Skin Photoprotection and Anti-Aging Benefits of a Combination of Rosemary and Grapefruit Extracts: Evidence from In Vitro Models and Human Study
Abstract
:1. Introduction
2. Results
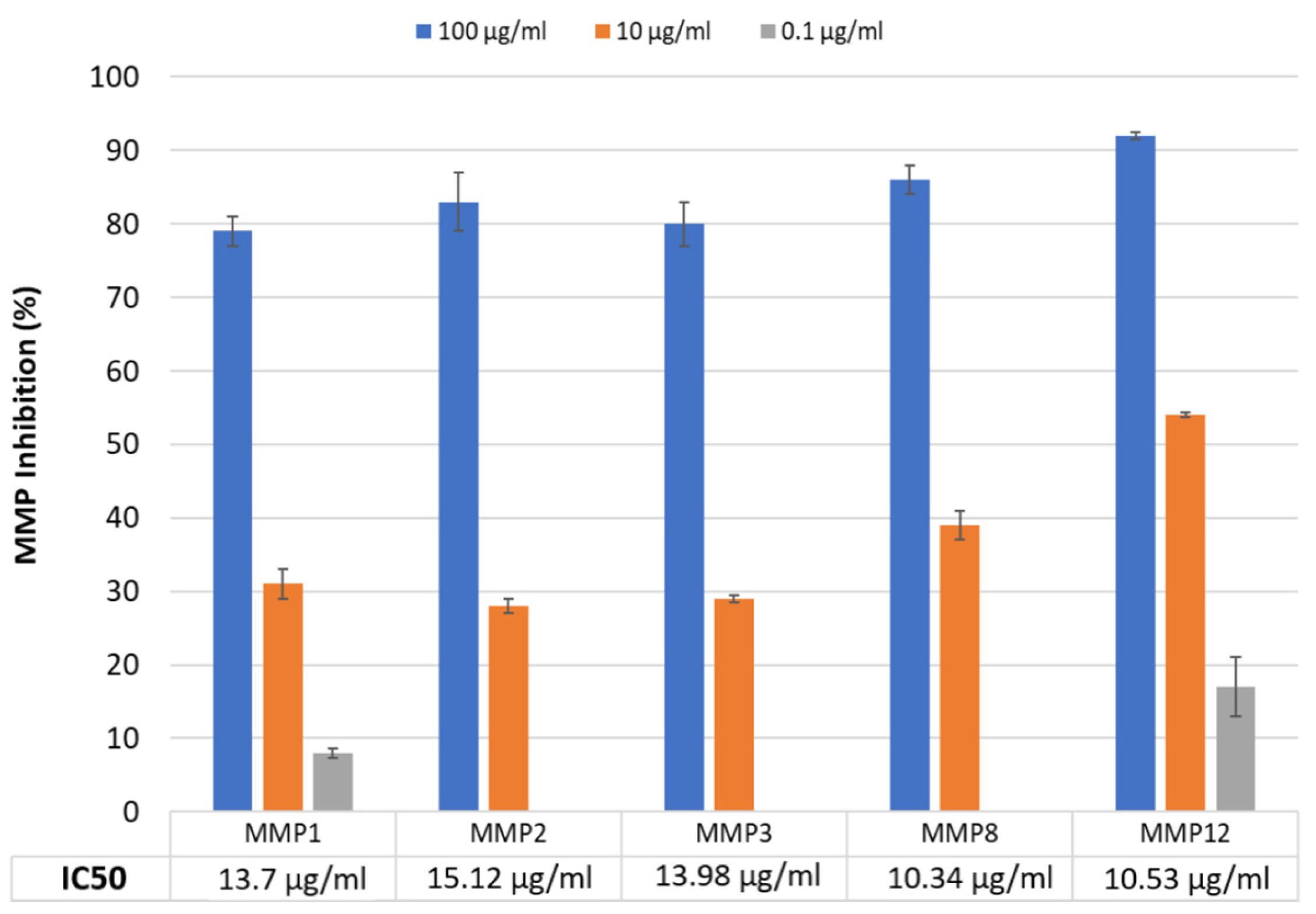
2.1. MMP Inhibition Study
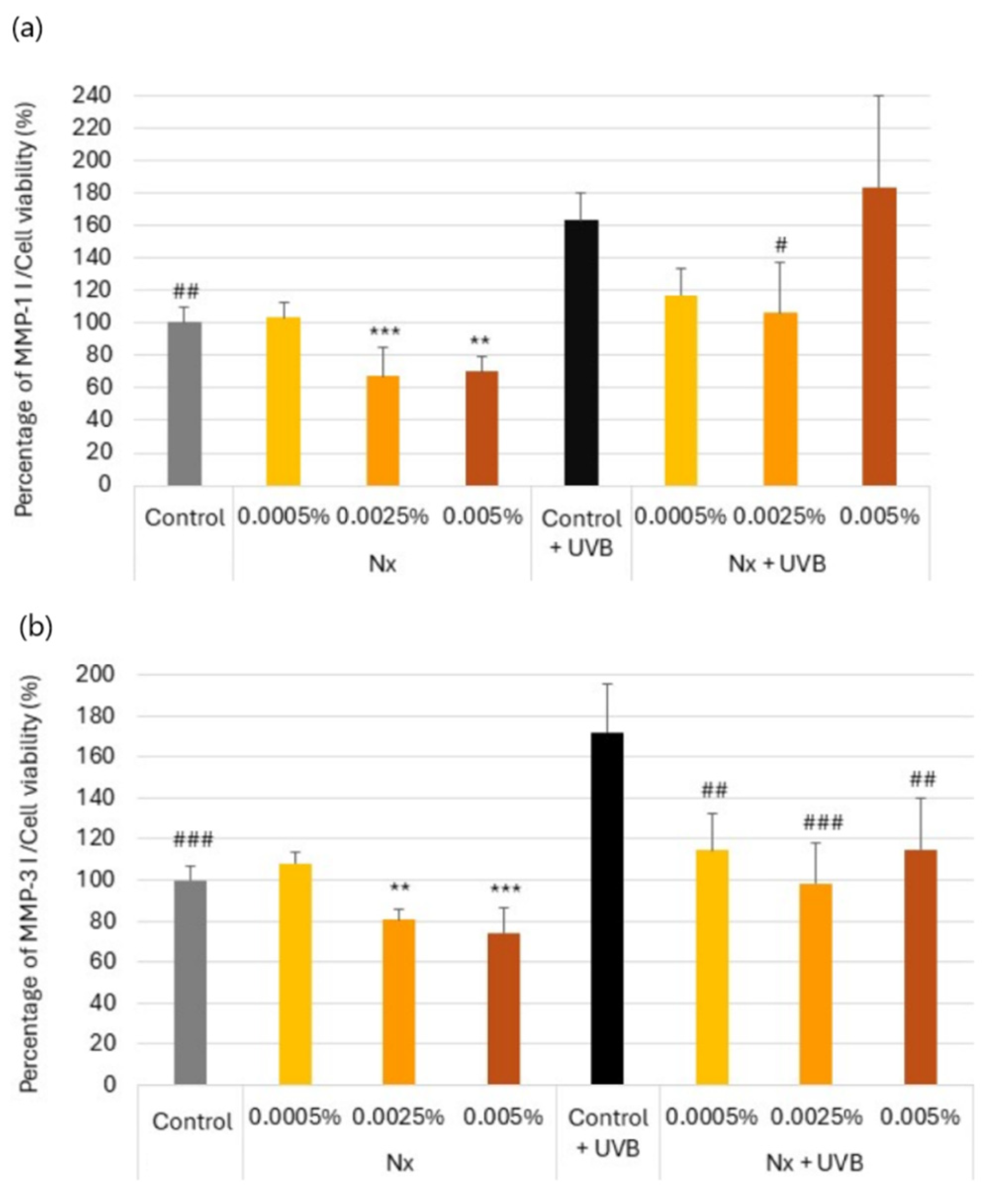
2.2. Regulation of MMP-1 and MMP-3 Secretion by Nutroxsun® in UV-Irradiated and Non-Irradiated Human Dermal Fibroblasts
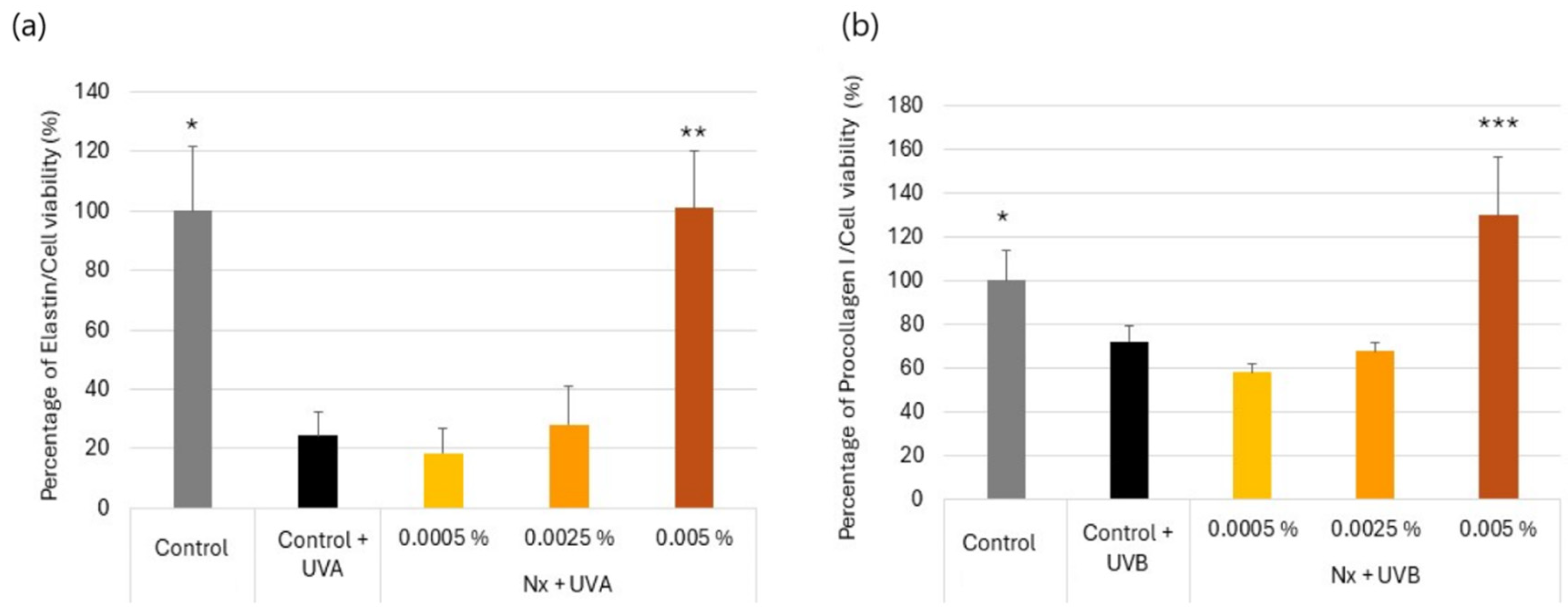
2.3. Effect of Nutroxsun® on the Secretion of Elastin and Procollagen Proteins in UVR-Exposed Human Dermal Fibroblasts
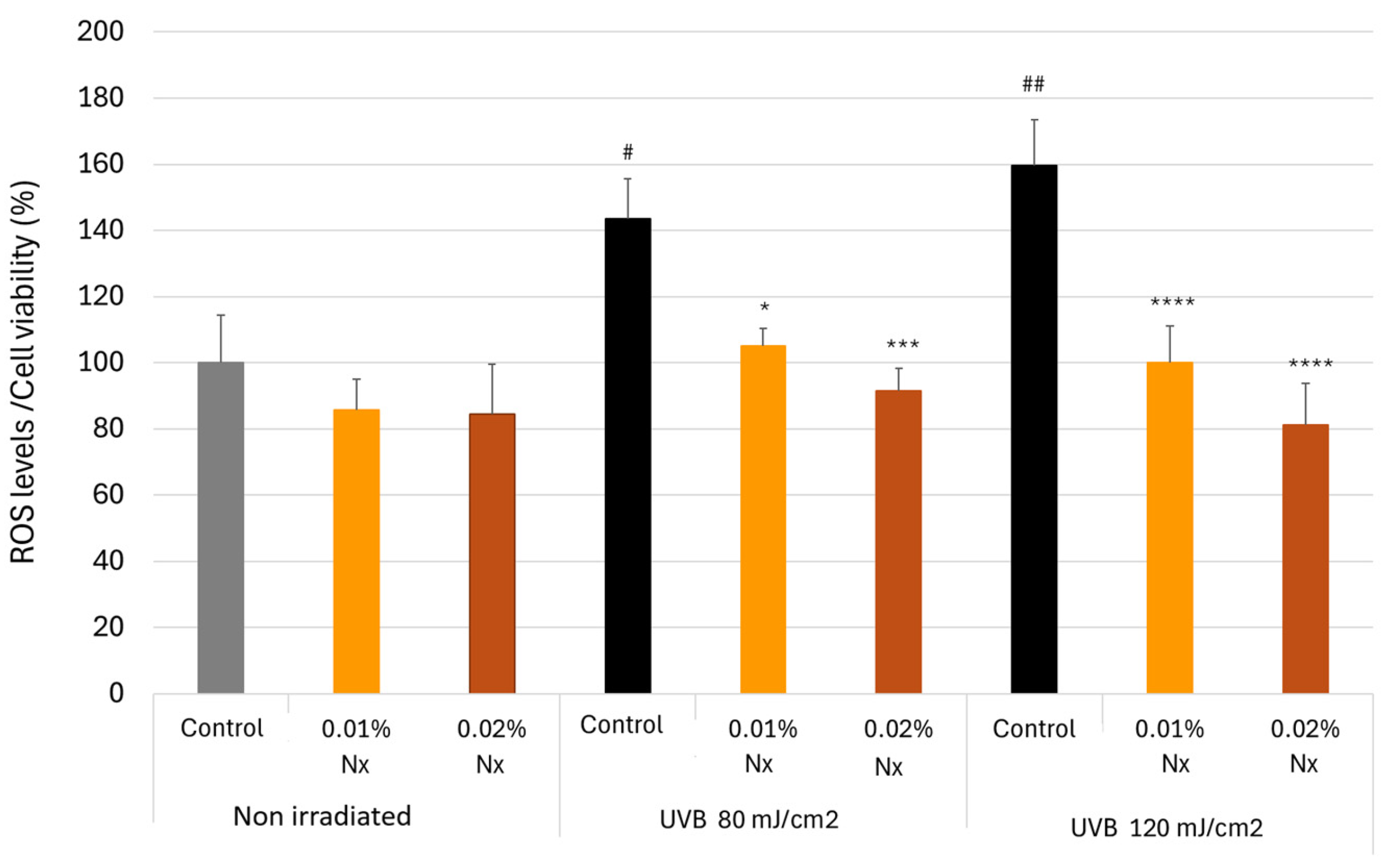
2.4. Impact of Nutroxsun® on ROS Generation in UVB-Irradiated Keratinocytes
2.5. Impact of Nutroxsun® on Interleukin Generation in UVB-Irradiated Keratinocytes
2.6. Placebo-Controlled Clinical Assessment Results
3. Discussion
4. Materials and Methods
4.1. Test Product
4.2. MMP Inhibition Study
4.3. Cellular Assays
4.3.1. Cell Cultures
4.3.2. Cell Viability
- For procollagen type I, elastin, MMP-1 and MMP-3 protein quantification (ELISA), and NHDFs were treated with the test product for 48 h.
- For the antioxidant and anti-inflammatory studies, HaCaT were treated with the test product for 2 h.
4.3.3. UV Radiation
4.3.4. Quantitation of MMP-1 and MMP-3 in Normal Human Dermal Fibroblasts
4.3.5. Quantification of Pro-Collagen I
4.3.6. Quantification of Elastin
4.3.7. Antioxidant Assessment by UVB-Induced Oxidative Stress
4.3.8. Anti-Inflammatory Assessment by UVB-Induced Cytokines
4.3.9. Data Processing and Statistical Analysis
4.4. Placebo-Controlled Clinical–Instrumental Assessment
4.4.1. Study Design
4.4.2. Subjects
4.4.3. Intervention
4.4.4. Sample Size
4.4.5. Randomization and Blinding
4.4.6. Efficacy Evaluation. Skin Redness Recovery
4.4.7. Safety
4.4.8. Statistical Analysis and Analytical Plan
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holick, M.F. Sunlight and Vitamin D for Bone Health and Prevention of Autoimmune Diseases, Cancers, and Cardiovascular Disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.B. Sunlight Has Cardiovascular Benefits Independently of Vitamin D. Blood Purif. 2016, 41, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Krutmann, J.; Andersen, M.; Katta, R.; Zouboulis, C. Clinical and Biological Impact of the Exposome on the Skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 4–25. [Google Scholar] [CrossRef]
- Greaves, M.W.; Sondergaard, J. Pharmacologic Agents Released in Ultraviolet Inflammation Studied by Continuous Skin Pefusion. J. Investig. Dermatol. 1970, 54, 365–367. [Google Scholar] [CrossRef]
- Benrath, J.; Eschenfelder, C.; Zimmerman, M.; Gillardon, F. Calcitonin Gene-Related Peptide, Substance P and Nitric Oxide Are Involved in Cutaneous Inflammation Following Ultraviolet Irradiation. Eur. J. Pharmacol. 1995, 293, 87–96. [Google Scholar] [CrossRef]
- Endoh, I.; Di Girolamo, N.; Hampartzoumian, T.; Cameron, B.; Geczy, C.L.; Tedla, N. Ultraviolet B Irradiation Selectively Increases the Production of Interleukin-8 in Human Cord Blood-Derived Mast Cells. Clin. Exp. Immunol. 2007, 148, 161–167. [Google Scholar] [CrossRef]
- Urbanski, A.; Schwarz, T.; Neuner, P.; Krutmann, J.; Kirnbauer, R.; Köck, A.; Luger, T.A. Ultraviolet Light Induces Increased Circulating Interleukin-6 in Humans. J. Investig. Dermatol. 1990, 94, 808–811. [Google Scholar] [CrossRef]
- Pecorelli, A.; Valacchi, G. Oxidative-Stress-Sensitive microRNAs in UV-Promoted Development of Melanoma. Cancers 2022, 14, 3224. [Google Scholar] [CrossRef] [PubMed]
- Kucera, R.; Topolcan, O.; Treskova, I.; Kinkorova, J.; Windrichova, J.; Fuchsova, R.; Svobodova, S.; Treska, V.; Babuska, V.; Novak, J.; et al. Evaluation of IL-2, IL-6, IL-8 and IL-10 in Malignant Melanoma Diagnostics. Anticancer Res. 2015, 35, 3537–3541. [Google Scholar]
- Bridge, J.A.; Lee, J.C.; Daud, A.; Wells, J.W.; Bluestone, J.A. Cytokines, Chemokines, and Other Biomarkers of Response for Checkpoint Inhibitor Therapy in Skin Cancer. Front. Med. 2018, 5, 351. [Google Scholar] [CrossRef]
- Wei, M.; He, X.; Liu, N.; Deng, H. Role of Reactive Oxygen Species in Ultraviolet-Induced Photodamage of the Skin. Cell Div. 2024, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef]
- Lee, C.-H.; Wu, S.-B.; Hong, C.-H.; Yu, H.-S.; Wei, Y.-H. Molecular Mechanisms of UV-Induced Apoptosis and Its Effects on Skin Residential Cells: The Implication in UV-Based Phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.M.; Lozano, J. Skin Collagen through the Lifestages: Importance for Skin Health and Beauty. Plast. Aesthetic Res. 2021, 8, 2. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Freitas-Rodríguez, S.; Folgueras, A.R.; López-Otín, C. The Role of Matrix Metalloproteinases in Aging: Tissue Remodeling and Beyond. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef]
- Quan, T.; Little, E.; Quan, H.; Qin, Z.; Voorhees, J.J.; Fisher, G.J. Elevated Matrix Metalloproteinases and Collagen Fragmentation in Photodamaged Human Skin: Impact of Altered Extracellular Matrix Microenvironment on Dermal Fibroblast Function. J. Investig. Dermatol. 2013, 133, 1362–1366. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-Degrading Metalloproteinases in Photoaging. J. Investig. Dermatol. Symp. Proc. Soc. Investig. Dermatol. Inc Eur. Soc. Dermatol. Res. 2009, 14, 20. [Google Scholar] [CrossRef]
- Parrado, C.; Philips, N.; Gilaberte, Y.; Juarranz, A.; González, S. Oral Photoprotection: Effective Agents and Potential Candidates. Front. Med. 2018, 5, 188. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. Nutricosmetics: A Brief Overview. Phytother. Res. PTR 2019, 33, 3054–3063. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.F.; Cervantes, E.L.; Luna-Vital, D.A.; Mojica, L. Food-Derived Bioactive Compounds with Anti-Aging Potential for Nutricosmetic and Cosmeceutical Products. Crit. Rev. Food Sci. Nutr. 2021, 61, 3740–3755. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for Skin Care: A Comprehensive Review of Human Clinical Studies. Nutrients 2018, 10, 403. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Alcaraz, M.; Micol, V. Protective Effects of Citrus and Rosemary Extracts on UV-Induced Damage in Skin Cell Model and Human Volunteers. J. Photochem. Photobiol. B 2014, 136, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Nobile, V.; Michelotti, A.; Cestone, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Pérez-Sánchez, A.; Micol, V. Skin Photoprotective and Antiageing Effects of a Combination of Rosemary (Rosmarinus Officinalis) and Grapefruit (Citrus Paradisi) Polyphenols. Food Nutr. Res. 2016, 60, 31871. [Google Scholar] [CrossRef]
- Murphy, G.; Docherty, A.J. The Matrix Metalloproteinases and Their Inhibitors. Am. J. Respir. Cell Mol. Biol. 1992, 7, 120–125. [Google Scholar] [CrossRef]
- Woessner, J.F. Matrix Metalloproteinases and Their Inhibitors in Connective Tissue Remodeling. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1991, 5, 2145–2154. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. Matrix Metalloproteinases (MMPs): Chemical-Biological Functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef]
- Li, D.-Q.; Lee, S.-B.; Gunja-Smith, Z.; Liu, Y.; Solomon, A.; Meller, D.; Tseng, S.C.G. Overexpression of Collagenase (MMP-1) and Stromelysin (MMP-3) by Pterygium Head Fibroblasts. Arch. Ophthalmol. 2001, 119, 71–80. [Google Scholar]
- Weihermann, A.C.; Lorencini, M.; Brohem, C.A.; de Carvalho, C.M. Elastin Structure and Its Involvement in Skin Photoageing. Int. J. Cosmet. Sci. 2017, 39, 241–247. [Google Scholar] [CrossRef]
- Uitto, J. The Role of Elastin and Collagen in Cutaneous Aging: Intrinsic Aging versus Photoexposure. J. Drugs Dermatol. JDD 2008, 7, s12–s16. [Google Scholar] [PubMed]
- Fleischmajer, R.; Perlish, J.S.; Timpl, R. Collagen Fibrillogenesis in Human Skin. Ann. N. Y. Acad. Sci. 1985, 460, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Eghbali, S.; Askari, S.F.; Avan, R.; Sahebkar, A. Therapeutic Effects of Punica Granatum (Pomegranate): An Updated Review of Clinical Trials. J. Nutr. Metab. 2021, 2021, 5297162. [Google Scholar] [CrossRef]
- Heck, D.E.; Vetrano, A.M.; Mariano, T.M.; Laskin, J.D. UVB Light Stimulates Production of Reactive Oxygen Species: UNEXPECTED ROLE FOR CATALASE*. J. Biol. Chem. 2003, 278, 22432–22436. [Google Scholar] [CrossRef] [PubMed]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the Probe 2′,7′-Dichlorofluorescin as an Indicator of Reactive Oxygen Species Formation and Oxidative Stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef]
- Ansel, J.; Perry, P.; Brown, J.; Damm, D.; Phan, T.; Hart, C.; Luger, T.; Hefeneider, S. Cytokine Modulation of Keratinocyte Cytokines. J. Investig. Dermatol. 1990, 94, 101S–107S. [Google Scholar] [CrossRef]
- Meephansan, J.; Komine, M.; Tsuda, H.; Tominaga, S.-I.; Ohtsuki, M. Ultraviolet B Irradiation Induces the Expression of IL-33 mRNA and Protein in Normal Human Epidermal Keratinocytes. J. Dermatol. Sci. 2012, 65, 72–74. [Google Scholar] [CrossRef]
- Benrath, J.; Gillardon, F.; Zimmermann, M. Differential Time Courses of Skin Blood Flow and Hyperalgesia in the Human Sunburn Reaction Following Ultraviolet Irradiation of the Skin. Eur. J. Pain Lond. Engl. 2001, 5, 155–167. [Google Scholar] [CrossRef]
- Ms, D.; Rf, W. Clinical Management of the Acute Sunburn Reaction. Cutis 2000, 66, 53–58. [Google Scholar]
- Kasamatsu, S.; Takano, K.; Aoki, M.; Takahashi, Y.; Suzuki, T. Rosemary Extract and Rosmarinic Acid Accelerate Elastic Fiber Formation by Increasing the Expression of Elastic Fiber Components in Dermal Fibroblasts. J. Dermatol. 2024, 51, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Okano, Y.; Masaki, H. An Ocimum Basilicum Extract Containing Rosmarinic Acid Restores the Disruption of Collagen Fibers Caused by Repetitive UVA Irradiation of Dermal Fibroblasts. J. Oleo Sci. 2020, 69, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Sutkowska, J.; Hupert, N.; Gawron, K.; Strawa, J.W.; Tomczyk, M.; Forlino, A.; Galicka, A. The Stimulating Effect of Rosmarinic Acid and Extracts from Rosemary and Lemon Balm on Collagen Type I Biosynthesis in Osteogenesis Imperfecta Type I Skin Fibroblasts. Pharmaceutics 2021, 13, 938. [Google Scholar] [CrossRef]
- Martin, R.; Pierrard, C.; Lejeune, F.; Hilaire, P.; Breton, L.; Bernerd, F. Photoprotective Effect of a Water-Soluble Extract of Rosmarinus Officinalis L. against UV-Induced Matrix Metalloproteinase-1 in Human Dermal Fibroblasts and Reconstructed Skin. Eur. J. Dermatol. EJD 2008, 18, 128–135. [Google Scholar] [PubMed]
- Park, M.; Han, J.; Lee, C.S.; Heung Soo, B.; Lim, K.-M.; Ha, H. Carnosic Acid, a Phenolic Diterpene from Rosemary, Prevents UV-Induced Expression of Matrix Metalloproteinases in Human Skin Fibroblasts and Keratinocytes. Exp. Dermatol. 2013, 22, 336–341. [Google Scholar] [CrossRef]
- Das, R.N.; Balupillai, A.; David, E.; Santhoshkumar, M.; Muruhan, S. Naringin, a Natural Flavonoid, Modulates UVB Radiation-Induced DNA Damage and Photoaging by Modulating NER Repair and MMPS Expression in Mouse Embryonic Fibroblast Cells. J. Environ. Pathol. Toxicol. Oncol. Off. Organ Int. Soc. Environ. Toxicol. Cancer 2020, 39, 191–199. [Google Scholar] [CrossRef]
- Young, A.R.; Tewari, A. Sunburn In UpToDate. Dellavalle R.P. (Ed), UpToDate, Waltham, MA, USA. Available online: https://www.uptodate.com/contents/sunburn?search=sunburn&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 13 November 2024).
- Doolaege, E.H.A.; Raes, K.; De Vos, F.; Verhé, R.; De Smet, S. Absorption, Distribution and Elimination of Carnosic Acid, A Natural Antioxidant from Rosmarinus Officinalis, in Rats. Plant Foods Hum. Nutr. 2011, 66, 196–202. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic Acid–Human Pharmacokinetics and Health Benefits. Planta Med. 2020, 87, 273–282. [Google Scholar] [CrossRef]
- Noguchi-Shinohara, M.; Ono, K.; Hamaguchi, T.; Iwasa, K.; Nagai, T.; Kobayashi, S.; Nakamura, H.; Yamada, M. Pharmacokinetics, Safety and Tolerability of Melissa Officinalis Extract Which Contained Rosmarinic Acid in Healthy Individuals: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0126422. [Google Scholar] [CrossRef]
- Mata-Bilbao, M.d.L.; Andrés-Lacueva, C.; Roura, E.; Jáuregui, O.; Escribano, E.; Torre, C.; Lamuela-Raventós, R.M. Absorption and Pharmacokinetics of Grapefruit Flavanones in Beagles. Br. J. Nutr. 2007, 98, 86–92. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Bounartzi, M.I.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the Citrus Flavanone Aglycones Hesperetin and Naringenin after Single Oral Administration in Human Subjects. Eur. J. Clin. Nutr. 2007, 61, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Strickland, I.; Rhodes, L.E.; Flanagan, B.F.; Friedmann, P.S. TNF-α and IL-8 Are Upregulated in the Epidermis of Normal Human Skin after UVB Exposure: Correlation with Neutrophil Accumulation and E-Selectin Expression. J. Investig. Dermatol. 1997, 108, 763–768. [Google Scholar] [CrossRef]
- Steude, J.; Kulke, R.; Christophers, E. Interleukin-1-Stimulated Secretion of Interleukin-8 and Growth-Related Oncogene-α Demonstrates Greatly Enhanced Keratinocyte Growth in Human Raft Cultured Epidermis. J. Investig. Dermatol. 2002, 119, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Mills, L.; Mian, B.; Tellez, C.; McCarty, M.; Yang, X.-D.; Gudas, J.M.; Bar-Eli, M. Fully Humanized Neutralizing Antibodies to Interleukin-8 (ABX-IL8) Inhibit Angiogenesis, Tumor Growth, and Metastasis of Human Melanoma. Am. J. Pathol. 2002, 161, 125–134. [Google Scholar] [CrossRef]
- Storey, A.; McArdle, F.; Friedmann, P.S.; Jackson, M.J.; Rhodes, L.E. Eicosapentaenoic Acid and Docosahexaenoic Acid Reduce UVB- and TNF-α-Induced IL-8 Secretion in Keratinocytes and UVB-Induced IL-8 in Fibroblasts. J. Investig. Dermatol. 2005, 124, 248–255. [Google Scholar] [CrossRef]
- Nasti, T.H.; Timares, L. Inflammasome Activation of IL-1 Family Mediators in Response to Cutaneous Photodamage. Photochem. Photobiol. 2012, 88, 1111. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T. UV Radiation and Cytokines. In Skin Cancer and UV Radiation; Altmeyer, P., Hoffmann, K., Stücker, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 219–226. [Google Scholar] [CrossRef]
- Yoon, Y.-C.; Choi, H.-J.; Park, J.-H.; Diniyah, N.; Shin, H.-A.; Kim, M.-Y. Combination of Grapefruit and Rosemary Extracts Has Skin Protective Effect through MMPs, MAPKs, and the NF-κB Signaling Pathway In Vitro and In Vivo UVB-Exposed Model. Korean J. Plant Resour. 2019, 32, 633–643. [Google Scholar] [CrossRef]
- Lim, H.W.; Arellano-Mendoza, M.-I.; Stengel, F. Current Challenges in Photoprotection. J. Am. Acad. Dermatol. 2017, 76, S91–S99. [Google Scholar] [CrossRef]
- Reis-Mansur, M.C.P.P.; da Luz, B.G.; dos Santos, E.P. Consumer Behavior, Skin Phototype, Sunscreens, and Tools for Photoprotection: A Review. Cosmetics 2023, 10, 39. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- AAT Bioquest, Inc. Quest GraphTM IC50 Calculator. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 29 October 2024).
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef] [PubMed]
- ISO 24444:2019. Available online: https://www.iso.org/standard/72250.html (accessed on 29 October 2024).
- Commission Internationale de l’’Éclairage (CIE). Colorimetry, 2nd ed.; CIE Publication No. 15.2; CIE: Vienna, Austria, 1976. [Google Scholar]

 product intake. Statistically significant vs. placebo is reported as * p < 0.05 and ** p < 0.01.
product intake. Statistically significant vs. placebo is reported as * p < 0.05 and ** p < 0.01.
 product intake. Statistically significant vs. placebo is reported as * p < 0.05 and ** p < 0.01.
product intake. Statistically significant vs. placebo is reported as * p < 0.05 and ** p < 0.01.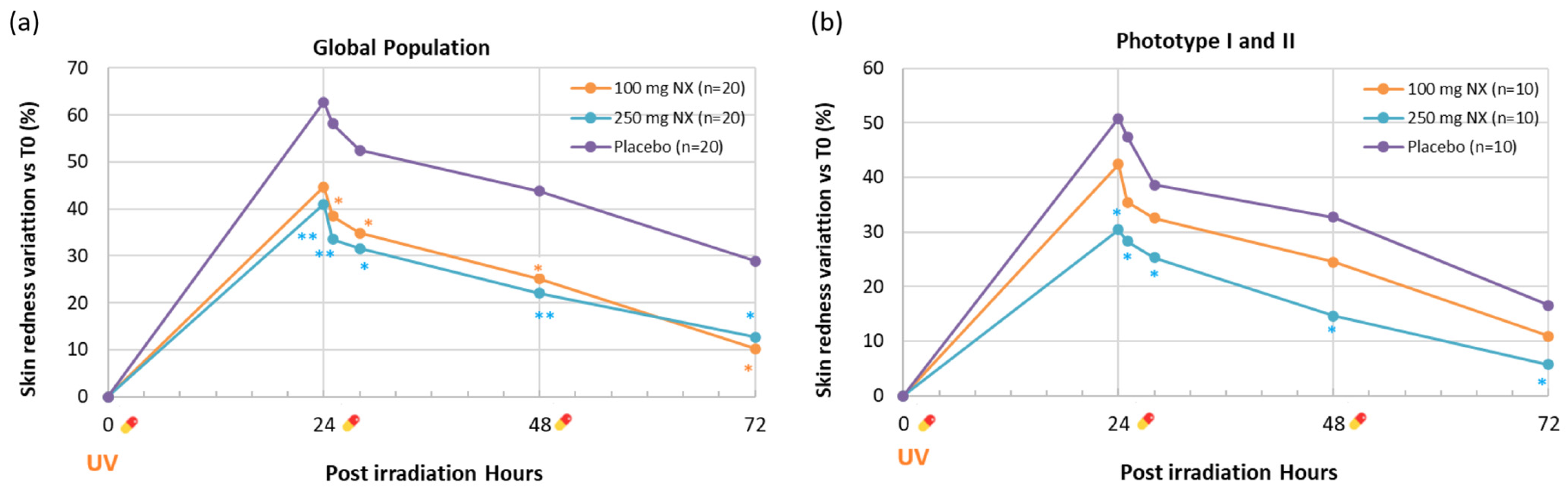
 information,
information,  physical examination,
physical examination,  informed consent signature,
informed consent signature,  eligibility check,
eligibility check,  randomization, R skin redness,
randomization, R skin redness,  product intake.
product intake.
 information,
information,  physical examination,
physical examination,  informed consent signature,
informed consent signature,  eligibility check,
eligibility check,  randomization, R skin redness,
randomization, R skin redness,  product intake.
product intake.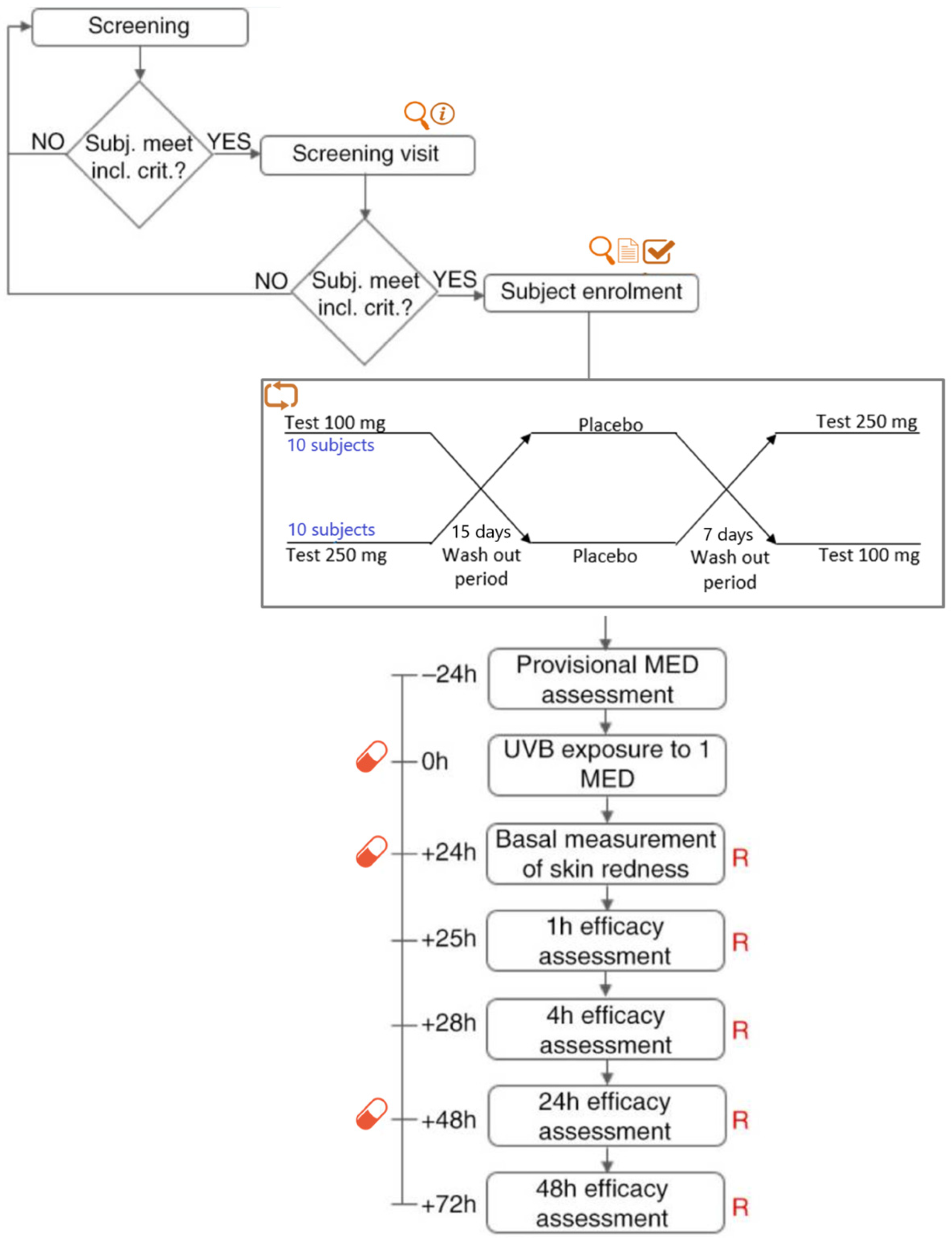
| Metalloproteinases | Substrates | |
|---|---|---|
| MMP-1 | Interstitial collagenase | Collagen I, II, III, VII, VIII, IX, gelatin, etaktyn, aggrecan |
| MMP-2 | Gelatinase A | Collagen I, IV, V, VII, X, XI, XIV, gelatin, fibronectin, laminin, agrecan, elastin |
| MMP-3 | Stromelysin 1 | Collagen III, IV, V, IX, X, XI, gelatin, laminin, fibronectin, elastin, agrecan, cazein, tenascin |
| MMP-8 | Neutrophil collagenase | Collagen types I, III, gelatin, fibronectin |
| MMP-12 | Macrophage elastase | Elastin, fibronectin, laminin, collagen types IV, V, gelatin, casein |
| Panel | Units | |
|---|---|---|
| Sex | ||
| Male | 5 | No. |
| Female | 15 | No. |
| Skin phototypes | ||
| I | 10% | % |
| II | 40% | % |
| III | 50% | % |
| Age | 45.1 ± 3.1 | years |
| Skin erythema (basal) | 8.1 ± 0.3 | a.u. |
| Global Panel | 0 h | 24 h | 25 h | 28 h | 48 h | 72 h |
|---|---|---|---|---|---|---|
| 100 mg NX (n = 20) | 8.1 ± 0.3 | 11.7 ± 0.5 | 11.2 ± 0.4 | 11.0 ± 0.5 | 10.2 ± 0.5 | 9.0 ± 0.5 |
| (+44.7%) | (+38.4% *) | (+34.8% *) | (+25.1% *) | (+10.3% *) | ||
| 250 mg NX (n = 20) | 8.3 ± 0.3 | 11.5 ± 0.4 | 11.0 ± 0.4 | 10.8 ± 0.5 | 10.1 ± 0.4 | 9.3 ± 0.5 |
| (+41.0% **) | (+33.5% **) | (+31.6% *) | (+22.0% **) | (+12.7% *) | ||
| Placebo (n = 20) | 8.0 ± 0.4 | 12.4 ± 0.2 | 12.1 ± 0.2 | 11.7 ± 0.2 | 11.0 ± 0.2 | 9.9 ± 0.2 |
| (+62.7%) | (+58.1%) | (+52.5%) | (+43.8%) | (+28.9%) | ||
| Phototype I and II subgroup | ||||||
| 100 mg NX (n = 10) | 8.5 ± 0.4 | 12.0 ± 0.6 | 11.5 ± 0.5 | 11.2 ± 0.6 | 10.6 ± 0.6 | 9.5 ± 0.6 |
| (+42.4%) | (+35.4%) | (+32.5%) | (+24.5%) | (+10.9%) | ||
| 250 mg NX (n =10) | 8.8 ± 0.4 | 11.3 ± 0.5 | 11.1 ± 0.4 | 10.8 ± 0.5 | 9.9 ± 0.4 | 9.2 ± 0.5 |
| (+30.4% *) | (+28.3% *) | (+25.3% *) | (+14.6% *) | (+5.7% *) | ||
| Placebo (n = 10) | 8.5 ± 0.6 | 12.4 ± 0.4 | 12.1 ± 0.4 | 11.4 ± 0.3 | 11.0 ± 0.3 | 9.7 ± 0.3 |
| (+50.8%) | (+47.4%) | (+38.6%) | (+32.7%) | (+16.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, P.; Castillo, J.; Jones, J.; García, A.; Caturla, N. Skin Photoprotection and Anti-Aging Benefits of a Combination of Rosemary and Grapefruit Extracts: Evidence from In Vitro Models and Human Study. Int. J. Mol. Sci. 2025, 26, 4001. https://doi.org/10.3390/ijms26094001
Navarro P, Castillo J, Jones J, García A, Caturla N. Skin Photoprotection and Anti-Aging Benefits of a Combination of Rosemary and Grapefruit Extracts: Evidence from In Vitro Models and Human Study. International Journal of Molecular Sciences. 2025; 26(9):4001. https://doi.org/10.3390/ijms26094001
Chicago/Turabian StyleNavarro, Pau, Julián Castillo, Jonathan Jones, Adrián García, and Nuria Caturla. 2025. "Skin Photoprotection and Anti-Aging Benefits of a Combination of Rosemary and Grapefruit Extracts: Evidence from In Vitro Models and Human Study" International Journal of Molecular Sciences 26, no. 9: 4001. https://doi.org/10.3390/ijms26094001
APA StyleNavarro, P., Castillo, J., Jones, J., García, A., & Caturla, N. (2025). Skin Photoprotection and Anti-Aging Benefits of a Combination of Rosemary and Grapefruit Extracts: Evidence from In Vitro Models and Human Study. International Journal of Molecular Sciences, 26(9), 4001. https://doi.org/10.3390/ijms26094001





