Google Scholar] [CrossRef]
- Jiao, J.; Xiong, W.; Wang, L.; Yang, J.; Qiu, P.; Hirai, H.; Shao, L.; Milewicz, D.; Chen, Y.E.; Yang, B. Differentiation defect in neural crest-derived smooth muscle cells in patients with aortopathy associated with bicuspid aortic valves. eBioMedicine 2016, 10, 282–290. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Marullo, A.G.M.; Madonna, M.; Cavarretta, E.; Allegra, A.; Cesarini, V.; Iaccarino, A.; Schiavon, S.; Peruzzi, M.; Greco, E.; et al. Deregulation of TLR4 signaling pathway characterizes Bicuspid Aortic valve syndrome. Sci. Rep. 2019, 9, 11028. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Tian, W.; Qiu, P.; Norton, E.L.; Wang, M.M.; Chen, Y.E.; Yang, B. Induced pluripotent stem cells with NOTCH1 gene mutation show impaired differentiation into smooth muscle and endothelial cells: Implications for bicuspid aortic valve-related aortopathy. J. Thorac. Cardiovasc. Surg. 2018, 156, 515–522.e1. [Google Scholar] [CrossRef]
- Harrison, O.J.; Visan, A.C.; Moorjani, N.; Modi, A.; Salhiyyah, K.; Torrens, C.; Ohri, S.; Cagampang, F.R. Defective NOTCH signaling drives increased vascular smooth muscle cell apoptosis and contractile differentiation in bicuspid aortic valve aortopathy: A review of the evidence and future directions. Trends Cardiovasc. Med. 2019, 29, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, S.; Pilato, M.; Mazzoccoli, G.; Pazienza, V.; Vinciguerra, M. Anti-correlation between longevity gene SirT1 and Notch signaling in ascending aorta biopsies from patients with bicuspid aortic valve disease. Heart Vessels 2013, 28, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Holliday, C.J.; Ankeny, R.F.; Jo, H.; Nerem, R.M. Discovery of shear- and side-specific mRNAs and miRNAs in human aortic valvular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H856–H867. [Google Scholar] [CrossRef]
- Heath, J.M.; Fernandez Esmerats, J.; Khambouneheuang, L.; Kumar, S.; Simmons, R.; Jo, H. Mechanosensitive microRNA-181b Regulates Aortic Valve Endothelial Matrix Degradation by Targeting TIMP3. Cardiovasc. Eng. Technol. 2018, 9, 141–150. [Google Scholar] [CrossRef]
- Poggio, P.; Songia, P.; Moschetta, D.; Valerio, V.; Myasoedova, V.; Perrucci, G.L.; Pompilio, G. MiRNA profiling revealed enhanced susceptibility to oxidative stress of endothelial cells from bicuspid aortic valve. J. Mol. Cell. Cardiol. 2019, 131, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; Petersen, J.; Neumann, N.; Ungelenk, M.; Kurth, I.; Reichenspurner, H.; Zeller, T. MiR-145 expression and rare NOTCH1 variants in bicuspid aortic valve-associated aortopathy. PLoS ONE 2018, 13, e0200205. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zheng, R.; Xiao, F.; Zhang, S.; He, K.; Zhang, J.; Shao, Y. Downregulated MicroRNA-195 in the Bicuspid Aortic Valve Promotes Calcification of Valve Interstitial Cells via Targeting SMAD7. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fan, G.-C. Extracellular/circulating microRNAs and their potential role in cardiovascular disease. Am. J. Cardiovasc. Dis. 2011, 1, 138. [Google Scholar]
- Rana, T.M. Illuminating the silence: Understanding the structure and function of small RNAs. Nat. Rev. Mol. Cell Biol. 2007, 8, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.; Wicik, Z.; De Rosa, S.; Eyileten, C.; Jakubik, D.; Spaccarotella, C.; Mongiardo, A.; Postula, M.; Indolfi, C. MicroRNAs fingerprint of bicuspid aortic valve. J. Mol. Cell. Cardiol. 2019, 134, 98–106. [Google Scholar] [CrossRef]
- Lin, Z.; Ge, J.; Wang, Z.; Ren, J.; Wang, X.; Xiong, H.; Gao, J.; Zhang, Y.; Zhang, Q. Let-7e modulates the inflammatory response in vascular endothelial cells through ceRNA crosstalk. Sci. Rep. 2017, 7, 42498. [Google Scholar] [CrossRef]
- Alanazi, A.; Barui, A.K.; Mohieldin, A.M.; Gupta, A.; Ramchandran, R.; Nauli, S.M. Identifying the roles of miR-17 in ciliogenesis and cell cycle. Front. Cell Dev. Biol. 2024, 12, 1397931. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Zhang, Y.; Zhang, L.; Weakley, S.M.; Yao, Q. MicroRNA-196: Critical roles and clinical applications in development and cancer. J. Cell. Mol. Med. 2011, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wang, D.D.; Zhang, Y.L.; He, S.; Chen, D.; Wu, Y.X.; Pang, Q.F. MiR-196a-5p hinders vascular smooth muscle cell proliferation and vascular remodeling via repressing BACH1 expression. Sci. Rep. 2024, 14, 16904. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.H.; Appoo, J.J.; Saczkowski, R.; Smith, H.N.; Ouzounian, M.; Gregory, A.J.; Herget, E.J.; Boodhwani, M. Association of Mortality and Acute Aortic Events with Ascending Aortic Aneurysm: A Systematic Review and Meta-analysis. JAMA Netw. Open 2018, 1, e181281. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Kalahasti, V.; Alkharabsheh, S.; Svensson, L.G.; Sabik, J.F.; Roselli, E.E.; Hammer, D.; Johnston, D.R.; Collier, P.; Rodriguez, L.L.; et al. Characteristics and long-term outcomes of contemporary patients with bicuspid aortic valves. J. Thorac. Cardiovasc. Surg. 2016, 151, 1650–1659.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, Y.; Lu, P.; Wu, B.; Zhou, B. NOTCH Signaling in Aortic Valve Development and Calcific Aortic Valve Disease. Front. Cardiovasc. Med. 2021, 8, 682298. [Google Scholar] [CrossRef] [PubMed]
- Soto-Navarrete, M.T.; López-Unzu, M.Á.; Durán, A.C.; Fernández, B. Embryonic development of bicuspid aortic valves. Prog. Cardiovasc. Dis. 2020, 63, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Freiholtz, D.; Bergman, O.; Lång, K.; Poujade, F.-A.; Paloschi, V.; Granath, C.; Lindeman, J.H.N.; Olsson, C.; Franco-Cereceda, A.; Eriksson, P.; et al. Bicuspid aortic valve aortopathy is characterized by embryonic epithelial to mesenchymal transition and endothelial instability. J. Mol. 2023, 101, 801–811. [Google Scholar] [CrossRef]
- Haunschild, J.; Schellinger, I.N.; Barnard, S.J.; von Aspern, K.; Davierwala, P.; Misfeld, M.; Petroff, D.; Borger, M.A.; Etz, C.D. Bicuspid aortic valve patients show specific epigenetic tissue signature increasing extracellular matrix destruction. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 937–943. [Google Scholar] [CrossRef]
- Peterson, J.C.; Chughtai, M.; Wisse, L.J.; Gittenberger-de Groot, A.C.; Feng, Q.; Goumans, M.-J.T.H.; Conny VanMunsteren, J.; Jongbloed, M.R.M.; DeRuiter, M.C. Bicuspid aortic valve formation: Nos3 mutation leads to abnormal lineage patterning of neural crest cells and the second heart field. Dis. Models Mech. 2018, 11, dmm034637. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.; Poujade, F.-A.; Bergman, O.; Gådin, J.R.; Simon, N.; Lång, K.; Franco-Cereceda, A.; Body, S.C.; Björck, H.M.; Eriksson, P. Endothelial/Epithelial Mesenchymal Transition in Ascending Aortas of Patients with Bicuspid Aortic Valve. Front. Cardiovasc. Med. 2019, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Agnese, V.; Coronnello, C.; Raffa, G.M.; Bellavia, D.; Conaldi, P.G.; Pilato, M.; Pasta, S. On the prospect of serum exosomal miRNA profiling and protein biomarkers for the diagnosis of ascending aortic dilatation in patients with bicuspid and tricuspid aortic valve. Int. J. Cardiol. 2018, 273, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Khairy, P.; Graham, D.A.; Colan, S.D.; Galvin, T.C.; Sanders, S.P.; Singh, M.N.; Bhatt, A.; Lacro, R.V. Bicuspid aortic valve and associated aortic dilation in the young. Heart 2012, 98, 1014–1019. [Google Scholar] [CrossRef]
- Krauze, A.; Procyk, G.; Gąsecka, A.; Garstka-Pacak, I.; Wrzosek, M. The Role of MicroRNAs in Aortic Stenosis-Lessons from Recent Clinical Research Studies. Int. J. Mol. Sci. 2023, 24, 13095. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Tan, J.; Lu, J.; Huang, J.; Li, X.; Xu, J.; Wang, X. MicroRNA-17-5p Promotes Vascular Calcification by Targeting ANKH. Curr. Neurovasc. Res. 2022, 19, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jing, X.; Li, Z.; Tian, Q.; Wang, Q.; Chen, X. Investigation of the role of the miR17-92 cluster in BMP9-induced osteoblast lineage commitment. J. Orthop. Surg. Res. 2021, 16, 652. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, L.; Zhang, L.; Zang, G.; Sun, Z.; Wang, Z. Role of endothelial cells in vascular calcification. Front. Cardiovasc. Med. 2022, 9, 895005. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Lighthouse, J.K.; Greif, D.M. Development and pathologies of the arterial wall. Cell. Mol. Life Sci. CMLS 2014, 71, 1977–1999. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ago, T.; Zhai, P.; Abdellatif, M.; Sadoshima, J. Thioredoxin 1 negatively regulates angiotensin II-induced cardiac hypertrophy through upregulation of miR-98/let-7. Circ. Res. 2011, 108, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Antequera-González, B.; Collell-Hernández, R.; Martínez-Micaelo, N.; Marimon-Blanch, C.; Carbonell-Prat, B.; Escribano, J.; Alegret, J.M. miR-130a expression is related to aortic dilation in bicuspid aortic valve children. Pediatr. Res. 2024, 95, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; Petersen, J.; Neumann, N.; Naito, S.; Gross, T.; Jagodzinski, A.; Reichenspurner, H.; Zeller, T. Novel Approaches for BAV Aortopathy Prediction-Is There a Need for Cohort Studies and Biomarkers? Biomolecules 2018, 8, 58. [Google Scholar] [CrossRef]
- Li, M.; Ding, Y.; Tuersong, T.; Chen, L.; Zhang, M.-L.; Li, T.; Feng, S.M.; Guo, Q. Let-7 family regulates HaCaT cell proliferation and apoptosis via the ΔNp63/PI3K/AKT pathway. Open Med. 2024, 19, 20240925. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-L.; Wen, Z.-X.; Mo, X.-Y.; Zhang, X.-Y.; Li, H.-N.; Cheung, W.-H.; Fu, D.; Zhang, S.H.; Wan, Y.; Chen, B.L. Bone-Metabolism-Related Serum microRNAs to Diagnose Osteoporosis in Middle-Aged and Elderly Women. Diagnostics 2022, 12, 2872. [Google Scholar] [CrossRef] [PubMed]
- Styrkarsdottir, U.; Stefansson, O.A.; Gunnarsdottir, K.; Thorleifsson, G.; Lund, S.H.; Stefansdottir, L.; Juliusson, K.; Agustsdottir, A.B.; Zink, F.; Halldorsson, G.H.; et al. GWAS of bone size yields twelve loci that also affect height, BMD, osteoarthritis or fractures. Nat. Commun. 2019, 10, 2054. [Google Scholar] [CrossRef]
- Takafuji, Y.; Tatsumi, K.; Kawao, N.; Okada, K.; Muratani, M.; Kaji, H. MicroRNA-196a-5p in Extracellular Vesicles Secreted from Myoblasts Suppresses Osteoclast-like Cell Formation in Mouse Cells. Calcif. Tissue Int. 2021, 108, 364–376. [Google Scholar] [CrossRef]
- Takafuji, Y.; Tatsumi, K.; Kawao, N.; Okada, K.; Muratani, M.; Kaji, H. Effects of fluid flow shear stress to mouse muscle cells on the bone actions of muscle cell-derived extracellular vesicless. PLoS ONE 2021, 16, e0250741. [Google Scholar] [CrossRef]
- Kara, R.; Vergara, C. Assessing turbulent effects in ascending aorta in presence of bicuspid aortic valve. Comput. Methods Biomech. Biomed. Eng. 2024, 27, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lv, H.; Liu, B.; Hao, M.; Taylor, H.S.; Zhang, X.; Li, D.; Huang, Y. Let-7 suppresses liver fibrosis by inhibiting hepatocyte apoptosis and TGF-β production. Mol. Metab. 2023, 78, 101828. [Google Scholar] [CrossRef] [PubMed]
- Torres-Do Rego, A.; Barrientos, M.; Ortega-Hernández, A.; Modrego, J.; Gómez-Gordo, R.; Álvarez-Sala, L.A.; Cachofeiro, V.; Gómez-Garre, D. Identification of a Plasma Microrna Signature as Biomarker of Subaneurysmal Aortic Dilation in Patients with High Cardiovascular Risk. J. Clin. Med. 2020, 9, 2783. [Google Scholar] [CrossRef]
- Bao, M.-H.; Feng, X.; Zhang, Y.-W.; Lou, X.-Y.; Cheng, Y.; Zhou, H.-H. Let-7 in cardiovascular diseases, heart development and cardiovascular differentiation from stem cells. Int. J. Mol. Sci. 2013, 14, 23086–23102. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Hua, S.; Zhang, J.; Xu, S. The MicroRNA Family Both in Normal Development and in Different Diseases: The miR-17-92 Cluster. BioMed Res. Int. 2019, 2019, 9450240. [Google Scholar] [CrossRef] [PubMed]
- Honda, N.; Jinnin, M.; Kajihara, I.; Makino, T.; Makino, K.; Masuguchi, S.; Fukushima, S.; Okamoto, Y.; Hasegawa, M.; Fujimoto, M.; et al. TGF-β-mediated downregulation of microRNA-196a contributes to the constitutive upregulated type I collagen expression in scleroderma dermal fibroblasts. J. Immunol. 2012, 188, 3323–3331. [Google Scholar] [CrossRef]
- Yang, L.-T.; Ye, Z.; Wajih Ullah, M.; Maleszewski, J.J.; Scott, C.G.; Padang, R.; Pislaru, S.V.; Nkomo, V.T.; Mankad, S.V.; Pellikka, P.A.; et al. Bicuspid aortic valve: Long-term morbidity and mortality. Eur. Heart J. 2023, 44, 4549–4562. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-T.; Tribouilloy, C.; Masri, A.; Bax, J.J.; Delgado, V.; Girdauskas, E.; Evangelista, A.; Sundt, T.M.; Svensson, L.G.; Enriquez-Sarano, M.; et al. Clinical presentation and outcomes of adults with bicuspid aortic valves: 2020 update. Prog. Cardiovasc. Dis. 2020, 63, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Pawade, T.; Clavel, M.-A.; Tribouilloy, C.; Dreyfus, J.; Mathieu, T.; Tastet, L.; Renard, C.; Gun, M.; Jenkins, W.S.A.; MacRon, L.; et al. Computed Tomography Aortic Valve Calcium Scoring in Patients with Aortic Stenosis. Circ. Cardiovasc. Imaging 2018, 11, e007146. [Google Scholar] [CrossRef]
- Davies, R.R.; Gallo, A.; Coady, M.A.; Tellides, G.; Botta, D.M.; Burke, B.; Coe, M.P.; Kopf, G.S.; Elefteriades, J.A. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann. Thorac. Surg. 2006, 81, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.A.; Li, Y.; Rizzo, J.A.; Charilaou, P.; Saeyeldin, A.; Velasquez, C.A.; Mansour, A.M.; Bin Mahmood, S.U.; Ma, W.G.; Brownstein, A.J.; et al. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J. Thorac. Cardiovasc. Surg. 2018, 155, 1938–1950. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Demographic | Total (n = 78) | Bicuspid Aortic Valve (n = 40) | Tricuspid Aortic Valve (n = 38) | p |
|---|---|---|---|---|
| Male (%) | 61 (78) | 29 (73) | 32 (84) | 0.27 |
| Female (%) | 17 (22) | 9 (23) | 8 (21) | 0.54 |
| Age (years) | 48 ± 12 | 54 ± 10 | 62 ± 13 | 0.007 |
| BMI (kg/m²) | 26 ± 5 | 26 ± 5 | 27 ± 4 | 0.20 |
| Laboratory Analysis Median (Min–Max) Q1, Q2, Q3 | ||||
| Hemoglobin (g/dL) | 14 (8.7–17.8) 12–14–16 | 12.2 (9.7–17.8) 13.1–14.2–16 | 14.2 (9.7–17.8) 13.1–14.2–15.7 | 0.62 |
| Leukocytes (×103/µL) | 7.6 (3.5–18.3) 6–7.1–8.5 | 7.1 (3.5–11.7) 6.3–7.1–8.4 | 7.1 (3.9–18.3) 5.4–7.1–9.1 | 0.95 |
| Lymphocytes (×103/µL) | 1.7 (0.4–9.3) 1.3–1.7–3.2 | 2.1 (0.7–4.2) 1.6–2.1–2.6 | 1.5 (0.4–9.3) 1.2–1.5–2.0 | 0.001 |
| Neutrophils (×103/µL) | 4.2 (1.1–14.1) 3.1–4.2–5.1 | 4.1 (1.1–7.7) 3.4–4.1–4.7) | 4.5 (2.0–14.1) 3.0–4.5–6.8 | 0.14 |
| Platelets (×103/µL) | 200 (86–453) 175–200–247 | 214 (98–377) 176–214–254 | 199 (86–453) 168–199–243) | 0.49 |
| Total Cholesterol (mg/dL) | 143 (46–246) 121–143–178 | 142 (46–246) 121–142–171 | 148 (76–218) 119–148–183 | 0.65 |
| HDL-C (mg/dL) | 40 (14.72) 33–40–46 | 40 (14–61) 33–40–45 | 42 (19–72) 33–42–51 | 0.35 |
| LDL-C (mg/dL) | 89 (24–190) 69–89–109 | 89 (26–190) 70–89–107 | 89 (23–150) 69–89–119 | 0.70 |
| Triglycerides (mg/dL) | 113 (53–245) 84–110–134 | 113 (55–190) 87–113–132 | 103 (53–245) 82–103–139 | 0.81 |
| Glucose (mg/dL) | 99 (77–199) 90–99–112 | 99 (77–189) 89–99–107 | 101 (79–199) 90–101–119 | 0.24 |
| Urea (mg/dL) | 22 (9.3–277) 16–22–29 | 21 (9–277) 16–21–29 | 22 (10–45) 16–22–28 | 0.77 |
| Creatinine (mg/dL) | 1.0 (1.0–6.0) 0.8–1.0–1.3 | 0.97 (1–6) 0.81–0.97–1.31 | 1.0 (1–2) 0.80–1.0–1.3 | 0.85 |
| Uric Acid (mg/dL) | 6 (2.3–13.1) 4.6–6–7.3 | 6 (3.7–10.1) 4.6–6–7.3 | 6 (2.3–13.1) 4.6–6–7.5 | 0.77 |
| ESR (mm/h) | 5 (1–59) 2.5–5–18 | 4 (1–6) 4–6–8 | 6 (2–59) 3–6–23 | 0.38 |
| CRP (mg/dL) | 5 (2–612) 1.3–5–26.4 | 6.7 (3–312) 1.4–6.7–16.1 | 4.3 (2–299) 1.2–4.3–72.5 | 0.83 |
| Comorbidities n (%) | ||||
| Diabetes Mellitus (%) | 12 (15.4) | 9 (23) | 3 (7.8) | 0.11 |
| Hypertension (%) | 29 (37.2) | 14 (35) | 15 (39) | 0.81 |
| Dyslipidemia (%) | 12 (15.4) | 6 (15) | 6 (16) | 0.58 |
| COPD (%) | 6 (7.7) | 1 (2.5) | 5 (13) | 0.10 |
| Previous MI (%) | 4 (5.1) | 2 (5) | 2 (5.1) | 0.67 |
| Total (n = 78) | Bicuspid Aortic Valve (n = 40) | Tricuspid Aortic Valve (n = 38) | p | |
|---|---|---|---|---|
| Native valve | 74 (95) | 36 (90) | 38 (100) | 0.11 |
| Aortic stenosis | 41 (53) | 27 (68) | 14 (37) | 0.01 |
| Aortic double lesion | 32 (41) | 18 (45) | 14 (37) | 0.49 |
| Aortic coarctation | 2 (2.5) | 2 (5) | 0 (0) | 0.49 |
| LVEF | 51 (15–74) 37–51–60 | 51 (15–74) 37–51–60 | 52 (20–62) 39–52–58 | 0.90 |
| PASP | 33 (10–98) 27–33–42 | 34 (10–80) 25–34–43 | 32 (10–98) 27–31–39 | 0.37 |
| Mean gradient | 31 (2–102) | 46.5 (3–102) 12.4–46.5–67.7 | 7 (2–90) 3–7–40 | 0.0001 |
| Valve area | 0.70 (0.20–4.20) 0.51–0.70–1.20 | 0.53 (0.20–1.0) 0.49–0.53–0.70 | 1.3 (0.38–4.9) 0.77–1.3–2.0 | 0.0001 |
| Indexed valve area | 0.40 (0.10–3.75) 0.29–0.40–0.90 | 0.30 (0.10–0.60) 0.22–0.30–0.40 | 0.99 (0.20–3.75) 0.50–0.99–1.60 | 0.0001 |
| Aortic annulus | 24 (9–40) 21–24–27 | 23 (9–34) 20–23–26 | 25 (18–40) 22–25–28 | 0.01 |
| Sinuses of Valsalva | 36 (17–79) 31–36–42 | 34 (17–78) 27–33–40 | 39 (20–79) 33–39–47 | 0.007 |
| Sinotubular junction | 31 (11–85) 27–31–40 | 29 (11–80) 26–29–38 | 35 (16–85) 28–35–44 | 0.02 |
| Ascending aorta | 39 (15–100) 34–39–50 | 36 (15–84) 34–36–43 | 46 (16–100) 35–46–59 | 0.01 |
| Total n = 78 | BAV n = 40 | TAV n = 38 | p | |
|---|---|---|---|---|
| Type of surgery | ||||
| Elective surgery | 56 (71.7) | 31 (79.5) | 25 (64.1) | 0.20 |
| Urgent surgery | 22 (28.2) | 8 (20.5) | 14 (35.9) | 0.20 |
| Aortopathy | ||||
| Aortic dissection | 21 (26.9) | 6 (15.4) | 15 (38.5) | 0.03 |
| Coarctation of the aorta | 2 (2.5) | 2 (5.1) | 0 (0) | 0.49 |
| Valvular calcification | 1501 (552–11,347) | 552 (0–7712) | 2655 (0–11,347) | |
| Complications | ||||
| Endocarditis | 4 (5.1) | 2 (5.1) | 2 (5.1) | 1 |
| Reintervention | 10 (12.8) | 2 (5.1) | 8 (20.5) | 0.08 |
| Death | 3 (3.8) | 3 (7.6) | 0 (0) | 0.24 |
| Type of Surgery | Total (n = 78) | Bicuspid Aortic Valve (n = 40) | Tricuspid Aortic Valve (n = 38) | p |
|---|---|---|---|---|
| Bentall and De Bono | 27 (34.6) | 9 (22.5) | 18 (47.3) | 0.01 |
| Bentall + Arch | 2 (2.5) | 0 (0) | 2 (5.2) | NS |
| Aortic valve replacement | 26 (33) | 19 (47.5) | 7 (18.4) | 0.008 |
| Aortic and mitral valve replacement | 1 (1.2) | 0 (0) | 1 (2.6) | NS |
| Wheat | 16 (20.5) | 10 (25) | 6 (15.7) | NS |
| Supracoronary replacement | 2 (2.5) | 0 (0) | 2 (5.2) | NS |
| Arch replacement | 1 (1.2) | 0 (0) | 1 (2.6) | NS |
| Ascending aorta replacement | 1 (1.2) | 1 (2.5) | 0 (0) | NS |
| Warden | 2 (2.5) | 0 (0) | 2 (2.6) | NS |
| Type of Implanted Valve | ||||
| Mechanical | 49 (63) | 28 (70) | 21 (55) | NS |
| Biological | 27 (35) | 10 (25) | 17 (45) | 0.005 |
| Surgical Times’ Median (Min–Max) Q1–Q2–Q3 | ||||
| Clamping | 146 (47–305) 97–146–188 | 114 (47–280) 95–114–168 | 152 (66–305) 115–152–214 | 0.001 |
| CPB | 183 (71–630) 135–183–288 | 150 (71–568) 124–150–240 | 224 (76–230) 153–224–350 | 0.005 |
| Bleeding | 410 (3–4900) 325–420–680 | 370 (3–790) 230–370–480 | 490 (190–4900) 360–490–850 | 0.004 |
| Case BAV | Age | Gender | BMI | Comorbidities | LVEF | AA | SV | STJ | Ao Asc | Type of Surgery | Type of Valve | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | M | 31 | SAH previous myocardial infarction | 46 | 26 | 51 | 38 | 34 | Bentall | Biological | Cardiogenic shock |
| 2 | 59 | M | 24 | SAH | 54 | 34 | 78 | 69 | 71 | Wheat | Biological | Cardiogenic shock |
| 3 | 64 | M | 23 | None | 54 | 24 | 46 | 40 | 46 | Wheat | Mechanics | Cardiogenic shock |
| BAV | Control | p1 | p2 | p3 | p4 | |||
|---|---|---|---|---|---|---|---|---|
| Non-AD n = 22 | AD n = 16 | Non-AD n = 18 | AD n = 20 | |||||
| Let-7e-5p | 5.18 (0.62–88.97) | 21.58 (1.50–101.89) | 1.84 (0.22–12.67) | 0.99 (0.12–12.37) | 0.02 | 0.12 | 0.10 | 0.01 |
| miR-17a-5p | 7.60 (0.0940–131.20) | 29.74 (0.78–131.40) | 120.52 (9.87–439.25) | 40.12 (6.21–232.11) | 0.01 | 0.02 | 0.01 | 0.20 |
| miR-196-5p | 5.53 (0.35–97.81) | 31.10 (1.67–111.75) | 3.17 (0.19–80.28) | 3.95 (0.11–14.82) | 0.01 | 0.64 | 0.19 | 0.01 |
| BAV | Control | p1 | p2 | |||
|---|---|---|---|---|---|---|
| Non-Stenosis n = 22 | Stenosis n = 16 | Non-Stenosis n = 18 | Stenosis n = 20 | |||
| Let-7e-5p | 6.61 (1.5–64.05) | 6.60 (0.62–155.43) | 1.17 (0.02–12.37) | 5.61 (0.01–12.67) | 0.001 | 0.16 |
| miR-17a-5p | 15.10 (0.78–63.99) | 9.43 (0.09–131.40) | 42.44 (9.58–439.25) | 142.98 (6.21–335.73) | 0.005 | 0.01 |
| miR-196–5p | 6.24 (1.33–33.03) | 6.94 (0.35–111.75) | 2.94 (0.17–14.82) | 4.14 (0.11–890.28) | 0.74 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Garcia, A.d.J.; Soule-Egea, M.; Fuentevilla-Alvarez, G.; Vargas-Alarcon, G.; Hernández-Mejia, B.I.; Martínez-Hernández, H.; Mora-Canela, S.L.; Santibanez-Escobar, F.; Ávila-Martinez, V.; Castrejón-Tellez, V.; et al. Role of miRNAs in Regulating Ascending Aortic Dilation in Bicuspid Aortic Valve Patients Operated for Aortic Stenosis. Int. J. Mol. Sci. 2025, 26, 779. https://doi.org/10.3390/ijms26020779
Sanchez-Garcia AdJ, Soule-Egea M, Fuentevilla-Alvarez G, Vargas-Alarcon G, Hernández-Mejia BI, Martínez-Hernández H, Mora-Canela SL, Santibanez-Escobar F, Ávila-Martinez V, Castrejón-Tellez V, et al. Role of miRNAs in Regulating Ascending Aortic Dilation in Bicuspid Aortic Valve Patients Operated for Aortic Stenosis. International Journal of Molecular Sciences. 2025; 26(2):779. https://doi.org/10.3390/ijms26020779
Chicago/Turabian StyleSanchez-Garcia, Antonio de Jesús, Mauricio Soule-Egea, Giovanny Fuentevilla-Alvarez, Gilberto Vargas-Alarcon, Benjamín Iván Hernández-Mejia, Humberto Martínez-Hernández, Sergio Luis Mora-Canela, Felipe Santibanez-Escobar, Valeria Ávila-Martinez, Vicente Castrejón-Tellez, and et al. 2025. "Role of miRNAs in Regulating Ascending Aortic Dilation in Bicuspid Aortic Valve Patients Operated for Aortic Stenosis" International Journal of Molecular Sciences 26, no. 2: 779. https://doi.org/10.3390/ijms26020779
APA StyleSanchez-Garcia, A. d. J., Soule-Egea, M., Fuentevilla-Alvarez, G., Vargas-Alarcon, G., Hernández-Mejia, B. I., Martínez-Hernández, H., Mora-Canela, S. L., Santibanez-Escobar, F., Ávila-Martinez, V., Castrejón-Tellez, V., Alvarez-León, E., de la Mora-Cervantes, R., Pérez-Torres, I., & Soto, M. E. (2025). Role of miRNAs in Regulating Ascending Aortic Dilation in Bicuspid Aortic Valve Patients Operated for Aortic Stenosis. International Journal of Molecular Sciences, 26(2), 779. https://doi.org/10.3390/ijms26020779






