TMEM160 Promotes Tumor Growth in Lung Adenocarcinoma and Cervical Adenocarcinoma Cell Lines
Abstract
1. Introduction
2. Results
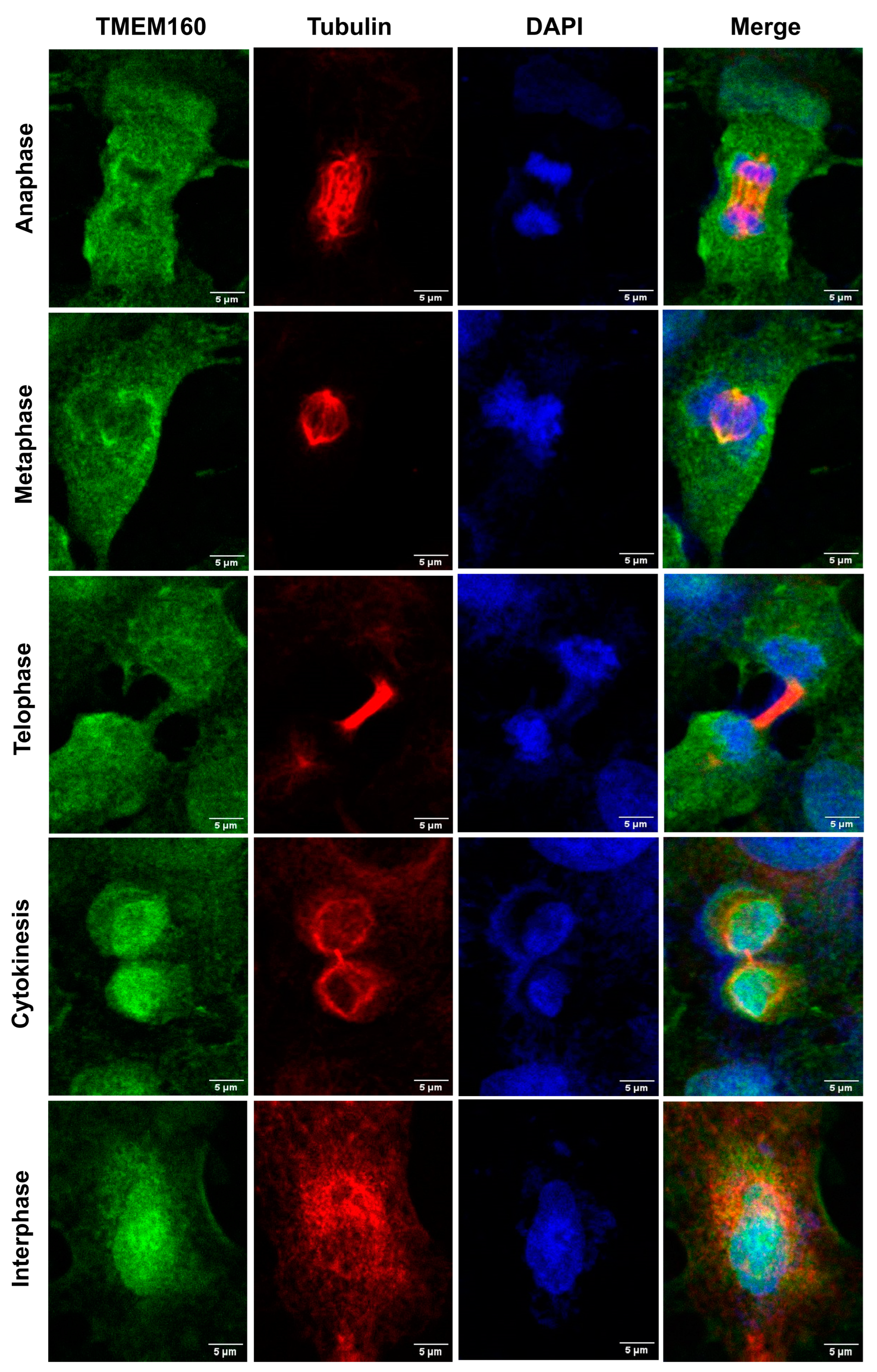
2.1. TMEM160 Is Localized in the Nucleus and Cytoplasm of the Tumor and Non-Tumor Cells of the Lung and Cervix
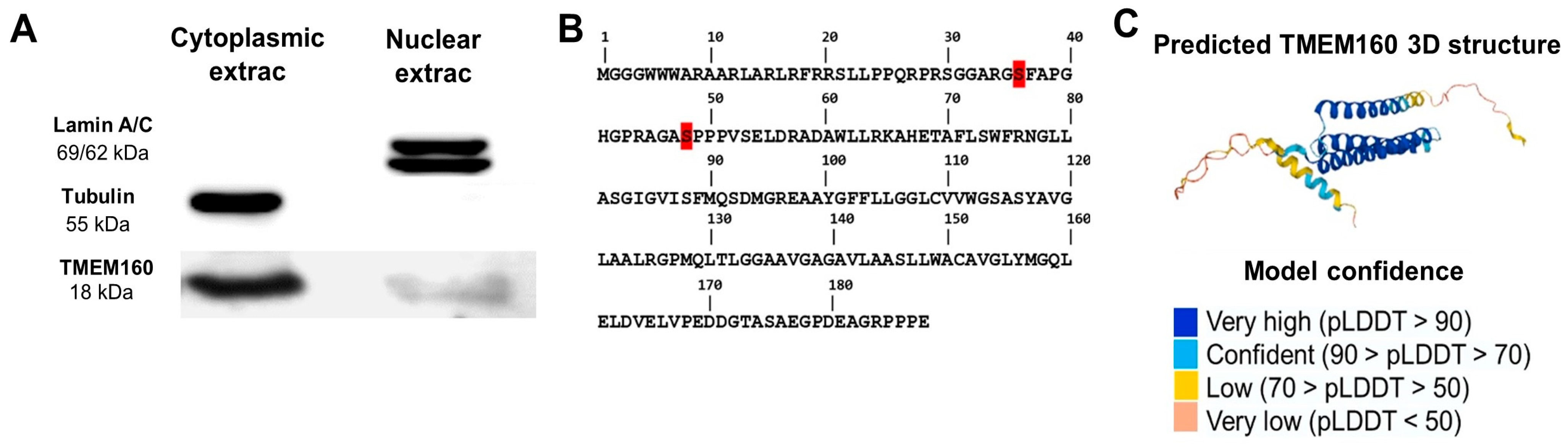
2.2. Confirmation of Nuclear and Cytoplasmatic Localization of TMEM160
2.3. TMEM160 Is Upregulated in LUAD and Cervical Cancer
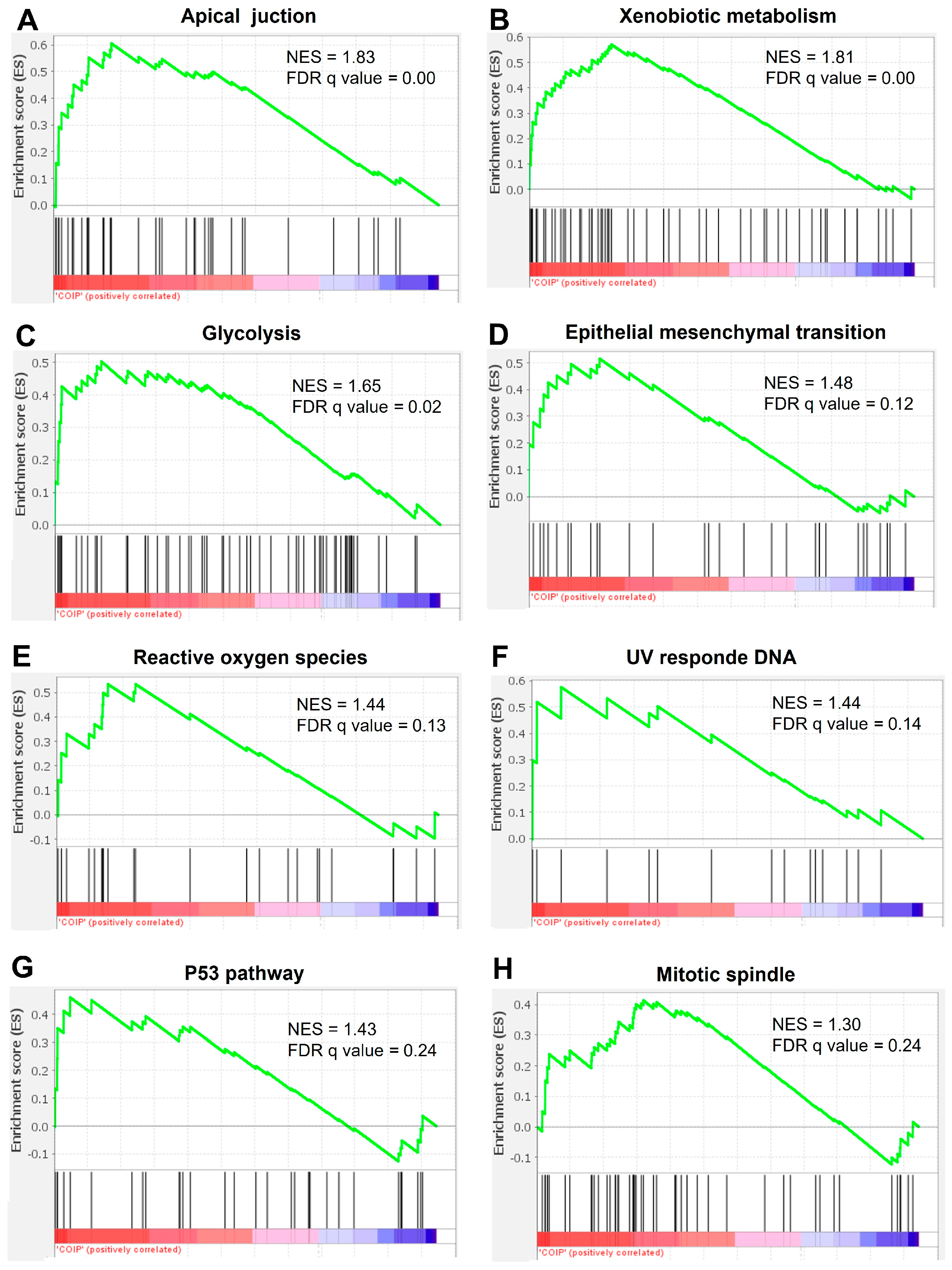
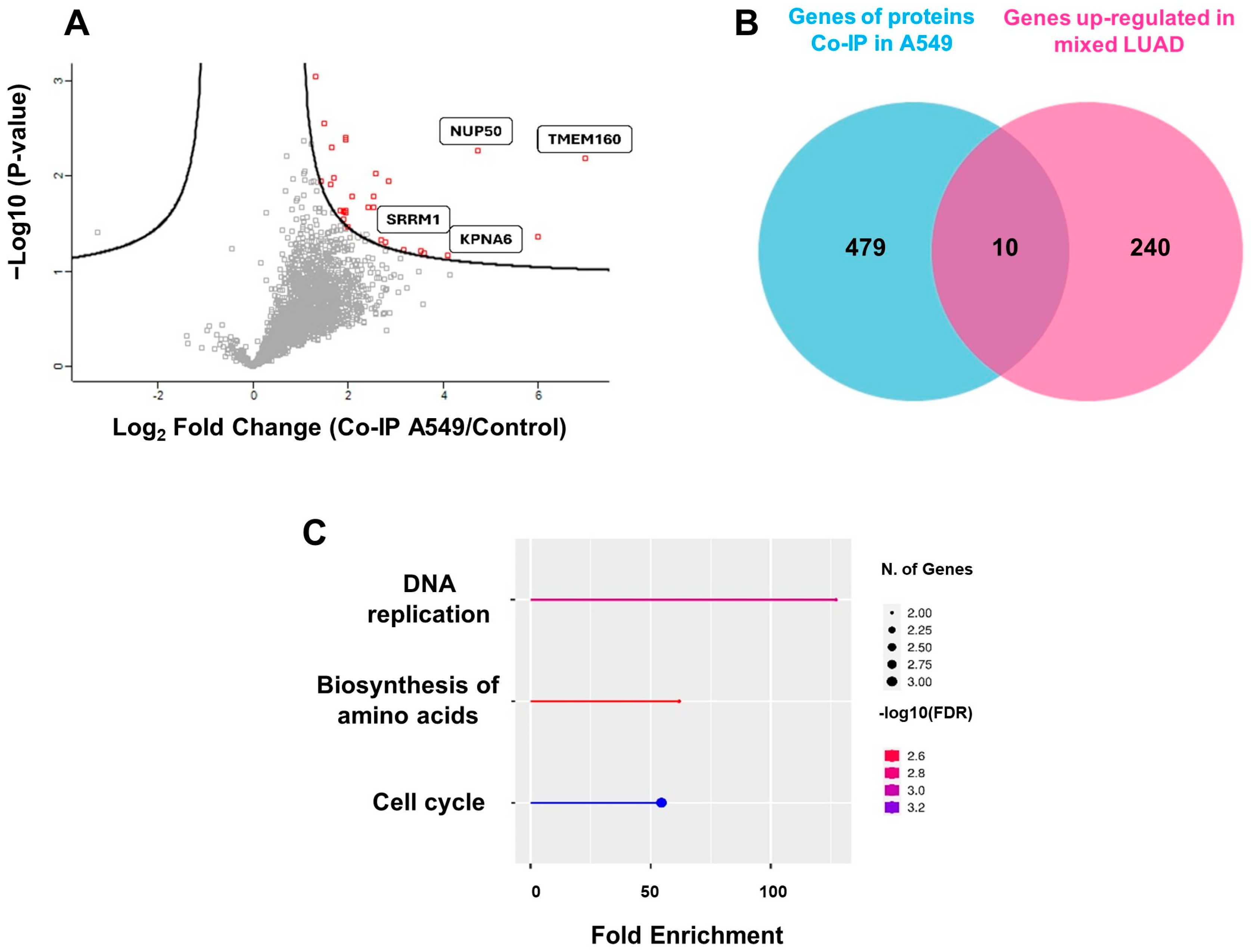
2.4. The Interactome of TMEM160 Is Related to Biological Processes and Protumorigenic Pathways Associated with Nuclear Proteins, DNA Replication, and the Cell Cycle in LUAD
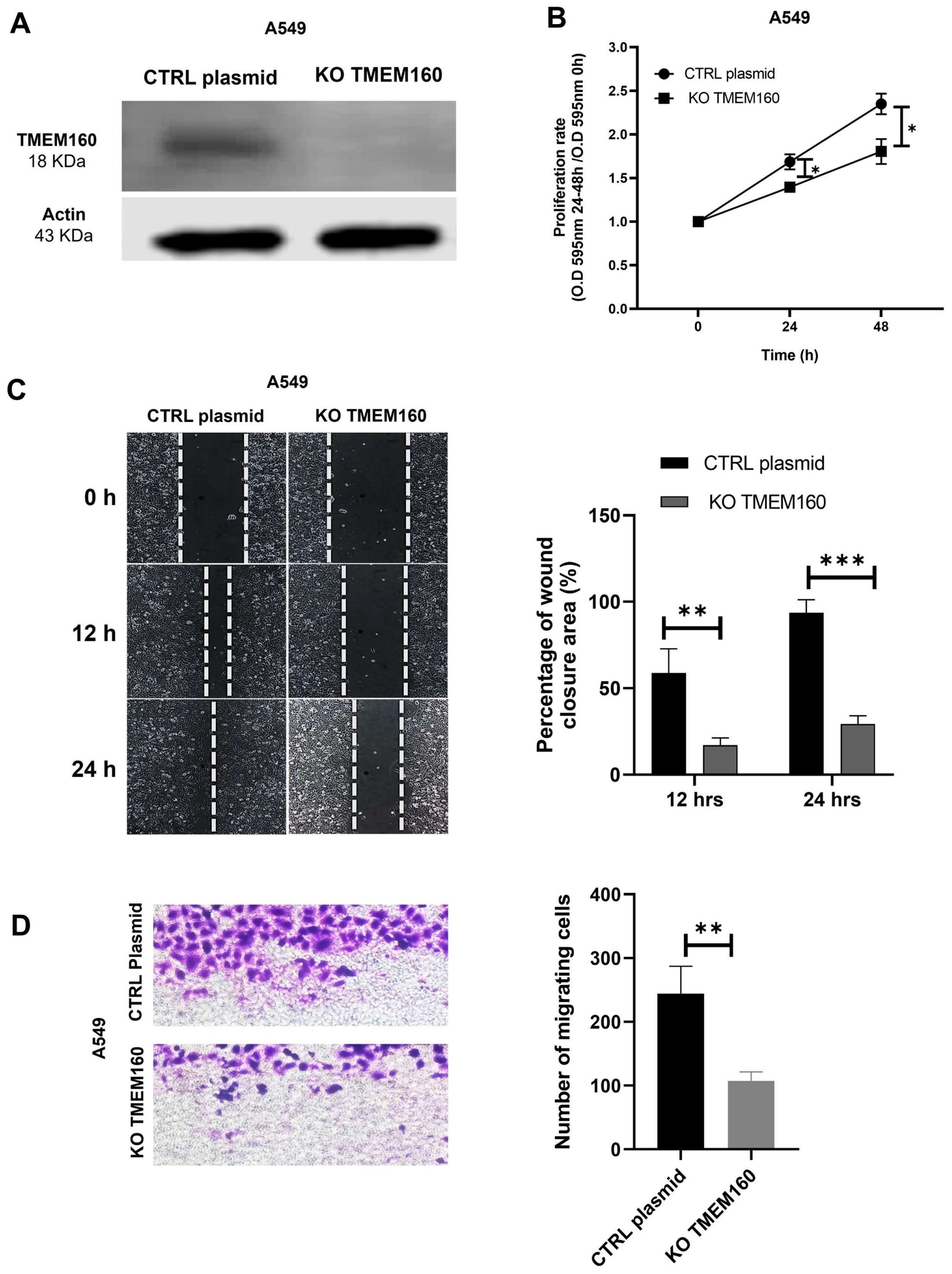
2.5. Knockout of TMEM160 Inhibits the Proliferation and Migration of LUAD Cells In Vitro
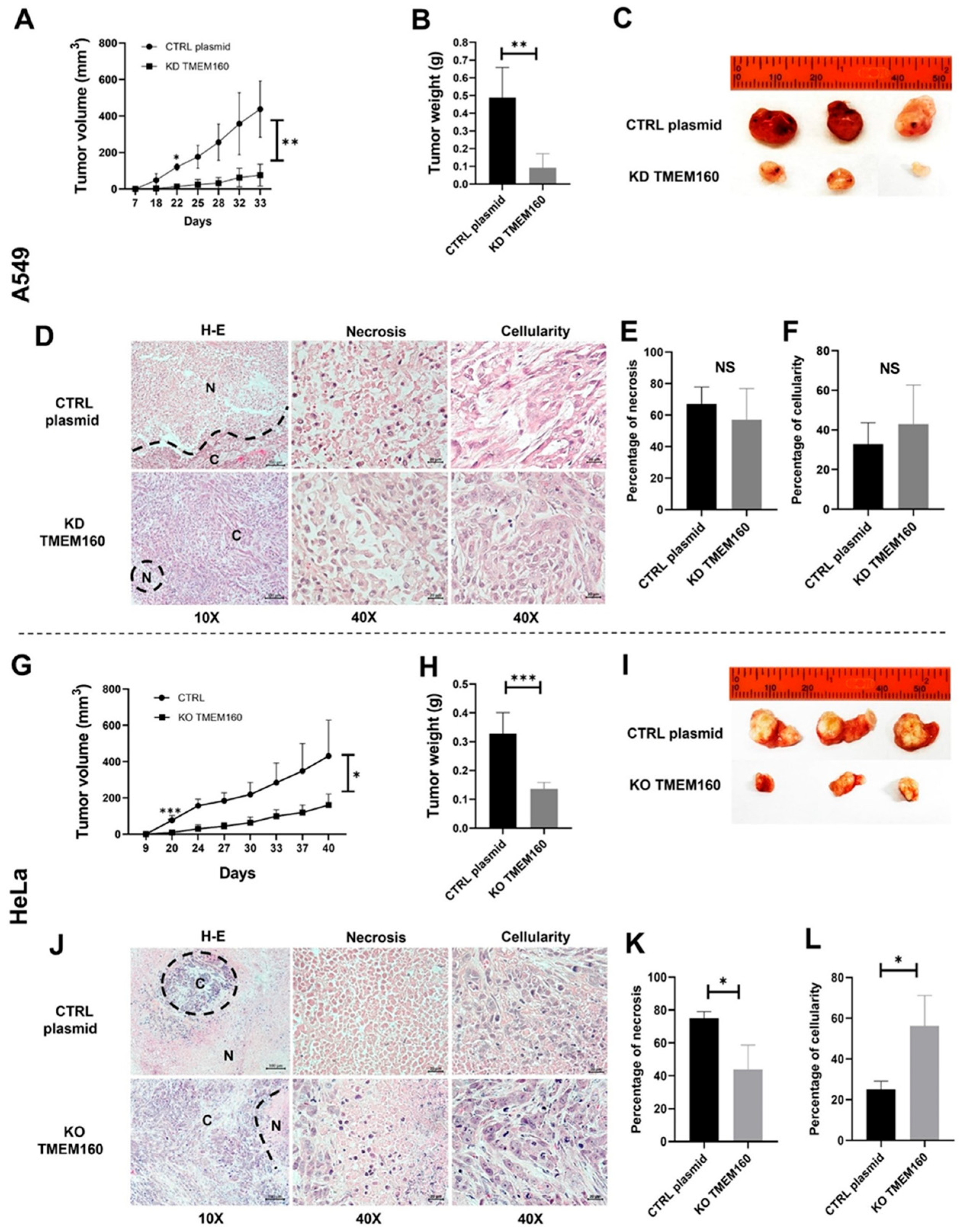
2.6. Silencing TMEM160 Inhibits Tumor Growth of LUAD and Cervical Cancer Cells In Vivo
3. Discussion
4. Materials and Methods
4.1. Patients and Tissue Samples
4.2. Immunohistochemistry (IHQ)
4.3. Cell Culture
4.4. Protein Extraction
4.5. Western Blot Analysis
4.6. CRISPR/Cas9 of the TMEM160 Gene and Cell Transfection
4.7. Cell Proliferation Assay
4.8. Wound Healing Assay
4.9. Transwell Migration Assay
4.10. Immunofluorescence (IF)
4.11. Isolation of Nuclear and Cytoplasmic Extract
4.12. Co-Inmunoprecipitation (Co-IP)
4.13. LC-MS/MS Analysis
4.14. Protein Identification and Data Analysis
4.15. Tumor Xenograft Mouse Model
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K. Lung cancer: Understanding its molecular pathology and the 2015 WHO classification. Front. Oncol. 2017, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef]
- Rodak, O.; Peris-Díaz, M.D.; Olbromski, M.; Podhorska-Okołów, M.; Dzięgiel, P. Current Landscape of Non-Small Cell Lung Cancer: Epidemiology, Histological Classification, Targeted Therapies, and Immunotherapy. Cancers 2021, 13, 4705. [Google Scholar] [CrossRef]
- Halle, M.K.; Sundaresan, A.; Zhang, J.; Pedamallu, C.S.; Srinivasasainagendra, V.; Blair, J.; Brooke, D.; Bertelsen, B.I.; Woie, K.; Shrestha, S.; et al. Genomic alterations associated with mutational signatures, DNA damage repair and chromatin remodeling pathways in cervical carcinoma. NPJ Genom. Med. 2021, 6, 82. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Burmeister, C.A.; Khan, S.F.; Schäfer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical cancer therapies: Current challenges and future perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef]
- Paik, Y.K.; Omenn, G.S.; Hancock, W.S.; Lane, L.; Overall, C.M. Advances in the Chromosome-Centric Human Proteome Project: Looking to the future. Expert Rev. Proteom. 2017, 14, 1059–1071. [Google Scholar] [CrossRef]
- Paik, Y.K.; Lane, L.; Kawamura, T.; Chen, Y.J.; Cho, J.Y.; Labaer, J.; Yoo, J.S.; Domont, G.; Corrales, F.; Omenn, G.S.; et al. Launching the C-HPP neXt-CP50 Pilot Project for Functional Characterization of Identified Proteins with No Known Function. J. Proteome Res. 2018, 17, 4042–4050. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, F.; Zhao, J. Transmembrane protein 45A regulates the proliferation, migration, and invasion of glioma cells through nuclear factor kappa-B. Anti-Cancer Drugs 2020, 31, 900–907. [Google Scholar] [CrossRef]
- Zhu, M.; Jiang, B.; Yan, D.; Wang, X.; Ge, H.; Sun, Y. Knockdown of TMEM45A overcomes multidrug resistance and epithelial–mesenchymal transition in human colorectal cancer cells through inhibition of TGF-β signalling pathway. Clin. Exp. Pharmacol. Physiol. 2020, 47, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.Y.; Wang, L.; Liu, Z.Y.; Song, W.X.; Zhou, M.; Xi, L. TMEM48 promotes cell proliferation and invasion in cervical cancer via activation of the Wnt/β-catenin pathway. J. Recept. Signal Transduct. 2021, 41, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Su, Z.; Ding, Q.; Shen, L.; Nie, X.; Pan, X.; Yan, A.; Yan, R.; Zhou, Y.; Li, L.; et al. Inhibition of proliferation by knockdown of transmembrane (TMEM) 168 in glioblastoma cells via suppression of Wnt/β-catenin pathway. Oncol. Res. 2019, 27, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Wu, X.; Zhou, X.; Deng, R.; Ma, Y. TMEM205 Is an Independent Prognostic Factor and Is Associated With Immune Cell Infiltrates in Hepatocellular Carcinoma. Front. Genet. 2020, 11, 575776. [Google Scholar] [CrossRef]
- Xu, D.; Qu, L.; Hu, J.; Li, G.; Lv, P.; Ma, D.; Guo, M.; Chen, Y. Transmembrane protein 106A is silenced by promoter region hypermethylation and suppresses gastric cancer growth by inducing apoptosis. J. Cell. Mol. Med. 2014, 18, 1655–1666. [Google Scholar] [CrossRef]
- Wu, C.; Xu, J.; Wang, H.; Zhang, J.; Zhong, J.; Zou, X.; Chen, Y.; Yang, G.; Zhong, Y.; Lai, D.; et al. TMEM106a is a novel tumor suppressor in human renal cancer. Kidney Blood Press. Res. 2018, 42, 853–864. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, H. TMEM106A inhibits cell proliferation, migration, and induces apoptosis of lung cancer cells. J. Cell. Biochem. 2019, 120, 7825–7833. [Google Scholar] [CrossRef]
- Zhang, Z.; Shang, J.; Dai, Z.; Yao, Y.; Shi, Y.; Zhong, D.; Liang, Y.; Lai, C.; Yang, Q.; Feng, T.; et al. Transmembrane Protein 170B is a Prognostic Biomarker and Associated With Immune Infiltrates in Pancreatic Adenocarcinoma. Front. Genet. 2022, 13, 848391. [Google Scholar] [CrossRef]
- Li, M.; Han, Y.; Zhou, H.; Li, X.; Lin, C.; Zhang, E.; Chi, X.; Hu, J.; Xu, H. Transmembrane protein 170B is a novel breast tumorigenesis suppressor gene that inhibits the Wnt/β-catenin pathway. Cell Death Dis. 2018, 9, 91. [Google Scholar] [CrossRef]
- Anzai, T.; Matsumura, Y. Topological analysis of TMEM180, a newly identified membrane protein that is highly expressed in colorectal cancer cells. Biochem. Biophys. Res. Commun. 2019, 520, 566–572. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, X.; Jiang, G.; Miao, Y.; Wang, L.; Zhang, Y.; Liu, Y.; Fan, C.; Lin, X.; Dong, Q.; et al. Cytosolic TMEM88 promotes invasion and metastasis in lung cancer cells by binding DVLS. Cancer Res. 2015, 75, 4527–4537. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, W.; Zhang, D.; Lin, B.; Li, B. TMEM158 expression is negatively regulated by AR signaling and associated with favorite survival outcomes in prostate cancers. Front. Oncol. 2022, 12, 1023455. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, T.; Ikeda, K.; Tsukada, Y.; Nishizawa, Y.; Sasaki, T.; Ito, M.; Kojima, M.; Ishii, G.; Tsumura, R.; Saijou, S.; et al. High expression of TMEM180, a novel tumour marker, is associated with poor survival in stage III colorectal cancer. BMC Cancer 2021, 21, 302. [Google Scholar] [CrossRef]
- Mei, S.; Ke, J.; Tian, J.; Ying, P.; Yang, N.; Wang, X.; Zou, D.; Peng, X.; Yang, Y.; Zhu, Y.; et al. A functional variant in the boundary of a topological association domain is associated with pancreatic cancer risk. Mol. Carcinog. 2019, 58, 1855–1862. [Google Scholar] [CrossRef]
- Wu, S.G.; Xu, J.Y.; Lei, J.; Hu, M.; Zhou, J. Tumor-Suppressor Gene Transmembrane Protein 98 Promotes Proliferation and Inhibits Apoptosis in Ovarian Cancer. Front. Biosci.-Landmark 2022, 27, 210. [Google Scholar] [CrossRef]
- Fu, W.; Cheng, Y.; Zhang, Y.; Mo, X.; Li, T.; Liu, Y.; Wang, P.; Pan, W.; Chen, Y.; Xue, Y.; et al. The Secreted Form of Transmembrane Protein 98 Promotes the Differentiation of T Helper 1 Cells. J. Interferon Cytokine Res. 2015, 35, 720–733. [Google Scholar] [CrossRef]
- Yamashita, K.; Haraguchi, M.; Yano, M. Knockdown of TMEM160 leads to an increase in reactive oxygen species generation and the induction of the mitochondrial unfolded protein response. FEBS Open Bio 2022, 12, 2179–2190. [Google Scholar] [CrossRef]
- Dai, X.; Wu, Z.; Ruan, R.; Chen, J.; Huang, C.; Lei, W.; Yao, Y.; Li, L.; Tang, X.; Xiong, J.; et al. TMEM160 promotes tumor immune evasion and radiotherapy resistance via PD-L1 binding in colorectal cancer. Cell Commun. Signal. 2024, 22, 168. [Google Scholar] [CrossRef]
- Vuzman, D.; Levy, Y. Intrinsically disordered regions as affinity tuners in protein–DNA interactions. Mol. Biosyst. 2011, 8, 47–57. [Google Scholar] [CrossRef]
- Bekker-Jensen, D.B.; Kelstrup, C.D.; Batth, T.S.; Larsen, S.C.; Haldrup, C.; Bramsen, J.B.; Sørensen, K.D.; Høyer, S.; Ørntoft, T.F.; Andersen, C.L.; et al. An Optimized Shotgun Strategy for the Rapid Generation of Comprehensive Human Proteomes. Cell Syst. 2017, 4, 587–599.e4. [Google Scholar] [CrossRef]
- Geiger, T.; Wehner, A.; Schaab, C.; Cox, J.; Mann, M. Comparative Proteomic Analysis of Eleven Common Cell Lines Reveals Ubiquitous but Varying Expression of Most Proteins. Mol. Cell. Proteom. 2012, 11, M111.014050. [Google Scholar] [CrossRef] [PubMed]
- TMEM160—Transmembrane Protein 160—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/Q9NX00/entry (accessed on 8 September 2024).
- Herrera-Quiterio, G.A.; Encarnación-Guevara, S. The transmembrane proteins (TMEM) and their role in cell proliferation, migration, invasion, and epithelial-mesenchymal transition in cancer. Front. Oncol. 2023, 13, 1244740. [Google Scholar] [CrossRef] [PubMed]
- Schmit, K.; Michiels, C. TMEM Proteins in Cancer: A Review. Front. Pharmacol. 2018, 9, 1345. [Google Scholar] [CrossRef] [PubMed]
- Köhler, M.; Speck, C.; Christiansen, M.; Bischoff, F.R.; Prehn, S.; Haller, H.; Görlich, D.; Hartmann, E. Evidence for distinct substrate specificities of importin α family members in nuclear protein import. Mol. Cell. Biol. 1999, 19, 7782–7791. [Google Scholar] [CrossRef]
- Nardozzi, J.D.; Lott, K.; Cingolani, G. Phosphorylation meets nuclear import: A review. Cell Commun. Signal. 2010, 8, 32. [Google Scholar] [CrossRef]
- Harreman, M.T.; Kline, T.M.; Milford, H.G.; Harben, M.B.; Hodel, A.E.; Corbett, A.H. Regulation of nuclear import by phosphorylation adjacent to nuclear localization signals. J. Biol. Chem. 2004, 279, 20613–20621. [Google Scholar] [CrossRef]
- Lewander, A.; Gao, J.; Carstensen, J.; Arbman, G.; Zhang, H.; Sun, X.F. NF-κB p65 phosphorylated at serine-536 is an independent prognostic factor in Swedish colorectal cancer patients. Int. J. Color. Dis. 2012, 27, 447–452. [Google Scholar] [CrossRef]
- Singh, V.; Ram, M.; Kumar, R.; Prasad, R.; Roy, B.K.; Singh, K.K. Phosphorylation: Implications in Cancer. Protein J. 2017, 36, 1–6. [Google Scholar] [CrossRef]
- Wang, R.; Brattain, M.G. The maximal size of protein to diffuse through the nuclear pore is larger than 60 kDa. FEBS Lett. 2007, 581, 3164–3170. [Google Scholar] [CrossRef]
- Trivedi, R.; Nagarajaram, H.A. Intrinsically Disordered Proteins: An Overview. Int. J. Mol. Sci. 2022, 23, 14050. [Google Scholar] [CrossRef]
- Struhl, K. Intrinsically disordered regions (IDRs): A vague and confusing concept for protein function. Mol. Cell 2024, 84, 1186–1187. [Google Scholar] [CrossRef] [PubMed]
- Babu, M.M. The contribution of intrinsically disordered regions to protein function, cellular complexity, and human disease. Biochem. Soc. Trans. 2016, 44, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Yang, W.; Wang, X.; Gao, G. TMEΜ45B Interacts with Sindbis Virus Nsp1 and Nsp4 and Inhibits Viral Replication. J. Virol. 2022, 96, e0091922. [Google Scholar] [CrossRef]
- Foulquier, F.; Amyere, M.; Jaeken, J.; Zeevaert, R.; Schollen, E.; Race, V.; Bammens, R.; Morelle, W.; Rosnoblet, C.; Legrand, D.; et al. TMEM165 Deficiency Causes a Congenital Disorder of Glycosylation. Am. J. Hum. Genet. 2012, 91, 15–26. [Google Scholar] [CrossRef]
- Yu, C.; Wang, L.; Lv, B.; Lu, Y.; Zeng, L.; Chen, Y.; Ma, D.; Shi, T.; Wang, L. TMEM74, a lysosome and autophagosome protein, regulates autophagy. Biochem. Biophys. Res. Commun. 2008, 369, 622–629. [Google Scholar] [CrossRef]
- Kanamoto, T.; Mizuhashi, K.; Terada, K.; Minami, T.; Yoshikawa, H.; Furukawa, T. Isolation and characterization of a novel plasma membrane protein, osteoblast induction factor (obif), associated with osteoblast differentiation. BMC Dev. Biol. 2009, 9, 70. [Google Scholar] [CrossRef]
- Palpant, N.J.; Pabon, L.; Rabinowitz, J.S.; Hadland, B.K.; Stoick-Cooper, C.L.; Paige, S.L.; Bernstein, I.D.; Moon, R.T.; Murry, C.E. Transmembrane protein 88: A Wnt regulatory protein that specifies cardiomyocyte development. Development 2013, 140, 3799–3808. [Google Scholar] [CrossRef]
- Hayez, A.; Roegiers, E.; Malaisse, J.; Balau, B.; Sterpin, C.; Achouri, Y.; De Rouvroit, C.L.; Poumay, Y.; Michiels, C.; De Backer, O. TMEM45A is dispensable for epidermal morphogenesis, keratinization and barrier formation. PLoS ONE 2016, 11, e0147069. [Google Scholar] [CrossRef]
- Marx, S.; Dal Maso, T.; Chen, J.W.; Bury, M.; Wouters, J.; Michiels, C.; Le Calvé, B. Transmembrane (TMEM) protein family members: Poorly characterized even if essential for the metastatic process. Semin. Cancer Biol. 2020, 60, 96–106. [Google Scholar] [CrossRef]
- Ogawa, Y.; Miyamoto, Y.; Asally, M.; Oka, M.; Yasuda, Y.; Yoneda, Y. Two Isoforms of Npap60 (Nup50) Differentially Regulate Nuclear Protein Import. Mol. Biol. Cell 2010, 21, 630–638. [Google Scholar] [CrossRef]
- Buchwalter, A.L.; Liang, Y.; Hetzer, M.W. Nup50 is required for cell differentiation and exhibits transcription-dependent dynamics. Mol. Biol. Cell 2014, 25, 2472–2484. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.R.; Howa, A.C.; Werner, T.L.; Ullman, K.S. Nup153 and Nup50 promote recruitment of 53BP1 to DNA repair foci by antagonizing BRCA1-dependent events. J. Cell Sci. 2017, 130, 3347–3359. [Google Scholar] [CrossRef] [PubMed]
- Hajeri, V.A.; Little, B.A.; Ladage, M.L.; Padilla, P.A. NPP-16/Nup50 Function and CDK-1 Inactivation Are Associated with Anoxia-induced Prophase Arrest in Caenorhabditis elegans. Mol. Biol. Cell 2010, 21, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Yoshimaru, T.; Chen, Y.A.; Matsuo, T.; Komatsu, M.; Miyoshi, Y.; Tanaka, E.; Sasa, M.; Mizuguchi, K.; Katagiri, T. BIG3 Inhibits the Estrogen-Dependent Nuclear Translocation of PHB2 via Multiple Karyopherin-Alpha Proteins in Breast Cancer Cells. PLoS ONE 2015, 10, e0127707. [Google Scholar] [CrossRef]
- McCracken, S.; Longman, D.; Johnstone, I.L.; Cáceres, J.F.; Blencowe, B.J. An Evolutionarily Conserved Role for SRm160 in 3′-End Processing that Functions Independently of Exon Junction Complex Formation. J. Biol. Chem. 2003, 278, 44153–44160. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Kilari, D.; Guancial, E.; Kim, E.S. Role of copper transporters in platinum resistance. World J. Clin. Oncol. 2016, 7, 106–113. [Google Scholar] [CrossRef]
- Bansal, A.; Celeste Simon, M. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef]
- Gil, J.; Rezeli, M.; Lutz, E.G.; Kim, Y.; Sugihara, Y.; Malm, J.; Semenov, Y.R.; Yu, K.H.; Nguyen, N.; Wan, G.; et al. An observational study on the molecular profiling of primary melanomas reveals a progression dependence on mitochondrial activation. Cancers 2021, 13, 6066. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Quiterio, G.A.; Valencia-González, H.A.; de la Cruz-López, K.G.; Fernández-Coto, D.L.; Gil, J.; Marko-Varga, G.; Morales-Gálvez, J.; Sánchez, N.C.; Rodríguez-Bautista, R.; Avilés-Salas, A.; et al. TMEM160 Promotes Tumor Growth in Lung Adenocarcinoma and Cervical Adenocarcinoma Cell Lines. Int. J. Mol. Sci. 2025, 26, 1097. https://doi.org/10.3390/ijms26031097
Herrera-Quiterio GA, Valencia-González HA, de la Cruz-López KG, Fernández-Coto DL, Gil J, Marko-Varga G, Morales-Gálvez J, Sánchez NC, Rodríguez-Bautista R, Avilés-Salas A, et al. TMEM160 Promotes Tumor Growth in Lung Adenocarcinoma and Cervical Adenocarcinoma Cell Lines. International Journal of Molecular Sciences. 2025; 26(3):1097. https://doi.org/10.3390/ijms26031097
Chicago/Turabian StyleHerrera-Quiterio, Gloria Angelina, Heriberto Abraham Valencia-González, Karen Griselda de la Cruz-López, Diana Lashidua Fernández-Coto, Jeovanis Gil, György Marko-Varga, Josué Morales-Gálvez, Nilda C. Sánchez, Rubén Rodríguez-Bautista, Alejandro Avilés-Salas, and et al. 2025. "TMEM160 Promotes Tumor Growth in Lung Adenocarcinoma and Cervical Adenocarcinoma Cell Lines" International Journal of Molecular Sciences 26, no. 3: 1097. https://doi.org/10.3390/ijms26031097
APA StyleHerrera-Quiterio, G. A., Valencia-González, H. A., de la Cruz-López, K. G., Fernández-Coto, D. L., Gil, J., Marko-Varga, G., Morales-Gálvez, J., Sánchez, N. C., Rodríguez-Bautista, R., Avilés-Salas, A., Arrieta, O., García-Carrancá, A., & Encarnación-Guevara, S. (2025). TMEM160 Promotes Tumor Growth in Lung Adenocarcinoma and Cervical Adenocarcinoma Cell Lines. International Journal of Molecular Sciences, 26(3), 1097. https://doi.org/10.3390/ijms26031097







