Cellular and Molecular Mechanisms Modulated by Genistein in Cancer
Abstract
1. Introduction
2. Chemistry
3. Sources
4. Bioavailability and Metabolism
5. Genistein and Angiogenesis
6. Genistein Inhibits Cancer Invasion and Metastases
7. Genistein and Epithelial Mesenchymal Transition
8. Genistein Eradicates Cancer Stem Cells
9. Genistein and Cell Cycle Arrest
10. Programmed Cell Death
10.1. Breast Cancer
10.2. Prostate Cancer
10.3. Combinatorial Strategy
10.4. Autophagy and Ferroptosis
11. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AIF | apoptosis-inducing factor |
| Akt | protein kinase B |
| AMPK | AMP-activated protein kinase |
| ARHI/DIRAS3 | Aplasia Ras Homology I |
| ART | ataxia telangiectasia and Rad3-related kinase |
| ASK1 | apoptosis signaling kinase 1 |
| Bax | BCL2-associated X, apoptosis regulator |
| Bcl-2 | B-cell lymphoma 2 |
| Bim | Bcl-2 Interacting Mediator of cell death |
| CCNH | cyclin H |
| CD | cluster of differentiation |
| cdc | cell division cycle |
| CDK | cyclin-dependent kinase |
| CDKN2A | cyclin-dependent kinase inhibitor 2A |
| CHEK2 | checkpoint kinase 2 |
| CHOP | C/EBP homologous protein |
| CRC | colorectal cancer |
| CSCs | cancer stem cells |
| CuZnSOD | copper–zinc superoxide dismutase |
| CXCL16 | C-X-C Motif Chemokine Ligand 16 |
| DNA | deoxyribonucleic acid |
| DNA-PKcs | DNA kinase catalytic subunit |
| ECM | extracellular matrix |
| EGFR | epidermal growth factor receptor |
| EMT | epithelial–mesenchymal transition |
| ERs | estrogen receptors |
| ERK | extracellular signal-related kinase |
| ESA | epithelial-specific antigen |
| FADD | FAS-associated protein with death domain |
| FAKs | focal adhesion kinases |
| FGF | fibroblast growth factor |
| FOXM1 | Forkhead box protein M1 |
| FSARE | flaxseed aglycone-rich extract FTH1 ferritin heavy chain 1 |
| 5-FU | 5-fluorouracil |
| Gli1 | GLI Family Zinc Finger 1 |
| GPR30 | G protein-coupled receptor 30 |
| GPX | glutathione peroxidase |
| GSK-3β | glycogen synthase kinase 3-beta |
| HAT1 | histone acetyl transferase 1 |
| HCC | hepatocellular carcinoma |
| HeLa | human cervical carcinoma cell |
| HERC5 | Hect domain and RLD5 |
| HIF-1α | hypoxia-inducible factor 1α |
| HOTAIR | HOX Antisense Intergenic RNA |
| HPCD | 2-hydroxpropyl-beta-cyclodextrin |
| ICAM1 | intercellular adhesion molecules-1 |
| IGF-IR | type I insulin growth factor receptor |
| JAK | Janus kinase |
| JNK | Jun N-terminal kinase |
| KCNK9 | potassium channel proteins containing two pore-forming P domains |
| MAPK | mitogen-activated protein kinase |
| MCM | minichromosome maintenance |
| MEK | MAP kinase-ERK kinase |
| miRNAs | microRNA |
| MMPs | metalloproteases |
| MnSOD | manganese muperoxide dismutase |
| mRNA | messenger ribonucleic acid |
| mTOR | mammalian target of rapamycin |
| ncRNAs | non-coding RNAs |
| NFAT1 | nuclear factor of activated T cells 1 |
| NF-kB | nuclear factor-kappa B |
| Notch | signal transducer and activator of transcription |
| NSCLC | non-small cell lung carcinoma |
| Oct | octamer-binding transcription factor |
| OPN | osteopontin |
| PAI-1 | plasminogen activator inhibitor-1 |
| PARP | poly-ADP ribose polymerase |
| PC | prostate cancer |
| PCD | programmed cell death |
| PCNA | proliferating cell nuclear antigen |
| PDGF | platelet-derived growth factor |
| PI3K | phosphatidylinositol-3-kinase |
| PLK-1 | polo-like kinase 1 |
| PTEN | phosphatase and tensin homolog |
| ROS | reactive oxygen species |
| SARE | soya aglycone-rich extract |
| SIRT1 | sirtuin 1 |
| Snail | Snail homolog 1/2 of drosophila |
| SHH | Sonic Hedgehog |
| Sox2 | SEX-determining region (SRY) homology box 2 |
| SPI | soy protein isolate |
| STAT | signal transducer and activator of transcription |
| TAZ | PDZ-binding motif |
| TGF-β | transforming growth factor-beta |
| TNF | tumor necrosis factor |
| TNFR | tumor necrosis factor receptor |
| TRAIL | TNF-related apoptosis-inducing ligand |
| TPA | tetradecanoylphorbol-13-acetate |
| TSA | trichostatin A |
| TIMP-1 | tissue inhibitor of metalloproteinases 1 |
| Trx | thioredoxin |
| TSP-1 | thrombospondin-1 |
| TWIST | Twist family bHLH transcription factor |
| ULK1 kinase | UNC51-like kinase-1 |
| uPA | urokinase-type plasminogen activator |
| VEGF | vascular endothelial growth factor |
| YAP | Hippo-Yes-associated protein |
| ZEB1/2 | zinc finger E-box binding homeobox 1/2 |
| ZO-1 | zonula occludens-1 |
References
- Zhu, L.; Yang, X.; Zhu, R.; Yu, L. Identifying Discriminative Biological Function Features and Rules for Cancer-Related Long Non-Coding RNAs. Front. Genet. 2020, 11, 598773. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Suhail, Y.; Cain, M.P.; Vanaja, K.; Kurywchak, P.A.; Levchenko, A.; Kalluri, R. Kshitiz Systems Biology of Cancer Metastasis. Cell Syst. 2019, 9, 109–127. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Kucuk, O. Cancer Chemoprevention. Cancer Metastasis Rev. 2002, 21, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Steward, W.P.; Brown, K. Cancer Chemoprevention: A Rapidly Evolving Field. Br. J. Cancer. 2013, 109, 1–7. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, F.; Pagliacci, M.C.; Migliorati, G.; Moraca, R.; Grignani, F.; Riccardi, C.; Nicoletti, I. The Natural Tyrosine Kinase Inhibitor Genistein Produces Cell Cycle Arrest and Apoptosis in Jurkat T-Leukemia Cells. Leuk. Res. 1994, 18, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Boutas, I.; Kontogeorgi, A.; Dimitrakakis, C.; Kalantaridou, S.N. Soy Isoflavones and Breast Cancer Risk: A Meta-Analysis. In Vivo 2022, 36, 556–562. [Google Scholar] [CrossRef]
- Sivoňová, M.K.; Kaplán, P.; Tatarková, Z.; Lichardusová, L.; Dušenka, R.; Jurečeková, J. Androgen Receptor and Soy Isoflavones in Prostate Cancer. Mol. Clin. Oncol. 2019, 10, 191–204. [Google Scholar] [CrossRef]
- Van der Eecken, H.; Joniau, S.; Berghen, C.; Rans, K.; De Meerleer, G. The Use of Soy Isoflavones in the Treatment of Prostate Cancer: A Focus on the Cellular Effects. Nutrients 2023, 15, 4856. [Google Scholar] [CrossRef]
- Pavese, J.M.; Farmer, R.L.; Bergan, R.C. Inhibition of Cancer Cell Invasion and Metastasis by Genistein. Cancer Metastasis Rev. 2010, 29, 465–482. [Google Scholar] [CrossRef]
- Bhat, S.S.; Prasad, S.K.; Shivamallu, C.; Prasad, K.S.; Syed, A.; Reddy, P.; Cull, C.A.; Amachawadi, R.G. Genistein: A Potent Anti-Breast Cancer Agent. Curr. Issues Mol. Biol. 2021, 43, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-Targeted Therapy of Cancer by Genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and Cancer: Current Status, Challenges, and Future Directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxidative Med. Cell. Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef]
- Smeriglio, A.; Calderaro, A.; Denaro, M.; Laganà, G.; Bellocco, E. Effects of Isolated Isoflavones Intake on Health. Curr. Med. Chem. 2019, 26, 5094–5107. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Akhtar, J.; Singh, S.P.; Ahsan, F. An Overview on Genistein and Its Various Formulations. Drug Res. 2019, 69, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Garbiec, E.; Cielecka-Piontek, J.; Kowalówka, M.; Hołubiec, M.; Zalewski, P. Genistein-Opportunities Related to an Interesting Molecule of Natural Origin. Molecules 2022, 27, 815. [Google Scholar] [CrossRef]
- Tham, D.M.; Gardner, C.D.; Haskell, W.L. Clinical Review 97: Potential Health Benefits of Dietary Phytoestrogens: A Review of the Clinical, Epidemiological, and Mechanistic Evidence. J. Clin. Endocrinol. Metab. 1998, 83, 2223–2235. [Google Scholar] [CrossRef]
- Akiyama, T.; Ishida, J.; Nakagawa, S.; Ogawara, H.; Watanabe, S.; Itoh, N.; Shibuya, M.; Fukami, Y. Genistein, a Specific Inhibitor of Tyrosine-Specific Protein Kinases. J. Biol. Chem. 1987, 262, 5592–5595. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Ho, C.T.; Pan, M.H. Bioavailability and Health Benefits of Major Isoflavone Aglycones and Their Metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Isoflavone Content of Selected Foods, Release 2.0; U.S. Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory: Beltsville, MD, USA, 2008. Available online: https://www.ars.usda.gov/ARSUserFiles/80400535/Data/isoflav/Isoflav_R2.pdf (accessed on 1 November 2024).
- Hou, S. Genistein: Therapeutic and Preventive Effects, Mechanisms, and Clinical Application in Digestive Tract Tumor. Evid.-Based Complement. Altern. Med. 2022, 2022, 5957378. [Google Scholar] [CrossRef]
- Mureșan, L.; Clapa, D.; Borsai, O.; Teodor, R.; Wang, T.T.Y.; Park, J.B. Potential Impacts of Soil Tillage System on Isoflavone Concentration of Soybean as Functional Food Ingredients. Land 2020, 9, 386. [Google Scholar] [CrossRef]
- Zaheer, K.; Humayoun Akhtar, M. An Updated Review of Dietary Isoflavones: Nutrition, Processing, Bioavailability and Impacts on Human Health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Leuner, O.; Havlik, J.; Hummelova, J.; Prokudina, E.; Novy, P.; Kokoska, L. Distribution of Isoflavones and Coumestrol in Neglected Tropical and Subtropical Legumes. J. Sci. Food Agric. 2013, 93, 575–579. [Google Scholar] [CrossRef]
- Liggins, J.; Bluck, L.J.C.; Runswick, S.; Atkinson, C.; Coward, W.A.; Bingham, S.A. Daidzein and Genistein Contents of Vegetables. Br. J. Nutr. 2000, 84, 717–725. [Google Scholar] [CrossRef]
- Sureda, A.; Sanches Silva, A.; Sánchez-Machado, D.I.; López-Cervantes, J.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. Hypotensive Effects of Genistein: From Chemistry to Medicine. Chem. Biol. Interact. 2017, 268, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Wang, T.; Luo, Y. A Review on Plant-Based Proteins from Soybean: Health Benefits and Soy Product Development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xu, B. An Insight into the Health Benefits of Fermented Soy Products. Food Chem. 2019, 271, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Kulling, S.E.; Schwartz, H.; Rowland, I.; Ruefer, C.E.; Rimbach, G.; Cassidy, A.; Magee, P.; Millar, J.; Hall, W.L.; et al. Analytical and Compositional Aspects of Isoflavones in Food and Their Biological Effects. Mol. Nutr. Food Res. 2009, 53 (Suppl. S2), S266–S309. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Sharma, A. Phytoestrogen “Genistein”: Its Extraction and Isolation from Soybean Seeds. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 1121–1126. [Google Scholar]
- Wang, D.; Khan, M.S.; Cui, L.; Song, X.; Zhu, H.; Ma, T.; Li, X.; Sun, R. A Novel Method for the Highly Efficient Biotransformation of Genistein from Genistin Using a High-Speed Counter-Current Chromatography Bioreactor. RSC Adv. 2019, 9, 4892–4899. [Google Scholar] [CrossRef]
- He, J.; Fan, P.; Feng, S.; Shao, P.; Sun, P. Isolation and Purification of Two Isoflavones from Hericium Erinaceum Mycelium by High-Speed Counter-Current Chromatography. Molecules 2018, 23, 560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.J.; Ka, M.N.; Luo, K.Q. Extraction and Purification of Isoflavones from Soybeans and Characterization of Their Estrogenic Activities. J. Agric. Food Chem. 2007, 55, 6940–6950. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, B.; Di Carro, M.D.; Magi, E. Phytoestrogens in Soy-Based Meat Substitutes: Comparison of Different Extraction Methods for the Subsequent Analysis by Liquid Chromatography-Tandem Mass Spectrometry. J. Mass Spectrom. 2018, 53, 862–870. [Google Scholar] [CrossRef]
- Rusin, A.; Krawczyk, Z.; Grynkiewicz, G.; Gogler, A.; Zawisza-Puchałka, J.; Szeja, W. Synthetic Derivatives of Genistein, Their Properties and Possible Applications. Acta Biochim. Pol. 2010, 57, 23–34. [Google Scholar] [CrossRef]
- Cai, J.S.; Feng, J.Y.; Ni, Z.J.; Ma, R.H.; Thakur, K.; Wang, S.; Hu, F.; Zhang, J.G.; Wei, Z.J. An Update on the Nutritional, Functional, Sensory Characteristics of Soy Products, and Applications of New Processing Strategies. Trends Food Sci. Technol. 2021, 112, 676–689. [Google Scholar] [CrossRef]
- de Oliveira, I.C.; Santana, D.C.; de Oliveira, J.L.G.; Silva, E.V.M.; da Silva Candido Seron, A.C.; Blanco, M.; Teodoro, L.P.R.; da Silva Júnior, C.A.; Baio, F.H.R.; Alves, C.Z.; et al. Flavonoids and Their Relationship with the Physiological Quality of Seeds from Different Soybean Genotypes. Sci. Rep. 2024, 14, 17008. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Zimmer-Nechemias, L.; Brashear, W.T.; Wolfe, B.E.; Kirschner, A.S.; Heubi, J.E. Evidence for Lack of Absorption of Soy Isoflavone Glycosides in Humans, Supporting the Crucial Role of Intestinal Metabolism for Bioavailability. Am. J. Clin. Nutr. 2002, 76, 447–453. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic Activity, Biological Effect and Bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, E.K.; Gioxari, A.; Dimitriou, M.; Panoutsopoulos, G.I.; Panagiotopoulos, A.A. Molecular Pathways of Genistein Activity in Breast Cancer Cells. Int. J. Mol. Sci. 2024, 25, 5556. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sherrard, M.; Pagadala, S.; Wixon, R.; Scott, R.A. Isoflavone Content among Maturity Group 0 to II Soybeans. J. Am. Oil Chem. Soc. 2000, 77, 483–487. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Katan, M.B. Dietary Flavonoids: Intake, Health Effects and Bioavailability. Food Chem. Toxicol. 1999, 37, 937–942. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary Flavonoids: Bioavailability, Metabolic Effects, and Safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Kwon, S.H.; Kang, M.J.; Huh, J.S.; Ha, K.W.; Lee, J.R.; Lee, S.K.; Lee, B.S.; Han, I.H.; Lee, M.S.; Lee, M.W.; et al. Comparison of Oral Bioavailability of Genistein and Genistin in Rats. Int. J. Pharm. 2007, 337, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Motlekar, N.; Khan, M.A.; Youan, B.B.C. Preparation and Characterization of Genistein Containing Poly(Ethylene Glycol) Microparticles. J. Appl. Polym. Sci. 2006, 101, 2070–2078. [Google Scholar] [CrossRef]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kubota, Y.; Kikuchi, M. Soy Isoflavone Aglycones Are Absorbed Faster and in Higher Amounts than Their Glucosides in Humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid Metabolism: The Interaction of Metabolites and Gut Microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.A.; Lai, J.F.; Halm, B.M. Absorption, Distribution, Metabolism, and Excretion of Isoflavonoids after Soy Intake. Arch. Biochem. Biophys. 2014, 559, 24. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Kulkarni, K.; Zhu, W.; Hu, M. Bioavailability and Pharmacokinetics of Genistein: Mechanistic Studies on Its ADME. Anti-Cancer Agents Med. Chem. 2012, 12, 1264–1280. [Google Scholar] [CrossRef]
- Kobayashi, S.; Shinohara, M.; Nagai, T.; Konishi, Y. Transport Mechanisms for Soy Isoflavones and Microbial Metabolites Dihydrogenistein and Dihydrodaidzein across Monolayers and Membranes. Biosci. Biotechnol. Biochem. 2013, 77, 2210–2217. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; An, Q.; Gong, L.; Yang, S.; Zhang, B.; Su, B.; Yang, D.; Zhang, L.; Lu, Y.; et al. Optimized Solubility and Bioavailability of Genistein Based on Cocrystal Engineering. Nat. Prod. Bioprospect. 2023, 13, 30. [Google Scholar] [CrossRef]
- Aditya, N.P.; Shim, M.; Lee, I.; Lee, Y.; Im, M.H.; Ko, S. Curcumin and Genistein Coloaded Nanostructured Lipid Carriers: In Vitro Digestion and Antiprostate Cancer Activity. J. Agric. Food Chem. 2013, 61, 1878–1883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, X.; Ye, T.; Chen, F.; Sun, X.; Kong, J.; Yang, X.; Pan, W.; Li, S. Design, Characterization, and in Vitro Cellular Inhibition and Uptake of Optimized Genistein-Loaded NLC for the Prevention of Posterior Capsular Opacification Using Response Surface Methodology. Int. J. Pharm. 2013, 454, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Stancanelli, R.; Mazzaglia, A.; Tommasini, S.; Calabrò, M.L.; Villari, V.; Guardo, M.; Ficarra, P.; Ficarra, R. The Enhancement of Isoflavones Water Solubility by Complexation with Modified Cyclodextrins: A Spectroscopic Investigation with Implications in the Pharmaceutical Analysis. J. Pharm. Biomed. Anal. 2007, 44, 980–984. [Google Scholar] [CrossRef]
- Adair, T.H.; Montani, J.-P. Angiogenesis; Morgan & Claypool Life Sciences, Ed.; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Liekens, S.; De Clercq, E.; Neyts, J. Angiogenesis: Regulators and Clinical Applications. Biochem. Pharmacol. 2001, 61, 253–270. [Google Scholar] [CrossRef]
- Kazerounian, S.; Lawler, J. Integration of Pro- and Anti-Angiogenic Signals by Endothelial Cells. J. Cell Commun. Signal. 2018, 12, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Groothuis, P.G. Angiogenesis and Vascular Remodelling in Female Reproductive Organs. Angiogenesis 2005, 8, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Moeller, B.J.; Cao, Y.; Vujaskovic, Z.; Li, C.Y.; Haroon, Z.A.; Dewhirst, M.W. The Relationship between Hypoxia and Angiogenesis. Semin. Radiat. Oncol. 2004, 14, 215–221. [Google Scholar] [CrossRef]
- García-Caballero, M.; Sokol, L.; Cuypers, A.; Carmeliet, P. Metabolic Reprogramming in Tumor Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 11052. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The Role of Microenvironment in Tumor Angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar] [CrossRef]
- Bridges, E.M.; Harris, A.L. The Angiogenic Process as a Therapeutic Target in Cancer. Biochem. Pharmacol. 2011, 81, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Varinska, L.; Gal, P.; Mojzisova, G.; Mirossay, L.; Mojzis, J. Soy and Breast Cancer: Focus on Angiogenesis. Int. J. Mol. Sci. 2015, 16, 11728–11749. [Google Scholar] [CrossRef] [PubMed]
- Mukund, V.; Saddala, M.S.; Farran, B.; Mannavarapu, M.; Alam, A.; Nagaraju, G.P. Molecular Docking Studies of Angiogenesis Target Protein HIF-1α and Genistein in Breast Cancer. Gene 2019, 701, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Masoud, G.N.; Li, W. HIF-1α Pathway: Role, Regulation and Intervention for Cancer Therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef]
- Uifalean, A.; Rath, H.; Hammer, E.; Ionescu, C.; Iuga, C.A.; Lalk, M. Influence of Soy Isoflavones in Breast Cancer Angiogenesis: A Multiplex Glass ELISA Approach. J. BUON 2018, 23, 53–59. [Google Scholar]
- Deng, W.; Liu, X.; Huang, S.; Wu, Z.; Alessandro, F.; Lin, Q.; Cai, Z.; Zhang, Z.; Huang, Y.; Wang, H.; et al. CXCL16 Promotes Tumor Metastasis by Regulating Angiogenesis in the Tumor Micro-Environment of BRAF V600E Mutant Colorectal Cancer. Transl. Oncol. 2024, 41, 101854. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H. Flavonoids Inhibit VEGF/BFGF-Induced Angiogenesis in Vitro by Inhibiting the Matrix-Degrading Proteases. J. Cell. Biochem. 2003, 89, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, S.A.; Alp, E.; Yar Saglam, A.S.; Konac, E.; Menevse, E.S. The Effects of Thymoquinone and Genistein Treatment on Telomerase Activity, Apoptosis, Angiogenesis, and Survival in Thyroid Cancer Cell Lines. J. Cancer Res. Ther. 2018, 14, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, M.T.; Bellanti, F.; Zadorozhna, M.; Fiocco, D.; Mangieri, D. Multi-Faceted Role of Luteolin in Cancer Metastasis: EMT, Angiogenesis, ECM Degradation and Apoptosis. Int. J. Mol. Sci. 2023, 24, 8824. [Google Scholar] [CrossRef] [PubMed]
- Brassart-Pasco, S.; Brézillon, S.; Brassart, B.; Ramont, L.; Oudart, J.B.; Monboisse, J.C. Tumor Microenvironment: Extracellular Matrix Alterations Influence Tumor Progression. Front. Oncol. 2020, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Basset, P.; Okada, A.; Chenard, M.P.; Kannan, R.; Stoll, I.; Anglard, P.; Bellocq, J.P.; Rio, M.C. Matrix Metalloproteinases as Stromal Effectors of Human Carcinoma Progression: Therapeutic Implications. Matrix Biol. 1997, 15, 535–541. [Google Scholar] [CrossRef]
- John, A.; Tuszynski, G. The Role of Matrix Metalloproteinases in Tumor Angiogenesis and Tumor Metastasis. Pathol. Oncol. Res. 2001, 7, 14–23. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Matrix Metalloproteinases and Tumor Metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef]
- Kousidou, O.C.; Mitropoulou, T.N.; Roussidis, A.E.; Kletsas, D.; Theocharis, A.D.; Karamanos, N.K. Genistein Suppresses the Invasive Potential of Human Breast Cancer Cells through Transcriptional Regulation of Metalloproteinases and Their Tissue Inhibitors. Int. J. Oncol. 2005, 26, 1101–1109. [Google Scholar] [CrossRef]
- Rajendran, P. Unveiling the Power of Flavonoids: A Dynamic Exploration of Their Impact on Cancer through Matrix Metalloproteinases Regulation. Biomedicine 2024, 14, 12–28. [Google Scholar] [CrossRef]
- Han, L.; Zhang, H.W.; Zhou, W.P.; Chen, G.M.; Guo, K.J. The Effects of Genistein on Transforming Growth Factor-Β1-Induced Invasion and Metastasis in Human Pancreatic Cancer Cell Line Panc-1 in Vitro. Chin. Med. J. 2012, 125, 2032–2040. [Google Scholar] [PubMed]
- Wang, Z.; Ahmad, A.; Banerjee, S.; Azmi, A.; Kong, D.; Li, Y.; Sarkar, F.H. FoxM1 Is a Novel Target of a Natural Agent in Pancreatic Cancer. Pharm. Res. 2010, 27, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liu, Z.; Wang, R.; Wang, J.; Zhang, S.; Cai, X.; Wu, K.; Bergan, R.C.; Xu, L.; Fan, D. Genistein Suppresses FLT4 and Inhibits Human Colorectal Cancer Metastasis. Oncotarget 2015, 6, 3225–3239. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Harish, G.; Prabhu, S.A.; Mohsin, J.; Khan, M.A.; Rizvi, T.A.; Sharma, C. Inhibitory Effect of Genistein on the Invasive Potential of Human Cervical Cancer Cells via Modulation of Matrix Metalloproteinase-9 and Tissue Inhibitors of Matrix Metalloproteinase-1 Expression. Cancer Epidemiol. 2012, 36, e387–e393. [Google Scholar] [CrossRef] [PubMed]
- Saykali, B.A.; El-Sibai, M. Invadopodia, Regulation, and Assembly in Cancer Cell Invasion. Cell Commun. Adhes. 2014, 21, 207–212. [Google Scholar] [CrossRef]
- Brullo, C.; Tasso, B. New Insights on Fak and Fak Inhibitors. Curr. Med. Chem. 2021, 28, 3318–3338. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhu, C.F.; Iwamoto, H.; Chen, J.S. Genistein Inhibits Invasive Potential of Human Hepatocellular Carcinoma by Altering Cell Cycle, Apoptosis, and Angiogenesis. World J. Gastroenterol. 2005, 11, 6512–6517. [Google Scholar] [CrossRef]
- Malik, P.; Singh, R.; Kumar, M.; Malik, A.; Mukherjee, T.K. Understanding the Phytoestrogen Genistein Actions on Breast Cancer: Insights on Estrogen Receptor Equivalence, Pleiotropic Essence and Emerging Paradigms in Bioavailability Modulation. Curr. Top. Med. Chem. 2023, 23, 1395–1413. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen Receptors Alpha (ERα) and Beta (ERβ): Subtype-Selective Ligands and Clinical Potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef]
- Chan, K.K.L.; Siu, M.K.Y.; Jiang, Y.X.; Wang, J.J.; Leung, T.H.Y.; Ngan, H.Y.S. Estrogen Receptor Modulators Genistein, Daidzein and ERB-041 Inhibit Cell Migration, Invasion, Proliferation and Sphere Formation via Modulation of FAK and PI3K/AKT Signaling in Ovarian Cancer. Cancer Cell Int. 2018, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Yin, L.; Ding, J.; Jin, H.; Feng, Y. Genistein Inhibits Placental Choriocarcinoma Cell Line JAR Invasion through ERβ/MTA3/Snail/E-Cadherin Pathway. Oncol. Lett. 2011, 2, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK Pathway for Cancer Therapy: From Mechanism to Clinical Studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, Y.; Chen, S.; Ruan, X.; Liao, H.; Zhang, Y.; Sun, J.; Gao, J.; Deng, G. Genistein Inhibits Migration and Invasion of Cervical Cancer HeLa Cells by Regulating FAK-Paxillin and MAPK Signaling Pathways. Taiwan J. Obstet. Gynecol. 2020, 59, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Hong, S.; Lin, S.; Chen, K. Genistein Inhibits the Proliferation, Migration and Invasion of the Squamous Cell Carcinoma Cells via Inhibition of MEK/ERK and JNK Signalling Pathways. J. BUON 2020, 25, 1172–1177. [Google Scholar] [PubMed]
- Cui, S.; Wang, J.; Wu, Q.; Qian, J.; Yang, C.; Bo, P. Genistein Inhibits the Growth and Regulates the Migration and Invasion Abilities of Melanoma Cells via the FAK/Paxillin and MAPK Pathways. Oncotarget 2017, 8, 21674–21691. [Google Scholar] [CrossRef]
- Danciu, C.; Borcan, F.; Bojin, F.; Zupko, I.; Dehelean, C. Effect of the Isoflavone Genistein on Tumor Size, Metastasis Potential and Melanization in a B16 Mouse Model of Murine Melanoma. Nat. Prod. Commun. 2013, 8, 343–346. [Google Scholar] [CrossRef]
- Hung, C.Y.; Lee, C.H.; Chiou, H.L.; Line, C.L.; Chen, P.N.; Lin, M.T.; Hsieh, Y.H.; Chou, M.C. Praeruptorin-B Inhibits 12-O-Tetradecanoylphorbol-13-Acetate-Induced Cell Invasion by Targeting AKT/NF-ΚB via Matrix Metalloproteinase-2/-9 Expression in Human Cervical Cancer Cells. Cell Physiol. Biochem. 2019, 52, 1255–1266. [Google Scholar] [CrossRef]
- Wang, S.D.; Chen, B.C.; Kao, S.T.; Liu, C.J.; Yeh, C.C. Genistein Inhibits Tumor Invasion by Suppressing Multiple Signal Transduction Pathways in Human Hepatocellular Carcinoma Cells. BMC Complement. Altern. Med. 2014, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Khongsti, K.; Das, K.B.; Das, B. MAPK Pathway and SIRT1 Are Involved in the Down-Regulation of Secreted Osteopontin Expression by Genistein in Metastatic Cancer Cells. Life Sci. 2021, 265, 118787. [Google Scholar] [CrossRef]
- Panda, V.K.; Mishra, B.; Nath, A.N.; Butti, R.; Yadav, A.S.; Malhotra, D.; Khanra, S.; Mahapatra, S.; Mishra, P.; Swain, B.; et al. Osteopontin: A Key Multifaceted Regulator in Tumor Progression and Immunomodulation. Biomedicines 2024, 12, 1527. [Google Scholar] [CrossRef] [PubMed]
- Touny, L.H.E.; Banerjee, P.P. Identification of a Biphasic Role for Genistein in the Regulation of Prostate Cancer Growth and Metastasis. Cancer Res. 2009, 69, 3695–3703. [Google Scholar] [CrossRef]
- Xu, L.; Bergan, R.C. Genistein Inhibits Matrix Metalloproteinase Type 2 Activation and Prostate Cancer Cell Invasion by Blocking the Transforming Growth Factor Beta-Mediated Activation of Mitogen-Activated Protein Kinase-Activated Protein Kinase 2-27-KDa Heat Shock Protein Pathway. Mol. Pharmacol. 2006, 70, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Wang, Y.; Kurita, T.; Adomat, H.; Cunha, G.R.; Wang, Y. Genistein Increases Epidermal Growth Factor Receptor Signaling and Promotes Tumor Progression in Advanced Human Prostate Cancer. PLoS ONE 2011, 6, e20034. [Google Scholar] [CrossRef]
- Xu, L.; Ding, Y.; Catalona, W.J.; Yang, X.J.; Anderson, W.F.; Jovanovic, B.; Wellman, K.; Killmer, J.; Huang, X.; Scheidt, K.A.; et al. MEK4 Function, Genistein Treatment, and Invasion of Human Prostate Cancer Cells. J. Natl. Cancer Inst. 2009, 101, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- Javed, Z.; Khan, K.; Herrera-Bravo, J.; Naeem, S.; Iqbal, M.J.; Sadia, H.; Qadri, Q.R.; Raza, S.; Irshad, A.; Akbar, A.; et al. Genistein as a Regulator of Signaling Pathways and MicroRNAs in Different Types of Cancers. Cancer Cell Int. 2021, 21, 388. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.M.; LaSpina, M.; Long, J.; Ho, S.M. Expression of Estrogen Receptor (ER)-Alpha and ER-Beta in Normal and Malignant Prostatic Epithelial Cells: Regulation by Methylation and Involvement in Growth Regulation. Cancer Res. 2000, 60, 3175–3182. [Google Scholar]
- Patel, A.J.; Lazdunski, M. The 2P-Domain K+ Channels: Role in Apoptosis and Tumorigenesis. Pflug. Arch. 2004, 448, 261–273. [Google Scholar] [CrossRef]
- Kim, C.J.; Cho, Y.G.; Jeong, S.W.; Kim, Y.S.; Kim, S.Y.; Nam, S.W.; Lee, S.H.; Yoo, N.J.; Lee, J.Y.; Park, W.S. Altered Expression of KCNK9 in Colorectal Cancers. APMIS 2004, 112, 588–594. [Google Scholar] [CrossRef]
- Cheng, Y.; Tang, Y.; Tan, Y.; Li, J.; Zhang, X. KCNK9 Mediates the Inhibitory Effects of Genistein on Hepatic Metastasis from Colon Cancer. Clinics 2023, 78, 100141. [Google Scholar] [CrossRef]
- Kotula, E.; Berthault, N.; Agrario, C.; Lienafa, M.C.; Simon, A.; Dingli, F.; Loew, D.; Sibut, V.; Saule, S.; Dutreix, M. DNA-PKcs Plays Role in Cancer Metastasis through Regulation of Secreted Proteins Involved in Migration and Invasion. Cell Cycle 2015, 14, 1961–1972. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Liu, B.; Zheng, X.; Li, P.; Zhao, T.; Jin, X.; Ye, F.; Zhang, P.; Chen, W.; et al. Genistein Inhibits Radiation-Induced Invasion and Migration of Glioblastoma Cells by Blocking the DNA-PKcs/Akt2/Rac1 Signaling Pathway. Radiother. Oncol. 2021, 155, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Flavahan, W.A.; Gaskell, E.; Bernstein, B.E. Epigenetic Plasticity and the Hallmarks of Cancer. Science 2017, 357, eaal2380. [Google Scholar] [CrossRef]
- Huang, M.; Chen, C.; Geng, J.; Han, D.; Wang, T.; Xie, T.; Wang, L.; Wang, Y.; Wang, C.; Lei, Z.; et al. Targeting KDM1A Attenuates Wnt/β-Catenin Signaling Pathway to Eliminate Sorafenib-Resistant Stem-like Cells in Hepatocellular Carcinoma. Cancer Lett. 2017, 398, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ren, J.; Tang, L. Genistein Inhibits Invasion and Migration of Colon Cancer Cells by Recovering WIF1 Expression. Mol. Med. Rep. 2018, 17, 7265–7273. [Google Scholar] [CrossRef]
- Xia, J.; Cheng, L.; Mei, C.; Ma, J.; Shi, Y.; Zeng, F.; Wang, Z.; Wang, Z. Genistein Inhibits Cell Growth and Invasion through Regulation of MiR-27a in Pancreatic Cancer Cells. Curr. Pharm. Des. 2014, 20, 5348–5353. [Google Scholar] [CrossRef]
- Hirata, H.; Ueno, K.; Nakajima, K.; Tabatabai, Z.L.; Hinoda, Y.; Ishii, N.; Dahiya, R. Genistein Downregulates Onco-MiR-1260b and Inhibits Wnt-Signalling in Renal Cancer Cells. Br. J. Cancer 2013, 108, 2070–2078. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Y.; Gu, J.; Liang, P.; Shen, M.; Xi, J.; Qin, J. Anti-Invasive Effect and Pharmacological Mechanism of Genistein against Colorectal Cancer. Biofactors 2020, 46, 620–628. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Jakobsen, T.; Hager, H.; Kjems, J. The Emerging Roles of CircRNAs in Cancer and Oncology. Nat. Rev. Clin. Oncol. 2022, 19, 188–206. [Google Scholar] [CrossRef]
- Pu, Y.; Han, Y.; Ouyang, Y.; Li, H.; Li, L.; Wu, X.; Yang, L.; Gao, J.; Zhang, L.; Zhou, J.; et al. Kaempferol Inhibits Colorectal Cancer Metastasis through Circ_0000345 Mediated JMJD2C/β-Catenin Signalling Pathway. Phytomedicine 2024, 128, 155261. [Google Scholar] [CrossRef]
- Wang, W.; Xie, Y.; Malhotra, A. Potential of Curcumin and Quercetin in Modulation of Premature Mitochondrial Senescence and Related Changes during Lung Carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2021, 40, 53–60. [Google Scholar] [CrossRef]
- Liao, G.B.; Li, X.Z.; Zeng, S.; Liu, C.; Yang, S.M.; Yang, L.; Hu, C.J.; Bai, J.Y. Regulation of the Master Regulator FOXM1 in Cancer. Cell Commun. Signal. 2018, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xing, Y.; Zhang, Q.; Zhang, Q.; Huang, S.; Li, X.; Gao, C. Soy Isoflavone Genistein Inhibits Hsa_circ_0031250/MiR-873-5p/FOXM1 Axis to Suppress Non-Small-Cell Lung Cancer Progression. IUBMB Life 2021, 73, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Qi, J.; Li, X.-T.; Zhou, K.; Xu, J.-H.; Zhou, Y.; Zhang, G.-Q.; Xu, J.-P.; Zhou, R.-J. ATRA and Genistein Synergistically Inhibit the Metastatic Potential of Human Lung Adenocarcinoma Cells. Int. J. Clin. Exp. Med. 2015, 8, 4220–4227. [Google Scholar] [PubMed]
- Erdogan, M.A.; Yılmaz, O.A. Rottlerin and Genistein Inhibit Neuroblastoma Cell Proliferation and Invasion through EF2K Suppression and Related Protein Pathways. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 2481–2500. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular Mechanisms of Epithelial-Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Huang, R.Y.J.; Guilford, P.; Thiery, J.P. Early Events in Cell Adhesion and Polarity during Epithelial-Mesenchymal Transition. J. Cell Sci. 2012, 125, 4417–4422. [Google Scholar] [CrossRef]
- Gonzalez, D.M.; Medici, D. Signaling Mechanisms of the Epithelial-Mesenchymal Transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [CrossRef]
- Akrida, I.; Bravou, V.; Papadaki, H. The Deadly Cross-Talk between Hippo Pathway and Epithelial-Mesenchymal Transition (EMT) in Cancer. Mol. Biol. Rep. 2022, 49, 10065–10076. [Google Scholar] [CrossRef] [PubMed]
- Khanbabaei, H.; Ebrahimi, S.; García-Rodríguez, J.L.; Ghasemi, Z.; Pourghadamyari, H.; Mohammadi, M.; Kristensen, L.S. Non-Coding RNAs and Epithelial Mesenchymal Transition in Cancer: Molecular Mechanisms and Clinical Implications. J. Exp. Clin. Cancer Res. 2022, 41, 278. [Google Scholar] [CrossRef] [PubMed]
- Debnath, P.; Huirem, R.S.; Dutta, P.; Palchaudhuri, S. Epithelial-Mesenchymal Transition and Its Transcription Factors. Biosci. Rep. 2022, 42, BSR20211754. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Xing, T.; Yang, Z.; Dudek, R.; Lu, Q.; Chen, Y.H. Epithelial Mesenchymal Transition in Embryonic Development, Tissue Repair and Cancer: A Comprehensive Overview. J. Clin. Med. 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Kozak, J.; Forma, A.; Czeczelewski, M.; Kozyra, P.; Sitarz, E.; Radzikowska-büchner, E.; Sitarz, M.; Baj, J. Inhibition or Reversal of the Epithelial-Mesenchymal Transition in Gastric Cancer: Pharmacological Approaches. Int. J. Mol. Sci. 2020, 22, 277. [Google Scholar] [CrossRef]
- Hsieh, P.L.; Liao, Y.W.; Hsieh, C.W.; Chen, P.N.; Yu, C.C. Soy Isoflavone Genistein Impedes Cancer Stemness and Mesenchymal Transition in Head and Neck Cancer through Activating MiR-34a/RTCB Axis. Nutrients 2020, 12, 1924. [Google Scholar] [CrossRef]
- Ma, J.; Zeng, F.; Ma, C.; Pang, H.; Fang, B.; Lian, C.; Yin, B.; Zhang, X.; Wang, Z.; Xia, J. Synergistic Reversal Effect of Epithelial-to-Mesenchymal Transition by MiR-223 Inhibitor and Genistein in Gemcitabine-Resistant Pancreatic Cancer Cells. Am. J. Cancer Res. 2016, 6, 1384–1395. [Google Scholar]
- Kim, Y.S.; Choi, K.C.; Hwang, K.A. Genistein Suppressed Epithelial-Mesenchymal Transition and Migration Efficacies of BG-1 Ovarian Cancer Cells Activated by Estrogenic Chemicals via Estrogen Receptor Pathway and Downregulation of TGF-β Signaling Pathway. Phytomedicine 2015, 22, 993–999. [Google Scholar] [CrossRef]
- Dai, W.; Wang, F.; He, L.; Lin, C.; Wu, S.; Chen, P.; Zhang, Y.; Shen, M.; Wu, D.; Wang, C.; et al. Genistein Inhibits Hepatocellular Carcinoma Cell Migration by Reversing the Epithelial-Mesenchymal Transition: Partial Mediation by the Transcription Factor NFAT1. Mol. Carcinog. 2015, 54, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wang, C.; Hu, Z.; Chen, W.; Qi, W.; Li, A. Genistein Induces Apoptosis of Colon Cancer Cells by Reversal of Epithelial-to-Mesenchymal via a Notch1/NF-ΚB/Slug/E-Cadherin Pathway. BMC Cancer 2017, 17, 813. [Google Scholar] [CrossRef]
- Zhang, L.L.; Li, L.; Wu, D.P.; Fan, J.H.; Li, X.; Wu, K.J.; Wang, X.Y.; He, D.L. A Novel Anti-Cancer Effect of Genistein: Reversal of Epithelial Mesenchymal Transition in Prostate Cancer Cells. Acta Pharmacol. Sin. 2008, 29, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lv, B.; Yi, C.; Cui, X.; Sui, S.; Li, X.; Qi, M.; Hao, C.; Han, B.; Liu, Z. Genistein Inhibits Human Papillary Thyroid Cancer Cell Detachment, Invasion and Metastasis. J. Cancer 2019, 10, 737–748. [Google Scholar] [CrossRef]
- Ayob, A.Z.; Ramasamy, T.S. Cancer Stem Cells as Key Drivers of Tumour Progression. J. Biomed. Sci. 2018, 25, 20. [Google Scholar] [CrossRef]
- Atashzar, M.R.; Baharlou, R.; Karami, J.; Abdollahi, H.; Rezaei, R.; Pourramezan, F.; Zoljalali Moghaddam, S.H. Cancer Stem Cells: A Review from Origin to Therapeutic Implications. J. Cell. Physiol. 2020, 235, 790–803. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting Cancer Stem Cell Pathways for Cancer Therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-Catenin Signaling Pathway in Cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Lian, I.; Kim, J.; Okazawa, H.; Zhao, J.; Zhao, B.; Yu, J.; Chinnaiyan, A.; Israel, M.A.; Goldstein, L.S.B.; Abujarour, R.; et al. The Role of YAP Transcription Coactivator in Regulating Stem Cell Self-Renewal and Differentiation. Genes Dev. 2010, 24, 1106–1118. [Google Scholar] [CrossRef]
- Ajani, J.A.; Song, S.; Hochster, H.S.; Steinberg, I.B. Cancer Stem Cells: The Promise and the Potential. Semin. Oncol. 2015, 42 (Suppl. S1), S3–S17. [Google Scholar] [CrossRef]
- Sharpe, B.; Beresford, M.; Bowen, R.; Mitchard, J.; Chalmers, A.D. Searching for Prostate Cancer Stem Cells: Markers and Methods. Stem Cell Rev. Rep. 2013, 9, 721–730. [Google Scholar] [CrossRef]
- Song, S.Y.; Seo, D. Cancer Stem Cells: Biological Features and Targeted Therapeutics. Hanyang Med. Rev. 2015, 35, 250. [Google Scholar] [CrossRef]
- Naujokat, C.; McKee, D.L. The “Big Five” Phytochemicals Targeting Cancer Stem Cells: Curcumin, EGCG, Sulforaphane, Resveratrol and Genistein. Curr. Med. Chem. 2021, 28, 4321–4342. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Jiao, M.; Wu, D.; Wu, K.; Li, X.; Zhu, G.; Yang, L.; Wang, X.; Hsieh, J.T.; et al. Genistein Inhibits the Stemness Properties of Prostate Cancer Cells through Targeting Hedgehog-Gli1 Pathway. Cancer Lett. 2012, 323, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Naujokata, C.; Laufer, S. Targeting Cancer Stem Cells with Defined Compounds and Drugs. J. Cancer Res. Updates 2013, 2, 36–67. [Google Scholar] [CrossRef]
- Chae, H.S.; Xu, R.; Won, J.Y.; Chin, Y.W.; Yim, H. Molecular Targets of Genistein and Its Related Flavonoids to Exert Anticancer Effects. Int. J. Mol. Sci. 2019, 20, 2420. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.A.; Hwang, K.A.; Choi, K.C. Roles of Dietary Phytoestrogens on the Regulation of Epithelial-Mesenchymal Transition in Diverse Cancer Metastasis. Toxins 2016, 8, 162. [Google Scholar] [CrossRef]
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its Role in Metabolic Diseases and Cancer. Crit. Rev. Oncol. Hematol. 2017, 119, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Fan, S.; Wang, H.; Mao, J.; Shi, Y.; Ibrahim, M.M.; Ma, W.; Yu, X.; Hou, Z.; Wang, B.; et al. Genistein Decreases the Breast Cancer Stem-like Cell Population through Hedgehog Pathway. Stem Cell Res. Ther. 2013, 4, 146. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, T.; Wang, S.; Chen, H.; Su, D.; Fu, X.; Zhang, Q.; Kang, X. Genistein-Induced Differentiation of Breast Cancer Stem/Progenitor Cells through a Paracrine Mechanism. Int. J. Oncol. 2016, 48, 1063–1072. [Google Scholar] [CrossRef]
- Yu, D.; Shin, H.S.; Lee, Y.S.; Lee, D.; Kim, S.; Lee, Y.C. Genistein Attenuates Cancer Stem Cell Characteristics in Gastric Cancer through the Downregulation of Gli1. Oncol. Rep. 2014, 31, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wan, C.; Luo, Q.; Huang, Z.; Luo, Q. Genistein-Inhibited Cancer Stem Cell-like Properties and Reduced Chemoresistance of Gastric Cancer. Int. J. Mol. Sci. 2014, 15, 3432–3443. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Zhang, T.A.O.; Sun, X.; Li, Y.; Geng, H.A.O.; Yu, D.; Zhong, C. Sonic Hedgehog Pathway Mediates Genistein Inhibition of Renal Cancer Stem Cells. Oncol. Lett. 2019, 18, 3081–3091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cao, W.S.; Wang, X.Q.; Zhang, M.; Lu, X.M.; Chen, J.Q.; Chen, Y.; Ge, M.M.; Zhong, C.Y.; Han, H.Y. Genistein Inhibits Nasopharyngeal Cancer Stem Cells through Sonic Hedgehog Signaling. Phytother. Res. 2019, 33, 2783–2791. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ren, K.; Song, Z.; Li, D.; Quan, M.; Zheng, Y.; Cao, J.; Zeng, W.; Zou, H. 7-Difluoromethoxyl-5,4′-Di-n-Octyl Genistein Inhibits the Stem-like Characteristics of Gastric Cancer Stem-like Cells and Reverses the Phenotype of Epithelial-Mesenchymal Transition in Gastric Cancer Cells. Oncol. Rep. 2016, 36, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Xu, M.; Cao, X.; Chen, X.; Luo, X. Inactivation of AKT, ERK and NF-ΚB by Genistein Derivative, 7-Difluoromethoxyl-5,4′-Di-n-Octylygenistein, Reduces Ovarian Carcinoma Oncogenicity. Oncol. Rep. 2017, 38, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.X.; Li, Q.X.; Ren, K.Q.; Quan, M.F.; Cao, J.G. 7-Difluoromethoxyl-5,4′-Di-n-Octyl Genistein Inhibits Ovarian Cancer Stem Cell Characteristics through the Downregulation of FOXM1. Oncol. Lett. 2014, 8, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Cao, X.; Liu, L.; Cao, X.; Cui, Y.; Li, X.; Quan, M.; Ren, K.; Chen, A.; Xu, C.; et al. Genistein Inhibits Lung Cancer Cell Stem-like Characteristics by Modulating MnSOD and FoxM1 Expression. Oncol. Lett. 2020, 20, 2506–2515. [Google Scholar] [CrossRef]
- Montales, M.T.E.; Rahal, O.M.; Kang, J.; Rogers, T.J.; Prior, R.L.; Wu, X.; Simmen, R.C.M. Repression of Mammosphere Formation of Human Breast Cancer Cells by Soy Isoflavone Genistein and Blueberry Polyphenolic Acids Suggests Diet-Mediated Targeting of Cancer Stem-like/Progenitor Cells. Carcinogenesis 2012, 33, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, E.S.; Singh, A.T.K. Cell-Cycle Checkpoints and Aneuploidy on the Path to Cancer. In Vivo 2018, 32, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Barnum, K.J.; O’Connell, M.J. Cell Cycle Regulation by Checkpoints. Methods Mol. Biol. 2014, 1170, 29. [Google Scholar] [CrossRef]
- Ding, L.; Cao, J.; Lin, W.; Chen, H.; Xiong, X.; Ao, H.; Yu, M.; Lin, J.; Cui, Q. The Roles of Cyclin-Dependent Kinases in Cell-Cycle Progression and Therapeutic Strategies in Human Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1960. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Cell Cycle, CDKs and Cancer: A Changing Paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Stewart, Z.A.; Westfall, M.D.; Pietenpol, J.A. Cell-Cycle Dysregulation and Anticancer Therapy. Trends Pharmacol. Sci. 2003, 24, 139–145. [Google Scholar] [CrossRef]
- Pietenpol, J.A.; Stewart, Z.A. Cell Cycle Checkpoint Signaling: Cell Cycle Arrest versus Apoptosis. Toxicology 2002, 181, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.L.; Southgate, H.; Tweddle, D.A.; Curtin, N.J. DNA Damage Checkpoint Kinases in Cancer. Expert Rev. Mol. Med. 2020, 22, e2. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, M.H.; Muthugounder, S.; Presser, N.; Viswanathan, S. Anticancer Therapeutic Potential of Soy Isoflavone, Genistein. Adv. Exp. Med. Biol. 2004, 546, 121–165. [Google Scholar] [CrossRef]
- Farghadani, R.; Naidu, R. The Anticancer Mechanism of Action of Selected Polyphenols in Triple-Negative Breast Cancer (TNBC). Biomed. Pharmacother. 2023, 165, 115170. [Google Scholar] [CrossRef]
- Mukund, V. Genistein: Its Role in Breast Cancer Growth and Metastasis. Curr. Drug Metab. 2020, 21, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Cha, H.J.; Lee, H.; Hwang-Bo, H.; Ji, S.Y.; Kim, M.Y.; Hong, S.H.; Jeong, J.W.; Han, M.H.; Choi, S.H.; et al. Induction of G2/M Cell Cycle Arrest and Apoptosis by Genistein in Human Bladder Cancer T24 Cells through Inhibition of the ROS-Dependent PI3k/Akt Signal Transduction Pathway. Antioxidants 2019, 8, 327. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhang, G.Q.; Yang, Y.; Zhang, C.Y.; Fu, R.X.; Yang, Y.M. Genistein Induces G2/M Arrest in Gastric Cancer Cells by Increasing the Tumor Suppressor PTEN Expression. Nutr. Cancer 2013, 65, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Robak, E.; Gerlicz-Kowalczuk, Z.; Dziankowska-Bartkowiak, B.; Wozniacka, A.; Bogaczewicz, J. Genistein-Triggered Anticancer Activity against Liver Cancer Cell Line HepG2 Involves ROS Generation, Mitochondrial Apoptosis, G2/M Cell Cycle Arrest and Inhibition of Cell Migration. Arch. Med. Sci. 2019, 15, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.L.; Min, M.; Shen, W.; Liu, Y. Genistein Induced Anticancer Effects on Pancreatic Cancer Cell Lines Involves Mitochondrial Apoptosis, G0/G1cell Cycle Arrest and Regulation of STAT3 Signalling Pathway. Phytomedicine 2018, 39, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy Isoflavones and Prostate Cancer: A Review of Molecular Mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Katiyar, S.K. Cell Cycle Control as a Basis for Cancer Chemoprevention through Dietary Agents. Front. Biosci. 2008, 13, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, V.; Fioravanti, L.; Miodini, P.; Di Fronzo, G. Genistein Blocks Breast Cancer Cells in the G(2)M Phase of the Cell Cycle. J. Cell. Biochem. 2000, 79, 594–600. [Google Scholar] [CrossRef]
- Alatawi, F.S.; Faridi, U. Anticancer and Anti-Metastasis Activity of 1,25 Dihydroxycholecalciferols and Genistein in MCF-7 and MDA-MB-231 Breast Cancer Cell Lines. Heliyon 2023, 9, e21975. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Z.; Hill, D.L.; Chen, X.; Wang, H.; Zhang, R. Genistein, a Dietary Isoflavone, down-Regulates the MDM2 Oncogene at Both Transcriptional and Posttranslational Levels. Cancer Res. 2005, 65, 8200–8208. [Google Scholar] [CrossRef] [PubMed]
- Oki, T.; Sowa, Y.; Hirose, T.; Takagaki, N.; Horinaka, M.; Nakanishi, R.; Yasuda, C.; Yoshida, T.; Kanazawa, M.; Satomi, Y.; et al. Genistein Induces Gadd45 Gene and G2/M Cell Cycle Arrest in the DU145 Human Prostate Cancer Cell Line. FEBS Lett. 2004, 577, 55–59. [Google Scholar] [CrossRef]
- Majid, S.; Kikuno, N.; Nelles, J.; Noonan, E.; Tanaka, Y.; Kawamoto, K.; Hirata, H.; Li, L.C.; Zhao, H.; Okino, S.T.; et al. Genistein Induces the P21WAF1/CIP1 and P16INK4a Tumor Suppressor Genes in Prostate Cancer Cells by Epigenetic Mechanisms Involving Active Chromatin Modification. Cancer Res. 2008, 68, 2736–2744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, B.; Zhou, K.; Li, H.; Li, D.; Gao, H.; Zhang, T.; Wei, D.; Li, Z.; Diao, Y. Potential Therapeutic Mechanism of Genistein in Breast Cancer Involves Inhibition of Cell Cycle Regulation. Mol. Med. Rep. 2015, 11, 1820–1826. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Q.; Wang, X.; Yang, X.; Wang, X.; Huang, Z.; Jiao, Y.; Wang, J. Quantitative Phosphoproteomics Reveals Genistein as a Modulator of Cell Cycle and DNA Damage Response Pathways in Triple-Negative Breast Cancer Cells. Int. J. Oncol. 2016, 48, 1016. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, W.; Mo, B.; Hu, C.; Liu, H.; Qi, H.; Wang, X.; Xu, J. Caffeine Overcomes Genistein-Induced G2/M Cell Cycle Arrest in Breast Cancer Cells. Nutr. Cancer 2008, 60, 382–388. [Google Scholar] [CrossRef]
- Balabhadrapathruni, S.; Thomas, T.J.; Yurkow, E.J.; Amenta, P.S.; Thomas, T. Effects of Genistein and Structurally Related Phytoestrogens on Cell Cycle Kinetics and Apoptosis in MDA-MB-468 Human Breast Cancer Cells. Oncol. Rep. 2000, 7, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Takeshima, M.; Nishi, A.; Higuchi, T.; Nakano, S. Genistein Suppresses V-Src-Driven Proliferative Activity by Arresting the Cell-Cycle at G2/M through Increasing P21 Level in Src-Activated Human Gallbladder Carcinoma Cells. Nutr. Cancer 2021, 73, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, C.; Jin, X.; Li, P.; Ye, F.; Zhao, T.; Gong, L.; Li, Q. Genistein Enhances the Radiosensitivity of Breast Cancer Cells via G2/M Cell Cycle Arrest and Apoptosis. Molecules 2013, 18, 13200–13217. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Li, Z.; Wei, C. Genistein Inhibits the S-Phase Kinase-Associated Protein 2 Expression in Breast Cancer Cells. Exp. Ther. Med. 2018, 15, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhou, W.; He, W.; Liu, X.; Ding, Q.; Ling, L.; Zha, X.; Wang, S. Genistein Inhibits MDA-MB-231 Triple-Negative Breast Cancer Cell Growth by Inhibiting NF-ΚB Activity via the Notch-1 Pathway. Int. J. Mol. Med. 2012, 30, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; Mo, B.; Hu, C.; Liu, H.; Qi, H.; Wang, X.; Xu, J. Genistein Induces G2/M Cell Cycle Arrest via Stable Activation of ERK1/2 Pathway in MDA-MB-231 Breast Cancer Cells. Cell Biol. Toxicol. 2008, 24, 401–409. [Google Scholar] [CrossRef]
- Kim, G.Y.; Suh, J.; Jang, J.-H.; Kim, D.-H.; Park, O.J.; Park, S.K.; Surh, Y.-J. Genistein Inhibits Proliferation of BRCA1 Mutated Breast Cancer Cells: The GPR30-Akt Axis as a Potential Target. J. Cancer Prev. 2019, 24, 197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Xiang, N.; Domann, F.E.; Zhong, W. Effects of Selenite and Genistein on G2/M Cell Cycle Arrest and Apoptosis in Human Prostate Cancer Cells. Nutr. Cancer 2009, 61, 397–407. [Google Scholar] [CrossRef]
- Raffoul, J.J.; Wang, Y.; Kucuk, O.; Forman, J.D.; Sarkar, F.H.; Hillman, G.G. Genistein Inhibits Radiation-Induced Activation of NF-KappaB in Prostate Cancer Cells Promoting Apoptosis and G2/M Cell Cycle Arrest. BMC Cancer 2006, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.J.; Kim, B.S.; Chun, S.Y.; Park, Y.K.; Kang, K.S.; Kwon, T.G. Apoptotic Effects of Genistein, Biochanin-A and Apigenin on LNCaP and PC-3 Cells by P21 through Transcriptional Inhibition of Polo-like Kinase-1. J. Korean Med. Sci. 2011, 26, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.M.; Brown-Clay, J.D.; Fornace, A.J. Gadd45 in Stress Signaling, Cell Cycle Control, and Apoptosis. Adv. Exp. Med. Biol. 2013, 793, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Humayun, A.; Fornace, A.J. GADD45 in Stress Signaling, Cell Cycle Control, and Apoptosis. Adv. Exp. Med. Biol. 2022, 1360, 1–22. [Google Scholar] [CrossRef]
- Rabiau, N.; Kossaï, M.; Braud, M.; Chalabi, N.; Satih, S.; Bignon, Y.J.; Bernard-Gallon, D.J. Genistein and Daidzein Act on a Panel of Genes Implicated in Cell Cycle and Angiogenesis by Polymerase Chain Reaction Arrays in Human Prostate Cancer Cell Lines. Cancer Epidemiol. 2010, 34, 200–206. [Google Scholar] [CrossRef]
- Shenouda, N.S.; Zhou, C.; Browning, J.D.; Ansell, P.J.; Sakla, M.S.; Lubahn, D.B.; MacDonald, R.S. Phytoestrogens in Common Herbs Regulate Prostate Cancer Cell Growth in Vitro. Nutr. Cancer 2004, 49, 200–208. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Hernandez-Montes, E.; Vauzour, D.; Schönthal, A.H.; Rice-Evans, C.; Cadenas, E.; Spencer, J.P.E. The Intracellular Genistein Metabolite 5,7,3′,4′-Tetrahydroxyisoflavone Mediates G2-M Cell Cycle Arrest in Cancer Cells via Modulation of the P38 Signaling Pathway. Free Radic. Biol. Med. 2006, 41, 1225–1239. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Raji, I.; Cinar, B.; Kucuk, O.; Oyelere, A.K. Design, Synthesis, and Evaluation of the Antiproliferative Activity of Hydantoin-Derived Antiandrogen-Genistein Conjugates. Bioorg. Med. Chem. 2018, 26, 1481. [Google Scholar] [CrossRef]
- Tsuboy, M.S.; Marcarini, J.C.; De Souza, A.O.; De Paula, N.A.; Dorta, D.J.; Mantovani, M.S.; Ribeiro, L.R. Genistein at Maximal Physiologic Serum Levels Induces G0/G1 Arrest in MCF-7 and HB4a Cells, But Not Apoptosis. J. Med. Food 2014, 17, 218–225. [Google Scholar] [CrossRef]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Iriti, M.; Wojtyczka, R.D.; Buszman, E.; Stojko, J. Flavonoids, Bioactive Components of Propolis, Exhibit Cytotoxic Activity and Induce Cell Cycle Arrest and Apoptosis in Human Breast Cancer Cells MDA-MB-231 and MCF-7—A Comparative Study. Cell. Mol. Biol. 2018, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Hou, Y.C.; Lin, C.H.; Hsu, Y.A.; Sheu, J.J.C.; Lai, C.H.; Chen, B.H.; Lee Chao, P.D.; Wan, L.; Tsai, F.J. Puerariae Radix Isoflavones and Their Metabolites Inhibit Growth and Induce Apoptosis in Breast Cancer Cells. Biochem. Biophys. Res. Commun. 2009, 378, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Pons, D.G.; Nadal-Serrano, M.; Blanquer-Rossello, M.M.; Sastre-Serra, J.; Oliver, J.; Roca, P. Genistein Modulates Proliferation and Mitochondrial Functionality in Breast Cancer Cells Depending on ERalpha/ERbeta Ratio. J. Cell. Biochem. 2014, 115, 949–958. [Google Scholar] [CrossRef]
- Mai, Z.; Blackburn, G.L.; Zhou, J.R. Genistein Sensitizes Inhibitory Effect of Tamoxifen on the Growth of Estrogen Receptor-Positive and HER2-Overexpressing Human Breast Cancer Cells. Mol. Carcinog. 2007, 46, 534–542. [Google Scholar] [CrossRef]
- Kaufman-Szymczyk, A.; Jalmuzna, J.; Lubecka-Gajewska, K. Soy-Derived Isoflavones as Chemo-Preventive Agents Targeting Multiple Signalling Pathways for Cancer Prevention and Therapy. Br. J. Pharmacol. 2024. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Goel, N. Intrinsic and Extrinsic Pathways of Apoptosis: Role in Cancer Development and Prognosis. Adv. Protein Chem. Struct. Biol. 2021, 125, 73–120. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting Apoptosis in Cancer Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Kiraz, Y.; Adan, A.; Kartal Yandim, M.; Baran, Y. Major Apoptotic Mechanisms and Genes Involved in Apoptosis. Tumour Biol. 2016, 37, 8471–8486. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Liao, M.; Qin, R.; Zhu, S.; Peng, C.; Fu, L.; Chen, Y.; Han, B. Regulated Cell Death (RCD) in Cancer: Key Pathways and Targeted Therapies. Signal Transduct. Target. Ther. 2022, 7, 286. [Google Scholar] [CrossRef]
- Edlich, F. BCL-2 Proteins and Apoptosis: Recent Insights and Unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 Family Isoforms in Apoptosis and Cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-ΚB Signaling Pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Mukhtar, E.; Mustafa Adhami, V.; Khan, N.; Mukhtar, H. Apoptosis and Autophagy Induction as Mechanism of Cancer Prevention by Naturally Occurring Dietary Agents. Curr. Drug Targets 2012, 13, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Shahar, N.; Larisch, S. Inhibiting the Inhibitors: Targeting Anti-Apoptotic Proteins in Cancer and Therapy Resistance. Drug Resist. Updates 2020, 52, 100712. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.Y.; Lin, L.T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad Targeting of Resistance to Apoptosis in Cancer. Semin. Cancer Biol. 2015, 35, S78–S103. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.; Rehman, K.; Shahid, M.; Suhail, S.; Akash, M.S.H. Therapeutic Potentials of Genistein: New Insights and Perspectives. J. Food Biochem. 2022, 46, e14228. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.; Arnould, S.; Scalbert, A.; Manach, C. Isoflavones and the Prevention of Breast and Prostate Cancer: New Perspectives Opened by Nutrigenomics. Br. J. Nutr. 2008, 99 (Suppl. S1), ES78–ES108. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, J.A.; Takahashi, Y.; Chandramouli, G.V.R.; Liu, H.; Perkins, S.N.; Hursting, S.D.; Wang, T.T.Y. Concentration-Dependent Effects of Genistein on Global Gene Expression in MCF-7 Breast Cancer Cells: An Oligo Microarray Study. Breast Cancer Res. Treat. 2008, 110, 85–98. [Google Scholar] [CrossRef]
- Tophkhane, C.; Yang, S.; Bales, W.; Archer, L.; Osunkoya, A.; Thor, A.D.; Yang, X. Bcl-2 Overexpression Sensitizes MCF-7 Cells to Genistein by Multiple Mechanisms. Int. J. Oncol. 2007, 31, 867–874. [Google Scholar] [CrossRef]
- Choi, E.J.; Jung, J.Y.; Kim, G.H. Genistein Inhibits the Proliferation and Differentiation of MCF-7 and 3T3-L1 Cells via the Regulation of ERα Expression and Induction of Apoptosis. Exp. Ther. Med. 2014, 8, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Ju, J.H.; Jang, K.; Shin, I. Induction of Apoptotic Cell Death by Phytoestrogens by Up-Regulating the Levels of Phospho-P53 and P21 in Normal and Malignant Estrogen Receptor α-Negative Breast Cells. Nutr. Res. 2011, 31, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.X.; Zhao, M.; Parris, A.B.; Xing, Y.; Yang, X. Genistein Targets the Cancerous Inhibitor of PP2A to Induce Growth Inhibition and Apoptosis in Breast Cancer Cells. Int. J. Oncol. 2016, 49, 1203–1210. [Google Scholar] [CrossRef]
- Sakamoto, T.; Horiguchi, H.; Oguma, E.; Kayama, F. Effects of Diverse Dietary Phytoestrogens on Cell Growth, Cell Cycle and Apoptosis in Estrogen-Receptor-Positive Breast Cancer Cells. J. Nutr. Biochem. 2010, 21, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, C.; Yong, W.; Ye, Y.; Huang, Z. Calycosin and Genistein Induce Apoptosis by Inactivation of HOTAIR/p-Akt Signaling Pathway in Human Breast Cancer MCF-7 Cells. Cell. Physiol. Biochem. 2015, 35, 722–728. [Google Scholar] [CrossRef]
- Hakami, M.A.; Hazazi, A.; Abdulaziz, O.; Almasoudi, H.H.; Alhazmi, A.Y.M.; Alkhalil, S.S.; Alharthi, N.S.; Alhuthali, H.M.; Almalki, W.H.; Gupta, G.; et al. HOTAIR: A Key Regulator of the Wnt/β-Catenin Signaling Cascade in Cancer Progression and Treatment. Pathol. Res. Pract. 2024, 253, 154957. [Google Scholar] [CrossRef]
- Chen, F.P.; Chien, M.H. Phytoestrogens Induce Apoptosis through a Mitochondria/Caspase Pathway in Human Breast Cancer Cells. Climacteric 2014, 17, 385–392. [Google Scholar] [CrossRef]
- Chan, M.M.; Chen, R.; Fong, D. Targeting Cancer Stem Cells with Dietary Phytochemical—Repositioned Drug Combinations. Cancer Lett. 2018, 433, 53–64. [Google Scholar] [CrossRef]
- Seo, H.S.; Choi, H.S.; Choi, H.S.; Choi, Y.K.; Um, J.Y.; Choi, I.; Shin, Y.C.; Ko, S.G. Phytoestrogens Induce Apoptosis via Extrinsic Pathway, Inhibiting Nuclear Factor-KappaB Signaling in HER2-Overexpressing Breast Cancer Cells. Anti-Cancer Res. 2011, 31, 3301–3313. [Google Scholar]
- Li, Z.; Li, J.; Mo, B.; Hu, C.; Liu, H.; Qi, H.; Wang, X.; Xu, J. Genistein Induces Cell Apoptosis in MDA-MB-231 Breast Cancer Cells via the Mitogen-Activated Protein Kinase Pathway. Toxicol Vitr. 2008, 22, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Duan, Y.; Zhang, X.; Ye, Y.; Ge, B.; Chen, J. Genistein Induces Apoptosis by the Inactivation of the IGF-1R/p-Akt Signaling Pathway in MCF-7 Human Breast Cancer Cells. Food Funct. 2015, 6, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Sarg, N.H.; Zaher, D.M.; Abu Jayab, N.N.; Mostafa, S.H.; Ismail, H.H.; Omar, H.A. The Interplay of P38 MAPK Signaling and Mitochondrial Metabolism, a Dynamic Target in Cancer and Pathological Contexts. Biochem. Pharmacol. 2024, 225, 116307. [Google Scholar] [CrossRef]
- Shim, H.Y.; Park, J.H.; Paik, H.D.; Nah, S.Y.; Kim, D.S.H.L.; Han, Y.S. Genistein-Induced Apoptosis of Human Breast Cancer MCF-7 Cells Involves Calpain-Caspase and Apoptosis Signaling Kinase 1-P38 Mitogen-Activated Protein Kinase Activation Cascades. Anti-Cancer Drugs 2007, 18, 649–657. [Google Scholar] [CrossRef]
- Sergeev, I.N. Genistein Induces Ca2+ -Mediated, Calpain/Caspase-12-Dependent Apoptosis in Breast Cancer Cells. Biochem. Biophys. Res. Commun. 2004, 321, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiang, Z.; Malaviarachchi, P.A.; Yan, Y.; Baltz, N.J.; Emanuel, P.D.; Liu, Y.L. PTEN Is Indispensable for Cells to Respond to MAPK Inhibitors in Myeloid Leukemia. Cell Signal. 2018, 50, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Waite, K.A.; Sinden, M.R.; Eng, C. Phytoestrogen Exposure Elevates PTEN Levels. Hum. Mol. Genet. 2005, 14, 1457–1463. [Google Scholar] [CrossRef]
- De La Parra, C.; Castillo-Pichardo, L.; Cruz-Collazo, A.; Cubano, L.; Redis, R.; Calin, G.A.; Dharmawardhane, S. Soy Isoflavone Genistein-Mediated Downregulation of MiR-155 Contributes to the Anticancer Effects of Genistein. Nutr. Cancer 2016, 68, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Dave, B.; Eason, R.R.; Till, S.R.; Geng, Y.; Velarde, M.C.; Badger, T.M.; Simmen, R.C.M. The Soy Isoflavone Genistein Promotes Apoptosis in Mammary Epithelial Cells by Inducing the Tumor Suppressor PTEN. Carcinogenesis 2005, 26, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Prietsch, R.F.; Monte, L.G.; Da Silva, F.A.; Beira, F.T.; Del Pino, F.A.B.; Campos, V.F.; Collares, T.; Pinto, L.S.; Spanevello, R.M.; Gamaro, G.D.; et al. Genistein Induces Apoptosis and Autophagy in Human Breast MCF-7 Cells by Modulating the Expression of Proapoptotic Factors and Oxidative Stress Enzymes. Mol. Cell. Biochem. 2014, 390, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.F.; Ahmad, A.; Zubair, H.; Khan, H.Y.; Wang, Z.; Sarkar, F.H.; Hadi, S.M. Soy Isoflavone Genistein Induces Cell Death in Breast Cancer Cells through Mobilization of Endogenous Copper Ions and Generation of Reactive Oxygen Species. Mol. Nutr. Food Res. 2011, 55, 553–559. [Google Scholar] [CrossRef]
- Hwang, C.S.; Kwak, H.S.; Lim, H.J.; Lee, S.H.; Kang, Y.S.; Choe, T.B.; Hur, H.G.; Han, K.O. Isoflavone Metabolites and Their in Vitro Dual Functions: They Can Act as an Estrogenic Agonist or Antagonist Depending on the Estrogen Concentration. J. Steroid Biochem. Mol. Biol. 2006, 101, 246–253. [Google Scholar] [CrossRef]
- Sotoca, A.M.; Sollewijn Gelpke, M.D.; Boeren, S.; Ström, A.; Gustafsson, J.-Å.; Murk, A.J.; Rietjens, I.M.C.M.; Vervoort, J. Quantitative Proteomics and Transcriptomics Addressing the Estrogen Receptor Subtype-Mediated Effects in T47D Breast Cancer Cells Exposed to the Phytoestrogen Genistein. Mol. Cell. Proteom. 2011, 10, M110.002170. [Google Scholar] [CrossRef] [PubMed]
- Obiorah, I.E.; Fan, P.; Jordan, V.C. Breast Cancer Cell Apoptosis with Phytoestrogens Is Dependent on an Estrogen-Deprived State. Cancer Prev. Res. 2014, 7, 939–949. [Google Scholar] [CrossRef]
- Chen, F.P.; Chien, M.H. Effects of Phytoestrogens on the Activity and Growth of Primary Breast Cancer Cells Ex Vivo. J. Obstet. Gynaecol. Res. 2019, 45, 1352–1362. [Google Scholar] [CrossRef]
- Rajah, T.T.; Peine, K.J.; Du, N.; Serret, C.A.; Drews, N.R. Physiological Concentrations of Genistein and 17β-Estradiol Inhibit MDA-MB-231 Breast Cancer Cell Growth by Increasing BAX/BCL-2 and Reducing PERK1/2. Anti-Cancer Res. 2012, 32, 1181–1191. [Google Scholar]
- Choi, E.J.; Kim, G.H. Antiproliferative Activity of Daidzein and Genistein May Be Related to ERα/c-ErbB-2 Expression in Human Breast Cancer Cells. Mol. Med. Rep. 2013, 7, 781–784. [Google Scholar] [CrossRef]
- Ock, P.J. Comparison of Estrogen and Genistein in Their Antigenotoxic Effects, Apoptosis and Signal Transduction Protein Expression Patterns. Biofactors 2004, 21, 379–382. [Google Scholar] [CrossRef]
- Park, O.J.; Shin, J.I. Proapoptotic Potentials of Genistein under Growth Stimulation by Estrogen. Ann. N. Y. Acad. Sci. 2004, 1030, 410–418. [Google Scholar] [CrossRef]
- Chen, F.P.; Chien, M.H.; Chern, I.Y.Y. Impact of Lower Concentrations of Phytoestrogens on the Effects of Estradiol in Breast Cancer Cells. Climacteric 2015, 18, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Lucki, N.C.; Sewer, M.B. Genistein Stimulates MCF-7 Breast Cancer Cell Growth by Inducing Acid Ceramidase (ASAH1) Gene Expression. J. Biol. Chem. 2011, 286, 19399–19409. [Google Scholar] [CrossRef]
- Schmidt, S.; Michna, H.; Diel, P. Combinatory Effects of Phytoestrogens and 17beta-Estradiol on Proliferation and Apoptosis in MCF-7 Breast Cancer Cells. J. Steroid Biochem. Mol. Biol. 2005, 94, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Privat, M.; Aubel, C.; Arnould, S.; Communal, Y.; Ferrara, M.; Bignon, Y.J. Breast Cancer Cell Response to Genistein Is Conditioned by BRCA1 Mutations. Biochem. Biophys. Res. Commun. 2009, 379, 785–789. [Google Scholar] [CrossRef]
- Privat, M.; Aubel, C.; Arnould, S.; Communal, Y.; Ferrara, M.; Bignon, Y.J. AKT and P21 WAF1/CIP1 as Potential Genistein Targets in BRCA1-Mutant Human Breast Cancer Cell Lines. Anti-Cancer Res. 2010, 30, 2049–2054. [Google Scholar]
- Dutta, S.; Kharkar, P.S.; Sahu, N.U.; Khanna, A. Molecular Docking Prediction and in Vitro Studies Elucidate Anti-Cancer Activity of Phytoestrogens. Life Sci. 2017, 185, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Stocco, B.; Toledo, K.A.; Fumagalli, H.F.; Bianchini, F.J.; Fortes, V.S.; Fonseca, M.J.V.; Toloi, M.R.T. Biotransformed Soybean Extract Induces Cell Death of Estrogen-Dependent Breast Cancer Cells by Modulation of Apoptotic Proteins. Nutr. Cancer 2015, 67, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, G.; Saidijam, M.; Tayebinia, H.; Khodadadi, I. Beneficial Effects of Genistein in Suppression of Proliferation, Inhibition of Metastasis, and Induction of Apoptosis in PC3 Prostate Cancer Cells. Arch. Physiol. Biochem. 2022, 128, 694–702. [Google Scholar] [CrossRef]
- Kumi-Diaka, J.K.; Hassanhi, M.; Merchant, K.; Horman, V. Influence of Genistein Isoflavone on Matrix Metalloproteinase-2 Expression in Prostate Cancer Cells. J. Med. Food 2006, 9, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Che, M.; Bhagat, S.; Ellis, K.L.; Kucuk, O.; Doerge, D.R.; Abrams, J.; Cher, M.L.; Sarkar, F.H. Regulation of Gene Expression and Inhibition of Experimental Prostate Cancer Bone Metastasis by Dietary Genistein. Neoplasia 2004, 6, 354–363. [Google Scholar] [CrossRef]
- Karsli-Ceppioglu, S.; Ngollo, M.; Adjakly, M.; Dagdemir, A.; Judes, G.; Lebert, A.; Boiteux, J.P.; Penault-Llorca, F.; Bignon, Y.J.; Guy, L.; et al. Genome-Wide DNA Methylation Modified by Soy Phytoestrogens: Role for Epigenetic Therapeutics in Prostate Cancer? OMICS J. Integr. Biol. 2015, 19, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Saif Zaman, M.; Deng, G.; Majid, S.; Saini, S.; Liu, J.; Tanaka, Y.; Dahiya, R. MicroRNAs 221/222 and Genistein-Mediated Regulation of ARHI Tumor Suppressor Gene in Prostate Cancer. Cancer Prev. Res. 2011, 4, 76–86. [Google Scholar] [CrossRef]
- Chiyomaru, T.; Yamamura, S.; Fukuhara, S.; Hidaka, H.; Majid, S.; Saini, S.; Arora, S.; Deng, G.; Shahryari, V.; Chang, I.; et al. Genistein Up-Regulates Tumor Suppressor MicroRNA-574-3p in Prostate Cancer. PLoS ONE 2013, 8, e58929. [Google Scholar] [CrossRef]
- Phillip, C.J.; Giardina, C.K.; Bilir, B.; Cutler, D.J.; Lai, Y.H.; Kucuk, O.; Moreno, C.S. Genistein Cooperates with the Histone Deacetylase Inhibitor Vorinostat to Induce Cell Death in Prostate Cancer Cells. BMC Cancer 2012, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Chiyomaru, T.; Yamamura, S.; Fukuhara, S.; Yoshino, H.; Kinoshita, T.; Majid, S.; Saini, S.; Chang, I.; Tanaka, Y.; Enokida, H.; et al. Genistein Inhibits Prostate Cancer Cell Growth by Targeting MiR-34a and Oncogenic HOTAIR. PLoS ONE 2013, 8, e70372. [Google Scholar] [CrossRef] [PubMed]
- Terzioglu-Usak, S.; Yildiz, M.T.; Goncu, B.; Ozten-Kandas, N. Achieving the Balance: Biphasic Effects of Genistein on PC-3 Cells. J. Food Biochem. 2019, 43, e12951. [Google Scholar] [CrossRef]
- Szliszka, E.; Krol, W. Soy Isoflavones Augment the Effect of TRAIL-Mediated Apoptotic Death in Prostate Cancer Cells. Oncol. Rep. 2011, 26, 533–541. [Google Scholar] [CrossRef]
- Khamesi, S.M.; Barough, M.S.; Zargan, J.; Shayesteh, M.; Banaee, N.; Noormohammadi, A.H.; Alikhani, H.K.; Mousavi, M. Evaluation of Anticancer and Cytotoxic Effects of Genistein on PC3 Prostate Cell Line under Three-Dimensional Culture Medium. Iran. Biomed. J. 2022, 26, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; El Oirdi, M.; Aatif, M.; Nahvi, I.; Muteeb, G.; Alam, M.W. Soy Isoflavones Induce Cell Death by Copper-Mediated Mechanism: Understanding Its Anticancer Properties. Molecules 2023, 28, 2925. [Google Scholar] [CrossRef]
- Bosland, M.C.; Huang, J.; Schlicht, M.J.; Enk, E.; Xie, H.; Kato, I. Impact of 18-Month Soy Protein Supplementation on Steroid Hormones and Serum Biomarkers of Angiogenesis, Apoptosis, and the Growth Hormone/IGF-1 Axis: Results of a Randomized, Placebo-Controlled Trial in Males Following Prostatectomy. Nutr. Cancer 2022, 74, 110–121. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Kong, D.; Li, R.; Sarkar, S.H.; Sarkar, F.H. Regulation of Akt/FOXO3a/GSK-3beta/AR Signaling Network by Isoflavone in Prostate Cancer Cells. J. Biol. Chem. 2008, 283, 27707–27716. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.; Bray, T.M.; Helferich, W.G.; Doerge, D.R.; Ho, E. Differential Effects of Whole Soy Extract and Soy Isoflavones on Apoptosis in Prostate Cancer Cells. Exp. Biol. Med. 2010, 235, 90–97. [Google Scholar] [CrossRef]
- Tepper, C.G.; Vinall, R.L.; Wee, C.B.; Xue, L.; Shi, X.B.; Burich, R.; Mack, P.C.; White, R.W.D.V. GCP-Mediated Growth Inhibition and Apoptosis of Prostate Cancer Cells via Androgen Receptor-Dependent and -Independent Mechanisms. Prostate 2007, 67, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Vinall, R.L.; Hwa, K.; Ghosh, P.; Pan, C.X.; Lara, P.N.; De Vere White, R.W. Combination Treatment of Prostate Cancer Cell Lines with Bioactive Soy Isoflavones and Perifosine Causes Increased Growth Arrest and/or Apoptosis. Clin. Cancer Res. 2007, 13, 6204–6216. [Google Scholar] [CrossRef] [PubMed]
- Burich, R.A.; Holland, W.S.; Vinall, R.L.; Tepper, C.; DeVere White, R.W.; Mack, P.C. Genistein Combined Polysaccharide Enhances Activity of Docetaxel, Bicalutamide and Src Kinase Inhibition in Androgen-Dependent and Independent Prostate Cancer Cell Lines. BJU Int. 2008, 102, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Bemis, D.L.; Capodice, J.L.; Desai, M.; Buityan, R.; Katz, A.E. A Concentrated Aglycone Isoflavone Preparation (GCP) That Demonstrates Potent Anti-Prostate Cancer Activity in Vitro and in Vivo. Clin. Cancer Res. 2004, 10, 5282–5292. [Google Scholar] [CrossRef] [PubMed]
- Vaishampayan, U.; Hussain, M.; Banerjee, M.; Seren, S.; Sarkar, F.H.; Fontana, J.; Forman, J.D.; Cher, M.L.; Powell, I.; Pontes, J.E.; et al. Lycopene and Soy Isoflavones in the Treatment of Prostate Cancer. Nutr. Cancer 2007, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ardito, F.; Di Gioia, G.; Pellegrino, M.R.; Muzio, L. Lo Genistein as a Potential Anticancer Agent Against Head and Neck Squamous Cell Carcinoma. Curr. Top. Med. Chem. 2018, 18, 174–181. [Google Scholar] [CrossRef]
- Das, R.; Woo, J. Identifying the Multitarget Pharmacological Mechanism of Action of Genistein on Lung Cancer by Integrating Network Pharmacology and Molecular Dynamic Simulation. Molecules 2024, 29, 1913. [Google Scholar] [CrossRef]
- Hsiao, Y.C.; Peng, S.F.; Lai, K.C.; Liao, C.L.; Huang, Y.P.; Lin, C.C.; Lin, M.L.; Liu, K.C.; Tsai, C.C.; Ma, Y.S.; et al. Genistein Induces Apoptosis in Vitro and Has Antitumor Activity against Human Leukemia HL-60 Cancer Cell Xenograft Growth in Vivo. Environ. Toxicol. 2019, 34, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, Y.; Amrán, D.; Fernández, C.; De Blas, E.; Aller, P. Genistein Selectively Potentiates Arsenic Trioxide-Induced Apoptosis in Human Leukemia Cells via Reactive Oxygen Species Generation and Activation of Reactive Oxygen Species-Inducible Protein Kinases (P38-MAPK, AMPK). Int. J. Cancer 2008, 123, 1205–1214. [Google Scholar] [CrossRef]
- Raynal, N.J.M.; Momparler, L.; Charbonneau, M.; Momparler, R.L. Antileukemic Activity of Genistein, a Major Isoflavone Present in Soy Products. J. Nat. Prod. 2008, 71, 3–7. [Google Scholar] [CrossRef]
- Xue, J.P.; Wang, G.; Zhao, Z.B.; Wang, Q.; Shi, Y. Synergistic Cytotoxic Effect of Genistein and Doxorubicin on Drug-Resistant Human Breast Cancer MCF-7/Adr Cells. Oncol. Rep. 2014, 32, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ahmed, F.; Ali, S.; Philip, P.A.; Kucuk, O.; Sarkar, F.H. Inactivation of Nuclear Factor KappaB by Soy Isoflavone Genistein Contributes to Increased Apoptosis Induced by Chemotherapeutic Agents in Human Cancer Cells. Cancer Res. 2005, 65, 6934–6942. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xu, X.; Liang, C.; Chen, X.; Yu, X.; Zhong, H.; Xu, W.; Cheng, Y.; Wang, W.; Wu, Y.; et al. ERβ Modulates Genistein’s Cisplatin-Enhancing Activities in Breast Cancer MDA-MB-231 Cells via P53-Independent Pathway. Mol. Cell. Biochem. 2019, 456, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.J.; Xie, M.Y.; Kluxen, F.M.; Diel, P. Genistein Modulates the Anti-Tumor Activity of Cisplatin in MCF-7 Breast and HT-29 Colon Cancer Cells. Arch. Toxicol. 2014, 88, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, X.; Min, J.; Chen, X.; Liu, R.; Cui, X.; Cheng, J.; Xie, M.; Diel, P.; Hu, X. Genistein Interferes with Antitumor Effects of Cisplatin in an Ovariectomized Breast Cancer Xenograft Tumor Model. Toxicol. Lett. 2022, 355, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Pons, D.G.; Nadal-Serrano, M.; Torrens-Mas, M.; Oliver, J.; Roca, P. The Phytoestrogen Genistein Affects Breast Cancer Cells Treatment Depending on the ERα/ERβ Ratio. J. Cell. Biochem. 2016, 117, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Pan, S.L.; Guh, J.H.; Teng, C.M. Genistein Inversely Affects Tubulin-Binding Agent-Induced Apoptosis in Human Breast Cancer Cells. Biochem. Pharmacol. 2004, 67, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Chen, Z.; Kim, S.; Iqbal, S.; Chi, A.; Ritenour, C.; Wang, Y.A.; Kucuk, O.; Wu, D. Genistein Enhances the Efficacy of Cabazitaxel Chemotherapy in Metastatic Castration-Resistant Prostate Cancer Cells. Prostate 2013, 73, 1681–1689. [Google Scholar] [CrossRef]
- Hörmann, V.; Kumi-Diaka, J.; Durity, M.; Rathinavelu, A. Anticancer Activities of Genistein-Topotecan Combination in Prostate Cancer Cells. J. Cell. Mol. Med. 2012, 16, 2631–2636. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Shyam, H.; Agarwal, S.; Sharma, R.; Nag, T.C.; Dwivedi, A.K.; Balapure, A.K. Genistein Potentiates Centchroman Induced Antineoplasticity in Breast Cancer via PI3K/Akt Deactivation and ROS Dependent Induction of Apoptosis. Life Sci. 2019, 239, 117073. [Google Scholar] [CrossRef] [PubMed]
- Swami, S.; Krishnan, A.V.; Peehl, D.M.; Feldman, D. Genistein Potentiates the Growth Inhibitory Effects of 1,25-Dihydroxyvitamin D3 in DU145 Human Prostate Cancer Cells: Role of the Direct Inhibition of CYP24 Enzyme Activity. Mol. Cell. Endocrinol. 2005, 241, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.Y.; Leem, J.; Yoon, S.J.; Yoon, S.; Hong, S.J. Lipid Raft Cholesterol and Genistein Inhibit the Cell Viability of Prostate Cancer Cells via the Partial Contribution of EGFR-Akt/P70S6k Pathway and down-Regulation of Androgen Receptor. Biochem. Biophys. Res. Commun. 2010, 393, 319–324. [Google Scholar] [CrossRef]
- Chang, K.L.; Cheng, H.L.; Huang, L.W.; Hsieh, B.S.; Hu, Y.C.; Chih, T.T.; Shyu, H.W.; Su, S.J. Combined Effects of Terazosin and Genistein on a Metastatic, Hormone-Independent Human Prostate Cancer Cell Line. Cancer Lett. 2009, 276, 14–20. [Google Scholar] [CrossRef]
- Hillman, G.G.; Wang, Y.; Kucuk, O.; Che, M.; Doerge, D.R.; Yudelev, M.; Joiner, M.C.; Marples, B.; Forman, J.D.; Sarkar, F.H. Genistein Potentiates Inhibition of Tumor Growth by Radiation in a Prostate Cancer Orthotopic Model. Mol. Cancer Ther. 2004, 3, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.X.; Ejima, Y.; Sasaki, R.; Zheng, S.S.; Demizu, Y.; Soejima, T.; Sugimura, K. Combination of Genistein with Ionizing Radiation on Androgen-Independent Prostate Cancer Cells. Asian J. Androl. 2004, 6, 285–290. [Google Scholar] [PubMed]
- Ferenc, P.; Solár, P.; Kleban, J.; Mikeš, J.; Fedoročko, P. Down-Regulation of Bcl-2 and Akt Induced by Combination of Photoactivated Hypericin and Genistein in Human Breast Cancer Cells. J. Photochem. Photobiol. B 2010, 98, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Ma, J.; Sun, J.; Yang, L.; Yang, F.; Zhang, W.; Li, R.; Wang, L.; Wang, Y.; Wang, H. Genistein and AG1024 Synergistically Increase the Radiosensitivity of Prostate Cancer Cells. Oncol. Rep. 2018, 40, 579–588. [Google Scholar] [PubMed]
- Bezerra, P.H.A.; Amaral, C.; Almeida, C.F.; Correia-da-Silva, G.; Torqueti, M.R.; Teixeira, N. In Vitro Effects of Combining Genistein with Aromatase Inhibitors: Concerns Regarding Its Consumption during Breast Cancer Treatment. Molecules 2023, 28, 4893. [Google Scholar] [CrossRef]
- Davis, D.A.; Sarkar, S.H.; Hussain, M.; Li, Y.; Sarkar, F.H. Increased Therapeutic Potential of an Experimental Anti-Mitotic Inhibitor SB715992 by Genistein in PC-3 Human Prostate Cancer Cell Line. BMC Cancer 2006, 6, 22. [Google Scholar] [CrossRef][Green Version]
- Lubecka, K.; Kaufman-Szymczyk, A.; Cebula-Obrzut, B.; Smolewski, P.; Szemraj, J.; Fabianowska-Majewska, K. Novel Clofarabine-Based Combinations with Polyphenols Epigenetically Reactivate Retinoic Acid Receptor Beta, Inhibit Cell Growth, and Induce Apoptosis of Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3970. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yan, N.; Wu, D.; Dou, Y.; Liu, Z.; Hu, X.; Chen, C. Combination Inhibition of Triple-Negative Breast Cancer Cell Growth with CD36 SiRNA-Loaded DNA Nanoprism and Genistein. Nanotechnology 2021, 32, 395101. [Google Scholar] [CrossRef]
- Fan, Y.-J.; Huang, N.-S.; Xia, L. Genistein Synergizes with RNA Interference Inhibiting Survivin for Inducing DU-145 of Prostate Cancer Cells to Apoptosis. Cancer Lett. 2009, 284, 189–197. [Google Scholar] [CrossRef]
- Kumi-Diaka, J.; Saddler-Shawnette, S.; Aller, A.; Brown, J. Potential Mechanism of Phytochemical-Induced Apoptosis in Human Prostate Adenocarcinoma Cells: Therapeutic Synergy in Genistein and Beta-Lapachone Combination Treatment. Cancer Cell Int. 2004, 4, 5. [Google Scholar] [CrossRef][Green Version]
- Dong, X.; Xu, W.; Sikes, R.A.; Wu, C. Combination of Low Dose of Genistein and Daidzein Has Synergistic Preventive Effects on Isogenic Human Prostate Cancer Cells When Compared with Individual Soy Isoflavone. Food Chem. 2013, 141, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Ejima, K.; Higuchi, T.; Takeshima, M.; Wakimoto, R.; Nakano, S. Equol Enhances Apoptosis-Inducing Activity of Genistein by Increasing Bax/Bcl-XL Expression Ratio in MCF-7 Human Breast Cancer Cells. Nutr. Cancer 2017, 69, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Jeune, M.A.L.; Kumi-Diaka, J.; Brown, J. Anticancer Activities of Pomegranate Extracts and Genistein in Human Breast Cancer Cells. J. Med. Food 2005, 8, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Li, Y.; Tollefsbol, T.O. The Effects of Combinatorial Genistein and Sulforaphane in Breast Tumor Inhibition: Role in Epigenetic Regulation. Int. J. Mol. Sci. 2018, 19, 1754. [Google Scholar] [CrossRef]
- Sharma, M.; Tollefsbol, T.O. Combinatorial Epigenetic Mechanisms of Sulforaphane, Genistein and Sodium Butyrate in Breast Cancer Inhibition. Exp. Cell Res. 2022, 416, 113160. [Google Scholar] [CrossRef] [PubMed]
- Arzuk, E.; Armağan, G. Genistein and Daidzein Induce Ferroptosis in MDA-MB-231 Cells. J. Pharm. Pharmacol. 2024, 76, 1599–1608. [Google Scholar] [CrossRef]
- Yin, W.; Wang, J.; Jiang, L.; James Kang, Y. Cancer and Stem Cells. Exp. Biol. Med. 2021, 246, 1791. [Google Scholar] [CrossRef] [PubMed]
- Sauter, E.R. Cancer Prevention and Treatment Using Combination Therapy with Natural Compounds. Expert Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Di Domizio, A.; Limonta, P. Natural Compounds in Prostate Cancer Prevention and Treatment: Mechanisms of Action and Molecular Targets. Cells 2020, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Izzo, S.; Naponelli, V.; Bettuzzi, S. Flavonoids as Epigenetic Modulators for Prostate Cancer Prevention. Nutrients 2020, 12, 1010. [Google Scholar] [CrossRef]
- Montano, L.; Maugeri, A.; Volpe, M.G.; Micali, S.; Mirone, V.; Mantovani, A.; Navarra, M.; Piscopo, M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. Int. J. Mol. Sci. 2022, 23, 1568. [Google Scholar] [CrossRef]
- Alzate-Yepes, T.; Pérez-Palacio, L.; Martínez, E.; Osorio, M. Mechanisms of Action of Fruit and Vegetable Phytochemicals in Colorectal Cancer Prevention. Molecules 2023, 28, 4322. [Google Scholar] [CrossRef] [PubMed]
- Perabo, F.G.E.; Von Löw, E.C.; Ellinger, J.; von Rücker, A.; Müller, S.C.; Bastian, P.J. Soy Isoflavone Genistein in Prevention and Treatment of Prostate Cancer. Prostate Cancer Prostatic Dis. 2008, 11, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Feng, H.; Qluwakemi, B.; Wang, J.; Yao, S.; Cheng, G.; Xu, H.; Qiu, H.; Zhu, L.; Yuan, M. Phytoestrogens and Risk of Prostate Cancer: An Updated Meta-Analysis of Epidemiologic Studies. Int. J. Food Sci. Nutr. 2017, 68, 28–42. [Google Scholar] [CrossRef]
- Taylor, C.K.; Levy, R.M.; Elliott, J.C.; Burnett, B.P. The Effect of Genistein Aglycone on Cancer and Cancer Risk: A Review of in Vitro, Preclinical, and Clinical Studies. Nutr. Rev. 2009, 67, 398–415. [Google Scholar] [CrossRef]
- Ferrado, J.B.; Perez, A.A.; Baravalle, M.E.; Renna, M.S.; Ortega, H.H.; Santiago, L.G. Genistein Loaded in Self-Assembled Bovine Serum Albumin Nanovehicles and Their Effects on Mouse Mammary Adenocarcinoma Cells. Colloids Surf. B Biointerfaces 2021, 204, 111777. [Google Scholar] [CrossRef]
- Tian, J.Y.; Chi, C.L.; Bian, G.; Xing, D.; Guo, F.J.; Wang, X.Q. PSMA Conjugated Combinatorial Liposomal Formulation Encapsulating Genistein and Plumbagin to Induce Apoptosis in Prostate Cancer Cells. Colloids Surf. B Biointerfaces 2021, 203, 111723. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.; Walters, J.; Brownlow, B.; Elbayoumi, T. Enhanced Cytotoxicity of Optimized Liposomal Genistein via Specific Induction of Apoptosis in Breast, Ovarian and Prostate Carcinomas. J. Drug Target. 2013, 21, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Guo, F.; Chen, Y.; Li, Y.; Yu, B.; Li, Y. Nanoliposomal Formulation Encapsulating Celecoxib and Genistein Inhibiting COX-2 Pathway and Glut-1 Receptors to Prevent Prostate Cancer Cell Proliferation. Cancer Lett. 2019, 448, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.P.; Dewangan, J.; Urandur, S.; Banala, V.T.; Diwedi, M.; Sharma, S.; Agrawal, S.; Rath, S.K.; Trivedi, R.; Mishra, P.R. Multifunctional Hybrid Nanoconstructs Facilitate Intracellular Localization of Doxorubicin and Genistein to Enhance Apoptotic and Anti-Angiogenic Efficacy in Breast Adenocarcinoma. Biomater. Sci. 2020, 8, 1298–1315. [Google Scholar] [CrossRef] [PubMed]
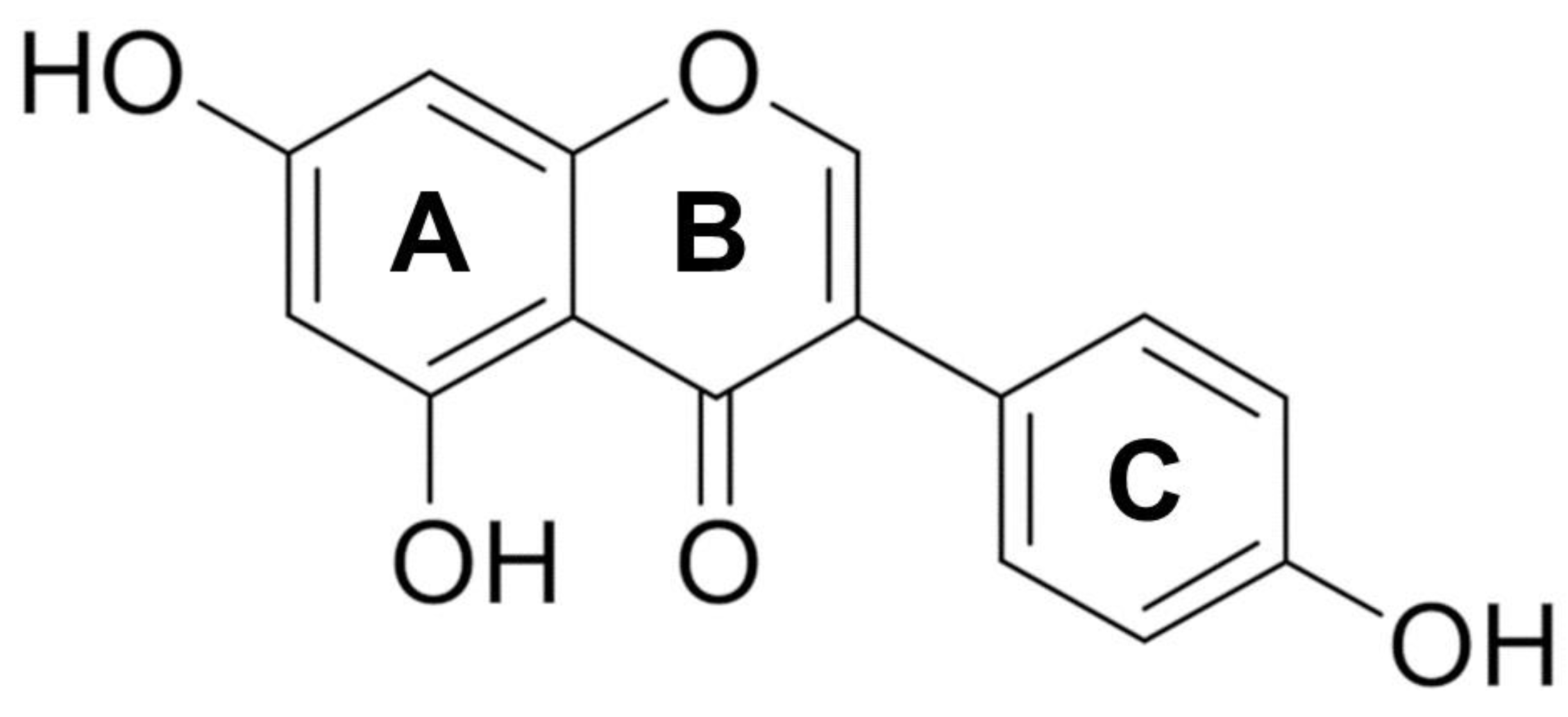

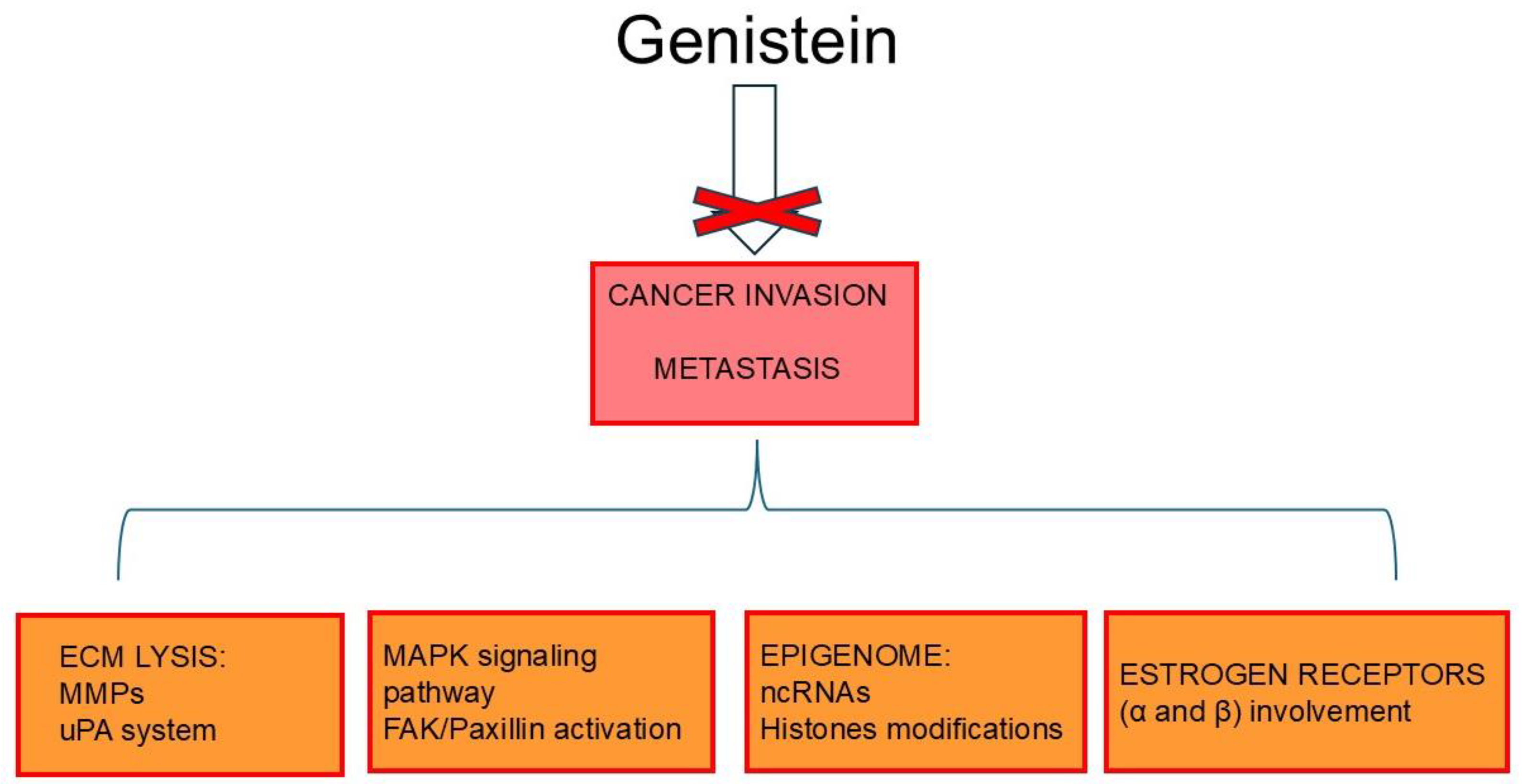
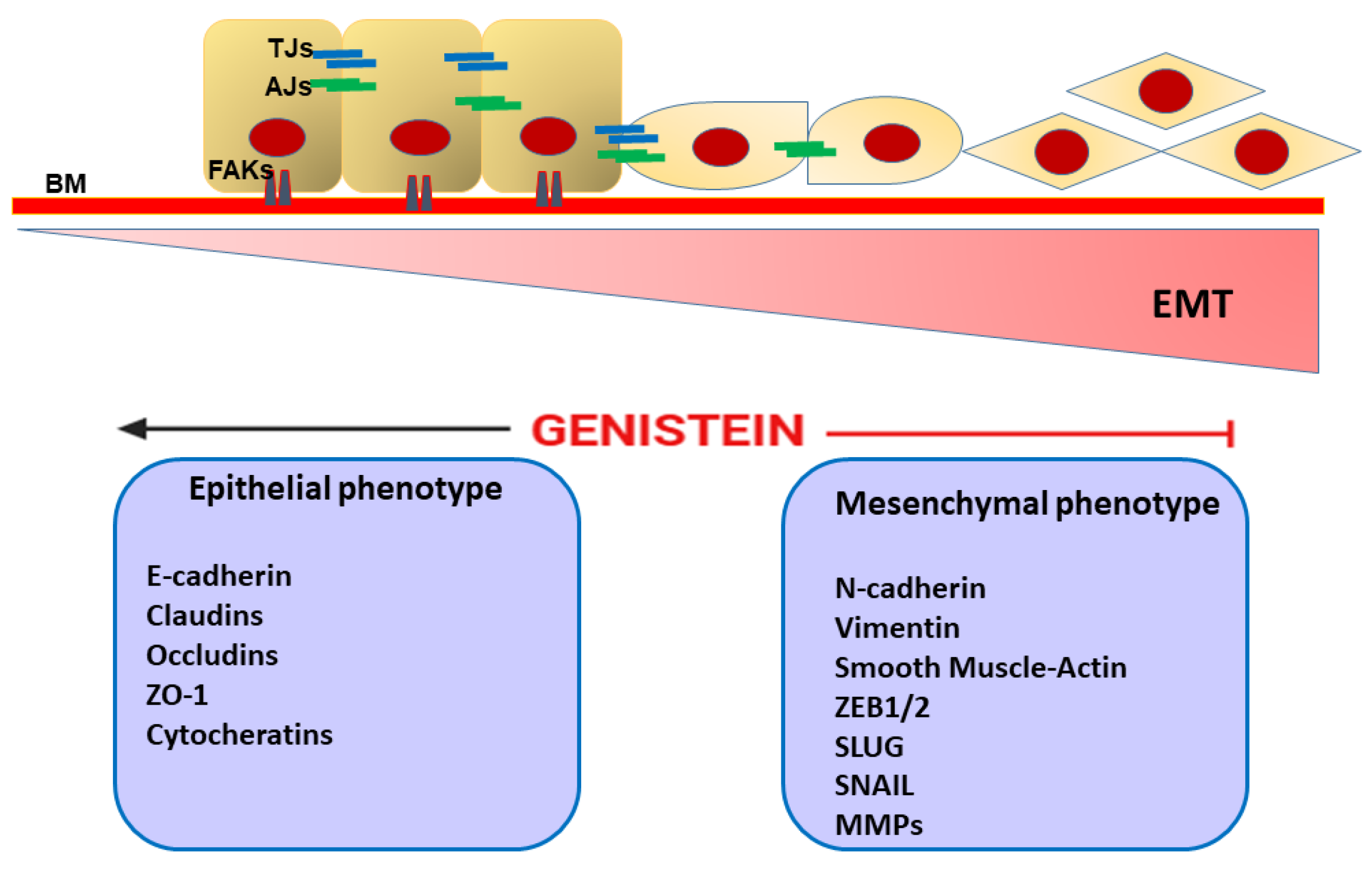
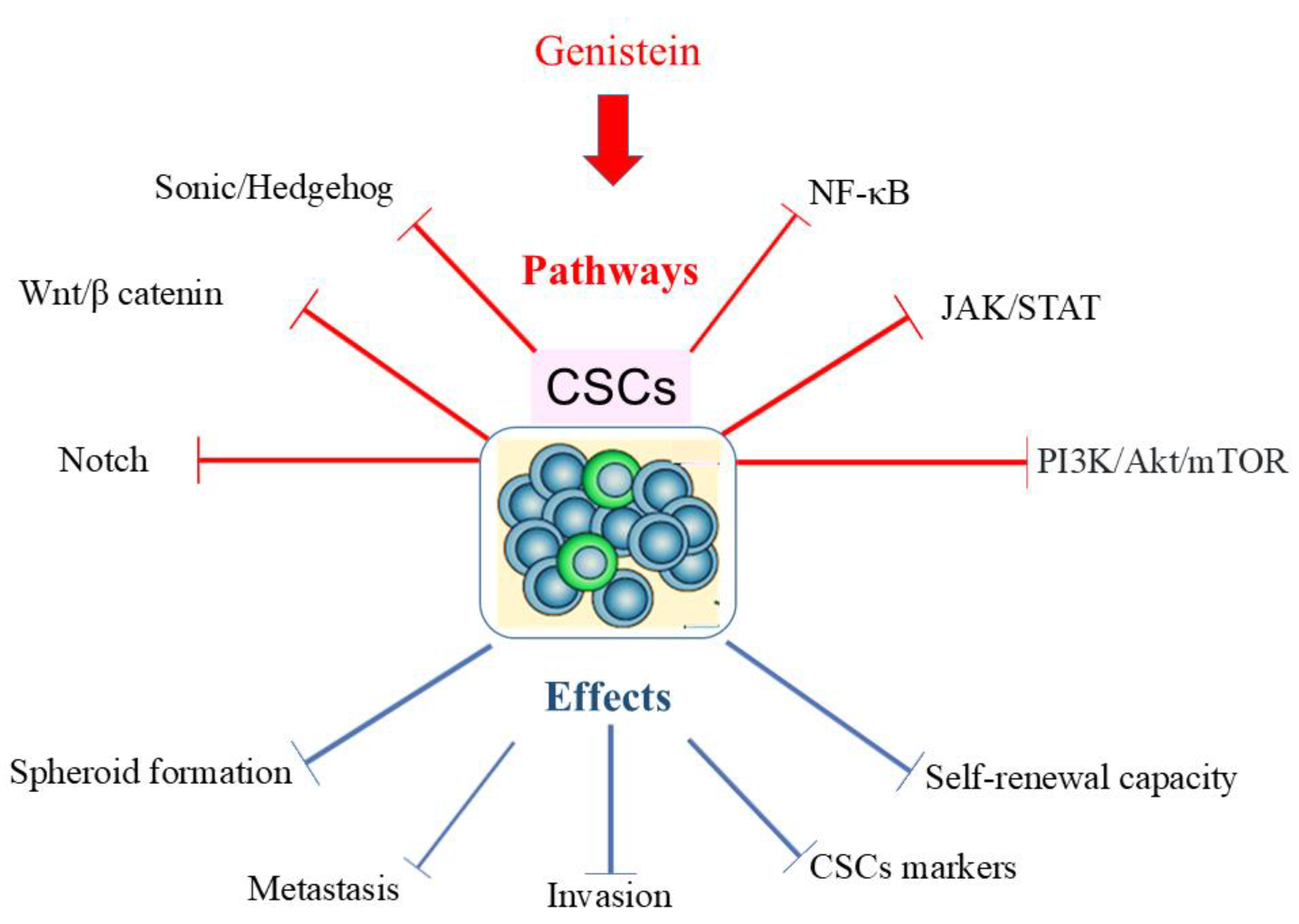
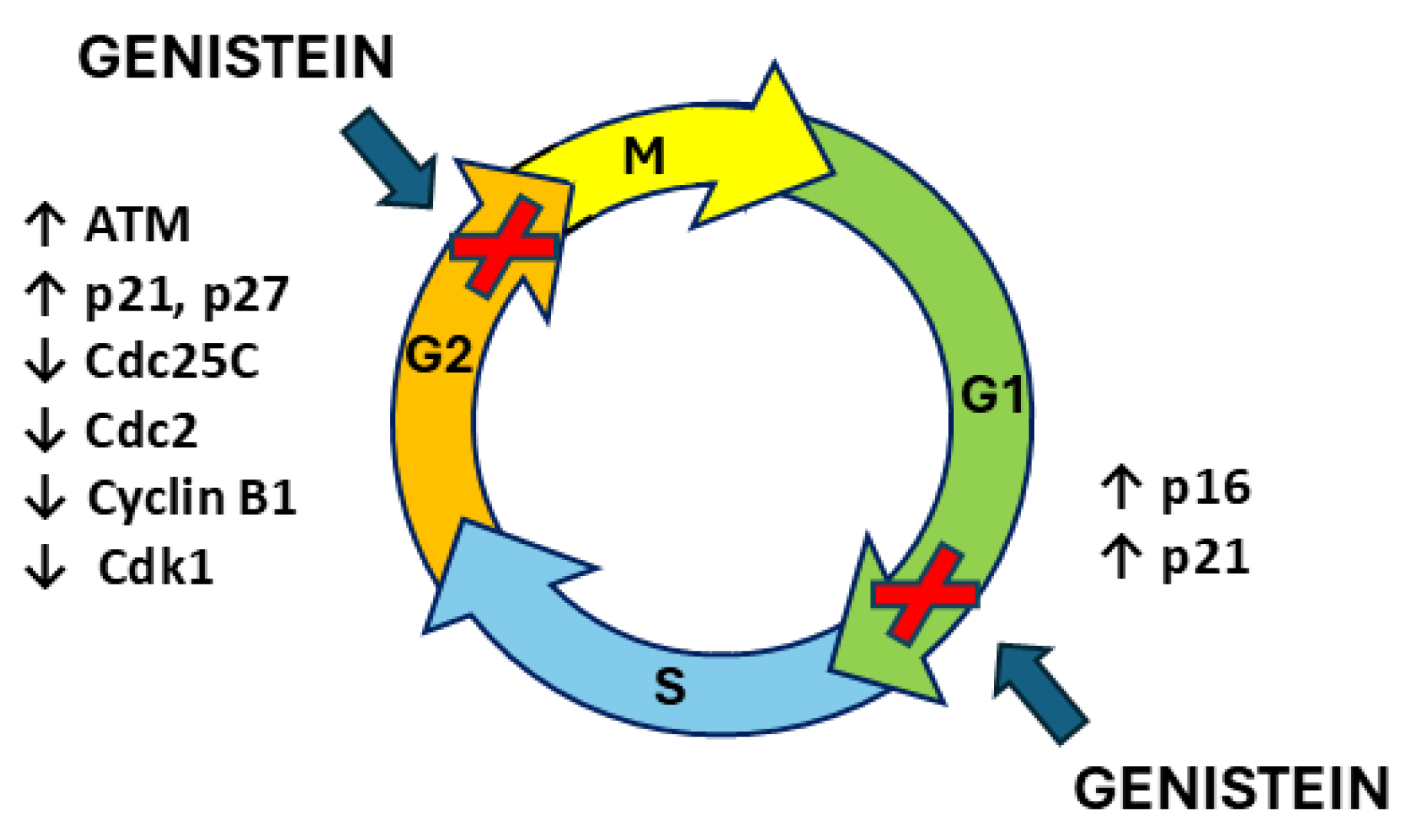
| Source | Content (mg/100 g) |
|---|---|
| Soybeans seeds | 5.56–267.2 |
| Miso | 33.69–67.20 |
| Natto | 21.52–59.37 |
| Tempeh | 1.11–112.21 |
| Pistachio nuts | 0.10–3.40 |
| Chickpeas | 0.069–0.214 |
| Peanuts | 0.02–0.39 |
| Lentils | 0.00–0.36 |
| Parsley | 0.057 |
| Almonds | 0.00–0.01 |
| Genistein-Treated Cells/Animals | Molecular Pathway/Protein | Effect | Ref. | |
|---|---|---|---|---|
| Breast cancer | MCF-7 overexpressing Bcl-2 | ↑ Bcl-2; ↑ p85; ↑ cyt c | ↑ apoptosis | [229] |
| MCF-7 | ↓ Bcl-2; ↑ Bax; ERα | ↑ apoptosis | [230] | |
| MDA-MB-231 | ↑ p-p53; ↑ p21; ↓ Bcl-xL; ↓ cyclin B1 | ↑ apoptosis | [231] | |
| MCF-7 | ↓ Bcl-2/Bax | ↑ apoptosis | [233] | |
| MDA-MB-231 and SKBR3 | ↓S kp2; ↑ p21; ↑ p27 | ↑ apoptosis | [197] | |
| MCF-7 | ↓ MDM2; ↑ p21 | ↑ apoptosis | [188] | |
| MCF-7 | ↓ p-Akt; ↓ HOTAIR | ↑ apoptosis | [234] | |
| MCF-7-caspase-3 and T47D | ↓ CIP2A; ↑ caspase-3; ↑ c-PARP | ↑ apoptosis | [232] | |
| MCF-7 | ↓ PI3K; ↓ Akt; ↑ Fas; ↑ FADD; ↑ cyt c; ↑ t-Bid; ↑ caspase-9; ↑ caspase-3; | ↑ apoptosis | [236] | |
| MCF-7 overexpressing HER2 | ↑ p53; ↑ Fas receptor; ↑ c-PARP; ↑ caspase-9; ↓ p-IκBα; ↓ NF-κB | ↑ apoptosis | [238] | |
| MDA-MB-231 | ↓ NF-κB; ↓ Notch-1; ↓ cyclin B1, ↓ Bcl-2; ↓ Bcl-xL, | ↑ apoptosis | [198] | |
| MDA-MB-231 | ↓ MEK5; ↓ ERK5; ↓ p-ERK5; ↓ NF-κB/p65; ↑ Bax; ↓ Bcl-2; ↑ caspase-3 | ↑ apoptosis | [199] | |
| MCF-7 | ↓ Bcl-2/Bax; ↓ IGF-1R/p-Akt | ↑ apoptosis | [240] | |
| MCF-7 | ↑ calpain; ↑ Ca++; ↑ caspase-7; ↑ c-PARP; ↑ ASK1-p38 MAPK | ↑ apoptosis | [242] | |
| MCF-7 | ↑ Ca++; ↑ mu-calpain; ↑ caspase-12 | ↑ apoptosis | [243] | |
| MCF-7 | ↑ PTEN; ↓ p-Akt; ↑ p27 | ↑ apoptosis | [245] | |
| MDA-MB-435 and Hs578t | ↓ miR-155; ↑ FOXO3; ↑ PTEN; ↑ casein kinase; ↑ p27 | ↑ apoptosis | [246] | |
| Rats MCF-7 | ↑ PTEN; ↑ p21; ↑ Bax; ↑ Bok; ↑ PTEN | ↑ apoptosis ↑ apoptosis | [247] | |
| MCF-7 | ↓ Bcl-2/Bax; ↓ survivin; ↓ CuZnSOD; ↓ MnSOD; ↓ TrxR; ↑ GPx | ↑ apoptosis | [248] | |
| MDA-MB-231 and MDA-MB-468 | ↓ Bcl-2; ↑ Bax;↑ caspase-3; ↑ ROS; ↑ Cu | ↑ apoptosis | [249] | |
| T47D | ↑ IRE1α; ↑ CHOP; ↑ Bim; ↑ TNF; ↑ FAS; ↑ FADD | ↑ apoptosis | [252] | |
| Primary breast cancer cells +17β-estradiol | ↑ FADD; ↑ tBid; ↑ cyt c; ↑ caspase-8; ↑ caspase-3 | ↑ apoptosis ↓ apoptosis | [253] | |
| MDA-MB-231 GEN (<10 μM) + 17 β-estradiol GEN (10–100 μM) ± 17β-estradiol | ↓ Bax/Bcl-2; ↓ p-ERK1/2 | ↑ apoptosis ↑ apoptosis | [254] | |
| MCF-7 + 17β -estradiol T47D + 17β -estradiol | ↓ p-STAT3/STAT3 | ↑ proliferation ↑ apoptosis | [213] | |
| MCF-7 SK; BR 3 and ZR-75-1 GEN (<10 μM) GEN (10–100 μM) | ↑ ERα ↓ ERα and erbB2 | ↑ proliferation ↑ apoptosis | [255] | |
| MCF-7 GEN (50 μM) + 17β -estradiol GEN (100 μM) + 17β -estradiol | ↑ Cyclin B1 ↓ Cyclin B1 | ↓ apoptosis ↑ apoptosis | [256] | |
| MCF-7 +17 β-estradiol Female rats ±17 β-estradiol | ↓ Bcl-2; ↑ Bax; ↑ p21; ↑ p53 ↓ Bcl-2; ↑ Bax; | ↑ apoptosis No apoptosis ↓ tumor growth | [257] | |
| MCF-7 +17β -estradiol | ↑ cell nuclear antigen; ↑ PI3K; ↑ p-Akt; ↓ FADD; ↓ cyt c; ↓ tBid; ↓ caspase-9; ↓ caspase-3; ↓ ERβ | ↓ apoptosis | [258] | |
| MCF-7 20 nm GEN or 5 nm E2 | ↑ ASAH1; ↑ GRP30; ↑ p-ERK1/2; ↑ p-Erα; ↑ S1P | ↑ proliferation | [259] | |
| MCF-7 +17β -estradiol [10−10 M] | ↓ apoptosis | [260] | ||
| SUM1315MO2 (185delAG BRCA1) | ↓ ERβ | ↑ apoptosis | [261] | |
| HCC1937, SUM149, SUM1315 MDA-MB-231 | ↑ p21; ↓ Akt ↑ Akt; ↓ p21 | ↑ apoptosis ↓ apoptosis | [262] | |
| Prostate cancer | PC3 | ↑ caspase-3; ↓ p38MAPK; ↓ MMP-2 | ↑ apoptosis | [265] |
| PC3 and LNCaP | ↓ MMP-2 | ↑ apoptosis | [266] | |
| PC3 PC3-SCID mouse | ↓ MMP-2; ↓ MMP-11; ↓ MMP-13; ↓ MMP-14; ↓ MT-MMP; ↑ osteoprotegerin ↓ MMP-9n | ↑ apoptosis ↑ apoptosis | [267] | |
| PC3 PC3 xenograft | ↓ MMP-2; ↑ p21 | ↑ apoptosis ↓ tumor growth | [188] | |
| LNCaP and PC3 | ↓ PLK-1; ↑ p21 | ↑ apoptosis | [203] | |
| PC3, DU145 and LNCap | ↑ ARHI; ↑ HERC5; ↑ CDNK1A; ↑ GADD45A; ↓ miR-221; ↓ miR-222 | ↑ apoptosis | [269] | |
| DU145 and PC3 | ↑ miR-574-3p; ↓ Bcl-xL; ↑ caspase-9; ↑ caspase-3 | ↑ apoptosis | [270] | |
| PC3, DU145, ARCaP-E, ARCaP-M, and LNCaP | ↑ HAT1; ↑ H3K9 acetylation; ↑ SOX7 | ↑ apoptosis | [281] | |
| DU145 PC3 | ↑ HOTAIR; ↑ miR-34a ↑ HOTAIR; ↑ miR-34a | ↑ apoptosis no change | [272] | |
| PC3 GEN (≤10 μM) GEN (>10 μM) | ↑ CDKs, ↑ MAPKs; ↑ RPSKs, ↓ TGF-β; ↓ SMAD 2/3,4 | ↑ proliferation ↑ apoptosis | [273] | |
| LNCaP | ↑ TRAIL; ↓ MMP | ↑ apoptosis | [274] | |
| 3D culture PC3 | ↓ NO; ↑ catalase; ↑ GSH | ↑ apoptosis | [275] | |
| DU145 and LNCaP | ↑ ROS; ↑ CTR1; ↑ ATP7A | ↑ apoptosis | [276] | |
| LTL163a) xenograft NOD-SCID mouse | ↑ tyrosine kinases; ↑ EGFR; ↑ Src | ↑ proliferation ↓ apoptosis | [106] |
| Genistein-Treated Cells/Animals | Combination with Genistein | Molecular Pathway/Protein | Effect | Ref. |
|---|---|---|---|---|
| MCF-7/Adr | doxorubicin | ↓ Her2/neu | ↑ apoptosis | [290] |
| PC3 and MDA-MB-231 PC3 SCID mice | cisplatin, docetaxel, or doxorubicin docetaxel | ↓ NF-κB | ↑ apoptosis ↑ growth inhibition | [291] |
| ERα- MDA-MB-231 | cisplatin | ↓ Bcl-2/Bax; ↑ p21 | ↑ apoptosis | [292] |
| MCF-7s | cisplatin (-17β-estradiol) | ↑ Bax/Bcl-xl | ↓ apoptosis | [293] |
| EMT6 xenograft mice | Cisplatin | ↑ Bcl-2/Bax | ↓ apoptosis | [294] |
| MCF-7 (high ERa/Erb) T47D (low ERa/Erb) MCF7 + ERβ | cisplatin | ↓ ROS | ↓ apoptosis ↑ apoptosis ↑ apoptosis | [295] |
| PC3, DU145, ARCaP-E, ARCaP-M, and LNCaP | vorinostat | ↓ BIRC7/Livin; ↓ TGFB1I1/ARA55; ↓ HES1; ↓ SLUG | ↑ apoptosis | [271] |
| MCF-7 and MDA-MB-231 | paclitaxel or vincristine | ↓ p-Bcl-2; ↓ cyclin B1; ↓ CDC2 | ↓ apoptosis | [296] |
| mCRPC | cabazitaxel | ↑ Bax | ↑ apoptosis | [297] |
| LNCaP | topotecan | ↑ caspase-3; ↑ caspase-9; ↑ ROS | ↑ apoptosis | [298] |
| BT-474-overexpressing ER+/HER2 | tamoxifen | ↓ survivin; ↓ EGFR; ↓ Her2; ↓ ERα | ↑ apoptosis | [214] |
| MCF-7 and MDA-MB-231 mouse 4Ti breast tumor | centchroman | ↑ ROS; ↑ Bax/Bcl-2; ↑ caspase-3; ↑ caspase-7; ↑ caspase-9; ↑ c-PARP; ↓ p-PI3K; ↓ p-Akt; ↓ NF-κB | ↑ apoptosis | [299] |
| MCF-7 and MDA-MB-231 | 1,25(OH)2D3 | ↑ Bax and ↑ caspase-3; ↓ Bcl-2 | ↑ apoptosis | [187] |
| DU145 | 1,25(OH)2D3 | ↓ CYP24; ↑ VDR | ↑ growth inhibition | [300] |
| LNCaP | HPCD | c-PARP; caspase-3; ↓ EGFR-Akt-GSK-3beta-p70S6k | ↑ apoptosis | [301] |
| DU145 | terazosin | ↓ Bcl-xl | ↑ apoptosis | [302] |
| PC3 | radiation | ↓ NF-κB; ↓ cyclin B and/or ↑ p21; ↑ c-PARP | ↑ apoptosis | [202] |
| MCF-7 and MDA-MB-231 | X-ray irradiation | ↑ Bax; ↑ p73; ↓ Bcl-2 | ↑ apoptosis | [196] |
| prostate cancer orthotopic model | radiation | ↑ apoptosis | [303] | |
| DU145 | ionizing radiation | ↓ damage repair | ↑ apoptosis | [304] |
| MCF-7 and MDA-MB-231 | hypericin | ↓ Bcl-2; ↑ Bax; ↓ p-Akt; ↓ p-Erk1/2 | ↑ apoptosis | [305] |
| DU145 | tyrphostin AG1024 and X-irradiation | ↑ apoptosis | [306] | |
| MCF7-aro and LTEDaro | Exe Exemestane | ↑ c-PARP/PARP | ↑ apoptosis | [307] |
| PC3 | KSP inhibitor SB715992 | ↑ apoptosis | [308] | |
| MCF7 and MDA-MB-231 | clofarabine | ↑ PTEN; ↑ RARB; ↑ CDKN1A | ↑ apoptosis | [309] |
| MDA-MB-231 | NP-siCD36 | ↓ p-p38 | ↑ growth inhibition | [310] |
| DU145 | survivin RNAi | ↑ caspase-3 | ↑ apoptosis | [311] |
| PC3 | beta-lapachone (bLap) | ↓ caspase-3; ↑ NQO1 | ↑ apoptosis | [312] |
| LNCaP; C4-2B | daidzein | ↑ apoptosis | [313] | |
| MCF-7 cells | equol | ↑ Bax/Bcl-xl | ↑ apoptosis | [314] |
| MCF-7 | pomegranate extracts | ↑ apoptosis | [315] | |
| MDA-MB-231; MCF-7 | sulforaphane | ↓ KLF4; ↓ HDAC2; ↓ HDAC3 | ↑ growth inhibition | [316] |
| MDA-MB-231 and MCF-7 | sulforaphane, sodium butyrate | ↓ DNMT; ↓ HDAC; ↓ HMT; ↑ HAT methyltransferases and histone methylases | ↑ apoptosis | [317] |
| LNCaP and p53-null PC3 | selenite | ↑ p21; ↑ Bax | ↑ apoptosis | [201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naponelli, V.; Piscazzi, A.; Mangieri, D. Cellular and Molecular Mechanisms Modulated by Genistein in Cancer. Int. J. Mol. Sci. 2025, 26, 1114. https://doi.org/10.3390/ijms26031114
Naponelli V, Piscazzi A, Mangieri D. Cellular and Molecular Mechanisms Modulated by Genistein in Cancer. International Journal of Molecular Sciences. 2025; 26(3):1114. https://doi.org/10.3390/ijms26031114
Chicago/Turabian StyleNaponelli, Valeria, Annamaria Piscazzi, and Domenica Mangieri. 2025. "Cellular and Molecular Mechanisms Modulated by Genistein in Cancer" International Journal of Molecular Sciences 26, no. 3: 1114. https://doi.org/10.3390/ijms26031114
APA StyleNaponelli, V., Piscazzi, A., & Mangieri, D. (2025). Cellular and Molecular Mechanisms Modulated by Genistein in Cancer. International Journal of Molecular Sciences, 26(3), 1114. https://doi.org/10.3390/ijms26031114








