Plant Biostimulants to Enhance Abiotic Stress Resilience in Crops
Abstract
:1. Introduction
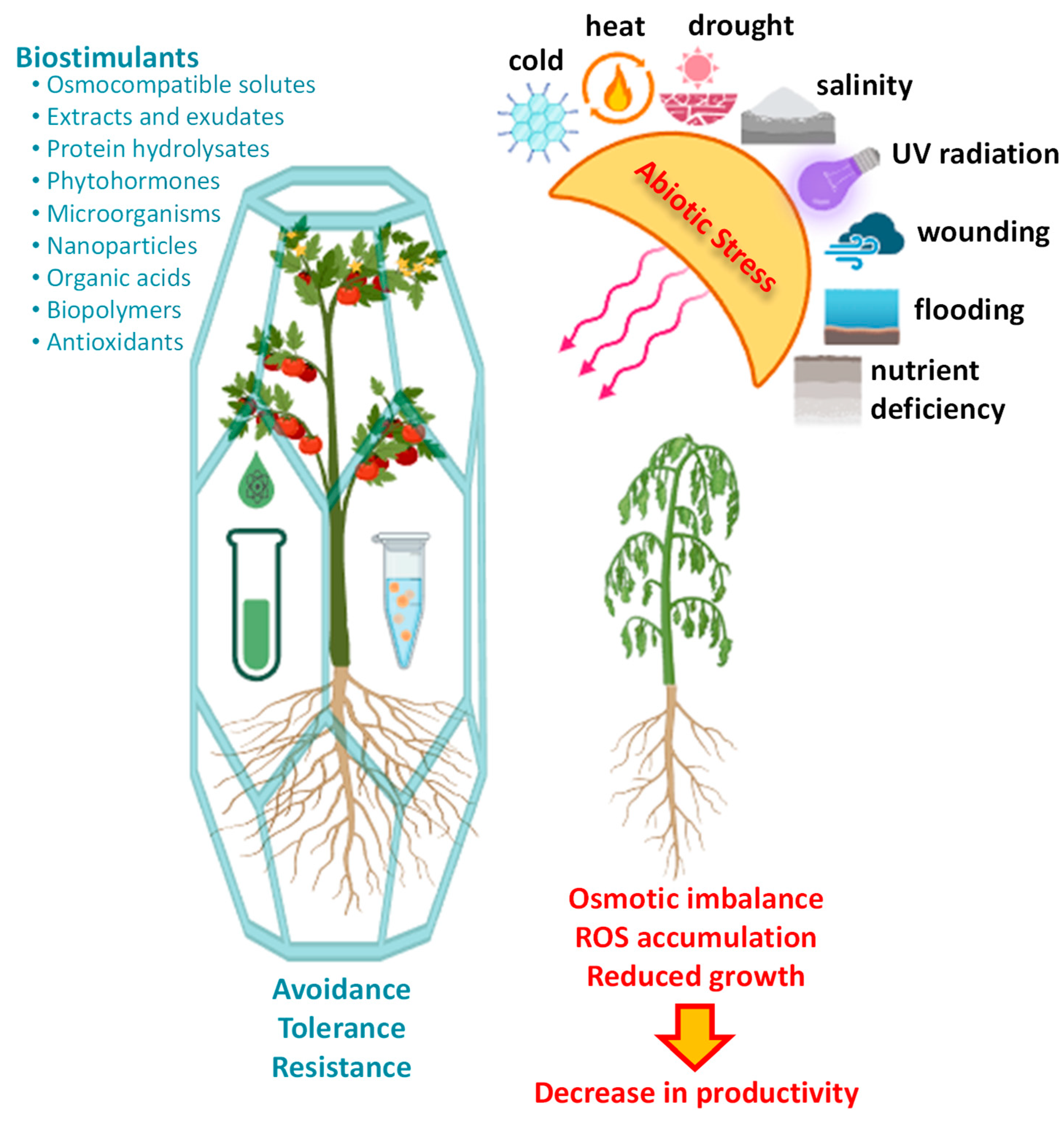
2. Abiotic Stress in Crops: Challenges and Implications
3. Biostimulants to Tackle Abiotic Stress in Crops
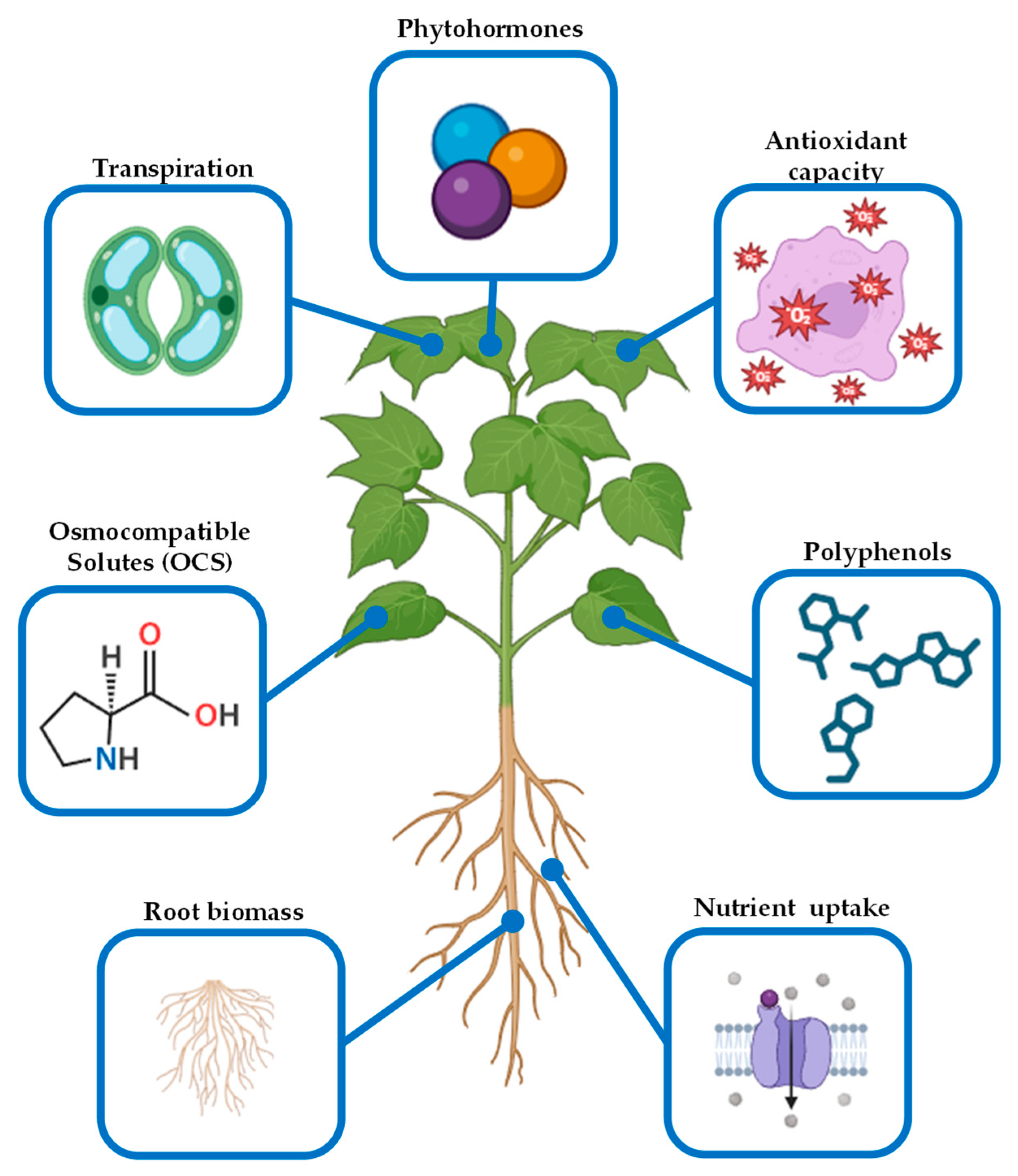
3.1. Osmocompatible Solutes (OCSs)
3.2. Antioxidants
3.3. Phytohormones
3.4. Extracts, Exudates and Protein Hydrolysates
4. Plant-Derived Biostimulants
| Biostimulant Source | Biostimulant | Treated Crop | Improvement in | Reference |
|---|---|---|---|---|
| Abscisic acid | Nicotiana benthamiana | Drought stress tolerance | [71] | |
| Vitis vinifera | Ripening and fruit quality | [72] | ||
| Antioxidant capacity | [73,74] | |||
| Melatonin | Gossypium hirsutum | Germination/antioxidant capacity | [79] | |
| Stevia rebaudiana | [80] | |||
| Solanum lycopersicum | Root growth/nutrient use efficiency | [81] | ||
| Cucumis sativus | [82] | |||
| Glycine max | [83] | |||
| Triticum aestivum | Drought stress tolerance | [84] | ||
| Polygonum cuspidatum | Drought stress tolerance/resveratrol levels | [85] | ||
| Zea mays | Drought stress tolerance | [86] | ||
| Oryza sativa | [87] | |||
| Actinidia chinensis | Flood stress tolerance | [88] | ||
| Triticum aestivum | Flood stress tolerance/antioxidant capacity | [89] | ||
| Protein hydrolysates | Begonia tuberhybrida | Nutrient use efficiency/plant growth | [92] | |
| Pelargonium peltatum | ||||
| Viola cornuta | ||||
| Spinacia oleracea | [93] | |||
| Valerianella locusta | ||||
| Cannabis sativa | [94] | |||
| Extracts | Vitis vinifera | Polyphenol content | [98] | |
| Coriandrum sativum | [99] | |||
| Capsicum chinensis | [100] | |||
| Vitis vinifera | Terpene and norisoprenoid content | [101,102] | ||
| Mentha Arvensis | Menthol content | [103] | ||
| Pelargonium graveolens | Geraniol, linalool, and citronellol content | [104] | ||
| Seaweeds | Fucans | Nicotiana tabacum | Biotic stress tolerance | [106] |
| Carrageenans | Zea mays/Cicer arietinum | Plant growth | [107] | |
| Nicotiana tabacum | [108] | |||
| Alginates | Foeniculum vulgare | Plant growth and development | [109] | |
| Carrageenans | Pinus radiata | Plant growth | [110] | |
| Eucalyptus globulus | [111] | |||
| Alginates | Papaver somniferum | Plant growth and development | [112] | |
| Oryza sativa | [113] | |||
| Arachis hypogaea | ||||
| Triticum aestivum | Drought stress tolerance | [114] | ||
| A. nodosum extract | Solanum lycopersicum | Heat stress tolerance | [115] | |
| G. rugosa extract | Drought stress tolerance | [116] | ||
| Microalgae | Nannochloris sp. extract | Solanum lycopersicum | Drought stress tolerance | [117] |
| Spirulina platensis | Carica papaya | Plant growth | [118] | |
| Solanum melongena | [119] | |||
| A. platensis and Scenedesmus sp. | Petunia hybrida | [120] | ||
| A. fusiformis | Allium sativum | [121] | ||
| Spirulina platensis | Capsicum annuum | Fruit yield and quality | [122] | |
| Bacteria | PGPR | Phoenix dactylifera | Salt stress tolerance | [123] |
| Oryza sativa | Salt stress tolerance | [124] | ||
| Amaranthus viridis | Salt stress tolerance | [125] | ||
| Hordeum vulgare | Drought stress tolerance | [126] | ||
| Zea mays | Salt stress tolerance | [127] | ||
| Solanum lycopersicon | Plant growth/fruit yield | [128] | ||
| PSB | Zea mays | Nutrient use efficiency/salt tolerance | [129] | |
| Quercus Brantii | Drought stress tolerance | [130] | ||
| Arachis hypogaea | Salt stress tolerance | [131] | ||
| Solanum tuberosum | Plant growth | [132] | ||
| Lycopersicon esculentum | Drought stress tolerance | [133] |
5. Biostimulants from Seaweeds (Macroalgae) and Microalgae
6. Bacteria-Derived Plant Biostimulant
7. Conclusions
8. Future Perspectives: Optimizing Biostimulants for Sustainable Crop Production
Author Contributions
Funding
Conflicts of Interest
References
- Xiong, W.; Reynolds, M.; Xu, Y. Climate change challenges plant breeding. Curr. Opin. Plant Biol. 2022, 70, 102308. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Blumwald, E. Genetic engineering for modern agriculture: Challenges and perspectives. Annu. Rev. Plant Biol. 2010, 61, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Anand, U.; López-Bucio, J.; Kumar, M.; Lal, M.K.; Tiwari, R.K.; Dey, A. Biostimulants and environmental stress mitigation in crops: A novel and emerging approach for agricultural sustainability under climate change. Environ. Res. 2023, 233, 116357. [Google Scholar] [CrossRef]
- Ma, Y.; Freitas, H.; Dias, M.C. Strategies and prospects for biostimulants to alleviate abiotic stress in plants. Front. Plant Sci. 2022, 13, 1024243. [Google Scholar] [CrossRef]
- Bhupenchandra, I.; Chongtham, S.K.; Devi, E.L.; Choudhary, A.K.; Salam, M.D.; Sahoo, M.R.; Khaba, C.I. Role of biostimulants in mitigating the effects of climate change on crop performance. Front. Plant Sci. 2022, 13, 967665. [Google Scholar] [CrossRef] [PubMed]
- Pugnaire, F.I.; Morillo, J.A.; Peñuelas, J.; Reich, P.B.; Bardgett, R.D.; Gaxiola, A.; Van Der Putten, W.H. Climate change effects on plant-soil feedbacks and consequences for biodiversity and functioning of terrestrial ecosystems. Sci. Adv. 2019, 5, eaaz1834. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.C.; Oppenheimer, M.; Warren, R.; Hallegatte, S.; Kopp, R.E.; Pörtner, H.O.; Yohe, G. IPCC reasons for concern regarding climate change risks. Nat. Clim. Change 2017, 7, 28–37. [Google Scholar] [CrossRef]
- Buono, D.D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef] [PubMed]
- Ramankutty, N.; Mehrabi, Z.; Waha, K.; Jarvis, L.; Kremen, C.; Herrero, M.; Rieseberg, L.H. Trends in global agricultural land use: Implications for environmental health and food security. Annu. Rev. Plant Biol. 2018, 69, 789–815. [Google Scholar] [CrossRef]
- Espeland, E.K.; Kettenring, K.M. Strategic plant choices can alleviate climate change impacts: A review. J. Environ. Manag. 2018, 222, 316–324. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of the European Union. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing regulation (EC) No 2003/2003 (text with EEA relevance). Off. J. Eur. Union 2019, 170, 1–114. [Google Scholar]
- García-Segura, S.; Pardos, J.A. Osmoprotectants in plant stress adaptation and signaling. Front. Plant Sci. 2018, 9, 1303. [Google Scholar]
- Wang, Z.; Liang, X.; Wang, H.; Liu, Y. Osmoprotectants as plant biostimulants: A review of their mechanisms and applications. J. Plant Physiol. 2023, 226, 173–191. [Google Scholar]
- Hasegawa, P.M.; Zhu, J.K. Roles of osmolytes in plant responses to abiotic stress. Ann. Bot. 2015, 115, 1131–1146. [Google Scholar]
- Zhang, J.; Zhao, X.; Zhang, Z.; Li, B. Osmoprotectants as plant biostimulants: Current progress and future perspectives. Plant Growth Regul. 2023, 120, 75–94. [Google Scholar]
- Benedito, T.P.; Pereira, L.G.; Machado, A.C.; de Oliveira, C.A.; Soares, M.A. Glycerol as a compatible solute in improving the tolerance of soybean seedlings to water deficit and salt stress. Plant Growth Regul. 2023, 149, 101583. [Google Scholar]
- Liu, F.; Ma, Y.; Li, J.; Li, N.; Wang, J.; Zhang, W. Osmoprotectants improve salt tolerance in rice seedlings by regulating stress-inducible gene expression and antioxidant systems. Plant Physiol. Biochem. 2023, 156, 265–277. [Google Scholar]
- Sahoo, S.K.; Singh, M.; Panda, D.K. Proline alleviates drought stress in wheat seedlings: A role of reactive oxygen species, antioxidant enzymes, and photosynthesis. Front. Plant Sci. 2023, 14, 723582. [Google Scholar]
- Xu, L.; Wang, Y.; Wang, D. Molecular mechanisms of osmotic stress-responsive osmoprotectants in plant stress tolerance and development. Front. Plant Sci. 2023, 14, 796746. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Mehla, N.; Sindhi, V.; Josula, D.; Bisht, P.; Wani, S.H. An introduction to antioxidants and their roles in plant stress tolerance. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation Under Abiotic Stress; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–23. [Google Scholar]
- Singh, A.; Kumar, A.; Yadav, S.; Singh, I.K. Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene 2019, 18, 100173. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop Stress and Its Management: Perspectives and Strategies; Springer: Berlin/Heidelberg, Germany, 2012; pp. 261–315. [Google Scholar]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kaur, J.; Grewal, S.K.; Singh, I. Effect of heat stress on antioxidative defense system and its amelioration by heat acclimation and salicylic acid pre-treatments in three pigeonpea genotypes. Indian J. Agric. Biochem. 2019, 32, 106–110. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Awasthi, R. Antioxidants and their role in plant abiotic stress tolerance: A review. J. Stress Physiol. Biochem. 2022, 18, 9. [Google Scholar]
- Ashraf, M.; Mehmood, K.; Akhtar, M.S. Antioxidants as natural biostimulants to mitigate abiotic stress in plants. Plant Physiol. Biochem. 2023, 162, 104686. [Google Scholar]
- Zhang, L.; Li, J.; Chen, W.; Wang, Y. Enhancing plant resistance to abiotic stress using antioxidants: A review. Front. Plant Sci. 2023, 14, 983619. [Google Scholar]
- Liu, H.; Chen, H.; Li, X.; Wang, Z.; Wang, X. Vitamin C improves drought tolerance and metabolic flexibility in maize seedlings by regulating antioxidant responses and stress signaling pathways. J. Plant Physiol. 2023, 226, 105457. [Google Scholar]
- Akhter, M.S.; Shah, M.T. Phytohormones and abiotic stress: A review of their role and regulation in plant resilience. Plant Physiol. Biochem. 2022, 158, 231–245. [Google Scholar]
- Li, H.; Zhang, D.; Li, H. Phytohormones in enhancing plant abiotic stress tolerance: Mechanisms of action and application strategies. Front. Plant Sci. 2023, 14, 917246. [Google Scholar]
- Khan, A.; Zhang, W.; Ashraf, M. Phytohormones and their role in plant abiotic stress tolerance: A review. Front. Plant Sci. 2023, 14, 868884. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Abscisic acid: A guardian of plant resilience under abiotic stress. Plant Physiol. 2022, 188, 1289–1305. [Google Scholar]
- Dey, U.; Kumar, R. Salicylic acid: A crucial player in plant abiotic stress resilience and defense responses. J. Plant Physiol. 2023, 235, 178–199. [Google Scholar]
- Guo, Y.; Wu, J.; Liu, W.; Xie, Y. Gibberellins in plant abiotic stress tolerance: Current understanding and future directions. Plant Cell Environ. 2022, 45, 3720–3737. [Google Scholar]
- Lee, J.S.; Kim, J.H. Cytokinins in plant abiotic stress tolerance: Mechanisms of action and application strategies. Front. Plant Sci. 2023, 14, 936300. [Google Scholar]
- Shahzad, S.; Zia, A.; Anwar, F.; Siddiqui, M.S. Auxins in mediating plant responses to abiotic stress. Plant Sci. 2022, 296, 111655. [Google Scholar]
- Johnson, R.; Joel, J.M.; Puthur, J.T. Biostimulants: The futuristic sustainable approach for alleviating crop productivity and abiotic stress tolerance. J. Plant Growth Regul. 2023, 43, 659–674. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Lucini, L.; Miras-Moreno, B.; Colla, G.; Bonini, P.; Cardarelli, M. Metabolomic responses of maize shoots and roots elicited by combinatorial seed treatments with microbial and non-microbial biostimulants. Front. Microbiol. 2020, 11, 664. [Google Scholar] [CrossRef]
- Braun, J.C.; Colla, L.M. Use of microalgae for the development of biofertilizers and biostimulants. BioEnergy Res. 2023, 16, 289–310. [Google Scholar] [CrossRef]
- Mzibra, A.; Aasfar, A.; Benhima, R.; Khouloud, M.; Boulif, R.; Douira, A.; Meftah Kadmiri, I. Biostimulants derived from Moroccan seaweeds: Seed germination metabolomics and growth promotion of tomato plant. J. Plant Growth Regul. 2021, 40, 353–370. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Understanding the biostimulant action of vegetal-derived protein hydrolysates by high-throughput plant phenotyping and metabolomics: A case study on tomato. Front. Plant Sci. 2019, 10, 47. [Google Scholar] [CrossRef]
- Abraham, R.E.; Su, P.; Puri, M.; Raston, C.L.; Zhang, W. Optimisation of biorefinery production of alginate, fucoidan and laminarin from brown seaweed Durvillaea potatorum. Algal Res. 2019, 38, 101389. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Torres, M.D.; González-Muñoz, M.J.; Domínguez, H. Recovery of bioactive and gelling extracts from edible brown seaweed Laminaria ochroleuca by non-isothermal autohydrolysis. Food Chem. 2019, 277, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, K.; Ervin, E.H. Optimizing dosages of seaweed extract-based cytokinins and zeatin riboside for improving creeping bentgrass heat tolerance. Crop Sci. 2010, 50, 316–320. [Google Scholar] [CrossRef]
- Blunden, G.; Jenkins, T.; Liu, Y.W. Enhanced leaf chlorophyll levels in plants treated with seaweed extract. J. Appl. Phycol. 1996, 8, 535–543. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H. Impact of seaweed extract-based cytokinins and zeatin riboside on creeping bentgrass heat tolerance. Crop Sci. 2008, 48, 364–370. [Google Scholar] [CrossRef]
- Deolu-Ajayi, A.O.; van der Meer, I.M.; Van der Werf, A.; Karlova, R. The power of seaweeds as plant biostimulants to boost crop production under abiotic stress. Plant Cell Environ. 2022, 45, 2537–2553. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patel, J.S. Seaweed extract: Biostimulator of plant defense and plant productivity. Int. J. Environ. Sci. Technol. 2020, 17, 553–568. [Google Scholar] [CrossRef]
- Lewandowska, S.; Marczewski, K.; Kozak, M.; Ohkama-Ohtsu, N.; Łabowska, M.; Detyna, J.; Michalak, I. Impact of freshwater macroalga (Cladophora glomerata) extract on the yield and morphological responses of Glycine max (L.) Merr. Agriculture 2022, 12, 685. [Google Scholar] [CrossRef]
- Johnson, R.; Puthur, J.T. Biostimulant priming in Oryza sativa: A novel approach to reprogram the functional biology under nutrient-deficient soil. Cereal Res. Commun. 2021, 50, 45–52. [Google Scholar] [CrossRef]
- Merwad, A.R.M. Mitigation of salinity stress effects on growth, yield and nutrient uptake of wheat by application of organic extracts. Commun. Soil Sci. Plant Anal. 2020, 51, 1150–1160. [Google Scholar] [CrossRef]
- Di Stasio, E.; Cirillo, V.; Raimondi, G.; Giordano, M.; Esposito, M.; Maggio, A. Osmo-priming with seaweed extracts enhances yield of salt-stressed tomato plants. Agronomy 2020, 10, 1559. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Caruso, G.; Cozzolino, E.; De Pascale, S.; Kyriacou, M.C.; Rouphael, Y. Stand-alone and combinatorial effects of plant-based biostimulants on the production and leaf quality of perennial wall rocket. Plants 2020, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’Aversana, E.; El-Nakhel, C.; Giordano, M.; Rouphael, Y. Morphological and physiological responses induced by protein hydrolysate-based biostimulant and nitrogen rates in greenhouse spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Semida, W.M.; Rady, M.M. Moringa leaf extract as biostimulant improves water use efficiency, physio-biochemical attributes of squash plants under deficit irrigation. Agric. Water Manag. 2017, 193, 46–54. [Google Scholar] [CrossRef]
- Nalia, A.; Sengupta, K. Effect of humic acid on the growth and yield of rabi pigeon pea (Cajanus cajan (L.) Millsp) in the New Alluvial Zone of West Bengal. J. Crop Weed 2019, 15, 205–208. [Google Scholar]
- Hassan, S.M.; Ashour, M.; Sakai, N.; Zhang, L.; Hassanien, H.A.; Gaber, A.; Ammar, G. Impact of seaweed liquid extract biostimulant on growth, yield, and chemical composition of cucumber (Cucumis sativus). Agriculture 2021, 11, 320. [Google Scholar] [CrossRef]
- Canellas, L.P.; Canellas, N.O.; da Silva, R.M.; Spaccini, R.; Mota, G.P.; Olivares, F.L. Biostimulants using humic substances and plant-growth-promoting bacteria: Effects on cassava (Manihot esculentus) and okra (Abelmoschus esculentus) yield. Agronomy 2022, 13, 80. [Google Scholar] [CrossRef]
- Martínez-Lorente, S.E.; Martí-Guillén, J.M.; Pedreño, M.Á.; Almagro, L.; Sabater-Jara, A.B. Higher Plant-Derived Biostimulants: Mechanisms of Action and Their Role in Mitigating Plant Abiotic Stress. Antioxidants 2024, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef]
- Zhang, X.; Schmidt, R.E. Hormone-containing products’ impact on antioxidant status of tall fescue and creeping bentgrass subjected to drought. Crop Sci. 2000, 40, 1344–1349. [Google Scholar] [CrossRef]
- Xu, X.B.; Liu, H.; Praat, M.; Pizzio, G.A.; Jiang, Z.; Driever, S.M.; Wang, R.; Van De Cotte, B.; Villers, S.L.Y.; Gevaert, K.; et al. Stomatal opening under high temperatures is controlled by the OST1-regulated TOT3-AHA1 module. Nat. Plants 2024, 11, 105–117. [Google Scholar] [CrossRef]
- Pizzio, G.A.; Mayordomo, C.; Illescas-Miranda, J.; Coego, A.; Bono, M.; Sanchez-Olvera, M.; Martin-Vasquez, C.; Samantara, K.; Merilo, E.; Forment, J.; et al. Basal ABA signaling balances transpiration and photosynthesis. Physiol. Plant 2024, 176, e14494. [Google Scholar] [CrossRef] [PubMed]
- Pilati, S.; Bagagli, G.; Sonego, P.; Moretto, M.; Brazzale, D.; Castorina, G.; Simoni, L.; Tonelli, C.; Guella, G.; Engelen, K.; et al. Abscisic Acid Is a Major Regulator of Grape Berry Ripening Onset: New Insights into ABA Signaling Network. Front. Plant Sci. 2017, 8, 1093. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.M.; Mazzeo, A.; Matarrese, A.M.S.; Pacucci, C.; Pacifico, A.; Gambacorta, G.; Faccia, M.; Trani, A.; Gallo, V.; Cafagna, I.; et al. Application of Abscisic Acid (S-ABA) to ’Crimson Seedless’ Grape Berries in a Mediterranean Climate: Effects on Color, Chemical Characteristics, Metabolic Profile, and S-ABA Concentration. J. Plant Growth Regul. 2013, 32, 491–505. [Google Scholar] [CrossRef]
- Villalobos-Gonzalez, L.; Peña-Neira, A.; Ibañez, F.; Pastenes, C. Long-term effects of abscisic acid (ABA) on the grape berry phenylpropanoid pathway: Gene expression and metabolite content. Plant Physiol. Biochem. 2016, 105, 213–223. [Google Scholar] [CrossRef]
- Zocchi, E.; Hontecillas, R.; Leber, A.; Einerhand, A.; Carbo, A.; Bruzzone, S.; Tubau-Juni, N.; Philipson, N.; Zoccoli-Rodriguez, V.; Sturla, L.; et al. Abscisic Acid: A Novel Nutraceutical for Glycemic Control. Front. Nutr. 2017, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Pizzio, G.A. Potential Implications of the Phytohormone Abscisic Acid in Human Health Improvement at the Central Nervous System. Ann. Epidemiol. Public Health 2022, 5, 1090. [Google Scholar]
- Bono, M.; Ferrer-Gallego, R.; Pou, A.; Rivera-Moreno, M.; Benavente, J.L.; Mayordomo, C.; Deis, L.; Carbonell-Bejerano, P.; Pizzio, G.A.; Navarro-Payá, D.; et al. Chemical activation of ABA signaling in grapevine through the iSB09 and AMF4 ABA receptor agonists enhances water use efficiency. Physiol. Plant 2024, 176, e14635. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, Y.; Wang, Y.; Zhang, L.; Li, L.; Looi, L.J.; Zhang, Z. The potential of melatonin and its crosstalk with other hormones in the fight against stress. Front. Plant Sci. 2024, 15, 1492036. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, L.; Wang, H.; Li, D.; Bai, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Li, C. Exogenous melatonin accelerates seed germination in cotton (Gossypium hirsutum L.). PLoS ONE 2019, 14, e0216575. [Google Scholar] [CrossRef] [PubMed]
- Simlat, M.; Ptak, A.; Skrzypek, E.; Warchoł, M.; Morańska, E.; Piórkowska, E. Melatonin significantly influences seed germination and seedling growth of Stevia rebaudiana Bertoni. PeerJ 2018, 6, e5009. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, G.; Dou, J.; Niu, Y.; Li, R.; An, W.; Tang, Z.; Yu, J. Melatonin modulates tomato root morphology by regulating key genes and endogenous hormones. Plants 2024, 13, 383. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, R.; Li, S.; Ran, S.; Wang, J.; Zhou, Y.; Gao, H.; Zhong, F. The mechanism of melatonin promotion on cucumber seedling growth at different nitrogen levels. Plant Physiol. Biochem. 2024, 206, 108263. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, C.; Cao, L.; Jin, X.; Wang, M.; Zhang, M.; Zhao, Q.; Li, H.; Zhang, Y.; Yu, G.; et al. The mechanisms underlying melatonin improved soybean seedling growth at different nitrogen levels. Funct. Plant Biol. 2021, 48, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wang, D.; Delaplace, P.; Pan, Y.; Zhou, Y.; Tang, W.; Chen, K.; Chen, J.; Xu, Z.; Ma, Y.; et al. Melatonin enhances drought tolerance by affecting jasmonic acid and lignin biosynthesis in wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2023, 202, 107974. [Google Scholar] [CrossRef]
- Shi, R.; Ye, M.; Liu, Y.; Wu, Q.; Allah, E.F.; Zhou, N. Exogenous Melatonin Regulates Physiological Responses and Active Ingredient Levels in Polygonum cuspidatum under Drought Stress. Plants 2023, 12, 2141. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Yang, L.; Ahmad, S.; Farooq, S.; Khan, A.; Muhammad, N.; Ullah, S.; Adnan, M.; Ali, S.; Liang, Q.P.; et al. Melatonin-priming enhances maize seedling drought tolerance by regulating the antioxidant defense system. Plant Physiol. 2023, 191, 2301–2315. [Google Scholar] [CrossRef]
- Li, R.; Yang, R.; Zheng, W.; Wu, L.; Zhang, C.; Zhang, H. Melatonin promotes SGT1-involved signals to ameliorate drought stress adaption in rice. Int. J. Mol. Sci. 2022, 23, 599. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Wang, H.; Wang, Q.; Gao, Y.; Xu, K.; Sun, X. Exogenous treatment with melatonin enhances waterlogging tolerance of kiwifruit plants. Front. Plant Sci. 2022, 13, 1081787. [Google Scholar] [CrossRef]
- Ma, S.; Gai, P.; Geng, B.; Wang, Y.; Ullah, N.; Zhang, W.; Zhang, H.; Fan, Y.; Huang, Z. Exogenous melatonin improves waterlogging tolerance in wheat through promoting antioxidant enzymatic activity and carbon assimilation. Agronomy 2022, 12, 2876. [Google Scholar] [CrossRef]
- Li, J.; Lardon, R.; Mangelinckx, S.; Geelen, G. A practical guide to the discovery of biomolecules with biostimulant activity. J. Exp. Bot. 2024, 75, 3797–3817. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent advances in the molecular effects of biostimulants in plants: An overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, C.; Rouphael, Y.; Pannico, A.; El-Nakhel, C.; Colla, G.; De Pascale, S. Application of Protein Hydrolysate-Based Biostimulant as New Approach to Improve Performance of Bedding Plants. In Proceedings of the ISHS Acta Horticulturae 1215: International Symposium on Greener Cities for More Efficient Ecosystem Services in a Climate Changing World, Bologna, Italy, 12–15 September 2017; pp. 443–448. [Google Scholar]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Nocerino, S.; Rouphael, Y.; Colla, G.; El-Nakhel, C.; Mori, M. Nitrogen Use and Uptake Efficiency and Crop Performance of Baby Spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) Grown under Variable Sub-Optimal N Regimes Combined with Plant-Based Biostimulant Application. Agronomy 2020, 10, 278. [Google Scholar] [CrossRef]
- Wise, K.; Selby-Pham, J.; Chai, X.; Simovich, T.; Gupta, S.; Gill, H. Fertiliser Supplementation with a Biostimulant Complex of Fish Hydrolysate, Aloe Vera Extract, and Kelp Alters Cannabis Root Architecture to Enhance Nutrient Uptake. Sci. Hortic. 2024, 323, 112483. [Google Scholar] [CrossRef]
- Ceccarelli, A.V.; Miras-Moreno, B.; Buffagni, V.; Senizza, B.; Pii, Y.; Cardarelli, M.; Rouphael, Y.; Colla, G.; Lucini, L. Foliar Application of Different Vegetal-Derived Protein Hydrolysates Distinctively Modulates Tomato Root Development and Metabolism. Plants 2021, 10, 326. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Ciriello, M.; Formisano, L.; El-Nakhel, C.; Ganugi, P.; Fiorini, A.; Miras Moreno, B.; Zhang, L.; Cardarelli, M.; et al. Copper Boosts the Biostimulant Activity of a Vegetal-Derived Protein Hydrolysate in Basil: Morpho-Physiological and Metabolomics Insights. Front. Plant Sci. 2023, 14, 1235686. [Google Scholar] [CrossRef]
- Lucini, L.; Miras-Moreno, B.; Rouphael, Y.; Cardarelli, M.; Colla, G. Combining Molecular Weight Fractionation and Metabolomics to Elucidate the Bioactivity of Vegetal Protein Hydrolysates in Tomato Plants. Front. Plant Sci. 2020, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Pardo-García, A.I.; Martínez-Gil, A.M.; Cadahía, E.; Pardo, F.; Alonso, G.L.; Salinas, M.R. Oak extract application to grapevines as a plant biostimulant to increase wine polyphenols. Food Res. Int. 2014, 55, 150–160. [Google Scholar] [CrossRef]
- Mazrou, R.M. Moringa leaf extract application as a natural biostimulant improves the volatile oil content, radical scavenging activity, and total phenolics of coriander. J. Med. Plant Stud. 2019, 7, 45–51. [Google Scholar]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Zalacain, A.; Pardo, F.; Alonso, G.L.; Salinas, M.R. Moscatel Vine-Shoot Extracts as a Grapevine Biostimulant to Enhance Wine Quality. Food Res. Int. 2017, 98, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gómez, R.; Torregrosa, L.; Zalacain, A.; Ojeda, H.; Bouckenooghe, V.; Schneider, R.; Alonso, G.L.; Salinas, M.R. The Microvine, a Plant Model to Study the Effect of Vine-Shoot Extract on the Accumulation of Glycosylated Aroma Precursors in Grapes. J. Sci. Food Agric. 2018, 98, 3031–3040. [Google Scholar] [CrossRef]
- Haider, F.; Bagchi, G.D.; Singh, A.K. Effect of Calliterpenone on Growth, Herb Yield and Oil Quality of Mentha Arvensis. Int. J. Integr. Biol. 2009, 7, 53–57. [Google Scholar]
- Ali, E.F.; Hassan, F.A.S.; Elgimabi, M. Improving the Growth, Yield and Volatile Oil Content of Pelargonium graveolens L. Herit by Foliar Application with Moringa Leaf Extract through Motivating Physiological and Biochemical Parameters. S. Afr. J. Bot. 2018, 119, 383–389. [Google Scholar]
- De Diego, N.; Spíchal, L. Presence and future of plant phenotyping approaches in biostimulant research and development. J. Exp. Bot. 2022, 73, 5199–5212. [Google Scholar] [PubMed]
- Klarzynski, O.; Descamps, V.; Plesse, B.; Yvin, J.C.; Kloareg, B.; Fritig, B. Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Mol. Plant-Microbe Interact. 2003, 16, 115–122. [Google Scholar] [PubMed]
- Bi, F.; Iqbal, S.; Arman, M.; Ali, A.; Hassan, M. Carrageenan as an elicitor of induced secondary metabolites and its effects on various growth characters of chickpea and maize plants. J. Saudi Chem. Soc. 2011, 15, 269–273. [Google Scholar]
- Castro, J.; Vera, J.; González, A.; Moenne, A. Oligo-carrageenans stimulate growth by enhancing photosynthesis, basal metabolism, and cell cycle in tobacco plants (var. Burley). J. Plant Growth Regul. 2012, 31, 173–185. [Google Scholar]
- Hashmi, N.; Khan, M.M.A.; Idrees, M.; Khan, Z.H.; Ali, A.; Varshney, L. Depolymerized carrageenan ameliorates growth, physiological attributes, essential oil yield and active constituents of Foeniculum vulgare Mill. Carbohydr. Polym. 2012, 90, 407–412. [Google Scholar]
- Saucedo, S.; Contreras, R.A.; Moenne, A. Oligo-carrageenan kappa increases C, N and S assimilation, auxin and gibberellin contents, and growth in Pinus radiata trees. J. For. Res. 2015, 26, 635–640. [Google Scholar]
- Gonzalez, A.; Contreras, R.A.; Moenne, A. Oligo-carrageenans enhance growth and contents of cellulose, essential oils and polyphenolic compounds in Eucalyptus globulus trees. Molecules 2013, 18, 8740–8751. [Google Scholar] [CrossRef]
- Khan, Z.H.; Khan, M.M.A.; Aftab, T.; Idrees, M.; Naeem, M. Influence of alginate oligosaccharides on growth, yield and alkaloid production of opium poppy (Papaver somniferum L.). Front. Agric. China 2011, 5, 122–127. [Google Scholar]
- Hien, N.Q.; Nagasawa, N.; Tham, L.X.; Yoshii, F.; Dang, V.H.; Mitomo, H.; Kume, T. Growth-promotion of plants with depolymerized alginates by irradiation. Radiat. Phys. Chem. 2000, 59, 97–101. [Google Scholar]
- Liu, H.; Zhang, Y.H.; Yin, H.; Wang, W.X.; Zhao, X.M.; Du, Y.G. Alginate oligosaccharides enhanced Triticum aestivum L. tolerance to drought stress. Plant Physiol. Biochem. 2013, 62, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Carmody, N.; Goñi, O.; Łangowski, Ł.; O’Connell, S. Ascophyllum nodosum extract biostimulant processing and its impact on enhancing heat stress tolerance during tomato fruit set. Front. Plant Sci. 2020, 11, 807. [Google Scholar] [CrossRef]
- Morales-Sierra, S.; Luis, J.C.; Jiménez-Arias, D.; Rancel-Rodríguez, N.M.; Coego, A.; Rodriguez, P.L.; Cueto, M.; Borges, A.A. Biostimulant activity of Galaxaura rugosa seaweed extracts against water deficit stress in tomato seedlings involves activation of ABA signaling. Front. Plant Sci. 2023, 14, 1251442. [Google Scholar] [CrossRef] [PubMed]
- Oancea, F.; Velea, S.; Fãtu, V.; Mincea, C.; Ilie, L. Micro-algae based plant biostimulant and its effect on water stressed tomato plants. Rom. J. Plant Prot. 2013, 6, 104–117. [Google Scholar]
- Guedes, W.A.; Araújo, R.H.C.R.; Rocha, J.L.A.; Lima, J.F.D.; Dias, G.A.; Oliveira, Á.M.F.D.; Lima, R.F.; Oliveira, L.M. Production of papaya seedlings using Spirulina platensis as a biostimulant applied on leaf and root. J. Exp. Agric. Int. 2018, 28, 1–9. [Google Scholar] [CrossRef]
- Dias, G.A.; Rocha, R.H.C.; Araújo, J.L.; Lima, J.F.; Guedes, W.A. Growth, yield, and postharvest quality in eggplant produced under different foliar fertilizer (Spirulina platensis) treatments. Semin. Ciências Agrárias 2016, 37, 3893–3902. [Google Scholar] [CrossRef]
- Plaza, B.M.; Gómez-Serrano, C.; Acién-Fernández, F.G.; Jimenez-Becker, S. Effect of microalgae hydrolysate foliar application (Arthrospira platensis and Scenedesmus sp.) on Petunia x hybrida growth. J. Appl. Phycol. 2018, 30, 2359–2365. [Google Scholar] [CrossRef]
- Shalaby, T.A.; El-Ramady, H. Effect of foliar application of bio-stimulants on growth, yield, components, and storability of garlic (Allium sativum L.). Aust. J. Crop Sci. 2014, 8, 271–275. [Google Scholar]
- Seğmen, E.; Özdamar Ünlü, H. Effects of foliar applications of commercial seaweed and Spirulina platensis extracts on yield and fruit quality in pepper (Capsicum annuum L.). Cogent Food Agric. 2023, 9, 2233733. [Google Scholar] [CrossRef]
- Anli, M.; Baslam, M.; Tahiri, A.; Raklami, A.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Toubali, S.; Ait Rahou, Y.; et al. Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm. Front. Plant Sci. 2020, 11, 516818. [Google Scholar] [CrossRef]
- Ji, J.; Yuan, D.; Jin, C.; Wang, G.; Li, X.; Guan, C. Enhancement of growth and salt tolerance of rice seedlings (Oryza sativa L.) by regulating ethylene production with a novel halotolerant PGPR strain Glutamicibacter sp. YD01 containing ACC deaminase activity. Acta Physiol. Plant. 2020, 42, 42. [Google Scholar] [CrossRef]
- Patel, M.; Vurukonda, S.S.K.P.; Patel, A. Multi-trait halotolerant plant growth-promoting bacteria mitigate induced salt stress and enhance growth of Amaranthus viridis. J. Soil Sci. Plant Nutr. 2023, 23, 1860–1883. [Google Scholar] [CrossRef]
- Slimani, A.; Raklami, A.; Oufdou, K.; Meddich, A. Isolation and characterization of PGPR and their potential for drought alleviation in barley plants. Gesunde Pflanz. 2023, 75, 377–391. [Google Scholar] [CrossRef]
- Ali, B.; Hafeez, A.; Afridi, M.S.; Javed, M.A.; Sumaira; Suleman, F.; Nadeem, M.; Ali, S.; Alwahibi, M.S.; Elshikh, M.S.; et al. Bacterial-Mediated Salinity Stress Tolerance in Maize (Zea mays L.): A Fortunate Way toward Sustainable Agriculture. ACS Omega 2023, 23, 20471–20487. [Google Scholar] [CrossRef]
- Gashash, E.A.; Osman, N.A.; Alsahli, A.A.; Hewait, H.M.; Ashmawi, A.E.; Alshallash, K.S.; El-Taher, A.M.; Azab, E.S.; Abd El-Raouf, H.S.; Ibrahim, M.F. Effects of plant-growth-promoting rhizobacteria (PGPR) and cyanobacteria on botanical characteristics of tomato (Solanum lycopersicon L.) plants. Plants 2022, 11, 2732. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, R.; Rezaei, K.; Fayyaz, P.; Naghiha, R.; Namvar, Z. The effect of indigenous phosphate-solubilizing bacteria on Quercus brantii seedlings under water stress. J. Sustain. For. 2021, 40, 733–747. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, P.; Wang, T.; Chi, X.; Wang, M.; Chen, M.; Chen, N.; Pan, L. Role of halotolerant phosphate-solubilising bacteria on growth promotion of peanut (Arachis hypogaea) under saline soil. Ann. Appl. Biol. 2019, 174, 20–30. [Google Scholar] [CrossRef]
- Jahangir, G.Z.; Arshad, Q.U.A.; Shah, A.; Younas, A.; Naz, S.; Ali, Q. Bio-fertilizing efficiency of phosphate solubilizing bacteria in natural environment: A trial field study on stress tolerant potato (Solanum tuberosum L.). Appl. Ecol. Environ. Res. 2019, 17, 10845. [Google Scholar] [CrossRef]
- Shintu, P.V.; Jayaram, K.M. Phosphate solubilising bacteria (Bacillus polymyxa)-An effective approach to mitigate drought in tomato (Lycopersicon esculentum Mill.). Trop. Plant Res. 2015, 2, 2349–9265. [Google Scholar]
- Sagervanshi, A.; Priyanka, K.; Anju, N.; Ashwani, K. Isolation and characterization of phosphate solubilizing bacteria from and agriculture soil. Int. J. Life Sci. Pharma Res. 2012, 23, 256–266. [Google Scholar]
- Bello, A.S.; Saadaoui, I.; Ben-Hamadou, R. “Beyond the source of bioenergy”: Microalgae in modern agriculture as a biostimulant, biofertilizer, and anti-abiotic stress. Agronomy 2021, 11, 1610. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae for human and animal nutrition. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 461–503. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. Algaebase: Listing the World’s Algae. The Irish Scientist 2005, World-Wide Electronic Publication, National University of Ireland, Galway. pp. 74–75. Available online: https://www.algaebase.orgYearbook (accessed on 26 December 2024).
- Kumar, M.; Kumari, P.; Reddy, C.R.K.; Jha, B. Salinity and desiccation induced oxidative stress acclimation in seaweeds. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 91–123. [Google Scholar]
- Karsten, U. Seaweed acclimation to salinity and desiccation stress. In Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization; Springer: Berlin/Heidelberg, Germany, 2012; pp. 87–107. [Google Scholar]
- Davison, I.R.; Pearson, G.A. Stress tolerance in intertidal seaweeds. J. Phycol. 1996, 32, 197–211. [Google Scholar] [CrossRef]
- Kirst, G.O. Salinity tolerance of eukaryotic marine algae. Annu. Rev. Plant Biol. 1990, 41, 21–53. [Google Scholar] [CrossRef]
- Bischof, K.; Rautenberger, R. Seaweed responses to environmental stress: Reactive oxygen and antioxidative strategies. In Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization; Springer: Berlin/Heidelberg, Germany, 2012; pp. 109–132. [Google Scholar]
- Kumar, M.; Reddy, C.R.K. Oxidative Stress Tolerance Mechanisms in Marine Macroalgae (Seaweeds): Oxidative Stress in Seaweeds; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2012; 160p. [Google Scholar]
- Xing, G.; Li, J.; Li, W.; Lam, S.M.; Yuan, H.; Shui, G.; Yang, J. AP2/ERF and R2R3-MYB family transcription factors: Potential associations between temperature stress and lipid metabolism in Auxenochlorella protothecoides. Biotechnol. Biofuels 2021, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and utilization of seaweed pigments in food processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Belghit, I.; Rasinger, J.D.; Heesch, S.; Biancarosa, I.; Liland, N.; Torstensen, B.; Bruckner, C.G. In-depth metabolic profiling of marine macroalgae confirms strong biochemical differences between brown, red and green algae. Algal Res. 2017, 26, 240–249. [Google Scholar] [CrossRef]
- Sharma, M.; Dubey, A.; Pareek, A. Algal flora on degrading polythene waste. CIBTech J. Microbiol. 2014, 3, 43–47. [Google Scholar]
- Saito, K.; Matsuda, F. Metabolomics for functional genomics, systems biology, and biotechnology. Annu. Rev. Plant Biol. 2010, 61, 463–489. [Google Scholar] [CrossRef] [PubMed]
- Klejdus, B.; Lojková, L.; Plaza, M.; Šnóblová, M.; Štěrbová, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7956–7965. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Munro, M.H.; Blunt, J.W.; Puga, J.; Jesus, B.; Calado, R.; Madeira, C. Biogeography and biodiscovery hotspots of macroalgal marine natural products. Nat. Prod. Rep. 2013, 30, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, K.H.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Pinto, E. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef]
- Murtic, S.; Oljaca, R.; Murtic, M.S.; Vranac, A.; Koleska, I.; Karic, L. Effects of seaweed extract on the growth, yield and quality of cherry tomato under different growth conditions. Acta Agric. Slov. 2018, 111, 315–325. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 462648. [Google Scholar] [CrossRef] [PubMed]
- Markets & Markets. Biostimulants Market by Active Ingredient (Humic Substances, Amino Acids, Seaweed Extracts, Microbial Amendments), Crop Type (Fruits & Vegetables, Cereals, Turf & Ornamentals), Application Method, Form, and Region—Global Forecast to 2025; Markets & Markets: Pune, India, 2019. [Google Scholar]
- Mac Monagail, M.; Cornish, L.; Morrison, L.; Araújo, R.; Critchley, A.T. Sustainable harvesting of wild seaweed resources. Eur. J. Phycol. 2017, 52, 371–390. [Google Scholar] [CrossRef]
- Papenfus, H.B.; Stirk, W.A.; Finnie, J.F.; Van Staden, J. Seasonal variation in the polyamines of Ecklonia maxima. Bot. Mar. 2012, 55, 539–546. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, R.E.; Roy, P.D.; Torrescano-Valle, N.; Cabanillas-Terán, N.; Carrillo-Domínguez, S.; Collado-Vides, L.; van Tussenbroek, B.I. Element concentrations in pelagic Sargassum along the Mexican Caribbean coast in 2018–2019. PeerJ 2020, 8, e8667. [Google Scholar] [CrossRef]
- Nabti, E.; Jha, B.; Hartmann, A. Impact of seaweeds on agricultural crop production as biofertilizer. Int. J. Environ. Sci. Technol. 2017, 14, 1119–1134. [Google Scholar] [CrossRef]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Raja, R.; Ravikumar, R.; Carvalho, I.S. Microalgae taxonomy and breeding. In Biofuel Crops: Production, Physiology and Genetics; CABI: Wallingford, UK, 2013; pp. 44–53. [Google Scholar]
- Rachidi, F.; Benhima, R.; Sbabou, L.; El Arroussi, H. Microalgae polysaccharides bio-stimulating effect on tomato plants: Growth and metabolic distribution. Biotechnol. Rep. 2020, 25, e00426. [Google Scholar] [CrossRef] [PubMed]
- Chanda, M.J.; Merghoub, N.; El Arroussi, H. Microalgae polysaccharides: The new sustainable bioactive products for the development of plant bio-stimulants? World J. Microbiol. Biotechnol. 2019, 35, 177. [Google Scholar] [CrossRef]
- Farid, R.; Mutale-Joan, C.; Redouane, B.; Mernissi Najib, E.L.; Abderahime, A.; Laila, S.; Arroussi; Hicham, E.L. Effect of microalgae polysaccharides on biochemical and metabolomics pathways related to plant defense in Solanum lycopersicum. Appl. Biochem. Biotechnol. 2019, 188, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Baglieri, A.; Stevanato, P.; Broccanello, C.; Bertoldo, G.; Bertaggia, M.; Cagnin, M.; Pizzeghello, D.; Moliterni, V.M.C.; Mandolino, G.; et al. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.). J. Appl. Phycol. 2018, 30, 1061–1071. [Google Scholar] [CrossRef]
- El Arroussi, H.; Benhima, R.; Elbaouchi, A.; Sijilmassi, B.; El Mernissi, N.; Aafsar, A.; Meftah-Kadmiri, I.; Bendaou, N.; Smouni, A. Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J. Appl. Phycol. 2018, 30, 2929–2941. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, C.U.; Aoyagi, H.; Uchiyama, H. Photobioreactors for mass cultivation of algae. Bioresour. Technol. 2008, 99, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.P.; Meireles, L.A.; Malcata, F.X. Microalgal reactors: A review of enclosed system designs and performances. Biotechnol. Prog. 2006, 22, 1490–1506. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- García, J.R.; Fernández, F.A.; Sevilla, J.F. Development of a process for the production of L-amino-acids concentrates from microalgae by enzymatic hydrolysis. Bioresour. Technol. 2012, 112, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Gil-Chávez, J.; Villa, J.A.; Ayala-Zavala, F.; Heredia, B.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compreh. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Olguín, E.J.; Sánchez-Galván, G.; Arias-Olguín, I.I.; Melo, F.J.; González-Portela, R.E.; Cruz, L.; De Philippis, R.; Adessi, A. Microalgae-based biorefineries: Challenges and future trends to produce carbohydrate enriched biomass, high-added value products and bioactive compounds. Biology 2022, 11, 1146. [Google Scholar] [CrossRef] [PubMed]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Park, E.; Yu, H.; Lim, J.H.; Choi, J.H.; Park, K.J.; Lee, J. Seaweed metabolomics: A review on its nutrients, bioactive compounds, and changes in climate change. Food Res. Int. 2023, 163, 112221. [Google Scholar] [CrossRef]
- Elarroussia, H.; Elmernissia, N.; Benhimaa, R.; El Kadmiria, I.M.; Bendaou, N.; Smouni, A.; Wahbya, I. Microalgae polysaccharides: A promising plant growth biostimulant. J. Algal Biomass Utln. 2016, 7, 55–63. [Google Scholar]
- Priyadarshani, I.; Rath, B. Commercial and industrial applications of microalgae–A review. J. Algal Biomass Util. 2012, 3, 89–100. [Google Scholar]
- Belkhadir, Y.; Yang, L.; Hetzel, J.; Dangl, J.L.; Chory, J. The growth–defense pivot: Crisis management in plants mediated by LRR-RK surface receptors. Trends Biochem. Sci. 2014, 39, 447–456. [Google Scholar] [CrossRef]
- González, A.; Castro, J.; Vera, J.; Moenne, A. Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation, basal metabolism, and cell division. J. Plant Growth Regul. 2013, 32, 443–448. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, Z.Y.; Mora-Garcia, S.; Li, J.; Yoshida, S.; Asami, T.; Chory, J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 2002, 109, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, K.; Zielony, T.; Gajewski, M. Effect of Aminoplant and Asahi on yield and quality of lettuce grown on rockwool. In Proceedings of the Conference of Biostimulators in Modern Agricultura, Warsaw, Poland, 7–8 February 2008; pp. 7–8. [Google Scholar]
- Coppens, J.; Grunert, O.; Van Den Hende, S.; Vanhoutte, I.; Boon, N.; Haesaert, G.; De Gelder, L. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 2016, 28, 2367–2377. [Google Scholar] [CrossRef]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable sources of plant biostimulation: Microalgae as a sustainable means to improve crop performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef]
- Lemoine, Y.; Schoefs, B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: A multifunctional response to stress. Photosynth. Res. 2010, 106, 155–177. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, E.; Roussis, V. Natural products from seaweeds. In Plant-Derived Natural Products: Synthesis, Function, and Application; Springer: New York, NY, USA, 2009; pp. 51–81. [Google Scholar]
- Colla, G.; Rouphael, Y.; Lucini, L.; Canaguier, R.; Stefanoni, W.; Fiorillo, A.; Cardarelli, M. Protein hydrolysate-based biostimulants: Origin, biological activity, and application methods. Acta Hortic. 2016, 1148, 27–34. [Google Scholar] [CrossRef]
- Colla, G.; Svecová, E.; Cardarelli, M.; Rouphael, Y.; Reynaud, H.; Canaguier, R.; Planques, B. Effectiveness of a plant-derived protein hydrolysate to improve crop performances under different growing conditions. Acta Hortic. 2013, 1009, 175–179. [Google Scholar] [CrossRef]
- Stirk, W.A.; Bálint, P.; Tarkowská, D.; Novák, O.; Strnad, M.; Ördög, V.; Van Staden, J. Hormone profiles in microalgae: Gibberellins and brassinosteroids. Plant Physiol. Biochem. 2013, 70, 348–353. [Google Scholar] [CrossRef]
- Stirk, W.A.; Ördög, V.; Novák, O.; Rolčík, J.; Strnad, M.; Bálint, P.; van Staden, J. Auxin and cytokinin relationships in 24 microalgal strains. J. Phycol. 2013, 49, 459–467. [Google Scholar] [CrossRef]
- Tate, J.J.; Gutierrez-Wing, M.T.; Rusch, K.A.; Benton, M.G. The effects of plant growth substances and mixed cultures on growth and metabolite production of green algae Chlorella sp.: A review. J. Plant Growth Regul. 2013, 32, 417–428. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Balasubramanian, R.; Iyer, C.S.P. Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu (II) from aqueous solutions. Bioresour. Technol. 2007, 98, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Mutale-joan, C.; Rachidi, F.; Mohamed, H.A.; Mernissi, N.E.; Aasfar, A.; Barakate, M.; Mohammed, D.; Sbabou, L.; Arroussi, H.E. Microalgae-cyanobacteria–based biostimulant effect on salinity tolerance mechanisms, nutrient uptake, and tomato plant growth under salt stress. J. Appl. Phycol. 2021, 33, 3779–3795. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, K. Microbes as a source of new plant biostimulants: From the rhizosphere to the endosphere. Plant Soil 2015, 387, 11–34. [Google Scholar]
- Azcón-Aguilar, C.; Navarro-Racinés, P.; Gianinazzi, S. The role of microorganisms as elicitors of plant defense responses: From genes to field application. Plant Soil 2017, 412, 5–26. [Google Scholar]
- Bishnoi, U. PGPR interaction: An ecofriendly approach promoting the sustainable agriculture system. Adv. Bot. Res. 2015, 75, 81–113. [Google Scholar]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Alberton, D.; Valdameri, G.; Moure, V.R.; Monteiro, R.A.; Pedrosa, F.D.O.; Müller-Santos, M.; de Souza, E.M. What did we learn from plant growth-promoting rhizobacteria (PGPR)-grass associations studies through proteomic and metabolomic approaches? Front. Sustain. Food Syst. 2020, 4, 607343. [Google Scholar] [CrossRef]
- Gray, E.J.; Smith, D.L. Intracellular and extracellular PGPR: Commonalities and distinctions in the plant–bacterium signaling processes. Soil Biol. Biochem. 2005, 37, 395–412. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Petropoulos, S.A.; Sun, W. Survey of the influences of microbial biostimulants on horticultural crops: Case studies and successful paradigms. Horticulturae 2023, 9, 193. [Google Scholar] [CrossRef]
- Camelo, M.; Vera, S.P.; Bonilla, R.R. Mechanisms of action of plant growth-promoting rhizobacteria. Cienc. Tecnol. Agropecuaria 2011, 12, 159–166. [Google Scholar] [CrossRef]
- Dash, N.P.; Kumar, A.; Kaushik, M.S.; Abraham, G.; Singh, P.K. Agrochemicals influencing nitrogenase, biomass of N2-fixing cyanobacteria and yield of rice in wetland cultivation. Biocatal. Agric. Biotechnol. 2017, 9, 28–34. [Google Scholar] [CrossRef]
- Raymond, J.; Siefert, J.L.; Staples, C.R.; Blankenship, R.E. The natural history of nitrogen fixation. Mol. Biol. Evol. 2004, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Wunnava, A.; Verma, P.; Chandra, A.; Sharma, R.K. Strategies to mitigate the adverse effect of drought stress on crop plants—Influences of soil bacteria: A review. Pedosphere 2021, 31, 496–509. [Google Scholar] [CrossRef]
- Ilyas, N.; Mazhar, R.; Yasmin, H.; Khan, W.; Iqbal, S.; Enshasy, H.E.; Dailin, D.J. Rhizobacteria isolated from saline soil induce systemic tolerance in wheat (Triticum aestivum L.) against salinity stress. Agronomy 2020, 10, 989. [Google Scholar] [CrossRef]
- Ashry, N.M.; Alaidaroos, B.A.; Mohamed, S.A.; Badr, O.A.; El-Saadony, M.T.; Esmael, A. Utilization of drought-tolerant bacterial strains isolated from harsh soils as plant growth-promoting rhizobacteria (PGPR). Saudi J. Biol. Sci. 2022, 29, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial plant biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Ayuso-Calles, M.; García-Estévez, I.; Jiménez-Gómez, A.; Flores-Félix, J.D.; Escribano-Bailón, M.T.; Rivas, R. Rhizobium laguerreae improves productivity and phenolic compound content of lettuce (Lactuca sativa L.) under saline stress conditions. Foods 2020, 9, 1166. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Alqarawi, A.A.; Radhakrishnan, R.; Al-Arjani, A.B.F.; Aldehaish, H.A.; Egamberdieva, D.; Abd Allah, E.F. Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J. Biol. Sci. 2018, 25, 1102–1114. [Google Scholar] [CrossRef]
- Janati, W.; Bouabid, R.; Mikou, K.; Ghadraoui, L.E.; Errachidi, F. Phosphate solubilizing bacteria from soils with varying environmental conditions: Occurrence and function. PLoS ONE 2023, 18, e0289127. [Google Scholar] [CrossRef]
- Mosimann, C.; Oberhänsli, T.; Ziegler, D.; Nassal, D.; Kandeler, E.; Boller, T.; Mäder, P.; Thonar, C. Tracing of two Pseudomonas strains in the root and rhizoplane of maize, as related to their plant growth-promoting effect in contrasting soils. Front. Microbiol. 2017, 7, 2150. [Google Scholar] [CrossRef]
- Rizvi, A.; Ahmed, B.; Khan, M.S.; Umar, S.; Lee, J. Psychrophilic bacterial phosphate-biofertilizers: A novel extremophile for sustainable crop production under cold environment. Microorganisms 2021, 9, 2451. [Google Scholar] [CrossRef]
- Liang, J.L.; Liu, J.; Jia, P.; Yang, T.T.; Zeng, Q.W.; Zhang, S.C.; Liao, B.; Shu, W.S.; Li, J.T. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef] [PubMed]
- Tagele, S.B.; Kim, S.W.; Lee, H.G.; Kim, H.S.; Lee, Y.S. Effectiveness of multi-trait Burkholderia contaminans KNU17BI1 in growth promotion and management of banded leaf and sheath blight in maize seedling. Microbiol. Res. 2018, 214, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Dhuldhaj, U.P.; Malik, N. Global perspective of phosphate solubilizing microbes and phosphatase for improvement of soil, food and human health. Cell. Mol. Biomed. Rep. 2022, 2, 173–186. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- De Zutter, N.; Ameye, M.; Bekaert, B.; Verwaeren, J.; De Gelder, L.; Audenaert, K. Uncovering new insights and misconceptions on the effectiveness of phosphate solubilizing rhizobacteria in plants: A meta-analysis. Front. Plant Sci. 2022, 13, 858804. [Google Scholar] [CrossRef] [PubMed]
- Raymond, N.S.; Gómez-Muñoz, B.; van der Bom, F.J.; Nybroe, O.; Jensen, L.S.; Müller-Stöver, D.S.; Oberson, A.; Richardson, A.E. Phosphate-solubilising microorganisms for improved crop productivity: A critical assessment. New Phytol. 2021, 229, 1268–1277. [Google Scholar] [CrossRef]
- Adnan, M.; Fahad, S.; Zamin, M.; Shah, S.; Mian, I.A.; Danish, S.; Zafar-ul-Hye, M.; Battaglia, M.L.; Naz, R.M.M.; Saeed, B.; et al. Coupling phosphate-solubilizing bacteria with phosphorus supplements improve maize phosphorus acquisition and growth under lime induced salinity stress. Plants 2020, 9, 900. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for using phosphate-solubilizing microorganisms as natural fertilizers in agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef]
- Rafi, M.M.; Krishnaveni, M.S.; Charyulu, P.B.B.N. Phosphate-solubilizing microorganisms and their emerging role in sustainable agriculture. In Recent Developments in Applied Microbiology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–233. [Google Scholar]
- Wu, W.; Li, X.; Liang, X.; Wang, D. Microorganisms as plant biostimulants: A review of their mechanisms and applications. J. Plant Physiol. 2023, 226, 384–403. [Google Scholar]
- Wang, Z.; Liang, X.; Wang, H.; Liu, Y. Microorganism-based biostimulants: A new strategy for enhancing crop productivity and abiotic stress resilience. Adv. Agron. 2023, 158, 1–31. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Sario, L.; Boeri, P.; Matus, J.T.; Pizzio, G.A. Plant Biostimulants to Enhance Abiotic Stress Resilience in Crops. Int. J. Mol. Sci. 2025, 26, 1129. https://doi.org/10.3390/ijms26031129
Di Sario L, Boeri P, Matus JT, Pizzio GA. Plant Biostimulants to Enhance Abiotic Stress Resilience in Crops. International Journal of Molecular Sciences. 2025; 26(3):1129. https://doi.org/10.3390/ijms26031129
Chicago/Turabian StyleDi Sario, Luciana, Patricia Boeri, José Tomás Matus, and Gastón A. Pizzio. 2025. "Plant Biostimulants to Enhance Abiotic Stress Resilience in Crops" International Journal of Molecular Sciences 26, no. 3: 1129. https://doi.org/10.3390/ijms26031129
APA StyleDi Sario, L., Boeri, P., Matus, J. T., & Pizzio, G. A. (2025). Plant Biostimulants to Enhance Abiotic Stress Resilience in Crops. International Journal of Molecular Sciences, 26(3), 1129. https://doi.org/10.3390/ijms26031129








