Exploring the Characters of Non-Coding RNAs in Spermatogenesis and Male Infertility
Abstract
1. Introduction
| Congenital Factors | Acquired Factors | Idiopathic Risk Factors |
|---|---|---|
| Anorchia | Varicocele | Smoking |
| Congenital absence of vas deferens | Testicular trauma or torsion | Alcohol |
| Cryptorchidism | Systemic diseases | Recreational drugs |
| Y chromosome microdeletions | Germ cell tumors | Obesity |
| Chromosomal abnormalities genetic abnormalities | Acquired hypogonadotropic hypogonadism Postinflammatory conditions (epididymitis, mumps, orchitis) | Psychological stress Dietary factors |
| Klinefelter syndrome and its variants | Recurrent urogenital infections (prostatitis, prostatovesciculitis) | Advanced paternal age |
| Kallmann syndrome Mild androgen insensitivity syndrome | Urogenital tract obstruction Anti-sperm antibodies | Environmental exposure to toxins (organic contaminants, industrial and environmental chemicals, heavy metals, organic solvents, pesticides and endocrine disrupting chemicals) |
| Robertsonian translocation | Surgeries that can comprise vascularization of the testis Sexual dysfunction (erectile or ejaculatory dysfunction) Exogenous factors (chemotherapy, medications, radiation, heat) Systemic diseases (live cirrhosis, renal failure) | Occupational exposure to toxins |
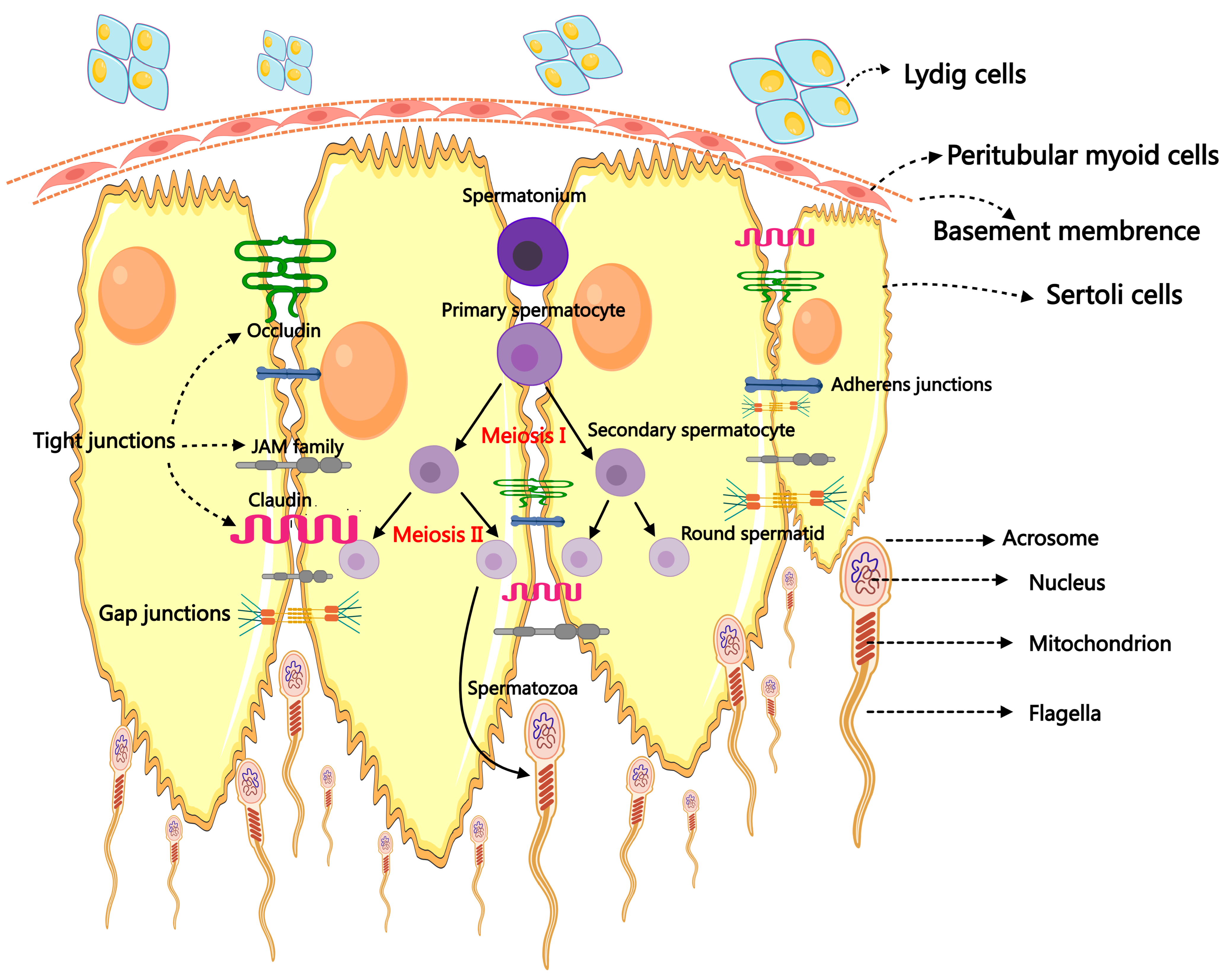
2. Spermatogenesis and Male Infertility
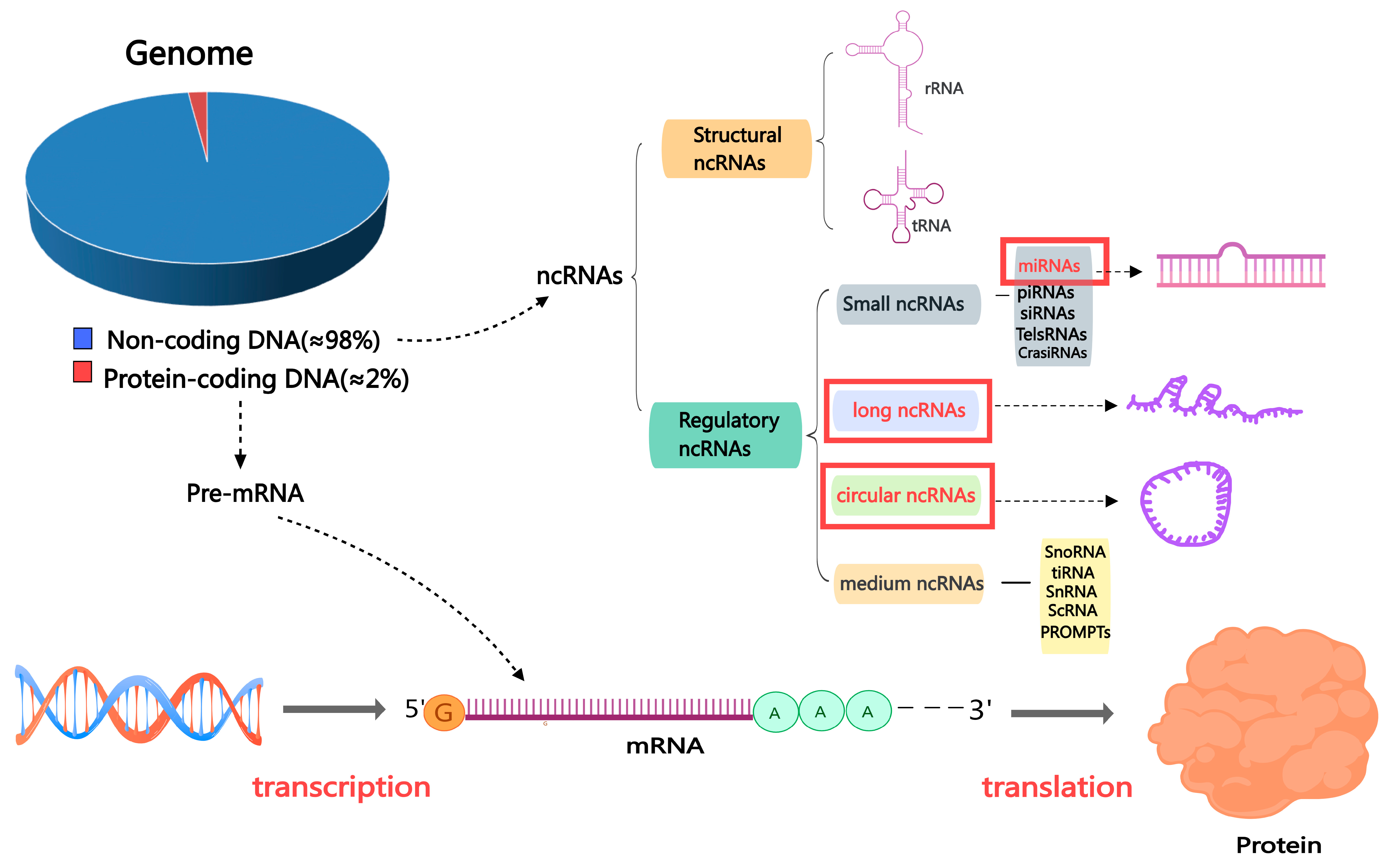
3. The Non-Coding RNA Family
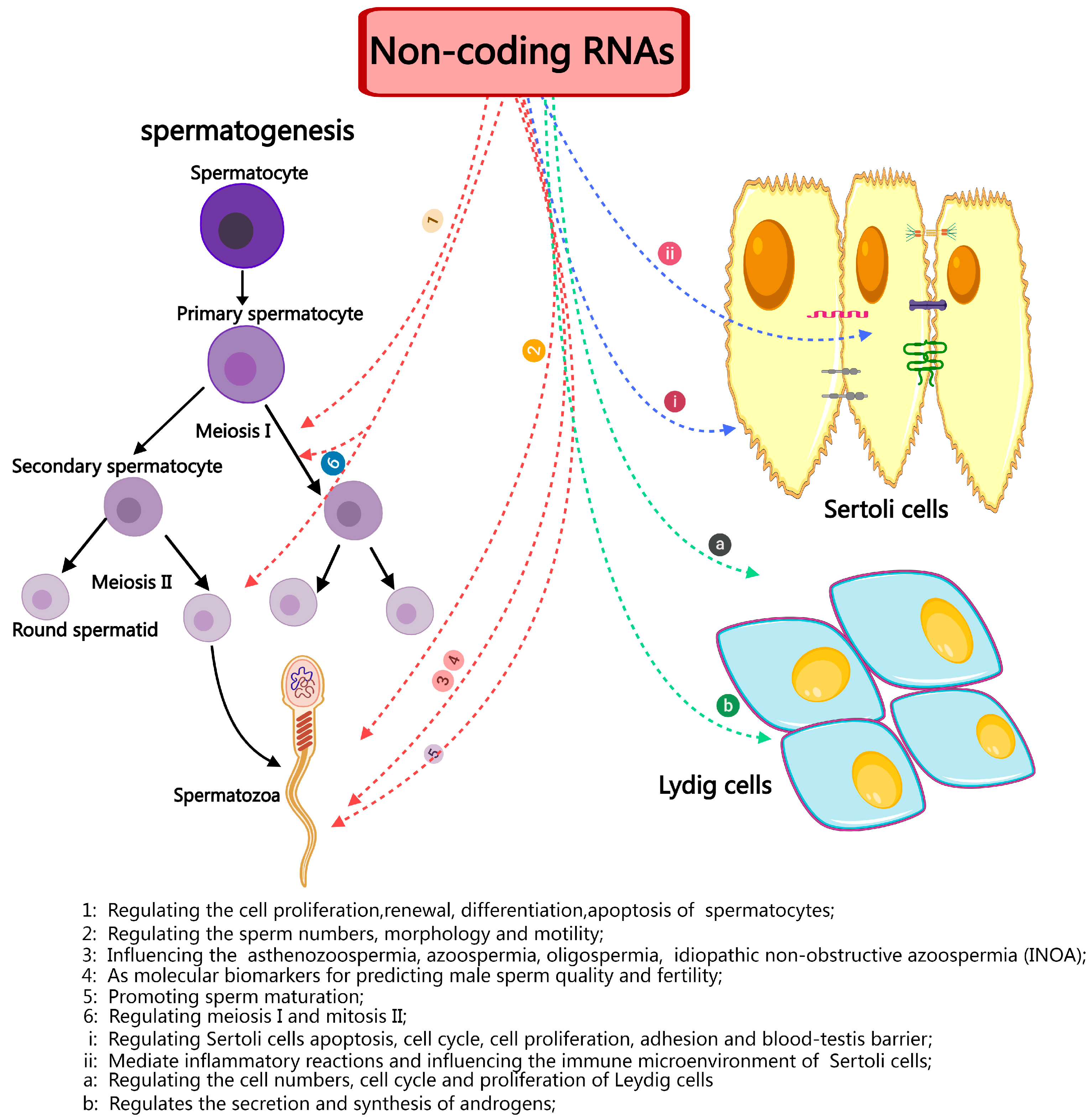
4. The Function of ncRNAs in Spermatogenesis
4.1. MicroRNAs in Spermatogenesis
4.2. CircRNAs in Spermatogenesis
4.3. LncRNAs in Spermatogenesis
| Name | Functions | Reactive Sites | Organism | Reference |
|---|---|---|---|---|
| NLC1-C | Inhibiting apoptosis promotes cell growth | Spermatogonia, spermatocytes | Human | [77] |
| Tug1 lncRNA | Regulating sperm numbers and morphology | Sperms | Human, Mouse | [78] |
| Mrhl | Cell adhesion, differentiation, signaling, and development | Spermatogonial cells | Mouse | [66] |
| Tsx | Regulating meiosis | Pachytene spermatocytes, | Mouse | [79] |
| Tesra | Regulating meiosis | LCs | Mouse | [80] |
| Drm | Regulating switching between mitosis and meiosis | SCs, germ cells | Mouse | [69] |
| Spga-lncRNAs | Maintaining stemness of spermatogonia | Spermatogonia, pachytene spermatocytes, round spermatids | Mouse | [81] |
| lncRNA-Tcam1 | Immune response | SSCs | Mouse | [82] |
| LncRNA033862 | Regulating SSC self-renewal | SSCs, spermatogonia | Mouse | [83] |
| AK015322 | Maintaining SSC self-renewal capacity, promoting the proliferation of SSCs | Germ cells | Mouse | [84] |
| Gm2044 | Regulating germ cell transition, regulating meiotic progression | Pachytene spermatocytes | Mouse | [85] |
| HongrES2 | Promotes sperm maturation | Sperms in epididymis | Rat | [75] |
5. ncRNAs in Testicular Somatic Cells
5.1. ncRNAs in Sertoli Cells
5.2. miRNAs in Sertoli Cells
5.3. circRNAs in Sertoli Cells
5.4. LncRNAs in Sertoli Cells
5.5. ncRNAs in Leydig Cells
5.6. miRNAs in Leydig Cells
5.7. circRNAs in Leydig Cells
5.8. LncRNAs in Leydig Cells
6. Other Important ncRNAs in Spermatogenesis and Male Infertility
7. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ncRNA | non-coding RNA |
| lncRNA | long non-coding RNA |
| miRNA | microRNA |
| mRNA | messenger RNA |
| piRNA | PIWI-interacting RNA |
| tRNA | transfer RNA |
| rRNA | ribosomal RNA |
| SSCs | spermatogonial stem cells |
| LCs | Leydig cells |
| SCs | Sertoli cells |
| STs | seminiferous tubules |
| BTB | blood–testis barrier |
| PMCs | peritubular myoid cells |
| SPGF | spermatogenic failure |
| PGCs | primordial germ cells |
| RBPs | RNA-binding proteins |
| sncRNAs | small or short non-coding RNAs |
References
- Sun, H.; Gong, T.T.; Jiang, Y.T.; Zhang, S.; Zhao, Y.H.; Wu, Q.J. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: Results from a global burden of disease study, 2017. Aging 2019, 11, 10952–10991. [Google Scholar] [CrossRef] [PubMed]
- Slade, P.; O’Neill, C.; Simpson, A.J.; Lashen, H. The relationship between perceived stigma, disclosure patterns, support and distress in new attendees at an infertility clinic. Hum. Reprod. 2007, 22, 2309–2317. [Google Scholar] [CrossRef]
- Akhter, A.; Momen, S.H.M.; Fatema, K.; Nath, S.D. Prevalence of Abnormal Semen Parameters among the Infertile Couples Seeking Infertility Treatment. Mymensingh Med. J. 2024, 33, 586–591. [Google Scholar] [PubMed]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Hombach, S.; Kretz, M. Non-coding RNAs: Classification, Biology and Functioning. Adv. Exp. Med. Biol. 2016, 937, 3–17. [Google Scholar] [CrossRef]
- Robles, V.; Valcarce, D.G.; Riesco, M.F. Non-coding RNA regulation in reproduction: Their potential use as biomarkers. Non-Coding RNA Res. 2019, 4, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S. Non-coding RNAs: The architects of eukaryotic complexity. EMBO Rep. 2001, 2, 986–991. [Google Scholar] [CrossRef]
- Zhou, F.; Chen, W.; Jiang, Y.; He, Z. Regulation of long non-coding RNAs and circular RNAs in spermatogonial stem cells. Reproduction 2019, 158, R15–R25. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhang, M.; Zhang, J.; Feng, Y.; Xie, Z.; Liu, S.; Zhu, D.; Luo, Y. The gene regulatory role of non-coding RNAs in non-obstructive azoospermia. Front. Endocrinol. 2022, 13, 959487. [Google Scholar] [CrossRef]
- Du, L.; Chen, W.; Cheng, Z.; Wu, S.; He, J.; Han, L.; He, Z.; Qin, W. Novel Gene Regulation in Normal and Abnormal Spermatogenesis. Cells 2021, 10, 666. [Google Scholar] [CrossRef] [PubMed]
- Staub, C.; Johnson, L. Review: Spermatogenesis in the bull. Anim. Int. J. Anim. Biosci. 2018, 12, s27–s35. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Rajender, S. Long non-coding RNAs (lncRNAs) in spermatogenesis and male infertility. Reprod. Biol. Endocrinol. 2020, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; He, X.; Qi, K.; Wang, T.; Qi, Y.; Cui, L.; Wang, F.; Song, M. Effects of environmental contaminants on fertility and reproductive health. J. Environ. Sci. 2019, 77, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Zhang, Y.; Wang, Q.; Yuan, L. Autophagy: A Double-Edged Sword in Male Reproduction. Int. J. Mol. Sci. 2022, 23, 15273. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Mruk, D.D.; Cheng, Y.H.; Tang, E.I.; Han, D.; Lee, W.M.; Wong, E.W.; Cheng, C.Y. Actin binding proteins, spermatid transport and spermiation. Semin. Cell Dev. Biol. 2014, 30, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Schlatt, S.; Ehmcke, J. Regulation of spermatogenesis: An evolutionary biologist’s perspective. Semin. Cell Dev. Biol. 2014, 29, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Khawar, M.B.; Li, W. Autophagy in Reproduction. Adv. Exp. Med. Biol. 2019, 1206, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Matzuk, M.M.; Lamb, D.J. The biology of infertility: Research advances and clinical challenges. Nat. Med. 2008, 14, 1197–1213. [Google Scholar] [CrossRef] [PubMed]
- Neto, F.T.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Kimmins, S.; Sassone-Corsi, P. Chromatin remodelling and epigenetic features of germ cells. Nature 2005, 434, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Plant, T.M.; Ramaswamy, S.; Simorangkir, D.; Marshall, G.R. Postnatal and pubertal development of the rhesus monkey (Macaca mulatta) testis. Ann. N. Y. Acad. Sci. 2005, 1061, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.M.; Atanassova, N.; McKinnell, C.; Parte, P.; Turner, K.J.; Fisher, J.S.; Kerr, J.B.; Groome, N.P.; Macpherson, S.; Millar, M.R.; et al. Abnormalities in functional development of the Sertoli cells in rats treated neonatally with diethylstilbestrol: A possible role for estrogens in Sertoli cell development. Biol. Reprod. 1998, 59, 1084–1094. [Google Scholar] [CrossRef][Green Version]
- Guerri, G.; Maniscalchi, T.; Barati, S.; Busetto, G.M.; Del Giudice, F.; De Berardinis, E.; Cannarella, R.; Calogero, A.E.; Bertelli, M. Non-syndromic monogenic male infertility. Acta Bio-Med. Atenei Parm. 2019, 90, 62–67. [Google Scholar] [CrossRef]
- Bond, A.M.; Vangompel, M.J.; Sametsky, E.A.; Clark, M.F.; Savage, J.C.; Disterhoft, J.F.; Kohtz, J.D. Balanced gene regulation by an embryonic brain ncRNA is critical for adult hippocampal GABA circuitry. Nat. Neurosci. 2009, 12, 1020–1027. [Google Scholar] [CrossRef]
- Yang, F.; Deng, X.; Ma, W.; Berletch, J.B.; Rabaia, N.; Wei, G.; Moore, J.M.; Filippova, G.N.; Xu, J.; Liu, Y.; et al. The lncRNA Firre anchors the inactive X chromosome to the nucleolus by binding CTCF and maintains H3K27me3 methylation. Genome Biol. 2015, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- Panni, S.; Lovering, R.C.; Porras, P.; Orchard, S. Non-coding RNA regulatory networks. Biochim. Biophys. Acta. Gene Regul. Mech. 2020, 1863, 194417. [Google Scholar] [CrossRef]
- Beermann, J.; Piccoli, M.T.; Viereck, J.; Thum, T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol. Rev. 2016, 96, 1297–1325. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, M.; Fan, Y.; Li, S.; Lai, Z.; Huang, Y.; Lan, X.; Lei, C.; Chen, H.; Dang, R. Identification and characterization of circular RNAs in Qinchuan cattle testis. R. Soc. Open Sci. 2018, 5, 180413. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular RNA: Metabolism, functions and interactions with proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.; Moreno, C.; Zhang, W.C. Non-Coding RNAs in Lung Tumor Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2774. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lei, X.; Chen, Z.; Mo, Z. The roles of cirRNA in the development of germ cells. Acta Histochem. 2020, 122, 151506. [Google Scholar] [CrossRef] [PubMed]
- Paronetto, M.P.; Sette, C. Role of RNA-binding proteins in mammalian spermatogenesis. Int. J. Androl. 2010, 33, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, D.L.; Roote, J.; Kennison, J.A. Anent the genomics of spermatogenesis in Drosophila melanogaster. PLoS ONE 2013, 8, e55915. [Google Scholar] [CrossRef] [PubMed]
- de Mateo, S.; Sassone-Corsi, P. Regulation of spermatogenesis by small non-coding RNAs: Role of the germ granule. Semin. Cell Dev. Biol. 2014, 29, 84–92. [Google Scholar] [CrossRef]
- Mukherjee, A.; Koli, S.; Reddy, K.V. Regulatory non-coding transcripts in spermatogenesis: Shedding light on ’dark matter’. Andrology 2014, 2, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Grosshans, H.; Filipowicz, W. Molecular biology: The expanding world of small RNAs. Nature 2008, 451, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Sayed, D.; Abdellatif, M. MicroRNAs in development and disease. Physiol. Rev. 2011, 91, 827–887. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Goodyear, S.M.; Rao, S.; Wu, X.; Tobias, J.W.; Avarbock, M.R.; Brinster, R.L. MicroRNA-21 regulates the self-renewal of mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. USA 2011, 108, 12740–12745. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Jiang, J.; Kokkinaki, M.; Tang, L.; Zeng, W.; Gallicano, I.; Dobrinski, I.; Dym, M. MiRNA-20 and mirna-106a regulate spermatogonial stem cell renewal at the post-transcriptional level via targeting STAT3 and Ccnd1. Stem Cells 2013, 31, 2205–2217. [Google Scholar] [CrossRef] [PubMed]
- Huszar, J.M.; Payne, C.J. MicroRNA 146 (Mir146) modulates spermatogonial differentiation by retinoic acid in mice. Biol. Reprod. 2013, 88, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.E.; Racicot, K.E.; Kaucher, A.V.; Oatley, M.J.; Oatley, J.M. MicroRNAs 221 and 222 regulate the undifferentiated state in mammalian male germ cells. Development 2013, 140, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.H.; Mitchell, D.A.; McGowan, S.D.; Evanoff, R.; Griswold, M.D. Two miRNA clusters, Mir-17-92 (Mirc1) and Mir-106b-25 (Mirc3), are involved in the regulation of spermatogonial differentiation in mice. Biol. Reprod. 2012, 86, 72. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Hu, Z.; Qin, Y.; Dong, J.; Dai, J.; Lu, C.; Zhang, W.; Shen, H.; Xia, Y.; Wang, X. Seminal plasma microRNAs: Potential biomarkers for spermatogenesis status. Mol. Hum. Reprod. 2012, 18, 489–497. [Google Scholar] [CrossRef]
- Lian, J.; Zhang, X.; Tian, H.; Liang, N.; Wang, Y.; Liang, C.; Li, X.; Sun, F. Altered microRNA expression in patients with non-obstructive azoospermia. Reprod. Biol. Endocrinol. 2009, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Sanger, H.L.; Klotz, G.; Riesner, D.; Gross, H.J.; Kleinschmidt, A.K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. USA 1976, 73, 3852–3856. [Google Scholar] [CrossRef] [PubMed]
- Wilusz, J.E. A 360° view of circular RNAs: From biogenesis to functions. Wiley Interdiscip. Rev. RNA 2018, 9, e1478. [Google Scholar] [CrossRef]
- Chen, J.; Chen, T.; Zhu, Y.; Li, Y.; Zhang, Y.; Wang, Y.; Li, X.; Xie, X.; Wang, J.; Huang, M.; et al. circPTN sponges miR-145-5p/miR-330-5p to promote proliferation and stemness in glioma. J. Exp. Clin. Cancer Res. 2019, 38, 398. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Yang, Y.; Niu, G.; Tang, Z.; Li, K. Genome-wide profiling of Sus scrofa circular RNAs across nine organs and three developmental stages. DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes 2017, 24, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Amano, T.; Zhang, J.; Chen, Y.E.; Tian, X.C. Acceptance of embryonic stem cells by a wide developmental range of mouse tetraploid embryos. Biol. Reprod. 2010, 83, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Megosh, H.B.; Cox, D.N.; Campbell, C.; Lin, H. The role of PIWI and the miRNA machinery in Drosophila germline determination. Curr. Biol. 2006, 16, 1884–1894. [Google Scholar] [CrossRef]
- Dong, W.W.; Li, H.M.; Qing, X.R.; Huang, D.H.; Li, H.G. Identification and characterization of human testis derived circular RNAs and their existence in seminal plasma. Sci. Rep. 2016, 6, 39080. [Google Scholar] [CrossRef]
- Rasmussen, K.D.; Helin, K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016, 30, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Gòdia, M.; Castelló, A.; Rocco, M.; Cabrera, B.; Rodríguez-Gil, J.E.; Balasch, S.; Lewis, C.; Sánchez, A.; Clop, A. Identification of circular RNAs in porcine sperm and evaluation of their relation to sperm motility. Sci. Rep. 2020, 10, 7985. [Google Scholar] [CrossRef]
- Saberiyan, M.; Karimi, E.; Safi, A.; Movahhed, P.; Dehdehi, L.; Haririan, N.; Mirfakhraie, R. Circular RNAs: Novel Biomarkers in Spermatogenesis Defects and Male Infertility. Reprod. Sci. 2023, 30, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Luo, Y.; Bo, H.; Gong, G.; Tang, R.; Fan, J.; Zhang, H.; Liu, G.; Zhu, W.; Tan, Y.; et al. Trace the profile and function of circular RNAs in Sertoli cell only syndrome. Genomics 2021, 113, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, F.; Wen, Z.; Li, T.; Lv, M.; Zhao, X.; Zhang, W.; Liu, J.; Wang, L.; Ma, X. Preliminary investigation of the function of hsa_circ_0049356 in nonobstructive azoospermia patients. Andrologia 2020, 52, e13814. [Google Scholar] [CrossRef]
- Ge, P.; Zhang, J.; Zhou, L.; Lv, M.Q.; Li, Y.X.; Wang, J.; Zhou, D.X. CircRNA expression profile and functional analysis in testicular tissue of patients with non-obstructive azoospermia. Reprod. Biol. Endocrinol. 2019, 17, 100. [Google Scholar] [CrossRef]
- Batista, P.J.; Chang, H.Y. Long noncoding RNAs: Cellular address codes in development and disease. Cell 2013, 152, 1298–1307. [Google Scholar] [CrossRef]
- Fang, Y.; Fullwood, M.J. Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genom. Proteom. Bioinform. 2016, 14, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Nishant, K.T.; Ravishankar, H.; Rao, M.R. Characterization of a mouse recombination hot spot locus encoding a novel non-protein-coding RNA. Mol. Cell. Biol. 2004, 24, 5620–5634. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, G.; Rao, S.M. A novel noncoding RNA processed by Drosha is restricted to nucleus in mouse. RNA 2008, 14, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Arun, G.; Akhade, V.S.; Donakonda, S.; Rao, M.R. mrhl RNA, a long noncoding RNA, negatively regulates Wnt signaling through its protein partner Ddx5/p68 in mouse spermatogonial cells. Mol. Cell. Biol. 2012, 32, 3140–3152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, H.; Xin, D.; Cheng, H.; Zhou, R. A novel ncRNA gene from mouse chromosome 5 trans-splices with Dmrt1 on chromosome 19. Biochem. Biophys. Res. Commun. 2010, 400, 696–700. [Google Scholar] [CrossRef]
- Ottolenghi, C.; Veitia, R.; Barbieri, M.; Fellous, M.; McElreavey, K. The human doublesex-related gene, DMRT2, is homologous to a gene involved in somitogenesis and encodes a potential bicistronic transcript. Genomics 2000, 64, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Agbor, V.A.; Tao, S.; Lei, N.; Heckert, L.L. A Wt1-Dmrt1 transgene restores DMRT1 to sertoli cells of Dmrt1(-/-) testes: A novel model of DMRT1-deficient germ cells. Biol. Reprod. 2013, 88, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, C.; Yang, S.; Tian, R.; Wang, J.; Yuan, Q.; Dong, H.; He, Z.; Wang, S.; Li, Z. Dynamics of the Transcriptome during Human Spermatogenesis: Predicting the Potential Key Genes Regulating Male Gametes Generation. Sci. Rep. 2016, 6, 19069. [Google Scholar] [CrossRef] [PubMed]
- Rolland, A.D.; Evrard, B.; Darde, T.A.; Le Béguec, C.; Le Bras, Y.; Bensalah, K.; Lavoué, S.; Jost, B.; Primig, M.; Dejucq-Rainsford, N.; et al. RNA profiling of human testicular cells identifies syntenic lncRNAs associated with spermatogenesis. Hum. Reprod. 2019, 34, 1278–1290. [Google Scholar] [CrossRef]
- Wichman, L.; Somasundaram, S.; Breindel, C.; Valerio, D.M.; McCarrey, J.R.; Hodges, C.A.; Khalil, A.M. Dynamic expression of long noncoding RNAs reveals their potential roles in spermatogenesis and fertility. Biol. Reprod. 2017, 97, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, F.; Fu, J.; Zhang, P.; Wang, Y.; Zeng, X. Systematic identification and characterization of long non-coding RNAs in mouse mature sperm. PLoS ONE 2017, 12, e0173402. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, Y.; Gao, Y.; Lin, Z.; Yang, S.; Wang, T.; Wang, Q.; Xie, N.; Hua, R.; Liu, M.; et al. Single-cell RNA-seq uncovers dynamic processes and critical regulators in mouse spermatogenesis. Cell Res. 2018, 28, 879–896. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.J.; Hu, Z.H.; Liu, Q.; Liu, M.F.; Lu, M.H.; Zhang, J.S.; Zhang, L.; Zhang, Y.L. Identification and characterization of a novel non-coding RNA involved in sperm maturation. PLoS ONE 2011, 6, e26053. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, C.; Guo, F.; Zhang, Y.; Ju, Z.; Jiang, Q.; Zhao, X.; Liu, Y.; Zhao, H.; Wang, J.; et al. Integrated analysis of mRNAs and long noncoding RNAs in the semen from Holstein bulls with high and low sperm motility. Sci. Rep. 2019, 9, 2092. [Google Scholar] [CrossRef] [PubMed]
- Lü, M.; Tian, H.; Cao, Y.X.; He, X.; Chen, L.; Song, X.; Ping, P.; Huang, H.; Sun, F. Downregulation of miR-320a/383-sponge-like long non-coding RNA NLC1-C (narcolepsy candidate-region 1 genes) is associated with male infertility and promotes testicular embryonal carcinoma cell proliferation. Cell Death Dis. 2015, 6, e1960. [Google Scholar] [CrossRef]
- Lewandowski, J.P.; Dumbović, G.; Watson, A.R.; Hwang, T.; Jacobs-Palmer, E.; Chang, N.; Much, C.; Turner, K.M.; Kirby, C.; Rubinstein, N.D.; et al. The Tug1 lncRNA locus is essential for male fertility. Genome Biol. 2020, 21, 237. [Google Scholar] [CrossRef]
- Anguera, M.C.; Ma, W.; Clift, D.; Namekawa, S.; Kelleher, R.J., 3rd; Lee, J.T. Tsx produces a long noncoding RNA and has general functions in the germline, stem cells, and brain. PLoS Genet. 2011, 7, e1002248. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Takei, N.; Kawamura, S.; Takahashi, N.; Kotani, T.; Kimura, A.P. A novel testis-specific long noncoding RNA, Tesra, activates the Prss42/Tessp-2 gene during mouse spermatogenesis†. Biol. Reprod. 2019, 100, 833–848. [Google Scholar] [CrossRef]
- Chan, W.Y.; Lee, T.L.; Wu, S.M.; Ruszczyk, L.; Alba, D.; Baxendale, V.; Rennert, O.M. Transcriptome analyses of male germ cells with serial analysis of gene expression (SAGE). Mol. Cell. Endocrinol. 2006, 250, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, M.; Otsuka, K.; Matsubara, S.; Shiraishi, A.; Satake, H.; Kimura, A.P. A Testis-Specific Long Non-Coding RNA, lncRNA-Tcam1, Regulates Immune-Related Genes in Mouse Male Germ Cells. Front. Endocrinol. 2017, 8, 299. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Wang, M.; Wu, X.; Geng, L.; Xue, Y.; Wei, X.; Jia, Y.; Wu, X. A long non-coding RNA interacts with Gfra1 and maintains survival of mouse spermatogonial stem cells. Cell Death Dis. 2016, 7, e2140. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Zhang, J.; Liang, M. LncRNA AK015322 promotes proliferation of spermatogonial stem cell C18-4 by acting as a decoy for microRNA-19b-3p. Vitr. Cell. Dev. Biol. Anim. 2017, 53, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Li, L.; Liao, Y.; Liang, M. LncRNA Gm2044 highly expresses in spermatocyte and inhibits Utf1 translation by interacting with Utf1 mRNA. Genes Genom. 2018, 40, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wu, J.; Liu, B.; Jiang, Y.; Chen, W.; Li, J.; He, Q.; He, Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell. Mol. Life Sci. 2019, 76, 2681–2695. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.M.; Shen, X.Y.; Niu, C.M.; Xia, J.; Sun, H.Y.; Zheng, Y. [MicroRNA regulates Sertoli cell proliferation and adhesion]. Yi Chuan = Hered. 2018, 40, 724–732. [Google Scholar] [CrossRef]
- Procópio, M.S.; de Avelar, G.F.; Costa, G.M.J.; Lacerda, S.; Resende, R.R.; de França, L.R. MicroRNAs in Sertoli cells: Implications for spermatogenesis and fertility. Cell Tissue Res. 2017, 370, 335–346. [Google Scholar] [CrossRef] [PubMed]
- França, L.R.; Hess, R.A.; Dufour, J.M.; Hofmann, M.C.; Griswold, M.D. The Sertoli cell: One hundred fifty years of beauty and plasticity. Andrology 2016, 4, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.A.; Renato de Franca, L. Spermatogenesis and cycle of the seminiferous epithelium. Adv. Exp. Med. Biol. 2008, 636, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.G.; Rato, L.; Carvalho, R.A.; Moreira, P.I.; Socorro, S.; Oliveira, P.F. Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell. Mol. Life Sci. 2013, 70, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, B.; Wang, J. Functions and mechanism of noncoding RNA in the somatic cells of the testis. Zygote 2020, 28, 87–92. [Google Scholar] [CrossRef]
- O’Shaughnessy, P.J. Hormonal control of germ cell development and spermatogenesis. Semin. Cell Dev. Biol. 2014, 29, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.M.; Yadav, R.P.; Da Ros, M.; Chalmel, F.; Zimmermann, C.; Toppari, J.; Nef, S.; Kotaja, N. DICER Regulates the Formation and Maintenance of Cell-Cell Junctions in the Mouse Seminiferous Epithelium. Biol. Reprod. 2015, 93, 139. [Google Scholar] [CrossRef][Green Version]
- Zimmermann, C.; Romero, Y.; Warnefors, M.; Bilican, A.; Borel, C.; Smith, L.B.; Kotaja, N.; Kaessmann, H.; Nef, S. Germ cell-specific targeting of DICER or DGCR8 reveals a novel role for endo-siRNAs in the progression of mammalian spermatogenesis and male fertility. PLoS ONE 2014, 9, e107023. [Google Scholar] [CrossRef]
- Jovanovic, M.; Hengartner, M.O. miRNAs and apoptosis: RNAs to die for. Oncogene 2006, 25, 6176–6187. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Georg, I.; Scherthan, H.; Merkenschlager, M.; Guillou, F.; Scherer, G.; Barrionuevo, F. Dicer is required for Sertoli cell function and survival. Int. J. Dev. Biol. 2010, 54, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, M.D.; Pitetti, J.L.; Ro, S.; Park, C.; Aubry, F.; Schaad, O.; Vejnar, C.E.; Kühne, F.; Descombes, P.; Zdobnov, E.M.; et al. Sertoli cell Dicer is essential for spermatogenesis in mice. Dev. Biol. 2009, 326, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, M.D.; Lagarrigue, M.; Vejnar, C.E.; Rolland, A.D.; Kühne, F.; Aubry, F.; Schaad, O.; Fort, A.; Descombes, P.; Neerman-Arbez, M.; et al. Loss of Dicer in Sertoli cells has a major impact on the testicular proteome of mice. Mol. Cell. Proteom. 2011, 10, M900587mcp900200. [Google Scholar] [CrossRef]
- Panneerdoss, S.; Viswanadhapalli, S.; Abdelfattah, N.; Onyeagucha, B.C.; Timilsina, S.; Mohammad, T.A.; Chen, Y.; Drake, M.; Vuori, K.; Kumar, T.R.; et al. Cross-talk between miR-471-5p and autophagy component proteins regulates LC3-associated phagocytosis (LAP) of apoptotic germ cells. Nat. Commun. 2017, 8, 598. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Wang, J.; Wang, L.; Xiang, Z.; Li, D.; Han, X. Microcystin-Leucine Arginine Causes Cytotoxic Effects in Sertoli Cells Resulting in Reproductive Dysfunction in Male Mice. Sci. Rep. 2016, 6, 39238. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.J.; Tarulli, G.A.; Laven-Law, G.; Matthiesson, K.L.; Meachem, S.J.; McLachlan, R.I.; Dinger, M.E.; Stanton, P.G. Gonadotropin suppression in men leads to a reduction in claudin-11 at the Sertoli cell tight junction. Hum. Reprod. 2016, 31, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.H.; Cheng, C.Y. The blood-testis barrier: Its biology, regulation, and physiological role in spermatogenesis. Curr. Top. Dev. Biol. 2005, 71, 263–296. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mruk, D.D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar] [CrossRef]
- Lui, W.Y.; Lee, W.M.; Cheng, C.Y. Sertoli-germ cell adherens junction dynamics in the testis are regulated by RhoB GTPase via the ROCK/LIMK signaling pathway. Biol. Reprod. 2003, 68, 2189–2206. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Qin, L.; Lin, J.; Xie, X. CircRNA-9119 suppresses poly I:C induced inflammation in Leydig and Sertoli cells via TLR3 and RIG-I signal pathways. Mol. Med. 2019, 25, 28. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Ji, X.; Sun, J.; Zhang, Z. [Silencing circular RNA_ embryonic-lethal abnormal vision-like protein 2(circELAVL2) inhibits the proliferation and induces apoptosis of mouse testicular TM4 Sertoli cells]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi = Chin. J. Cell. Mol. Immunol. 2022, 38, 1005–1010. [Google Scholar]
- Wang, S.; Qian, Z.; Ge, X.; Li, C.; Xue, M.; Liang, K.; Ma, R.; Ouyang, L.; Zheng, L.; Jing, J.; et al. LncRNA Tug1 maintains blood-testis barrier integrity by modulating Ccl2 expression in high-fat diet mice. Cell. Mol. Life Sci. 2022, 79, 114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, D.; Niu, T.; Sun, Z.; Wang, Y.; Han, X.; Xiong, B.; Shen, W.; Sun, Q.; Zhao, Y.; et al. LncRNA5251 inhibits spermatogenesis via modification of cell-cell junctions. Biol. Direct 2023, 18, 31. [Google Scholar] [CrossRef]
- Basu, S.; Arya, S.P.; Usmani, A.; Pradhan, B.S.; Sarkar, R.K.; Ganguli, N.; Shukla, M.; Mandal, K.; Singh, S.; Sarda, K.; et al. Defective Wnt3 expression by testicular Sertoli cells compromise male fertility. Cell Tissue Res. 2018, 371, 351–363. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, Q.; Qiao, L.; Huang, S.; Dai, Z.; Yang, T.; Liu, L.; Zhao, Z. LncWNT3-IT affects the proliferation of Sertoli cells by regulating the expression of the WNT3 gene in goat testis. Reprod. Domest. Anim. = Zuchthyg. 2020, 55, 1061–1071. [Google Scholar] [CrossRef]
- Zhao, Z.; Qiao, L.; Dai, Z.; He, Q.; Lan, X.; Huang, S.; He, L. LncNONO-AS regulates AR expression by mediating NONO. Theriogenology 2020, 145, 198–206. [Google Scholar] [CrossRef]
- Danga, A.K.; Kour, S.; Kumari, A.; Rath, P.C. Cell-type specific and differential expression of LINC-RSAS long noncoding RNA declines in the testes during ageing of the rat. Biogerontology 2024, 25, 543–566. [Google Scholar] [CrossRef]
- Jiménez-Badillo, S.E.; Oviedo, N.; Hernández-Guzmán, C.; González-Mariscal, L.; Hernández-Sánchez, J. Catsper1 promoter is bidirectional and regulates the expression of a novel lncRNA. Sci. Rep. 2017, 7, 13351. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, F.; Li, F.; Ren, C.; Pang, J.; Wan, Y.; Wang, Z.; Feng, X.; Zhang, Y. Comprehensive analysis of long noncoding RNA and mRNA expression patterns in sheep testicular maturation. Biol. Reprod. 2018, 99, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Sun, M.; Yuan, Q.; Niu, M.; Chen, Z.; Hou, J.; Wang, H.; Wen, L.; Liu, Y.; Li, Z.; et al. MiRNA-133b promotes the proliferation of human Sertoli cells through targeting GLI3. Oncotarget 2016, 7, 2201–2219. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Chen, B.; Weng, B.; Tang, X.; Chen, Y.; Yang, A.; Chu, D.; Zeng, X.; Ran, M. miR-130a promotes immature porcine Sertoli cell growth by activating SMAD5 through the TGF-β-PI3K/AKT signaling pathway. FASEB J. 2020, 34, 15164–15179. [Google Scholar] [CrossRef]
- Xiao, Z.; Liang, J.; Huang, R.; Chen, D.; Mei, J.; Deng, J.; Wang, Z.; Li, L.; Li, Z.; Xia, H.; et al. Inhibition of miR-143-3p Restores Blood-Testis Barrier Function and Ameliorates Sertoli Cell Senescence. Cells 2024, 13, 313. [Google Scholar] [CrossRef]
- Potter, S.J.; DeFalco, T. Role of the testis interstitial compartment in spermatogonial stem cell function. Reproduction 2017, 153, R151–R162. [Google Scholar] [CrossRef] [PubMed]
- Huhtaniemi, I.T. Andropause--lessons from the European Male Ageing Study. Ann. D’endocrinologie 2014, 75, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Matzkin, M.E.; Yamashita, S.; Ascoli, M. The ERK1/2 pathway regulates testosterone synthesis by coordinately regulating the expression of steroidogenic genes in Leydig cells. Mol. Cell. Endocrinol. 2013, 370, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.S.; Hardy, M.P. Variation in the end products of androgen biosynthesis and metabolism during postnatal differentiation of rat Leydig cells. Endocrinology 1998, 139, 3787–3795. [Google Scholar] [CrossRef] [PubMed]
- Stanley, E.; Lin, C.Y.; Jin, S.; Liu, J.; Sottas, C.M.; Ge, R.; Zirkin, B.R.; Chen, H. Identification, proliferation, and differentiation of adult Leydig stem cells. Endocrinology 2012, 153, 5002–5010. [Google Scholar] [CrossRef]
- Laslett, A.L.; McFarlane, J.R.; Risbridger, G.P. Developmental response by Leydig cells to acidic and basic fibroblast growth factor. J. Steroid Biochem. Mol. Biol. 1997, 60, 171–179. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Zhang, L.; Liang, R.; Ge, R.S.; Zhang, Y.; Zhang, Q.; Xiang, Q.; Huang, Y.; Su, Z. Basic fibroblast growth factor promotes stem Leydig cell development and inhibits LH-stimulated androgen production by regulating microRNA expression. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt B, 483–491. [Google Scholar] [CrossRef]
- Shen, W.J.; Azhar, S.; Kraemer, F.B. SR-B1: A Unique Multifunctional Receptor for Cholesterol Influx and Efflux. Annu. Rev. Physiol. 2018, 80, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Shen, W.J.; Kraemer, F.B.; Azhar, S. MicroRNAs 125a and 455 repress lipoprotein-supported steroidogenesis by targeting scavenger receptor class B type I in steroidogenic cells. Mol. Cell. Biol. 2012, 32, 5035–5045. [Google Scholar] [CrossRef]
- Geng, X.J.; Zhao, D.M.; Mao, G.H.; Tan, L. MicroRNA-150 regulates steroidogenesis of mouse testicular Leydig cells by targeting STAR. Reproduction 2017, 154, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Hu, S.; Yao, G.; Xie, S.; Ni, M.; Liu, Q.; Gao, X.; Zhang, J.; Huang, X.; Zhang, Y. An androgen receptor-microrna-29a regulatory circuitry in mouse epididymis. J. Biol. Chem. 2013, 288, 29369–29381. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Pu, J.; Teng, Y.; Zhu, Q.; Guo, L.; Zhao, J.; Ding, H.; Fang, Y.; Ma, X.; Liu, H.; et al. Melatonin Inhibits Testosterone Synthesis in Rooster Leydig Cells by Targeting CXCL14 through miR-7481-3p. Int. J. Mol. Sci. 2023, 24, 16552. [Google Scholar] [CrossRef]
- Mei, X.; Chen, S.Y. Circular RNAs in cardiovascular diseases. Pharmacol. Ther. 2022, 232, 107991. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Li, X.; Jiang, C.; Wang, K.; Zuo, T.; He, G.; Qin, L.; Xu, W. Testis-enriched circular RNA circ-Bbs9 plays an important role in Leydig cell proliferation by regulating a CyclinD2-dependent pathway. Reprod. Fertil. Dev. 2020, 32, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Y.; Zhang, G.; Li, J.; Wu, X.; Ma, X.; Shan, G.; Mei, Y. Long noncoding RNA EMS connects c-Myc to cell cycle control and tumorigenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 14620–14629. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.E.I.; Xiang-Liang, T.; Yu, Z.; Bin, L.; Lian-Ju, S.; Chun-Lan, L.; Tao, L.I.N.; Da-Wei, H.E.; Sheng-de, W.U.; Guang-Hui, W.E.I. DEHP exposure destroys blood-testis barrier (BTB) integrity of immature testes through excessive ROS-mediated autophagy. Genes Dis. 2018, 5, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Lian, F.Z.; Leng, X.; Wang, S.M.; Li, Y.B.; Wang, Z.Y.; Li, K.R.; Gao, Z.X.; Jiang, Y.G. Microarray expression profiling and co-expression network analysis of circulating LncRNAs and mRNAs associated with neurotoxicity induced by BPA. Environ. Sci. Pollut. Res. Int. 2018, 25, 15006–15018. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, J.; Zhao, T.; Wei, Y.; Han, L.; Shen, L.; Long, C.; Wu, S.; Wei, G. LncRNAs activate longevity regulation pathway due to aging of Leydig cells caused by DEHP exposure: A transcriptome-based study. Ecotoxicol. Environ. Saf. 2021, 209, 111798. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Huang, F.J.; Du, P.J.; Wang, J.; Guo, F.; Shao, M.W.; Song, Y.; Liu, Y.X.; Qin, G.J. Long noncoding RNA MIR22HG promotes Leydig cell apoptosis by acting as a competing endogenous RNA for microRNA-125a-5p that targets N-Myc downstream-regulated gene 2 in late-onset hypogonadism. Lab. Investig. A J. Tech. Methods Pathol. 2021, 101, 1484–1493. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Wang, J.; Huang, F.; Du, P.; Wu, L.; Guo, F.; Song, Y.; Qin, G. LncRNA FENDRR promotes apoptosis of Leydig cells in late-onset hypogonadism by facilitating the degradation of Nrf2. Cell Tissue Res. 2021, 386, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Matsubara, S.; Shiraishi, A.; Takei, N.; Satoh, Y.; Terao, M.; Takada, S.; Kotani, T.; Satake, H.; Kimura, A.P. A Testis-Specific Long Noncoding RNA, Start, Is a Regulator of Steroidogenesis in Mouse Leydig Cells. Front. Endocrinol. 2021, 12, 665874. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, X.; Zhang, X.; Yang, Y.; Gao, H.; Gao, J.; Bao, H.; Zhao, L.; Yang, G.; Zhang, Y.; et al. The long noncoding RNA CIRBIL is a regulator of steroidogenesis in mice. Reprod. Biol. 2023, 23, 100783. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Ning, Z.; Li, L.; Cui, Z.; Du, X.; Amevor, F.K.; Tian, Y.; Shu, G.; Du, X.; Han, X.; et al. High expression of miR-22-3p in chicken hierarchical follicles promotes granulosa cell proliferation, steroidogenesis, and lipid metabolism via PTEN/PI3K/Akt/mTOR signaling pathway. Int. J. Biol. Macromol. 2023, 253, 127415. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Sethi, S.; Mehta, P.; Kumari, A.; Rajender, S. Small RNAs, spermatogenesis, and male infertility: A decade of retrospect. Reprod. Biol. Endocrinol. 2023, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, I.; Colpan, C.; Arif, A.; Cecchini, K.; Zamore, P.D. A Single Mechanism of Biogenesis, Initiated and Directed by PIWI Proteins, Explains piRNA Production in Most Animals. Mol. Cell 2018, 71, 775–790.e5. [Google Scholar] [CrossRef] [PubMed]
- Meikar, O.; Da Ros, M.; Korhonen, H.; Kotaja, N. Chromatoid body and small RNAs in male germ cells. Reproduction 2011, 142, 195–209. [Google Scholar] [CrossRef]
- Choi, H.; Wang, Z.; Dean, J. Sperm acrosome overgrowth and infertility in mice lacking chromosome 18 pachytene piRNA. PLoS Genet. 2021, 17, e1009485. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Fu, Y.; Cecchini, K.; Özata, D.M.; Arif, A.; Yu, T.; Colpan, C.; Gainetdinov, I.; Weng, Z.; Zamore, P.D. The evolutionarily conserved piRNA-producing locus pi6 is required for male mouse fertility. Nat. Genet. 2020, 52, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Liu, M. Knockout Gene-Based Evidence for PIWI-Interacting RNA Pathway in Mammals. Front. Cell Dev. Biol. 2021, 9, 681188. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi-Miyagawa, S.; Watanabe, T.; Gotoh, K.; Totoki, Y.; Toyoda, A.; Ikawa, M.; Asada, N.; Kojima, K.; Yamaguchi, Y.; Ijiri, T.W.; et al. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev. 2008, 22, 908–917. [Google Scholar] [CrossRef]
- Carmell, M.A.; Girard, A.; van de Kant, H.J.; Bourc’his, D.; Bestor, T.H.; de Rooij, D.G.; Hannon, G.J. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev. Cell 2007, 12, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Lin, H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev. Cell 2002, 2, 819–830. [Google Scholar] [CrossRef]
- Dai, P.; Wang, X.; Gou, L.T.; Li, Z.T.; Wen, Z.; Chen, Z.G.; Hua, M.M.; Zhong, A.; Wang, L.; Su, H.; et al. A Translation-Activating Function of MIWI/piRNA during Mouse Spermiogenesis. Cell 2019, 179, 1566–1581.e16. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; James, E.R.; Aston, K.I.; Carrell, D.T.; Jenkins, T.G.; Yeste, M. The role of miRNAs in male human reproduction: A systematic review. Andrology 2020, 8, 7–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xie, D.; Zhao, X.; Xu, M.; Luo, L.; Deng, D.; Chen, M. Enhanced Expression of miR-34c in Peripheral Plasma Associated with Diabetic Foot Ulcer in Type 2 Diabetes Patients. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 4263–4273. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; Wang, Q. Exploring the Characters of Non-Coding RNAs in Spermatogenesis and Male Infertility. Int. J. Mol. Sci. 2025, 26, 1128. https://doi.org/10.3390/ijms26031128
Yan Q, Wang Q. Exploring the Characters of Non-Coding RNAs in Spermatogenesis and Male Infertility. International Journal of Molecular Sciences. 2025; 26(3):1128. https://doi.org/10.3390/ijms26031128
Chicago/Turabian StyleYan, Qiu, and Qi Wang. 2025. "Exploring the Characters of Non-Coding RNAs in Spermatogenesis and Male Infertility" International Journal of Molecular Sciences 26, no. 3: 1128. https://doi.org/10.3390/ijms26031128
APA StyleYan, Q., & Wang, Q. (2025). Exploring the Characters of Non-Coding RNAs in Spermatogenesis and Male Infertility. International Journal of Molecular Sciences, 26(3), 1128. https://doi.org/10.3390/ijms26031128







