Cell Wall Microdomains Analysis in the Quadrifids of Utricularia dichotoma
Abstract
:1. Introduction
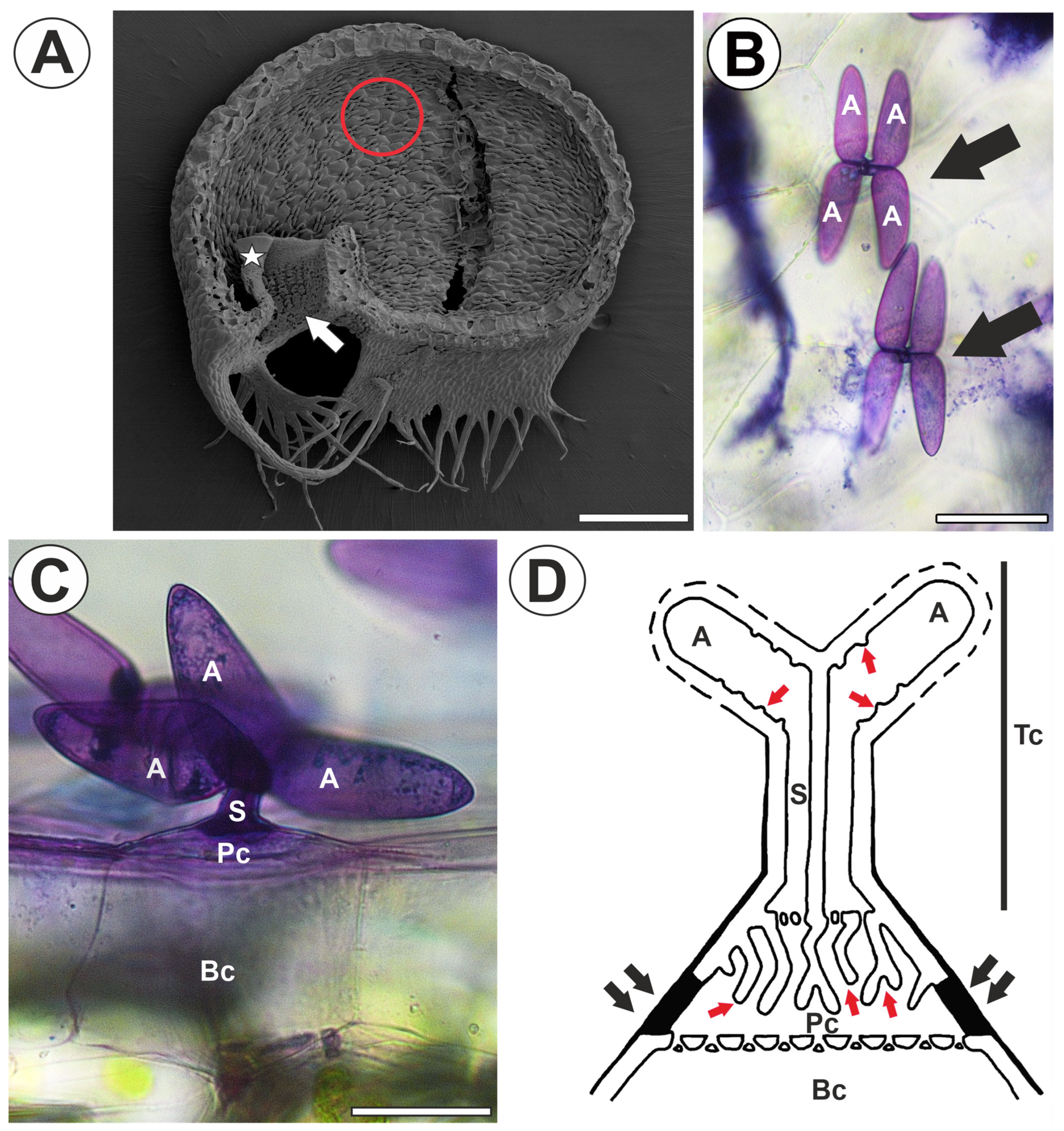
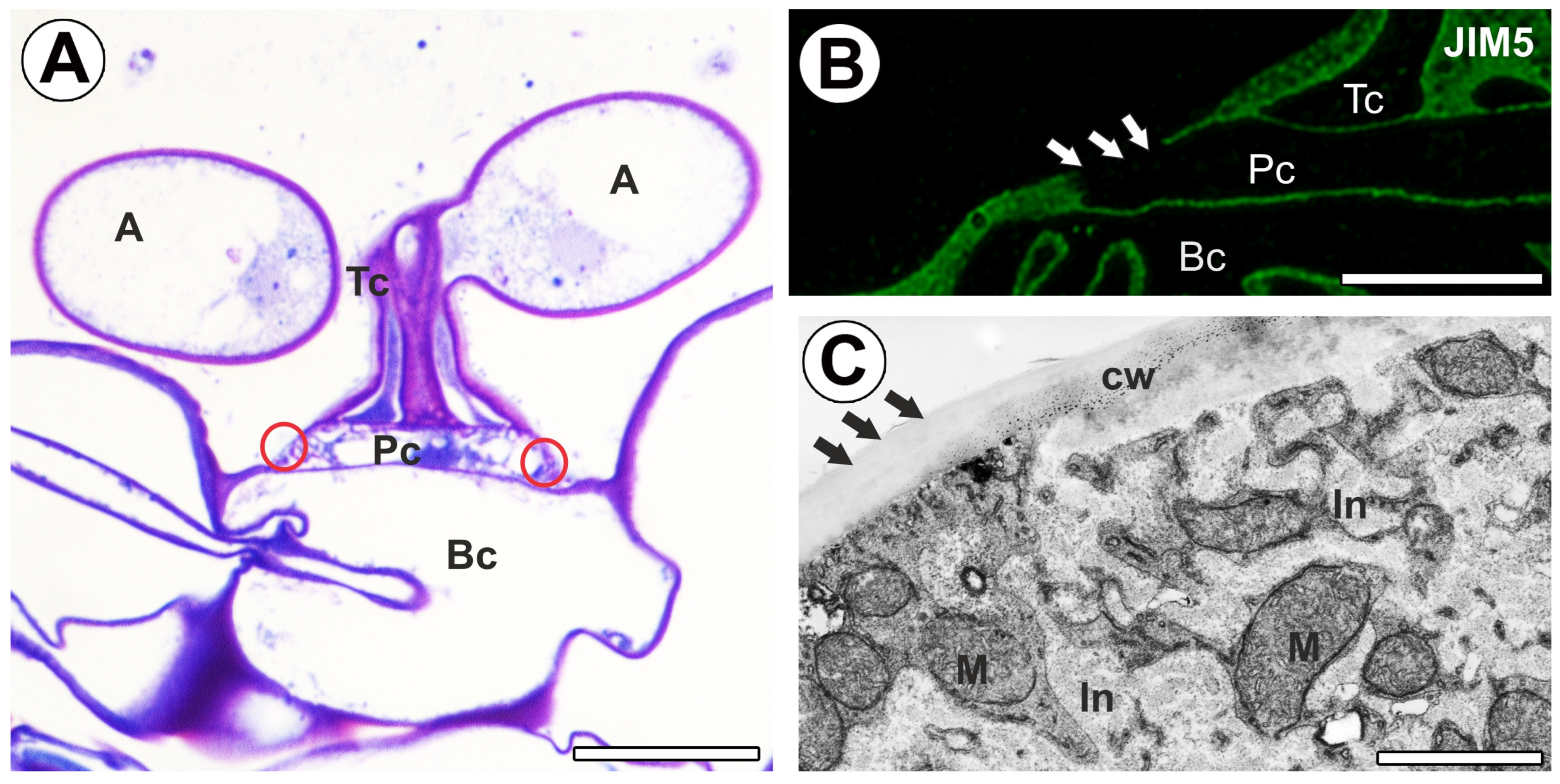
2. Results
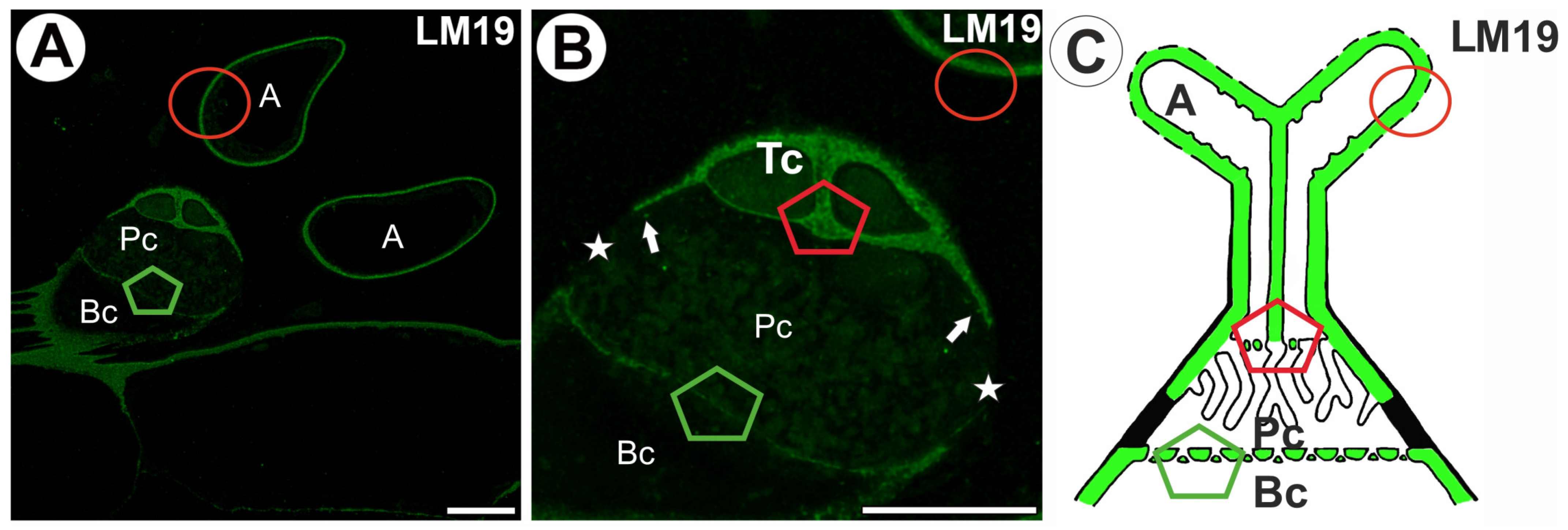
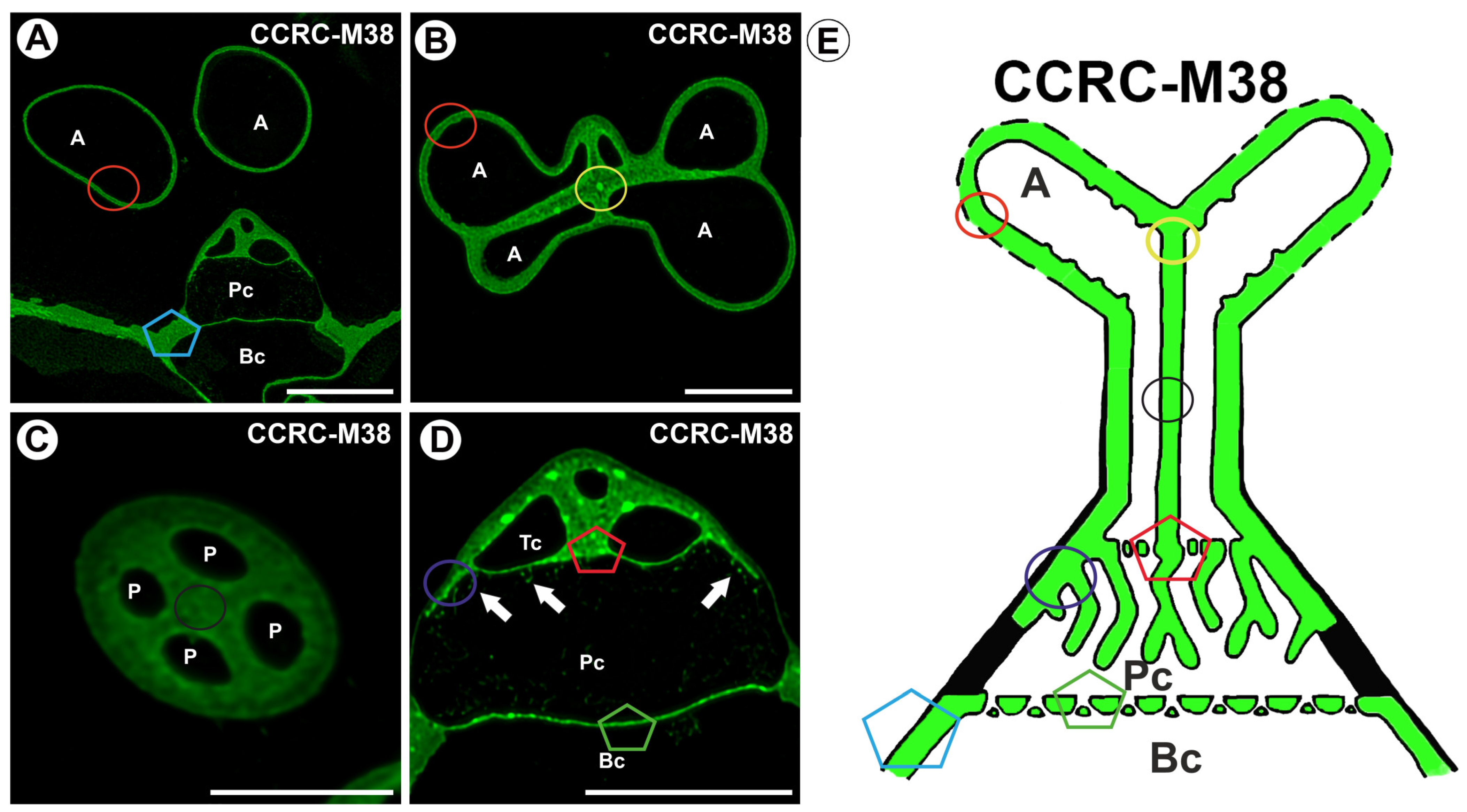
2.1. Homogalacturonan Distribution
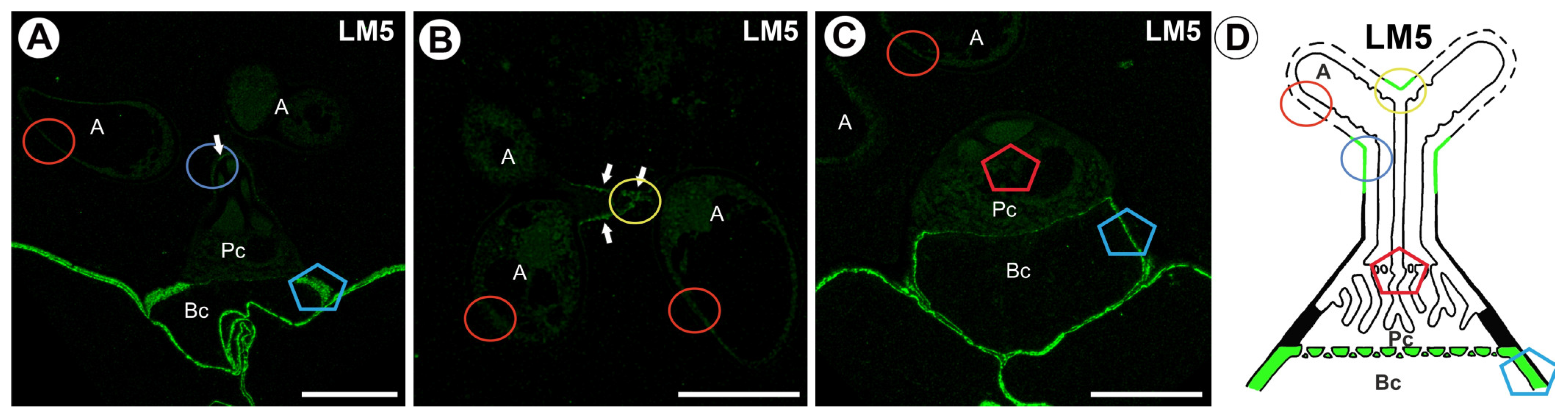
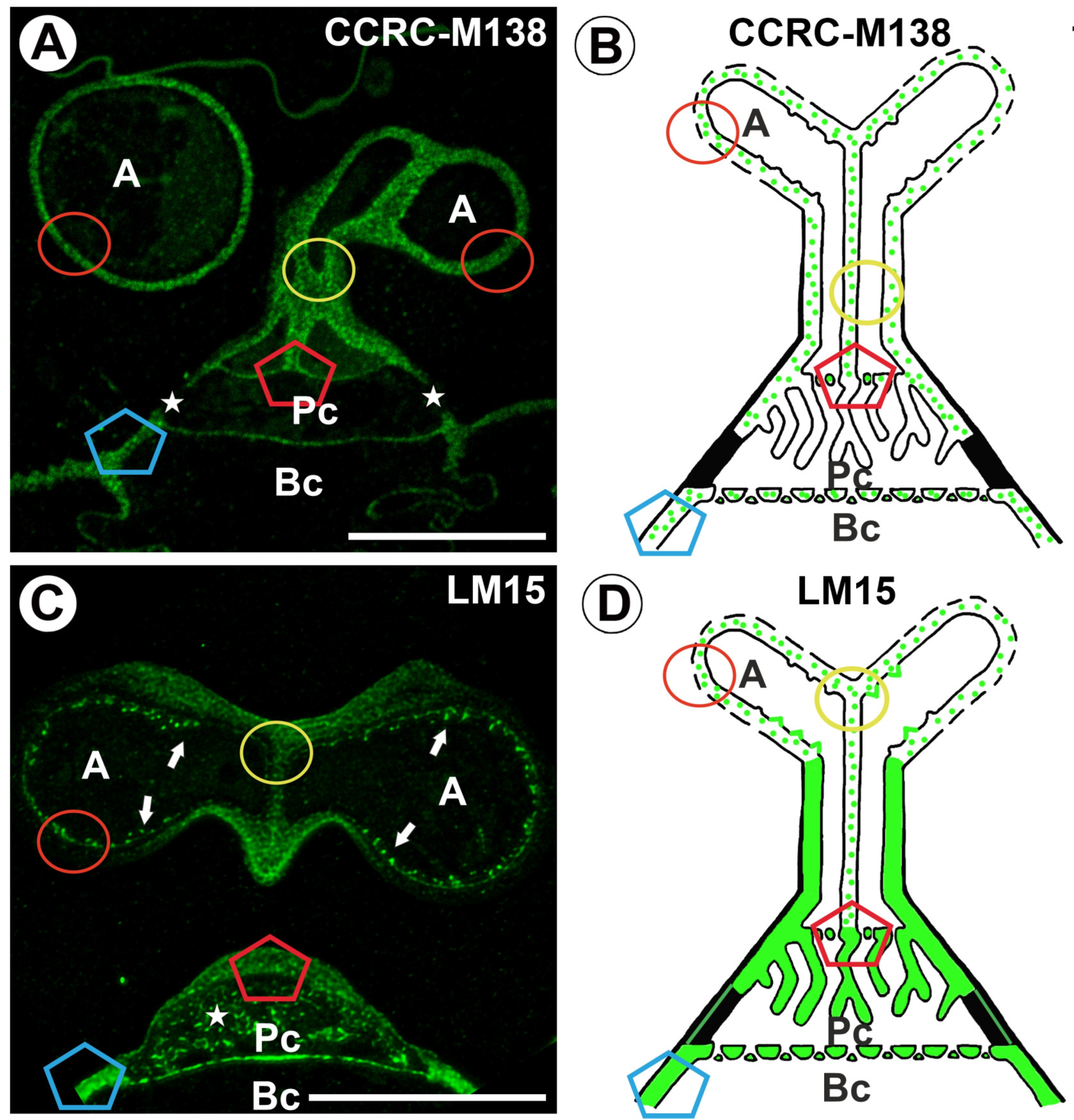
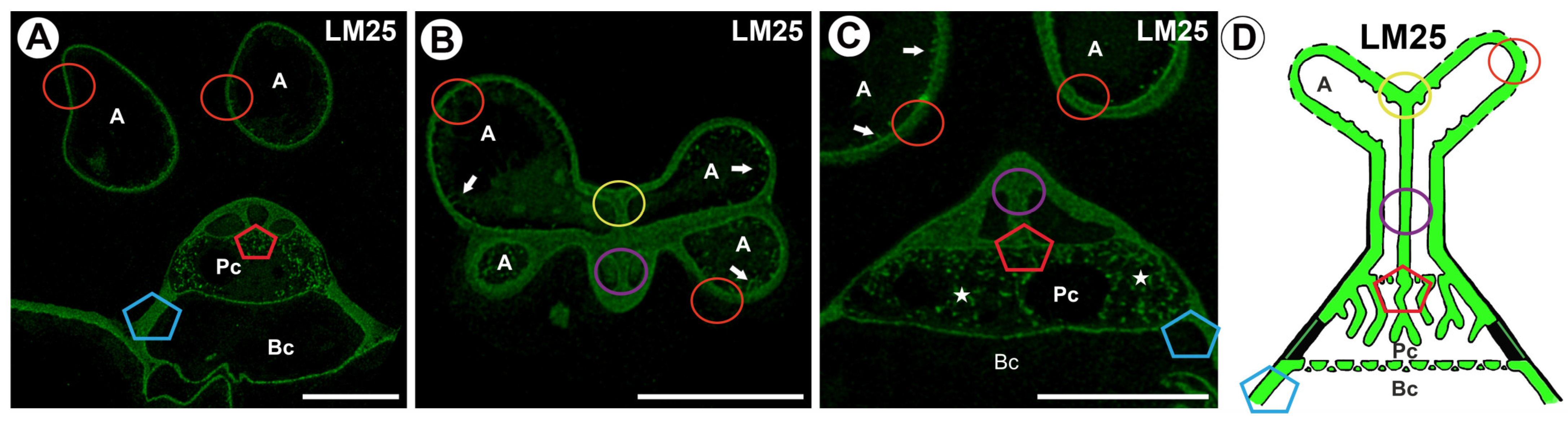
2.2. Hemicellulose Distribution
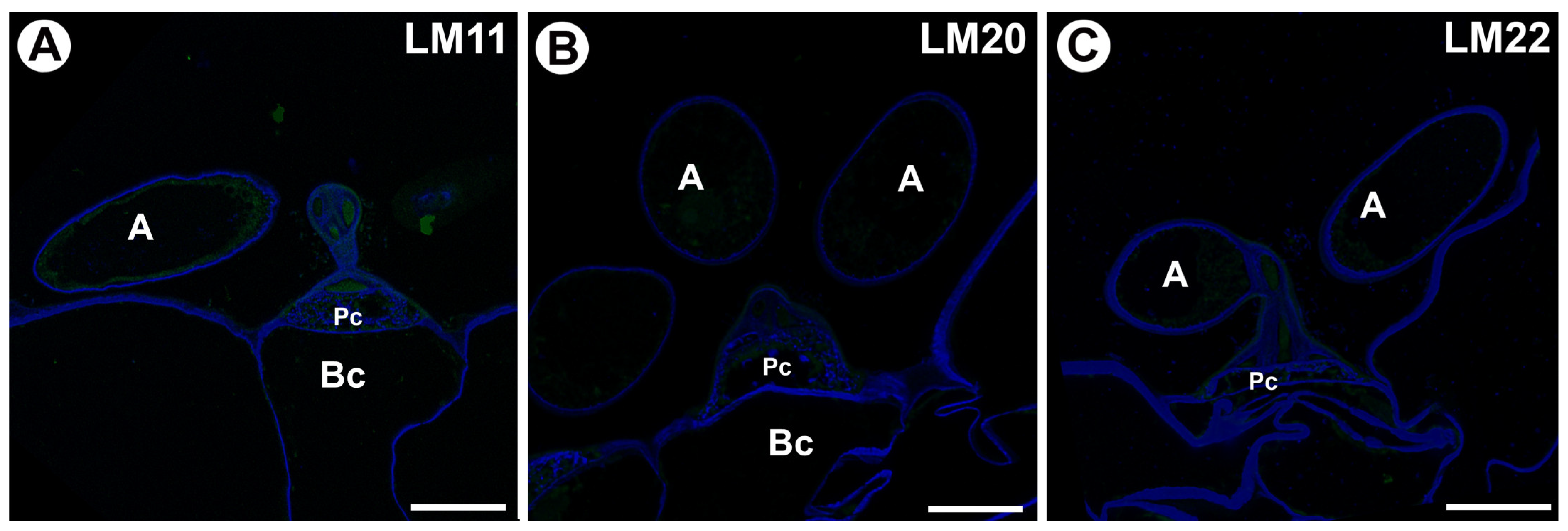
2.3. Mannan Distribution
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Histological and Immunochemical Analysis
4.3. Scanning Transmission Electron Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juniper, B.E.; Robbins, R.J.; Joel, D.M. The Carnivorous Plants; Academic Press: London, UK, 1989. [Google Scholar]
- Król, E.; Płachno, B.J.; Adamec, L.; Stolarz, M.; Dziubińska, H.; Trębacz, K. Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Ann. Bot. 2012, 109, 47–64. [Google Scholar] [CrossRef]
- Ellison, A.M.; Adamec, L. Carnivorous Plants: Physiology, Ecology, and Evolution; Oxford University Press: Oxford, UK, 2018; 510p. [Google Scholar]
- Darwin, C. Insectivorous Plants, 1st ed.; John Murray: London, UK, 1875. [Google Scholar]
- Lloyd, F.E. The range of structural and functional variety in the traps of Utricularia and Polypompholyx. Flora 1932, 126, 303–328. [Google Scholar] [CrossRef]
- Poppinga, S.; Masselter, T.; Speck, T. Faster than their prey: New insights into the rapid movements of active carnivorous plants traps. BioEssays 2013, 35, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Poppinga, S.C.; Weisskopf, A.S.; Westermeier, T.; Masselter, T.; Speck, T. Fastest predators in the plant kingdom: Functional morphology and biomechanics of suction traps found in the largest genus of carnivorous plants. AoB Plants 2016, 8, plv140. [Google Scholar] [CrossRef] [PubMed]
- Rutishauser, R.; Isler, B. Developmental genetics and morphological evolution of flowering plants, especially bladderworts (Utricularia): Fuzzy Arberian Morphology complements Classical Morphology. Ann. Bot. 2001, 88, 1173–1202. [Google Scholar] [CrossRef]
- Rutishauser, R. Evolution of unusual morphologies in Lentibulariaceae (bladderworts and allies) and Podostemaceae (river-weeds): A pictorial report at the interface of developmental biology and morphological diversification. Ann. Bot. 2016, 117, 811–832. [Google Scholar] [CrossRef]
- Reut, M.S.; Płachno, B.J. Unusual developmental morphology and anatomy of vegetative organs in Utricularia dichotoma—Leaf, shoot and root dynamics. Protoplasma 2020, 257, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Reut, M.S.; Płachno, B.J. Development, Diversity and Dynamics of Plant Architecture in Utricularia subgenus Polypompholyx—Towards Understanding Evolutionary Processes in the Lentibulariaceae. Bot. Rev. 2023, 89, 201–236. [Google Scholar] [CrossRef]
- Miranda, V.F.O.; Silva, S.R.; Reut, M.S.; Dolsan, H.; Stolarczyk, P.; Rutishauser, R.; Płachno, B.J. A Historical Perspective of Bladderworts (Utricularia): Traps, Carnivory and Body Architecture. Plants 2021, 10, 2656. [Google Scholar] [CrossRef]
- Rutishauser, R. EvoDevo: Past and Future of Continuum and Process Plant Morphology. Philosophies 2020, 5, 41. [Google Scholar] [CrossRef]
- Płachno, B.J.; Świątek, P. Unusual embryo structure in viviparous Utricularia nelumbifolia, with remarks on embryo evolution in genus Utricularia. Protoplasma 2010, 239, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Carretero-Paulet, L.; Librado, P.; Chang, T.H.; Ibarra-Laclette, E.; Herrera-Estrella, L.; Rozas, J.; Albert, V.A. High gene family turnover rates and gene space adaptation in the compact genome of the carnivorous plant Utricularia gibba. Mol. Biol. Evol. 2015, 32, 1284–1295. [Google Scholar] [CrossRef]
- Lan, T.; Renner, T.; Ibarra-Laclette, E.; Farr, K.M.; Chang, T.H.; Cervantes-Pérez, S.A.; Zheng, C.; Sankoff, D.; Tang, H.; Purbojati, R.W.; et al. Long-read sequencing uncovers the adaptive topography of a carnivorous plant genome. Proc. Natl. Acad. Sci. USA 2017, 114, E4435–E4441. [Google Scholar] [CrossRef]
- Renner, T.; Lan, T.; Farr, K.M.; Ibarra-Laclette, E.; Herrera-Estrella, L.; Schuster, S.C.; Hasebe, M.; Fukushima, K.; Albert, V.A. Carnivorous plant genomes. In Carnivorous Plants: Physiology, Ecology, and Evolution; Oxford University Press: New York, NY, USA, 2018; pp. 135–153. [Google Scholar]
- Hepler, N.K.; Bowman, A.; Carey, R.E.; Cosgrove, D.J. Expansin gene loss is a common occurrence during adaptation to an aquatic environment. Plant J. 2020, 101, 666–680. [Google Scholar] [CrossRef]
- Laspisa, D.; Bang, S.; Schmitz, R.J.; Parrott, W.; Wallace, J. Mining the Utricularia gibba genome for insulator-like elements for genetic engineering. Front. Plant Sci. 2023, 14, 1279231. [Google Scholar] [CrossRef]
- Ibarra-Laclette, E.; Albert, V.A.; Perez-Torres, C.A.; Zamundio-Hernandez, F.; Ortega-Estrada, M.J.; Herrera-Estrella, A.; Herrera-Estrella, L. Transcriptomics and molecular evolutionary rate analysis of the bladderwort (Utricularia), a carnivorous plant with a minimal genome. BMC Plant Biol. 2011, 11, 101. [Google Scholar] [CrossRef]
- Ibarra-Laclette, E.; Lyons, E.; Hernández-Guzmán, G.; Pérez-Torres, C.; Carretero-Paulet, L.; Chang, T.; Lan, T.; Welch, A.J.; Juárez, M.J.; Simpson, J.; et al. Architecture and evolution of a minute plant genome. Nature 2013, 498, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Veleba, A.; Bureš, P.; Adamec, L.; Šmarda, P.; Lipnerová, I.; Horová, L. Genome size and genomic GC content evolution in the miniature genome-sized family Lentibulariaceae. New Phytol. 2014, 203, 22–28. [Google Scholar] [CrossRef]
- Carretero-Paulet, L.; Chang, T.H.; Librado, P.; Ibarra-Laclette, E.; Herrera-Estrella, L.; Rozas, J.; Albert, V.A. Genome-wide analysis of adaptive molecular evolution in the carnivorous plant Utricularia gibba. Genome Biol. Evol. 2015, 7, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Fang, X.; Alvarez-Ponce, D.; Cai, H.; Carretero-Paulet, L.; Chen, C.; Chang, T.H.; Farr, K.M.; Fujita, T.; Hiwatashi, Y.; et al. Genome of the pitcher plant Cephalotus reveals genetic changes associated with carnivory. Nat. Ecol. Evol. 2017, 1, 0059. [Google Scholar] [CrossRef]
- Palfalvi, G.; Hackl, T.; Terhoeven, N.; Shibata, T.F.; Nishiyama, T.; Ankenbrand, M.; Becker, D.; Förster, F.; Freund, M.; Iosip, A.; et al. Genomes of the Venus Flytrap and Close Relatives Unveil the Roots of Plant Carnivory. Curr. Biol. 2020, 30, 2312–2320.e5. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Preick, M.; Abelt, S.; Scheffel, A.; Hofreiter, M. Annotated genome sequences of the carnivorous plant Roridula gorgonias and a non-carnivorous relative, Clethra arborea. BMC Res. Notes 2020, 13, 426. [Google Scholar] [CrossRef]
- Matos, R.G.; Silva, S.R.; Płachno, B.J.; Adamec, L.; Michael, T.P.; Varani, A.M.; Miranda, V.F.O. The complete mitochondrial genome of carnivorous Genlisea tuberosa (Lentibulariaceae): Structure and evolutionary aspects. Gene 2022, 824, 146391. [Google Scholar] [CrossRef]
- Saul, F.; Scharmann, M.; Wakatake, T.; Rajaraman, S.; Marques, A.; Freund, M.; Bringmann, G.; Channon, L.; Becker, D.; Carroll, E.; et al. Subgenome dominance shapes novel gene evolution in the decaploid pitcher plant Nepenthes gracilis. Nat. Plants 2023, 9, 2000–2015. [Google Scholar] [CrossRef]
- Fu, C.N.; Wicke, S.; Zhu, A.D.; Li, D.; Gao, L. Distinctive plastome evolution in carnivorous angiosperms. BMC Plant Biol. 2023, 23, 660. [Google Scholar] [CrossRef] [PubMed]
- Baharin, A.; Ting, T.-Y.; Goh, H.-H. Omics Approaches in Uncovering Molecular Evolution and Physiology of Botanical Carnivory. Plants 2023, 12, 408. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Pareek, A.; Dkhar, J. Genetic Basis of Carnivorous Leaf Development. Front. Plant Sci. 2022, 12, 825289. [Google Scholar] [CrossRef] [PubMed]
- Whitewoods, C.; Gonçalves, B.; Cheng, J.; Cui, M.; Kennaway, R.; Lee, K.; Bushell, C.; Yu, M.; Piao, C.; Coen, E. Evolution of carnivorous traps from planar leaves through simple shifts in gene expression. Science 2019, 367, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Zedek, F.; Šmerda, J.; Halasová, A.; Adamec, L.; Veleba, A.; Plačková, K.; Bureš, P. The smallest angiosperm genomes may be the price for effective traps of bladderworts. Ann. Bot. 2024, 134, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, F.E. The Carnivorous Plants; Ronald Press: New York, NY, USA, 1942. [Google Scholar]
- Fineran, B.A.; Lee, M.S. Organization of quadrifid and bifid hairs in the trap of Utricularia monanthos. Protoplasma 1975, 84, 43–70. [Google Scholar] [CrossRef]
- Taylor, P. The Genus Utricularia: A Taxonomic Monograph; Kew: Kew Bulletin, Additional Series; Her Majesty’s Stationery Office: London, UK, 1989; Volume 4, pp. 1–724.
- Płachno, B.J.; Jankun, A. Transfer cell wall architecture in secretory hairs of Utricularia intermedia traps. Acta Biol. Cracov. Ser. Bot. 2004, 46, 193–200. [Google Scholar]
- Yang, Y.-P.; Liu, H.-Y.; Chao, Y.-S. Trap gland morphology and its systematic implications in Taiwan Utricularia (Lentibulariaceae). Flora-Morphol. Distrib. Funct. Ecol. Plants 2009, 204, 692–699. [Google Scholar] [CrossRef]
- Płachno, B.J.; Świątek, P.; Adamec, L.; Carvalho, S.; Miranda, V.F.O. The Trap Architecture of Utricularia multifida and Utricularia westonii (subg. Polypompholyx). Front. Plant Sci. 2019, 10, 336. [Google Scholar] [CrossRef]
- Fineran, B.A. Glandular trichomes in Utricularia: A review of their structure and function. Isr. J. Bot. 1985, 34, 295–330. [Google Scholar]
- Płachno, B.J.; Kapusta, M. The Localization of Cell Wall Components in the Quadrifids of Whole-Mount Immunolabeled Utricularia dichotoma Traps. Int. J. Mol. Sci. 2024, 25, 56. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Feldo, M.; Świątek, P. Do Arabinogalactan Proteins Occur in the Transfer Cells of Utricularia dichotoma? Int. J. Mol. Sci. 2024, 25, 6623. [Google Scholar] [CrossRef] [PubMed]
- Pate, J.S.; Gunning, B.E.S. Transfer cells. Annual Review. Plant Physiol. 1972, 23, 173–196. [Google Scholar] [CrossRef]
- Fineran, B.A.; Lee, M.S.L. Transfer cells in traps of the carnivorous plant Utricularia monanthos. J. Ultrast. Res. 1974, 48, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Offler, C.E.; Patrick, J.W. Transfer cells: What regulates the development of their intricate wall labyrinths? New Phytol. 2020, 228, 427–444. [Google Scholar] [CrossRef]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Wolf, S.; Mouille, G.; Pelloux, J. Homogalacturonan Methyl-esterification and plant development. Mol. Plant 2009, 5, 851–860. [Google Scholar] [CrossRef]
- Hasegawa, K.; Kamada, S.; Takehara, S.; Takeuchi, H.; Nakamura, A.; Satoh, S.; Iwai, H. Rice Putative Methyltransferase Gene OsPMT16 Is Required for Pistil Development Involving Pectin Modification. Front. Plant Sci. 2020, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Anderson, C.T.; Xiao, C.W. Dynamics of pectic homogalacturonan in cellular morphogenesis and adhesion, wall integrity sensing and plant development. Nat. Plants 2022, 8, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, V.; Cervone, F.; Bellincampi, D. Methyl esterification of pectin plays a role during plant-pathogen interactions and affects plant resistance to diseases. J. Plant Physiol. 2012, 169, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Weraduwage, S.M.; Kim, S.J.; Renna, L.; Anozie, F.; Sharkey, T.; Brandizzi, F. Pectin Methylesterification Impacts the Relationship between Photosynthesis and Plant Growth. Plant Physiol. 2016, 171, 833–848. [Google Scholar]
- Hasegawa, K.; Ichikawa, A.; Takeuchi, H.; Nakamura, A.; Iwai, H. Maintenance of Methyl-Esterified Pectin Level in Pollen Mother-Cell Stages Is Required for Microspore Development. Plants 2023, 12, 1717. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, X.; Peng, G.; Liu, M.; Zhang, S.; Chen, M.; Liao, S.; Wei, X.; Xu, P.; Tan, X.; et al. Methylesterification of cell-wall pectin controls the diurnal flower-opening times in rice. Mol. Plant 2022, 15, 956–972. [Google Scholar] [CrossRef]
- Dash, L.; Swaminathan, S.; Šimura, J.; Gonzales, C.L.P.; Montes, C.; Solanki, N.; Mejia, L.; Ljung, K.; Zabotina, O.A.; Kelley, D.R. Changes in cell wall composition due to a pectin biosynthesis enzyme GAUT10 impact root growth. Plant Physiol. 2023, 193, 2480–2497. [Google Scholar] [PubMed]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Wójciak, M.; Świątek, P. Immunocytochemical Analysis of Bifid Trichomes in Aldrovanda vesiculosa L. Traps. Int. J. Mol. Sci. 2023, 24, 3358. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P. Stellate Trichomes in Dionaea muscipula Ellis (Venus Flytrap) Traps, Structure and Functions. Int. J. Mol. Sci. 2022, 24, 553. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P.; Strzemski, M.; Miranda, V.F.O. Immunocytochemical Analysis of the Wall Ingrowths in the Digestive Gland Transfer Cells in Aldrovanda vesiculosa L. (Droseraceae). Cells 2022, 11, 2218. [Google Scholar] [CrossRef] [PubMed]
- Liners, F.; Letesson, J.J.; Didembourg, C.; Van Cutsem, P. Monoclonal Antibodies against Pectin: Recognition of a Conformation Induced by Calcium. Plant Physiol. 1989, 91, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P.; Lichtscheidl, I. Differences in the Occurrence of Cell Wall Components between Distinct Cell Types in Glands of Drosophyllum lusitanicum. Int. J. Mol. Sci. 2023, 24, 15045. [Google Scholar] [CrossRef]
- Vaughn, K.C.; Talbot, M.J.; Offler, C.E.; McCurdy, D.W. Wall ingrowths in epidermal transfer cells of Vicia faba cotyledons are modified primary walls marked by localized accumulations of arabinogalactan proteins. Plant Cell Physiol. 2007, 48, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.S.; Renzaglia, K.S. The placenta of Physcomitrium patens: Transfer cell wall polymers compared across the three bryophyte groups. Diversity 2021, 13, 378. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.S.; Ligrone, R.; Vaughn, K.C.; Lopez, R.A.; Renzaglia, K.S. Cell wall polymers in the Phaeoceros placenta reflect developmental and functional differences across generations. Bryophyt. Divers. Evol. 2021, 43, 265–283. [Google Scholar] [CrossRef]
- Fineran, B.A.; Gilbertson, J.M. Application of lanthanum and uranyl salts as tracers to demonstrate apoplastic pathways for transport in glands of the carnivorous plant Utricularia monanthos. Eur. J. Cell Biol. 1980, 23, 66–72. [Google Scholar] [PubMed]
- Pattathil, S.; Avci, U.; Miller, J.S.; Hahn, M.G. Immunological approaches to plant cell wall and biomass characterization: Glycome profiling. In Biomass Conversion: Methods and Protocols; Himmel, M., Ed.; Springer Science + Business Media, LLC: New York, NY, USA, 2012; pp. 61–72. [Google Scholar]
- Marzec-Schmidt, K.; Ludwikow, A.; Wojciechowska, N.; Kasprowicz-Maluski, A.; Mucha, J.; Bagniewska-Zadworna, A. Xylem cell wall formation in pioneer roots and stems of Populus trichocarpa (Torr. & Gray). Front. Plant Sci. 2019, 10, 1419. [Google Scholar]
- Płachno, B.J.; Adamec, L.; Świątek, P.; Kapusta, M.; Miranda, V.F.O. Life in the Current: Anatomy and Morphology of Utricularia neottioides. Int. J. Mol. Sci. 2020, 21, 4474. [Google Scholar] [CrossRef] [PubMed]
- McCartney, L.; Ormerod, A.P.; Gidley, M.J.; Knox, J.P. Temporal and spatial regulation of pectic (1→4)-β-d-galactan in cell walls of developing pea cotyledons: Implications for mechanical properties. Plant J. 2000, 22, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ulvskov, P.; Wium, H.; Bruce, D.; Jørgensen, B.; Qvist, K.B.; Skjøt, M.; Hepworth, D.; Borkhardt, B.; Sørensen, S.O. Biophysical consequences of remodeling the neutral side chains of rhamnogalacturonan I in tubers of transgenic potatoes. Planta 2005, 220, 609–620. [Google Scholar] [CrossRef]
- Obro, J.; Borkhardt, B.; Harholt, J.; Skjot, M.; Willats, W.G.; Ulvskov, P. Simultaneous in vivo truncation of pectic side chains. Transgenic Res. 2009, 18, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Michalak, K.M.; Wojciechowska, N.; Marzec-Schmidt, K.; Bagniewska-Zadworna, A. Conserved autophagy and diverse cell wall composition: Unifying features of vascular tissues in evolutionarily distinct plants. Ann. Bot. 2024, 133, 559–572. [Google Scholar] [CrossRef]
- Lee, K.J.; Sakata, Y.; Mau, S.-L.; Pettolino, F.; Bacic, A.; Quatrano, R.S.; Knight, C.D.; Knox, J.P. Diversity in the distribution of polysaccharide and glyco-protein epitopes in the cell walls of bryophytes: New evidence for multiple evolution of water-conducting cells. New Phytol. 2002, 156, 491–508. [Google Scholar]
- Mansouri, K. Comparative Ultrastructure of Apical Cells and Derivatives in Bryophytes, with Special Reference to Plasmodesmata. Ph.D. Thesis, Southern Illinois University at Carbondale, Carbondale, IL, USA, 2012. [Google Scholar]
- Yan, J.; Liu, Y.; Yang, L.; He, H.; Huang, Y.; Fang, L.; Scheller, H.V.; Jiang, M.; Zhang, A. Cell wall β-1,4-galactan regulated by the BPC1/BPC2-GALS1 module aggravates salt sensitivity in Arabidopsis thaliana. Mol. Plant 2021, 14, 411–425. [Google Scholar] [CrossRef]
- Moneo-Sánchez, M.; Alonso-Chico, A.; Knox, J.P.; Dopico, B.; Labrador, E.; Martín, I. β-(1,4)-Galactan remodelling in Arabidopsis cell walls affects the xyloglucan structure during elongation. Planta 2019, 249, 351–362. [Google Scholar] [CrossRef]
- Torode, T.A.; O’Neill, R.; Marcus, S.E.; Cornuault, V.; Pose, S.; Lauder, R.P.; Kračun, S.K.; Rydahl, M.G.; Andersen, M.C.F.; Willats, W.G.T.; et al. Branched pectic galactan in phloem sieve element cell walls: Implications for cell mechanics. Plant Physiol. 2018, 176, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Ligrone, R.; Vaughn, K.C.; Rascio, N. A cytochemical and immunocytochemical analysis of the wall labyrinth apparatus in leaf transfer cells in Elodea canadensis. Ann. Bot. 2011, 107, 717–722. [Google Scholar] [CrossRef]
- Henry, J.S.; Lopez, R.A.; Renzaglia, K.S. Differential localization of cell wall polymers across generations in the placenta of Marchantia polymorpha. J. Plant Res. 2020, 133, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Chanliaud, E.; Burrows, K.M.; Jeronimidis, G.; Gidley, M.J. Mechanical properties of primary plant cell wall analogues. Planta 2002, 215, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Braybrook, S.A.; Jönsson, H. Shifting foundations: The mechanical cell wall and development. Curr. Opin. Plant Biol. 2016, 29, 115–120. [Google Scholar] [CrossRef]
- Delmer, D.; Dixon, R.A.; Keegstra, K.; Mohnen, D. The plant cell wall—Dynamic, strong, and adaptable—Is a natural shapeshifter. Plant Cell 2024, 36, 1257–1311. [Google Scholar] [CrossRef]
- Curry, T.M.; Peña, M.J.; Urbanowicz, B.R. An update on xylan structure, biosynthesis, and potential commercial applications. Cell Surf. 2023, 9, 100101. [Google Scholar] [CrossRef] [PubMed]
- Peňa, M.J.; Ryden, P.; Madson, M.; Smith, A.C.; Carpita, N.C. The galactose residues of xyloglucan are essential to maintain mechanical strength of the primary cell walls in Arabidopsis during growth. Plant Physiol. 2004, 134, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Obel, N.; Neumetzler, L.; Pauly, M. Hemicelluloses and cell expansion. In Plant Cell Monographs the Expanding Cell; Verbelen, J.-P., Vissenberg, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 5, pp. 57–88. [Google Scholar]
- Dauphin, B.G.; Ranocha, P.; Dunand, C.; Burlat, V. Cell-wall microdomain remodeling controls crucial developmental processes. Trends Plant Sci. 2022, 27, 1033–1048. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Feldo, M.; Świątek, P. Homogalacturonans and Hemicelluloses in the External Glands of Utricularia dichotoma Traps. Int. J. Mol. Sci. 2024, 25, 13124. [Google Scholar] [CrossRef] [PubMed]
- Jobson, R.W.; Baleeiro, P.C. Radiations of fairy-aprons (Utricularia dichotoma, Lentibulariaceae) in Australia and New Zealand: Molecular evidence and proposal of new subspecies. Aust. Syst. Bot. 2020, 33, 278–310. [Google Scholar] [CrossRef]
- Paul Knox, PhD, University of Leeds. Available online: https://www.kerafast.com/cat/799/paul-knox-phd (accessed on 13 November 2023).
- Knox, J.P.; Day, S.; Roberts, K. A set of cell surface glycoproteins forms an early marker of cell position, but not cell type, in the root apical meristem of Daucus carota L. Development 1989, 106, 47–56. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Ordaz-Ortiz, J.J.; Knox, J.P. An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr. Res. 2009, 28, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Pattathil, S.; Avci, U.; Baldwin, D.; Swennes, A.G.; McGill, J.A.; Popper, Z.; Bootten, T.; Albert, A.; Davis, R.H.; Chennareddy, C.; et al. A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol. 2010, 153, 514–525. [Google Scholar] [CrossRef]
- McCartney, L.; Marcus, S.E.; Knox, J.P. Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J. Histochem. Cytochem. 2005, 53, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.E.; Verhertbruggen, Y.; Hervé, C.; Ordaz-Ortiz, J.J.; Farkas, V.; Pedersen, H.L.; Willats, W.G.; Knox, J.P. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 2008, 22, 60. [Google Scholar] [CrossRef]
- Available online: https://www.kerafast.com/item/1603/anti-heteroxylan-lm11-antibody (accessed on 26 November 2024).
- Marcus, S.E.; Blake, A.W.; Benians, T.A.S.; Lee, K.J.D.; Poyser, C.; Donaldson, L.; Leroux, O.; Rogowski, A.; Petersen, H.L.; Boraston, A.; et al. Restricted access of proteins to mannan polysaccharides in intact plant cell walls. Plant J. 2010, 64, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Pauly, M.; Keegstra, K. Biosynthesis of the plant cell wall matrix polysaccharide xyloglucan. Annu. Rev. Plant Biol. 2017, 67, 235–259. [Google Scholar] [CrossRef]
- Płachno, B.J.; Świątek, P.; Jobson, R.W.; Małota, K.; Brutkowski, W. Serial block face SEM visualization of unusual plant nuclear tubular extensions in a carnivorous plant (Utricularia, Lentibulariaceae). Ann. Bot. 2017, 120, 673–680. [Google Scholar] [CrossRef]
- Available online: https://www.kerafast.com/item/1604/anti-glucuronoxylan-lm28-antibody (accessed on 26 November 2024).
- Cornuault, V.; Buffetto, F.; Rydahl, M.G.; Marcus, S.E.; Torode, T.A.; Xue, J.; Crépeau, M.J.; Faria-Blanc, N.; Willats, W.G.; Dupree, P.; et al. Monoclonal antibodies indicate low-abundance links between heteroxylan and other glycans of plant cell walls. Planta 2015, 242, 1321–1334. [Google Scholar] [CrossRef]



| Cell Wall Polysaccharides | Monoclonal Antibody | Specificity |
|---|---|---|
| pectins | JIM5 | low methylesterified HGs |
| LM19 | low methylesterified HGs | |
| JIM7 | highly esterified HGs | |
| CCRC-M38 | a fully de-esterified HG | |
| LM5 | galactan | |
| hemicelluloses | LM25 | galactoxyloglucan (XLLG, XXLG, XXXG modules) |
| LM15 | xyloglucan (XXXG module) | |
| CCRC-M138 | xylan | |
| LM11 | heteroxylan, unsubstituted and relatively low-substituted xylans | |
| LM20 | heteromannan | |
| LM22 | heteromannan, glucomannan, β-(1→4)-manno-oligosaccharides from DP2 to DP5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płachno, B.J.; Kapusta, M.; Feldo, M.; Świątek, P. Cell Wall Microdomains Analysis in the Quadrifids of Utricularia dichotoma. Int. J. Mol. Sci. 2025, 26, 832. https://doi.org/10.3390/ijms26020832
Płachno BJ, Kapusta M, Feldo M, Świątek P. Cell Wall Microdomains Analysis in the Quadrifids of Utricularia dichotoma. International Journal of Molecular Sciences. 2025; 26(2):832. https://doi.org/10.3390/ijms26020832
Chicago/Turabian StylePłachno, Bartosz J., Małgorzata Kapusta, Marcin Feldo, and Piotr Świątek. 2025. "Cell Wall Microdomains Analysis in the Quadrifids of Utricularia dichotoma" International Journal of Molecular Sciences 26, no. 2: 832. https://doi.org/10.3390/ijms26020832
APA StylePłachno, B. J., Kapusta, M., Feldo, M., & Świątek, P. (2025). Cell Wall Microdomains Analysis in the Quadrifids of Utricularia dichotoma. International Journal of Molecular Sciences, 26(2), 832. https://doi.org/10.3390/ijms26020832







