Analysis of Protein Inhibitors of Trypsin in Quinoa, Amaranth and Lupine Seeds. Selection and Deep Structure–Function Characterization of the Amaranthus caudatus Species
Abstract
1. Introduction
2. Results
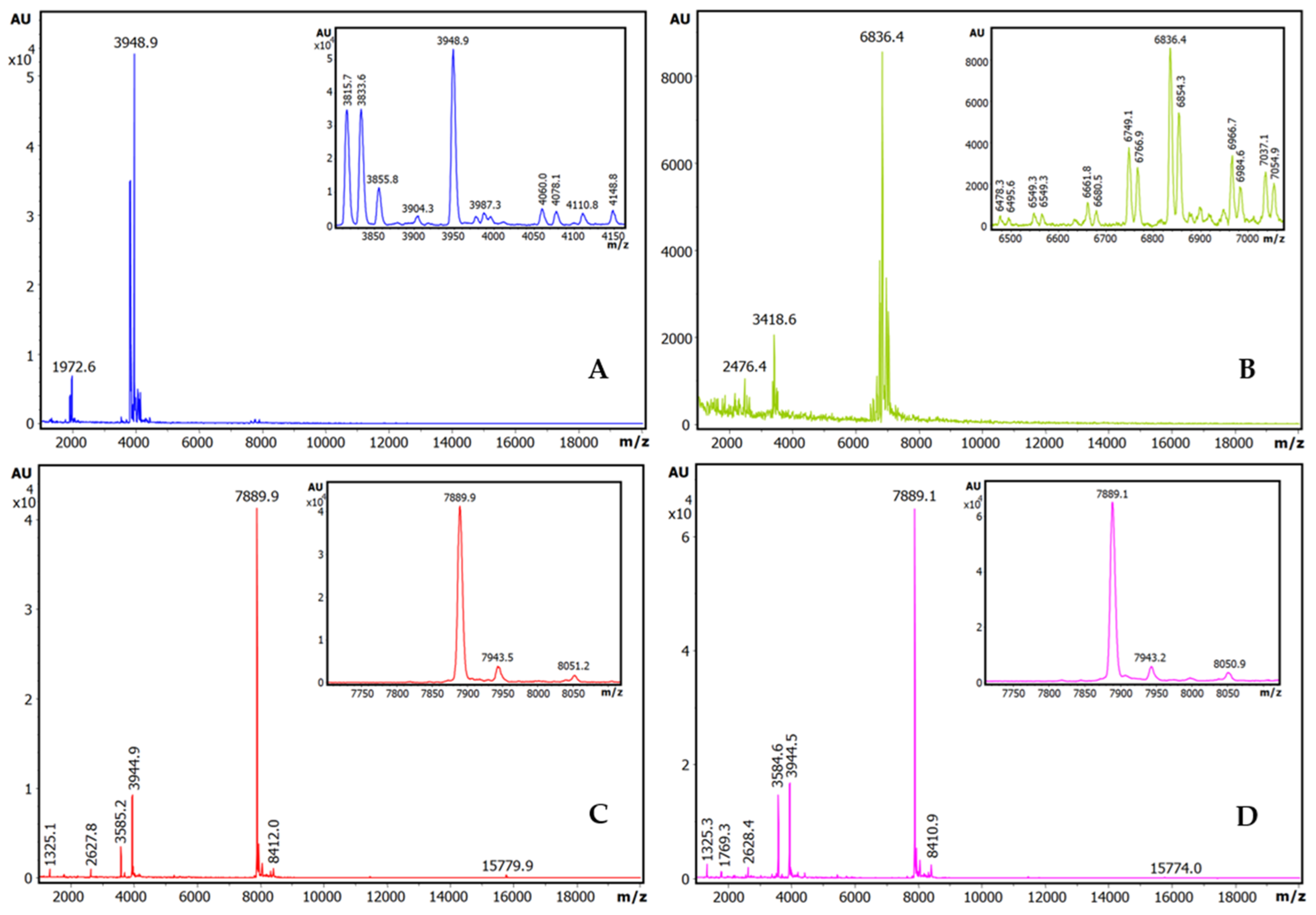
2.1. Trypsin Inhibition Dose–Response Curves, Inhibitory Capabilities, and Identification of Inhibitory Species
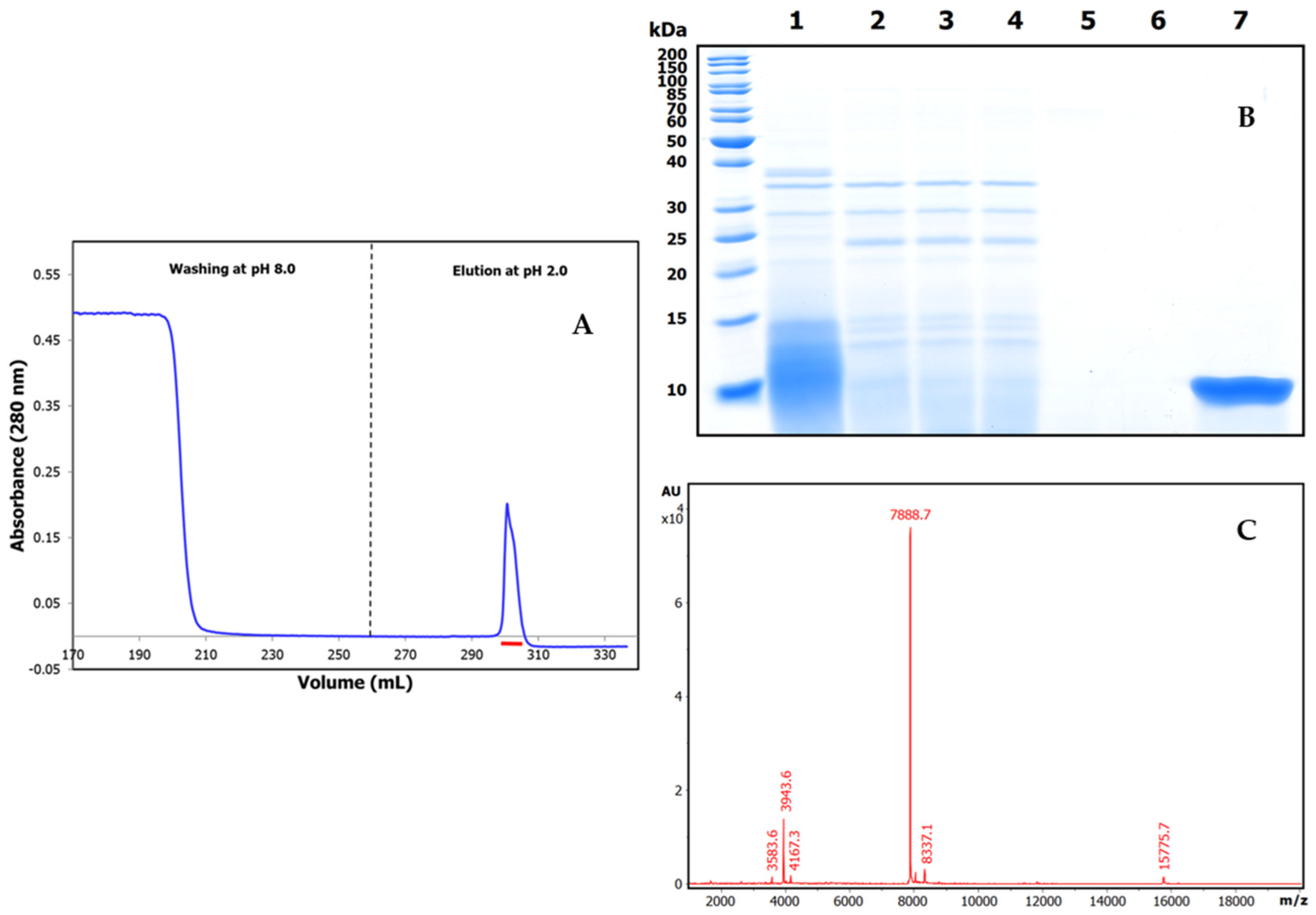
2.2. Purification and Mass Characterization of Natural ATSI
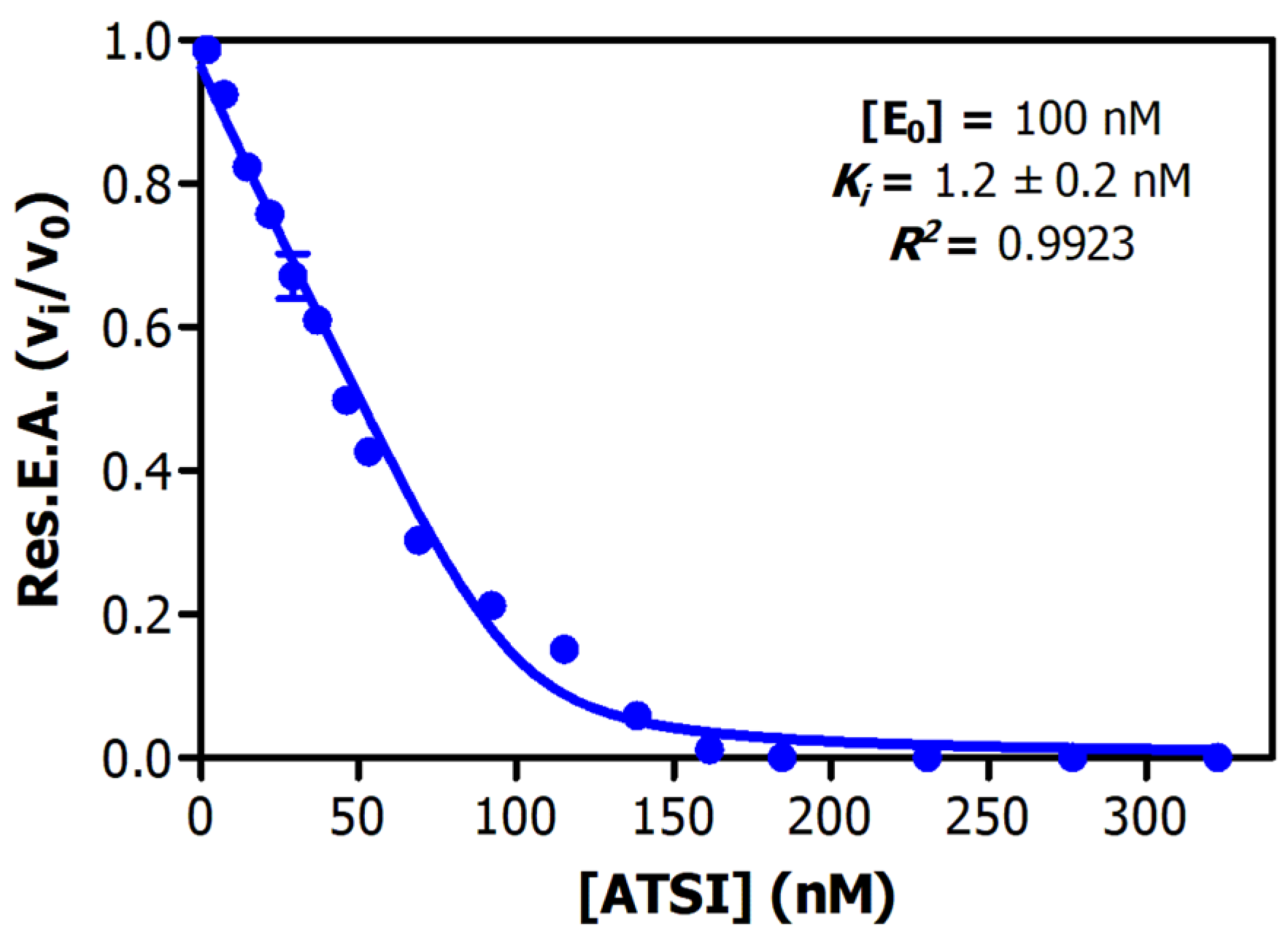
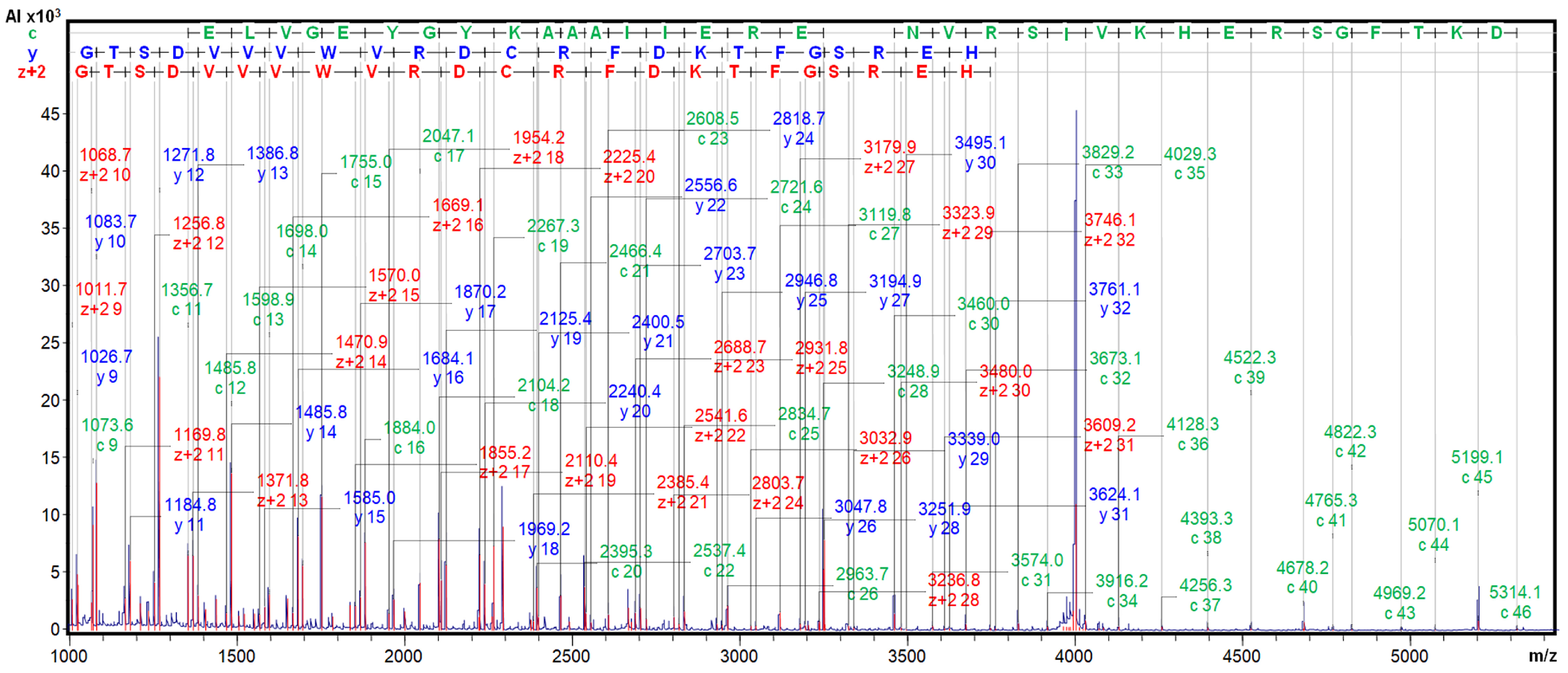
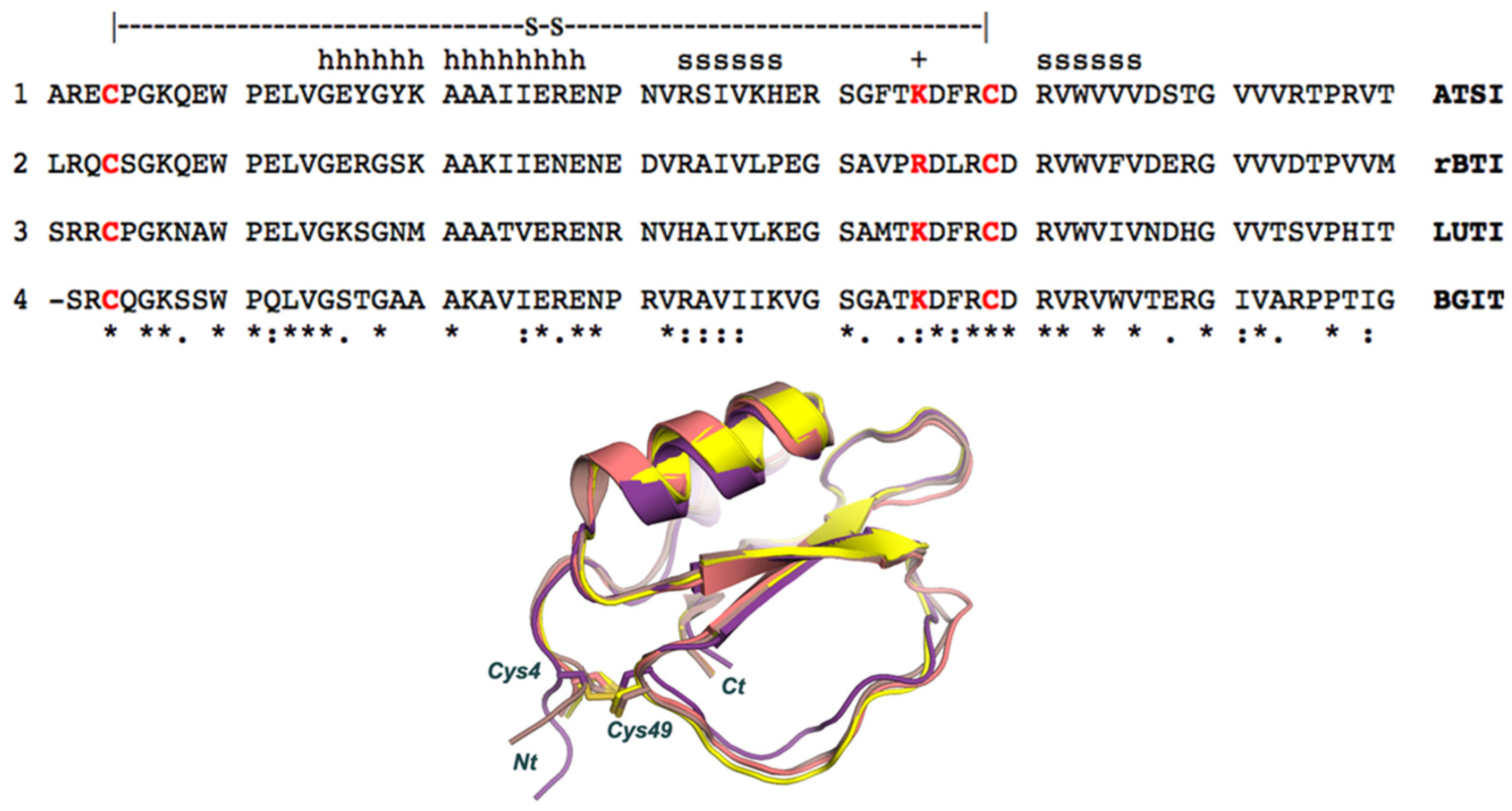
2.3. Determination of Inhibitory Activities, Ki Value, Number of Cysteines, and Top-Down MS Sequencing Analysis
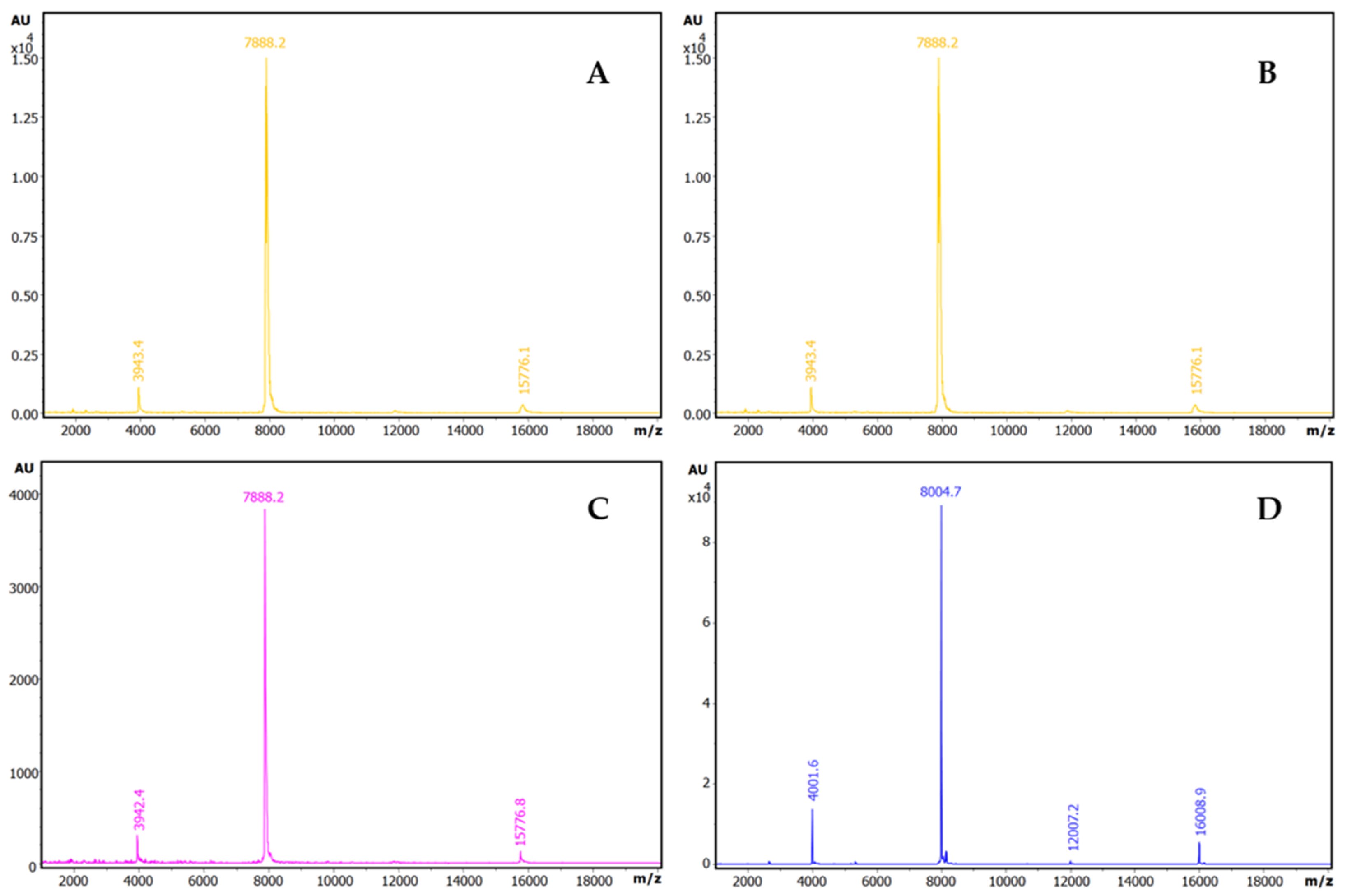
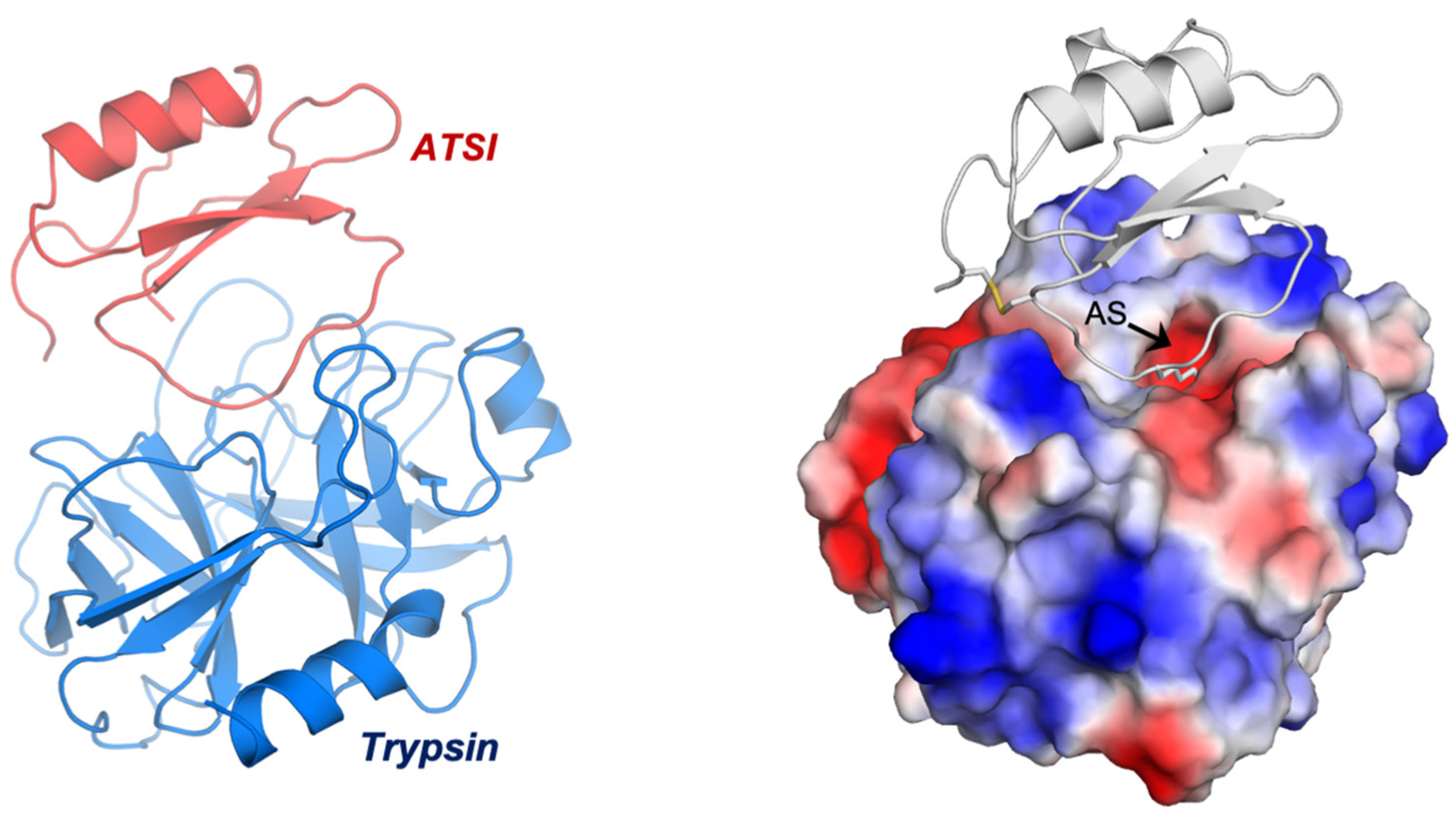
2.4. Formation and Isolation of the ATSI-Bovine Trypsin Complex
2.5. Three-Dimensional Crystal Structure Analysis of the ATSI-bTrypsin Complex
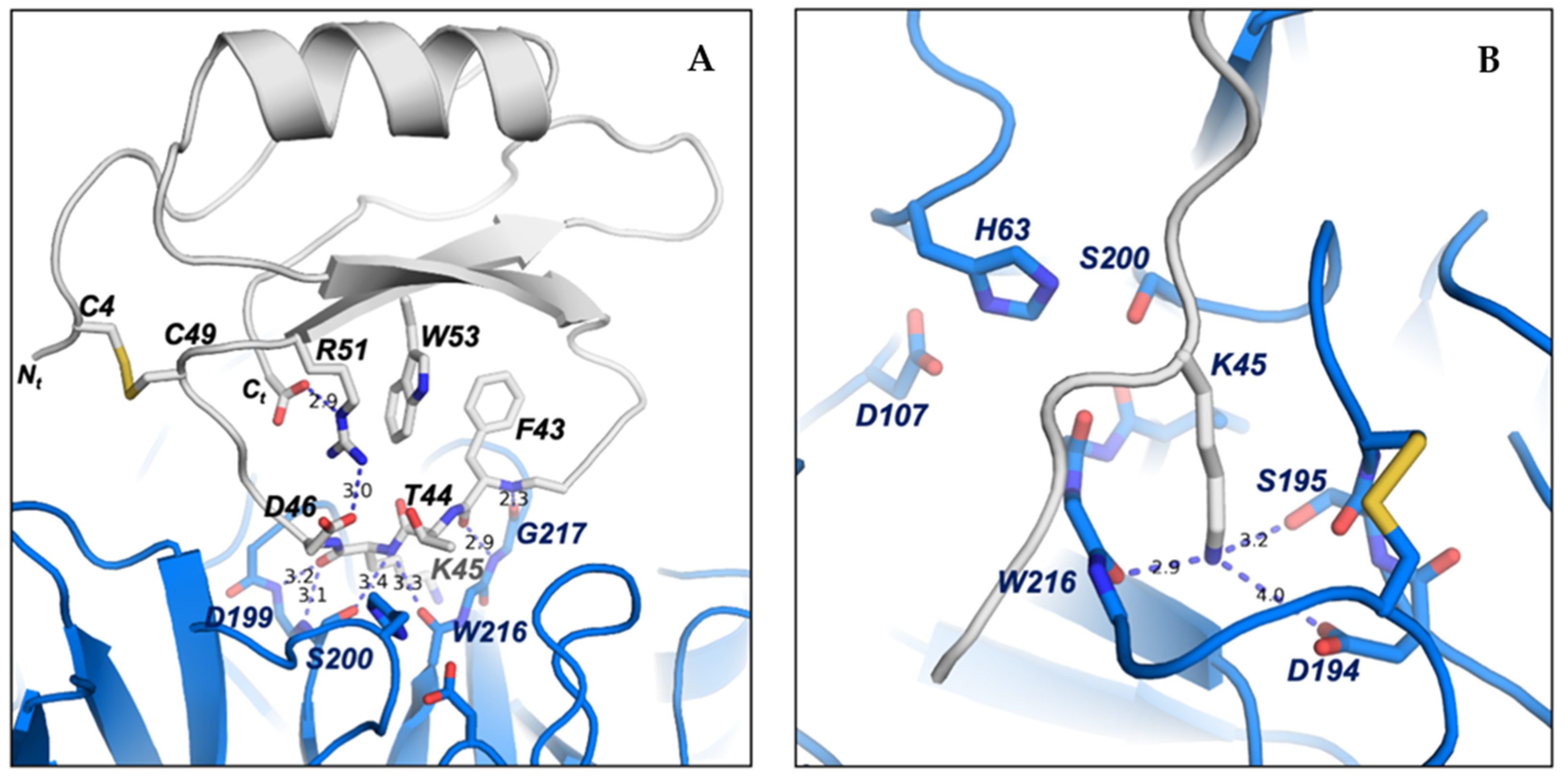
2.6. Structure–Function Details of the ATSI-Trypsin Complex
2.7. Assaying the Capabilities of ATSI to Act as an Antimicrobial in Cell Cultures
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Extraction from Seeds
4.2.2. Enzymatic Characterization of Proteolytic and Inhibitory Activities
4.2.3. Molecular Characterization of Protease Inhibitors
Determination of the Number of Free and Total Cysteine Residues
MALDI Top-Down Sequencing Using ISD for Natural ATS
4.2.4. Preparation of ATSI-Trypsin Complex, Crystallization, and Structure Determination
4.2.5. Cell Culture and in Cellulo Inhibitory Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, D.C.; Bhattacharjee, M. Revisiting the significance of natural protease inhibitors: A comprehensive review. Int. J. Biol. Macromol. 2024, 280 Pt 3, 135899. [Google Scholar] [CrossRef]
- Hellinger, R.; Gruber, C.W. Peptide-based protease inhibitors from plants. Drug Discov. Today 2019, 24, 1877–1889. [Google Scholar] [CrossRef]
- Cotabarren, J.; Lufrano, D.; Parisi, M.G.; Obregón, W.D. Biotechnological, biomedical, and agronomical applications of plant protease inhibitors with high stability: A systematic review. Plant Sci. 2020, 292, 110398. [Google Scholar] [CrossRef] [PubMed]
- Cid-Gallegos, M.S.; Corzo-Ríos, L.J.; Jiménez-Martínez, C.; Sánchez-Chino, X.M. Protease Inhibitors from Plants as Therapeutic Agents—A Review. Plant Foods Hum. Nutr. 2022, 77, 20–29. [Google Scholar] [CrossRef]
- Parisi, M.G.; Ozón, B.; Vera González, S.M.; García-Pardo, J.; Obregón, W.D. Plant Protease Inhibitors as Emerging Antimicrobial Peptide Agents: A Comprehensive Review. Pharmaceutics 2024, 16, 582. [Google Scholar] [CrossRef] [PubMed]
- Geisslitz, S.; Weegels, P.; Shewry, P.; Zevallos, V.; Masci, S.; Sorrells, M.; Gregorini, A.; Colomba, M.; Jonkers, D.; Huang, X.; et al. Wheat amylase/trypsin inhibitors (ATIs): Occurrence, function and health aspects. Eur. J. Nutr. 2022, 61, 2873–2880. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Bateman, A. How to use the MEROPS database and website to help understand peptidase specificity. Protein Sci. 2020, 30, 83–92. [Google Scholar] [CrossRef]
- Joshi, D.C.; Sood, S.; Hosahatti, R.; Kant, L.; Pattanayak, A.; Kumar, A.; Yadav, D.; Stetter, M.G. From zero to hero: The past, present and future of grain amaranth breeding. Theor. Appl. Genet. 2018, 131, 1807–1823. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Guleria, S.; Kimta, N.; Dhalaria, R.; Nepovimova, E.; Dhanjal, D.S.; Alomar, S.Y.; Kuca, K. Amaranth and buckwheat grains: Nutritional profile, development of functional foods, their pre-clinical cum clinical aspects and enrichment in feed. Curr. Res. Food Sci. 2024, 9, 100836. [Google Scholar] [CrossRef]
- Covaleda, G.; Trejo, S.A.; Salas-Sarduy, E.; del Rivero, M.A.; Chavez, M.A.; Aviles, F.X. Intensity fading MALDI-TOF mass spectrometry and functional proteomics assignments to identify protease inhibitors in marine invertebrates. J. Proteom. 2017, 165, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Hejgaard, J.; Dam, J.; Petersen, L.C.; Bjørn, S.E. Primary structure and specificity of the major serine proteinase inhibitor of amaranth (Amaranthus caudatus L.) seeds. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzym. 1994, 1204, 68–74. [Google Scholar] [CrossRef]
- Villanueva, J.; Yanes, O.; Querol, E.; Serrano, L.; Aviles, F.X. Identification of protein ligands in complex biological samples using intensity-fading MALDI-TOF mass spectrometry. Anal. Chem. 2003, 75, 3385–3395. [Google Scholar] [CrossRef]
- Yanes, O.; Villanueva, J.; Querol, E.; Aviles, F.X. Detection of non-covalent protein interactions by ’intensity fading’ MAL-DI-TOF mass spectrometry: Applications to proteases and protease inhibitors. Nat. Protoc. 2007, 2, 119–130. [Google Scholar] [CrossRef]
- Morrison, J.F. The slow-binding and slow, tight-binding inhibition of enzyme-catalysed reactions. Trends Biochem. Sci. 1982, 7, 102–105. [Google Scholar] [CrossRef]
- Copeland, R.A.; Lombardo, D.; Giannaras, J.; Decicco, C.P. Estimating KI values for tight binding inhibitors from dose-response plots. Bioorganic Med. Chem. Lett. 1995, 5, 1947–1952. [Google Scholar] [CrossRef]
- Smith, R.L.; Shaw, E. Pseudotrypsin. A modified bovine trypsin produced by limited autodigestion. J. Biol. Chem. 1969, 244, 4704–4712. [Google Scholar] [CrossRef] [PubMed]
- Perutka, Z.; Šebela, M. Pseudotrypsin: A Little-Known Trypsin Proteoform. Molecules 2018, 23, 2637. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, F.; Li, M.; Zhang, H.; Gao, Y.; Cao, P.; Pan, X.; Wang, Z.; Chang, W. Conformational changes of rBTI from buckwheat upon binding to trypsin: Implications for the role of the P8′ residue in the potato inhibitor I family. PLoS ONE 2011, 6, e20950. [Google Scholar] [CrossRef] [PubMed]
- Becker-Ritt, A.B.; Carlini, C.R. Fungitoxic and insecticidal plant polypeptides. Biopolymers 2012, 98, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Maiyo, Z.C.; Ngure, R.M.; Matasyoh, J.C.; Chepkorir, R. Phytochemical constituents and antimicrobial activity of leaf extracts of three Amaranthus plant species. Afr. J. Biotechnol. 2010, 9, 3178–3182. [Google Scholar]
- Montesinos, L.; Gascón, B.; Ruz, L.; Badosa, E.; Planas, M.; Feliu, L.; Montesinos, E. A bifunctional synthetic peptide with antimicrobial and plant defence elicitation properties that protects tomato plants from bacterial and fungal infections. Front. Plant Sci. 2021, 12, 756357. [Google Scholar] [CrossRef]
- Badosa, E.; Moiset, G.; Montesinos, L.; Talleda, M.; Bardají, E.; Feliu, L.; Planas, M.; Montesinos, E. Derivatives of the Antimicrobial Peptide BP100 for Expression in Plant Systems. PLoS ONE 2013, 8, e85515. [Google Scholar] [CrossRef] [PubMed]
- Puig, M.; Moragrega, C.; Ruz, L.; Calderón, C.E.; Cazorla, F.M.; Montesinos, E.; Llorente, I. Interaction of antifungal peptide BP15 with Stemphylium vesicarium, the causal agent of brown spot of pear. Fungal Biol. 2016, 120, 61–71. [Google Scholar] [CrossRef]
- Sippel, K.H.; Venkatakrishnan, B.; Boehlein, S.K.; Sankaran, B.; Quirit, J.G.; Govindasamy, L.; Agbandje-McKenna, M.; Goodison, S.; Rosser, C.J.; McKenna, R. Insights into Mycoplasma genitalium metabolism revealed by the structure of MG289, an extracytoplasmic thiamine binding lipoprotein. Proteins 2011, 79, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Sprankel, L.; Vizarraga, D.; Martín, J.; Manger, S.; Meier-Credo, J.; Marcos, M.; Julve, J.; Rotllan, N.; Scheffer, M.P.; Escolà-Gil, J.C.; et al. Essential protein P116 extracts cholesterol and other indispensable lipids for Mycoplasmas. Nat. Struct. Mol. Biol. 2023, 30, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Quilis, J.; Meynard, D.; Vila, L.; Avilés, F.X.; Guiderdoni, E.; San Segundo, B. A potato carboxypeptidase inhibitor gene provides pathogen resistance in transgenic rice. Plant Biotechnol. J. 2007, 5, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Rodríguez, A.; Gómez-Favela, M.A.; Reyes-Moreno, C.; Milán-Carrillo, J.; González de Mejía, E. Identification of Bioactive Peptide Sequences from Amaranth (Amaranthus hypochondriacus) Seed Proteins and Their Potential Role in the Prevention of Chronic Diseases. Compr. Rev. Food Sci. Food Saf. 2015, 14, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Chen, Z. Plant Protease Inhibitors in Therapeutics-Focus on Cancer Therapy. Front. Pharmacol. 2016, 7, 470. [Google Scholar] [CrossRef] [PubMed]
- de Souza Nascimento, A.M.; de Oliveira Segundo, V.H.; Felipe Camelo Aguiar, A.J.; Piuvezam, G.; Souza Passos, T.; Florentino da Silva Chaves Damasceno, K.S.; de Araújo Morais, A.H. Antibacterial action mechanisms and mode of trypsin inhibitors: A systematic review. J. Enzym. Inhib. Med. Chem. 2022, 37, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal grains: Nutritional value, health benefits and current applications for the development of gluten-free foods. Food Chem. Toxicol. 2020, 137, 111178. [Google Scholar] [CrossRef]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Rüssel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells. Gastroenterology 2017, 152, 1100–1113.e12. [Google Scholar] [CrossRef] [PubMed]
- Scarafoni, A.; Consonni, A.; Galbusera, V.; Negri, A.; Tedeschi, G.; Rasmussen, P.; Magni, C.; Duranti, M. Identification and characterization of a Bowman–Birk inhibitor active towards trypsin but not chymotrypsin in Lupinus albus seeds. Phytochemistry 2008, 69, 1820–1825. [Google Scholar] [CrossRef]
- Pesoti, A.R.; de Oliveira, B.M.; de Oliveira, A.C.; Pompeu, D.G.; Gonçalves, D.B.; Marangoni, S.; da Silva, J.A.; Granjeiro, P.A. Extraction, purification and characterization of inhibitor of trypsin from Chenopodium quinoa seeds. Food Sci. Technol. 2015, 35, 588–597. [Google Scholar] [CrossRef]
- Das, S. Amaranthus: A Promising Crop of Future; Springer: Singapore, 2016; ISBN 978-9-81-101468-0. [Google Scholar] [CrossRef]
- Villacrés, E.; Quelal, M.B.; Jácome, X.; Cueva, G.; Rosell, C.M. Effect of debittering and solid-state fermentation processes on the nutritional content of lupine, Lupinus mutabilis, sweet. Int. J. Food Sci. Technol. (LWT) 2020, 55, 2589–2598. [Google Scholar] [CrossRef]
- Carvajal-Larenas, F.E.; Linnemann, A.R.; Nout, M.J.; Koziol, M.; van Boekel, M.A. Lupinus mutabilis: Composition, Uses, Toxicology, and Debittering. Crit. Rev. Food Sci. Nutr. 2015, 56, 1454–1487. [Google Scholar] [CrossRef] [PubMed]
- Luis, G.M.; Hernández Hernández, B.R.; Peña Caballero, V.; Torres López, G.; Martínez Espinoza, V.A.; Ramírez Pacheco, L. Current and potential uses of Amaranth (Amaranthus Spp.). J. Negat. No Posit. Results 2018, 3, 423–436. [Google Scholar] [CrossRef]
- Baraniak, J.; Kania-Dobrowolska, M. The Dual Nature of Amaranth—Functional Food and Potential Medicine. Foods 2022, 11, 618. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Rodriguez, S.; Segura-Nieto, M.; Chagolla-Lopez, A.; Verver y Vargas-Cortina, A.; Martinez-Gallardo, N.; Blanco-Labra, A. Purification, characterization, and complete amino acid sequence of a trypsin inhibitor from amaranth (Amaranthus hypochondriacus) seeds. Plant Physiol. 1993, 103, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Asgher, M.; Sher, F.; Hussain, S.M.; Nazish, N.; Joshi, N.; Sharma, A.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M.N. Exploring Marine as a Rich Source of Bioactive Peptides: Challenges and Opportunities from Marine Pharmacology. Mar. Drugs 2022, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Rodríguez, S.; Blanco-Labra, A.; Gutiérrez-Benicio, G.; Boradenenko, A.; Herrera-Estrella, A.; Simpson, J. Cloning and characterization of a trypsin inhibitor cDNA from amaranth (Amaranthus hypochondriacus) seeds. Plant Mol. Biol. 1999, 41, 15–23. [Google Scholar] [CrossRef]
- Adhikary, D.; Khatri-Chhetri, U.; Slaski, J. Amaranth: An Ancient and High-Quality Wholesome Crop. In Nutritional Value of Amaranth; Waisundara, V.Y., Ed.; IntechOpen Ltd.: London, UK, 2020. [Google Scholar] [CrossRef]
- Otlewski, J.; Jelen, F.; Zakrzewska, M.; Oleksy, A. The many faces of protease–protein inhibitor interaction. EMBO J. 2005, 24, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamun, M.A.; Husna, J.; Khatun, M.; Hasan, R.; Kamruzzaman, M.; Hoque, K.M.F.; Abu Reza, M.; Ferdousi, Z. Assessment of antioxidant, anticancer and antimicrobial activity of two vegetable species of Amaranthus in Bangladesh. BMC Complement. Altern. Med. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; Marien, W.; Terras, F.R.G.; De Bolle, M.F.C.; Proost, P.; Van Damme, J.; Dillen, L.; Claeys, M.; Rees, S.B. Antimicrobial peptides from Amaranthus caudatus seeds with sequence homology to the cysteine/glycine-rich domain of chitin-binding proteins. Biochemistry 1992, 31, 4308–4314. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Toxicity and Antimicrobial Activities of Amaranthus caudatus L. (Amaranthaceae) Harvested From Formulated Soils at Different Growth Stages. J. Evidence-Based Integr. Med. 2020, 25, 2515690X20971578. [Google Scholar] [CrossRef] [PubMed]
- Long, J.Y.; Chen, Y.H.; Xia, J.R. First Report of a Group 16SrI Phytoplasma Associated with Amaranthus hypochondriacus Cladodes in China. Plant Dis. 2011, 95, 871. [Google Scholar] [CrossRef] [PubMed]
- Dejkriengkraikhul, P.; Wilairat, P. Requirement of malarial protease in the invasion of human red cells by merozoites of Plasmodium falciparum. Z. Parasitenkd. 1983, 69, 313–317. [Google Scholar] [CrossRef]
- Bastianelli, G.; Bouillon, A.; Nguyen, C.; Le-Nguyen, D.; Nilges, M.; Barale, J.-C. Computational Design of Protein-Based Inhibitors of Plasmodium vivax Subtilisin-Like 1 Protease. PLoS ONE 2014, 9, e109269. [Google Scholar] [CrossRef] [PubMed]
- Lidumniece, E.; Withers-Martinez, C.; Hackett, F.; Blackman, M.J.; Jirgensons, A. Subtilisin-like Serine Protease 1 (SUB1) as an Emerging Antimalarial Drug Target: Current Achievements in Inhibitor Discovery. J. Med. Chem. 2022, 65, 12535–12545. [Google Scholar] [CrossRef] [PubMed]
- Appiah-Kubi, Z.; Adomako, J.; Dwamena, H.; Obeng, E.A.; Adu-Gyamfi, R.; Osekre, E.A.; Quain, M.; Kwoseh, C. Survey of fungal diseases associated with amaranth (Amaranthus species) in peri-urban vegetable farms in Kumasi and Tamale metropolis of Ghana. Afr. J. Agric. Res. 2022, 18, 792–802. [Google Scholar] [CrossRef]
- Ochoa-Sánchez, J.C.; Parra-Cota, F.I.; Aviña-Padilla, K.; Délano-Frier, J.; Martínez-Soriano, J.P. Amaranthus spp.: A new host of “Candidatus Phytoplasma aurantifolia”. Phytoparasitica 2009, 37, 381–384. [Google Scholar] [CrossRef]
- Guisán Jose, M. Agarose-aldehyde gels as supports for immobilization-stabilization of enzymes. Enzym. Microb. Technol. 1988, 10, 375–382. [Google Scholar] [CrossRef]
- Orrego, A.H.; Romero-Fernández, M.; Millán-Linares, M.D.C.; Yust, M.D.M.; Guisán, J.M.; Rocha-Martin, J. Stabilization of Enzymes by Multipoint Covalent Attachment on Aldehyde-Supports: 2-Picoline Borane as an Alternative Reducing Agent. Catalysts 2018, 8, 333. [Google Scholar] [CrossRef]
- Ruiz-Tapia, T.E.; Jácome-Camacho, G.R.; Sinche-Serra, M.V.; Castillo-Domínguez, J.P.; Avilés, F.X.; Hernández de la Torre, M. Purification and characterization of papain inhibitors from amaranto (Amaranthus caudatus) and frijol (Phaseolus vulgaris) seeds. Agrociencia 2020, 54, 731–745. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Alonso-Del-Rivero, M.; Trejo, S.A.; Reytor, M.L.; Rodriguez-de-la-Vega, M.; Delfin, J.; Diaz, J.; González-González, Y.; Canals, F.; Chavez, M.A.; Aviles, F.X. Tri-domain bifunctional inhibitor of metallocarboxypeptidases A and serine proteases isolated from marine annelid Sabellastarte magnifica. J. Biol. Chem. 2012, 287, 15427–15438. [Google Scholar] [CrossRef]
- Bieth, J.G. Theoretical and practical aspects of proteinase inhibition kinetics. Methods Enzymol. 1995, 248, 59–84. [Google Scholar] [CrossRef] [PubMed]
- Erlanger, B.F.; Kokowsky, N.; Cohen, W. The preparation and properties of two new chromogenic substrates of trypsin. Arch. Biochem. Biophys. 1961, 95, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Bru, R.; Walde, P. Product inhibition of alpha-chymotrypsin in reverse micelles. Eur. J. Biochem. 1991, 199, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Lyublinskaya, L.A.; Belyaev, S.V.; Strongin, A.Y.; Matyash, L.F.; Levin, E.D.; Stepanov, V.M. A new chromogenic substrate for subtilisin. Anal. Biochem. 1974, 62, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Yanes, O.; Villanueva, J.; Querol, E.; Aviles, F.X. Functional screening of serine protease inhibitors in the medical leech Hirudo medicinalis monitored by intensity fading MALDI-TOF MS. Mol. Cell. Proteom. 2005, 4, 1602–1613. [Google Scholar] [CrossRef]
- Resemann, A.; Jabs, W.; Wiechmann, A.; Wagner, E.; Colas, O.; Evers, W.; Belau, E.; Vorwerg, L.; Evans, C.; Beck, A.; et al. Full validation of therapeutic antibody sequences by middle-up mass measurements and middle-down protein sequencing. mAbs 2015, 8, 318–330. [Google Scholar] [CrossRef]
- Juanhuix, J.; Gil-Ortiz, F.; Cuní, G.; Colldelram, C.; Nicolás, J.; Lidón, J.; Boter, E.; Ruget, C.; Ferrer, S.; Benach, J. Developments in optics and performance at BL13-XALOC, the macromolecular crystallography beamline at the Alba Synchrotron. J. Synchrotron Radiat. 2014, 21 Pt 4, 679–689. [Google Scholar] [CrossRef]
- Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 133–144. [Google Scholar] [CrossRef]
- Agirre, J.; Atanasova, M.; Bagdonas, H.; Ballard, C.B.; Baslé, A.; Beilsten-Edmands, J.; Borges, R.J.; Brown, D.G.; Burgos-Mármol, J.J.; Berrisford, J.M.; et al. The CCP4 suite: Integrative software for macromolecular crystallography. Acta Crystallogr. Sect. D Struct. Biol. 2023, 79, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.-W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, D.; Torres-Puig, S.; Ratera, M.; Querol, E.; Piñol, J.; Pich, O.Q.; Fita, I. Mycoplasma genitalium adhesin P110 binds sialic-acid human receptors. Nat. Commun. 2018, 9, 4471. [Google Scholar] [CrossRef]
- Cabrera-Muñoz, A.; Sierra-Gómez, Y.; Covaleda-Cortés, G.; Reytor, M.L.; González-González, Y.; Bautista, J.M.; Avilés, F.X.; Alonso-Del-Rivero, M. Isolation and Characterization of NpCI, a New Metallocarboxypeptidase Inhibitor from the Marine Snail Nerita peloronta with Anti-Plasmodium falciparum Activity. Mar. Drugs 2023, 21, 94. [Google Scholar] [CrossRef]
- Karr, J.R.; Sanghvi, J.C.; Macklin, D.N.; Gutschow, M.V.; Jacobs, J.M.; Bolival, B., Jr.; Assad-Garcia, N.; Glass, J.I.; Covert, M.W. A whole-cell computational model predicts phenotype from genotype. Cell 2012, 150, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthi, R.; Gong, Y.X.; Richardson, M. A new protein inhibitor of trypsin and activated hageman factor from pumpkin (Cucurbita maxima) seeds. FEBS Lett. 1990, 273, 163–167. [Google Scholar] [CrossRef] [PubMed]


| Total Protein (mg) | Inhibitory Activity (U) | Specific Inhibitory Activity (U/mg) | Yield (%) | Purification (Fold) | |
|---|---|---|---|---|---|
| Crude extract | 186.5 | 11.1 ± 1.5 | 0.059 ± 0.008 | 100 | 1.0 |
| Peak from affinity chromatography | 1.88 | 8.5 ± 0.7 | 4.5 ± 0.3 | 76.4 | 75.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández de la Torre, M.; Covaleda-Cortés, G.; Montesinos, L.; Covaleda, D.; Ortiz, J.C.; Piñol, J.; Bautista, J.M.; Castillo, J.P.; Reverter, D.; Avilés, F.X. Analysis of Protein Inhibitors of Trypsin in Quinoa, Amaranth and Lupine Seeds. Selection and Deep Structure–Function Characterization of the Amaranthus caudatus Species. Int. J. Mol. Sci. 2025, 26, 1150. https://doi.org/10.3390/ijms26031150
Hernández de la Torre M, Covaleda-Cortés G, Montesinos L, Covaleda D, Ortiz JC, Piñol J, Bautista JM, Castillo JP, Reverter D, Avilés FX. Analysis of Protein Inhibitors of Trypsin in Quinoa, Amaranth and Lupine Seeds. Selection and Deep Structure–Function Characterization of the Amaranthus caudatus Species. International Journal of Molecular Sciences. 2025; 26(3):1150. https://doi.org/10.3390/ijms26031150
Chicago/Turabian StyleHernández de la Torre, Martha, Giovanni Covaleda-Cortés, Laura Montesinos, Daniela Covaleda, Juan C. Ortiz, Jaume Piñol, José M. Bautista, J. Patricio Castillo, David Reverter, and Francesc Xavier Avilés. 2025. "Analysis of Protein Inhibitors of Trypsin in Quinoa, Amaranth and Lupine Seeds. Selection and Deep Structure–Function Characterization of the Amaranthus caudatus Species" International Journal of Molecular Sciences 26, no. 3: 1150. https://doi.org/10.3390/ijms26031150
APA StyleHernández de la Torre, M., Covaleda-Cortés, G., Montesinos, L., Covaleda, D., Ortiz, J. C., Piñol, J., Bautista, J. M., Castillo, J. P., Reverter, D., & Avilés, F. X. (2025). Analysis of Protein Inhibitors of Trypsin in Quinoa, Amaranth and Lupine Seeds. Selection and Deep Structure–Function Characterization of the Amaranthus caudatus Species. International Journal of Molecular Sciences, 26(3), 1150. https://doi.org/10.3390/ijms26031150







