Exploring the Genetic and Functional Diversity of Porphyromonas gingivalis Survival Factor RagAB
Abstract
:1. Introduction
2. Results
2.1. Developing a Universal RagA-Gene-Directed PCR Method
2.2. Genome Mapping Results
2.3. Phylogenetic Tree Building
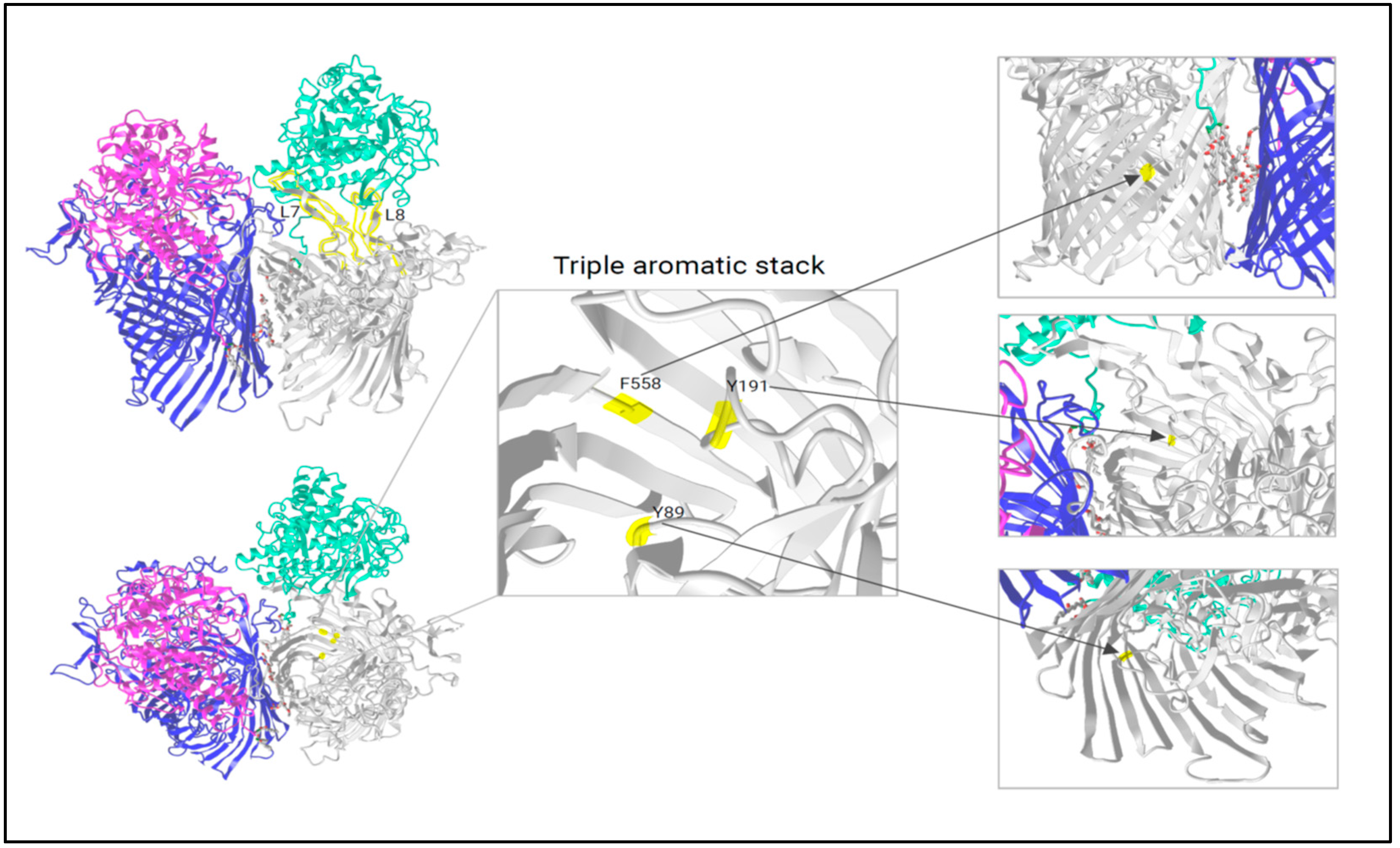
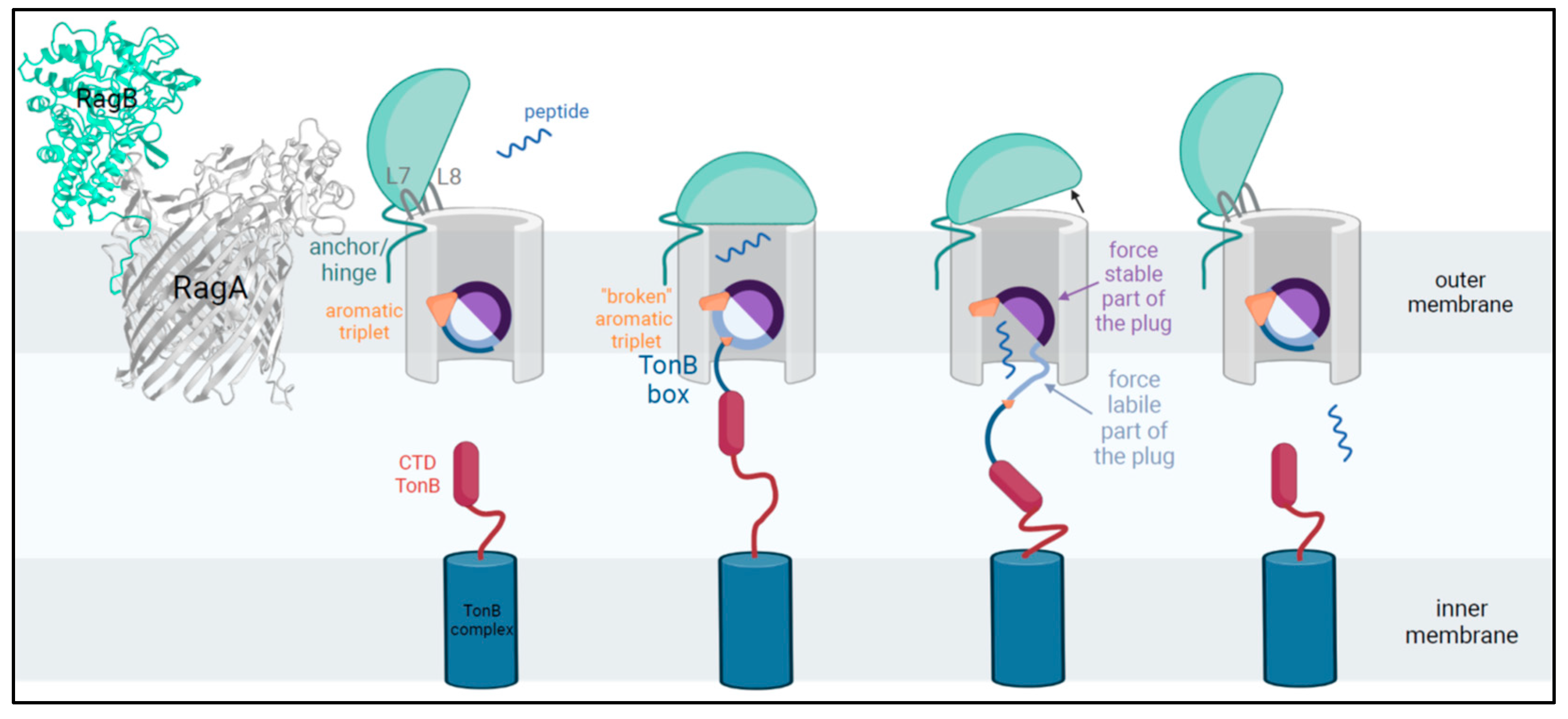
2.4. Analysis of RagA Functional Domains
2.5. Analysis of RagB Functional Domains
3. Discussion
3.1. Benefits and Limits of Universal RagA-Gene-Directed PCR Method
3.2. New Insights into RagAB Phylogeny and Evolution
3.3. RagAB as Factors of Virulence and Fitness
3.4. Substrate Specificity and Binding/Uptake Capacity as Explanation for Virulence
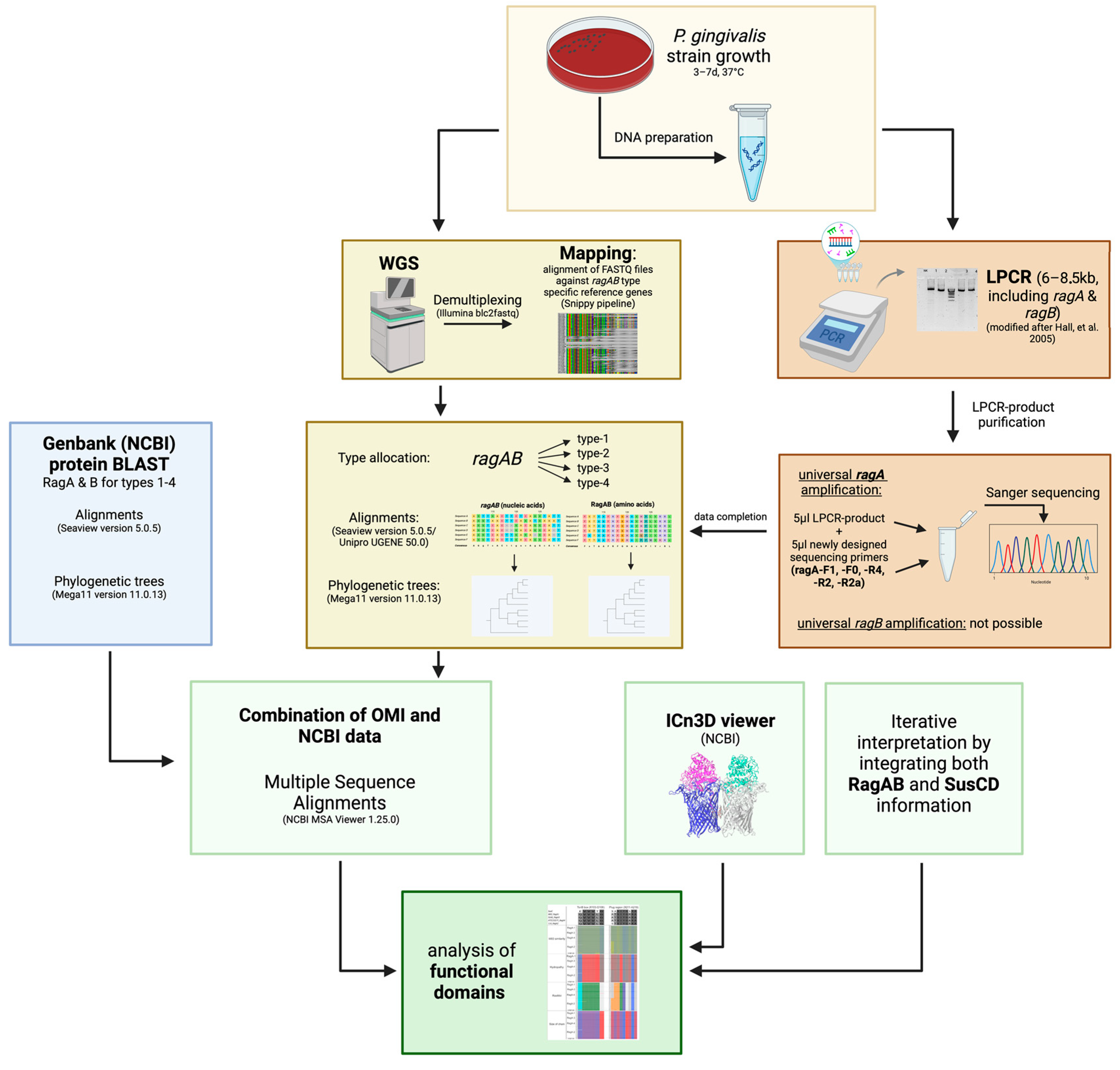
4. Material and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Genomic DNA (gDNA) Extraction
4.3. Comparative Genomics and Phylogeny of RagAB
4.4. Development of Universal RagA-Gene-Directed PCR Method
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conrads, G.; Nagy, E.; Könönen, E. Bacteroides, Porphyromonas, Prevotella, Fusobacterium, and Other Anaerobic Gram-Negative Rods. In Manual of Clinical Microbiology, 12th ed.; Caroll, K., Pfaller, M.A., Eds.; ASM Press: Washington, DC, USA, 2019. [Google Scholar]
- Henry, L.G.; McKenzie, R.M.; Robles, A.; Fletcher, H.M. Oxidative stress resistance in Porphyromonas gingivalis. Future Microbiol. 2012, 7, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.J.; France, J.; Crean, S.; Singhrao, S.K. The Porphyromonas gingivalis/host interactome shows enrichment in GWASdb genes related to Alzheimer’s Disease, Diabetes and Cardiovascular Diseases. Front. Aging Neurosci. 2017, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Potempa, J.; Mydel, P.; Koziel, J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat. Rev. Rheumatol. 2017, 13, 606–620. [Google Scholar] [CrossRef]
- Miyauchi, M.; Ao, M.; Furusho, H.; Chea, C.; Nagasaki, A.; Sakamoto, S.; Ando, T.; Inubushi, T.; Kozai, K.; Takata, T. Galectin-3 plays an important role in preterm birth caused by dental infection of Porphyromonas gingivalis. Sci. Rep. 2018, 8, 2867. [Google Scholar] [CrossRef]
- Meyer, H.L.; Abdelbary, M.M.H.; Buhl, E.M.; Kuppe, C.; Conrads, G. Exploring the genetic and functional diversity of Porphyromonas gingivalis long fimbriae. Mol. Oral. Microbiol. 2023, 38, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Potempa, J.; Madej, M.; Scott, D.A. The RagA and RagB proteins of Porphyromonas gingivalis. Mol. Oral. Microbiol. 2021, 36, 225–232. [Google Scholar] [CrossRef]
- Shi, X.; Hanley, S.A.; Faray-Kele, M.C.; Fawell, S.C.; Aduse-Opoku, J.; Whiley, R.A.; Curtis, M.A.; Hall, L.M. The rag locus of Porphyromonas gingivalis contributes to virulence in a murine model of soft tissue destruction. Infect. Immun. 2007, 75, 2071–2074. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, E.V.; Poulsen, K.; Curtis, M.A.; Kilian, M. Evidence of recombination in Porphyromonas gingivalis and random distribution of putative virulence markers. Infect. Immun. 2001, 69, 4479–4485. [Google Scholar] [CrossRef]
- Hanley, S.A.; Aduse-Opoku, J.; Curtis, M.A. A 55-kilodalton immunodominant antigen of Porphyromonas gingivalis W50 has arisen via horizontal gene transfer. Infect. Immun. 1999, 67, 1157–1171. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Hanley, S.A.; Aduse-Opoku, J. The rag locus of Porphyromonas gingivalis: A novel pathogenicity island. J. Periodontal Res. 1999, 34, 400–405. [Google Scholar] [CrossRef]
- Nagano, K.; Murakami, Y.; Nishikawa, K.; Sakakibara, J.; Shimozato, K.; Yoshimura, F. Characterization of RagA and RagB in Porphyromonas gingivalis: Study using gene-deletion mutants. J. Med. Microbiol. 2007, 56, 1536–1548. [Google Scholar] [CrossRef] [PubMed]
- Dashper, S.G.; Mitchell, H.L.; Seers, C.A.; Gladman, S.L.; Seemann, T.; Bulach, D.M.; Chandry, P.S.; Cross, K.J.; Cleal, S.M.; Reynolds, E.C. Porphyromonas gingivalis uses specific domain rearrangements and allelic exchange to generate diversity in surface virulence factors. Front. Microbiol. 2017, 8, 48. [Google Scholar] [CrossRef]
- Hall, L.M.; Fawell, S.C.; Shi, X.; Faray-Kele, M.C.; Aduse-Opoku, J.; Whiley, R.A.; Curtis, M.A. Sequence diversity and antigenic variation at the rag locus of Porphyromonas gingivalis. Infect. Immun. 2005, 73, 4253–4262. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Wang, L.; Guo, Y.; Xiao, S. Prevalence of Porphyromonas gingivalis four rag locus genotypes in patients of orthodontic gingivitis and periodontitis. PLoS ONE 2013, 8, e61028. [Google Scholar] [CrossRef] [PubMed]
- Madej, M.; White, J.B.R.; Nowakowska, Z.; Rawson, S.; Scavenius, C.; Enghild, J.J.; Bereta, G.P.; Pothula, K.; Kleinekathoefer, U.; Basle, A.; et al. Structural and functional insights into oligopeptide acquisition by the RagAB transporter from Porphyromonas gingivalis. Nat. Microbiol. 2020, 5, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Nakayama, K.; Okamoto, K.; Abe, N.; Baba, A.; Shi, Y.; Ratnayake, D.B.; Yamamoto, K. Porphyromonas gingivalis proteinases as virulence determinants in progression of periodontal diseases. J. Biochem. 2000, 128, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Reeves, A.R.; Wang, G.R.; Salyers, A.A. Characterization of four outer membrane proteins that play a role in utilization of starch by Bacteroides thetaiotaomicron. J. Bacteriol. 1997, 179, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Kommedal, O.; Karlsen, B.; Saebo, O. Analysis of mixed sequencing chromatograms and its application in direct 16S rRNA gene sequencing of polymicrobial samples. J. Clin. Microbiol. 2008, 46, 3766–3771. [Google Scholar] [CrossRef] [PubMed]
- Böcher, S.; Meyer, H.L.; Dafni, E.; Conrads, G. Prevalence and phylogenetic analysis of lipoprotein-Gene ragB-1 of Porphyromonas gingivalis-A pilot study. Antibiotics 2023, 12, 1458. [Google Scholar] [CrossRef] [PubMed]
- White, J.B.R.; Silale, A.; Feasey, M.; Heunis, T.; Zhu, Y.; Zheng, H.; Gajbhiye, A.; Firbank, S.; Basle, A.; Trost, M.; et al. Outer membrane utilisomes mediate glycan uptake in gut Bacteroidetes. Nature 2023, 618, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Montz, P. 2025. Figure 1: RagAB Functional Domains During Substrate Transport. Created in BioRender. Available online: https://BioRender.com/o50b721 (accessed on 21 January 2025).
- Montz, P. 2025. Figure 2: TonB-Dependent Peptide-Substrate Transport in Porphyromonas gingivalis. Created in BioRender. Available online: https://BioRender.com/u76z058 (accessed on 21 January 2025).
- Goulas, T.; Garcia-Ferrer, I.; Hutcherson, J.A.; Potempa, B.A.; Potempa, J.; Scott, D.A.; Gomis-Ruth, F.X. Structure of RagB, a major immunodominant outer-membrane surface receptor antigen of Porphyromonas gingivalis. Mol. Oral. Microbiol. 2016, 31, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Chiu, C.H.; Nagano, K. Transcriptional analysis of the mfa-cluster genes in Porphyromonas gingivalis strains with one and two mfa5 genes. Mol. Oral. Microbiol. 2023, 38, 41–47. [Google Scholar] [CrossRef]
- Snyder, T.M.; Tse, B.N.; Liu, D.R. Effects of template sequence and secondary structure on DNA-templated reactivity. J. Am. Chem. Soc. 2008, 130, 1392–1401. [Google Scholar] [CrossRef]
- Conrads, G.; Klomp, T.; Deng, D.; Wenzler, J.S.; Braun, A.; Abdelbary, M.M.H. The Antimicrobial Susceptibility of Porphyromonas gingivalis: Genetic Repertoire, Global Phenotype, and Review of the Literature. Antibiotics 2021, 10, 1438. [Google Scholar] [CrossRef] [PubMed]
- Bivand, J.M.; Dyrhovden, R.; Sivertsen, A.; Tellevik, M.G.; Patel, R.; Kommedal, O. Broad-range amplification and sequencing of the rpoB gene: A novel assay for bacterial identification in clinical microbiology. J. Clin. Microbiol. 2024, 62, e0026624. [Google Scholar] [CrossRef] [PubMed]
- Izydorczyk, C.; Waddell, B.J.; Thornton, C.S.; Conly, J.M.; Rabin, H.R.; Somayaji, R.; Surette, M.G.; Church, D.L.; Parkins, M.D. Stenotrophomonas maltophilia natural history and evolution in the airways of adults with cystic fibrosis. Front. Microbiol. 2023, 14, 1205389. [Google Scholar] [CrossRef]
- Baishya, J.; Wakeman, C.A. Selective pressures during chronic infection drive microbial competition and cooperation. NPJ Biofilms Microbiomes 2019, 5, 16. [Google Scholar] [CrossRef]
- Abdelbary, M.M.H.; Hatting, M.; Dahlhausen, A.; Bott, A.; Conrads, G. Insights into Within-Host Evolution and Dynamics of Oral and Intestinal Streptococci Unveil Niche Adaptation. Int. J. Mol. Sci. 2024, 25, 13507. [Google Scholar] [CrossRef] [PubMed]
- Diard, M.; Hardt, W.D. Evolution of bacterial virulence. FEMS Microbiol. Rev. 2017, 41, 679–697. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, G.; Basic, A.; Bylund, J. Importance of virulence factors for the persistence of oral bacteria in the inflamed gingival crevice and in the pathogenesis of Periodontal Disease. J. Clin. Med. 2019, 8, 1339. [Google Scholar] [CrossRef]
- Fujiwara-Takahashi, K.; Watanabe, T.; Shimogishi, M.; Shibasaki, M.; Umeda, M.; Izumi, Y.; Nakagawa, I. Phylogenetic diversity in fim and mfa gene clusters between Porphyromonas gingivalis and Porphyromonas gulae, as a potential cause of host specificity. J. Oral. Microbiol. 2020, 12, 1775333. [Google Scholar] [CrossRef]
- Kim, Y.H.; O’Neill, H.M.; Whitehead, J.P. Carboxypeptidase X-1 (CPX-1) is a secreted collagen-binding glycoprotein. Biochem. Biophys. Res. Commun. 2015, 468, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Glenwright, A.J.; Pothula, K.R.; Bhamidimarri, S.P.; Chorev, D.S.; Basle, A.; Firbank, S.J.; Zheng, H.; Robinson, C.V.; Winterhalter, M.; Kleinekathofer, U.; et al. Structural basis for nutrient acquisition by dominant members of the human gut microbiota. Nature 2017, 541, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Pollet, R.M.; Martin, L.M.; Koropatkin, N.M. TonB-dependent transporters in the Bacteroidetes: Unique domain structures and potential functions. Mol. Microbiol. 2021, 115, 490–501. [Google Scholar] [CrossRef]
- Hutcherson, J.A.; Bagaitkar, J.; Nagano, K.; Yoshimura, F.; Wang, H.; Scott, D.A. Porphyromonas gingivalis RagB is a proinflammatory signal transducer and activator of transcription 4 agonist. Mol. Oral. Microbiol. 2015, 30, 242–252. [Google Scholar] [CrossRef]
- Gray, D.A.; White, J.B.R.; Oluwole, A.O.; Rath, P.; Glenwright, A.J.; Mazur, A.; Zahn, M.; Basle, A.; Morland, C.; Evans, S.L.; et al. Insights into SusCD-mediated glycan import by a prominent gut symbiont. Nat. Commun. 2021, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Montz, P. 2025. Figure 3. Graphical Abstract of Methodological Flow of Study. Created in BioRender. Available online: https://BioRender.com/m93c482 (accessed on 21 January 2025).
- Gouy, M.; Tannier, E.; Comte, N.; Parsons, D.P. Seaview Version 5: A multiplatform software for multiple sequence alignment, molecular phylogenetic analyses, and tree reconciliation. Methods Mol. Biol. 2021, 2231, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Copley, R.R.; Russell, R.B.; Ponting, C.P. Sialidase-like Asp-boxes: Sequence-similar structures within different protein folds. Protein Sci. 2001, 10, 285–292. [Google Scholar] [CrossRef]

| Primer | Sequence | Length | |
|---|---|---|---|
| PCR | LPCR-F | 5′ CAA AGT CCT GCC ACG AGT AGC 3′ | 6–8.5 kb |
| primer | LPCR-R | 5′ CGT TTT CTC GCC ACT TTC GTC 3′ | |
| Position Start/End | |||
| Sequencing | ragA-F1 | 5′ ATG AAA AGA ATG ACG CTA TTC TTC C 3′ | 50/1170 |
| Primer | ragA-F0 | 5′ GGT CAG GTA GCC GGT ATG CAG GTT AT 3′ | 480/1540 |
| ragA-R4 | 5′ CCR GGR ACA TAC CAC A 3′ | 2500/1370 | |
| ragA-R2 | 5′ TTA RAA MGA MAN YTG RAT ACC 3′ | 3030/2000 | |
| ragA-R2a | 5′ GGG TCR AAR CCT TTR WAC TT 3′ | 2980/1900 (type-4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montz, P.G.; Dafni, E.; Neumann, B.; Deng, D.; Abdelbary, M.M.H.; Conrads, G. Exploring the Genetic and Functional Diversity of Porphyromonas gingivalis Survival Factor RagAB. Int. J. Mol. Sci. 2025, 26, 1073. https://doi.org/10.3390/ijms26031073
Montz PG, Dafni E, Neumann B, Deng D, Abdelbary MMH, Conrads G. Exploring the Genetic and Functional Diversity of Porphyromonas gingivalis Survival Factor RagAB. International Journal of Molecular Sciences. 2025; 26(3):1073. https://doi.org/10.3390/ijms26031073
Chicago/Turabian StyleMontz, Pauline G., Evdokia Dafni, Bernd Neumann, Dongmei Deng, Mohamed M. H. Abdelbary, and Georg Conrads. 2025. "Exploring the Genetic and Functional Diversity of Porphyromonas gingivalis Survival Factor RagAB" International Journal of Molecular Sciences 26, no. 3: 1073. https://doi.org/10.3390/ijms26031073
APA StyleMontz, P. G., Dafni, E., Neumann, B., Deng, D., Abdelbary, M. M. H., & Conrads, G. (2025). Exploring the Genetic and Functional Diversity of Porphyromonas gingivalis Survival Factor RagAB. International Journal of Molecular Sciences, 26(3), 1073. https://doi.org/10.3390/ijms26031073





