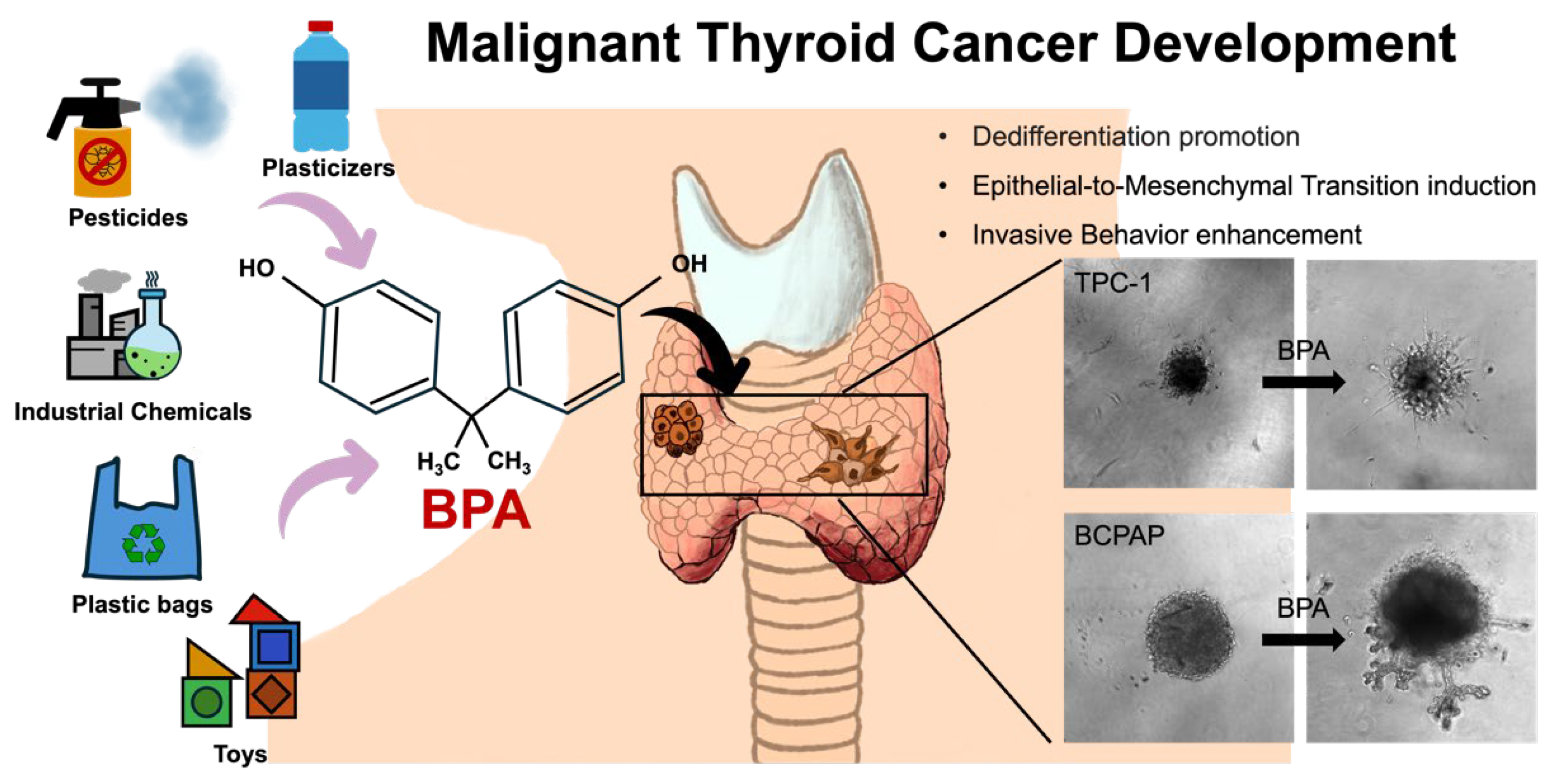
Environmental Exposure to Bisphenol A Enhances Invasiveness in Papillary Thyroid Cancer
Abstract
1. Introduction
2. Results and Discussions
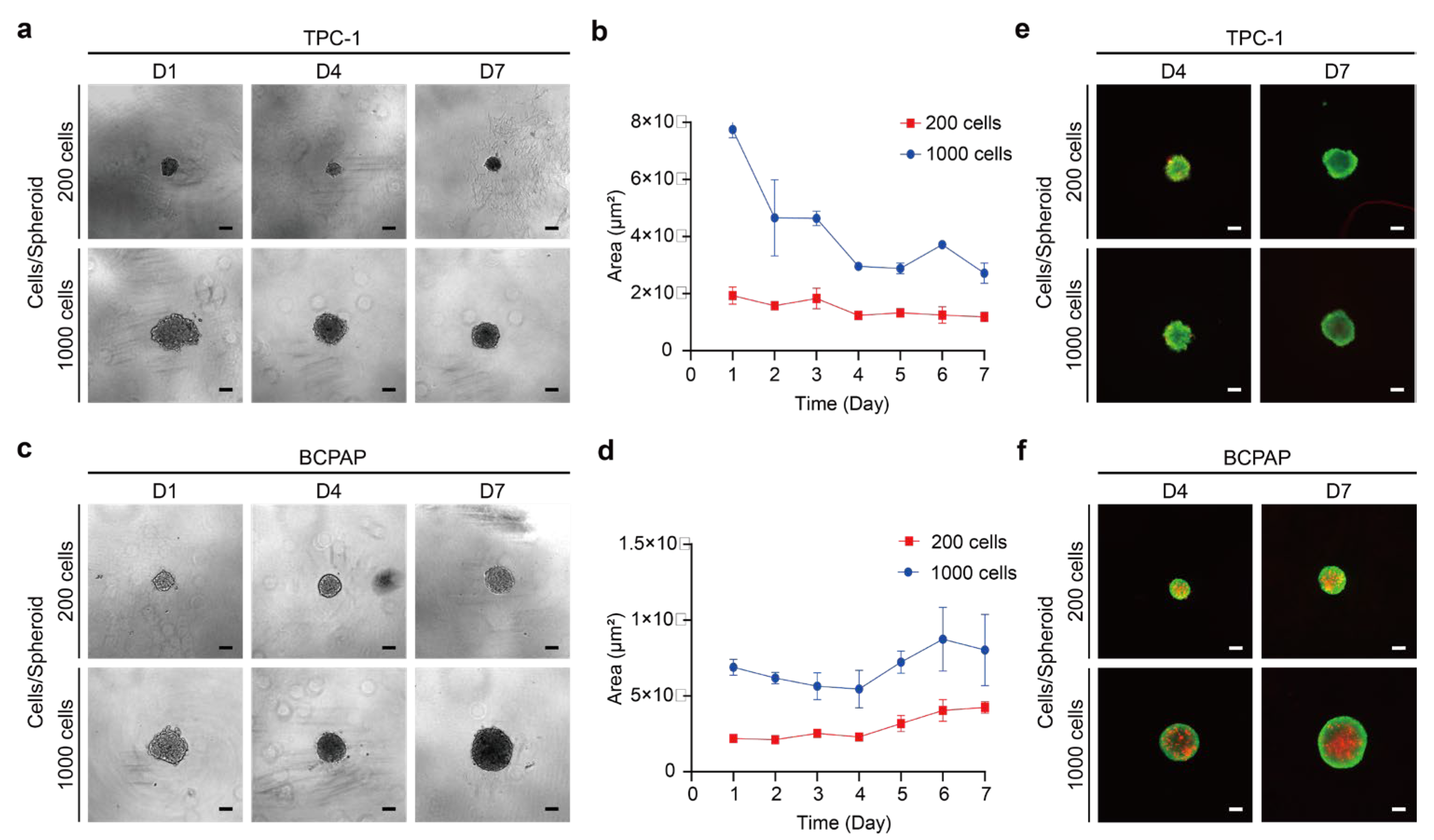
2.1. Establishment of 3D Thyroid Cancer Spheroids
2.1.1. Analysis of Thyroid Cancer Spheroid Morphology and Growths
2.1.2. Cell Viability and Structure of Thyroid Cancer Spheroids
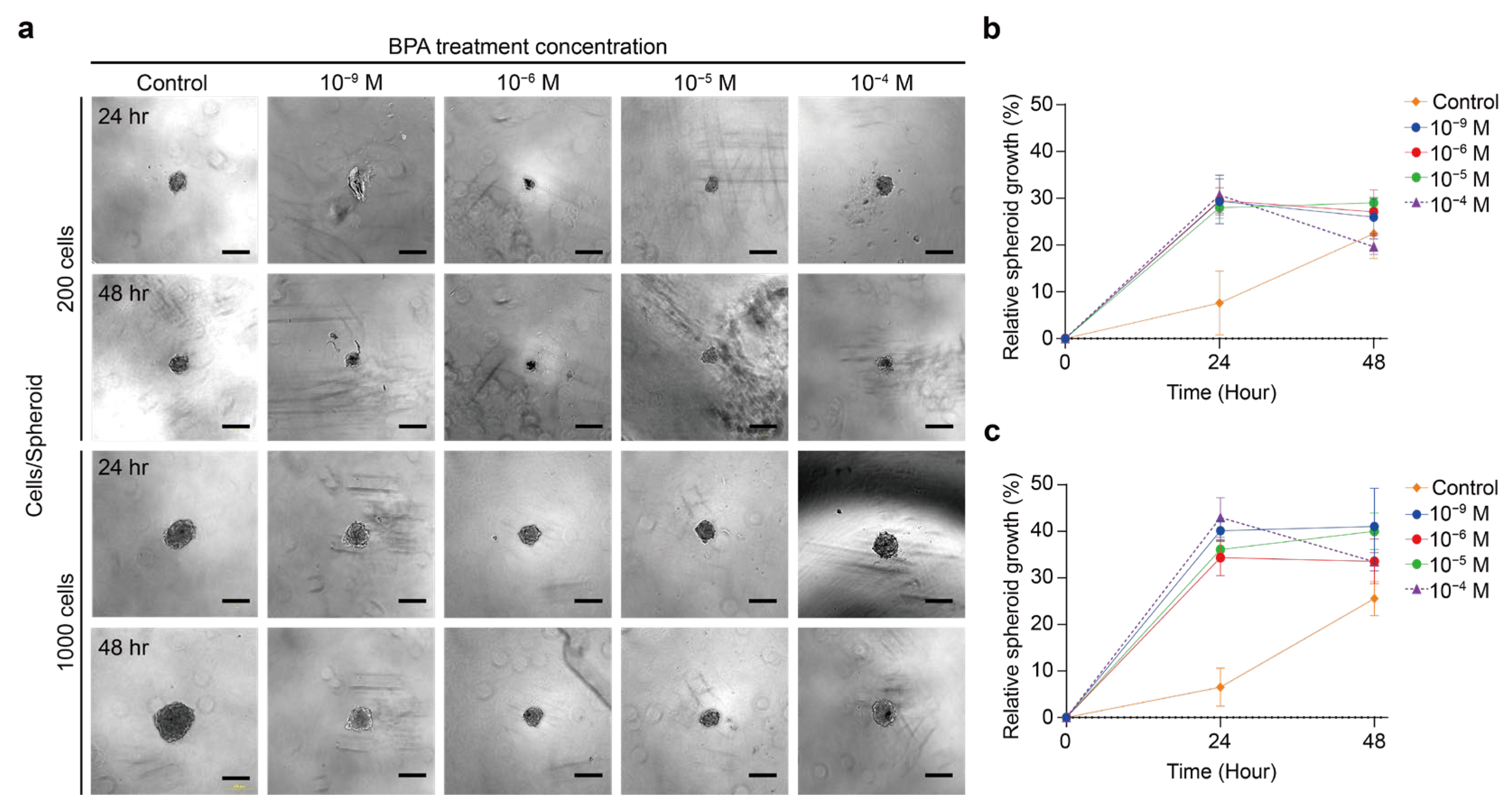
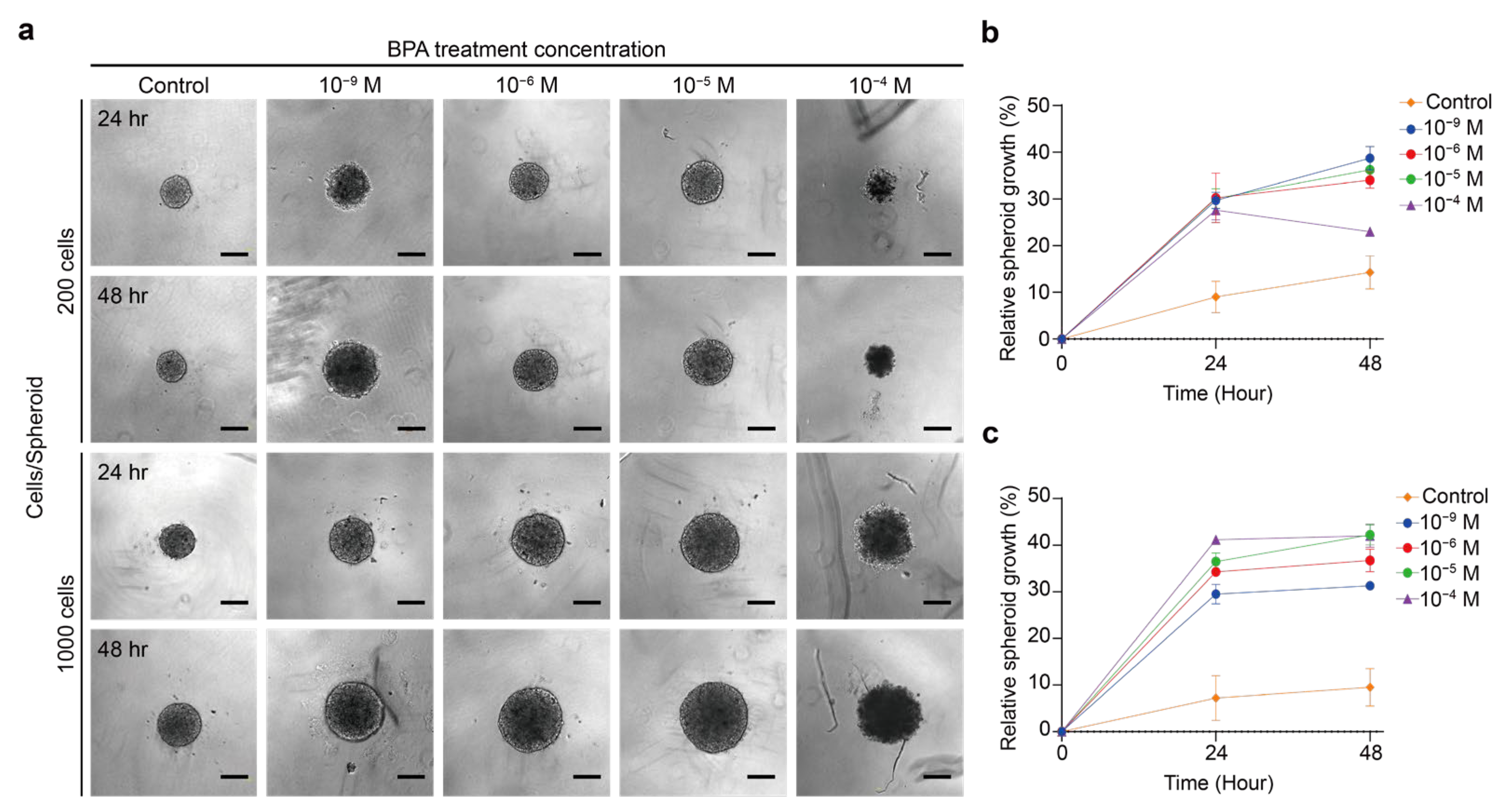
2.2. Effect of BPA on the Growth of Thyroid Cancer Spheroid
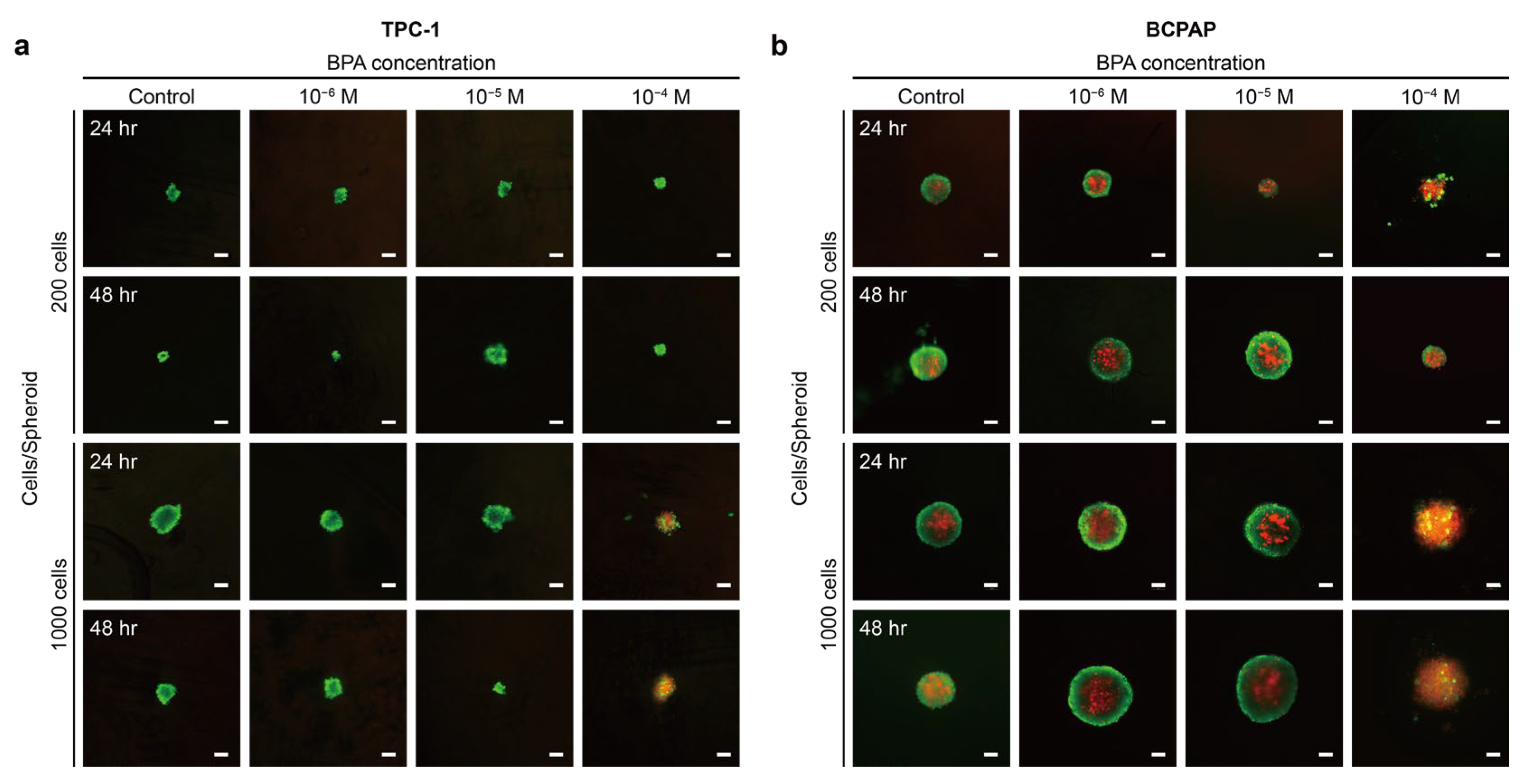
2.2.1. BPA Induces the Proliferation or Apoptosis of Thyroid Cancer
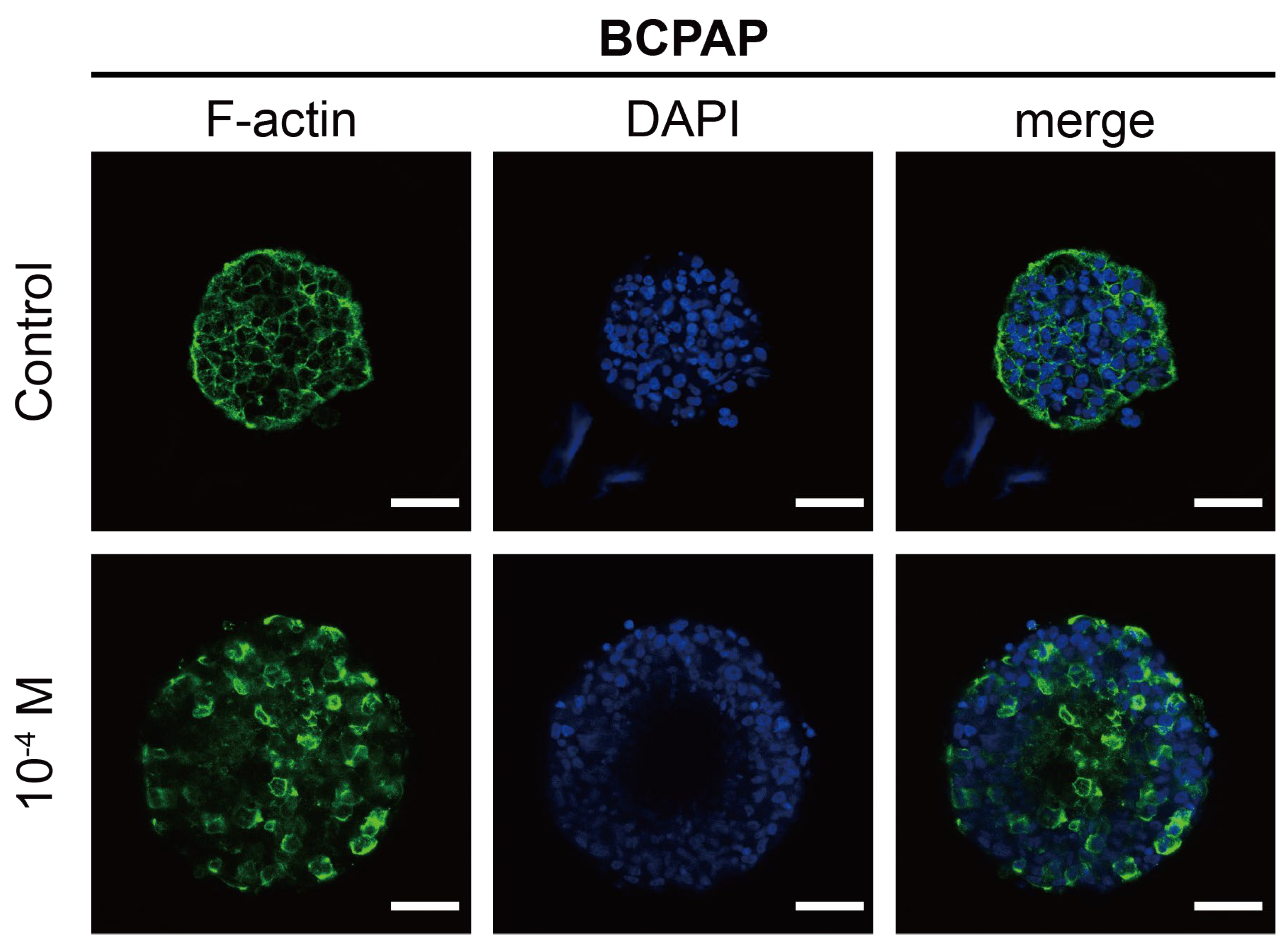
2.2.2. Morphological Analysis of F-Actin
2.3. Effects of BPA on Thyroid Function in PTC
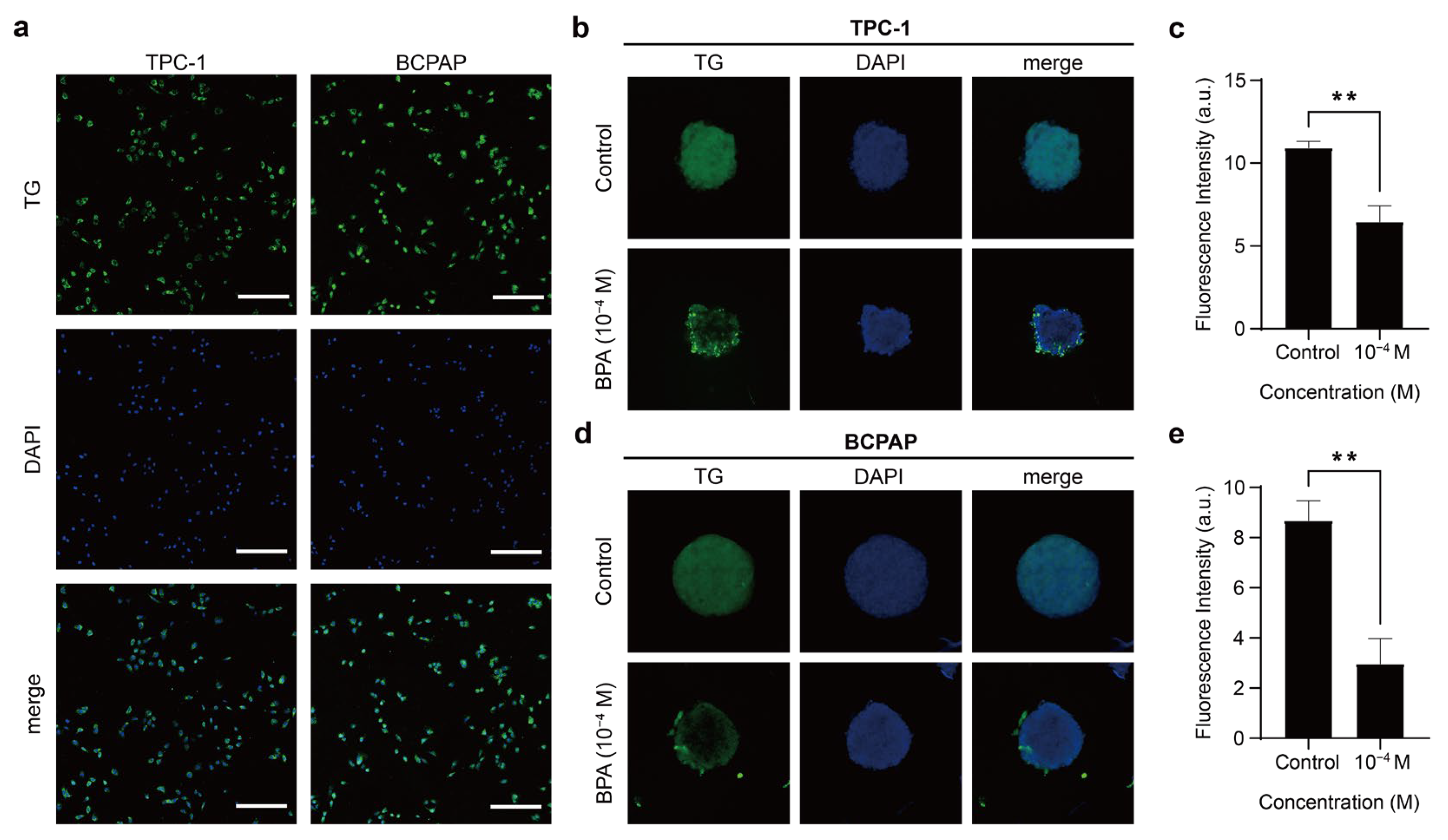
2.3.1. TG Expression in 2D Thyroid Cancer Cells
2.3.2. BPA Resulted in Decreased TG Expression of PTC Spheroids
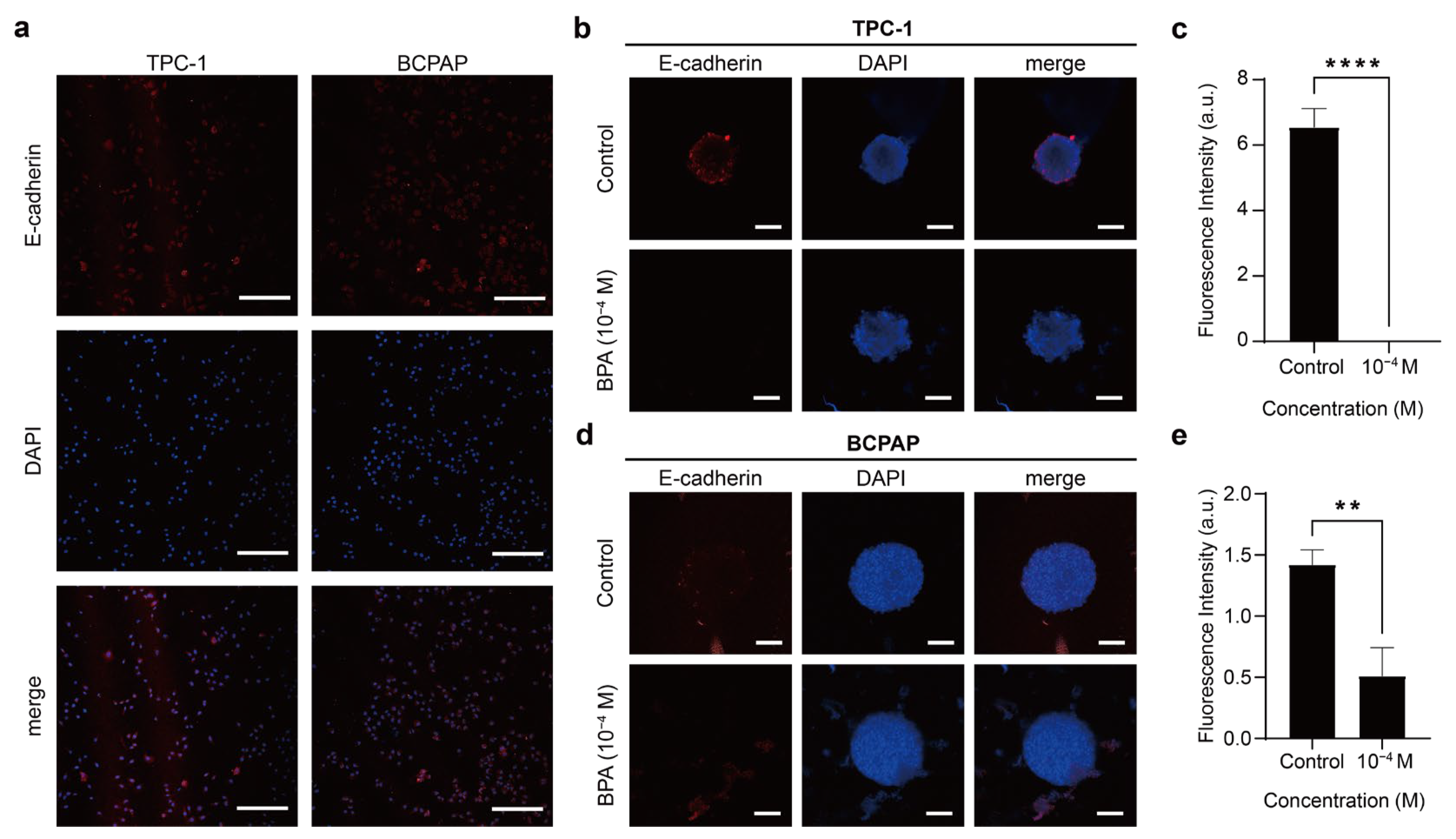
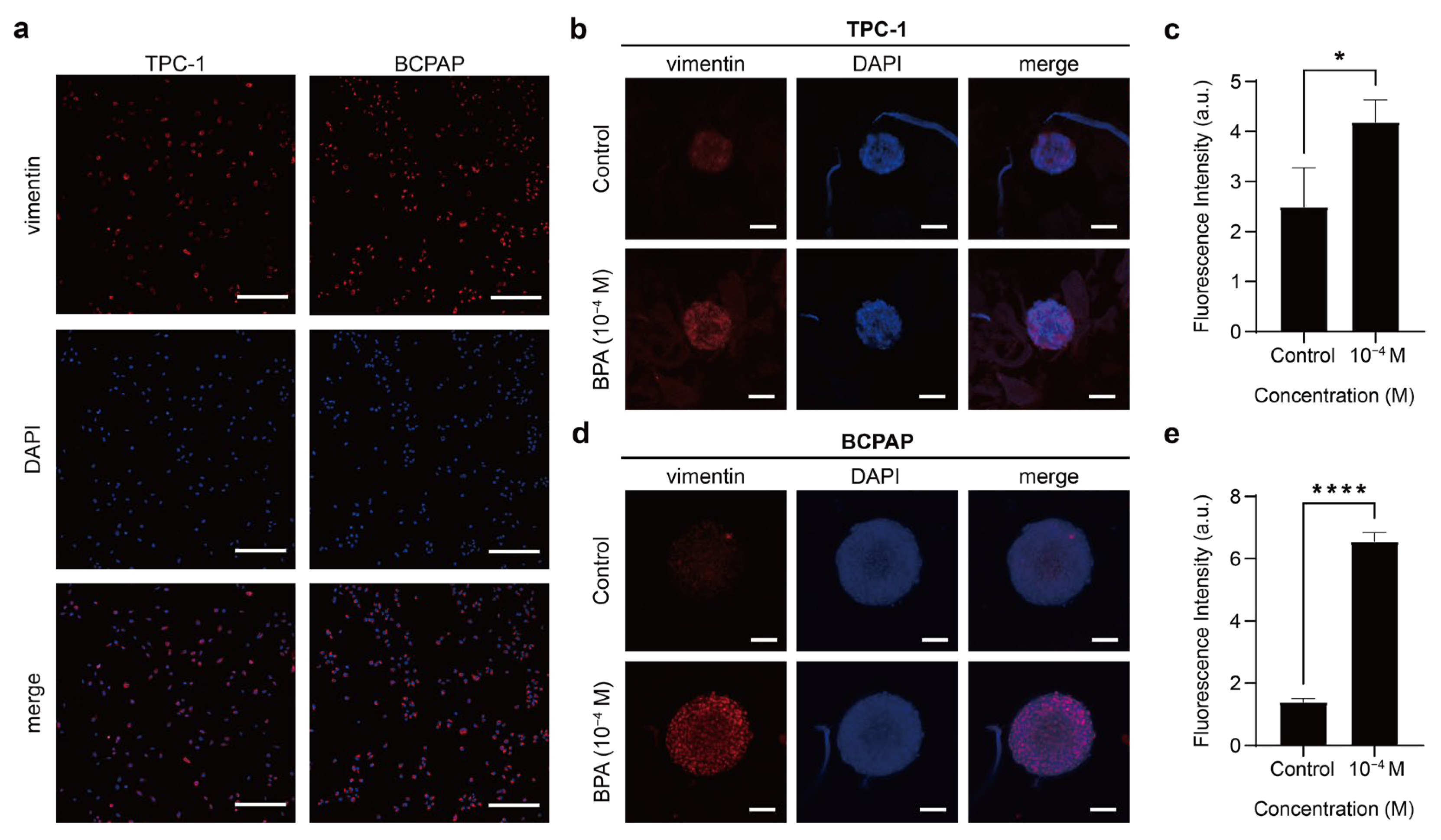
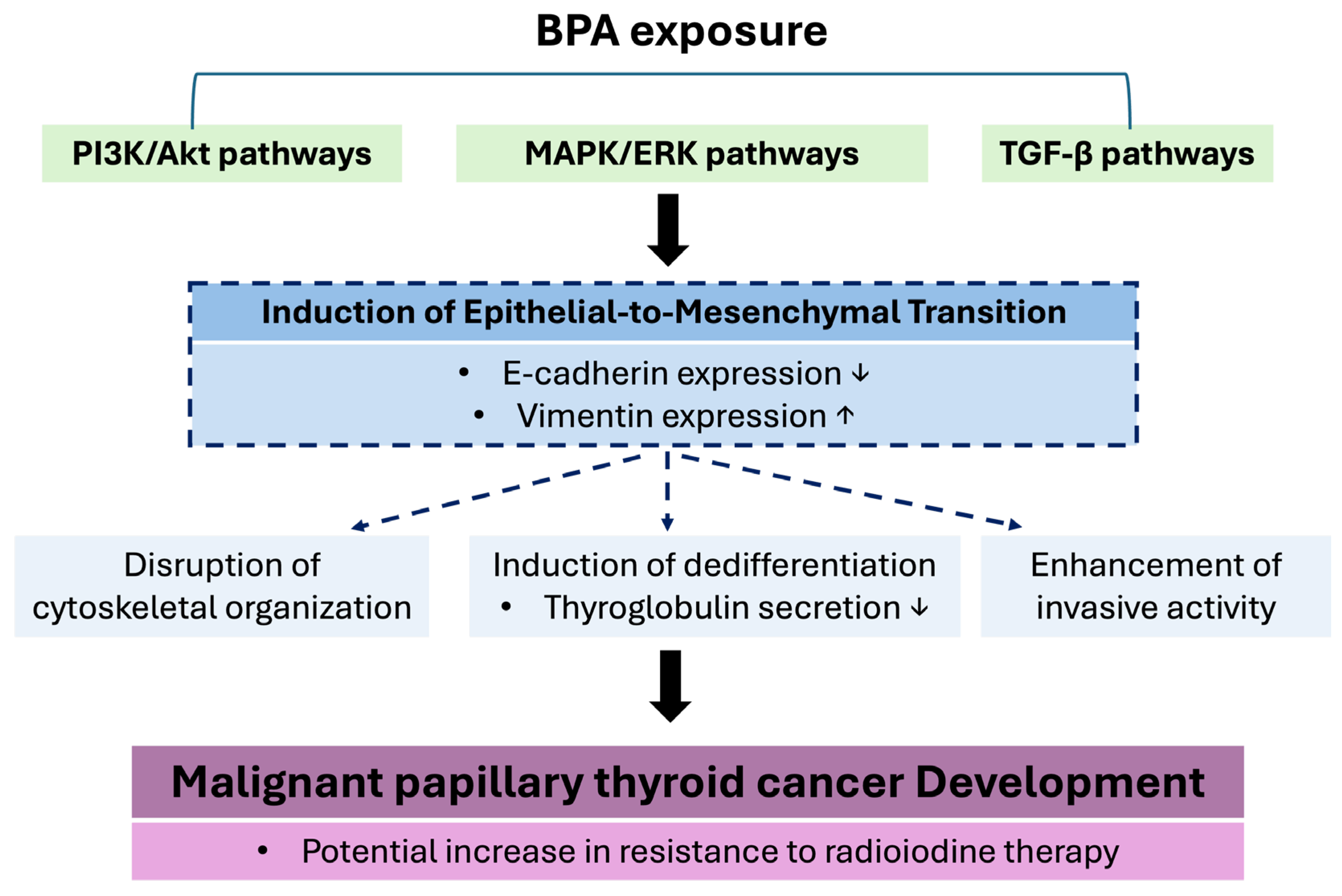
2.4. Effect of BPA on Cancer Characteristics of PTC
BPA Promotes the Occurrence of EMT in PTC
2.5. Effects of BPA on Thyroid Cancer Spheroid in ECM Model
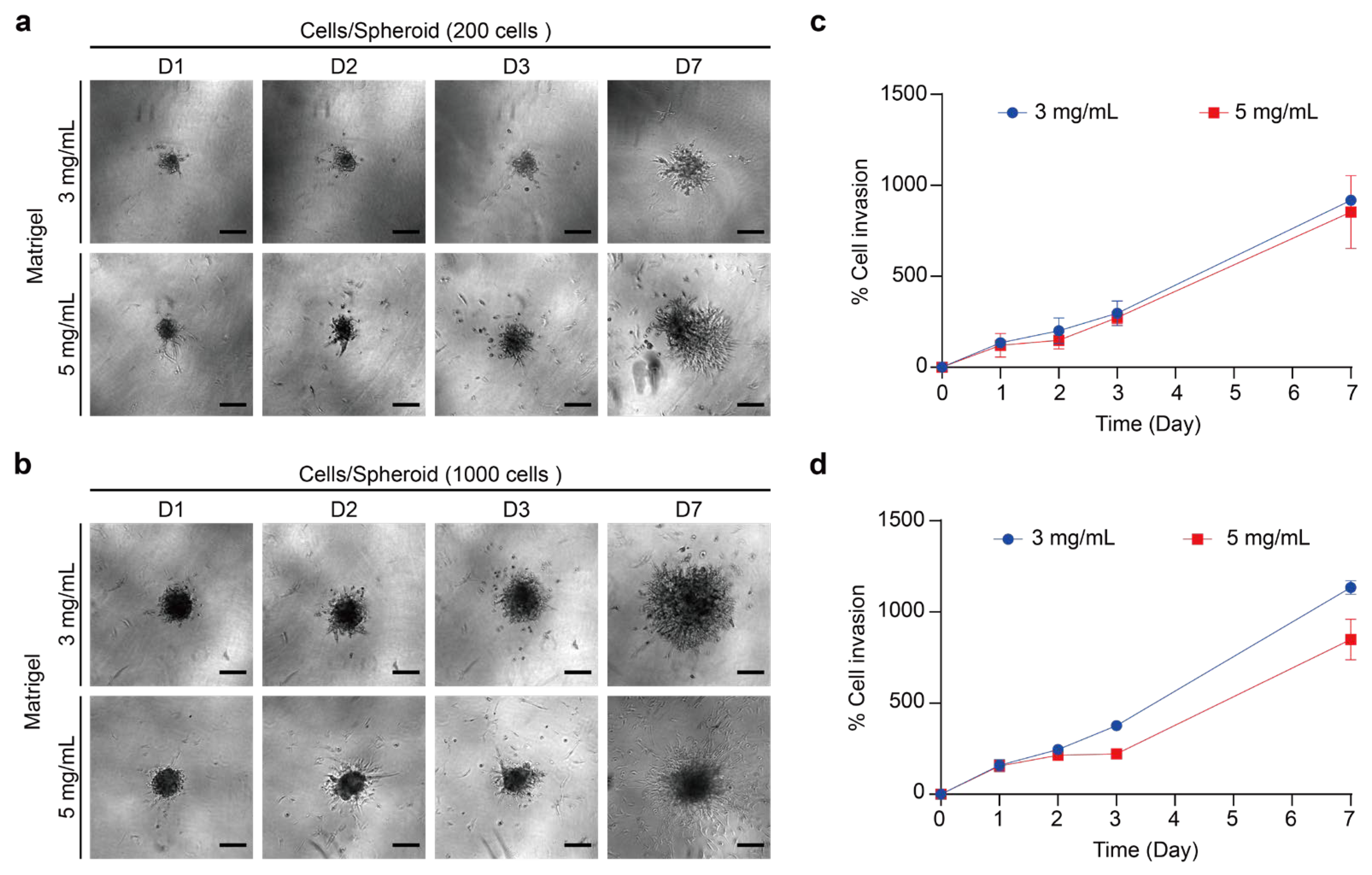
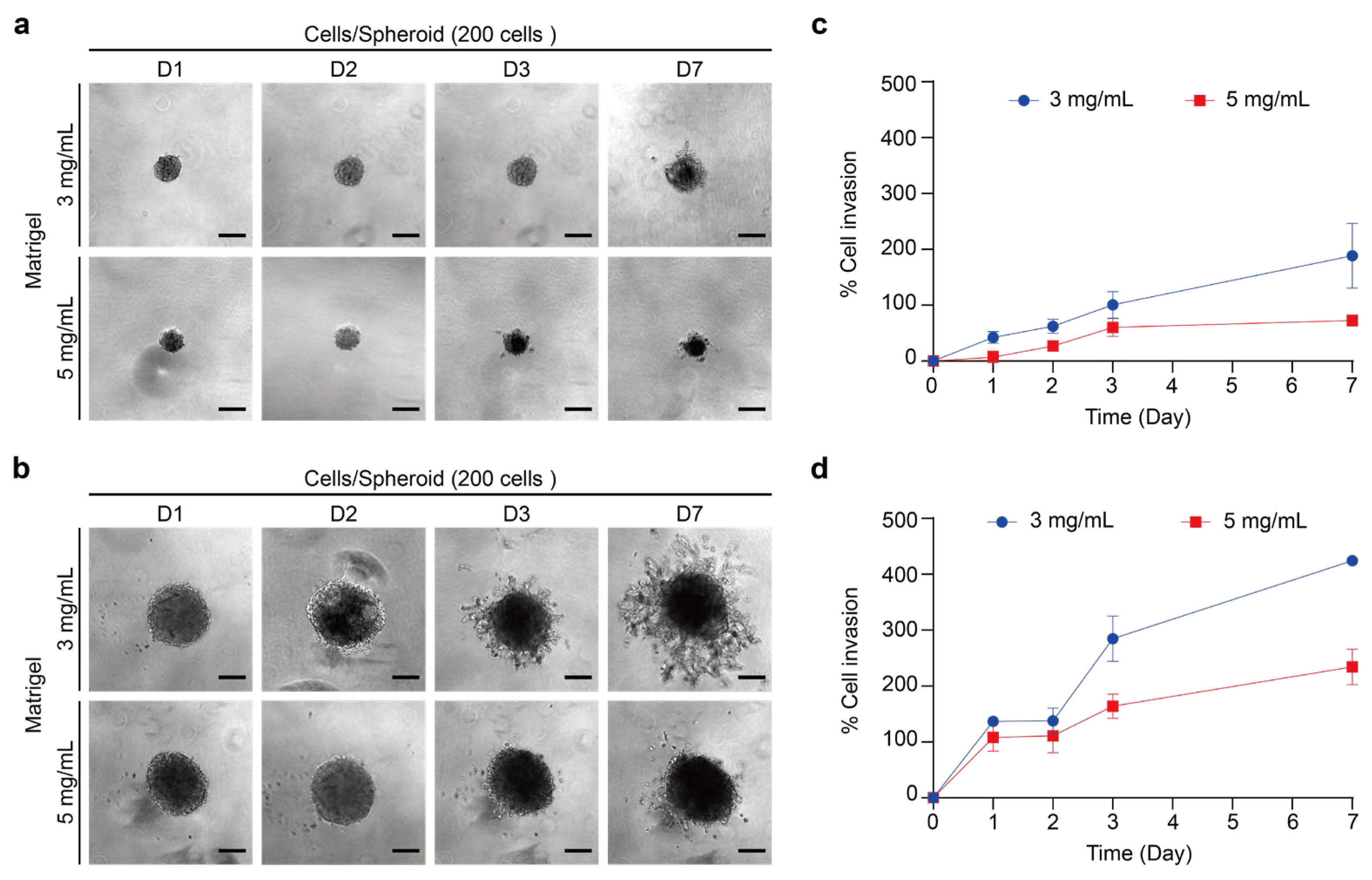
2.5.1. Invasion Assay of 3D Thyroid Cancer Spheroids into Matrigel
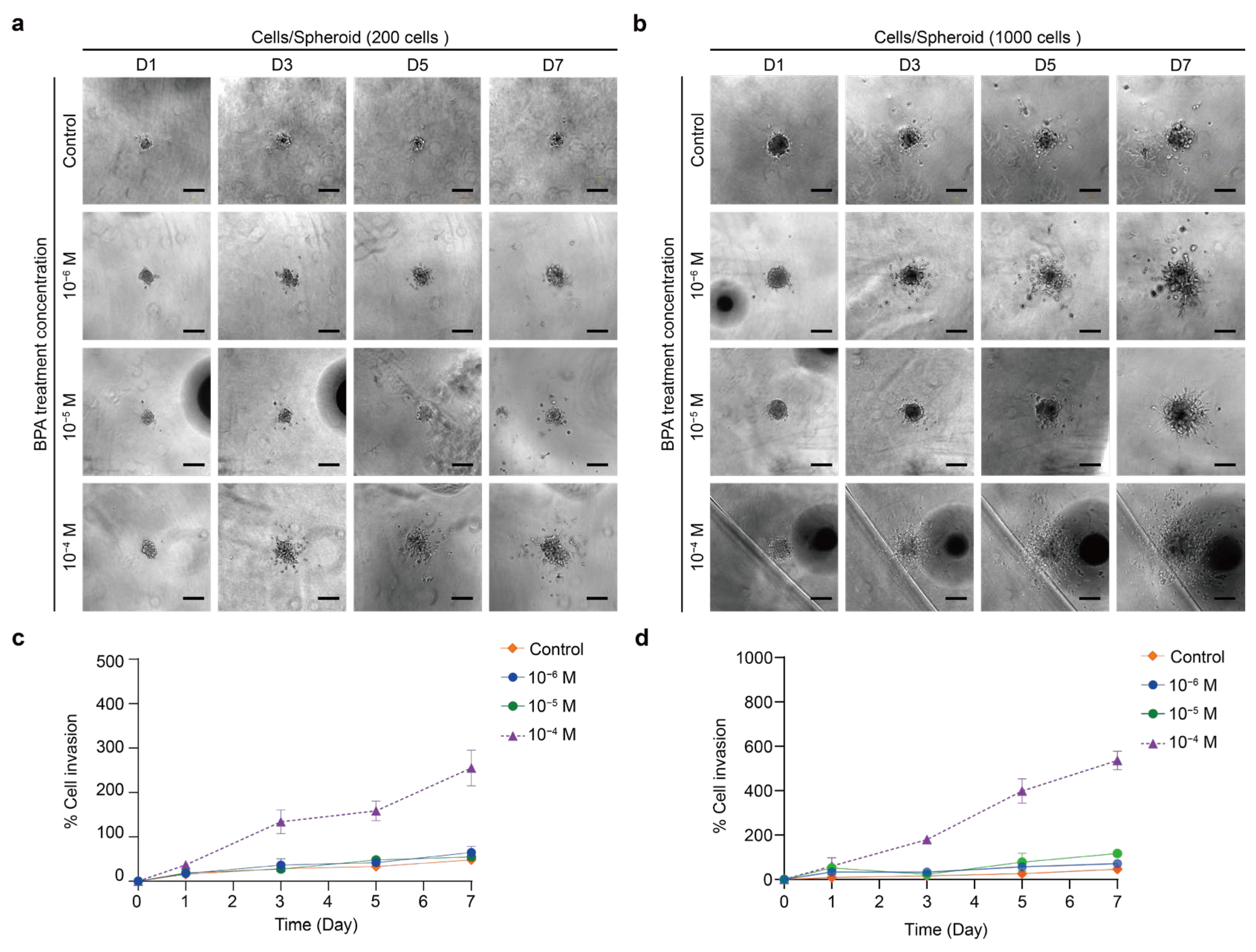
2.5.2. Effect of BPA on the Invasion of PTC Spheroids in the ECM Model
3. Materials and Methods
3.1. Cell Culture
3.2. Spheroid Cultures
3.3. BPA Exposure
3.4. Spheroid Embedding in the ECM Model
3.5. Live/Dead Cell Viability Assay
3.6. Cytoskeleton Analysis
3.7. Immunofluorescence Staining
3.8. Statistics and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Marotta, V.; Grumetto, L.; Neri, I.; Russo, G.; Tortora, A.; Izzo, G.; Panariello, I.; Rocco, D.; Pezzullo, L.; Vitale, M. Exposure to Bisphenol A increases malignancy risk of thyroid nodules in overweight/obese patients. Environ. Pollut. 2023, 316, 120478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Fan, S.; Zhong, Y.; Li, J.; Zhao, Y.; Ni, S.; Liu, J.; Wu, Y. A case-control study of urinary concentrations of bisphenol A, bisphenol F, and bisphenol S and the risk of papillary thyroid cancer. Chemosphere 2023, 312, 137162. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, Y.J. Bisphenols and thyroid hormone. Endocrinol. Metab. 2019, 34, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Kang, Y.; Tae, K.; Bae, G.-U.; Park, J.Y.; Cho, Y.H.; Yang, M. Proteomic Biomarkers for Bisphenol A—Early Exposure and Women’s Thyroid Cancer. Cancer Res. Treat 2017, 50, 111–117. [Google Scholar] [CrossRef]
- Gorini, F.; Bustaffa, E.; Coi, A.; Iervasi, G.; Bianchi, F. Bisphenols as Environmental Triggers of Thyroid Dysfunction: Clues and Evidence. Int. J. Environ. Res. Public Health 2020, 17, 2654. [Google Scholar] [CrossRef]
- Norouzi, F.; Alizadeh, I.; Faraji, M. Human exposure to pesticides and thyroid cancer: A worldwide systematic review of the literatures. Thyroid Res. 2023, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Ikezuki, Y.; Tsutsumi, O.; Takai, Y.; Kamei, Y.; Taketani, Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 2002, 17, 2839–2841. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, X.; Lu, J.; Zou, R.; Ding, R.; Hua, X. Assessment of five typical environmental endocrine disruptors and thyroid cancer risk: A meta-analysis. Front. Endocrinol. 2023, 14, 1283087. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Zhang, J.; Gao, X.; Jin, H.; Liu, R.; Zhang, Z.; Zhang, X.; Wang, X.; Qu, P.; et al. Bisphenol A at a human exposed level can promote epithelial-mesenchymal transition in papillary thyroid carcinoma harbouring BRAFV600E mutation. J. Cell. Mol. Med. 2021, 25, 1739–1749. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Ropero, A.B.; Soriano, S.; García-Arévalo, M.; Ripoll, C.; Fuentes, E.; Quesada, I.; Nadal, Á. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol. Cell. Endocrinol. 2012, 355, 201–207. [Google Scholar] [CrossRef]
- Khan, N.G.; Correia, J.; Adiga, D.; Rai, P.S.; Dsouza, H.S.; Chakrabarty, S.; Kabekkodu, S.P. A comprehensive review on the carcinogenic potential of bisphenol A: Clues and evidence. Environ. Sci. Pollut. Res. 2021, 28, 19643–19663. [Google Scholar] [CrossRef]
- Hoffmann, M.; Rak, A.; Ptak, A. Bisphenol A and its derivatives decrease expression of chemerin, which reverses its stimulatory action in ovarian cancer cells. Toxicol. Lett. 2018, 291, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-F.; Zhang, J.; Shuai, H.-L.; Guan, B.-Z.; Luo, X.; Yan, R.-L. IKKβ/NF-κB mediated the low doses of bisphenol A induced migration of cervical cancer cells. Arch. Biochem. Biophys. 2015, 573, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Weuve, J.; Ye, X.; Jia, L.T.; Hu, H.; Ringer, S.; Huttner, K.; Hauser, R. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ. Health Perspect. 2009, 117, 639–644. [Google Scholar] [CrossRef]
- Alsen, M.; Sinclair, C.; Cooke, P.; Ziadkhanpour, K.; Genden, E.; van Gerwen, M. Endocrine Disrupting Chemicals and Thyroid Cancer: An Overview. Toxics 2021, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Nomiri, S.; Hoshyar, R.; Ambrosino, C.; Tyler, C.R.; Mansouri, B. A mini review of bisphenol A (BPA) effects on cancer-related cellular signaling pathways. Environ. Sci. Pollut. Res. 2019, 26, 8459–8467. [Google Scholar] [CrossRef]
- Sterian Ward, L.; Rabi, L.T.; Nascimento, M.; Teixeira, E.S.; Dal’ Bó Cruz, I.F. ODP276 Thyroid Cancer Cells Are More Resistant Than Normal Cells to Bisphenol-A Cytotoxic Effect. J. Endocr. Soc. 2022, 6, A435–A436. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, N.; Jin, H.; Liu, R.; Zhang, Z.; Cheng, C.; Fan, Z.; Zhang, G.; Xiao, M.; Wu, S.; et al. Bisphenol A drives di(2-ethylhexyl) phthalate promoting thyroid tumorigenesis via regulating HDAC6/PTEN and c-MYC signaling. J. Hazard. Mater. 2022, 425, 127911. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, F.; Zhang, J.; Hao, L.; Jiang, J.; Dang, L.; Mei, D.; Fan, S.; Yu, Y.; Jiang, L. Bisphenol A and estrogen induce proliferation of human thyroid tumor cells via an estrogen-receptor-dependent pathway. Arch. Biochem. Biophys. 2017, 633, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef]
- Huang, B.-W.; Gao, J.-Q. Application of 3D cultured multicellular spheroid tumor models in tumor-targeted drug delivery system research. J. Control. Release 2018, 270, 246–259. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Katt, M.E.; Placone, A.L.; Wong, A.D.; Xu, Z.S.; Searson, P.C. In vitro tumor models: Advantages, disadvantages, variables, and selecting the right platform. Front. Bioeng. Biotechnol. 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures–a comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Melnik, D.; Sahana, J.; Corydon, T.J.; Kopp, S.; Nassef, M.Z.; Wehland, M.; Infanger, M.; Grimm, D.; Krüger, M. Dexamethasone inhibits spheroid formation of thyroid cancer cells exposed to simulated microgravity. Cells 2020, 9, 367. [Google Scholar] [CrossRef]
- Däster, S.; Amatruda, N.; Calabrese, D.; Ivanek, R.; Turrini, E.; Droeser, R.A.; Zajac, P.; Fimognari, C.; Spagnoli, G.C.; Iezzi, G. Induction of hypoxia and necrosis in multicellular tumor spheroids is associated with resistance to chemotherapy treatment. Oncotarget 2017, 8, 1725. [Google Scholar] [CrossRef]
- Salinas-Vera, Y.M.; Valdés, J.; Pérez-Navarro, Y.; Mandujano-Lazaro, G.; Marchat, L.A.; Ramos-Payán, R.; Nuñez-Olvera, S.I.; Pérez-Plascencia, C.; López-Camarillo, C. Three-Dimensional 3D Culture Models in Gynecological and Breast Cancer Research. Front. Oncol. 2022, 12, 826113. [Google Scholar] [CrossRef]
- Jamieson, L.E.; Harrison, D.J.; Campbell, C. Chemical analysis of multicellular tumour spheroids. Analyst 2015, 140, 3910–3920. [Google Scholar]
- Castillo-Rivera, F.; Ondo-Méndez, A.; Guglielmi, J.; Guigonis, J.-M.; Jing, L.; Lindenthal, S.; Gonzalez, A.; López, D.; Cambien, B.; Pourcher, T. Tumor microenvironment affects exogenous sodium/iodide symporter expression. Transl. Oncol. 2021, 14, 100937. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef]
- Saiselet, M.; Floor, S.; Tarabichi, M.; Dom, G.; Hébrant, A.; van Staveren, W.; Maenhaut, C. Thyroid cancer cell lines: An overview. Front. Endocrinol. 2012, 3, 133. [Google Scholar] [CrossRef] [PubMed]
- Dini, S.; Binder, B.; Fischer, S.; Mattheyer, C.; Schmitz, A.; Stelzer, E.; Bean, N.; Green, J. Identifying the necrotic zone boundary in tumour spheroids with pair-correlation functions. J. R. Soc. Interface 2016, 13, 20160649. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.I.; Lassalle, S.; Long-Mira, E.; Hofman, V.; Zangari, J.; Bénaim, G.; Bozec, A.; Guevara, N.; Haudebourg, J.; Birtwisle-Peyrottes, I.; et al. In papillary thyroid carcinoma, TIMP-1 expression correlates with BRAFV600Emutation status and together with hypoxia-related proteins predicts aggressive behavior. Virchows Arch. 2013, 463, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Frasca, F.; Nucera, C.; Pellegriti, G.; Gangemi, P.; Attard, M.; Stella, M.; Loda, M.; Vella, V.; Giordano, C.; Trimarchi, F.; et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr.-Relat. Cancer 2008, 15, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Melillo, R.M.; Castellone, M.D.; Guarino, V.; De Falco, V.; Cirafici, A.M.; Salvatore, G.; Caiazzo, F.; Basolo, F.; Giannini, R.; Kruhoffer, M. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J. Clin. Investig. 2005, 115, 1068–1081. [Google Scholar] [CrossRef] [PubMed]
- Henderson, Y.C.; Ahn, S.-H.; Ryu, J.; Chen, Y.; Williams, M.D.; El-Naggar, A.K.; Gagea, M.; Schweppe, R.E.; Haugen, B.R.; Lai, S.Y.; et al. Development and Characterization of Six New Human Papillary Thyroid Carcinoma Cell Lines. J. Clin. Endocrinol. Metab. 2015, 100, E243–E252. [Google Scholar] [CrossRef]
- Ferrante, A.; Rainaldi, G.; Indovina, P.; Indovina, P.L.; Santini, M.T. Increased cell compaction can augment the resistance of HT-29 human colon adenocarcinoma spheroids to ionizing radiation. Int. J. Oncol. 2006, 28, 111–118. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Lim, J.; Jung, H.-I.; Castellani, G.; Piccinini, F.; Kwak, B. Optimization of Tumor Spheroid Preparation and Morphological Analysis for Drug Evaluation. BioChip J. 2024, 18, 160–169. [Google Scholar] [CrossRef]
- Li, M.; Chai, H.-F.; Peng, F.; Meng, Y.-T.; Zhang, L.-Z.; Zhang, L.; Zou, H.; Liang, Q.-L.; Li, M.-M.; Mao, K.-G.; et al. Estrogen receptor β upregulated by lncRNA-H19 to promote cancer stem-like properties in papillary thyroid carcinoma. Cell Death Dis. 2018, 9, 1120. [Google Scholar] [CrossRef]
- Gajda, E.; Godlewska, M.; Mariak, Z.; Nazaruk, E.; Gawel, D. Combinatory Treatment with miR-7-5p and Drug-Loaded Cubosomes Effectively Impairs Cancer Cells. Int. J. Mol. Sci. 2020, 21, 5039. [Google Scholar] [CrossRef]
- De Rose, F.; Braeuer, M.; Braesch-Andersen, S.; Otto, A.M.; Steiger, K.; Reder, S.; Mall, S.; Nekolla, S.; Schwaiger, M.; Weber, W.A.; et al. Galectin-3 Targeting in Thyroid Orthotopic Tumors Opens New Ways to Characterize Thyroid Cancer. J. Nucl. Med. 2019, 60, 770. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.M.; Gangadaran, P.; Rajendran, R.L.; Hong, C.M.; Lee, J.; Ahn, B.-C. Different expression of thyroid-specific proteins in thyroid cancer cells between 2-dimensional (2D) and 3-dimensional (3D) culture environment. Cells 2022, 11, 3559. [Google Scholar] [CrossRef]
- Wang, X.; Nag, R.; Brunton, N.P.; Siddique, M.A.B.; Harrison, S.M.; Monahan, F.J.; Cummins, E. Human health risk assessment of bisphenol A (BPA) through meat products. Environ. Res. 2022, 213, 113734. [Google Scholar] [CrossRef]
- Heimeier, R.A.; Das, B.; Buchholz, D.R.; Shi, Y.-B. The Xenoestrogen Bisphenol A Inhibits Postembryonic Vertebrate Development by Antagonizing Gene Regulation by Thyroid Hormone. Endocrinology 2009, 150, 2964–2973. [Google Scholar] [CrossRef]
- Aysin, F. Bisphenol promotes cell death in healthy respiratory system cells through inhibition of cell proliferation and induction of G2/M cell cycle arrest. Environ. Toxicol. 2024, 39, 3264–3273. [Google Scholar] [CrossRef]
- Urriola-Muñoz, P.; Lagos-Cabré, R.; Patiño-García, D.; Reyes, J.G.; Moreno, R.D. Bisphenol-A and Nonylphenol Induce Apoptosis in Reproductive Tract Cancer Cell Lines by the Activation of ADAM17. Int. J. Mol. Sci. 2018, 19, 2238. [Google Scholar] [CrossRef]
- Lee, M.A.; Bergdorf, K.N.; Phifer, C.J.; Jones, C.Y.; Byon, S.Y.; Sawyer, L.M.; Bauer, J.A.; Weiss, V.L. Novel three-dimensional cultures provide insights into thyroid cancer behavior. Endocr.-Relat. Cancer 2020, 27, 111–121. [Google Scholar] [CrossRef]
- Yoo, M.H.; Lee, S.-J.; Kim, W.; Kim, Y.; Kim, Y.-B.; Moon, K.S.; Lee, B.-S. Bisphenol A impairs renal function by reducing Na+/K+-ATPase and F-actin expression, kidney tubule formation in vitro and in vivo. Ecotoxicol. Environ. Saf. 2022, 246, 114141. [Google Scholar] [CrossRef]
- Yoo, M.H.; Lee, A.R.; Kim, W.; Yu, W.-J.; Lee, B.-S. Bisphenol A is more potent than bisphenol S in influencing the physiological and pathological functions of lungs via inducing lung fibrosis and stimulating metastasis. Ecotoxicol. Environ. Saf. 2023, 264, 115479. [Google Scholar] [CrossRef]
- Ong, M.S.; Deng, S.; Halim, C.E.; Cai, W.; Tan, T.Z.; Huang, R.Y.-J.; Sethi, G.; Hooi, S.C.; Kumar, A.P.; Yap, C.T. Cytoskeletal Proteins in Cancer and Intracellular Stress: A Therapeutic Perspective. Cancers 2020, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Ditto, M.; Jacho, D.; Eisenmann, K.M.; Yildirim-Ayan, E. Extracellular Mechanical Stimuli Alters the Metastatic Progression of Prostate Cancer Cells within 3D Tissue Matrix. Bioengineering 2023, 10, 1271. [Google Scholar] [CrossRef]
- Suresh, S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007, 3, 413–438. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, S.; Guise, T.A.; Chirgwin, J. The critical role of the bone microenvironment in cancer metastases. Mol. Cell. Endocrinol. 2009, 310, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Steurer, S.; Schneider, J.; Büscheck, F.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Hinsch, A.; Höflmayer, D.; Weidemann, S.; Fraune, C.; et al. Immunohistochemically detectable thyroglobulin expression in extrathyroidal cancer is 100% specific for thyroidal tumor origin. Ann. Diagn. Pathol. 2021, 54, 151793. [Google Scholar] [CrossRef]
- Watson-Brown, P.; Anderson, D. Differentiated thyroid cancer: A guide to survivorship care. Aust. J. Gen. Pract. 2023, 52, 47–51. [Google Scholar] [CrossRef]
- Evans, C.; Tennant, S.; Perros, P. Thyroglobulin in differentiated thyroid cancer. Clin. Chim. Acta 2015, 444, 310–317. [Google Scholar] [CrossRef]
- Lin, J.-D. Thyroglobulin and human thyroid cancer. Clin. Chim. Acta 2008, 388, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, H.; Karlsen, M.; Karlton, E.; Zedenius, J.; Larsson, C.; Ihre Lundgren, C.; Juhlin, C.C.; Shabo, I. Thyroglobulin expression, Ki-67 index, and lymph node ratio in the prognostic assessment of papillary thyroid cancer. Sci. Rep. 2023, 13, 1070. [Google Scholar] [CrossRef]
- Indrasena, B.S.H. Use of thyroglobulin as a tumour marker. World J. Biol. Chem. 2017, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Peiris, A.N.; Medlock, D.; Gavin, M. Thyroglobulin for Monitoring for Thyroid Cancer Recurrence. JAMA 2019, 321, 1228. [Google Scholar] [CrossRef]
- Tous, C.; Muñoz-Redondo, C.; Gavilán, A.; Bravo-Gil, N.; Baco-Antón, F.; Navarro-González, E.; Antiñolo, G.; Borrego, S. Delving into the Role of lncRNAs in Papillary Thyroid Cancer: Upregulation of LINC00887 Promotes Cell Proliferation, Growth and Invasion. Int. J. Mol. Sci. 2024, 25, 1587. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Sun, F.; Xing, L.; Sun, X. Towards an era of precise diagnosis and treatment: Role of novel molecular modification-based imaging and therapy for dedifferentiated thyroid cancer. Front. Endocrinol. 2022, 13, 980582. [Google Scholar] [CrossRef]
- Luo, H.; Xia, X.; Kim, G.D.; Liu, Y.; Xue, Z.; Zhang, L.; Shu, Y.; Yang, T.; Chen, Y.; Zhang, S.; et al. Characterizing dedifferentiation of thyroid cancer by integrated analysis. Sci. Adv. 2021, 7, eabf3657. [Google Scholar] [CrossRef]
- Zelinskaya, A. Immunocytochemical characteristics of thyrocytes in radioiodine refractory metastases of papillary thyroid cancer. Exp. Oncol. 2019, 41, 342–345. [Google Scholar] [CrossRef]
- Fabien, N.; Fusco, A.; Santoro, M.; Barbier, Y.; Dubois, P.-M.; Paulin, C. Description of a human papillary thyroid carcinoma cell line. Morphologic study and expression of tumoral markers. Cancer 1994, 73, 2206–2212. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kuzuya, H.; Nakao, K. Thyroid Hormone Action Is Disrupted by Bisphenol A as an Antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, K.; Guo, J.; Yang, J.; Su, Y. Bisphenol A exposure and thyroid dysfunction during pregnancy: A systematic review. Reprod. Toxicol. 2024, 129, 108680. [Google Scholar] [CrossRef]
- Ryszawy, D.; Pudełek, M.; Kochanowski, P.; Janik-Olchawa, N.; Bogusz, J.; Rąpała, M.; Koczurkiewicz, P.; Mikołajczyk, J.; Borek, I.; Kędracka-Krok, S.; et al. High bisphenol A concentrations augment the invasiveness of tumor cells through Snail-1/Cx43/ERRγ-dependent epithelial-mesenchymal transition. Toxicol. Vitro 2020, 62, 104676. [Google Scholar] [CrossRef]
- Liu, J.; Tian, M.; Qin, H.; Chen, D.; Mzava, S.M.; Wang, X.; Bigambo, F.M. Maternal bisphenols exposure and thyroid function in children: A systematic review and meta-analysis. Front. Endocrinol. 2024, 15, 1420540. [Google Scholar] [CrossRef]
- Li, L.; Ying, Y.; Zhang, C.; Wang, W.; Li, Y.; Feng, Y.; Liang, J.; Song, H.; Wang, Y. Bisphenol A exposure and risk of thyroid nodules in Chinese women: A case-control study. Environ. Int. 2019, 126, 321–328. [Google Scholar] [CrossRef]
- Kogai, T.; Brent, G.A. The sodium iodide symporter (NIS): Regulation and approaches to targeting for cancer therapeutics. Pharmacol. Ther. 2012, 135, 355–370. [Google Scholar] [CrossRef]
- Chung, J.-K.; Youn, H.W.; Kang, J.H.; Lee, H.Y.; Kang, K.W. Sodium Iodide Symporter and the Radioiodine Treatment of Thyroid Carcinoma. Nucl. Med. Mol. Imaging 2010, 44, 4–14. [Google Scholar] [CrossRef]
- Lin, H.; Li, H.; Lu, G.; Chen, Z.; Sun, W.; Shi, Y.; Fu, Z.; Huang, B.; Zhu, X.; Lu, W.; et al. Low Dose of Bisphenol A Modulates Ovarian Cancer Gene Expression Profile and Promotes Epithelial to Mesenchymal Transition via Canonical Wnt Pathway. Toxicol. Sci. 2018, 164, 527–538. [Google Scholar] [CrossRef]
- Wu, J.-s.; Jiang, J.; Chen, B.-j.; Wang, K.; Tang, Y.-l.; Liang, X.-h. Plasticity of cancer cell invasion: Patterns and mechanisms. Transl. Oncol. 2021, 14, 100899. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, J.; Zhong, C.; Yu, L.; Zhu, Y.; Xi, X.; Du, B. Polyphyllin VII as a potential medication for targeting epithelial mesenchymal transitionin in thyroid cancer. J. Pharmacol. Sci. 2024, 156, 49–56. [Google Scholar] [CrossRef]
- Vasko, V.; Espinosa, A.V.; Scouten, W.; He, H.; Auer, H.; Liyanarachchi, S.; Larin, A.; Savchenko, V.; Francis, G.L.; de la Chapelle, A.; et al. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc. Natl. Acad. Sci. USA 2007, 104, 2803–2808. [Google Scholar] [CrossRef] [PubMed]
- Buehler, D.; Hardin, H.; Shan, W.; Montemayor-Garcia, C.; Rush, P.S.; Asioli, S.; Chen, H.; Lloyd, R.V. Expression of epithelial-mesenchymal transition regulators SNAI2 and TWIST1 in thyroid carcinomas. Mod. Pathol. 2013, 26, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xiao, J.; Yi, D.; Ding, H.; Wang, R.; Duan, Z.; Liu, Z.; Shi, X.; Shen, M.; Sang, J. Cytosolic Cadherin 4 promotes angiogenesis and metastasis in papillary thyroid cancer by suppressing the ubiquitination/degradation of β-catenin. J. Transl. Med. 2024, 22, 201. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cai, Y.; Song, F.; Zhu, L.; Hu, Y.; Liu, Y.; Ma, W.; Ge, J.; Zeng, Q.; Ding, L.; et al. TESC promotes differentiated thyroid cancer development by activating ERK and weakening NIS and radioiodine uptake. Endocrine 2023, 81, 503–512. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Gong, Y.; Deng, J. E-cadherin on epithelial–mesenchymal transition in thyroid cancer. Cancer Cell Int. 2021, 21, 695. [Google Scholar] [CrossRef]
- Huang, Y.L.; Ma, Y.; Wu, C.; Shiau, C.; Segall, J.E.; Wu, M. Tumor spheroids under perfusion within a 3D microfluidic platform reveal critical roles of cell-cell adhesion in tumor invasion. Sci. Rep. 2020, 10, 9648. [Google Scholar] [CrossRef] [PubMed]
- Aghajani, M.J.; Yang, T.; Schmitz, U.; James, A.; McCafferty, C.E.; de Souza, P.; Niles, N.; Roberts, T.L. Epithelial-to-mesenchymal transition and its association with PD-L1 and CD8 in thyroid cancer. Endocr. Connect. 2020, 9, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Berr, A.L.; Wiese, K.; dos Santos, G.; Koch, C.M.; Anekalla, K.R.; Kidd, M.; Davis, J.M.; Cheng, Y.; Hu, Y.-S.; Ridge, K.M. Vimentin is required for tumor progression and metastasis in a mouse model of non–small cell lung cancer. Oncogene 2023, 42, 2074–2087. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Miranda, J.; Gallego-Gutiérrez, H.; Cano-Cortina, M.; Amaya, E. Relationship between apical junction proteins, gene expression and cancer. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183278. [Google Scholar] [CrossRef]
- Usman, S.; Waseem, N.H.; Nguyen, T.K.N.; Mohsin, S.; Jamal, A.; Teh, M.-T.; Waseem, A. Vimentin is at the heart of epithelial mesenchymal transition (EMT) mediated metastasis. Cancers 2021, 13, 4985. [Google Scholar] [CrossRef]
- Patel, B.B.; Kasneci, A.; Bolt, A.M.; Di Lalla, V.; Di Iorio, M.R.; Raad, M.; Mann, K.K.; Chalifour, L.E. Chronic Exposure to Bisphenol A Reduces Successful Cardiac Remodeling After an Experimental Myocardial Infarction in Male C57bl/6n Mice. Toxicol. Sci. 2015, 146, 101–115. [Google Scholar] [CrossRef]
- Öz, E.; Tüylü Küçükkılınç, T. Combined effect of fulvestrant and low dose BPA: Comparative implications on EMT, apoptosis, and TGF-β1 signaling in HepG2 cells. Drug Chem. Toxicol. 2022, 45, 2285–2291. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Hwang, K.-A.; Hyun, S.-H.; Nam, K.-H.; Lee, C.-K.; Choi, K.-C. Bisphenol A and Nonylphenol Have the Potential to Stimulate the Migration of Ovarian Cancer Cells by Inducing Epithelial–Mesenchymal Transition via an Estrogen Receptor Dependent Pathway. Chem. Res. Toxicol. 2015, 28, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-Y.; Zheng, C.-C.; Pan, Q.; Wu, S.-S.; Su, X.; Li, L.; Wu, J.-H.; Sun, Z.-Y. Oral exposure of low-dose bisphenol A promotes proliferation of dorsolateral prostate and induces epithelial–mesenchymal transition in aged rats. Sci. Rep. 2018, 8, 490. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, T.; Kremers, G.-J.; Seynhaeve, A.L.B.; ten Hagen, T.L.M. A microcarrier-based spheroid 3D invasion assay to monitor dynamic cell movement in extracellular matrix. Biol. Proced. Online 2020, 22, 3. [Google Scholar] [CrossRef]
- Gkretsi, V.; Stylianopoulos, T. Cell Adhesion and Matrix Stiffness: Coordinating Cancer Cell Invasion and Metastasis. Front. Oncol. 2018, 8, 145. [Google Scholar] [CrossRef]
- Slater, K.; Partridge, J.; Nadivada, H. Tuning the Elastic Moduli of Corning® Matrigel® and Collagen I 3D Matrices by Varying the Protein Concentration. Corning. 2017. Available online: https://www.corning.com/catalog/cls/documents/application-notes/CLS-AC-AN-449.pdf (accessed on 15 January 2025).
- Vincent, L.; Engler, A.J. 5.5 Effect of Substrate Modulus on Cell Function and Differentiation. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2017; pp. 88–101. [Google Scholar]
- Vinci, M.; Box, C.; Eccles, S.A. Three-dimensional (3D) tumor spheroid invasion assay. JoVE (J. Vis. Exp.) 2015, 99, e52686. [Google Scholar] [CrossRef]
- Brechbuhl, H.M.; Barrett, A.S.; Kopin, E.; Hagen, J.C.; Han, A.L.; Gillen, A.E.; Finlay-Schultz, J.; Cittelly, D.M.; Owens, P.; Horwitz, K.B. Fibroblast subtypes define a metastatic matrisome in breast cancer. JCI Insight 2020, 5, e130751. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fang, Z.; Ma, J. Regulatory mechanisms and clinical significance of vimentin in breast cancer. Biomed. Pharmacother. 2021, 133, 111068. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, R.; Nikiforov, Y.E. RET/PTC Rearrangements and BRAF Mutations in Thyroid Tumorigenesis. Endocrinology 2007, 148, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Caccia, D.; Miccichè, F.; Cassinelli, G.; Mondellini, P.; Casalini, P.; Bongarzone, I. Dasatinib reduces FAK phosphorylation increasing the effects of RPI-1 inhibition in a RET/PTC1-expressing cell line. Mol. Cancer 2010, 9, 278. [Google Scholar] [CrossRef]
- Qin, T.; Zhang, X.; Guo, T.; Yang, T.; Gao, Y.; Hao, W.; Xiao, X. Epigenetic Alteration Shaped by the Environmental Chemical Bisphenol A. Front. Genet. 2021, 11, 618966. [Google Scholar] [CrossRef] [PubMed]
- Kakadia, S.; Yarlagadda, N.; Awad, R.; Kundranda, M.; Niu, J.; Naraev, B.; Mina, L.; Dragovich, T.; Gimbel, M.; Mahmoud, F. Mechanisms of resistance to BRAF and MEK inhibitors and clinical update of US Food and Drug Administration-approved targeted therapy in advanced melanoma. OncoTargets Ther. 2018, 11, 7095–7107. [Google Scholar] [CrossRef] [PubMed]
- Swaika, A.; Crozier, J.A.; Joseph, R.W. Vemurafenib: An evidence-based review of its clinical utility in the treatment of metastatic melanoma. Drug Des. Dev. Ther. 2014, 8, 775–787. [Google Scholar]
- Mohammadi, M.; Hedayati, M. A brief review on the molecular basis of medullary thyroid carcinoma. Cell J. (Yakhteh) 2016, 18, 485. [Google Scholar]
- Regua, A.T.; Najjar, M.; Lo, H.-W. RET signaling pathway and RET inhibitors in human cancer. Front. Oncol. 2022, 12, 932353. [Google Scholar] [CrossRef]
- Cuomo, D.; Porreca, I.; Cobellis, G.; Tarallo, R.; Nassa, G.; Falco, G.; Nardone, A.; Rizzo, F.; Mallardo, M.; Ambrosino, C. Carcinogenic risk and Bisphenol A exposure: A focus on molecular aspects in endoderm derived glands. Mol. Cell. Endocrinol. 2017, 457, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.G.; Fortunati, N.; Boccuzzi, G. Epigenetics modifications and therapeutic prospects in human thyroid cancer. Front. Endocrinol. 2012, 3, 40. [Google Scholar]
- Alavian-Ghavanini, A.; Lin, P.-I.; Lind, P.M.; Risén Rimfors, S.; Halin Lejonklou, M.; Dunder, L.; Tang, M.; Lindh, C.; Bornehag, C.-G.; Rüegg, J. Prenatal Bisphenol A Exposure is Linked to Epigenetic Changes in Glutamate Receptor Subunit Gene Grin2b in Female Rats and Humans. Sci. Rep. 2018, 8, 11315. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Nuñez, F.; Bussaglia, E.; Mauricio, D.; Ybarra, J.; Vilar, M.; Lerma, E.; Leiva, A.d.; Matias-Guiu, X. PTEN Promoter Methylation in Sporadic Thyroid Carcinomas. Thyroid® 2006, 16, 17–23. [Google Scholar] [CrossRef]
- Ptak, A.; Hoffmann, M.; Gruca, I.; Barć, J. Bisphenol A induce ovarian cancer cell migration via the MAPK and PI3K/Akt signalling pathways. Toxicol. Lett. 2014, 229, 357–365. [Google Scholar] [CrossRef]
- Wang, K.-H.; Kao, A.-P.; Chang, C.-C.; Lin, T.-C.; Kuo, T.-C. Bisphenol A-induced epithelial to mesenchymal transition is mediated by cyclooxygenase-2 up-regulation in human endometrial carcinoma cells. Reprod. Toxicol. 2015, 58, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Ye, G.; Tu, X.; Luo, Y.; Cui, W.; Ma, Y.; Wei, L.; Tian, X.; Wang, Q. Roles of ERRα and TGF-β signaling in stemness enhancement induced by 1 µM bisphenol A exposure via human neural stem cells. Exp. Ther. Med. 2022, 23, 164. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, R.; Yin, N.; Faiola, F. In vivo and in vitro transcriptomics meta-analyses reveal that BPA may affect TGF-beta signaling regardless of the toxicology system employed. Environ. Pollut. 2021, 285, 117472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, D.; Lai, Y.; Liu, Y.; Tao, X.; Wang, Q.; Zhao, G.; Gu, H.; Liao, H.; Zhu, Y.; et al. Estrogen induces endometrial cancer cell proliferation and invasion by regulating the fat mass and obesity-associated gene via PI3K/AKT and MAPK signaling pathways. Cancer Lett. 2012, 319, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Goetze, S.; Bungenstock, A.; Czupalla, C.; Eilers, F.; Stawowy, P.; Kintscher, U.; Spencer-Hänsch, C.; Graf, K.; Nürnberg, B.; Law, R.E.; et al. Leptin Induces Endothelial Cell Migration Through Akt, Which Is Inhibited by PPARγ-Ligands. Hypertension 2002, 40, 748–754. [Google Scholar] [CrossRef]
- Niu, L.; Jia, J.; Yang, H.; Liu, S.; Wang, H.; Yan, Y.; Li, Q.; Dong, Q.; Zhang, H.; Zhao, G.; et al. Bisphenol A: Unveiling Its Role in Glioma Progression and Tumor Growth. Int. J. Mol. Sci. 2024, 25, 2504. [Google Scholar] [CrossRef]
- Kwon, Y. Potential Pro-Tumorigenic Effect of Bisphenol A in Breast Cancer via Altering the Tumor Microenvironment. Cancers 2022, 14, 3021. [Google Scholar] [CrossRef]
- Focaccetti, C.; Nardozi, D.; Benvenuto, M.; Lucarini, V.; Angiolini, V.; Carrano, R.; Scimeca, M.; Servadei, F.; Mauriello, A.; Mancini, P.; et al. Bisphenol-A in Drinking Water Accelerates Mammary Cancerogenesis and Favors an Immunosuppressive Tumor Microenvironment in BALB–neuT Mice. Int. J. Mol. Sci. 2024, 25, 6259. [Google Scholar] [CrossRef]
- Xu, J.; Huang, G.; Guo, T.L. Developmental Bisphenol A Exposure Modulates Immune-Related Diseases. Toxics 2016, 4, 23. [Google Scholar] [CrossRef]
- Ratajczak-Wrona, W.; Rusak, M.; Nowak, K.; Dabrowska, M.; Radziwon, P.; Jablonska, E. Effect of bisphenol A on human neutrophils immunophenotype. Sci. Rep. 2020, 10, 3083. [Google Scholar] [CrossRef]
- Sowers, M.L.; Tang, H.; Tian, B.; Goldblum, R.; Midoro-Horiuti, T.; Zhang, K. Bisphenol A Activates an Innate Viral Immune Response Pathway. J. Proteome Res. 2020, 19, 644–654. [Google Scholar] [CrossRef]
- Xia, B.; Qiu, L.; Yue, J.; Si, J.; Zhang, H. The metabolic crosstalk of cancer-associated fibroblasts and tumor cells: Recent advances and future perspectives. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2024, 1879, 189190. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Xie, R.-H.; Li, P.-H.; Chen, C.-Y.; You, B.-H.; Sun, Y.-C.; Chou, C.-K.; Chang, Y.-H.; Lin, W.-C.; Chen, G.-Y. Environmental Exposure to Bisphenol A Enhances Invasiveness in Papillary Thyroid Cancer. Int. J. Mol. Sci. 2025, 26, 814. https://doi.org/10.3390/ijms26020814
Huang C-Y, Xie R-H, Li P-H, Chen C-Y, You B-H, Sun Y-C, Chou C-K, Chang Y-H, Lin W-C, Chen G-Y. Environmental Exposure to Bisphenol A Enhances Invasiveness in Papillary Thyroid Cancer. International Journal of Molecular Sciences. 2025; 26(2):814. https://doi.org/10.3390/ijms26020814
Chicago/Turabian StyleHuang, Chien-Yu, Ren-Hao Xie, Pin-Hsuan Li, Chong-You Chen, Bo-Hong You, Yuan-Chin Sun, Chen-Kai Chou, Yen-Hsiang Chang, Wei-Che Lin, and Guan-Yu Chen. 2025. "Environmental Exposure to Bisphenol A Enhances Invasiveness in Papillary Thyroid Cancer" International Journal of Molecular Sciences 26, no. 2: 814. https://doi.org/10.3390/ijms26020814
APA StyleHuang, C.-Y., Xie, R.-H., Li, P.-H., Chen, C.-Y., You, B.-H., Sun, Y.-C., Chou, C.-K., Chang, Y.-H., Lin, W.-C., & Chen, G.-Y. (2025). Environmental Exposure to Bisphenol A Enhances Invasiveness in Papillary Thyroid Cancer. International Journal of Molecular Sciences, 26(2), 814. https://doi.org/10.3390/ijms26020814








