Abstract
Epilepsy affects 50 million people worldwide and is drug-resistant in approximately one-third of cases. Even when a structural lesion is identified as the epileptogenic focus, understanding the underlying genetic causes is crucial to guide both counseling and treatment decisions. Both somatic and germline DNA variants may contribute to the lesion itself and/or influence the severity of symptoms. We therefore used whole exome sequencing (WES) to search for potentially pathogenic somatic DNA variants in brain samples from children with lesional epilepsy who underwent epilepsy surgery. WES was performed on 20 paired DNA samples extracted from both lesional brain tissue and reference tissue from the same patient, such as leukocytes or fibroblasts. The paired WES data were jointly analyzed using GATK Mutect2 to identify somatic single nucleotide variants (SNVs) or insertions/deletions (InDels), which were subsequently evaluated in silico for their disease-causing potential using MutationTaster2021. We identified known pathogenic somatic variants in five patients (25%) with variant allele frequencies (VAF) ranging from 3–35% in the genes MTOR, TSC2, PIK3CA, FGFR1, and PIK3R1 as potential causes of cortical malformations or central nervous system (CNS) tumors. Depending on the VAF, we used different methods such as Sanger sequencing, allele-specific qPCR, or targeted ultra-deep sequencing (amplicon sequencing) to confirm the variant. In contrast to the usually straightforward confirmation of germline variants, the validation of somatic variants is more challenging because current methods have limitations in sensitivity, specificity, and cost-effectiveness. In our study, WES identified additional somatic variant candidates in additional genes with VAFs ranging from 0.7–7.0% that could not be validated by an orthogonal method. This highlights the importance of variant validation, especially for those with very low allele frequencies.
1. Introduction
Epilepsy is one of the most common neurological disorders, affecting approximately 50 million people affected worldwide []. The use of anti-seizure medication (ASM) is typically the first-line treatment for epilepsy. However, when seizures persist despite the administration of two appropriately selected ASMs, the condition is classified as “drug-resistant epilepsy” (DRE) []. In such cases, epilepsy surgery becomes a potential alternative, especially for structural DRE. Unfortunately, even when surgery is performed with curative intent and believed to be performed appropriately, approximately 30% of patients fail to achieve seizure freedom, sometimes with recurrences years after the initial surgery []. The reasons why surgery does not result in a cure in approximately one-third of these cases are the subject of active research and remain partly unclear [,].
Several hypotheses have been proposed for the molecular basis of DRE, including the “genetic variation hypothesis”, the “therapeutic target hypothesis”, and the “drug transporter hypothesis” []. It has been hypothesized that changes in the expression or function of drug-metabolizing enzymes, drug targets, or drug transporters may predispose to DRE, but this has been demonstrated in only a few examples. Such changes could be induced either directly by genetic alterations in the respective coding genes, or indirectly by the activation of inflammatory pathways, ultimately leading to secondary changes in gene expression [,].
In our study, we searched for pathogenic or likely pathogenic somatic variants in brain tissue excised during epilepsy surgery in children with focal structural epilepsy. For a description of the patient cohort, see Table 1. The identification of potentially pathogenic germline variants using WES of DNA extracted from blood was not the focus of this study but has been previously investigated. We depicted these results in Table 1. WES data from these previous investigations were used in the present study solely for paired data analysis in order to identify somatic variants and to exclude germline variants. In general, structural brain abnormalities can include malformations of cortical development (MCD), tumors, and hippocampal sclerosis (HS), although the latter is more commonly observed in adults. All of these abnormalities have been shown to play a role in DRE []. The first two conditions—(i) focal cortical dysplasia (FCD), hemimegalencephaly, and polymicrogyria as common forms of MCD, and (ii) common tumor types such as dysembryoplastic neuroepithelial tumors (DNET) and gangliogliomas (GG)—are particularly important in the pediatric population. Seizures are often the only symptom associated with these slow-growing, typically benign tumors, which are hence referred to as long-term epilepsy-associated tumors (LEATs) [,]. In addition, lesions acquired early in life, such as those resulting from perinatal infarction or hemorrhage, are a further common cause of lesional epilepsy in children. FCDs are classified by the International League Against Epilepsy (ILAE) into three different types based on their histopathologic appearance [,]: (i) FCDI is an isolated lesion with focal abnormalities of vertical (Ia), horizontal (Ib), or both vertical and horizontal (Ic) cortical lamination, (ii) FCDII is characterized by dysmorphic neurons with (IIB) or without (IIa) balloon cells, and (iii) FCDIII denotes malformed cortical lamination plus other lesions, such as hippocampal sclerosis (IIIa), tumors (IIIb), vascular malformations (IIIc), or other, e.g., post-traumatic or inflammatory lesions (IIId).

Table 1.
List of all subjects included in the study with accompanying additional information, allocated to the following etiologic groups: (A) inflammation, (B) malformations of cortical development, (C) hippocampal sclerosis, (D) vascular malformation, (E) brain tumors, and (F) post-traumatic lesions. Investigated patient material: (1) subdural (not cortical) sampling; (2) cortical biopsy, (3) HC biopsy. Abbreviations: DNET, dysembryoplastic neuroepithelial tumor; FCD, focal cortical dysplasia; GNT, glioneuronal tumor; HC, hippocampus; het, heterozygous; HMEG, hemimegalencephaly; HS, hippocampal sclerosis; ICA, internal carotid artery; LGGNT, low-grade glioneuronal tumor; MCA, middle cerebral artery; MCD, malformation of cortical development; MVNT, multinodular vacuolating neuronal tumor; nd, not determined; PMG, polymicrogyria; RE, Rasmussen encephalitis; RGNT, rosette-forming glioneuronal tumor; TSC, tuberous sclerosis; WES, whole exome sequencing. * For detailed information on somatic variants, see Table 2.
Epilepsy surgery aims to remove the epileptogenic tissue and often leads to significant improvement in seizure control, even in very young children [,,,]. However, despite surgical intervention, understanding the underlying genetic causes of these epileptogenic lesions remains critical, especially when seizures persist after surgery. Historically, the identification of epilepsy-related genes has focused on inherited germline mutations []. The first epilepsy genes discovered were those encoding ion channels, such as the CHRNA4 gene [], which encodes a nicotinic acetylcholine receptor, and the KCNQ2 gene [], which encodes a voltage-gated ion channel. These findings introduced the concept of monogenic “channelopathies”, in which mutations in a single ion channel gene would cause an increase in neuronal excitability and subsequently generalized epilepsy []. While this mechanism explained only a small fraction of cases, it provided a compelling basis for understanding the genetics of epilepsy. Subsequently, the identification of other epilepsy-associated genes beyond ion channels revealed novel pathogenic principles. Advances in sequencing technologies, particularly next-generation sequencing (NGS), have since expanded the understanding of the genetic drivers of epilepsy. As of September 2024, the latest Genomics England Panel [] for early onset or syndromic epilepsy (v6.4) includes 857 genes known to cause epilepsy in a monogenic, Mendelian fashion. In recent years, research efforts have focused on the detection of a wider range of genetic variants, including somatic variants, which are usually not inherited. These are genetic alterations that arise postzygotically and are restricted to a specific cell type or tissue, e.g., brain tissue, and which have been identified as contributing to the pathogenesis of both MCD and tumors [,,,,], thus representing an important cause of focal structural epilepsy.
One of the most studied genetic pathways implicated in developmental lesions is the mechanistic target of the rapamycin (mTOR) pathway. Somatic variants in genes within this pathway, such as MTOR, TSC1/2, DEPDC5, and AKT3 have been frequently identified in patients with epilepsy-associated MCD [,,,,]. LEATs are often associated with mutations in genes of the mitogen-activated protein kinase (MAPK) pathway, including BRAF and FGFR1 [,].
The aim of the present study was to detect pathogenic somatic small variants, especially SNVs and InDels, in brain biopsy specimens that might have contributed to the (drug-resistant) epilepsy in our patients. We applied WES to DNA extracted from fresh-frozen brain tissue and from blood leukocytes or fibroblasts. Our data analysis considered mechanisms related to both drug resistance and the development of structural lesions or LEATs.
2. Results
2.1. Technical Summary
In all cases included in the study, the quantity and quality of brain tissue samples were adequate for further analysis. Histopathology was not available for two subjects. DNA extracted from brain tissue was used for WES at 100× coverage (for 15 samples) or 500× coverage (for 5 samples) (Table 1). Sequencing at a mean depth of 100× yielded an average of 172 SNVs and small InDels after Mutect2 filtering steps (Figure 1), and sequencing at a higher depth of 500× yielded an average of 11.942 SNVs and small InDels. The higher coverage was chosen during the study to increase the reliable detection of low-frequency variants.
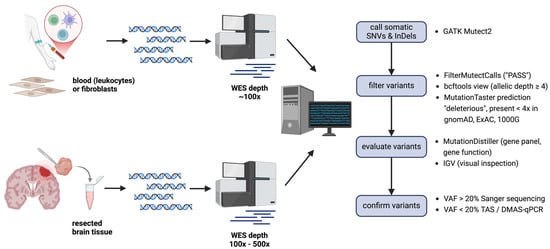
Figure 1.
Single steps performed to identify pathogenic somatic variants in brain biopsy specimens. Mutect2: GATK tool for somatic variant calling; FilterMutectCalls: GATK tool to filter real Mutect2 calls from errors, artifacts, germline variants, and more, probable somatic variants are given the flag “PASS”; bcftools: a tool to manipulate vcf files. It was used to keep only calls with an allelic depth of ≥4); MutationTaster: online variant prediction tool to distinguish pathogenic from benign variants, this tool was also used to filter out common variants from gnomAD, ExAC, and 1000G; MutationDistiller: online variant prioritization tool, used to apply different virtual gene panels taking into account the clinical phenotype of the patients and to prioritize variants based on assumed gene function (pathway-based); IGV: genome browser to display WES data. Abbreviations: DMAS-qPCR, double-mismatch allele-specific quantitative polymerase chain reaction; InDel, insertion and deletion, SNV, single nucleotide variant; TAS, targeted amplicon sequencing; VAF, variant allele frequency; WES, whole exome sequencing. The image was created with BioRender.
2.2. Description of the Patient Cohort
The study included 20 children with structural epilepsy (Table 1). Patients underwent presurgical evaluation as previously described [] and received epilepsy surgery between the ages of 3 months and 17 years. Patients were grouped according to the histopathology of resected tissue (if available) and/or radiologic findings: (A) inflammation, (B) MCD, (C) hippocampal sclerosis, (D) vascular malformation, (E) brain tumors, and (F) post-traumatic lesions.
2.3. Targeted Variant Search by Etiology, Based on Histopathologic/Radiologic Findings
We first searched for pathogenic somatic SNVs in genes known to be involved in each patient’s pathology using dedicated virtual gene panels. Depending on the group to which patients were assigned, we used the following virtual gene panels: (A) inflammation/autoinflammation panel (Table S5), (B) MCD/MCD panel (Table S1), (C) hippocampal sclerosis/epilepsy panel (Table S4), (D) vascular malformation/vascular malformation panel (Table S3), (E) brain tumor/tumorigenesis panel (Table S2), or (F) post-traumatic lesion/epilepsy panel (Table S4). We detected pathogenic somatic variants in genes of the mTOR pathway in three out of ten individuals with MCD (30%) and in two out of four patients with CNS tumors (50%) (for a summary see Table 2).

Table 2.
List of pathogenic somatic variants identified in brain samples that were confirmed by at least one orthogonal method. Description of etiologic groups: (B) malformations of cortical development, (E) brain tumors. fb, fibroblasts, DMAS, double-mismatch allele-specific, Sanger = Sanger sequencing, TAS, targeted amplicon sequencing, VAF, variant allele frequency, WES, whole exome sequencing.
2.3.1. MTOR
In brain sample 3, we identified a previously reported somatic missense variant in MTOR (c.6644C>T, p.S2215F, NM_004958) with a variant allele frequency (VAF) of 13% in the WES data (Figure S1). Confirmatory TAS showed a brain VAF of 7.5% (Figure S2i) and DMAS qPCR of 1.6% (Figure S3). Clinically, the patient had structural DRE with up to ten focal-onset seizures per day since the age of 7 months, despite ASM with lamotrigine, lacosamide, levetiracetam, and valproate. Radiology revealed a hemimegalencephaly (HMEG) (Figure 2a), and the patient underwent a hemispherotomy at the age of 2.5 years. Histopathology revealed reactive gliosis. The patient remained seizure-free for two months post-surgery while still on ASM but was lost to further follow-up. Blood WES did not identify a pathogenic germline variant. The somatic MTOR variant has been previously reported as a somatic variant in brain biopsy specimens from individuals with FCDIIb [] and HMEG. According to the additional specifications to the ACMG/AMP Sequence Variant Interpretation Guidelines [] to be used for somatic variants [], the variant can be classified as “pathogenic”. It is also listed in ClinVar (ID 156703), where it is classified as “pathogenic” for germline occurrences and it is a known hotspot mutation []. We believe that the variant identified in our patient’s brain sample is most likely associated with the formation of the HMEG as an epileptogenic lesion, as single gain-of-function variants in positive regulators of the mTOR pathway (e.g., in PIK3CA, MTOR, or RHEB) are sufficient to cause FCDII or HMEG [,]. This finding again emphasizes the central role of mTOR dysregulation in the pathogenesis of focal MCD such as HMEG.
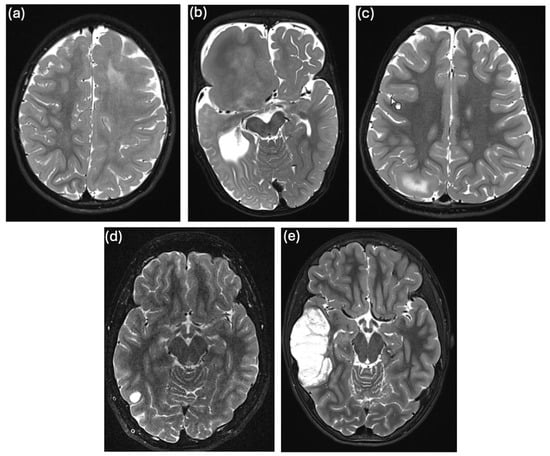
Figure 2.
T2-weighted cMRI images of individuals in whom we detected pathogenic somatic variants: (a) individual 3 with left-sided HMEG, (b) individual 4 with right-sided focal megalencephaly, (c) individual 4 with FCDIIb in the right-sided precuneal area, (d) individual 15 with a small right-sided, temporo-occipital lesion suspicious of LGGNT, and (e) individual 16 with a right-sided, temporal DNET.
2.3.2. PIK3CA
A previously reported somatic missense variant in PIK3CA (c.1624G>A, p.E542K, NM_006218) was detected in brain sample 4, with a VAF of 29% in WES data (Figure S1) and confirmed by Sanger sequencing (Figure S2i). Clinically, the patient presented with macrocephaly and DRE, with up to five focal-onset seizures of altered consciousness per day since the age of 4 months. Other features included syndactyly and polydactyly and the patient was diagnosed with Greig cephalopolysyndactyly syndrome (GCPS, OMIM #175700 []). A radiologic examination revealed right-sided focal hemimegalencephaly and an extensive migration disorder (Figure 2b). EEG showed unilateral nonconvulsive status epilepticus. Medication with oxcarbazepine, lamotrigine, levetiracetam, and clonazepam was ineffective. The patient underwent a hemispherotomy at the age of 1.9 years and has been seizure-free to date, recently without taking any ASM. Histologic analysis of a brain biopsy, which included medullary and ependymal tissue but not the cortex, showed predominantly reactive tissue changes. WES with DNA extracted from blood identified a paternally inherited heterozygous pathogenic germline deletion of 10 bp in GLI3 (ClinVar variation ID 972686). Of note, the patient’s father and grandfather were also diagnosed with GCPS, but had no history of seizures. This PIK3CA variant has been previously reported as a somatic variant in brain biopsy specimens from patients with HMEG [,,]. According to the additional specifications of the ACMG/AMP Sequence Variant Interpretation Guidelines [] to be used for somatic variants [], the variant can be classified as “pathogenic”. It is also listed in ClinVar (ID 31944) and marked as “pathogenic” in germline cases. Greig cephalopolysyndactyly syndrome is typically characterized by frontal bossing, polydactyly and variable syndactyly, peculiar cranial shape, and hypertelorism with variable expressivity. The GLI3 variant in our patient explains the cephalopolysyndactyly phenotype but not necessarily the epileptogenic brain malformation. However, since it has been shown that somatic variants in PIK3CA lead to the formation of HMEG, and the p.E542K variant identified in our patient is located in a mutational hotspot of the gene [], we believe that the somatic PIK3CA variant detected in our patient causes the epileptogenic brain lesion.
2.3.3. TSC2
In brain sample 5, a previously reported nonsense variant in TSC2 (c.3442C>T, p.Q1148*, NM_000548) was detected with a VAF of 3% in the WES data (Figure S1), 2.5% in the confirmatory TAS (Figure S2i) and 1.7% in DMAS-qPCR (Figure S3). Interestingly, the same TSC2 variant was also identified in fibroblasts from a skin punch biopsy with a higher VAF of 6% in DMAS-qPCR (Figure S3) and 7.5% in TAS (Figure S2i). Since both skin fibroblasts and CNS neurons are derived from the same germ cell layer, the ectoderm, we hypothesize that the somatic variant occurred in a common ectodermal progenitor cell. Clinically, the patient had DRE with up to five focal aware seizures per day since the age of 2 years. cMRI revealed focal cortical dysplasia (FCD) in the right precuneal area (Figure 2c). Interictal EEG showed normal background activity with epileptiform discharges in the right occipital region. Seizure control was not achieved with treatment using levetiracetam and oxcarbazepine. The patient underwent epilepsy surgery at the age of 3.5 years with complete resection of the FCD. Histopathology was consistent with FCDIIb. Postoperatively, the patient experienced four additional seizures with the previously known semiology but is now seizure-free for more than two years on lamotrigine monotherapy. Blood WES revealed a heterozygous probable pathogenic nonsense variant in ATP1A2 (ClinVar variation ID 2498438), which was, however, maternally inherited. Variants in ATP1A2 are associated with various forms of migraine, and with epilepsy and developmental and epileptic encephalopathy type 98 (DEE98) [,,]. Homozygous truncating mutations in ATP1A2 have been associated with early lethal hydrops fetalis, arthrogryposis, microcephaly, and polymicrogyria [], although this seems to be a very rare case. Of note, the patient’s mother suffers from migraine, but not from epilepsy. The somatic TSC2 variant has been previously reported as a germline variant in individuals with tuberous sclerosis (TSC) [,,] and is listed in ClinVar (ID 50087) as “pathogenic” for germline occurrences. Current knowledge suggests that brain lesion formation typically requires a “double hit” mechanism involving both a germline and a somatic loss-of-function variant in mTOR pathway repressors, such as TSC2 []. To investigate a potential “second hit” in mTOR repressors in our patient, in addition to the somatic TSC2 variant, we reanalyzed leukocyte the WES data for pathogenic SNVs, small InDels, or larger deletions/CNVs but we did not identify any additional relevant pathogenic germline variant in mTOR pathway genes, except for the ATP1A2 variant. Given reports of pathogenic somatic heterozygous variants in TSC1 or TSC2 causing FCDII without a second hit [,], we hypothesize a similar mechanism in this patient, possibly resulting from haploinsufficiency.
2.3.4. FGFR1 & PIK3R1
In brain sample 15, we detected previously reported pathogenic somatic variants in FGFR1 and PIK3R1. The FGFR1 variant (c.1966A>G, p.K656E, NM_023110) had a VAF of 36% in the WES data (Figure S1) and was confirmed by Sanger sequencing (Figure S2ii). The PIK3R1 variant (c.1690A>G, p.N564D, NM_181523) had a VAF of 33% in brain WES (Figure S1), also confirmed by Sanger sequencing (Figure S2ii). Clinically, the patient had a single bilateral tonic-clonic seizure of unknown origin at the age of 16 years. cMRI revealed a lesion in the right occipital gyrus, suspected to be a low-grade glioneuronal tumor (Figure 2d). The interictal EEG was normal. The patient underwent a lesionectomy shortly after the seizure, based on a suspected tumor diagnosis, despite epilepsy not being officially classified as drug-refractory. He is seizure-free to date. Histopathologic analysis revealed a low-grade glioneuronal tumor (LGGNT). In genome-wide methylation analysis, the DNA methylation profile could not be assigned to any known methylation class, but the highest classifier score was obtained for the rosette-forming glioneuronal tumor (RGNT) class (score slightly below the cutoff for classification). WES on fibroblast DNA was performed for joint calling of somatic variants in the brain sample, without additional evaluation of germline variants. The FGFR1 variant is listed in ClinVar (ID 224897) as “pathogenic” or “likely pathogenic” for germline occurrences. It is a known gain-of-function hotspot mutation [] and has been reported as a somatic variant in RGNTs and other cancers []. The PIK3R1 variant is also listed in ClinVar (ID 376261) as “pathogenic” or “likely pathogenic” for somatic origin and has been frequently detected in vascular malformations and various cancers [].
2.3.5. FGFR1 & NF1
In brain sample 16, we identified the same pathogenic somatic variant in the FGFR1 gene (c.1966A>G, p.K656E, NM_023110) as in brain sample 15. WES revealed a VAF of 24% (Figure S1), the variant was also confirmed by Sanger sequencing (Figure S2ii). In addition, we detected a pathogenic variant in the NF1 gene (c.2824delA, p.S942Afs*, NM_001042492, ClinVar ID 834401) with a VAF of 4.5% in the WES data, although this variant could not be confirmed by TAS (Table S10). Clinically, the patient had epilepsy characterized by focal-onset seizures with progression to generalized tonic-clonic seizures beginning at the age of eight years. These seizures persisted despite treatment with levetiracetam. Cranial MRI showed a right temporal lesion, most likely consistent with a dysembryoplastic neuroepithelial tumor (DNET) (Figure 2e). Interictal EEG showed epileptiform discharges in the right temporal region, and ictal EEG confirmed seizure activity in the same area. Due to progressive tumor growth, the patient underwent lesionectomy at the age of 13.9 years. He remained seizure-free for 14 months after surgery. No further follow-up data were available. Histopathologic analysis was consistent with a diagnosis of DNET or RGNT, WHO grade I. WES on fibroblast DNA was performed for joint calling of somatic variants in the brain sample, without additional evaluation of germline variants.
2.4. Search for Variants in Drug Absorption, Distribution, Metabolism, and Excretion (ADME) Genes
To identify pathogenic somatic variants in genes associated with drug resistance, we used a previously published [,] panel of genes involved in drug absorption, distribution, metabolism, and excretion (ADME) to select relevant variants (Table S6). In total, seven somatic variants predicted to be pathogenic were identified in brain WES from two patients. After visual inspection in IGV and further evaluation, two of these variants, a variant in METAP1 (c.1037C>T, p.A346V, NM_015143) with VAF 0.8% in brain WES of patient 18 with CNS tumor and a variant in TRPV4 (c.1796C>T, p.T599M, NM_021625) with VAF 0,72% in brain WES of patient 10 with TSC, qualified for confirmatory TAS. However, neither variant was confirmed (Table S10).
2.5. Search for Variants in Novel Genes Guided by the Patient Phenotype
We also aimed to identify pathogenic somatic variants in genes not previously associated with any of the etiologies listed in Figure 1. To this end, we applied a filtering strategy based on suspected gene function or involved pathways, guided by the patient’s specific phenotype. Filtering can be done by using the appropriate function in MutationDistiller [] (filter for GO, WikiPathways, or Reactome terms). This identified additional brain somatic variants that were considered potentially pathogenic but were not confirmed by TAS (Table S10). These included a nonsense variant in DAB1 (c.1000C>T, p.Q334*, NM_001365792) with a VAF of 2.7% in the brain WES dataset of patient 13 with complex malformations of cortical development (MCD) and hippocampal sclerosis, a missense variant in PAK6 (c.1916T>A, p.L639Q, ENST00000560346) with a VAF of 1.0% in the brain WES data from patient 14 with unilateral venous cavernous malformation, and a missense variant in APC2 (c.1940G>A, p.G647D, ENST00000233607.2) with VAF of 1.0% was found in brain WES of patient 11 with FCDIIb. As mentioned above, none of the variants were confirmed by TAS.
3. Discussion
The aim of this study was to further deepen our understanding of the genetic basis of lesional DRE by applying WES to DNA from brain biopsy specimens of affected individuals. Our focus was to identify pathogenic somatic variants associated with the formation of specific lesions, such as MCD and CNS tumors, as well as variants that may contribute to drug resistance. Using both histopathologic and radiologic findings, we stratified patients into six etiologic groups, allowing for targeted study using virtual gene panels relevant to each pathology. This approach allowed focused analysis of genetic alterations associated with specific structural etiologies and revealed known pathogenic somatic SNVs in established lesional epilepsy or tumorigenesis genes in 30% of patients with MCD and 50% of patients with CNS tumors, respectively.
We confirmed somatic variants in the PI3K-AKT-mTOR pathway in patients with MCD, specifically in the MTOR, PIK3CA, and TSC2 genes. In all cases presented here, the findings provided further molecular understanding of the patients’ phenotypes, which had not been fully explained by other diagnostic methods. Our findings are consistent with previous studies demonstrating the involvement of postzygotically acquired variants in genes of this pathway in the formation of FCDII and HMEG [,]. However, some studies have reported even higher diagnostic rates within the MCD subgroup. The lower diagnostic rate observed in our study may be due to the fact that the optimization of sample collection and pre-analytic handling for both neuropathologic examination and DNA extraction, according to published guidelines [], was implemented only partway through our study. As a result, it cannot be confirmed consistently that the brain biopsy samples used for DNA extraction were indeed taken from the most affected areas of the lesion.
To date, there is no clear genotype–phenotype correlation between the severity of MCD and the VAF for a specific somatic variant. However, a recent study in mice examined differences and similarities in the effects of somatic variants in either inhibitors (Depdc5, Tsc1, Pten) or activators (Rheb, Mtor) of the mTOR pathway on neuronal morphology, membrane excitability, and excitatory synaptic transmission []. The authors found that both the activation of the activators Rheb or Mtor or inactivation of the inhibitors Depdc5, Tsc1, or Pten—all of which boost mTORC1 activity—cause similar increases in neuronal soma size and mispositioned neurons. However, they differently affected excitatory synaptic transmission in a gene-specific manner: Tsc1 knock-out neurons showed an increase in the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) without a change in their amplitude. In contrast, neurons with activating mutations in Rheb, Mtor, and Pten showed an increased amplitude of sEPSCs but no change in their frequency. The underlying mechanisms for these differences remain unclear, but given the complexity of the mTOR pathway, additional signaling pathways may be involved. Identification of these additional pathways may have practical implications for more targeted therapeutic strategies.
In our study, pathogenic variants in FGFR1 and PIK3R1 were identified in two patients with neuroepithelial tumors, with VAFs ranging from 24–36%. These higher VAFs contrast with the lower VAFs of 3–29% observed in epilepsy lesions without tumor components. This disparity likely reflects the selective growth advantage conferred by somatic variants within tumor cells, highlighting the differences in mutational clonality and selective pressure between tumor-associated and non-tumor-associated epileptic lesions.
Given the difficulty in distinguishing LEAT entities by radiologic and histopathologic methods alone, insights into their genetic biomarkers are valuable and increasingly being applied. In the two cases presented here, molecular genetic findings further supported the histopathologic diagnoses. In brain sample 15, we identified somatic variants in FGFR1 and PIK3R1, strongly supporting the diagnosis of RGNT, as variants in these genes are part of the established molecular profile for RGNTs [,,]. Histopathologic analysis suggested a low-grade neuroepithelial tumor without further specification, while genome-wide methylation analysis, although not an exact match to any known methylation class, was most consistent with RGNT. The molecular genetic findings offer additional confirmation, reinforcing the histopathologic and methylation-based indications. In brain sample 16, a pathogenic somatic variant in FGFR1 was identified, complementing the histopathologic findings suggestive of DNET, which is characterized by somatic FGFR1 variants [].
Overall, we identified pathogenic somatic variants in 50% of CNS tumors. Other studies have reported high diagnostic yields of up to 100% in LEATs, especially when somatic CNV analysis was included. A recent study further highlighted the role of somatic structural variations (SVs) in pediatric brain tumors []. The lower diagnostic yield observed in the CNS tumor subgroup in our study may be due to the fact that we focused our analysis only on SNVs and small indels, and did not include CNV and SV analysis.
We have also investigated pathogenic variants in genes not previously associated with epilepsy lesions and DRE and have identified several candidates by WES. However, confirmation of variants, especially those with very low VAF, has proven challenging. Variant validation primarily aims to distinguish false positives from bona fide somatic variants using an orthogonal method. Different methods are available (e.g., Sanger sequencing, TAS, ddPCR (digital droplet PCR), DMAS-qPCR, and others). Key considerations for our choice of a specific method comprised VAF, experimental effort and cost, and the number of variants to be validated. We used Sanger sequencing for higher VAF variants due to its cost-effectiveness, and commercial TAS for lower VAF variants due to its higher sensitivity, despite its higher cost. In our study, none of the very low-VAF variants could be validated by TAS. This limitation in detecting low-VAF somatic variants in epilepsy lesions using alternative methods has been mentioned by other authors as well [] and has been attributed to sequencing errors in WES or artifacts during variant calling. In addition, it is possible that targeted amplicon sequencing as a validation method lacks sensitivity to detect very low abundance variants.
We first attempted allele-specific double mismatch qPCR (DMAS-qPCR) for variant confirmation, a technique known for its sensitivity, with detection thresholds for variant frequencies as low as 1% or even less []. DMAS-qPCR is widely used for SNP genotyping and mutation detection in cancer research and is most effective when controls for known hotspot mutations are available. It has also been used for confirmation of somatic variants in brain malformation studies []. In our case, we wanted to validate novel mutations without available controls. Relying on differences in Ct-values between affected DNA from brain biopsy specimens and reference DNA from fibroblasts or leukocytes from the same patient proved reduced reliability in our setting, as reflected by differences in VAF between DMAS-qPCR and WES/TAS in the two cases where we used both methods. In addition, primer design and PCR optimization posed significant challenges, as the 3’ ends of the oligonucleotide primers had to be positioned at the variant site, limiting our primer design options. For these reasons, DMAS-qPCR was unsuitable for large-scale variant validation, leading us to adopt TAS as an alternative method. Although amplicon sequencing is theoretically capable of detecting very low VAF, it still presents significant technical challenges. The benefit of high coverage comes at the cost of nearly identical duplicate reads, which add limited information and are susceptible to amplification bias and PCR error propagation, even when a high-fidelity polymerase is used in the initial PCR steps for amplicon generation. To address these issues, it is increasingly recommended to introduce unique molecular identifiers (UMIs) as barcodes prior to the amplification step. This approach allows monitoring of amplification imbalances, elimination of random errors, and reliable identification of true variants at very low VAFs []. It should also be noted that although Illumina sequencing generates data with very low error rates (around 0.1 to 0.5%), making it reliable for most purposes, even these low error rates hinder the accurate detection of somatic variants with VAFs around or below 0.5%. Despite the challenges of variant confirmation, we confirmed pathogenic somatic variants in 25% of our patients. The diagnostic yield of our study is in line with previous publications reporting overall diagnostic yields between 10–56% or even higher for specific subgroups such as HMEG, FCDIIb, or LEATs [,,,].
4. Materials and Methods
4.1. Patient Cohort
We included 20 children with lesional epilepsy who were treated at the German Epilepsy Center for Children and Adolescents at the Charité in Berlin, and who eventually underwent epilepsy surgery. Drug resistance was defined according to the consensus proposal of the ILAE Commission as “failure of adequate trials of two tolerated, appropriately chosen and used antiepileptic drug regimens” []. Patients were phenotyped by pediatric neurologists and all patients underwent additional diagnostic procedures including EEG and cranial MRI. In addition, WES and, in some cases, microarray analysis of blood-derived DNA was performed to address putative underlying germline variants and genetic rearrangements as genetic causes or predisposing factors for epilepsy, the importance of which has been outlined previously []. When blood WES was not available, we performed WES using DNA extracted from skin fibroblasts as the reference tissue for somatic variant calling. Patients were grouped according to histopathology of resected tissue (if available) and/or radiological findings: (A) inflammation (2 patients), (B) malformations of cortical development (10 patients), (C) hippocampal sclerosis (1 patient), (D) vascular malformations (1 patient), (E) brain tumors (4 patients), and (F) post-traumatic lesions (2 patients).
4.2. Sample Processing and DNA Extraction
As part of the study, specimens were taken from the site of lesional alteration during epilepsy surgery for DNA extraction and histopathologic examination. Of note, genomic DNA was not extracted from the exact same biopsy specimen as used for histopathology. Genomic DNA was extracted from fresh-frozen brain tissue using the phenol-chloroform isolation method to obtain high molecular weight genomic DNA with fragment sizes of above 100 kb. Genomic DNA from blood samples was extracted using the salt extraction method according to standard protocols. Genomic DNA from fibroblasts was extracted using the NucleoSpin® Tissue Kit from Macherey–Nagel (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instructions.
4.3. Whole Exome Sequencing
WES was performed on DNA isolated from freshly frozen bulk brain tissue and peripheral blood leukocytes or from cultured skin fibroblasts as described above. Sequencing was performed by commercial sequencing service providers. For blood samples, exonic sequences and flanking intronic regions were captured using Agilent SureSelectXT (Agilent, Santa Clara, CA, USA), Twist Human Core Exome, Twist Human Comprehensive Exome + Mitochondrial Genome (Twist Bioscience, South San Francisco, CA, USA), or company-specific in-solution hybridization techniques not further specified and sequenced on Illumina machines (NextSeq, NovaSeq or HiSeq, Illumina, San Diego, CA, USA) yielding 100 bp or 150 bp paired-end reads. For brain and fibroblast samples, exonic sequences and flanking intronic regions were captured using BGI’s proprietary DNBSeq library and sequenced on a BGISEQ-500 machine, yielding 100 bp paired-end reads (BGI, Shenzhen, China). The coverage achieved was >100× on average for blood and fibroblast samples. For the brain samples, we initially targeted at least 100× coverage on average and later increased this to at least 500× to achieve better coverage of low-VAF variants. The resulting unaligned reads were further processed in-house as described below.
4.3.1. Bioinformatic Processing of WES Data and Variant Calling
For all samples, FASTQ reads were aligned to the human_g1k_v37.fasta genomic reference using BWA-MEM2 v0.7.17-r1188 []. SNVs and small indels were then called following the Genome Analysis ToolKit (GATK) best practices for germline (if applicable) and somatic variant calling using GATK-4.4 HaplotypeCaller [] (for germline variants) and GATK-4.3.0.0 Mutect2 [] (for somatic variants). The search for potentially pathogenic germline variants was not the focus of this study but was previously investigated, with the results added in Table 1. In the present study, blood WES data in the form of .bam files from these prior investigations were used exclusively for paired data analysis to identify somatic variants, as detailed below. In cases where no blood WES was available or no search for pathogenic germline variants intended, we performed WES on DNA from skin fibroblasts to serve as the reference tissue for somatic variant calling only. For somatic variant detection, WES data on DNA from both brain tissue and a reference tissue were analyzed as a pair in Mutect2’s matched-normal mode. This approach allows differentiation between likely germline mutations and brain-specific somatic mutations not present in the reference tissue, achieved by the tool’s built-in algorithm, which also uses a Bayesian somatic likelihood model to calculate the log odds of alleles being somatic variants vs. sequencing errors. We enabled the Mutect2 option to exclude soft-clipped bases from variant calling, as these had led to numerous likely false positives during the optimization of the data analysis protocol.
Public GATK resources were used for a Panel of Normals (PoN) (https://gatk.broadinstitute.org/hc/en-us/articles/360035890631-Panel-of-Normals-PON, accessed on 22 December 2022) to capture recurrent technical artifacts and to mark common germline variants along with their allele frequencies (af-only-gnomad.raw.sites.grch37.vcf.gz from https://storage.googleapis.com/gatk-best-practices/somatic-b37/af-only-gnomad.raw.sites.vcf, accessed on 14 November 2022). To identify high-quality somatic variants, we followed previously published recommendations []. Resulting variants were filtered with GATK-4.3.0.0 FilterMutectCalls and selected with SelectVariants, requiring the filter flag PASS. As an additional hard filter, alternative alleles were required to be supported by at least 4 reads. The remaining variants were analyzed and further filtered using MutationTaster2021 (version of 2021, accessed on several dates between December 2022 and December 2024) [] and MutationDistiller (version of 2019, accessed on several dates between December 2022 and December 2024) [], excluding common polymorphisms found in large-scale sequencing projects such as the 1000 Genomes Project [], ExAC [], or gnomAD []. Nonsense, missense, or splice site variants predicted to be disease-causing were further prioritized. All samples were screened for variants in genes potentially associated with drug resistance. For this purpose, we used a virtual gene panel (Table S1) that lists genes involved in drug absorption, distribution, metabolism, and excretion (ADME). This gene panel has been previously used and published by other research groups involved in pharmacogenetic studies of ASM [,].
Variants were also prioritized according to the individual patient phenotype, e.g., for genes known to be involved in MCD (Table S1), in tumorigenesis (Table S2), and in vascular malformations (Table S3). For patients with post-traumatic lesions, a broad panel of all known epilepsy genes according to the Genomics England Panel App was used (Table S4). To extend our screening beyond known genes, we further prioritized variants based on putative gene function using Gene Ontology (GO), WikiPathways, and Reactome terms via the corresponding feature in MutationDistiller. Candidate variants were visually inspected using the Integrative Genomic Viewer (IGV) to sort out read-end artifacts and putative false positives in clusters of low-quality bases indicative of sequencing artifacts. CNVpytor-1.3.1 [] was used for CNV analysis, when necessary.
4.3.2. Variant Confirmation
Shortlisted somatic variants in DNA isolated from brain tissue were confirmed by an alternative method depending on the VAF. For variants with a VAF above 20%, we used PCR followed by automated Sanger sequencing [] using the BigDye® v3.1 Terminator protocol (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA) on the ABI 3500 Genetic Analyzer to confirm the presence of the variants (Table S7 for custom primers). For variants with a VAF below 20%, we used double-mismatch allele-specific quantitative polymerase chain reaction (DMAS-qPCR) [] and/or ultra-deep targeted amplicon sequencing (TAS). For DMAS-qPCR, we designed allele-specific primers using batchprimer3 (https://wheat.pw.usda.gov/demos/BatchPrimer3/, accessed on 9 August 2023) (Table S8 for custom primers) according to published guidelines [] and calculated the likely VAF based on the ∆∆Ct method (∆∆Ct = ∆Ct (wild-type allele) − ∆Ct (variant allele)) []. DMAS qPCR was later abandoned due to its difficult primer design, comparatively labor-intensive setup procedure, and partly inconclusive results.
For amplicon sequencing, we used Primer3Plus (https://www.primer3plus.com/, accessed on 24 April 2024) to design oligonucleotide primers that flank the putative variant in a fragment of 150–400 bp, with the variant ideally located between 50–80 bp on the forward strand (Table S9 for custom primers). Amplicon-EZ sequencing was then performed by a commercial service provider (Genewiz®/Azenta Life Sciences, Leipzig, Germany). We found it most effective to run PCR reactions in triplicates using 100 ng of template genomic DNA per 50 µL PCR reaction volume and to purify the triplicates on a single PCR purification column (Monarch® Spin PCR & DNA Cleanup Kit, #T1130S, New England Biolabs, Ipswich, MA, USA) to achieve the recommended amount of DNA (500 ng DNA with a concentration of at least 20 ng/µL). In the case of unspecific PCR products, the desired band was excised from the gel and purified using a gel extraction kit (Monarch® DNA Gel Extraction Kit, New England Biolabs, #T1020L). The amount of DNA was measured using a QubitTM 3.0 fluorometer and a QubitTM dsDNA HS assay kit (#Q32851, InvitrogenTM, Thermo Fisher Scientific, Waltham, MA, USA). A high-fidelity polymerase (Q5® High-Fidelity DNA Polymerase, #M0491S, New England Biolabs, Ipswich, MA, USA) was used.
4.3.3. Bioinformatic Processing of TAS Data
The FASTQ reads were trimmed using cutadapt-4.9 [] to remove low-quality read ends, quality filtered using the fastq filter for an average PHRED score of at least 35 (https://github.com/LUMC/fastq-filter, accessed on 31 July 2024), and then aligned to the human G1Kv37 genomic reference sequence using BWA-MEM v0.7.17. Reads were visualized using the Integrative Genomic Viewer (IGV) to inspect the position of each variant.
5. Conclusions
In conclusion, our study elucidates the genetic basis of lesional epilepsy by analyzing somatic mutations in brain tissue samples. By integrating WES with radiologic and histopathologic data, we identified brain pathogenic somatic variants in the following, already well-established lesional epilepsy genes of the PI3K-AKT-mTOR axis: MTOR (in patient 3 with HMEG), PIK3CA (in patient 4 with focal megalencephaly), and TSC2 (in patient 5 with FCD), highlighting their role in focal cortical dysplasia and in other epilepsy-related malformations. Moreover, we identified pathogenic somatic variants in the following genes associated with CNS tumors: FGFR1 (in patient 15 with LGGNT and patient 16 with DNET) and PIK3R1 (in patient 15 with LGGNT).
Identification of the molecular etiology of an epilepsy lesion primarily offers benefits to patients and affected families. These include knowledge about the cause and natural course of their disease when known somatic variants are detected. In addition, counseling regarding the risk of recurrence is relevant, since somatic variants are not passed on to offspring. Understanding pathogenic somatic variants can further clarify differences in clinical presentation in cases where both a parent and their child carry the same germline variant. In such cases, the additional somatic variant may explain the variation in symptom severity. Furthermore, although we could not demonstrate it, pathogenic somatic variants could, in principle, contribute to drug resistance. In our study, the identification of somatic variants had no direct therapeutic implications because all patients had undergone epilepsy surgery, and epileptogenic lesions were completely resected in the individuals in whom the pathogenic somatic variants were identified. However, in the case of incomplete resection or when somatic variants are detected in biopsy samples, the identification of pathogenic somatic variants may be relevant for more personalized epilepsy treatments, including the selection of ASM, pathway-specific treatments such as mTOR inhibitors, and therapeutic strategies for associated tumor management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26020815/s1.
Author Contributions
Conceptualization, A.M.K. and M.S.; methodology, J.M.S., A.M.K. and M.S.; software, J.M.S. and M.S.; sample acquisition, J.M.S., J.F., L.-L.B., U.-W.T., A.T. and E.K.; formal analysis, J.M.S.; investigation, J.M.S.; validation, J.M.S., M.W. and S.M.-G.; resources, A.M.K. and M.S.; data curation, J.M.S.; writing—original draft preparation, J.M.S.; writing—review and editing, J.M.S., E.K., L.-L.B., U.-W.T., A.T., A.M.K. and M.S.; visualization J.M.S.; supervision, A.M.K. and M.S.; project administration, J.M.S., A.M.K. and M.S.; funding acquisition, A.M.K. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Einstein Stiftung Fellowship through the Günter Endres Fond, the Sonnenfeld-Stiftung, and the Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) as part of the German Center for Child and Adolescent Health (DZKJ), and by the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation under Germany’s Excellence Strategy, EXC-2049-390688087) to M.S.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Charité–Universitätsmedizin Berlin (EA2/086/20, 27 November 2020).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study or subsets thereof are available on reasonable request from the corresponding author due to strict data protection rules for patient-derived genetic data. Human genomic data cannot be deposited in public repositories without consent, which we do not have.
Acknowledgments
We thank Ingrid Scheffer and Sam Berkovic for their valuable discussions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mesraoua, B.; Brigo, F.; Lattanzi, S.; Abou-Khalil, B.; Al Hail, H.; Asadi-Pooya, A.A. Drug-Resistant Epilepsy: Definition, Pathophysiology, and Management. J. Neurol. Sci. 2023, 452, 120766. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of Drug Resistant Epilepsy: Consensus Proposal by the Ad Hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Jehi, L.; Yehia, L.; Peterson, C.; Niazi, F.; Busch, R.; Prayson, R.; Ying, Z.; Bingaman, W.; Najm, I.; Eng, C. Preliminary Report: Late Seizure Recurrence Years after Epilepsy Surgery May Be Associated with Alterations in Brain Tissue Transcriptome. Epilepsia Open 2018, 3, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.M.; McCray, A.; Hom, K.; Teti, S.; Cohen, N.T.; Gaillard, W.D.; Oluigbo, C.O. Outcomes of Stereoelectroencephalography Following Failed Epilepsy Surgery in Children. Childs Nerv. Syst. 2024, 40, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Yardi, R.; Morita-Sherman, M.E.; Fitzgerald, Z.; Punia, V.; Bena, J.; Morrison, S.; Najm, I.; Bingaman, W.; Jehi, L. Long-Term Outcomes of Reoperations in Epilepsy Surgery. Epilepsia 2020, 61, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Hartz, A.M.S.; Bauer, B. Drug-Resistant Epilepsy: Multiple Hypotheses, Few Answers. Front. Neurol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Blumcke, I.; Spreafico, R.; Haaker, G.; Coras, R.; Kobow, K.; Bien, C.G.; Pfäfflin, M.; Elger, C.; Widman, G.; Schramm, J.; et al. Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N. Engl. J. Med. 2017, 377, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Luyken, C.; Blümcke, I.; Fimmers, R.; Urbach, H.; Elger, C.E.; Wiestler, O.D.; Schramm, J. The Spectrum of Long-Term Epilepsy-Associated Tumors: Long-Term Seizure and Tumor Outcome and Neurosurgical Aspects. Epilepsia 2003, 44, 822–830. [Google Scholar] [CrossRef]
- Rosemberg, S. Long-Term Epilepsy Associated-Tumors (LEATs): What Is New? Arq. Neuropsiquiatr. 2023, 81, 1146–1151. [Google Scholar] [CrossRef]
- Najm, I.; Lal, D.; Alonso Vanegas, M.; Cendes, F.; Lopes-Cendes, I.; Palmini, A.; Paglioli, E.; Sarnat, H.B.; Walsh, C.A.; Wiebe, S.; et al. The ILAE Consensus Classification of Focal Cortical Dysplasia: An Update Proposed by an Ad Hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia 2022, 63, 1899–1919. [Google Scholar] [CrossRef] [PubMed]
- Blümcke, I.; Thom, M.; Aronica, E.; Armstrong, D.D.; Vinters, H.V.; Palmini, A.; Jacques, T.S.; Avanzini, G.; Barkovich, A.J.; Battaglia, G.; et al. The Clinicopathologic Spectrum of Focal Cortical Dysplasias: A Consensus Classification Proposed by an Ad Hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia 2011, 52, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.; McDermott, M.P.; Wiebe, S.; Langfitt, J.T.; Stern, J.M.; Dewar, S.; Sperling, M.R.; Gardiner, I.; Erba, G.; Fried, I.; et al. Early Surgical Therapy for Drug-Resistant Temporal Lobe Epilepsy: A Randomized Trial. JAMA 2012, 307, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, S.; Blume, W.T.; Girvin, J.P.; Eliasziw, M. A Randomized, Controlled Trial of Surgery for Temporal-Lobe Epilepsy. N. Engl. J. Med. 2001, 345, 311–318. [Google Scholar] [CrossRef]
- Dwivedi, R.; Ramanujam, B.; Chandra, P.S.; Sapra, S.; Gulati, S.; Kalaivani, M.; Garg, A.; Bal, C.S.; Tripathi, M.; Dwivedi, S.N.; et al. Surgery for Drug-Resistant Epilepsy in Children. N. Engl. J. Med. 2017, 377, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Makridis, K.L.; Atalay, D.A.; Thomale, U.-W.; Tietze, A.; Elger, C.E.; Kaindl, A.M. Epilepsy Surgery in the First Six Months of Life: A Systematic Review and Meta-Analysis. Seizure 2022, 96, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Steinlein, O.K. Genetics and Epilepsy. Dialogues Clin. Neurosci. 2008, 10, 29–38. [Google Scholar] [CrossRef]
- Steinlein, O.K.; Mulley, J.C.; Propping, P.; Wallace, R.H.; Phillips, H.A.; Sutherland, G.R.; Scheffer, I.E.; Berkovic, S.F. A Missense Mutation in the Neuronal Nicotinic Acetylcholine Receptor Alpha 4 Subunit Is Associated with Autosomal Dominant Nocturnal Frontal Lobe Epilepsy. Nat. Genet. 1995, 11, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Biervert, C.; Schroeder, B.C.; Kubisch, C.; Berkovic, S.F.; Propping, P.; Jentsch, T.J.; Steinlein, O.K. A Potassium Channel Mutation in Neonatal Human Epilepsy. Science 1998, 279, 403–406. [Google Scholar] [CrossRef]
- Mulley, J.C.; Scheffer, I.E.; Petrou, S.; Berkovic, S.F. Channelopathies as a Genetic Cause of Epilepsy. Curr. Opin. Neurol. 2003, 16, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; Williams, E.; Foulger, R.E.; Leigh, S.; Daugherty, L.C.; Niblock, O.; Leong, I.U.S.; Smith, K.R.; Gerasimenko, O.; Haraldsdottir, E.; et al. PanelApp Crowdsources Expert Knowledge to Establish Consensus Diagnostic Gene Panels. Nat. Genet. 2019, 51, 1560–1565. [Google Scholar] [CrossRef]
- Lai, D.; Gade, M.; Yang, E.; Koh, H.Y.; Lu, J.; Walley, N.M.; Buckley, A.F.; Sands, T.T.; Akman, C.I.; Mikati, M.A.; et al. Somatic Variants in Diverse Genes Leads to a Spectrum of Focal Cortical Malformations. Brain 2022, 145, 2704–2720. [Google Scholar] [CrossRef] [PubMed]
- Bedrosian, T.A.; Miller, K.E.; Grischow, O.E.; Schieffer, K.M.; LaHaye, S.; Yoon, H.; Miller, A.R.; Navarro, J.; Westfall, J.; Leraas, K.; et al. Detection of Brain Somatic Variation in Epilepsy-Associated Developmental Lesions. Epilepsia 2022, 63, 1981–1997. [Google Scholar] [CrossRef] [PubMed]
- Carton, R.J.; Doyle, M.G.; Kearney, H.; Steward, C.A.; Lench, N.J.; Rogers, A.; Heinzen, E.L.; McDonald, S.; Fay, J.; Lacey, A.; et al. Somatic Variants as a Cause of Drug-Resistant Epilepsy Including Mesial Temporal Lobe Epilepsy with Hippocampal Sclerosis. Epilepsia 2024, 65, 1451–1461. [Google Scholar] [CrossRef]
- Rivera, B.; Gayden, T.; Carrot-Zhang, J.; Nadaf, J.; Boshari, T.; Faury, D.; Zeinieh, M.; Blanc, R.; Burk, D.L.; Fahiminiya, S.; et al. Germline and Somatic FGFR1 Abnormalities in Dysembryoplastic Neuroepithelial Tumors. Acta Neuropathol. 2016, 131, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.J.; Keeley, A.; Virasami, A.; Harkness, W.; Tisdall, M.; Izquierdo Delgado, E.; Gutteridge, A.; Brooks, T.; Kristiansen, M.; Chalker, J.; et al. Comprehensive Molecular Characterisation of Epilepsy-Associated Glioneuronal Tumours. Acta Neuropathol. 2018, 135, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Krochmalnek, E.; Accogli, A.; St-Onge, J.; Addour-Boudrahem, N.; Prakash, G.; Kim, S.-H.; Brunette-Clement, T.; Alhajaj, G.; Mougharbel, L.; Bruneau, E.; et al. mTOR Pathway Somatic Pathogenic Variants in Focal Malformations of Cortical Development. Neurol. Genet. 2023, 9, e200103. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Yang, X.; Bae, T.; Vong, K.I.; Mittal, S.; Donkels, C.; Westley Phillips, H.; Li, Z.; Marsh, A.P.L.; Breuss, M.W.; et al. Comprehensive Multi-Omic Profiling of Somatic Mutations in Malformations of Cortical Development. Nat. Genet. 2023, 55, 209–220. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, W.; Kang, H.-C.; Kim, S.H.; Park, A.H.; Park, E.K.; Cho, Y.-W.; Kim, S.; Kim, H.M.; Kim, J.A.; et al. Brain Somatic Mutations in MTOR Cause Focal Cortical Dysplasia Type II Leading to Intractable Epilepsy. Nat. Med. 2015, 21, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Wang, X.; Duan, Z.; Luan, G. Low-Grade Epilepsy-Associated Neuroepithelial Tumors: Tumor Spectrum and Diagnosis Based on Genetic Alterations. Front. Neurosci. 2023, 16, 1071314. [Google Scholar] [CrossRef]
- Becker, L.-L.; Makridis, K.L.; Abad-Perez, A.T.; Thomale, U.-W.; Tietze, A.; Elger, C.E.; Horn, D.; Kaindl, A.M. The Importance of Routine Genetic Testing in Pediatric Epilepsy Surgery. Epilepsia Open 2024, 9, 800–807. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. Off. J. Am. Coll. Med. Genet. 2015, 17, 405. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.; Soucy, A.; El Achkar, C.M.; Barkovich, A.J.; Cao, Y.; DiStefano, M.; Evenson, M.; Guerrini, R.; Knight, D.; Lee, Y.-S.; et al. The ClinGen Brain Malformation Variant Curation Expert Panel: Rules for Somatic Variants in AKT3, MTOR, PIK3CA, and PIK3R2. Genet. Med. 2022, 24, 2240–2248. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, F.; Berkseth, M.; Shear, R.; Gonzalez, L.; Timms, A.E.; Sulc, J.; Pao, E.; Oyama, N.; Forzano, F.; Conti, V.; et al. Profiling PI3K-AKT-MTOR Variants in Focal Brain Malformations Reveals New Insights for Diagnostic Care. Brain 2022, 145, 925. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.A.B.; Carvalho, S.C.S.; Yang, X.; Ball, L.L.; George, R.D.; James, K.N.; Stanley, V.; Breuss, M.W.; Thomé, U.; Santos, M.V.; et al. mTOR Pathway Somatic Variants and the Molecular Pathogenesis of Hemimegalencephaly. Epilepsia Open 2020, 5, 97–106. [Google Scholar] [CrossRef] [PubMed]
- MONDO:0008287; Disease ID for Polysyndactyly with Peculiar Skull Shape; Greig Cephalosyndactyly Syndrome; Greig Cephalopolysyndactyly Syndrome; Gcps; Polysyndactyly with Peculiars Skull shape; Greig Syndrome Greig Cephalopolysyndactyly Syndrome. Available online: http://purl.obolibrary.org/obo/MONDO_0008287 (accessed on 16 January 2025).
- Kurek, K.C.; Luks, V.L.; Ayturk, U.M.; Alomari, A.I.; Fishman, S.J.; Spencer, S.A.; Mulliken, J.B.; Bowen, M.E.; Yamamoto, G.L.; Kozakewich, H.P.W.; et al. Somatic Mosaic Activating Mutations in PIK3CA Cause CLOVES Syndrome. Am. J. Hum. Genet. 2012, 90, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- D’Gama, A.M.; Geng, Y.; Couto, J.A.; Martin, B.; Boyle, E.A.; LaCoursiere, C.M.; Hossain, A.; Hatem, N.E.; Barry, B.; Kwiatkowski, D.J.; et al. mTOR Pathway Mutations Cause Hemimegalencephaly and Focal Cortical Dysplasia. Ann. Neurol. 2015, 77, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Moya-Mendez, M.E.; Mueller, D.M.; Pratt, M.; Bonner, M.; Elliott, C.; Hunanyan, A.; Kucera, G.; Bock, C.; Prange, L.; Jasien, J.; et al. Early Onset Severe ATP1A2 Epileptic Encephalopathy: Clinical Characteristics and Underlying Mutations. Epilepsy Behav. 2021, 116, 107732. [Google Scholar] [CrossRef]
- Costa, C.; Prontera, P.; Sarchielli, P.; Tonelli, A.; Bassi, M.T.; Cupini, L.M.; Caproni, S.; Siliquini, S.; Donti, E.; Calabresi, P. A Novel ATP1A2 Gene Mutation in Familial Hemiplegic Migraine and Epilepsy. Cephalalgia 2014, 34, 68–72. [Google Scholar] [CrossRef]
- Córdoba, N.M.; Lince-Rivera, I.; Gómez, J.L.R.; Rubboli, G.; De la Rosa, S.O. ATP1A2-Related Epileptic Encephalopathy and Movement Disorder: Clinical Features of Three Novel Patients. Epileptic Disord. 2024, 26, 332–340. [Google Scholar] [CrossRef]
- Vetro, A.; Nielsen, H.N.; Holm, R.; Hevner, R.F.; Parrini, E.; Powis, Z.; Møller, R.S.; Bellan, C.; Simonati, A.; Lesca, G.; et al. ATP1A2- and ATP1A3-Associated Early Profound Epileptic Encephalopathy and Polymicrogyria. Brain 2021, 144, 1435–1450. [Google Scholar] [CrossRef]
- Qin, W.; Chan, J.A.; Vinters, H.V.; Mathern, G.W.; Franz, D.N.; Taillon, B.E.; Bouffard, P.; Kwiatkowski, D.J. Analysis of TSC Cortical Tubers by Deep Sequencing of TSC1, TSC2 and KRAS Demonstrates That Small Second-Hit Mutations in These Genes Are Rare Events. Brain Pathol. 2010, 20, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Shyamsundar, M.M.; Thomas, M.W.; Maynard, J.; Idziaszczyk, S.; Tomkins, S.; Sampson, J.R.; Cheadle, J.P. Comprehensive Mutation Analysis of TSC1 and TSC2-and Phenotypic Correlations in 150 Families with Tuberous Sclerosis. Am. J. Hum. Genet. 1999, 64, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Strizheva, G.D.; Carsillo, T.; Kruger, W.D.; Sullivan, E.J.; Ryu, J.H.; Henske, E.P. The Spectrum of Mutations in TSC1 and TSC2 in Women with Tuberous Sclerosis and Lymphangiomyomatosis. Am. J. Respir. Crit. Care Med. 2001, 163, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Gopalappa, R.; Kim, S.H.; Ramakrishna, S.; Lee, M.; Kim, W.; Kim, J.; Park, S.M.; Lee, J.; Oh, J.-H.; et al. Somatic Mutations in TSC1 and TSC2 Cause Focal Cortical Dysplasia. Am. J. Hum. Genet. 2017, 100, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Kurup, A.; Kovilapu, U.B.; Ranjan, R.; Sondhi, V. Somatic Mutations Involving TSC 1 and TSC2 Genes in Two Children with Focal Cortical Dysplasia. Brain Dev. 2022, 44, 166–172. [Google Scholar] [CrossRef]
- Hart, K.C.; Robertson, S.C.; Kanemitsu, M.Y.; Meyer, A.N.; Tynan, J.A.; Donoghue, D.J. Transformation and Stat Activation by Derivatives of FGFR1, FGFR3, and FGFR4. Oncogene 2000, 19, 3309–3320. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.-H.G.; Gupta, R.; Doo, P.; Lee, J.C.; Cadwell, C.R.; Ramani, B.; Hofmann, J.W.; Sloan, E.A.; Kleinschmidt-DeMasters, B.K.; Lee, H.S.; et al. Comprehensive Analysis of Diverse Low-Grade Neuroepithelial Tumors with FGFR1 Alterations Reveals a Distinct Molecular Signature of Rosette-Forming Glioneuronal Tumor. Acta Neuropathol. Commun. 2020, 8, 151. [Google Scholar] [CrossRef]
- Wolking, S.; Moreau, C.; McCormack, M.; Krause, R.; Krenn, M.; Berkovic, S.; Cavalleri, G.L.; Delanty, N.; Depondt, C.; Johnson, M.R.; et al. Assessing the Role of Rare Genetic Variants in Drug-resistant, Non-lesional Focal Epilepsy. Ann. Clin. Transl. Neurol. 2021, 8, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Wolking, S.; Schulz, H.; Nies, A.T.; McCormack, M.; Schaeffeler, E.; Auce, P.; Avbersek, A.; Becker, F.; Klein, K.M.; Krenn, M.; et al. Pharmacoresponse in Genetic Generalized Epilepsy: A Genome-Wide Association Study. Pharmacogenomics 2020, 21, 325–335. [Google Scholar] [CrossRef]
- Hombach, D.; Schuelke, M.; Knierim, E.; Ehmke, N.; Schwarz, J.M.; Fischer-Zirnsak, B.; Seelow, D. MutationDistiller: User-Driven Identification of Pathogenic DNA Variants. Nucleic Acids Res. 2019, 47, W114–W120. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, A.; Baldassari, S.; Baulac, S. mTOR Pathway: Insights into an Established Pathway for Brain Mosaicism in Epilepsy. Neurobiol. Dis. 2023, 182, 106144. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.W.C.B.; Koeleman, B.P.C.; Brilstra, E.H.; Jansen, F.E.; Baldassari, S.; Chipaux, M.; Sim, N.S.; Ko, A.; Kang, H.-C.; Blümcke, I.; et al. Somatic Variant Analysis of Resected Brain Tissue in Epilepsy Surgery Patients. Epilepsia 2024, 65, e209–e215. [Google Scholar] [CrossRef] [PubMed]
- Blümcke, I.; Aronica, E.; Miyata, H.; Sarnat, H.B.; Thom, M.; Roessler, K.; Rydenhag, B.; Jehi, L.; Krsek, P.; Wiebe, S.; et al. International Recommendation for a Comprehensive Neuropathologic Workup of Epilepsy Surgery Brain Tissue: A Consensus Task Force Report from the ILAE Commission on Diagnostic Methods. Epilepsia 2016, 57, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Xu, Y.; Nair, M.; Bordey, A. The mTOR Pathway Genes MTOR, Rheb, Depdc5, Pten, and Tsc1 Have Convergent and Divergent Impacts on Cortical Neuron Development and Function. eLife 2024, 12, RP91010. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, F.; Sun, Y.; Liu, X. Clinicopathological Analysis of Rosette-Forming Glioneuronal Tumors. Diagn. Pathol. 2024, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, L. Somatic Structural Variation Signatures in Pediatric Brain Tumors. Cell Rep. 2023, 42, 113276. [Google Scholar] [CrossRef] [PubMed]
- Lefever, S.; Rihani, A.; Van der Meulen, J.; Pattyn, F.; Van Maerken, T.; Van Dorpe, J.; Hellemans, J.; Vandesompele, J. Cost-Effective and Robust Genotyping Using Double-Mismatch Allele-Specific Quantitative PCR. Sci. Rep. 2019, 9, 2150. [Google Scholar] [CrossRef]
- Peng, X.; Dorman, K.S. Accurate Estimation of Molecular Counts from Amplicon Sequence Data with Unique Molecular Identifiers. Bioinformatics 2023, 39, btad002. [Google Scholar] [CrossRef] [PubMed]
- Baldassari, S.; Ribierre, T.; Marsan, E.; Adle-Biassette, H.; Ferrand-Sorbets, S.; Bulteau, C.; Dorison, N.; Fohlen, M.; Polivka, M.; Weckhuysen, S.; et al. Dissecting the Genetic Basis of Focal Cortical Dysplasia: A Large Cohort Study. Acta Neuropathol. 2019, 138, 885–900. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and Accurate Long-Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef]
- Poplin, R.; Ruano-Rubio, V.; DePristo, M.A.; Fennell, T.J.; Carneiro, M.O.; der Auwera, G.A.V.; Kling, D.E.; Gauthier, L.D.; Levy-Moonshine, A.; Roazen, D.; et al. Scaling Accurate Genetic Variant Discovery to Tens of Thousands of Samples. bioRxiv 2018. [Google Scholar] [CrossRef]
- Benjamin, D.; Sato, T.; Cibulskis, K.; Getz, G.; Stewart, C.; Lichtenstein, L. Calling Somatic SNVs and Indels with Mutect2. bioRxiv 2019. [Google Scholar] [CrossRef]
- Boßelmann, C.M.; Leu, C.; Lal, D. Technological and Computational Approaches to Detect Somatic Mosaicism in Epilepsy. Neurobiol. Dis. 2023, 184, 106208. [Google Scholar] [CrossRef] [PubMed]
- Steinhaus, R.; Proft, S.; Schuelke, M.; Cooper, D.N.; Schwarz, J.M.; Seelow, D. MutationTaster2021. Nucleic Acids Res. 2021, 49, W446–W451. [Google Scholar] [CrossRef]
- Genomes Project Consortium. The Genomes Project Consortium, A Global Reference for Human Genetic Variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of Protein-Coding Genetic Variation in 60,706 Humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The Mutational Constraint Spectrum Quantified from Variation in 141,456 Humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Suvakov, M.; Panda, A.; Diesh, C.; Holmes, I.; Abyzov, A. CNVpytor: A Tool for Copy Number Variation Detection and Analysis from Read Depth and Allele Imbalance in Whole-Genome Sequencing. GigaScience 2021, 10, giab074. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA Sequencing with Chain-Terminating Inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).