Multi-Omics Sequencing Dissects the Atlas of Seminal Plasma Exosomes from Semen Containing Low or High Rates of Sperm with Cytoplasmic Droplets
Abstract
:1. Introduction
2. Results
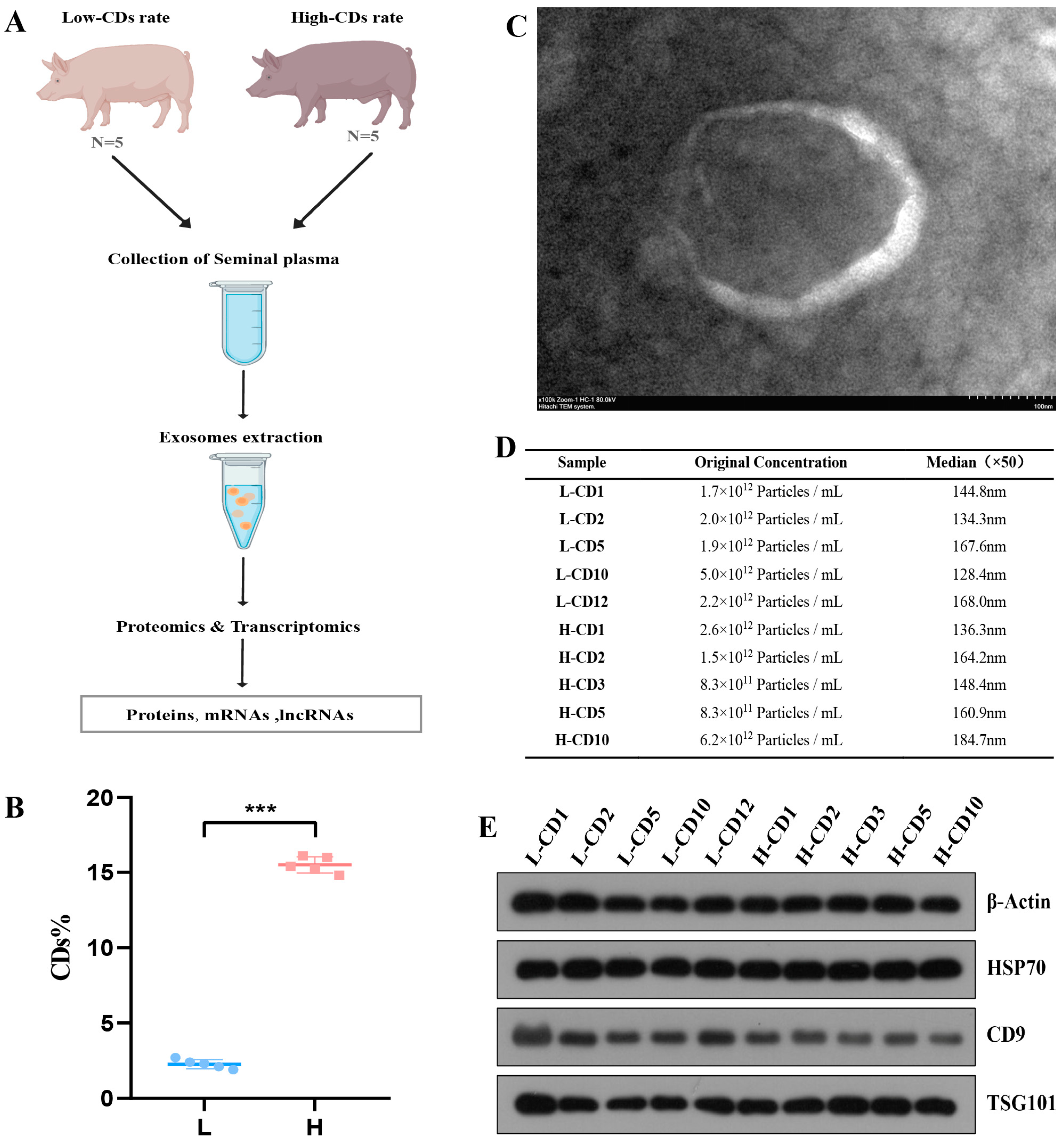
2.1. Characterization of Exosomes Derived from Seminal Plasma
2.2. Transcriptome Profile of Seminal Plasma Exosomes from Semen Containing Low or High Rates of Sperm with CDs
2.3. lncRNAs Profile of Seminal Plasma Exosomes from Semen Containing Low or High Rates of CDs
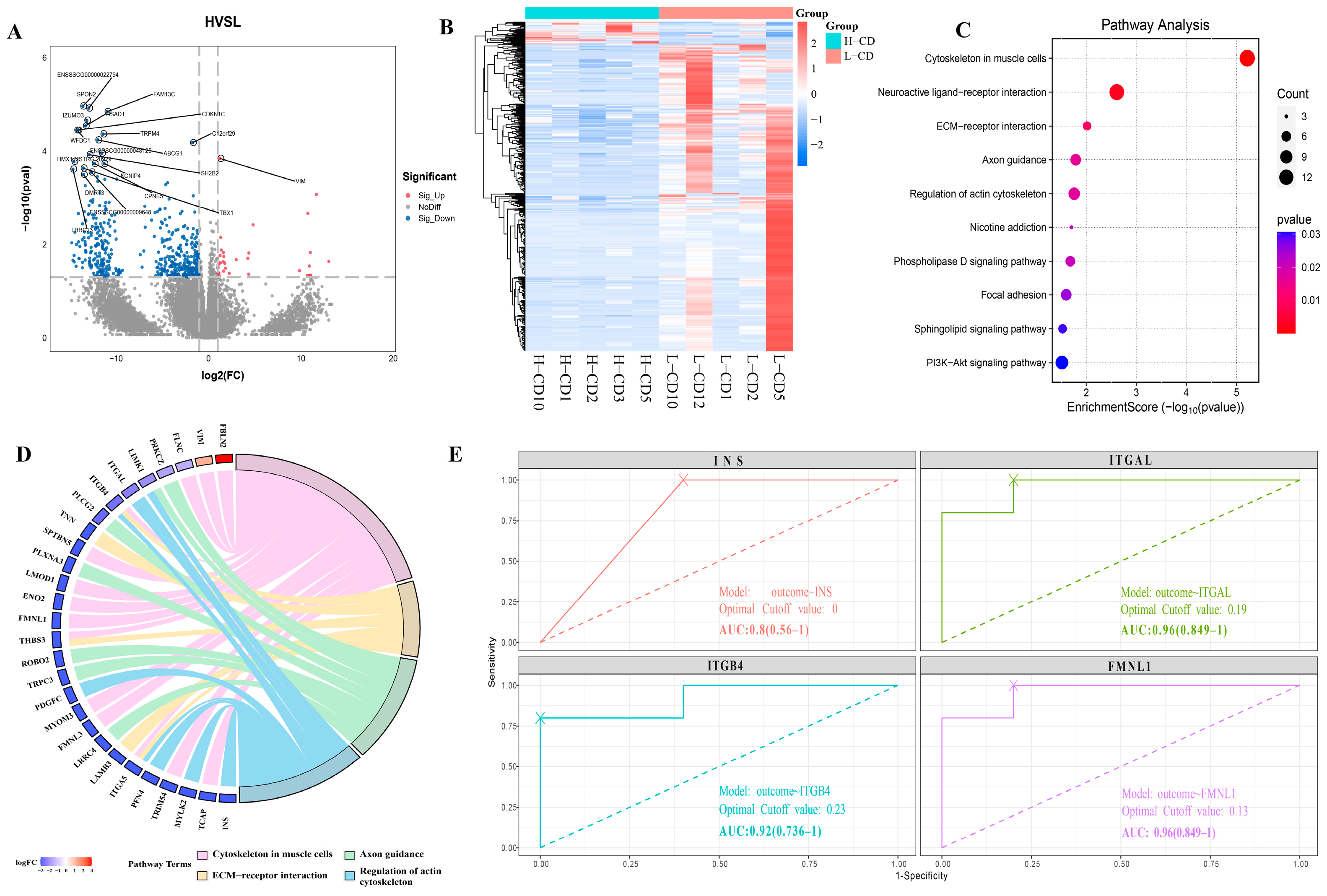
2.4. Proteins Profile of Seminal Plasma Exosomes from Semen Containing Low or High Rates of Sperm with CDs
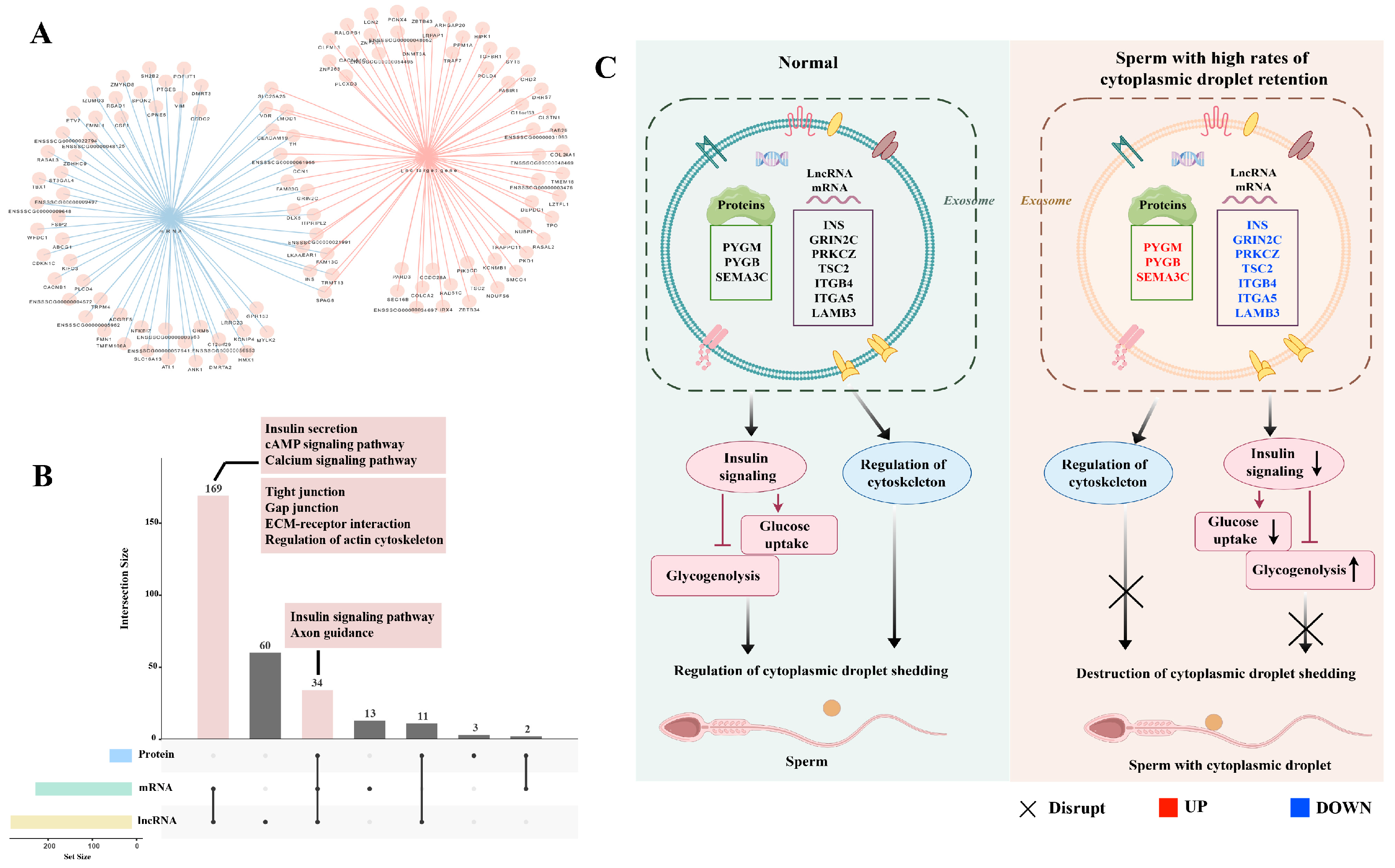
2.5. Integrative Analysis of Proteomics and Transcriptomics Datasets Derived from the Seminal Plasma Exosomes
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Isolation of Exosomes
4.3. Characterization of Exosomes
4.4. RNA Sequencing
4.5. Bioinformatic Analysis of the RNA-Seq Data
4.6. Proteomics Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDs | Cytoplasmic droplets |
| ECM | Extracellular matrix |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MCODE | Molecular Complex Detection |
| DIA | Data independent acquisition |
| ROC | Receiver operating characteristic |
| ITGAL | Integrin subunit alpha L |
| ITGB4 | Integrin β4 |
| FMNL1 | Formin-like 1 |
| INS | Insulin |
References
- Cooper, T.G. The epididymis, cytoplasmic droplets and male fertility. Asian J. Androl. 2011, 13, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Avidor-Reiss, T. Renaissance in sperm cytoplasmic contribution to infertility. J. Assist. Reprod. Genet. 2024, 41, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Hermo, L.; Pelletier, R.M.; Cyr, D.G.; Smith, C.E. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 3: Developmental changes in spermatid flagellum and cytoplasmic droplet and interaction of sperm with the zona pellucida and egg plasma membrane. Microsc. Res. Tech. 2010, 73, 320–363. [Google Scholar] [CrossRef] [PubMed]
- Au, C.E.; Hermo, L.; Byrne, E.; Smirle, J.; Fazel, A.; Kearney, R.E.; Smith, C.E.; Vali, H.; Fernandez-Rodriguez, J.; Simon, P.H.; et al. Compartmentalization of membrane trafficking, glucose transport, glycolysis, actin, tubulin and the proteasome in the cytoplasmic droplet/Hermes body of epididymal sperm. Open Biol. 2015, 5, 150080. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zheng, H.; Zheng, Z.; Yan, W. Proteomic analyses reveal a role of cytoplasmic droplets as an energy source during epididymal sperm maturation. PLoS ONE 2013, 8, e77466. [Google Scholar] [CrossRef]
- Hermo, L.; Oliveira, R.L.; Smith, C.E.; Au, C.E.; Bergeron, J.J.M. Dark side of the epididymis: Tails of sperm maturation. Andrology 2019, 7, 566–580. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Zhou, T.; Morris, D.; Chen, S.; Li, M.; Wang, Y.; Zheng, H.; Fu, W.; Yan, W. Small RNA shuffling between murine sperm and their cytoplasmic droplets during epididymal maturation. Dev. Cell 2023, 58, 779–790.e774. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, L.L.; Wei, H.K.; Zhou, Y.F.; Tan, J.J.; Sun, H.Q.; Jiang, S.W.; Peng, J. Logistic regression analysis of the related factors in discarded semen of boars in Southern China. Theriogenology 2019, 131, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Waberski, D.; Magnus, F.; Ardón, F.; Petrunkina, A.M.; Weitze, K.F.; Töpfer-Petersen, E. Binding of boar spermatozoa to oviductal epithelium in vitro in relation to sperm morphology and storage time. Reproduction 2006, 131, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Buder, S.; Rüdiger, K.; Beyerbach, M.; Waberski, D. Influences on semen traits used for selection of young AI boars. Anim. Reprod. Sci. 2014, 148, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Yunxiang, Z.; Changgen, X.; Li, Y.; Jinlong, Z. Analysis of the causes of high protoplasmic droplets in swine semen. Swine Ind. Sci. 2019, 36, 102–103. [Google Scholar]
- Schulze, M.; Ruediger, K.; Mueller, K.; Jung, M.; Well, C.; Reissmann, M. Development of an in vitro index to characterize fertilizing capacity of boar ejaculates. Anim. Reprod. Sci. 2013, 140, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Lovercamp, K.W.; Safranski, T.J.; Fischer, K.A.; Manandhar, G.; Sutovsky, M.; Herring, W.; Sutovsky, P. High resolution light microscopic evaluation of boar semen quality sperm cytoplasmic droplet retention in relationship with boar fertility parameters. Arch. Androl. 2007, 53, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Henning, H.; Luther, A.M.; Waberski, D. A High Incidence of Sperm with Cytoplasmic Droplets Affects the Response to Bicarbonate in Preserved Boar Semen. Animals 2021, 11, 2570. [Google Scholar] [CrossRef]
- Valencia, J.; Alzate, E.A.; Gómez, G.; Yeste, M.; Henao, F.J. Semen analysis of boars under intertropical conditions reveals the relevance of proximal and distal cytoplasm droplets for sperm functional integrity. Reprod. Domest. Anim. 2021, 56, 905–914. [Google Scholar] [CrossRef]
- Banaszewska, D.; Biesiada-Drzazga, B.; Andraszek, K. Frequency of Cytoplasmic Droplets Depends on the Breed and Age of Insemination Boars. Folia Biol. 2015, 63, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Stratton, C.J.; Morozumi, K.; Jin, J.; Yanagimachi, R.; Yan, W. Lack of Spem1 causes aberrant cytoplasm removal, sperm deformation, and male infertility. Proc. Natl. Acad. Sci. USA 2007, 104, 6852–6857. [Google Scholar] [CrossRef]
- Moore, K.; Lovercamp, K.; Feng, D.; Antelman, J.; Sutovsky, M.; Manandhar, G.; van Leyen, K.; Safranski, T.; Sutovsky, P. Altered epididymal sperm maturation and cytoplasmic droplet migration in subfertile male Alox15 mice. Cell Tissue Res. 2010, 340, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hermo, L.; Ding, D.; Wei, C.; Mann, J.M.; Yan, X.; Melnick, A.F.; Wu, Y.; Withrow, A.; Cibelli, J.; et al. SYPL1 defines a vesicular pathway essential for sperm cytoplasmic droplet formation and male fertility. Nat. Commun. 2023, 14, 5113. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, N.H.; Nguyen, T.; Gad, A.; Tesfaye, D.; Liao, S.F.; Willard, S.T.; Ryan, P.L.; Feugang, J.M. Characterization of Extracellular Vesicle-Coupled miRNA Profiles in Seminal Plasma of Boars with Divergent Semen Quality Status. Int. J. Mol. Sci. 2023, 24, 3194. [Google Scholar] [CrossRef]
- Del Giudice, P.T.; Belardin, L.B.; Camargo, M.; Zylbersztejn, D.S.; Carvalho, V.M.; Cardozo, K.H.; Bertolla, R.P.; Cedenho, A.P. Determination of testicular function in adolescents with varicocoele—A proteomics approach. Andrology 2016, 4, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, J.; Sun, J.; He, J.; Sun, Y.; Yuan, R.; Li, Z. Motility-related microRNAs identified in pig seminal plasma exosomes by high-throughput small RNA sequencing. Theriogenology 2024, 215, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Alvarez-Barrientos, A.; Parra, A.; Martínez-Díaz, P.; Lucas, X.; Roca, J. Immunophenotype profile by flow cytometry reveals different subtypes of extracellular vesicles in porcine seminal plasma. Cell Commun. Signal 2024, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Martin-DeLeon, P.A. Uterosomes: Exosomal cargo during the estrus cycle and interaction with sperm. Front. Biosci. 2016, 8, 115–122. [Google Scholar] [CrossRef]
- Sullivan, R.; Saez, F. Epididymosomes, prostasomes, and liposomes: Their roles in mammalian male reproductive physiology. Reproduction 2013, 146, R21–R35. [Google Scholar] [CrossRef]
- Guo, H.; Chang, Z.; Zhang, Z.; Zhao, Y.; Jiang, X.; Yu, H.; Zhang, Y.; Zhao, R.; He, B. Extracellular ATPs produced in seminal plasma exosomes regulate boar sperm motility and mitochondrial metabolism. Theriogenology 2019, 139, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, Y.; He, J.; Zhou, Q.; El-Ashram, S.; Yuan, S.; Chi, S.; Qin, J.; Huang, Z.; Ye, M.; et al. Small RNA expression patterns in seminal plasma exosomes isolated from semen containing spermatozoa with cytoplasmic droplets versus regular exosomes in boar semen. Theriogenology 2021, 176, 233–243. [Google Scholar] [CrossRef]
- Murdica, V.; Giacomini, E.; Alteri, A.; Bartolacci, A.; Cermisoni, G.C.; Zarovni, N.; Papaleo, E.; Montorsi, F.; Salonia, A.; Viganò, P.; et al. Seminal plasma of men with severe asthenozoospermia contain exosomes that affect spermatozoa motility and capacitation. Fertil. Steril. 2019, 111, 897–908.e892. [Google Scholar] [CrossRef]
- Mahdavinezhad, F.; Gilani, M.A.S.; Gharaei, R.; Ashrafnezhad, Z.; Valipour, J.; Nashtaei, M.S.; Amidi, F. Protective roles of seminal plasma exosomes and microvesicles during human sperm cryopreservation. Reprod. Biomed. Online 2022, 45, 341–353. [Google Scholar] [CrossRef]
- Du, J.; Shen, J.; Wang, Y.; Pan, C.; Pang, W.; Diao, H.; Dong, W. Boar seminal plasma exosomes maintain sperm function by infiltrating into the sperm membrane. Oncotarget 2016, 7, 58832–58847. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Stanger, S.J.; Anderson, A.L.; Bernstein, I.R.; De Iuliis, G.N.; McCluskey, A.; McLaughlin, E.A.; Dun, M.D.; Nixon, B. Mechanisms of tethering and cargo transfer during epididymosome-sperm interactions. BMC Biol. 2019, 17, 35. [Google Scholar] [CrossRef]
- Vilanova-Perez, T.; Jones, C.; Balint, S.; Dragovic, R.; Dustin, M.L.; Yeste, M.; Coward, K. Exosomes derived from HEK293T cells interact in an efficient and noninvasive manner with mammalian sperm in vitro. Nanomedicine 2020, 15, 1965–1980. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Siu, M.K.; Cheng, C.Y. Extracellular matrix: Recent advances on its role in junction dynamics in the seminiferous epithelium during spermatogenesis. Biol. Reprod. 2004, 71, 375–391. [Google Scholar] [CrossRef]
- Mathieu, M.; Isomursu, A.; Ivaska, J. Positive and negative durotaxis-Mechanisms and emerging concepts. J. Cell Sci. 2024, 137, jcs261919. [Google Scholar] [CrossRef]
- Valdivia, A.; Avalos, A.M.; Leyton, L. Thy-1 (CD90)-regulated cell adhesion and migration of mesenchymal cells: Insights into adhesomes, mechanical forces, and signaling pathways. Front. Cell Dev. Biol. 2023, 11, 1221306. [Google Scholar] [CrossRef] [PubMed]
- Siu, M.K.; Cheng, C.Y. Extracellular matrix and its role in spermatogenesis. Adv. Exp. Med. Biol. 2008, 636, 74–91. [Google Scholar] [CrossRef]
- Delgado-Buenrostro, N.L.; Mújica, A.; Chiquete-Felix, N.; Déciga-Alcaraz, A.; Medina-Reyes, E.I.; Uribe-Carvajal, S.; Chirino, Y.I. Role of Wasp and the small GTPases RhoA, RhoB, and Cdc42 during capacitation and acrosome reaction in spermatozoa of English guinea pigs. Mol. Reprod. Dev. 2016, 83, 927–937. [Google Scholar] [CrossRef]
- Breitbart, H.; Finkelstein, M. Actin cytoskeleton and sperm function. Biochem. Biophys. Res. Commun. 2018, 506, 372–377. [Google Scholar] [CrossRef]
- Rowland, A.F.; Fazakerley, D.J.; James, D.E. Mapping insulin/GLUT4 circuitry. Traffic 2011, 12, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Wal, P. Phytochemicals and their Potential Mechanisms against Insulin Resistance. Curr. Diabetes Rev. 2024, 20, e081123223322. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Hay, N. Akt isoforms and glucose homeostasis-The leptin connection. Trends Endocrinol. Metab. TEM 2011, 22, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Ueki, K.; Yamauchi, T.; Kubota, N. SnapShot: Insulin signaling pathways. Cell 2012, 148, 624. [Google Scholar] [CrossRef] [PubMed]
- Abu Aqel, Y.; Alnesf, A.; Aigha, I.I.; Islam, Z.; Kolatkar, P.R.; Teo, A.; Abdelalim, E.M. Glucokinase (GCK) in diabetes: From molecular mechanisms to disease pathogenesis. Cell Mol. Biol. Lett. 2024, 29, 120. [Google Scholar] [CrossRef]
- Matschinsky, F.M.; Wilson, D.F. The Central Role of Glucokinase in Glucose Homeostasis: A Perspective 50 Years After Demonstrating the Presence of the Enzyme in Islets of Langerhans. Front. Physiol. 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.; Kubota, T.; Kadowaki, T. Physiological and pathophysiological actions of insulin in the liver. Endocr. J. 2024, EJ24-0192. [Google Scholar] [CrossRef]
- Zańko, A.; Martynowicz, I.; Citko, A.; Konopka, P.; Paszko, A.; Pawłowski, M.; Szczerbiński, Ł.; Siewko, K.; Krętowski, A.J.; Kuczyński, W.; et al. The Influence of Lifestyle on Male Fertility in the Context of Insulin Resistance-Identification of Factors That Influence Semen Quality. J. Clin. Med. 2024, 13, 2797. [Google Scholar] [CrossRef] [PubMed]
- Andlib, N.; Sajad, M.; Kumar, R.; Thakur, S.C. Abnormalities in sex hormones and sexual dysfunction in males with diabetes mellitus: A mechanistic insight. Acta Histochem. 2023, 125, 151974. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Xu, X.; Chen, F.; Liu, Q.; Li, Z.; Zheng, X.; Zhao, Y. Multi-Omics Sequencing Dissects the Atlas of Seminal Plasma Exosomes from Semen Containing Low or High Rates of Sperm with Cytoplasmic Droplets. Int. J. Mol. Sci. 2025, 26, 1096. https://doi.org/10.3390/ijms26031096
Zhang Z, Xu X, Chen F, Liu Q, Li Z, Zheng X, Zhao Y. Multi-Omics Sequencing Dissects the Atlas of Seminal Plasma Exosomes from Semen Containing Low or High Rates of Sperm with Cytoplasmic Droplets. International Journal of Molecular Sciences. 2025; 26(3):1096. https://doi.org/10.3390/ijms26031096
Chicago/Turabian StyleZhang, Zilu, Xiaoxian Xu, Fumei Chen, Qingyou Liu, Zhili Li, Xibang Zheng, and Yunxiang Zhao. 2025. "Multi-Omics Sequencing Dissects the Atlas of Seminal Plasma Exosomes from Semen Containing Low or High Rates of Sperm with Cytoplasmic Droplets" International Journal of Molecular Sciences 26, no. 3: 1096. https://doi.org/10.3390/ijms26031096
APA StyleZhang, Z., Xu, X., Chen, F., Liu, Q., Li, Z., Zheng, X., & Zhao, Y. (2025). Multi-Omics Sequencing Dissects the Atlas of Seminal Plasma Exosomes from Semen Containing Low or High Rates of Sperm with Cytoplasmic Droplets. International Journal of Molecular Sciences, 26(3), 1096. https://doi.org/10.3390/ijms26031096






