Abstract
In addition to conventional treatments, there is growing interest in preventive and complementary therapies. Proper nutrition can prevent the manifestation of several chronic diseases such as obesity, diabetes, cardiovascular disease, and cancer, and can attenuate the severity of these diseases. Edible mushrooms have been used as nutrition and medicine for thousands of years. The spectrum and quantity of their medicinal compounds made them a widely investigated target both in basic research and clinical trials. The most abundant and medically important components are polysaccharides, terpenoids, phenols, and heterocyclic amines, but bioactive proteins, vitamins, including vitamin D, polyunsaturated fatty acids, and essential minerals are also important ingredients with noteworthy health benefits. Mushroom extracts have anti-diabetic, anti-hyperlipidemic, anti-inflammatory, antioxidant, cardioprotective, anti-osteoporotic, and anti-tumor effects and are well tolerated, even by cancer patients. In our previous review we detailed the molecular aspects of the development of type 2 diabetes, discussing the role of physical activity and diet, but we did not detail the role of medicinal mushrooms as part of nutrition. In this review, we aimed to summarize the most important medical mushrooms, along with their natural habitats, growing conditions, and components, that are presumably sufficient for the prevention and treatment of insulin resistance.
1. Introduction
In the last decade, there has been considerable interest in the efficiency of non-pharmacological treatments in disease prevention and their use as complementary treatments [1,2,3]. Sufficient physical activity and proper nutrition with adequate amounts of dietary components or, if needed, supplementation, are well-known regulators of homeostasis, and metabolic and immune processes, which enable the prevention and reduction of diseases and their progression [4,5,6,7,8,9,10].
Edible mushrooms are part of diets all over the world, and have also been used as medicine for thousands of years, mainly in Asia and Japan [11]. Their known pharmaceutical properties (i.e., anti-cancer, anti-inflammatory, antioxidant, and anti-diabetic) have made them a widely investigated target both in basic research and clinical trials [12,13,14]. The spectrum and quantity of medicinal compounds found in different edible mushrooms overlap to a large extent. The most abundant and medically important components are polysaccharides (among these α- and β-glucans), terpenoids, phenols, and heterocyclic amines [11,15]. Additionally, other important nutrients such as amino acids and proteins [16], unsaturated and polyunsaturated fatty acid (i.e., oleic and linoleic acids) [17], micronutrients like vitamin D (as ergosterol-the precursor of vitamin D2, or vitamin D2 itself) [12,18], vitamin B [19], and essential minerals (Zn, Fe, Mn, Ca, Mg), [12,20] as well as bioactive proteins (i.e., lectins, fungal immunomodulatory proteins (FIP), ribosome inactivating proteins (RIP), ribonucleases, laccases, and lentin) [21] are also important ingredients of edible mushrooms with noteworthy health benefits.
There is extensive knowledge in scientific literature from clinical, pre-clinical, and in vitro studies about the beneficial effects of mushrooms and their compounds against a variety of disorders and diseases. Reishi (Ganoderma lucidum) has several pharmacologically active compounds with antiviral, anti-inflammatory, antioxidant, cardioprotective, anti-osteoporotic, anti-tumor, neuroprotective, antidepressant, radio-protective effects and so on [12]. Mushroom extracts were well tolerated by cancer patients and found to improve quality of life (QoL) and immune outcomes (the molecular details of the effect on the immune system are not yet fully understood) and decrease anti-cancer treatment-related toxicities, thus increasing treatment adherence and improving outcomes without major adverse effects [13]. Sparassis crispa, which is sometimes called cauliflower fungus, has not only anti-cancer, but anti-inflammatory, anti-fungal, and antioxidant activities, based on the results of a meta-analysis where 33 randomized control trials (RCTs) were included [14]. Lentin from Shiitake (Lentinula edodes) inhibits HIV-1 reverse transcriptase and the proliferation of leukemia cells [22].
In our previous review, we detailed the molecular aspects of the development of type 2 diabetes (T2DM) along with possible preventive and complementary treatments, including physical activity and diet, but we did not discuss the role of medicinal mushrooms as part of nutrition [3]. In this review, we aimed to summarize those edible mushrooms that are considered and used as medical mushrooms due to their increased amount of medically effective composition. We detailed their components that are presumably sufficient for the prevention and treatment of insulin resistance and T2DM. We summarized their positive physiological roles and impact on specific and related signaling pathways. Additionally, we added supplementary information about the natural habitats and growing conditions of edible medical mushrooms (Supplementary Materials, Table S1).
2. Composition of Medical Mushrooms
Mushrooms are widely recognized for their dual role as both food and medicine, attributed to their rich composition of primary and secondary metabolites with notable health-promoting properties [23,24]. Key metabolites found in mushrooms included indole and phenolic compounds, carbohydrates, fatty acids, proteins, free amino acids, sterols, carotenoids, enzymes, and vitamins; alongside these, mushrooms are an important source of essential amino acids [25,26,27]. The indole (i.e., L-tryptophan, 5-hydroxy-L-tryptophan, tryptamine, 5-methyltryptamine, and melatonin) and phenolic derivatives (i.e., flavonoids, 4-hydroxybenzoic, ferulic, p-coumaric, protocatechuic, trans-cinnamic, and vanillic acid) of edible mushrooms have antioxidant properties [28].
The protein content of mushrooms is 12–35% of their dry weight and mushrooms are a highly digestible source of proteins, with a score of amino acids similar to milk and meat [29,30]. For instance, per 100 g of dry matter, mushrooms contain essential amino acids such as glutamic acid (130–240 mg), aspartic acid (91–120 mg), threonine (41–95 mg), arginine (37–140 mg), and valine (36–89 mg) [31]. However, some edible mushrooms are deficient in leucine, isoleucine, lysine, and tryptophan [32].
The fat content of mushrooms, measured on a dry matter basis, ranges from 1.1% to 8.3%, with an average of 4.0% [33]. This crude fat content comprises free fatty acids, mono-, di-, and triglycerides, sterols and their esters, and phospholipids [31,34].
Carbohydrates are another significant component, with their total content varying between 35% and 70% based on dry matter [30,33]. Digestible carbohydrates include glucose (<1%) and glycogen (5–10%). Additionally, mushrooms are a rich source of indigestible carbohydrates, such as oligosaccharides like trehalose and non-starch polysaccharides (NSPs) including chitin, β-glucans, and mannans [25,33].
Mushrooms are also valued for their vitamin content, including thiamine (vitamin B1), riboflavin (vitamin B2), niacin (vitamin B3), pantothenic acid (vitamin B5), biotin (vitamin B7), folate (vitamin B9), cobalamin (vitamin B12), ascorbic acid (vitamin C), and Vitamin D. Concentrations of certain vitamins per 100 g of dry matter include riboflavin (1.8–5.1 mg), folate (0.3–0.64 mg), and niacin (31–65 mg) [35,36].
The mineral composition of mushrooms is equally remarkable, with the ash content ranging from 7% to 17% based on dry matter [37]. Notable mineral concentrations per 100 g of dry matter include potassium (2700–4700 mg), phosphorus (500–1400 mg), magnesium (20–200 mg), zinc (4.7–9.2 mg), and copper (0.5–3.5 mg) [38,39].
Extensive research has been conducted on their therapeutic applications, revealing the significance of various bioactive compounds in their nutritional and medicinal benefits (Table 1) [21].

Table 1.
Medically active components of medical mushrooms. CAT—catalase; FFA—free fatty acid; GLUT4—glucose transporter type 4; GLP-1—glucagon-like peptide-1; GPR43—G-protein-coupled receptor 43; GSH—glutathione; GSH-Px—glutathione peroxidase; HDL-C—high-density lipoprotein cholesterol; HMG-CoA—3-hydroxy-3-methylglutaryl-CoA; HOMA-IR—Homeostatic Model Assessment for Insulin Resistance; IKKβ—inhibitor of κB kinase β; IκB—inhibitor of κB; IgA—immunoglobulin A; LDL-C—low-density lipoprotein cholesterol; MCP1—monocyte chemoattractant protein 1; MDA—malonaldehyde; NF-κB—nuclear factor kappa B; PI3K—phosphoinositide 3-kinase; PSA—prostate specific antigen; SCFAs—short-chain fatty acids; SHR—spontaneously hypertensive rats; SOD—superoxide-dismutase; TG—triglyceride; TGF-β1—transforming growth factor beta 1; TNF-α—tumor necrosis factor α; ZFR—Zucker fatty rats.
3. Medically Active Components with Positive Effects on Insulin Resistance
Insulin resistance (IR) is a clinical state [3], which is frequently diagnosed in obesity and T2DM [116,117]. It is developed under conditions when the human body continuously receives higher nutrient intake than it needs over a long period of time. In these circumstances the cells are unable to take up more glucose (impaired glucose tolerance or prediabetes), either because of a failure of insulin secretion or decreased insulin sensitivity [118]. The latter is partly the result of a negative feedback mechanism, where the nutrient overload, followed by the prolonged activation of insulin/Rheb/mTORC1/S6K1 signaling, induces Ser/Thr phosphorylation of IRS-1, resulting in its degradation blocking the activity of this pathway [119,120]. Although the terms “impaired glucose tolerance”, “glucose intolerance”, and “insulin resistance” suggest that only glucose homeostasis is involved in this physiological state, in fact, many other processes are involved.
Several in vivo and human studies discuss the beneficial effects of various doses of compounds from edible mushrooms on insulin resistance [121]. Important parts of glucose and lipid homeostasis where the compounds of medical mushrooms can effectively alter IR according to our recent scientific knowledge are listed below (Figure 1):
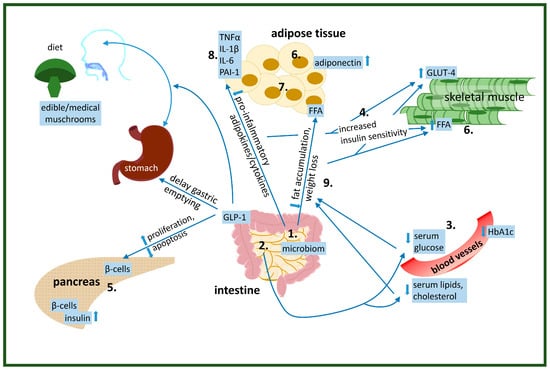
Figure 1.
Physiological effects of edible/medical mushroom components on the improvement of insulin resistance: 1. altered gut microbiome, 2. decreased glucose absorption, 3. lowered serum glucose levels, 4. increased glucose uptake by the cells, 5. increased/decreased insulin production by β-cells in pancreas, 6. altered lipid metabolism-increase utilization of FFA as an energy source in muscle, 7. altered adipose tissue function, 8. reduced pre-inflammatory cytokine levels, 9. reduced/induced weight loss.
- alter gut microbiom
- decrease glucose absorption
- lower serum glucose levels
- increase glucose uptake by the cells
- increase/decrease insulin production by β-cells in pancreas
- alter lipid metabolism-increase utilization of FFA as energy source in muscle
- alter adipose tissue function
- reduce pre-inflammatory cytokine levels
- reduce/induce weight loss
3.1. Altered Gut Microbiota
Mushrooms are considered a potential source of prebiotics due to their non-digestible, water-soluble, and insoluble polysaccharides, which stimulate the growth of beneficial bacteria in the colon [41]. The importance of gut microbiota in the development of obesity, T2DM, and insulin resistance has been known for a long time [122,123] but has recently come back into focus again as a potential target for improving insulin resistance using a comprehensive multi-omics strategy in humans [124]. Beneficial gut microbiota have important physiological functions, including the development of a proper immune system, regulation of intestinal barrier and endocrine function, and modulation of glucose and lipid homeostasis [125]. Moreover, it is not only the proportion of different bacterial genera that is important in the microbiota. The diversity of these bacteria is also important, as more of them can better digest food and produce essential amino acids or vitamins, thus reducing the risk of many diseases by increasing the absorption of nutrients necessary for maintaining health [126,127,128].
Microbiota derived metabolites such as short-chain fatty acids (SCFAs) acetate, propionate, and butyrate can suppress fat accumulation and improve energy expenditure in the liver and muscle through the G-protein-coupled receptor 43 (GPR43) [110]. However, SCFA supplementation itself significantly increased the GPR43 level in adipose tissues, with subsequent positive effects on altered gut microbiota, i.e., increased proportion of Bacteroidetes, decreased Firmicutes, and altered body composition, suggesting a positive feedback loop between SCFAs and gut microbiota [129]. Additionally, these SCFAs replacing carbohydrates may serve as an energy source and thus decrease insulin/IGF-1 signaling and further improve health [130].
The water extract of Ganoderma lucidum altered gut microbiota composition by increasing the variety of the above mentioned beneficial bacterial species (i.e., Parabacteroides goldsteinii, Bacteroides spp., Anaerotruncus colihominis, Roseburia hominis, Clostridium methylpentosum, Eubacterium coprostanoligenes), which correlated with improved body composition by reducing obesity [71]. Additionally, it restored the expression level of proteins, which are important for maintaining intestinal tight junction integrity. This alteration prevented the translocation of pro-inflammatory endotoxins into the circulation in high-fat diet-fed mice [48,71]. Fungal polysaccharides cannot be degraded in the human stomach and small intestine; these can be digested into SCFAs by intestinal bacteria in the colon, resulting in the induced secretion of GLP-1 from intestinal cells [131,132]. GLP-1 then influences the central nervous system, leading to reduction in appetite; it affects muscles, adipose tissue, liver, and stomach—in the latter it delays gastric emptying [48].
Similarly to the water extract of Ganoderma lucidum, Morchella esculenta polysaccharides also improved the composition of gut microbiota in a T2DM mice model, where the majority of the flora reverted to normal (i.e., increased abundance of Lactobacillaceae, Lachnospiraceae, and Enterobacteriaceae and decreased abundance of Staphylococcaceae and Corynebacteriaceae). Furthermore, similarly to the aqueous extract of Ganoderma lucidum, polysaccharides from Morchella esculenta restored intestinal permeability by inducing tight junction (ZO-1, occludin) protein levels [102].
An aqueous extract of Antrodia cinnamomea significantly increased the proportion of bacteria with anti-inflammatory properties in the normal intestinal flora (included the reduced Firmicutes/Bacteroidetes ratio), and also the levels of tight-junction protein ZO-1 and occludin, maintaining intestinal barrier integrity [56]. All of these resulted in reduced endotoxemia and chronic inflammation in treated mice fed a high-fat diet compared to untreated mice. Additionally, the antimicrobial peptide Reg3g and lysozyme C also were significantly elevated after the treatment in the ileum of high-fat diet-fed animals compared to their non-treated controls [56]. These antimicrobial effectors play an important role in maintaining homeostasis of gut microbiota [133], and their reduced expression was also demonstrated in mice fed a high-fat diet [134].
Extracts of Pleurotus ostreatus and Pleurotus eryngii, mainly containing glucans, were able to stimulate the probiotic growth of Lactobacillus ssp. and Bifidobacterium ssp., among others. [108]. Although the changes in intestinal microbiota were not evaluated in a double-blind, randomized, controlled crossover trial, oyster mushroom (Pleurotus ostreatus) powder rich in β-glucans, similar to the aforementioned polysaccharides from other mushrooms, increased the levels of GLP-1 and subsequently decreased appetite [112].
3.2. Decreased Glucose Absorption
The α-amylase and α-glycosidase enzymes are responsible for degrading carbohydrates into glucose, and through their activity they increase glucose absorption. The intracellular polysaccharides of Hericium erinaceus significantly inhibited the enzymes α-amylase and α-glucosidase, thus reducing glucose absorption [87]. Triterpenoids isolated from Ganoderma lucidum with chloroform or ethanol inhibited α-glucosidase enzymes and thus reduced glucose absorption from the intestine [76,77,78]. Similarly, the triterpenoids inotolactone A and B, extracted from submerged cultures of Inonotus obliquus, have inhibitory effects on α-glucosidase activity [96].
3.3. Lowered Serum Glucose Levels
The 500 and 750 mg/kg ethanol extract of White bottom mushroom Agaricus bisporus effectively reduced blood glucose levels in alloxan-induced diabetic rats compared to controls [40]. Consumption of 2 g/kg Agaricus bisporus per day was associated with significantly reduced glucose levels compared to controls in a human study [43]. Powder from this mushroom also significantly improved the glycemic index of T2DM patients after treatment in a randomized control trial [44]. Treatment with Morchella esculenta polysaccharides significantly decreased fasting glucose levels compared to the non-treated groups in T2DM mouse model [102,103]. Supplementation with an aqueous extract of Antrodia cinnamomea similarly reduced fasting glucose levels, as demonstrated by an oral glucose tolerance test, in treated mice fed a high-fat diet compared to their untreated counterparts [56]. In addition, administration of Antrodia cinnamomea powder at an optimal dose of 200 mg/kg body weight, which contains eburicoic acid, dehydroeburicoic acid, sulphurenic acid, dehydrosulphurenic acid, and ergostatrien-3β-ol, induced a significant decrease in plasma glucose levels at 30 and 60 min after administration compared to a control Wistar rat group [58]. A Grifola frondosa GF5000 water extract fraction (Mw > 5000D) significantly reduced fasting serum glucose levels in treated rats compared to diabetic controls [80]. Boletus polysaccharides significantly decreased fasting blood glucose levels in a treated group compared to controls in a T2DM rat model, in which the effect was comparable to metformin [65]. The FXM fraction of Grifola frondosa containing β-glucans (also found in cereals), which has been shown to be anti-diabetic according to several studies [135,136,137,138,139], significantly lowered the circulating glucose concentration in insulin-resistant KK mice at 8–12 and 16–18 h in an acute study, and on day 4 and 7 in a chronic study after oral gavage compared to controls [86]. Flammulina velotipes polysaccharides significantly decreased fasting serum glucose levels in treated compared to non-treated diabetic groups of mice [69]. After feeding streptozotocin-induced diabetic rats with Pleurotus ostreatus mushroom, their serum glucose levels decreased significantly [111,113,114], similarly to the clinical trial, where after consumption of Pleurotus ostreatus mushroom, a significant decrease in fasting and postprandial serum glucose level was observed both in healthy volunteers and diabetic patients [115]. Phthalaldehyde derivatives hericenal A, B, and C, from submerged cultures of Hericium erinaceus, have potential anti-hypergylcemic effects in diabetic patients [140]. Similarly, D-threitol, D-arabinitol, palmitic acid, and α-D-glucan from Hericium erinaceus also resulted in anti-hyperglycemic effects in diabetic rats [87,90]. Both methanol and aqueous extracts of Hericium erinaceus significantly reduced fasting serum glucose levels in streptozotocin-induced diabetic rats [90,91]. Moreover, extracellular polysaccharides from the Turkey tail mushroom after 4 weeks administration attenuated the elevation of blood glucose levels in a dose-dependent manner in T2DM rats [68].
3.4. Increased Glucose Uptake by Cells
SX-fraction (SXF) of Grifola frondosa significantly increased the glucose uptake in rat skeletal muscle L6 cells [81] and resulted in around a 30–63% decline in fasting blood glucose levels after treatment in diabetic patients [81,82]. Additionally, 8 weeks of treatment with Grifola frondosa inhibited the rise in blood glucose levels in spontaneously diabetic mice. This effect was further confirmed in a crossover experiment (an experiment where at the half-term of the treatments, groups were switched) [83]. Grifola frondosa polysaccharides induced significant glucose consumption in insulin-resistant HepG2 cells after 24 h of treatments compared to non-treated controls, suggesting improved insulin resistance [84]. A ReishiMax supplement containing polysaccharides and triterpenes from Ganoderma lucidum significantly increased glucose uptake by adipocytes through the activation of AMP-activated protein kinase (AMPK) [75]. Extracellular polysaccharopeptides obtained from Trametes versicolor induced glucose uptake in insulin-resistant HeG2 cells through activation of AMPK, insulin receptor substrate 2 (IRS-2), and increased levels of glucose transporter-1. Additionally, this compound significantly increased the glycogen content, suggesting that extracellular polysaccharopeptides regulate glucose uptake and glucose homeostasis in an insulin-independent manner [67].
3.5. Insulin Production and Effects on β-Cells in Pancreas
The alteration of gut microbiota by increased SCFAs subsequently induced the expression of GLP-1, which promoted proliferation and inhibited apoptosis in pancreatic β-cells [141,142].
Treatments with polysaccharide extracted from Morchella esculenta decreased fasting serum insulin levels in a T2DM mouse model [102]. Water extract from Antrodia cinnamomea similarly decreased fasting insulin levels in treated compared to untreated high-fat diet-fed mice, as confirmed by an insulin tolerance test [56]. Moreover, Antrodia cinnamomea powder containing eburicoic acid, dehydroeburicoic acid, sulphurenic acid, dehydrosulphurenic acid, and ergostatrien-3β-ol at an optimal dose of 200 mg/kg significantly increased plasma insulin levels at 30 min, and significantly decreased the HOMA-IR 60 min after administration compared to the control Wistar rat group [58]. Treatments with Grifola frondosa inhibited the rise in insulin levels in spontaneously diabetic mice compared to untreated groups, which was also confirmed by crossover experiments [83]. The FXM fraction containing β-glucan from Grifola frondosa significantly lowered the circulating insulin concentration in insulin-resistant KK mice on day 4 and 7 in a chronic study after oral gavage compared to controls [86]. Flammulina velotipes polysaccharide, similarly, significantly decreased fasting serum insulin levels after treatment compared the non-treated pairs [69]. The Mukitake mushroom Panellus serotinus alleviated the sever hyperinsulinemia in db/db mice after a 4 week feeding period, which was observed in control-fed db/db mice [105].
3.6. Altered Lipid Metabolism-Increase Utilization of FFA as an Energy Source in Muscle
Supplementation with the high molecular weight polysaccharide fraction of Pleurotus eryngii significantly increased the gene expression levels of LDL receptor (LDLR) and GPR43 in the liver and adipose tissue of mice fed a high-fat diet, respectively [143]. This finding supports the idea that microbiota-derived SCFA metabolites, through activation of GPR43 in adipocytes, prevent fat accumulation and increased energy utilization in other tissues, including muscle, where they improve glucose tolerance [110,144]. SCFAs can serve as an energy source instead of carbohydrates, thereby reducing insulin/IGF-1 signaling and improving health [106]. Morchella esculenta polysaccharides showed cholesterol-lowering effects by inducing the expression of CYP7A1 and LDLR and down-regulating HMG-CoA [103,104]. Fibers from food such as chitosan from mushrooms are not digested in the stomach nor in the small intestine. Thus, besides their bulking effects (i.e., delayed gastric empty and inducing satiety), binding dietary fats and bile acids inhibiting their absorption/enterohepatic circulation, consequently, reduce blood lipid and cholesterol levels [145,146]. Ganoderma lucidum polysaccharides reduced lipogenic gene expression in a dose-dependent way in high-fat diet-fed mice compared to non-treated pairs [71]. Water extract of Antrodia cinnamomea significantly decreased serum triglyceride levels in treated compared to non-treated high-fat diet-fed mice [56], and also significantly decreased triglyceride, LDL-C and total cholesterol in treated compared to non-treated obese mice [147]. Similarly, powder of Antrodia cinnamomea at an optimal dose of 200 mg/kg significantly decreased plasma FFA levels 60 min after administration compared to control Wistar rats [58]. In a human study, consumption of 2 g/kg Agaricus bisporus per day was associated with significantly reduced levels of total cholesterol, LDL-C, and TG and increased levels of HDL-C compared to controls [43]. Treatments with Grifola frondosa inhibited the rise in triglyceride levels compared to non-treated groups of spontaneously diabetic mice, which was also confirmed in crossover experiments [83]. Similarly, the GF5000 fraction of this mushroom significantly decreased total serum cholesterol and LDL-C levels in treated diabetic rats compared to diabetic controls [80]. Boletus polysaccharides significantly decreased total cholesterol, triglyceride, and LDL-C-cholesterol levels in treated groups compared to control T2DM rats, while HDL-C-cholesterol significantly and inversely changed after treatment. In addition, all levels were similar to normal untreated controls, indicating that boletus polysaccharides can be an effective alternative in the reduction in serum lipids [65]. Flammulina velotipes polysaccharides significantly decreased total cholesterol, triglyceride, LDL-C, and FFA and increased HDL-C levels in treated groups compared to non-treated streptozotocin-induced mice [69]. Extracts from Hericium erinaceus have potential anti-hypecholesterolemic effects in diabetic rats [90,92,93]. D-threitol, D-arabinitol, palmitic acid, and α-D-glucan from Hericium erinaceus, and both the methanol and aqueous extracts, significantly reduced the elevation of serum TG, total cholesterol [69], and additionally LDL-C, and increased HDL-C levels in treated compared to non-treated diabetic rats [91]. Extracts from Pleurotus ostreatus mushroom significantly decreased total cholesterol, TG, and LDL-C levels in streptozotocin-induced diabetic rats, while HDL-C levels were significantly increased after treatments compared to diabetic controls [111]. Similarly, a 25-week treatment with Lentinula edodes mushroom significantly decreased serum TG, total cholesterol, and LDL-C with an increased ratio of HDL-C/LDL-C in high-fat diet-fed C57BL/6 mice compared to controls [97]. Extracellular polysaccharides from Turkey tail mushroom after 4 weeks of administration attenuated the elevation of serum TG levels in a dose-dependent manner in T2DM rats [68].
3.7. Altered Adipose Tissue Function
It is well known that genes responsible for fatty acid synthesis (i.e., acetyl-CoA carboxylase-1 (ACC-1), fatty-acid synthase (FAS), sterol regulatory element-binding protein-1c (SREBP-1c)) are upregulated, while those regulating catabolism (i.e., PPARγ co-activator 1α (PGC-1α)) are downregulated in high-fat diet-fad mice [148]. Overexpression of GPR43 in the adipose tissue of high-fat diet-fed mice resulted in reduced white adipose tissue and body weight, and also in improved glucose tolerance [110,144].
Water extract of Antrodia cinnamomea attenuated ACC-1, FAS, and SREBP-1c and enhanced PGC-1α gene expression in treated compared to non-treated high-fat diet-fed mice [56]. A ReishiMax supplement containing polysaccharides and triterpenes from Ganoderma lucidum inhibited adipocyte differentiation by suppressing peroxisome proliferator-activated receptor-γ (PPAR-γ), SREBP-1c, and CCAAT/enhancer binding protein-α (C/EBP-α) transcription factors. Moreover, it suppressed FAS, acyl-CoA synthetase-1 (ACS1), fatty acid binding protein-4 (FABP4), fatty acid transport protein-1 (FATP1), and perilipin enzymes, which are responsible for lipid synthesis, transport, and storage [75]. Water extract of Ganoderma lucidum significantly reduced subcutaneous as well as liver fat accumulation by the alteration of gut microbiota in high-fat diet-fed mice. Additionally, it decreased the infiltration of anti-F4/80 and CD11b/CD11c-positive macrophages into the liver/adipose tissues and also increased the levels of Treg cells in the liver and adipose tissue in treated groups compared to non-treated mice [71]. Moreover, improvement in gut microbiota composition also resulted in a significantly decreased production of pro-inflammatory cytokines such as TNF-α, interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and plasminogen activator-inhibitor 1 (PAI-1) in adipocytes [48,71]. Antrodia cinnamomea similarly altered adipocyte function, as seen in the significantly decreased pro-inflammatory marker TNF-α, IL-1β, IL-6, and leptin levels, while adiponectin levels significantly increased in the treated groups compared to the non-treated high-fat diet-fed mice [56]. Twelve weeks of treatment with extract from Agaricus blazei Murill resulted in significantly decreased insulin levels and HOMA-IR in diabetic patients in parallel with significantly increased adiponectin levels [50]. The Mukitake mushroom Panellus serotinus significantly increased the serum adiponectin levels of db/db mice after a 4 week period of treatment compared to control-fed db/db mice. In addition, it also significantly decreased the serum levels of monocyte-attracting protein 1 (MCP1) [105].
3.8. Reduced Pro-Inflammatory Cytokine Levels and Immunomodulatory Properties
A decreased production of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and PAI-1 was detected after treatments of water extract of Ganoderma lucidum compared to non-treated high-fat diet-fed mice [48,71]. Similarly, a 5 g/day extract of α-glucans from Agaricus bisporus significantly decreased TNF-α levels compared to placebo controls [42]. Morchella esculenta polysaccharides also significantly reduced serum pro-inflammatory cytokine levels in a T2DM mouse model [102]. Furthermore, water extract of Antrodia cinnamomea decreased the serum levels of TNF-α, IL-1β, and IL-6 in treated mice compared to non-treated high-fat diet-fed mice [56]. In addition, Boletus polysaccharide treatment significantly decreased nuclear factor kappa B (NF-κB) expression, which is responsible for pro-inflammatory cytokine production, compared to the control T2DM group; the expression of TNF-α also significantly decreased to normal levels [65]. Flammulina velutipes polysaccharides have immunomodulatory and anti-inflammatory properties, based on recent studies [70]. Secondary metabolites from Hericium erinaceus, which have poor water solubility, have immunomodulatory properties as well [88,89]. A 25-week treatment with Lentinula edodes mushroom was associated with immunomodulation observed in an increased CD4+/CD8+ lymphocyte ratio and in a shift from pro- to anti-inflammatory cytokine production compared to high-fat diet-fed C57BL/6 control mice [97].
3.9. Induced/Reduced Weight Loss
A study investigating β-glucan supplementation in high-fat diet-fed induced obese rats for 6 weeks found that β-glucan, which is predominantly found in mushrooms and cereals, has beneficial therapeutic potential against obesity [149]. The treatment significantly reduced body weight and attenuated obesogenic markers such as hyperglycemia, dyslipidemia, and insulin resistance by altering the expression of PPAR-γ, SREBP-1c, FAS, HMG-CoA reductase, and Fab-4 in high-fat diet-fed induced obese rats. Water extract of Ganoderma lucidum induced significant weight loss in obese animals by altering gut microbiota composition, with this effect being primarily attributed to high molecular weight polysaccharides (>300 kDa) [48,71]. Concordantly, water extract of Antrodia cinnamomea induced a significant loss of body weight of around 10% in treated compared to untreated high-fat diet-fed mice [56]. SCFA supplementation altered gut microbiota, with an increased proportion of Bacteroidetes and decreased proportion of Firmicutes, and significantly increased GPR43 levels in adipose tissue, preventing high-fat-diet-induced obesity in mice [129]. Treatments with Grifola frondosa inhibited weight gain in spontaneously diabetic mice compared to non-treated groups, and this was confirmed by crossover experiments [83]. In a T2DM rat model, body weight was significantly decreased compared to the normal group but Boletus polysaccharide supplementation was able to revert this, resulting in attenuated weight loss in the treated group [65].
4. Supposed Signaling Mechanisms Targeted by Medicinal Mushroom Components
Increased endotoxin in the serum as the result of a leaky gut [150,151] induced JNK and NF-κB signaling activation [56], which inactivated IRS-1 by its inhibitory phosphorylation, leading to decreased insulin signaling and insulin resistance [152,153]. Ganoderma lucidum and Antrodia cinnamomea extracts could reduce endotoxemia, which could revert insulin signaling through IRS-1 reactivation [56,71]. Additionally, administration of Antrodia cinnamomea powder containing eburicoic acid, dehydroeburicoic acid, sulphurenic acid, dehydrosulphurenic acid, and ergostatrien-3β-ol, at an optimal dose of 200 mg/kg induced significant induction of insulin signaling with increased PI3K, IRS-1, and GLUT-4 levels compared to control Wistar rats [58].
Water-extracted mushroom polysaccharides increased SCFA levels in the intestine, inducing GLP-1 production, which led to increased expression of IGF-2/IGF-1R in an autocrine manner. This activation of the ligand-bound receptors then activated the Akt pathways in pancreatic β-cells, and, in a parallel process, inhibited apoptosis and induced proliferation in these β-cells [141].
SXF of Grifola frondosa significantly increased glucose uptake due to the reactivation of insulin signaling. In detail, SXF treatments decreased inhibitory serine phosphorylation of IRS-1 while increasing activating tyrosine phosphorylation, resulting in Akt activation and putative GLUT4 translocation to the plasma membrane [81]. Pleurotus ostreatus mushroom decreased hyperglycemia in streptozotocin-induced diabetic rats through increased p-AMPK levels and expression of GLUT4 in muscles and adipose tissues [113]. In insulin-resistant HepG2 cells, polysaccharides obtained from Grifola frondosa significantly increased Akt phosphorylation and thus inhibited glycogen synthase kinase-3 (GSK-3), the inhibitor of glycogen synthase, and subsequently increased glucose uptake and glycogen synthesis [84]. Moreover, novel heteropolysaccharides (GFP-N) from Grifola frondosa caused hypoglycemic effects in insulin-resistant HepG2 cells via activation of IRS-1, PI3K, and GLUT4 signaling and inhibition of JNK/p38 pathways [85]. Water extract of Coriolus versicolor significantly increased the mRNA expression of PI3K, p-Akt, Akt, p-p38 mitogen-activated protein kinase (MAPK), and p38 MAPK in rat skeletal muscles in vivo, in which signaling was reported to upregulate GLUT4, and thus reduce insulin resistance [66].
The molecular background of the anti-hyperlipidemic effects of Flammulina velutipes was demonstrated by the activation of the PI3K/Akt pathway in liver, where p-PI3K, p-Akt, GLUT4, and IRS-1 levels were significantly up-regulated after high-dose polysaccharide intervention in diabetic mice [69].
The GF5000 fraction from Grifola frondosa decreased pro-inflammatory cytokine levels, likely by suppressing the TLR4/MyD88/NF-κB pathway [80].
The signaling mechanisms targeted by the medicinal mushroom components described above are listed in Table 2.

Table 2.
Signaling pathways through which medical mushrooms improve insulin resistance.
5. The Role of the Vitamin D2 Component in Medical Mushrooms in the Context of Insulin Resistance
Vitamin D has a fundamental role in calcium homeostasis and bone metabolism [9]. However, its pleiotropic effects on immunity, cell growth, differentiation, and energy metabolism are now widely known due to the results of extensive studies [154,155] following the discovery of 25(OH)D [156], and then its hormonally active form, 1,25(OH)2D calcitriol [157].
Vitamin D is one of the fat-soluble vitamins and has two forms, ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3) [158,159]. Plants and mushrooms form vitamin D2 from its ergosterol precursor by ultraviolet B irradiation (UVB) [18], while vitamin D3 is synthesized in the epidermis from 7-dehydrocholesterol, also by UVB. Around 80% of vitamin D is produced by our skin and the remaining 20% is provided by our nutrition [160]. The content of ergosterol and vitamin D2 varies among different mushroom species [161].
UVB-exposed Agaricus bisporus consumption significantly increased serum 25(OH)D levels in patients to the same extent as supplementation with ergocalciferol or cholecalciferol [42]. Ergosterol did not alter the 25-hydroxylation process in either the HepG2 cells or the liver of ergosterol-supplemented mice, and the levels of 25(OH)D in serum and tissues were unchanged compared with cholecalciferol supplemented groups. It also did not change the concentration of 1,25(OH)2D and 24,25(OH)2D in the serum of treated animals compared to cholecalciferol supplementation. In addition, ergosterol did not alter liver lipid concentrations compared with cholecalciferol supplementation in treated mice [162]. In our previous review, we briefly discussed the positive role of vitamin D in insulin resistance [3], we discuss this in more detail below.
Primarily, the active metabolite, the hormone calcitriol (1,25(OH)2D), mediates the biological effects of vitamin D in organisms by binding to the vitamin D receptor (VDR) [2,154,158,163,164,165]. Upon binding to calcitriol, the nuclear receptor VDR forms a heterodimer with the retinoic acid X receptor (RXR) to enhance or inhibit the transcription of thousands of genes [154,163]. Although a membrane-associated VDR is also known to initiate membrane-signaling cascades [166], and non-genomic effects of 1,25(OH)2D do not necessarily require VDR [167], the physiology of the genomic and non-genomic effects overlaps to a large extent [154]. Interestingly, not only calcitriol, but also the inactive 25(OH)D form can bind to VDR, ensuring the intracrine effects of vitamin D in addition to 1,25(OH)2D produced locally and specifically in various tissues [168,169,170]. The presumed role of vitamin D was primarily probably the regulation of energy metabolism, and later it acquired new functions, namely the modulation of the innate and adaptive immunity, and the regulation of calcium and bone homeostasis [164].
Metabolic syndrome (MetS) is a complex metabolic disorder characterized by four main factors: hypertension, dyslipidemia, abdominal obesity, and IR, among others [3,118,171]. Several studies have shown that vitamin D levels (25(OH)D) are inversely associated with MetS factors [2,172,173]. Vitamin D deficiency has been linked to the earlier-onset and higher severity of T2DM, due to abnormal secretion of insulin and immune dysfunction [155]. However, vitamin D may prevent pancreatic β-cell destruction and the incidence of autoimmune diabetes, likely through the inhibition of pro-inflammatory cytokine release [174]. Furthermore, vitamin D supplementation was effective in improving T2DM-related conditions such as hyperglycemia and increased hemoglobin A1c (HbA1c) levels [175,176].
Vitamin D supplementation also significantly elevated the levels of SIRT1 and SIRT6, which play important roles in glucose homeostasis by increasing insulin secretion, inhibiting gluconeogenesis and lipogenesis and suppressing obesity-induced inflammation and insulin resistance [177,178,179]. Vitamin D increased glucose uptake by inducing the insulin-independent SIRT1/AMPK/IRS1/GLUT4 signaling pathway [180]. Additionally, vitamin D increased insulin sensitivity, presumably through an increase in Ca2+ influx, which stimulated insulin receptor expression, activation of the GLUT-4 glucose transporter, and activation of peroxisome proliferator-activated receptor delta (PPAR-δ) [181,182].
In addition to glucose homeostasis, vitamin D also supports optimal lipid homeostasis through increased expression of adiponectin and activation of AMPK in adipocytes [2,183]. Adiponectin has positive effects on both glucose and lipid metabolism, increasing glucose and FFA utilization in skeletal muscle and reducing blood glucose levels, as well as increasing HDL-C while decreasing TG levels [184]. Adiponectin is similarly important in balancing immune processes due to its anti-inflammatory properties [185,186].
Vitamin D also plays an important role in the direct regulation of innate and adaptive immune systems, through VDR, which is expressed in almost all immune cells [187]. Vitamin D modulates immune reactions by inducing anti-inflammatory cytokine production [70,188,189,190], through the suppression of TLR2 and TLR4 proteins and NF-κB signaling [187]. In addition, but partly through these aforementioned processes, it decreases low-grade chronic inflammation coexisting with IR [160]. Furthermore, vitamin D, as an epigenetic regulator, maintains the expression of DNA demethylases and thus prevents hypermethylation, which is an important characteristic of T2DM patients [191,192].
It is widely known that hyperglycemia causes oxidative stress through the overproduction of reactive oxidative species (ROS); however, vitamin D can protect cells from ROS overproduction and control mitochondrial respiration [193,194,195]. Additionally, in high-glucose-treated adipocytes, vitamin D inhibited oxidative stress as well through SIRT1/AMPK/GLUT4 signaling [196].
Calcification or vitamin D intoxication is an important consideration when using vitamin D supplementation as a preventive or complementary therapy. It should be noted that excessive exposure to sunlight cannot cause intoxication, as both the inactive and active form produced by the skin are photolabile and thus easily converted to biologically inactive products [197,198]. It is also important to note that levels of the inactive 25(OH)D form did not correlate with calcification related measures [199,200]. However, it is assumed that vitamin D2 is less effective in bone metabolism because it is not bone selective [201]. Moreover, patients with chronic kidney disease [202], vitamin D oversupply, and patients with vitamin D deficiency [203] with certain related health issues have increased risk, which may lead to calcification. Therefore, in these cases, additional laboratory parameters, i.e., measurement of serum calcium, phosphate, parathyroid hormone, creatinine, and alkaline phosphatase levels, should also be considered [10]. However, it is also important to know that convincing molecular evidence suggests that calcification is not only an active process but may also be reversible if treated at an early stage [202].
6. Summary
Edible mushrooms, including medical mushrooms, are an important part of nutrition. They are good source of fiber, vitamins, amino acids, and trace elements, but the most investigated are the water-soluble composites, polysaccharides. Polysaccharides have complex effects on the human body. They favorably modulate the intestinal microbiota, glucose, and lipid homeostasis, as well as the immune system, the combined effect of which can reduce insulin resistance. Thus, these effects make mushrooms, especially medicinal mushrooms, a potential part of complementary therapy for obesity and related diseases, such as type 2 diabetes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26020827/s1. References [12,14,23,25,97,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232] are referring to Supplementary Materials.
Author Contributions
Z.N.: idea, design, visualization, paper writing, and final approval; M.P.B.: idea, design, visualization, paper writing, and paper review; L.D.S.: paper review; G.S.: paper review; I.T.: paper review; L.S.-S.: paper review. All authors have read and agreed to the published version of the manuscript.
Funding
There was no funding related to this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACC-1 | acetyl-CoA carboxylase-1 |
| ACS1 | acyl-CoA synthetase-1 |
| AMPK | AMP-activated protein kinase |
| C/EBP-α | CCAAT/enhancer binding protein-α |
| CAT | catalase |
| FABP4 | fatty acid binding protein-4 |
| FAS | fatty-acid synthase |
| FATP1 | fatty acid transport protein-1 |
| FFA | free fatty acid |
| FIP | fungal immunomodulatory proteins |
| GLP-1 | glucagon-like peptide-1 |
| GLUT4 | glucose transporter type 4 |
| GPR43 | G-protein-coupled receptor 43 |
| GSH | glutathione |
| GSH-Px | glutathione peroxidase |
| GSK-3 | glycogen synthase kinase-3 |
| HbA1c | hemoglobin A1c |
| HDL-C | high-density lipoprotein cholesterol |
| HMG-CoA | 3-hydroxy-3-methylglutaryl-CoA |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| IgA | immunoglobulin A |
| IGF-1/IGF-2 | insulin growth factor 1/insulin growth factor 2 |
| IGF-1R | IGF-1 receptor |
| IKKβ | inhibitor of κB kinase β |
| IL-1β | interleukin-1 beta |
| IL-6 | interleukin-6 |
| IR | insulin resistance |
| IRS1/IRS2 | insulin receptor substrate 1/insulin receptor substrate 2 |
| IκB | inhibitor of κB |
| LDL-C | low-density lipoprotein cholesterol |
| LDLR | LDL receptor |
| MAPK | mitogen-activated protein kinase |
| MCP1 | monocyte attracting protein 1 |
| MCP1 | monocyte chemoattractant protein 1 |
| MDA | malonaldehyde |
| MetS | metabolic syndrome |
| MyD88 | myeloid differentiation primary response 88 |
| NF-κB | nuclear factor kappa B |
| NSPs | non-starch polysaccharides |
| PAI-1 | plasminogen activator-inhibitor 1 |
| PGC-1α | PPARγ co-activator 1α |
| PI3K | phosphoinositide 3-kinase |
| PPAR-γ | peroxisome proliferator-activated receptor-γ |
| PPAR-δ | peroxisome proliferator-activated receptor delta |
| PSA | prostate specific antigen |
| QoL | quality of life |
| RCTs | randomized control trials |
| RIP | ribosome inactivating proteins |
| ROS | reactive oxidative species |
| SCFAs | short-chain fatty acids |
| SHR | spontaneously hypertensive rats |
| SIRT1/SIRT6 | sirtuin 1/sirtuin 1 |
| SOD | superoxide-dismutase |
| SREBP-1c | sterol regulatory element-binding protein-1c |
| SXF | SX-fraction |
| T2DM | type 2 diabetes |
| TG | triglyceride |
| TGF-β1 | transforming growth factor beta 1 |
| TLR2/TLR4 | toll-like receptor 2/toll-like receptor 4 |
| TNF-α | tumor necrosis factor α |
| UVB | ultraviolet B irradiation |
| VDR | vitamin D receptor |
| ZFR | Zucker fatty rats |
References
- Nemeth, Z.; Kiss, E.; Takacs, I. The Role of Epigenetic Regulator SIRT1 in Balancing the Homeostasis and Preventing the Formation of Specific “Soil” of Metabolic Disorders and Related Cancers. Front. Biosci. Landmark Ed. 2022, 27, 253. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, Z.; Patonai, A.; Simon-Szabo, L.; Takacs, I. Interplay of Vitamin D and SIRT1 in Tissue-Specific Metabolism-Potential Roles in Prevention and Treatment of Non-Communicable Diseases Including Cancer. Int. J. Mol. Sci. 2023, 24, 6154. [Google Scholar] [CrossRef] [PubMed]
- Simon-Szabo, L.; Lizak, B.; Sturm, G.; Somogyi, A.; Takacs, I.; Nemeth, Z. Molecular Aspects in the Development of Type 2 Diabetes and Possible Preventive and Complementary Therapies. Int. J. Mol. Sci. 2024, 25, 9113. [Google Scholar] [CrossRef]
- Koelwyn, G.J.; Quail, D.F.; Zhang, X.; White, R.M.; Jones, L.W. Exercise-dependent regulation of the tumour microenvironment. Nat. Rev. Cancer 2017, 17, 620–632. [Google Scholar] [CrossRef]
- Kiss, E.; Forika, G.; Mohacsi, R.; Nemeth, Z.; Krenacs, T.; Dank, M. Methyl-Donors Can Induce Apoptosis and Attenuate Both the Akt and the Erk1/2 Mediated Proliferation Pathways in Breast and Lung Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 3598. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Forika, G.; Dank, M.; Krenacs, T.; Nemeth, Z. Methyl Donors Reduce Cell Proliferation by Diminishing Erk-Signaling and NFkB Levels, While Increasing E-Cadherin Expression in Panc-1 Cell Line. Int. J. Mol. Sci. 2022, 23, 2546. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Hajdu, A.; Forika, G.; Dank, M.; Krenacs, T.; Nemeth, Z. The Effect of Dietary Methyl-Donor Intake and Other Lifestyle Factors on Cancer Patients in Hungary. Cancers 2022, 14, 4432. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Muhl, D.; Mohacsi, R.; Dank, M.; Takacs, I.; Nemeth, Z. B Vitamin Intake and the Risk of Colorectal Cancer Development: A Systematic Review and Meta-Analysis of Observational Studies. Biomed. J. Sci. Tech. Res. 2022, 40, 12. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin D Sources, Metabolism, and Deficiency: Available Compounds and Guidelines for Its Treatment. Metabolites 2021, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Takacs, I.; Boyanov, M.; Belaya, Z.; Diaconu, C.C.; Mokhort, T.; Zherdova, N.; Rasa, I.; Payer, J.; Pilz, S. Clinical Practice in the Prevention, Diagnosis and Treatment of Vitamin D Deficiency: A Central and Eastern European Expert Consensus Statement. Nutrients 2022, 14, 1483. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Chen, C.M.; Mu, S.C.; Yang, S.H.; Ju, Y.M.; Li, S.C. Medicinal Components in Edible Mushrooms on Diabetes Mellitus Treatment. Pharmaceutics 2022, 14, 436. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Riaz, M.; Khan, A.; Aljamea, A.; Algheryafi, M.; Sewaket, D.; Alqathama, A. Ganoderma lucidum (Reishi) an edible mushroom; a comprehensive and critical review of its nutritional, cosmeceutical, mycochemical, pharmacological, clinical, and toxicological properties. Phytother. Res. 2021, 35, 6030–6062. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; de Mores, A.R.; Cohen, L.; Anwar, M.M.; Lazar, F.; Hicklen, R.; Lopez, G.; Yang, P.; Bruera, E. Medicinal Mushroom Supplements in Cancer: A Systematic Review of Clinical Studies. Curr. Oncol. Rep. 2023, 25, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Thi Nhu Ngoc, L.; Oh, Y.K.; Lee, Y.J.; Lee, Y.C. Effects of Sparassis crispa in Medical Therapeutics: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Mol. Sci. 2018, 19, 1487. [Google Scholar] [CrossRef] [PubMed]
- Thu, Z.M.; Myo, K.K.; Aung, H.T.; Clericuzio, M.; Armijos, C.; Vidari, G. Bioactive Phytochemical Constituents of Wild Edible Mushrooms from Southeast Asia. Molecules 2020, 25, 1972. [Google Scholar] [CrossRef] [PubMed]
- Surinrut, P.; Julshamn, K.; Rein Njaa, L. Protein, amino acids and some major and trace elements in Thai and Norwegian mushrooms. Plant Foods Hum. Nutr. 1987, 37, 117–125. [Google Scholar] [CrossRef]
- Gunc Ergonul, P.; Akata, I.; Kalyoncu, F.; Ergonul, B. Fatty acid compositions of six wild edible mushroom species. Sci. World J. 2013, 2013, 163964. [Google Scholar] [CrossRef]
- Hu, D.; Yang, X.; Hu, C.; Feng, Z.; Chen, W.; Shi, H. Comparison of Ergosterol and Vitamin D(2) in Mushrooms Agaricus bisporus and Cordyceps militaris Using Ultraviolet Irradiation Directly on Dry Powder or in Ethanol Suspension. ACS Omega 2021, 6, 29506–29515. [Google Scholar] [CrossRef] [PubMed]
- Zięba, P.; Sękara, A.; Bernaś, E.; Krakowska, A.; Sułkowska-Ziaja, K.; Kunicki, E.; Suchanek, M.; Muszyńska, B. Supplementation with Magnesium Salts-A Strategy to Increase Nutraceutical Value of Pleurotus djamor Fruiting Bodies. Molecules 2021, 26, 3273. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Copur, M.; Yildiz, D.; Akata, I. Metal concentration of wild edible mushrooms in Soguksu National Park in Turkey. Food Chem. 2011, 128, 731–734. [Google Scholar] [CrossRef]
- Xu, X.; Yan, H.; Chen, J.; Zhang, X. Bioactive proteins from mushrooms. Biotechnol. Adv. 2011, 29, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Ngai, P.H.; Ng, T.B. Lentin, a novel and potent antifungal protein from shitake mushroom with inhibitory effects on activity of human immunodeficiency virus-1 reverse transcriptase and proliferation of leukemia cells. Life Sci. 2003, 73, 3363–3374. [Google Scholar] [CrossRef]
- Zięba, P.; Sękara, A.; Sułkowska-Ziaja, K.; Muszyńska, B. Culinary and medicinal mushrooms: Insight into growing technologies. Acta Mycol. 2020, 55, 5526. [Google Scholar] [CrossRef]
- Cheraghi, Z.; Mirmiran, P.; Mansournia, M.A.; Moslehi, N.; Khalili, D.; Nedjat, S. The association between nutritional exposures and metabolic syndrome in the Tehran Lipid and Glucose Study (TLGS): A cohort study. Public Health 2016, 140, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Yadav, B.; Dalal, R.C.; Naorem, A.; Sinha, N.K.; Srinivasa Rao, C.; Dang, Y.P.; Patra, A.K.; Datta, S.P.; Subba Rao, A. Mushroom farming: A review Focusing on soil health, nutritional security and environmental sustainability. Farming Syst. 2024, 2, 100098. [Google Scholar] [CrossRef]
- Sande, D.; Oliveira, G.P.; Moura, M.; Martins, B.A.; Lima, M.; Takahashi, J.A. Edible mushrooms as a ubiquitous source of essential fatty acids. Food Res. Int. 2019, 125, 108524. [Google Scholar] [CrossRef]
- Kala, K.; Pajak, W.; Sulkowska-Ziaja, K.; Krakowska, A.; Lazur, J.; Fidurski, M.; Marzec, K.; Zieba, P.; Fijalkowska, A.; Szewczyk, A.; et al. Hypsizygus marmoreus as a Source of Indole Compounds and Other Bioactive Substances with Health-Promoting Activities. Molecules 2022, 27, 8917. [Google Scholar] [CrossRef] [PubMed]
- Podkowa, A.; Kryczyk-Poprawa, A.; Opoka, W.; Muszyńska, B. Culinary–medicinal mushrooms: A review of organic compounds and bioelements with antioxidant activity. Eur. Food Res. Technol. 2021, 247, 513–533. [Google Scholar] [CrossRef]
- Thakur, M.P. Advances in mushroom production: Key to food, nutritional and employment security: A review. Indian Phytopathol. 2020, 73, 377–395. [Google Scholar] [CrossRef]
- Tung, Y.T.; Pan, C.H.; Chien, Y.W.; Huang, H.Y. Edible Mushrooms: Novel Medicinal Agents to Combat Metabolic Syndrome and Associated Diseases. Curr. Pharm. Des. 2020, 26, 4970–4981. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K. A review on edible straw mushrooms: A source of high nutritional supplement, biologically active diverse structural polysaccharides. J. Sci. Res. 2020, 64, 295–304. [Google Scholar] [CrossRef]
- Manzi, P.; Gambelli, L.; Marconi, S.; Vivanti, V.; Pizzoferrato, L. Nutrients in edible mushrooms: An inter-species comparative study. Food Chem. 1999, 65, 477–482. [Google Scholar] [CrossRef]
- Longvah, T.; Deosthale, Y.G. Compositional and nutritional studies on edible wild mushroom from northeast India. Food Chem. 1998, 63, 331–334. [Google Scholar] [CrossRef]
- Kalac, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernandez-Perez, T.; Paredes-Lopez, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Konko, K.; Eurola, M.; Pihlava, J.M.; Astola, J.; Vahteristo, L.; Hietaniemi, V.; Kumpulainen, J.; Valtonen, M.; Piironen, V. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Bhambri, A.; Srivastava, M.; Mahale, V.G.; Mahale, S.; Karn, S.K. Mushrooms as Potential Sources of Active Metabolites and Medicines. Front. Microbiol. 2022, 13, 837266. [Google Scholar] [CrossRef]
- Uzun, Y.; Genccelep, H.; Kaya, A.; Akcay, M.E. The mineral contents of some wild edible mushrooms. Ekoloji 2011, 20, 6–12. [Google Scholar]
- Mallikarjuna, S.; Ranjini, A.; Haware, D.J.; Vijayalakshmi, M.; Shashirekha, M.; Rajarathnam, S. Mineral composition of four edible mushrooms. J. Chem. 2013, 2013, 805284. [Google Scholar] [CrossRef]
- Ekowati, N.; Yuniati, N.I.; Hernayanti, H.; Ratnaningtyas, N.I. Antidiabetic potentials of button mushroom (Agaricus bisporus) on alloxan-induced diabetic rats. Biosaintifika J. Biol. Biol. Educ. 2018, 10, 655–662. [Google Scholar] [CrossRef]
- Aida, F.M.N.A.; Shuhaimi, M.; Yazid, M.; Maaruf, A.G. Mushroom as a potential source of prebiotics: A review. Trends Food Sci. Technol. 2009, 20, 567–575. [Google Scholar] [CrossRef]
- Blumfield, M.; Abbott, K.; Duve, E.; Cassettari, T.; Marshall, S.; Fayet-Moore, F. Examining the health effects and bioactive components in Agaricus bisporus mushrooms: A scoping review. J. Nutr. Biochem. 2020, 84, 108453. [Google Scholar] [CrossRef]
- Abd-alwahab, W.I.; Al-dulaimi, F.K.; Abdulqader, A.T. Effect of mushroom cooked in olive oil on some physiological and biochemical parameters of human. EurAsian J. BioSci. 2018, 12, 393–397. [Google Scholar]
- Hashemi Yusefabad, H.; Hosseini, S.A.; Zakerkish, M.; Cheraghian, B.; Alipour, M. The effects of hot air-dried white button mushroom powder on glycemic indices, lipid profile, inflammatory biomarkers and total antioxidant capacity in patients with type-2 diabetes mellitus: A randomized controlled trial. J. Res. Med. Sci. 2022, 27, 49. [Google Scholar] [CrossRef] [PubMed]
- Cheskin, L.J.; Davis, L.M.; Lipsky, L.M.; Mitola, A.H.; Lycan, T.; Mitchell, V.; Mickle, B.; Adkins, E. Lack of energy compensation over 4 days when white button mushrooms are substituted for beef. Appetite 2008, 51, 50–57. [Google Scholar] [CrossRef]
- Calvo, M.S.; Mehrotra, A.; Beelman, R.B.; Nadkarni, G.; Wang, L.; Cai, W.; Goh, B.C.; Kalaras, M.D.; Uribarri, J. A Retrospective Study in Adults with Metabolic Syndrome: Diabetic Risk Factor Response to Daily Consumption of Agaricus bisporus (White Button Mushrooms). Plant Foods Hum. Nutr. 2016, 71, 245–251. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim. Biophys. Acta 2012, 1822, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Ojcius, D.M.; Chang, C.J.; Lin, C.S.; Lu, C.C.; Ko, Y.F.; Tseng, S.F.; Lai, H.C.; Young, J.D. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat. Rev. Endocrinol. 2017, 13, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Vitak, T.; Yurkiv, B.; Wasser, S.; Nevo, E.; Sybirna, N. Effect of medicinal mushrooms on blood cells under conditions of diabetes mellitus. World J. Diabetes 2017, 8, 187–201. [Google Scholar] [CrossRef]
- Hsu, C.H.; Liao, Y.L.; Lin, S.C.; Hwang, K.C.; Chou, P. The mushroom Agaricus Blazei Murill in combination with metformin and gliclazide improves insulin resistance in type 2 diabetes: A randomized, double-blinded, and placebo-controlled clinical trial. J. Altern. Complement. Med. 2007, 13, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Guterrez, Z.R.; Mantovani, M.S.; Eira, A.F.; Ribeiro, L.R.; Jordao, B.Q. Variation of the antimutagenicity effects of water extracts of Agaricus blazei Murrill in vitro. Toxicol. Vitr. 2004, 18, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Kido, T.; Takaku, T.; Sumiyoshi, M.; Baba, K. Isolation of an anti-angiogenic substance from Agaricus blazei Murill: Its antitumor and antimetastatic actions. Cancer Sci. 2004, 95, 758–764. [Google Scholar] [CrossRef]
- Ahn, W.-S.; Kim, D.-J.; Chae, G.-T.; Lee, J.-M.; Bae, S.-M.; Sin, J.-I.; Kim, Y.-W.; Namkoong, S.-E.; Lee, I. Natural killer cell activity and quality of life were improved by consumption of a mushroom extract, Agaricus blazei Murill Kyowa, in gynecological cancer patients undergoing chemotherapy. Int. J. Gynecol. Cancer 2004, 14, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Meng, Y.; Yan, J.; Wang, N.; Xue, Z.; Zhang, H.; Fan, Y. Polysaccharide-Enriched Fraction from Amillariella Mellea Fruiting Body Improves Insulin Resistance. Molecules 2018, 24, 46. [Google Scholar] [CrossRef] [PubMed]
- Manlai, U.; Chang, S.W.; Lee, S.C.; Ho, W.J.; Hsu, T.H.; Lin, J.G.; Lin, C.M.; Chen, Y.I.; Chang, S.L. Hypoglycemic Effect of Electroacupuncture Combined with Antrodia cinnamomea in Dexamethasone-Induced Insulin-Resistant Rats. Med. Acupunct. 2021, 33, 58–64. [Google Scholar] [CrossRef]
- Chang, C.J.; Lu, C.C.; Lin, C.S.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Wu, T.R.; Tsai, Y.H.; Yeh, T.S.; Lu, J.J.; et al. Antrodia cinnamomea reduces obesity and modulates the gut microbiota in high-fat diet-fed mice. Int. J. Obes. 2018, 42, 231–243. [Google Scholar] [CrossRef]
- Johnson, A.; Cheng, S.C.; Tsou, D.; Kong, Z.L. Attenuation of reproductive dysfunction in diabetic male rats with timber cultured Antrodia cinnamomea ethanol extract. Biomed. Pharmacother. 2019, 112, 108684. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Tzeng, C.Y.; Chen, Y.I.; Chang, S.W.; Hsu, T.H.; Ho, W.J.; Kuo, Y.H.; Hung, P.H.; Chang, S.L. Improving insulin resistance with Antrodia cinnamomea mycelium powder to induce a hypoglycemic effect in dexamethasone-induced insulin-resistant rats. Mol. Med. Rep. 2018, 17, 3260–3266. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Ganesan, K.; Xu, B. Insights into health-promoting effects of Jew’s ear (Auricularia auricula-judae). Trends Food Sci. Technol. 2021, 114, 552–569. [Google Scholar] [CrossRef]
- Hu, X.; Liu, C.; Wang, X.; Jia, D.; Lu, W.; Sun, X.; Liu, Y.; Yuan, L. Hpyerglycemic and anti-diabetic nephritis activities of polysaccharides separated from Auricularia auricular in diet-streptozotocin-induced diabetic rats. Exp. Ther. Med. 2017, 13, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Shen, M.; Fang, Z.; Xu, Y.; Yu, M.; Wang, S.; Zhang, Y.; Wang, W. Antidiabetic Effects of the Auricularia auricular Polysaccharides Simulated Hydrolysates in Experimental Type-2 Diabetic Rats. Nat. Product. Commun. 2018, 13, 1934578X1801300220. [Google Scholar] [CrossRef]
- Hu, J.L.; Nie, S.P.; Xie, M.Y. Antidiabetic Mechanism of Dietary Polysaccharides Based on Their Gastrointestinal Functions. J. Agric. Food Chem. 2018, 66, 4781–4786. [Google Scholar] [CrossRef]
- Wong, J.H.; Ng, T.B.; Chan, H.H.L.; Liu, Q.; Man, G.C.W.; Zhang, C.Z.; Guan, S.; Ng, C.C.W.; Fang, E.F.; Wang, H.; et al. Mushroom extracts and compounds with suppressive action on breast cancer: Evidence from studies using cultured cancer cells, tumor-bearing animals, and clinical trials. Appl. Microbiol. Biotechnol. 2020, 104, 4675–4703. [Google Scholar] [CrossRef] [PubMed]
- Saidu, S.; Eleazu, C.O.; Ebuka, D.; Ikechukwu, A.; Blessing, M.; Chibuike, N.; Chukwuma, C. Starch Hydrolysis, Polyphenol Contents, and In Vitro Alpha Amylase Inhibitory Properties of Some Nigerian Foods as Affected by Cooking. Front. Nutr. 2017, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, L.; Fan, Y.; Yan, P.; Li, S.; Zhou, X. The effect of boletus polysaccharides on diabetic hepatopathy in rats. Chem. Biol. Interact. 2019, 308, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Xian, H.M.; Che, H.; Qin, Y.; Yang, F.; Meng, S.Y.; Li, X.G.; Bai, Y.L.; Wang, L.H. Coriolus versicolor aqueous extract ameliorates insulin resistance with PI3K/Akt and p38 MAPK signaling pathways involved in diabetic skeletal muscle. Phytother. Res. 2018, 32, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.F.; Lee, C.H.; Hsu, T.H.; Lo, H.C. Potential activities and mechanisms of extracellular polysaccharopeptides from fermented Trametes versicolor on regulating glucose homeostasis in insulin-resistant HepG2 cells. PLoS ONE 2018, 13, e0201131. [Google Scholar] [CrossRef]
- Lo, H.C.; Hsu, T.H.; Lee, C.H. Extracellular Polysaccharopeptides from Fermented Turkey Tail Medicinal Mushroom, Trametes versicolor (Agaricomycetes), Mitigate Oxidative Stress, Hyperglycemia, and Hyperlipidemia in Rats with Type 2 Diabetes Mellitus. Int. J. Med. Mushrooms 2020, 22, 417–429. [Google Scholar] [CrossRef]
- Song, X.; Fu, H.; Chen, W. Effects of Flammulina velutipes polysaccharides on quality improvement of fermented milk and antihyperlipidemic on streptozotocin-induced mice. J. Funct. Foods 2021, 87, 104834. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H. Advances in the extraction, purification, structural-property relationships and bioactive molecular mechanism of Flammulina velutipes polysaccharides: A review. Int. J. Biol. Macromol. 2021, 167, 528–538. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Yan, R.; Kang, J.; Chen, R. Chemical Components of Ganoderma. Adv. Exp. Med. Biol. 2019, 1181, 59–106. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Lin, W.K.; Chang, S.H.; Tsai, G.J. Evaluation of the hypoglycaemic and antioxidant effects of submerged Ganoderma lucidum cultures in type 2 diabetic rats. Mycology 2020, 12, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Dutta, S.D.; Ganguly, K.; Cho, S.J.; Lim, K.T. Mushroom-Derived Bioactive Molecules as Immunotherapeutic Agents: A Review. Molecules 2021, 26, 1359. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan-Sahu, A.; Lane, B.; Sliva, D. ReishiMax, mushroom based dietary supplement, inhibits adipocyte differentiation, stimulates glucose uptake and activates AMPK. BMC Complement. Altern. Med. 2011, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, S.; Shimizu, K.; Kondo, R. Ganoderol B: A potent alpha-glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine 2011, 18, 1053–1055. [Google Scholar] [CrossRef]
- Satria, D.; Tamrakar, S.; Suhara, H.; Kaneko, S.; Shimizu, K. Mass Spectrometry-Based Untargeted Metabolomics and alpha-Glucosidase Inhibitory Activity of Lingzhi (Ganoderma lingzhi) During the Developmental Stages. Molecules 2019, 24, 2044. [Google Scholar] [CrossRef]
- Satria, D.; Amen, Y.; Niwa, Y.; Ashour, A.; Allam, A.E.; Shimizu, K. Lucidumol D, a new lanostane-type triterpene from fruiting bodies of Reishi (Ganoderma lingzhi). Nat. Prod. Res. 2019, 33, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Talpur, N.A.; Echard, B.W.; Fan, A.Y.; Jaffari, O.; Bagchi, D.; Preuss, H.G. Antihypertensive and metabolic effects of whole Maitake mushroom powder and its fractions in two rat strains. Mol. Cell. Biochem. 2002, 237, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Jiao, C.; Xie, Y.; Ye, L.; Li, Q.; Wu, Q. Grifola frondosa GF5000 improves insulin resistance by modulation the composition of gut microbiota in diabetic rats. J. Funct. Foods 2021, 77, 104313. [Google Scholar] [CrossRef]
- Konno, S.; Alexander, B.; Zade, J.; Choudhury, M. Possible hypoglycemic action of SX-fraction targeting insulin signal transduction pathway. Int. J. Gen. Med. 2013, 6, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Konno, S.; Tortorelis, D.G.; Fullerton, S.A.; Samadi, A.A.; Hettiarachchi, J.; Tazaki, H. A possible hypoglycaemic effect of maitake mushroom on Type 2 diabetic patients. Diabet. Med. 2001, 18, 1010. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Aoki, H.; Nanba, H. Anti-diabetic activity present in the fruit body of Grifola frondosa (Maitake). I. Biol. Pharm. Bull. 1994, 17, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, F.; Chen, Y.; Zhang, Y.; Hou, L.; Cao, X.; Wang, C. A polysaccharide from Grifola frondosa relieves insulin resistance of HepG2 cell by Akt-GSK-3 pathway. Glycoconj. J. 2014, 31, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, D.; Wang, D.; Lai, S.; Zhong, R.; Liu, Y.; Yang, C.; Liu, B.; Sarker, M.R.; Zhao, C. Hypoglycemic activity and gut microbiota regulation of a novel polysaccharide from Grifola frondosa in type 2 diabetic mice. Food Chem. Toxicol. 2019, 126, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Manohar, V.; Talpur, N.A.; Echard, B.W.; Lieberman, S.; Preuss, H.G. Effects of a water-soluble extract of maitake mushroom on circulating glucose/insulin concentrations in KK mice. Diabetes Obes. Metab. 2002, 4, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, J.; Hu, C.; Wang, J.; Zhang, J.; Ren, Z.; Song, X.; Jia, L. Antihyperglycaemic and organic protective effects on pancreas, liver and kidney by polysaccharides from Hericium erinaceus SG-02 in streptozotocin-induced diabetic mice. Sci. Rep. 2017, 7, 10847. [Google Scholar] [CrossRef] [PubMed]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 1–23. [Google Scholar] [CrossRef]
- Qian, F.; Xu, G.; Du, S.; Li, M. Isolation and identification of two new pyrone compounds from the culture of Herictum erinaceus. Yao Xue Xue Bao Acta Pharm. Sin. 1990, 25, 522–525. [Google Scholar]
- Wang, J.C.; Hu, S.H.; Wang, J.T.; Chen, K.S.; Chia, Y.C. Hypoglycemic effect of extract of Hericium erinaceus. J. Sci. Food Agric. 2005, 85, 641–646. [Google Scholar] [CrossRef]
- Liang, B.; Guo, Z.; Xie, F.; Zhao, A. Antihyperglycemic and antihyperlipidemic activities of aqueous extract of Hericium erinaceus in experimental diabetic rats. BMC Complement. Altern. Med. 2013, 13, 1–7. [Google Scholar] [CrossRef]
- Yang, B.-K.; Park, J.-B.; Song, C.-H. Hypolipidemic effect of exo-polymer produced in submerged mycelial culture of five different mushrooms. J. Microbiol. Biotechnol. 2002, 12, 957–961. [Google Scholar]
- Yang, B.-K.; Park, J.-B.; Song, C.-H. Hypolipidemic effect of an exo-biopolymer produced from a submerged mycelial culture of Hericium erinaceus. Biosci. Biotechnol. Biochem. 2003, 67, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Zeb, M.; Lee, C.H. Medicinal Properties and Bioactive Compounds from Wild Mushrooms Native to North America. Molecules 2021, 26, 251. [Google Scholar] [CrossRef]
- Chou, Y.-J.; Kan, W.-C.; Chang, C.-M.; Peng, Y.-J.; Wang, H.-Y.; Yu, W.-C.; Cheng, Y.-H.; Jhang, Y.-R.; Liu, H.-W.; Chuu, J.-J. Renal protective effects of low molecular weight of Inonotus obliquus polysaccharide (LIOP) on HFD/STZ-induced nephropathy in mice. Int. J. Mol. Sci. 2016, 17, 1535. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.M.; Zhang, L.Y.; Zhang, X.; Bai, H.B.; Liang, D.E.; Ma, L.F.; Shan, W.G.; Zhan, Z.J. Terpenoids with alpha-glucosidase inhibitory activity from the submerged culture of Inonotus obliquus. Phytochemistry 2014, 108, 171–176. [Google Scholar] [CrossRef]
- Drori, A.; Rotnemer-Golinkin, D.; Avni, S.; Drori, A.; Danay, O.; Levanon, D.; Tam, J.; Zolotarev, L.; Ilan, Y. Attenuating the rate of total body fat accumulation and alleviating liver damage by oral administration of vitamin D-enriched edible mushrooms in a diet-induced obesity murine model is mediated by an anti-inflammatory paradigm shift. BMC Gastroenterol. 2017, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Rivera, O.A.; Albarracín, W.; Lares, M. Bioactive components of shiitake (Lentinula edodes Berk. Pegler) and its impact on health. Arch. Venez. Farmacol. Ter. 2017, 36, 67–71. [Google Scholar]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Therapeutic potential of mushrooms in diabetes mellitus: Role of polysaccharides. Int. J. Biol. Macromol. 2020, 164, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Laurino, L.F.; Viroel, F.J.M.; Caetano, E.; Spim, S.; Pickler, T.B.; Rosa-Castro, R.M.; Vasconcelos, E.A.; Jozala, A.F.; Hataka, A.; Grotto, D.; et al. Lentinus edodes Exposure before and after Fetus Implantation: Materno-Fetal Development in Rats with Gestational Diabetes Mellitus. Nutrients 2019, 11, 2720. [Google Scholar] [CrossRef] [PubMed]
- Hussin, F.R.M.; Vitor II, R.J.S.; Joaquin, J.A.O.; Clerigo, M.M.; Paano, A.M.C. Anti-hyperglycemic effects of aqueous Lenzites betulina extracts from the Philippines on the blood glucose levels of the ICR mice (Mus musculus). Asian Pac. J. Trop. Biomed. 2016, 6, 155–158. [Google Scholar] [CrossRef]
- Rehman, A.U.; Siddiqui, N.Z.; Farooqui, N.A.; Alam, G.; Gul, A.; Ahmad, B.; Asim, M.; Khan, A.I.; Xin, Y.; Zexu, W.; et al. Morchella esculenta mushroom polysaccharide attenuates diabetes and modulates intestinal permeability and gut microbiota in a type 2 diabetic mice model. Front. Nutr. 2022, 9, 984695. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, J.; Li, J.; Liu, Y.; Park, H.J.; Yang, L. Recent Advances on Bioactive Ingredients of Morchella esculenta. Appl. Biochem. Biotechnol. 2021, 193, 4197–4213. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Y.; Lei, L.; Li, F.; Zhang, Y.; Chen, J.; Zhao, G.; Wu, S.; Yin, R.; Ming, J. Carboxymethylation of polysaccharide from Morchella angusticepes Peck enhances its cholesterol-lowering activity in rats. Carbohydr. Polym. 2017, 172, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Inoue, N.; Inafuku, M.; Shirouchi, B.; Morooka, T.; Nomura, S.; Nagamori, N.; Yanagita, T. Mukitake mushroom (Panellus serotinus) alleviates nonalcoholic fatty liver disease through the suppression of monocyte chemoattractant protein 1 production in db/db mice. J. Nutr. Biochem. 2010, 21, 418–423. [Google Scholar] [CrossRef]
- Jiang, X.; Meng, W.; Li, L.; Meng, Z.; Wang, D. Adjuvant Therapy with Mushroom Polysaccharides for Diabetic Complications. Front. Pharmacol. 2020, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Meng, G.; Zhang, C.; Lin, L.; Xu, N.; Liu, M.; Cui, F.; Jia, L. The antioxidative effects of acidic-, alkalic-, and enzymatic-extractable mycelium zinc polysaccharides by Pleurotus djamor on liver and kidney of streptozocin-induced diabetic mice. BMC Complement. Altern. Med. 2015, 15, 440. [Google Scholar] [CrossRef]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Jo, K.J.; Ghim, J.; Kim, J.; Lee, H.; Lee, T.G.; Kim, J.I.; Kim, Y.; Byun, J.W.; Min, B.S.; Son, J.S.; et al. Water Extract of Pleurotus eryngii var. ferulae Prevents High-Fat Diet-Induced Obesity by Inhibiting Pancreatic Lipase. J. Med. Food 2019, 22, 178–185. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, T.; Zhou, H.; Zhang, Y.; Jin, G.; Yang, Y. Antidiabetic effect of polysaccharides from Pleurotus ostreatus in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2016, 83, 126–132. [Google Scholar] [CrossRef]
- Dicks, L.; Jakobs, L.; Sari, M.; Hambitzer, R.; Ludwig, N.; Simon, M.C.; Stehle, P.; Stoffel-Wagner, B.; Helfrich, H.P.; Ahlborn, J.; et al. Fortifying a meal with oyster mushroom powder beneficially affects postprandial glucagon-like peptide-1, non-esterified free fatty acids and hunger sensation in adults with impaired glucose tolerance: A double-blind randomized controlled crossover trial. Eur. J. Nutr. 2022, 61, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Asrafuzzaman, M.; Rahman, M.M.; Mandal, M.; Marjuque, M.; Bhowmik, A.; Rokeya, B.; Hassan, Z.; Faruque, M.O. Oyster mushroom functions as an anti-hyperglycaemic through phosphorylation of AMPK and increased expression of GLUT4 in type 2 diabetic model rats. J. Taibah Univ. Med. Sci. 2018, 13, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Sangi, S.M.A.; Bawadekji, A.; Al Ali, M. Comparative effects of metformin, Pleurotus ostreatus, Nigella Sativa, and Zingiber officinale on the streptozotocin-induced diabetes mellitus in rats. Pharmacogn. Mag. 2018, 14, S268–S273. [Google Scholar] [CrossRef]
- Jayasuriya, W.B.N.; Wanigatunge, C.A.; Fernando, G.H.; Abeytunga, D.T.U.; Suresh, T.S. Hypoglycaemic activity of culinary Pleurotus ostreatus and P. cystidiosus mushrooms in healthy volunteers and type 2 diabetic patients on diet control and the possible mechanisms of action. Phytother. Res. 2015, 29, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Burillo, J.; Marques, P.; Jimenez, B.; Gonzalez-Blanco, C.; Benito, M.; Guillen, C. Insulin Resistance and Diabetes Mellitus in Alzheimer’s Disease. Cells 2021, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Inaishi, J.; Saisho, Y. Beta-Cell Mass in Obesity and Type 2 Diabetes, and Its Relation to Pancreas Fat: A Mini-Review. Nutrients 2020, 12, 3846. [Google Scholar] [CrossRef]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar] [CrossRef]
- Copps, K.D.; White, M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; D’Alessio, D.; Thomas, G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006, 3, 393–402. [Google Scholar] [CrossRef]
- Kundakovic, T.; Kolundzic, M. Therapeutic properties of mushrooms in managing adverse effects in the metabolic syndrome. Curr. Top. Med. Chem. 2013, 13, 2734–2744. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Neyrinck, A.M.; Backhed, F.; Cani, P.D. Targeting gut microbiota in obesity: Effects of prebiotics and probiotics. Nat. Rev. Endocrinol. 2011, 7, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Obin, M.S.; Zhao, L. The gut microbiota, obesity and insulin resistance. Mol. Asp. Med. 2013, 34, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y.; et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature 2023, 621, 389–395. [Google Scholar] [CrossRef]
- Lin, C.S.; Chang, C.J.; Lu, C.C.; Martel, J.; Ojcius, D.M.; Ko, Y.F.; Young, J.D.; Lai, H.C. Impact of the gut microbiota, prebiotics, and probiotics on human health and disease. Biomed. J. 2014, 37, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, Y. Gut Microbiota in Health and Disease. Ann. Nutr. Metab. 2017, 71, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Browne, P.D.; Claassen, E.; Cabana, M.D. Microbiota in Health and Disease: From Pregnancy to Childhood; Wageningen Academic Publishers: Wageningen, The Netherlands, 2017. [Google Scholar]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short Chain Fatty Acids Prevent High-fat-diet-induced Obesity in Mice by Regulating G Protein-coupled Receptors and Gut Microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Ojcius, D.M.; Ko, Y.F.; Chang, C.J.; Young, J.D. Antiaging effects of bioactive molecules isolated from plants and fungi. Med. Res. Rev. 2019, 39, 1515–1552. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Lee, J.H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef]
- Ostaff, M.J.; Stange, E.F.; Wehkamp, J. A ntimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol. Med. 2013, 5, 1465–1483. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Backhed, F.; Delzenne, N.M.; Schrenzel, J.; François, P.; Cani, P.D. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014, 8, 2116–2130. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, K.; Song, S.C.; Maruyama, A.; Kobayashi, A.; Kuzuhara, H.; Akaike, T. A new synthetic hypoglycaemic polysaccharide. Biochem. Biophys. Res. Commun. 1992, 188, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Wursch, P.; Pi-Sunyer, F.X. The role of viscous soluble fiber in the metabolic control of diabetes. A review with special emphasis on cereals rich in beta-glucan. Diabetes Care 1997, 20, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Pick, M.E.; Hawrysh, Z.J.; Gee, M.I.; Toth, E.; Garg, M.L.; Hardin, R.T. Oat bran concentrate bread products improve long-term control of diabetes: A pilot study. J. Am. Diet. Assoc. 1996, 96, 1254–1261. [Google Scholar] [CrossRef]
- Braaten, J.T.; Scott, F.W.; Wood, P.J.; Riedel, K.D.; Wolynetz, M.S.; Brule, D.; Collins, M.W. High beta-glucan oat bran and oat gum reduce postprandial blood glucose and insulin in subjects with and without type 2 diabetes. Diabet. Med. 1994, 11, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Gugolz, E.; Wursch, P. Effects of breakfast cereals containing various amounts of beta-glucan fibers on plasma glucose and insulin responses in NIDDM subjects. Diabetes Care 1996, 19, 831–834. [Google Scholar] [CrossRef]
- Vertesy, L.; Kurz, M.; Schindler, P.; Stump, H. Novel Phthalaldehyde Derivatives, Process for Their Preparation, and Their Use. CA2246928, 12 March 1999. [Google Scholar]
- Cornu, M.; Yang, J.Y.; Jaccard, E.; Poussin, C.; Widmann, C.; Thorens, B. Glucagon-like peptide-1 protects beta-cells against apoptosis by increasing the activity of an IGF-2/IGF-1 receptor autocrine loop. Diabetes 2009, 58, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, D.; Nan, C.; Mori, K.; Hanayama, M.; Kikuchi, H.; Hirai, S.; Egashira, Y. Effect of mushroom polysaccharides from Pleurotus eryngii on obesity and gut microbiota in mice fed a high-fat diet. Eur. J. Nutr. 2020, 59, 3231–3244. [Google Scholar] [CrossRef] [PubMed]
- Brockman, D.A.; Chen, X.; Gallaher, D.D. Consumption of a high beta-glucan barley flour improves glucose control and fatty liver and increases muscle acylcarnitines in the Zucker diabetic fatty rat. Eur. J. Nutr. 2013, 52, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J. Physiological and metabolic effects of dietary fiber. Fed. Proc. 1985, 44, 2902–2906. [Google Scholar] [PubMed]
- Topping, D.L. Soluble fiber polysaccharides: Effects on plasma cholesterol and colonic fermentation. Nutr. Rev. 1991, 49, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.N.; Ko, H.J.; Ng, L.T. Hypolipidemic effects of Antrodia cinnamomea extracts in high-fat diet-fed hamsters. J. Food Biochem. 2012, 36, 233–239. [Google Scholar] [CrossRef]
- Strable, M.S.; Ntambi, J.M. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Eraniappan, S.; Balasubramaniyan, P.; Uddandrao, V.V.S.; Roy, A.; Singaravel, S. Beta-glucan suppresses high-fat-diet-induced obesity by attenuating dyslipidemia and modulating obesogenic marker expressions in rats. Fundam. Clin. Pharmacol. 2023, 37, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- de Kort, S.; Keszthelyi, D.; Masclee, A.A. Leaky gut and diabetes mellitus: What is the link? Obes. Rev. 2011, 12, 449–458. [Google Scholar] [CrossRef]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D and Its Target Genes. Nutrients 2022, 14, 1354. [Google Scholar] [CrossRef]
- Lai, Y.H.; Fang, T.C. The pleiotropic effect of vitamin d. ISRN Nephrol. 2013, 2013, 898125. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Tanaka, Y.; DeLuca, H.F. Biological activity of 25-hydroxycholecalciferol, a metabolite of vitamin D3. Proc. Natl. Acad. Sci. USA 1968, 61, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W.; Myrtle, J.F.; Midgett, R.J.; Nowicki, H.G.; Williams, V.; Popjak, G. 1,25-dihydroxycholecalciferol: Identification of the proposed active form of vitamin D3 in the intestine. Science 1971, 173, 51–54. [Google Scholar] [CrossRef]
- Japelt, R.B.; Jakobsen, J. Vitamin D in plants: A review of occurrence, analysis, and biosynthesis. Front. Plant Sci. 2013, 4, 136. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Vitamin D in health and disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Szymczak-Pajor, I.; Drzewoski, J.; Sliwinska, A. The Molecular Mechanisms by Which Vitamin D Prevents Insulin Resistance and Associated Disorders. Int. J. Mol. Sci. 2020, 21, 6644. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Grasso, S.; Menon, A.; Brunton, N.P.; Lyng, J.G.; Jacquier, J.-C.; Bhuyan, D.J. Recovery of ergosterol and vitamin D2 from mushroom waste—Potential valorization by food and pharmaceutical industries. Trends Food Sci. Technol. 2020, 99, 351–366. [Google Scholar] [CrossRef]
- Baur, A.C.; Kuhn, J.; Brandsch, C.; Hirche, F.; Stangl, G.I. Intake of ergosterol increases the vitamin D concentrations in serum and liver of mice. J. Steroid Biochem. Mol. Biol. 2019, 194, 105435. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. A perspective on the beneficial effects of moderate exposure to sunlight: Bone health, cancer prevention, mental health and well being. In Comprehensive Series in Photosciences; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 11–37. [Google Scholar]
- Carlberg, C.; Munoz, A. An update on vitamin D signaling and cancer. Semin. Cancer Biol. 2022, 79, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Polly, P. Gene regulation by vitamin D3. Crit. Rev. Eukaryot. Gene Expr. 1998, 8, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.C.; Nemere, I. The ERp57/GRp58/1,25D3-MARRS receptor: Multiple functional roles in diverse cell systems. Curr. Med. Chem. 2007, 14, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Stavroulopoulos, A.; Cassidy, M.J.; Porter, C.J.; Hosking, D.J.; Roe, S.D. Vitamin D status in renal transplant recipients. Am. J. Transplant. 2007, 7, 2546–2552. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J. Clin. Endocrinol. Metab. 2001, 86, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and the intracrinology of innate immunity. Mol. Cell. Endocrinol. 2010, 321, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ambroselli, D.; Masciulli, F.; Romano, E.; Catanzaro, G.; Besharat, Z.M.; Massari, M.C.; Ferretti, E.; Migliaccio, S.; Izzo, L.; Ritieni, A.; et al. New Advances in Metabolic Syndrome, from Prevention to Treatment: The Role of Diet and Food. Nutrients 2023, 15, 640. [Google Scholar] [CrossRef]
- Melguizo-Rodriguez, L.; Costela-Ruiz, V.J.; Garcia-Recio, E.; De Luna-Bertos, E.; Ruiz, C.; Illescas-Montes, R. Role of Vitamin D in the Metabolic Syndrome. Nutrients 2021, 13, 830. [Google Scholar] [CrossRef]
- Theik, N.W.Y.; Raji, O.E.; Shenwai, P.; Shah, R.; Kalluri, S.R.; Bhutta, T.H.; Hannoodee, H.; Al Khalili, M.; Khan, S. Relationship and Effects of Vitamin D on Metabolic Syndrome: A Systematic Review. Cureus 2021, 13, e17419. [Google Scholar] [CrossRef]
- Zittermann, A.; Gummert, J.F. Nonclassical vitamin D action. Nutrients 2010, 2, 408–425. [Google Scholar] [CrossRef]
- Al-Timimi, D.J.; Ali, A.F. Serum 25(OH) D in Diabetes Mellitus Type 2: Relation to Glycaemic Control. J. Clin. Diagn. Res. 2013, 7, 2686–2688. [Google Scholar] [CrossRef] [PubMed]
- Chertow, B.S.; Sivitz, W.I.; Baranetsky, N.G.; Clark, S.A.; Waite, A.; Deluca, H.F. Cellular mechanisms of insulin release: The effects of vitamin D deficiency and repletion on rat insulin secretion. Endocrinology 1983, 113, 1511–1518. [Google Scholar] [CrossRef]
- Nikooyeh, B.; Hollis, B.W.; Neyestani, T.R. The effect of daily intake of vitamin D-fortified yogurt drink, with and without added calcium, on serum adiponectin and sirtuins 1 and 6 in adult subjects with type 2 diabetes. Nutr. Diabetes 2021, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ka, S.O.; Cha, H.N.; Chae, Y.N.; Kim, M.K.; Park, S.Y.; Bae, E.J.; Park, B.H. Myeloid Sirtuin 6 Deficiency Causes Insulin Resistance in High-Fat Diet-Fed Mice by Eliciting Macrophage Polarization Toward an M1 Phenotype. Diabetes 2017, 66, 2659–2668. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.J. Sirtuin 6, a possible therapeutic target for type 2 diabetes. Arch. Pharm. Res. 2017, 40, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Achari, A.E.; Jain, S.K. 1,25(OH)(2)-vitamin D(3) upregulates glucose uptake mediated by SIRT1/IRS1/GLUT4 signaling cascade in C2C12 myotubes. Mol. Cell. Biochem. 2018, 444, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Mitri, J.; Pittas, A.G. Vitamin D and diabetes. Endocrinol. Metab. Clin. N. Am. 2014, 43, 205–232. [Google Scholar] [CrossRef]
- Maestro, B.; Campion, J.; Davila, N.; Calle, C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr. J. 2000, 47, 383–391. [Google Scholar] [CrossRef]
- Shi, H.; Norman, A.W.; Okamura, W.H.; Sen, A.; Zemel, M.B. 1α, 25-Dihydroxyvitamin D3 modulates human adipocyte metabolism via nongenomic action. FASEB J. 2001, 15, 1–15. [Google Scholar] [CrossRef]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.; Wolf, D.; Rumpold, H.; Enrich, B.; Tilg, H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem. Biophys. Res. Commun. 2004, 323, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.C.; Liao, M.T.; Lu, K.C.; Wu, C.C. Role of vitamin D in insulin resistance. J. Biomed. Biotechnol. 2012, 2012, 634195. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Kim, Y. Vitamin D Insufficiency Exacerbates Adipose Tissue Macrophage Infiltration and Decreases AMPK/SIRT1 Activity in Obese Rats. Nutrients 2017, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H. Magnesium and Vitamin D Deficiency as a Potential Cause of Immune Dysfunction, Cytokine Storm and Disseminated Intravascular Coagulation in covid-19 patients. Mo. Med. 2021, 118, 68–73. [Google Scholar] [PubMed]
- Berridge, M.J. Vitamin D deficiency and diabetes. Biochem. J. 2017, 474, 1321–1332. [Google Scholar] [CrossRef]
- Pereira, F.; Barbachano, A.; Singh, P.K.; Campbell, M.J.; Munoz, A.; Larriba, M.J. Vitamin D has wide regulatory effects on histone demethylase genes. Cell Cycle 2012, 11, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Ricca, C.; Aillon, A.; Bergandi, L.; Alotto, D.; Castagnoli, C.; Silvagno, F. Vitamin D Receptor Is Necessary for Mitochondrial Function and Cell Health. Int. J. Mol. Sci. 2018, 19, 1672. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, M.; Viano, M.; Casarin, S.; Castagnoli, C.; Pescarmona, G.; Silvagno, F. Mitochondrial and lipogenic effects of vitamin D on differentiating and proliferating human keratinocytes. Exp. Dermatol. 2015, 24, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Achari, A.E.; Jain, S.K. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch. Biochem. Biophys. 2017, 615, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Holick, M. Sunlight and vitamin D: The bone and cancer connections. Radiat. Prot. Dosim. 2000, 91, 65–71. [Google Scholar] [CrossRef]
- Holick, M.F. McCollum Award Lecture, 1994: Vitamin D—New horizons for the 21st century. Am. J. Clin. Nutr. 1994, 60, 619–630. [Google Scholar] [CrossRef]
- Drueke, T.B.; Massy, Z.A. Role of vitamin D in vascular calcification: Bad guy or good guy? Nephrol. Dial. Transplant. 2012, 27, 1704–1707. [Google Scholar] [CrossRef]
- Calvo, M.S.; Babu, U.S.; Garthoff, L.H.; Woods, T.O.; Dreher, M.; Hill, G.; Nagaraja, S. Vitamin D2 from light-exposed edible mushrooms is safe, bioavailable and effectively supports bone growth in rats. Osteoporos. Int. 2013, 24, 197–207. [Google Scholar] [CrossRef]
- Weber, K.; Goldberg, M.; Stangassinger, M.; Erben, R.G. 1alpha-hydroxyvitamin D2 is less toxic but not bone selective relative to 1alpha-hydroxyvitamin D3 in ovariectomized rats. J. Bone Miner. Res. 2001, 16, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. The dualistic role of vitamin D in vascular calcifications. Kidney Int. 2011, 79, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Ellam, T.; Hameed, A.; ul Haque, R.; Muthana, M.; Wilkie, M.; Francis, S.E.; Chico, T.J. Vitamin D deficiency and exogenous vitamin D excess similarly increase diffuse atherosclerotic calcification in apolipoprotein E knockout mice. PLoS ONE 2014, 9, e88767. [Google Scholar] [CrossRef] [PubMed]
- Niksic, M.; Podgornik, B.B.; Berovic, M. Farming of Medicinal Mushrooms. Adv. Biochem. Eng. Biotechnol. 2023, 184, 29–76. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dai, J.; Wang, J.; Lu, C.; Luo, Z.; Zheng, X.; Lu, Z.; Yang, Z. Comparative Transcriptomic Analyses Propose the Molecular Regulatory Mechanisms Underlying 1,8-Cineole from Cinnamomum kanehirae Hay and Promote the Asexual Sporulation of Antrodia cinnamomea in Submerged Fermentation. Molecules 2023, 28, 7511. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Yuan, X.; Wang, J.; Yang, Y.; Zheng, Y. Transcriptome profiling of Antrodia cinnamomea fruiting bodies grown on Cinnamomum kanehirae and C. camphora wood substrates. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Liang, Z.; Zheng, K.; Zhao, Q.; Shao, W.; Li, C.; Wang, J.; Ma, C.; Kang, W. Structural identification and coagulation effect of Flammulina velutipes polysaccharides. Appl. Sci. 2021, 11, 1736. [Google Scholar] [CrossRef]
- Singh, C.; Pathak, P.; Chaudhary, N.; Rathi, A.; Dehariya, P.; Vyas, D. Mushrooms and mushroom composts in integrated farm management. Res. J. Agric. Sci. 2020, 11, 1436–1443. [Google Scholar]
- Szabó, L.G.; Babulka, P.; Fődi, A. A Pecsétviaszgomba (Ganoderma lucidum). Available online: https://www.dxntermekek.info.hu/a-pecsetviaszgomba-ganoderma-lucidum/ (accessed on 10 November 2024).
- Yang, Y.; Zhang, H.; Zuo, J.; Gong, X.; Yi, F.; Zhu, W.; Li, L. Advances in research on the active constituents and physiological effects of Ganoderma lucidum. Biomed. Dermatol. 2019, 3, 1–17. [Google Scholar] [CrossRef]
- Gregori, A.; Švagelj, M.; Voglar, D.; Berovic, M. Growth characteristics and ergosterol content of Grifola frondosa in various solid-state substrates. Chem. Biochem. Eng. Q. 2016, 30, 183–188. [Google Scholar] [CrossRef]
- Song, B.; Ye, J.; Sossah, F.L.; Li, C.; Li, D.; Meng, L.; Xu, S.; Fu, Y.; Li, Y. Assessing the effects of different agro-residue as substrates on growth cycle and yield of Grifola frondosa and statistical optimization of substrate components using simplex-lattice design. AMB Express 2018, 8, 46. [Google Scholar] [CrossRef]
- Sokol, S.; Golak-Siwulska, I.; Sobieralski, K.; Siwulski, M.; Górka, K. Biology, cultivation, and medicinal functions of the mushroom Hericium erinaceum. Acta Mycol. 2015, 50. [Google Scholar] [CrossRef]
- Petre, A.; Ene, M.; Vamanu, E. Submerged cultivation of Inonotus obliquus mycelium using statistical design of experiments and mathematical modeling to increase biomass yield. Appl. Sci. 2021, 11, 4104. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, Y.; Xue, Z.; Li, N.; Liu, J.; Chen, H. Recent Developments in Inonotus obliquus (Chaga mushroom) Polysaccharides: Isolation, Structural Characteristics, Biological Activities and Application. Polymers 2021, 13, 1441. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Wang, Z.; Chiu, W.; Lin, F.; Moore, D. An integrated study of individualism in Lentinula edodes in nature and its implication for cultivation strategy. Mycol. Res. 1999, 103, 651–660. [Google Scholar] [CrossRef]
- Wang, W.; Wang, K.; Wang, X.; Yang, R.; Li, Y.; Yao, Y. Investigation on natural resources and species conservation of Ophiocordyceps sinensis, the famous medicinal fungus endemic to the Tibetan Plateau. Protein Cell 2018, 9, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.H.; Yang, D.R.; Jiang, J.J.; Zhang, Q.J.; Liu, Y.; Liu, Y.L.; Zhang, Y.; Zhang, H.B.; Shi, C.; Tong, Y.; et al. The caterpillar fungus, Ophiocordyceps sinensis, genome provides insights into highland adaptation of fungal pathogenicity. Sci. Rep. 2017, 7, 1806. [Google Scholar] [CrossRef] [PubMed]
- Chellapandi, P.; Saranya, S. Ophiocordyceps sinensis: A potential caterpillar fungus for the production of bioactive compounds. Explor. Res. Hypothesis Med. 2024, 9, 236–249. [Google Scholar] [CrossRef]
- Woo, S.-I.; Ryoo, R.; Jang, Y.; Park, Y.; Jeong, Y.S.; Ka, K.-H. Mycelial culture and fruiting analysis of Panellus edulis strains collected in Korea. Korean J. Mycol. 2018, 46, 281–294. [Google Scholar]
- Bonito Lab. Panellus Serotinus. Available online: https://msu-prod.dotcmscloud.com/news/panellus-serotinus (accessed on 10 November 2024).
- Dai, Y.; Sun, L.; Yin, X.; Gao, M.; Zhao, Y.; Jia, P.; Yuan, X.; Fu, Y.; Li, Y. Pleurotus eryngii genomes reveal evolution and adaptation to the Gobi desert environment. Front. Microbiol. 2019, 10, 2024. [Google Scholar] [CrossRef]
- Li, J.; Han, L.-H.; Liu, X.-B.; Zhao, Z.-W.; Yang, Z.L. The saprotrophic Pleurotus ostreatus species complex: Late Eocene origin in East Asia, multiple dispersal, and complex speciation. IMA Fungus 2020, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geng, W.; Shen, Y.; Wang, Y.; Dai, Y.-C. Edible Mushroom Cultivation for Food Security and Rural Development in China: Bio-Innovation, Technological Dissemination and Marketing. Sustainability 2014, 6, 2961–2973. [Google Scholar] [CrossRef]
- Rathod, M.G. Mushroom Farming: Exploring Varieties, Cultivation Strategies, and Endless Possibilities. Res. Rev. Biotechnol. Biosci. 2023, 10, 1–11. [Google Scholar]
- Llanaj, X.; Toros, G.; Hajdu, P.; Abdalla, N.; El-Ramady, H.; Kiss, A.; Solberg, S.O.; Prokisch, J. Biotechnological Applications of Mushrooms under the Water-Energy-Food Nexus: Crucial Aspects and Prospects from Farm to Pharmacy. Foods 2023, 12, 2671. [Google Scholar] [CrossRef]
- Geösel, A.; Szabó, A. Modern Horticulture: Modern Mushroom Cultivation Technologies. Available online: http://kertesztananyag.hu/modern-mushroom-cultivation-technologies (accessed on 9 September 2024).
- Gupta, S.; Summuna, B.; Gupta, M.; Annepu, S.K. Edible mushrooms: Cultivation, bioactive molecules, and health benefits. Bioact. Mol. Food 2018, 1, 1–33. [Google Scholar]
- Singh, R.; Kim, J.; Shepherd Marion, W.; Luo, F.; Jiang, X. Determining Thermal Inactivation of Escherichia coli O157:H7 in Fresh Compost by Simulating Early Phases of the Composting Process. Appl. Environ. Microbiol. 2011, 77, 4126–4135. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.; Zied, D.C.; Pardo, J.E.; Preston, G.M.; Pardo-Giménez, A. Supplementation in mushroom crops and its impact on yield and quality. AMB Express 2018, 8, 146. [Google Scholar] [CrossRef]
- Berovic, M. Cultivation of Medicinal Mushroom Biomass by Solid-State Bioprocessing in Bioreactors. Adv. Biochem. Eng. Biotechnol. 2019, 169, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Sangeeta; Sharma, D.; Ramniwas, S.; Mugabi, R.; Uddin, J.; Nayik, G.A. Revolutionizing Mushroom processing: Innovative techniques and technologies. Food Chem. X 2024, 23, 101774. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).