Proanthocyanidins as Therapeutic Agents in Inflammation-Related Skin Disorders
Abstract
1. Introduction
2. Results and Discussion
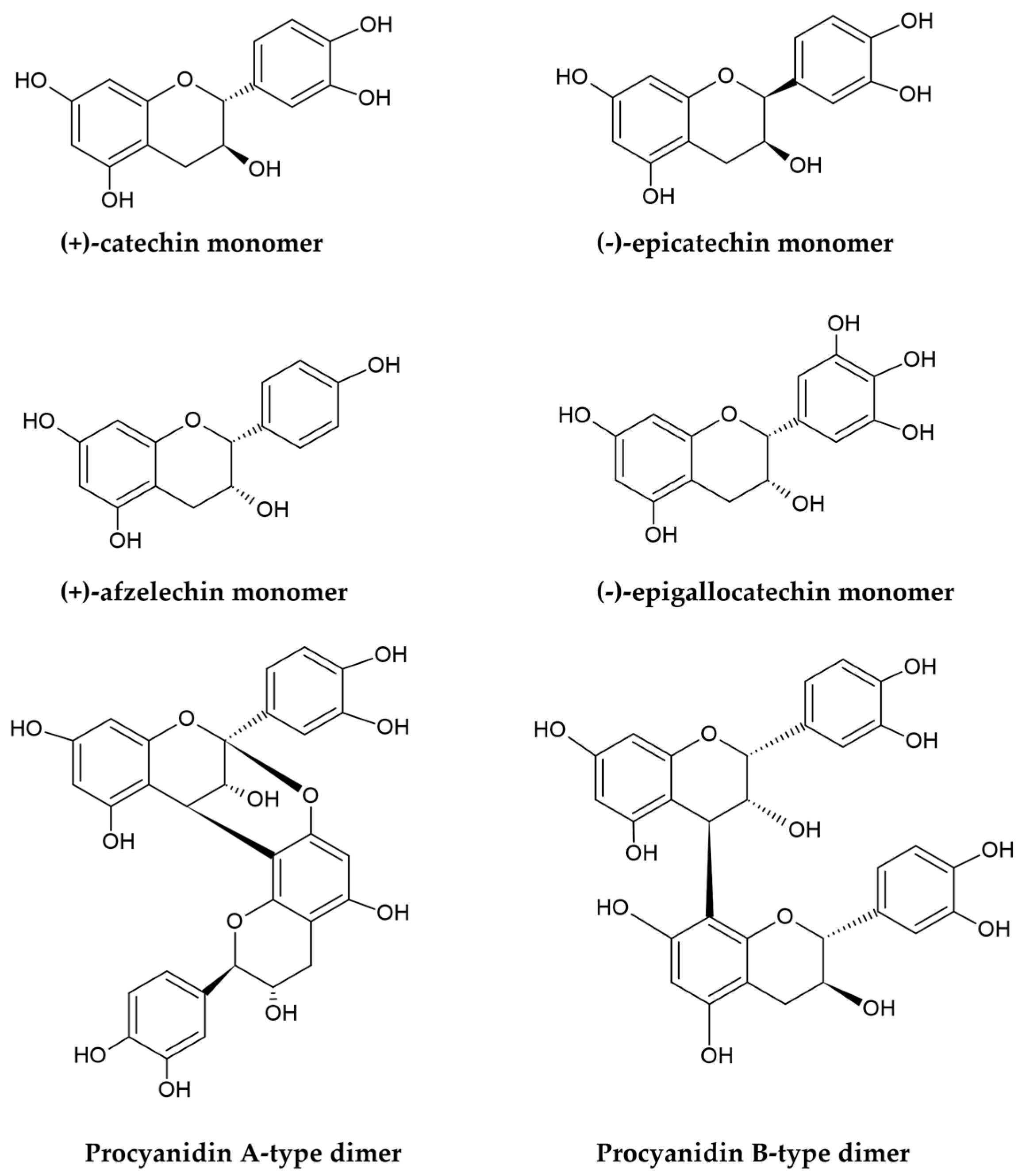
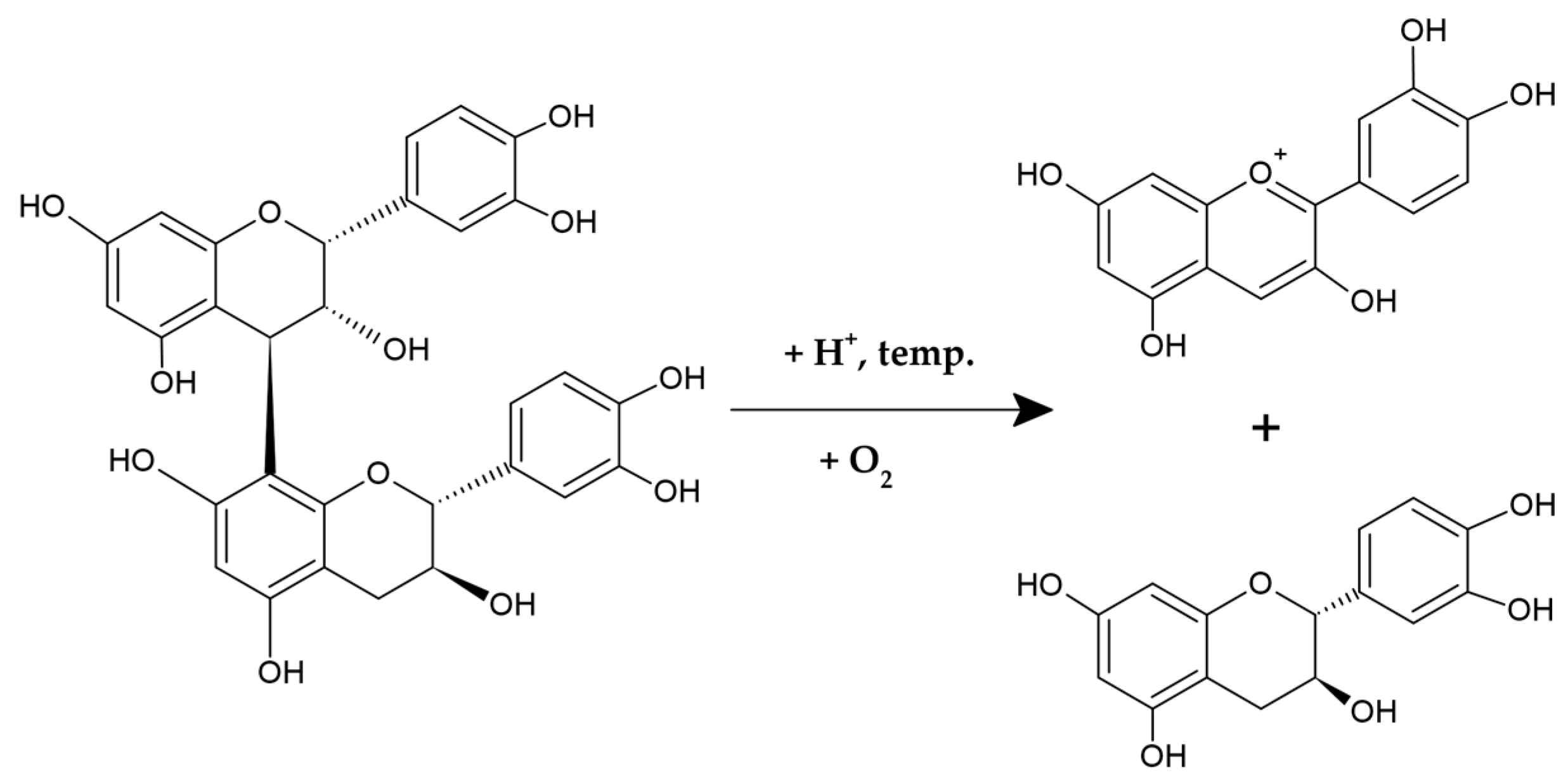
2.1. PACs—Chemical Structure and Distribution in Plants
2.1.1. Chemical Structure
2.1.2. Distribution in Plants
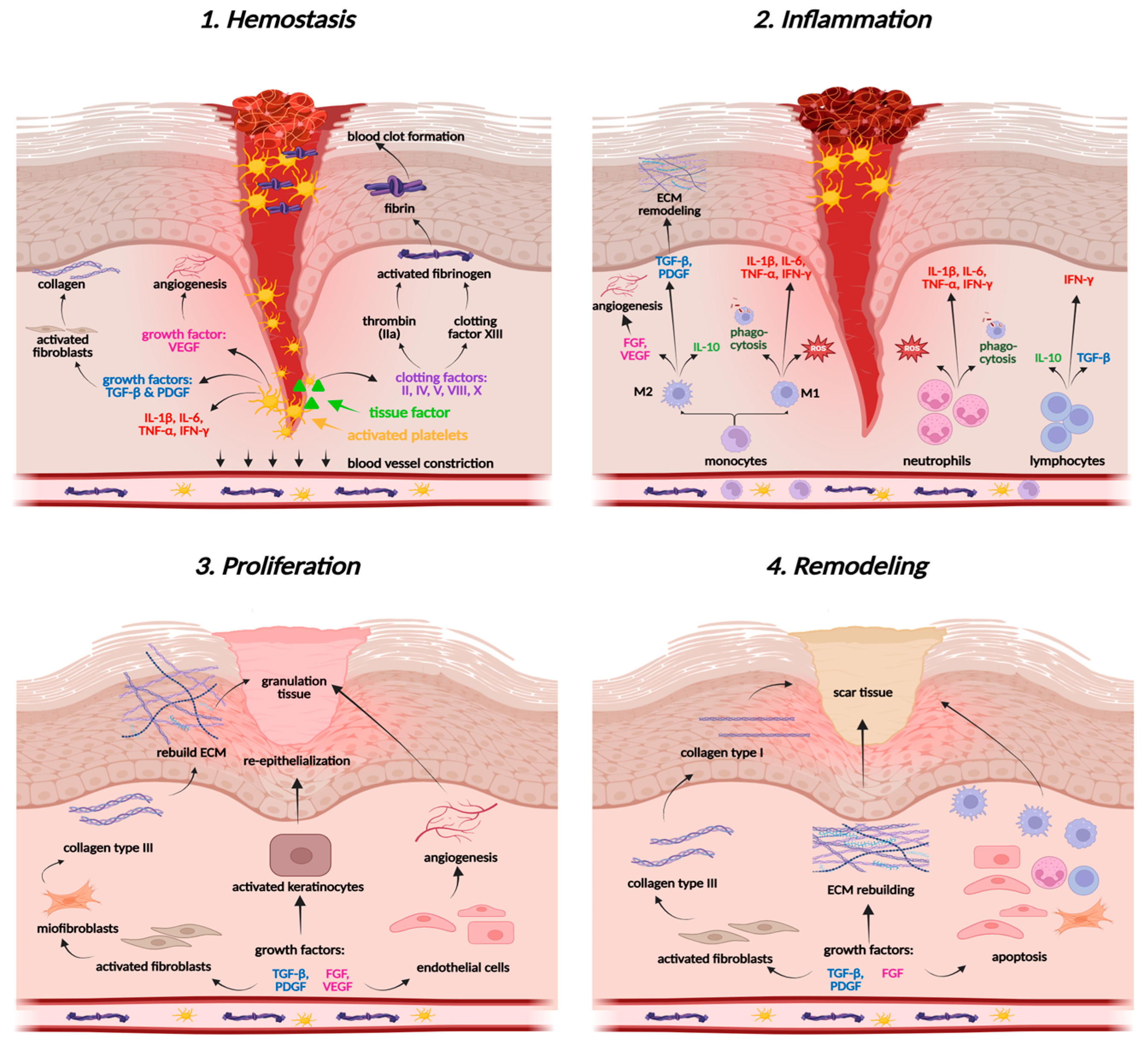
2.2. Therapeutic Potential of PACs in Wound Healing
2.2.1. Molecular Basis of Tissue Regeneration and Wound Healing
2.2.2. PACs—Wound Healing Activity
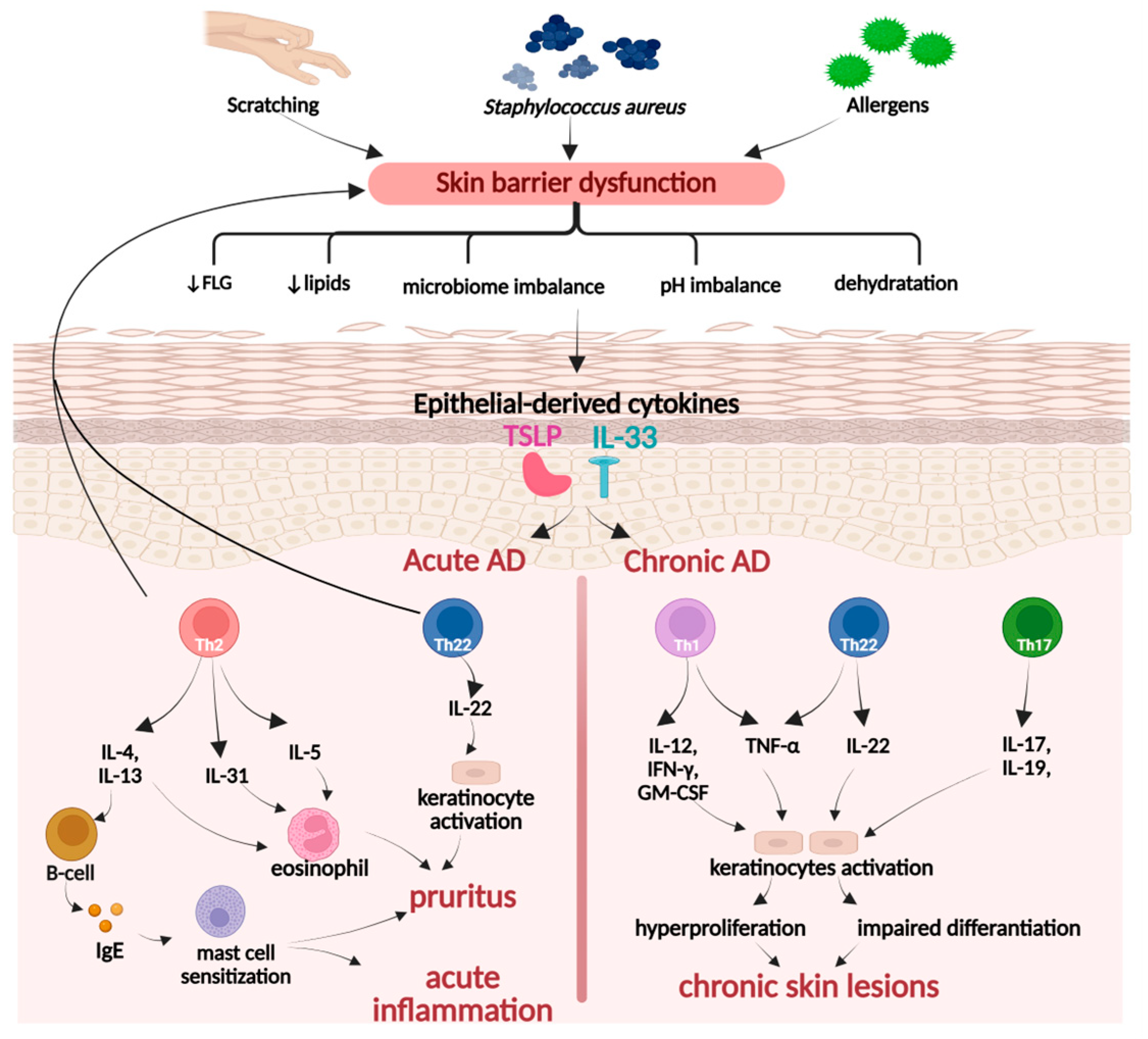
2.3. Therapeutic Potential of PACs in Inflammatory Skin Diseases
2.3.1. Molecular Basis of Skin Inflammation
Psoriasis
Atopic Dermatitis
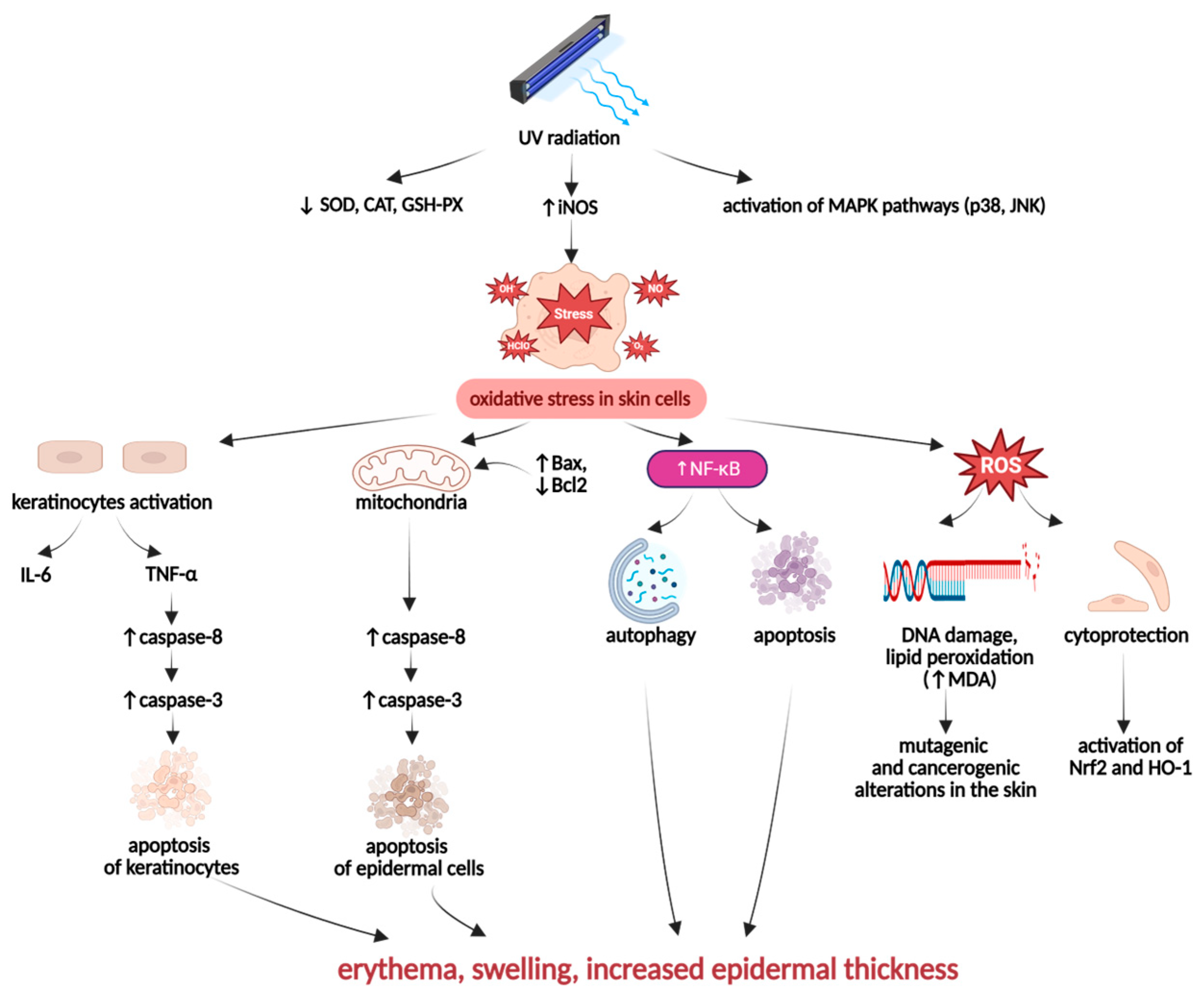
UV-Induced Skin Inflammation
2.3.2. PACs—Anti-Inflammatory Activity
2.3.3. PACs—Photoprotective Activity
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPs | Antimicrobial peptides |
| A549 | Human non-small-cell lung carcinoma cell line |
| CAT | Catalase |
| CE | Catechin equivalents |
| COX-2 | Cyclooxygenase-2 |
| CYE | Cyanidin equivalents |
| DEX | Dexamethasone |
| DMAC | 4-dimethylamino-cinnamaldehyde |
| ECM | Extracellular matrix |
| EMPB | Mallotus philippinensis bark extract |
| EPCs | Endothelial progenitor cells |
| FBS | Fetal bovine serum |
| FGF | Fibroblast growth factor |
| FLG | Filaggrin |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GSH | Glutathione |
| GSH-Px | Glutathione peroxidase |
| GSP | Grape seed extract, a commercially available product containing 95% dw PACs |
| GSPE | Grape seed extract, a commercially available product containing 54% dimeric, 13% trimeric, and 7% tetrameric PACs |
| GSSG | Glutathione disulfide |
| HaCaT | Immortalized human keratinocyte cell line |
| HAEC | Human aortic endothelial cells |
| HDFa | Human dermal fibroblasts |
| HFF | Human foreskin fibroblasts |
| HG | High-glucose |
| HO-1 | Heme oxygenase-1 |
| Hs27 | Human dermal fibroblasts |
| HUVECs | Human umbilical vein endothelial cells |
| IgE | Immunoglobulin E |
| IL | Interleukin |
| IFN-γ | Interferon gamma |
| IMQ | Imiquimod |
| iNOS | Inducible nitric oxide synthase |
| JNK | c-Jun N-terminal kinase |
| KUM6 | Mouse mesenchymal stem cells |
| L929 | Immortalized murine fibroblast cell line |
| LTB4 | Leukotriene B4 |
| MAPK | Mitogen-activated protein kinase |
| NHEKs | Normal human epidermal keratinocytes |
| NF-κB | Nuclear factor κB |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NIH-3T3 | Normal immortalized mouse embryonic fibroblasts |
| NIR | Near-infrared light |
| OPCs | Oligomeric procyanidins |
| OPCG | Oligomeric procyanidins from grape seeds |
| PAC(s) | Proanthocyanidin(s) |
| PB2 | Procyanidin B2 |
| PB3 | Procyanidin B3 |
| PB2E | Procyanidin B2 equivalents |
| PBO/PBOF | Hydrogel composed of polyvinyl alcohol (P), borax (B), oligomeric procyanidins (O), with or without ferric ion (F) |
| PGE2 | Prostaglandin E2 |
| PGES-1 | Microsomal prostaglandin E synthase-1 |
| pNHEK | Primary human epidermal keratinocytes |
| pNHD | Primary human dermal fibroblasts |
| PDGF | Platelet-derived growth factor |
| ProNPS | Bimetallic gold-silver nanoparticles modified with procyanidin B2 |
| RAW264.7 | Transformed murine macrophages |
| RRP | B-type catechin octamer from red-kerneled rice |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TF | Tissue factor |
| TGF-β | Transforming growth factor beta |
| Th | T-helper cells |
| TNF-α | Tumor necrosis factor alpha |
| TSLP | Thymic stromal lymphopoietin |
| UV | Ultraviolet radiation |
| VEGF | Vascular endothelial growth factor |
| 3-NT | 3-nitrotyrosine |
| 3T3-L1 | Immortalized mouse fibroblasts |
| 4-HNE | 4-hydroxynonenal |
| 5-LOX | 5-lipoxygenase |
References
- Fore, J.A. Review of Skin and the Effects of Aging on Skin Structure and Function. Ostomy Wound Manag. 2006, 52, 24–35. [Google Scholar]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin Barrier Immunity and Ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cui, L.; Li, J.; Guan, S.; Zhang, K.; Li, J. Aloe Vera: A Medicinal Plant Used in Skin Wound Healing. Tissue Eng. Part B Rev. 2021, 27, 455–474. [Google Scholar]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A Systematic Review of Natural Products for Skin Applications: Targeting Inflammation, Wound Healing, and Photo-Aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.J.; Jones, J.D.; Woessner, A.E.; Quinn, K.P. Skin Structure-Function Relationships and the Wound Healing Response to Intrinsic Aging. Adv. Wound Care 2020, 9, 127–143. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Structural Characteristics of the Aging Skin: A Review. Cutan. Ocul. Toxicol. 2007, 26, 343–357. [Google Scholar] [CrossRef]
- Jiao, Q.; Zhi, L.; You, B.; Wang, G.; Wu, N.; Jia, Y. Skin Homeostasis: Mechanism and Influencing Factors. J. Cosmet. Dermatol. 2024, 23, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The Dynamic Anatomy and Patterning of Skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The Immunological Anatomy of the Skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef]
- Veronese, S.; Picelli, A.; Smania, N.; Sbarbati, A. Hypodermis Involvement in Skin Disorders: Imaging and Functional Imaging Diagnostic Tools. Ski. Res. Technol. 2021, 27, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Yakupu, A.; Aimaier, R.; Yuan, B.; Chen, B.; Cheng, J.; Zhao, Y.; Peng, Y.; Dong, J.; Lu, S. The Burden of Skin and Subcutaneous Diseases: Findings from the Global Burden of Disease Study 2019. Front. Public Health 2023, 11, 1145513. [Google Scholar] [CrossRef]
- Mian, M.; Silfvast-Kaiser, A.S.; Paek, S.Y.; Kivelevitch, D.; Menter, A. A Review of the Most Common Dermatologic Conditions and Their Debilitating Psychosocial Impacts. Int. Arch. Intern. Med. 2019, 22, 3. [Google Scholar] [CrossRef]
- McGrath, L.N.; McGrath, L.G.; Edminister, J.R. A Year in Review: New Treatments and Expanded Indications in Dermatology in 2024. J. Dermatol. Treat. 2025, 36, 2456528. [Google Scholar] [CrossRef]
- Tabassum, N.; Hamdani, M. Plants Used to Treat Skin Diseases. Pharmacogn. Rev. 2014, 8, 52–60. [Google Scholar] [CrossRef]
- Melnyk, N.; Vlasova, I.; Skowrońska, W.; Bazylko, A.; Piwowarski, J.P.; Granica, S. Current Knowledge on Interactions of Plant Materials Traditionally Used in Skin Diseases in Poland and Ukraine with Human Skin Microbiota. Int. J. Mol. Sci. 2022, 23, 9644. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Chinigò, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and Where to Find Them: A Meta-Analytic Approach to Investigate Their Chemistry, Biosynthesis, Distribution and Effect on Human Health. Antioxidants 2021, 10, 1229. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, L.; Wang, K.; Renard, C.M.G.C.; Le Bourvellec, C.; Hu, Z.; Liu, X. A-Type Proanthocyanidins: Sources, Structure, Bioactivity, Processing, Nutrition, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2024, 23, 13352. [Google Scholar] [CrossRef]
- Tsuruya, M.; Niwano, Y.; Nakamura, K.; Kanno, T.; Nakashima, T.; Egusa, H.; Sasaki, K. Acceleration of Proliferative Response of Mouse Fibroblasts by Short-Time Pretreatment with Polyphenols. Appl. Biochem. Biotechnol. 2014, 174, 2223–2235. [Google Scholar] [CrossRef]
- Kisseih, E.; Lechtenberg, M.; Petereit, F.; Sendker, J.; Zacharski, D.; Brandt, S.; Agyare, C.; Hensel, A. Phytochemical Characterization and in Vitro Wound Healing Activity of Leaf Extracts from Combretum mucronatum Schum. & Thonn.: Oligomeric Procyanidins as Strong Inductors of Cellular Differentiation. J. Ethnopharmacol. 2015, 174, 628–636. [Google Scholar] [CrossRef]
- Chen, L.; Hao, L.; Yanshuo, C.; FangFang, W.; Daqin, C.; Weidong, X.; Jian, X.; Shaodong, C.; Hongyu, Z.; Ke, X. Grape Seed Proanthocyanidins Regulate Mitophagy of Endothelial Cells and Promote Wound Healing in Mice through P-JNK/FOXO3a/ROS Signal Pathway. Arch. Biochem. Biophys. 2023, 749, 109790. [Google Scholar] [CrossRef]
- Fan, J.; Liu, H.; Wang, J.; Zeng, J.; Tan, Y.; Wang, Y.; Yu, X.; Li, W.; Wang, P.; Yang, Z.; et al. Procyanidin B2 Improves Endothelial Progenitor Cell Function and Promotes Wound Healing in Diabetic Mice via Activating Nrf2. J. Cell. Mol. Med. 2021, 25, 652–665. [Google Scholar] [CrossRef]
- Khanna, S.; Roy, S.; Bagchi, D.; Bagchi, M.; Sen, C.K. Upregulation of oxidant-induced VEGF expression in cultured keratinocytes by a grape seed proanthocyanidin extract. Free Radic. Biol. Med. 2001, 31, 38–42. [Google Scholar] [CrossRef]
- Khanna, S.; Venojarvi, M.; Roy, S.; Sharma, N.; Trikha, P.; Bagchi, D.; Bagchi, M.; Sen, C.K. Dermal wound healing properties of redox-active grape seed proanthocyanidins. Free Radic. Biol. Med. 2002, 33, 1089–1096. [Google Scholar] [CrossRef]
- Esposito, D.; Overall, J.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Alaskan Berry Extracts Promote Dermal Wound Repair through Modulation of Bioenergetics and Integrin Signaling. Front. Pharmacol. 2019, 10, 1058. [Google Scholar] [CrossRef] [PubMed]
- Vilkickyte, G.; Zilius, M.; Petrikaite, V.; Raudone, L. Proanthocyanidins from Vaccinium Vitis-idaea L. Leaves: Perspectives in Wound Healing and Designing for Topical Delivery. Plants 2022, 11, 2615. [Google Scholar] [CrossRef]
- Kim, B.J.; Park, J.K.; Kim, B.K.; Park, S.J.; Kim, M.K.; Lee, C.W.; Choi, L.M.; Hur, J.A.; Kim, S.H.; Beom, J.; et al. Oligomeric Procyanidins (OPCs) Inhibit Procollagen Type I Secretion of Fibroblasts. Tissue Eng. Regen. Med. 2017, 14, 297–306. [Google Scholar] [CrossRef]
- Furumoto, T.; Ozawa, N.; Inami, Y.; Toyoshima, M.; Fujita, K.; Zaiki, K.; Sahara, S.; Akita, M.; Kitamura, K.; Nakaoji, K.; et al. Mallotus philippinensis Bark Extracts Promote Preferential Migration of Mesenchymal Stem Cells and Improve Wound Healing in Mice. Phytomedicine 2014, 21, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Kuge, K.; Ozawa, N.; Sahara, S.; Zaiki, K.; Nakaoji, K.; Hamada, K.; Takenaka, Y.; Tanahashi, T.; Tamai, K.; et al. Cinnamtannin B-1 Promotes Migration of Mesenchymal Stem Cells and Accelerates Wound Healing in Mice. PLoS ONE 2015, 10, e0144166. [Google Scholar] [CrossRef]
- Orlowski, P.; Zmigrodzka, M.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Pajak, B.; Slonska, A.; Cymerys, J.; Celichowski, G.; Grobelny, J.; Krzyzowska, M. Polyphenol-Conjugated Bimetallic Au@AgNPS for Improved Wound Healing. Int. J. Nanomed. 2020, 15, 4969–4990. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, C.; Chang, R.; He, Y.; Guan, F.; Yao, M. Ultra-Stretchable, Tissue-Adhesive, Shape-Adaptive, Self-Healing, on-Demand Removable Hydrogel Dressings with Multiple Functions for Infected Wound Healing in Regions of High Mobility. Acta Biomater. 2023, 166, 224–240. [Google Scholar] [CrossRef]
- Pinto, S.C.G.; Bueno, F.G.; Panizzon, G.P.; Morais, G.; Dos Santos, P.V.P.; Baesso, M.L.; De Souza Leite-Mello, E.V.; De Mello, J.C.P. Stryphnodendron adstringens: Clarifying Wound Healing in Streptozotocin-Induced Diabetic Rats. Planta Med. 2015, 81, 1090–1096. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, Y.; Li, X.; Song, J.; Guo, M.; Xian, D.; Zhong, J. The Protective Effect of Proanthocyanidins on the Psoriasis-like Cell Models via PI3K/AKT and HO-1. Redox Rep. 2022, 27, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Tsukayama, I.; Nagasaki, Y.; Konoike, Y.; Tamenobu, A.; Ganeko, N.; Ito, H.; Kawakami, Y.; Takahashi, Y.; Miki, Y.; et al. Red-Kerneled Rice Proanthocyanidin Inhibits Arachidonate 5-Lipoxygenase and Decreases Psoriasis-like Skin Inflammation. Arch. Biochem. Biophys. 2020, 689, 108307. [Google Scholar] [CrossRef] [PubMed]
- Tatsuno, T.; Jinno, M.; Arima, Y.; Kawabata, T.; Hasegawa, T.; Yahagi, N.; Takano, F.; Ohta, T. Anti-Inflammatory and Anti-Melanogenic Proanthocyanidin Oligomers from Peanut Skin. Biol. Pharm. Bull. 2012, 35, 909–916. [Google Scholar] [CrossRef]
- Park, K.H.; Choi, S.E.; Choi, Y.W.; Lee, D.I.; Joo, S.S.; Jeong, M.S.; Bang, H.; Lee, C.S.; Lee, M.K.; Seo, S.J.; et al. Topical Application of Two Condensed Tannins from the Root of Rosa multiflora Thunberg for the Treatment of Atopic Dermatitis (AD) in NC/Nga Mice. Phytother. Res. 2011, 25, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Shang, F.; Zhang, Y.; Wang, R.; Jia, Y.; Li, K. Persimmon Oligomeric Proanthocyanidins Alleviate Ultraviolet B-Induced Skin Damage by Regulating Oxidative Stress and Inflammatory Responses. Free Radic. Res. 2020, 54, 765–776. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, F.; McClements, D.J.; Xie, B.; Sun, Z.; Deng, Q. Oligomeric Procyanidin Nanoliposomes Prevent Melanogenesis and UV Radiation-Induced Skin Epithelial Cell (HFF-1) Damage. Molecules 2020, 25, 1458. [Google Scholar] [CrossRef]
- Matito, C.; Agell, N.; Sanchez-Tena, S.; Torres, J.L.; Cascante, M. Protective Effect of Structurally Diverse Grape Procyanidin Fractions against UV-Induced Cell Damage and Death. J. Agric. Food Chem. 2011, 59, 4489–4495. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and Hydrolysable Tannins: Occurrence, Dietary Intake and Pharmacological Effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- De La Iglesia, R.; Milagro, F.I.; Campión, J.; Boqué, N.; Martínez, J.A. Healthy Properties of Proanthocyanidins. BioFactors 2010, 36, 159–168. [Google Scholar] [CrossRef]
- Cires, M.J.; Wong, X.; Carrasco-Pozo, C.; Gotteland, M. The Gastrointestinal Tract as a Key Target Organ for the Health-Promoting Effects of Dietary Proanthocyanidins. Front. Nutr. 2017, 3, 57. [Google Scholar] [CrossRef]
- Xie, D.Y.; Dixon, R.A. Proanthocyanidin Biosynthesis—Still More Questions than Answers? Phytochemistry 2005, 66, 2127–2144. [Google Scholar] [CrossRef]
- Cosme, F.; Aires, A.; Pinto, T.; Oliveira, I.; Vilela, A.; Gonçalves, B. A Comprehensive Review of Bioactive Tannins in Foods and Beverages: Functional Properties, Health Benefits, and Sensory Qualities. Molecules 2025, 30, 800. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and Proanthocyanidins: Chemical Structures, Food Sources, Bioactivities, and Product Development. Food Rev. Int. 2023, 39, 4581–4609. [Google Scholar] [CrossRef]
- Verma, P.; Sen, R.; Bamanna, A.; Elhindawy, M.; Nagpal, K.; Krishnan, V. Structural Chemistry to Therapeutic Functionality: A Comprehensive Review on Proanthocyanidins. Biocatal. Agric. Biotechnol. 2024, 55, 102963. [Google Scholar] [CrossRef]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Song, H.; Yuan, C.; Li, J. Evaluation of Biological Activity and Prebiotic Properties of Proanthocyanidins with Different Degrees of Polymerization through Simulated Digestion and in Vitro Fermentation by Human Fecal Microbiota. Food Chem. 2024, 447, 139015. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Liu, L.; Cui, J.; Zhao, Y.; Zhang, D.; Zhou, D.; Wu, J.; Li, B.; Wang, T.; Li, M.; et al. Oligomeric Proanthocyanidins: An Updated Review of Their Natural Sources, Synthesis, and Potentials. Antioxidants 2023, 12, 1004. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Sarnala, S. Proanthocyanidin Biosynthesis—A Matter of Protection. Plant Physiol. 2020, 184, 579–591. [Google Scholar] [CrossRef]
- Wang, Y.; Harrington, P.D.B.; Chen, P. Metabolomic Profiling and Comparison of Major Cinnamon Species Using UHPLC–HRMS. Anal. Bioanal. Chem. 2020, 412, 7669–7681. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Nowak, S.; Michel, P.; Banaszczak, P.; Kicel, A. Assessment of the Content of Phenolics and Antioxidant Action of Inflorescences and Leaves of Selected Species from the Genus Sorbus Sensu Stricto. Molecules 2010, 15, 8769–8783. [Google Scholar] [CrossRef]
- Kicel, A.; Michel, P.; Owczarek, A.; Marchelak, A.; Zyzelewicz, D.; Budryn, G.; Oracz, J.; Olszewska, M.A. Phenolic Profile and Antioxidant Potential of Leaves from Selected Cotoneaster Medik. Species. Molecules 2016, 21, 688. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, P. Composition and Health Effects of Phenolic Compounds in Hawthorn (Crataegus spp.) of Different Origins. J. Sci. Food Agric. 2012, 92, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Rudikovskaya, E.G.; Dudareva, L.V.; Stavitskaya, Z.O.; Katysheva, N.B.; Vanina, L.S.; Rudikovskii, A. V Comparative Analysis of the Composition and Content of Phenolic Compounds in Fruits of Wild Species of Malus Native to Eastern Siberia and the Far East. Sci. Hortic. 2021, 289, 110432. [Google Scholar] [CrossRef]
- Scioneaux, A.N.; Schmidt, M.A.; Moore, M.A.; Lindroth, R.L.; Wooley, S.C.; Hagerman, A.E. Qualitative Variation in Proanthocyanidin Composition of Populus Species and Hybrids: Genetics Is the Key. J. Chem. Ecol. 2011, 37, 57–70. [Google Scholar] [CrossRef]
- De Freitas, V.A.P.; Glories, Y. Monique, Developmental Changes of Procyanidins in Grapes of Red Vitis vinifera Varieties and Their Composition in Respective Wines. Am. J. Enol. Vitic. 2000, 51, 397–403. [Google Scholar] [CrossRef]
- Suvanto, J.; Karppinen, K.; Riihinen, K.; Jaakola, L.; Salminen, J.P. Changes in the Proanthocyanidin Composition and Related Gene Expression in Bilberry (Vaccinium Myrtillus L.) Tissues. J. Agric. Food Chem. 2020, 68, 7378–7386. [Google Scholar] [CrossRef]
- Ferguson, A.; Carvalho, E.; Gourlay, G.; Walker, V.; Martens, S.; Salminen, J.-P.; Constabel, C.P. Phytochemical Analysis of Salal Berry (Gaultheria shallon Pursh.), a Traditionally-Consumed Fruit from Western North America with Exceptionally High Proanthocyanidin Content. Phytochemistry 2018, 147, 203–210. [Google Scholar] [CrossRef]
- Rutkowska, M.; Balcerczak, E.; Świechowski, R.; Dubicka, M.; Olszewska, M.A. Seasonal Variation in Phenylpropanoid Biosynthesis and in Vitro Antioxidant Activity of Sorbus Domestica Leaves: Harvesting Time Optimisation for Medicinal Application. Ind. Crops Prod. 2020, 156, 112858. [Google Scholar] [CrossRef]
- Hellström, J.K.; Törrönen, A.R.; Mattila, P.H. Proanthocyanidins in Common Food Products of Plant Origin. J. Agric. Food Chem. 2009, 57, 7899–7906. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, M.; Gaur, S.S.; Shams, R.; Dash, K.K.; Shaikh, A.M.; Béla, K. Valorization of Grape By-Products: Insights into Sustainable Industrial and Nutraceutical Applications. Future Foods 2025, 12, 100710. [Google Scholar] [CrossRef]
- Ramsay, A.; Mueller-Harvey, I. Procyanidins from Averrhoa Bilimbi Fruits and Leaves. J. Food Compos. Anal. 2016, 47, 16–20. [Google Scholar] [CrossRef]
- Rodríguez Montealegre, R.; Romero Peces, R.; Chacón Vozmediano, J.L.; Martínez Gascueña, J.; García Romero, E. Phenolic Compounds in Skins and Seeds of Ten Grape Vitis Vinifera Varieties Grown in a Warm Climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Ye, H.; Luo, L.; Wang, J.; Jiang, K.; Yue, T.; Yang, H. Highly Galloylated and A-Type Prodelphinidins and Procyanidins in Persimmon (Diospyros Kaki L.) Peel. Food Chem. 2022, 378, 131972. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, A.; Mueller-Harvey, I. Senna Alata Leaves Are a Good Source of Propelargonidins. Nat. Prod. Res. 2016, 30, 1548–1551. [Google Scholar] [CrossRef]
- Núñez, V.; Gómez-Cordovés, C.; Bartolomé, B.; Hong, Y.J.; Mitchell, A.E. Non-Galloylated and Galloylated Proanthocyanidin Oligomers in Grape Seeds from Vitus Vinifera L. Cv. Graciano, Tempranillo and Cabernet Sauvignon. J. Sci. Food Agric. 2006, 86, 915–921. [Google Scholar] [CrossRef]
- Ramsay, A.; Williams, A.R.; Thamsborg, S.M.; Mueller-Harvey, I. Galloylated Proanthocyanidins from Shea (Vitellaria Paradoxa) Meal Have Potent Anthelmintic Activity against Ascaris Suum. Phytochemistry 2016, 122, 146–153. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Wang, S.; Wei, L.; Cui, Y.Y.; Chen, Y.H. Proanthocyanidins: Components, Pharmacokinetics and Biomedical Properties. Am. J. Chin. Med. 2020, 48, 813–869. [Google Scholar] [CrossRef]
- García-Salas, P.; Gómez-Caravaca, A.M.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification and Quantification of Phenolic and Other Polar Compounds in the Edible Part of Annona cherimola and Its By-Products by HPLC-DAD-ESI-QTOF-MS. Food Res. Int. 2015, 78, 246–257. [Google Scholar] [CrossRef]
- Justino, A.B.; Franco, R.R.; Silva, H.C.G.; Saraiva, A.L.; Sousa, R.M.F.; Espindola, F.S. B Procyanidins of Annona crassiflora Fruit Peel Inhibited Glycation, Lipid Peroxidation and Protein-Bound Carbonyls, with Protective Effects on Glycated Catalase. Sci. Rep. 2019, 9, 19183. [Google Scholar] [CrossRef]
- Taheri, R.; Connolly, B.A.; Brand, M.H.; Bolling, B.W. Underutilized Chokeberry (Aronia Melanocarpa, Aronia Arbutifolia, Aronia Prunifolia) Accessions Are Rich Sources of Anthocyanins, Flavonoids, Hydroxycinnamic Acids, and Proanthocyanidins. J. Agric. Food Chem. 2013, 61, 8581–8588. [Google Scholar] [CrossRef]
- Gerasimov, M.A.; Perova, I.B.; Eller, K.I.; Akimov, M.Y.; Sukhanova, A.M.; Rodionova, G.M.; Ramenskaya, G.V. Investigation of Polyphenolic Compounds in Different Varieties of Black Chokeberry Aronia melanocarpa. Molecules 2023, 28, 4101. [Google Scholar] [CrossRef]
- Niroshani Wariyapperuma, W.A.M.; Kannangara, S.; Wijayasinghe, Y.S.; Subramanium, S.; Jayawardena, B. In Vitro Anti-Diabetic Effects and Phytochemical Profiling of Novel Varieties of Cinnamomum zeylanicum (L.) Extracts. PeerJ 2020, 8, e10070. [Google Scholar] [CrossRef] [PubMed]
- Elsadig Karar, M.G.; Kuhnert, N. UPLC-ESI-Q-TOF-MS/MS Characterization of Phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) Leaves, Fruits and Their Herbal Derived Drops (Crataegutt Tropfen). J. Chem. Biol. Ther. 2016, 1, 1000102. [Google Scholar] [CrossRef]
- Michel, P.; Granica, S.; Rosińska, K.; Glige, M.; Rojek, J.; Poraj, Ł.; Olszewska, M.A. The Effect of Standardised Leaf Extracts of Gaultheria procumbens on Multiple Oxidants, Inflammation-Related Enzymes, and Pro-Oxidant and Pro-Inflammatory Functions of Human Neutrophils. Molecules 2022, 27, 3357. [Google Scholar] [CrossRef]
- Lv, Q.; Luo, F.; Zhao, X.; Liu, Y.; Hu, G.; Sun, C.; Li, X.; Chen, K. Identification of Proanthocyanidins from Litchi (Litchi chinensis Sonn.) Pulp by LC-ESI-Q-TOF-MS and Their Antioxidant Activity. PLoS ONE 2015, 10, e0120480. [Google Scholar] [CrossRef]
- Navarro, M.; Moreira, I.; Arnaez, E.; Quesada, S.; Azofeifa, G.; Vargas, F.; Alvarado, D.; Chen, P. Polyphenolic Characterization and Antioxidant Activity of Malus domestica and Prunus domestica Cultivars from Costa Rica. Foods 2018, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Masumoto, S.; Moriichi, N.; Kanda, T.; Ohtake, Y. Apple (Malus pumila) Procyanidins Fractionated According to the Degree of Polymerization Using Normal-Phase Chromatography and Characterized by HPLC-ESI/MS and MALDI-TOF/MS. J. Chromatogr. A 2006, 1102, 206–213. [Google Scholar] [CrossRef]
- Marchelak, A.; Owczarek, A.; Matczak, M.; Pawlak, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Bioactivity Potential of Prunus spinosa L. Flower Extracts: Phytochemical Profiling, Cellular Safety, pro-Inflammatory Enzymes Inhibition and Protective Effects against Oxidative Stress in Vitro. Front. Pharmacol. 2017, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Matczak, M.; Marchelak, A.; Michel, P.; Owczarek, A.; Piszczan, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Sorbus Domestica L. Leaf Extracts as Functional Products: Phytochemical Profiling, Cellular Safety, pro-Inflammatory Enzymes Inhibition and Protective Effects against Oxidative Stress in Vitro. J. Funct. Foods 2018, 40, 207–218. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Identification and Quantification of Free and Bound Phenolic Compounds Contained in the High-Molecular Weight Melanoidin Fractions Derived from Two Different Types of Cocoa Beans by UHPLC-DAD-ESI-HR-MSn. Food Res. Int. 2019, 115, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.K.T.; Nguyen, H.V.H. Effects of Maturity at Harvest and Fermentation Conditions on Bioactive Compounds of Cocoa Beans. Plant Foods Hum. Nutr. 2019, 74, 54–60. [Google Scholar] [CrossRef]
- Grace, M.H.; Esposito, D.; Dunlap, K.L.; Lila, M.A. Comparative Analysis of Phenolic Content and Profile, Antioxidant Capacity, and Anti-Inflammatory Bioactivity in Wild Alaskan and Commercial Vaccinium Berries. J. Agric. Food Chem. 2014, 62, 4007–4017. [Google Scholar] [CrossRef]
- Merghem, R.; Jay, M.; Brun, N.; Voirin, B. Qualitative analysis and HPLC isolation and identification of procyanidins from Vicia faba. Phytochem. Anal. 2004, 15, 95–99. [Google Scholar] [CrossRef]
- Morazzoni, P.; Vanzani, P.; Santinello, S.; Gucciardi, A.; Zennaro, L.; Miotto, G.; Ursini, F. Grape Seeds Proanthocyanidins: Advanced Technological Preparation and Analytical Characterization. Antioxidants 2021, 10, 418. [Google Scholar] [CrossRef]
- Pérez-Navarro, J.; Izquierdo-Cañas, P.M.; Mena-Morales, A.; Martínez-Gascueña, J.; Chacón-Vozmediano, J.L.; García-Romero, E.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S. Phenolic Compounds Profile of Different Berry Parts from Novel Vitis vinifera L. Red Grape Genotypes and Tempranillo Using HPLC-DAD-ESI-MS/MS: A Varietal Differentiation Tool. Food Chem. 2019, 295, 350–360. [Google Scholar] [CrossRef]
- De Rosso, M.; Panighel, A.; Vedova, A.D.; Gardiman, M.; Flamini, R. Characterization of Non-Anthocyanic Flavonoids in Some Hybrid Red Grape Extracts Potentially Interesting for Industrial Uses. Molecules 2015, 20, 18095–18106. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef]
- Silveira, L.L.; Sarandy, M.M.; Novaes, R.D.; Morais-Santos, M.; Gonçalves, R.V. OxInflammation Affects Transdifferentiation to Myofibroblasts, Prolonging Wound Healing in Diabetes: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 8992. [Google Scholar] [CrossRef]
- Hassanshahi, A.; Moradzad, M.; Ghalamkari, S.; Fadaei, M.; Cowin, A.J.; Hassanshahi, M. Macrophage-Mediated Inflammation in Skin Wound Healing. Cells 2022, 11, 2953. [Google Scholar] [CrossRef]
- Gonzalez, A.C.D.O.; Costa, T.F.; Andrade, Z.D.A.; Medrado, A.R.A.P. Wound Healing—A Literature Review. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Raja, R. Wound Re-Epithelialization: Modulating Kerationcyte Migration in Wound Healing. Front. Biosci. 2007, 12, 2849. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, A.A.; Foroozan, M.; Houshmand, G.; Moosavi, Z.B.; Bahadoram, M.; Maram, N.S. The Topical Effect of Grape Seed Extract 2% Cream on Surgery Wound Healing. Glob. J. Health Sci. 2015, 7, 52–58. [Google Scholar] [CrossRef]
- Nicolaou, A. Eicosanoids in Skin Inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Calvello, R.; Porro, C.; Lofrumento, D.D.; Panaro, M.A. Inflammatory Skin Diseases: Focus on the Role of Suppressors of Cytokine Signaling (SOCS) Proteins. Cells 2024, 13, 505. [Google Scholar] [CrossRef]
- Bylund, S.; Von Kobyletzki, L.B.; Svalstedt, M.; Svensson, Å. Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm. Venereol. 2020, 100, 320–329. [Google Scholar] [CrossRef]
- Zhao, H.C.; Xiao, T.; Chen, Y.J. Ultraviolet Induced Skin Inflammation. Int. J. Dermatol. Venereol. 2021, 4, 229–235. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed]
- Sabat, R.; Wolk, K.; Loyal, L.; Döcke, W.D.; Ghoreschi, K. T Cell Pathology in Skin Inflammation. Semin. Immunopathol. 2019, 41, 359–377. [Google Scholar] [CrossRef]
- Somayaji, R.; Haber, R.M. Scratching the Surface: A Review of Dermatitis. Adv. Ski. Wound Care 2019, 32, 542–549. [Google Scholar]
- Furue, M.; Chiba, T.; Tsuji, G.; Ulzii, D.; Kido-Nakahara, M.; Nakahara, T.; Kadono, T. Atopic Dermatitis: Immune Deviation, Barrier Dysfunction, IgE Autoreactivity and New Therapies. Allergol. Int. 2017, 66, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, K.; Onodera, A.; Kiuchi, M.; Tsuji, K.; Hirahara, K.; Nakayama, T. Conventional and Pathogenic Th2 Cells in Inflammation, Tissue Repair, and Fibrosis. Front. Immunol. 2022, 13, 945063. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV Radiation-Induced Inflammation and Immunosuppression Accelerate the Aging Process in the Skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- NilamberLal Das, R.; Muruhan, S.; Nagarajan, R.P.; Balupillai, A. Naringin Prevents Ultraviolet-B Radiation-Induced Oxidative Damage and Inflammation through Activation of Peroxisome Proliferator-Activated Receptor γ in Mouse Embryonic Fibroblast (NIH-3T3) Cells. J. Biochem. Mol. Toxicol. 2019, 33, e23840. [Google Scholar] [CrossRef]
- Perluigi, M.; Di Domenico, F.; Blarzino, C.; Foppoli, C.; Cini, C.; Giorgi, A.; Grillo, C.; De Marco, F.; Butterfield, D.A.; Schininà, M.E.; et al. Effects of UVB-Induced Oxidative Stress on Protein Expression and Specific Protein Oxidation in Normal Human Epithelial Keratinocytes: A Proteomic Approach. Proteome Sci. 2010, 8, 13. [Google Scholar] [CrossRef]
- Zhong, J.L.; Edwards, G.P.; Raval, C.; Li, H.; Tyrrell, R.M. The Role of Nrf2 in Ultraviolet A Mediated Heme Oxygenase 1 Induction in Human Skin Fibroblasts. Photochem. Photobiol. Sci. 2010, 9, 18–24. [Google Scholar] [CrossRef]

| Plant Material | Extract/Sample | Identified PACs | Identification Method | PACs Content | Quantitation Method | Ref. |
|---|---|---|---|---|---|---|
| Annona cherimola Mille; fruit, pulp, peel, seed | Methanol-water (4:1, v/v) extracts of pulp/peel/seed powder | A-type dimers; B-type dimers, trimers, tetramers | HPLC-DAD-QTOF-MS | 38.30–46.80 mg/100 mg dw (pulp); 74.02–86.40 mg/100 mg dw (peel); 1.03–2.61 mg/100 mg dw (seed) | HPLC-DAD-QTOF-MS | [71] |
| Annona crassiflora Mart.; fruit peel | Ethyl acetate fraction of the ethanolic extract | B-type dimers, trimers, tetramers, pentamers | HPLC-ESI-MS/MS | 758 ± 12.00 mg catechin equivalents (CE)/g | Vanillin assay | [72] |
| Aronia arbutifolia (L.) Pers.; A. melanocarpa (Michx.) Elliott; A. mitschurinii A.K. Skvortsov and Maitul; A. prunifolia (Marshall) Rehder; fruit | Acidified acetone 70% extract from lyophilized berry powder | B-type dimers, trimers, oligomers (4–6 units, 7–10 units), polymers > 10 | HPLC-UV-MS | 9.28 ± 4.49 to 12.20 ± 7.28 mg cyanidin equivalents (CYE)/g dw; | 4-dimethylamino-cinnamaldehyde (DMAC) assay | [73] |
| 1.93 ± 0.14 to 2.17 ± 1.34 mg CYE/g dw | UHPLC-DAD-MS | |||||
| A. melanocarpa Michx. Elliott (various cultivars) A. prunifolia (Marshall) Rehder (cv. Aron); fruit | Ethanol-water (7:3, v/v) pulp extract from frozen berries | B-type dimers (B2, B5), trimers (C1) | HPLC-DAD-FLD | 1396 ± 24.00 to 2524 ± 37.70 mg procyanidin B2 equivalents (PB2E)/100 g fw | Bate–Smith assay | [74] |
| Cinnamomum cassia (L.) J.Presl; C. burmanni (Nees & T.Nees) Blume, C. loureiroi, C. verum J. Presl; bark | Methanol-water extract (6:4, v/v) from powdered cinnamon bark | A-type dimers, trimers, tetramers, pentamers; B-type dimers, trimers, tetramers, pentamers | UHPLC-HRMS | No available data | - | [52] |
| Cinnamomum zeylanicum (various cultivars); bark | Various extracts from bark (the highest content of PACs for decoction water extract) | No available data | - | 1.00 ± 0.01 to 40.10 ± 0.10 mg CE/g dw | Vanillin assay | [75] |
| Cotoneaster bullatus Bois; C. divaricatus Rehder and E.H.Wilson; C. dielsianus E. Pritz.; C. horizontalis Decne; C. hjelmqvistii Flinck et B. Hylmö; C. integerrimus Medik.; C. lucidus Schltdl.; C. melanocarpus Lodd. ex C.K. Schneid.; C. nanshan Mottet; C. tomentosus Lindl.; C. splendens Flinck et B. Hylmö; C. zabelii C.K. Schneid; leaf | Defatted methanol-water (7:3, v/v) leaf extracts | B-type dimers, trimers (C1), tetramers | UHPLC-PDA-ESI-QTOF-MS | 2.14 ± 0.03 to 15.00 ± 0.08% CYE (C. melanocarpus and C. bullatus, respectively) | Butanol/HCl assay | [54] |
| Crataegus laevigata (Poir.) DC., C. monogyna Jacq.; leaf, fruit | Ethanol-water (2:1, v/v) extracts from leaves and fruits | A-type dimer; B-type dimers and trimers | UPLC-ESI-Q-TOF-MS/MS | No available data | - | [76] |
| Gaultheria procumbens L.; leaf | Various extracts, including methanol-water (75:25, v/v) and ethyl acetate leaf extracts | A-type dimers, trimers, B-type dimers (B1, B2, C1), trimers | HPLC-PDA-ESI-MS3; | 36.90 ± 0.35 to 175 ± 2.35 mg CYE/g dw (ethyl acetate and methanol-water extracts, respectively) | Butanol/HCl assay | [77] |
| 9.67 ± 0.16 to 64.00 ± 2.01 mg CYE/g dw (ethyl acetate and methanol-water extracts, respectively) | HPLC-PDA | |||||
| Litchi chinensis Sonn. (cv. Hemaoli); fruit | Methanol-water (7:3, v/v) extract from pulp; | A-type dimers (A1, A2), trimers, tetramers; B-type dimers (B1, B2), trimers (C1), tetramers | LC-ESI-Q-TOF-MS; ESI-MS; NMR Spectroscopy | 12.10 ± 0.02 mg PB2E/g dw | DMAC assay | [78] |
| Malus domestica Borkh.; fruit | Acetone-water (7:3, v/v) extract from freeze-dried fruits | A-type dimers, trimers B-type dimers, trimers, tetramers, pentamers | UPLC-DAD-ESI-TQ-MS | No available data | - | [79] |
| Malus pumila Mill.; fruit | Methyl acetate fraction of extract from freeze-dried fruits | B-type dimers (B1, B2), trimers (C1), tetramers, oligomers (5–10 units) | HPLC-ESI/MS; MALDI-TOF/MS | No available data | - | [80] |
| Prunus spinosa L.; flower | Defatted methanol-water (7:3, v/v) flower extract and its various fractions | A-type dimers | UHPLC-PDA-ESI-MS3 | 45.10 ± 2.38 mg CYE/g dw (extract) 12.40 ± 0.25 mg CYE/g dw to 109.40 ± 3.71 mg CYE/g dw (water residue and ethyl acetate fraction, respectively) | Butanol/HCl assay | [81] |
| Sorbus domestica L.; leaf | Defatted methanol-water (7:3, v/v) leaf extract and its various fractions (the highest PAC content for n-butanol and ethyl acetate fractions) | B-type dimers, trimers (C1) | UHPLC-PDA-ESI-MS3 | 19.20 ± 0.90 to 183 ± 2.40 mg CYE/g dw | Butanol/HCl assay | [82] |
| Theobroma cacao L. (various cultivars); seed | Acidified methanolic extract from cocoa powder | B-type dimers (B2) | UHPLC-DAD-ESI-HR-MSn | 12.70 ± 0.11 to 25.50 ± 0.12 mg/100 g dw (procyanidin B2) | UHPLC-DAD-ESI-MSn after alkaline hydrolysis | [83] |
| Theobroma cacao L.; seed | Acidified methanolic extract from defatted cocoa powder | No available data | - | 16.10 ± 2.96 to 27.30 ± 0.78 g CE/100 g dw | Vanillin assay | [84] |
| Vaccinium angustifolium Aiton; V. macrocarpon Aiton; V. uliginosum L.; V. vitis idaea L.; fruit | Acidified methanol-water (7:3, v/v) extracts from freeze-dried fruits; | A- and B-type dimers, trimers, tetramers, pentamers, heptamers, polymers > 8 units | HPLC-FLD-DAD; | 27.30 ± 3.60 to 228 ± 4.20 mg CYE/g dw | HPLC-PDA; | [85] |
| Vicia faba L.; seed | Acetone-water extract (7:3, v/v) from seed coats | A-type dimer B-type dimers (B1, B2, B3) and trimer (C1) | ESI-MS | No available data | - | [86] |
| Vitis vinifera L.; seed | Aqueous extract from seeds | A-type dimers, trimers, tetramers, pentamers; B-type dimers (B2), trimers (C1), tetramers (D1), oligomers (5–13-mers) | LC-Chip/ESI-Q-TOF–MS | No available data | - | [87] |
| Vitis vinifera L.; fruit, fruit skin, seed | Methanol-water-formic acid (50:48.5:1.5, v/v) from whole fruit, skin and seeds | B-type dimers (B1, B2, B4), other dimers and oligomers (no additional data) | HPLC-MS-MRM; | 7.79 ± 1.07 to 12.9 ± 0.70 mg procyanidin B1 equivalents/kg grape fw (dimers; skin); 214 ± 9.45 to 333 ± 35.70 mg CE/kg grape fw (oligomers; skin); 199 ± 26.90 to 277 ± 16.30 mg procyanidin B1 equivalents/kg grape fw (dimers; seed) 0.82 ± 0.13 to 1.03 ± 0.10 mg CE/kg grape fw (oligomers; skin) | HPLC-MS-MRM | [88] |
| Vitis vinifera L. (various cultivars); fruit | Methanol-water (2:1, v/v) extract from fruits | B-type dimers (B1, B2, B3, B4, B5), trimers (T2, T3, T4, C1) | UHPLC/QTOF-MS | 5.40 to 20.60 mg/kg grapes | UHPLC/QTOF-MS | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokop, A.; Magiera, A.; Olszewska, M.A. Proanthocyanidins as Therapeutic Agents in Inflammation-Related Skin Disorders. Int. J. Mol. Sci. 2025, 26, 10116. https://doi.org/10.3390/ijms262010116
Prokop A, Magiera A, Olszewska MA. Proanthocyanidins as Therapeutic Agents in Inflammation-Related Skin Disorders. International Journal of Molecular Sciences. 2025; 26(20):10116. https://doi.org/10.3390/ijms262010116
Chicago/Turabian StyleProkop, Aleksandra, Anna Magiera, and Monika Anna Olszewska. 2025. "Proanthocyanidins as Therapeutic Agents in Inflammation-Related Skin Disorders" International Journal of Molecular Sciences 26, no. 20: 10116. https://doi.org/10.3390/ijms262010116
APA StyleProkop, A., Magiera, A., & Olszewska, M. A. (2025). Proanthocyanidins as Therapeutic Agents in Inflammation-Related Skin Disorders. International Journal of Molecular Sciences, 26(20), 10116. https://doi.org/10.3390/ijms262010116





