Different Efficacy of Five Soluble Dietary Fibers on Alleviating Loperamide-Induced Constipation in Mice: Influences of Different Structural Features
Abstract
1. Introduction
2. Results
2.1. Structural Information of the Fibers
2.2. Defecation Function
2.3. Gastrointestinal Hormone Levels
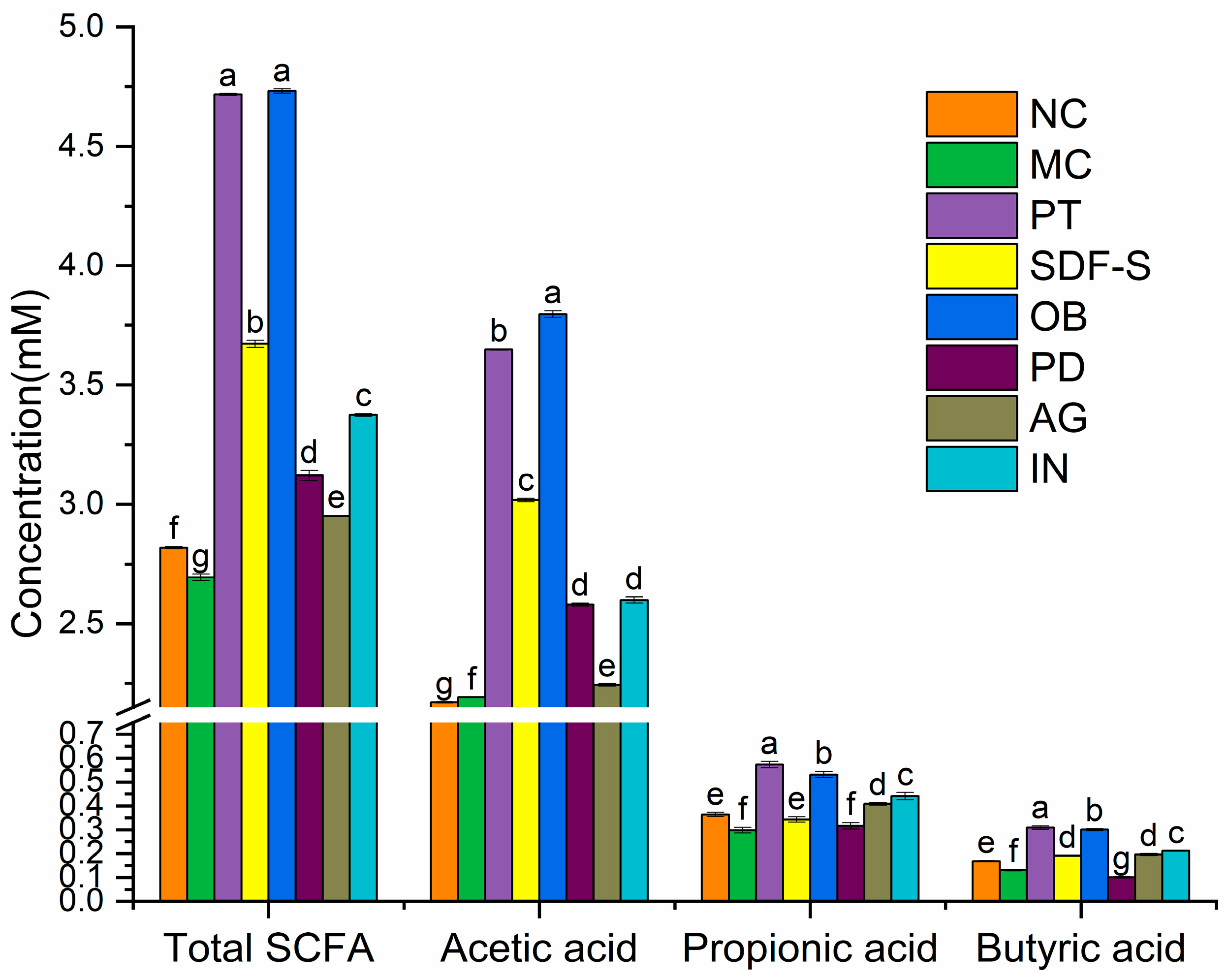
2.4. Fecal SCFA Profiles
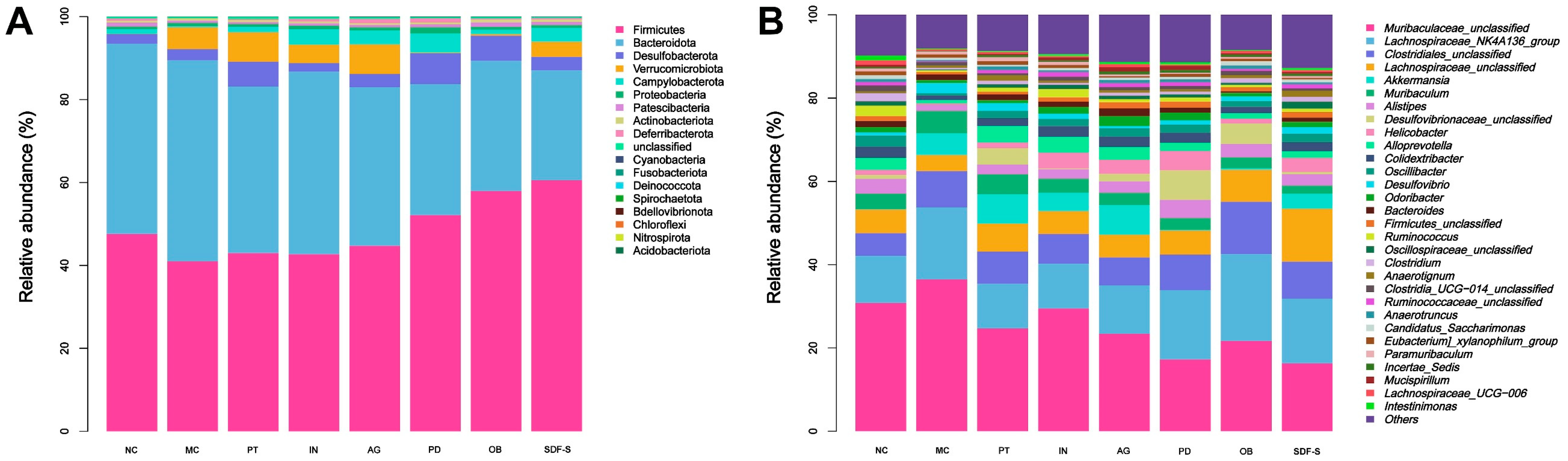
2.5. Gut Microbiota Structure
2.5.1. Diversity of Gut Microbial Communities
2.5.2. Community Structure of Gut Microbiota
2.6. Gut Microbiota Functional Prediction
2.7. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Determination of the Structural Properties of Fibers
4.3. Animals and Experimental Design
4.4. Assessing Effects of Polysaccharides on Constipation
4.4.1. SCFA in Feces
4.4.2. Defecation Parameters
Weight and Moisture of Stools
Small Intestinal Propulsion Trial
4.4.3. Serum Gastrointestinal Regulatory Hormones
4.4.4. 16S rDNA Gene Sequencing of Caecum Contents
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barberio, B.; Judge, C.; Savarino, E.V.; Ford, A.C. Global prevalence of functional constipation according to the Rome criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 638–648. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemianrad, M.; Ammari-Allahyari, M.; Rasoulpoor, S.; Shohaimi, S.; Mohammadi, M. Global prevalence of constipation in older adults: A systematic review and meta-analysis. Wien. Klin. Wochen. 2023, 135, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Staller, K.; Olen, O.; Soderling, J.; Roelstraete, B.; Tornblom, H.; Song, M.; Ludvigsson, J.F. Chronic Constipation as a Risk Factor for Colorectal Cancer: Results From a Nationwide, Case-Control Study. Clin. Gastroenterol. Hepatol. 2022, 20, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Molnar, M.Z.; Potukuchi, P.K.; Thomas, F.; Lu, J.L.; Yamagata, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Constipation and risk of death and cardiovascular events. Atherosclerosis 2019, 281, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Bouchoucha, M.; Faye, A.; Savarieau, B.; Arsac, M. Effect of an oral bulking agent and a rectal laxative administered alone or in combination for the treatment of constipation. Gastroenterol. Clin. Biol. 2004, 28, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, D.; Rao, S.S. Efficacy and safety of traditional medical therapies for chronic constipation: Systematic review. Am. J. Gastroenterol. 2005, 100, 936–971. [Google Scholar] [CrossRef]
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 718–727. [Google Scholar] [CrossRef]
- McRorie, J.J.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Collado, Y.L.; San, M.M.I.; Ciudad-Cabanas, M.J.; Calle-Puron, M.E.; Hernandez, C.M. Effectiveness of inulin intake on indicators of chronic constipation; a meta-analysis of controlled randomized clinical trials. Nutr. Hosp. 2014, 30, 244–252. [Google Scholar] [CrossRef]
- Huang, J.; Lin, B.; Zhang, Y.; Xie, Z.; Zheng, Y.; Wang, Q.; Xiao, H. Bamboo shavings derived O-acetylated xylan alleviates loperamide-induced constipation in mice. Carbohydr. Polym. 2022, 276, 118761. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Y.; Wen, P.; Chen, Y.; Ouyang, D.; Wang, D.; Zhang, B.; Deng, J.; Chen, Y.; Sun, Y.; et al. The Anti-Constipation Effects of Raffino-Oligosaccharide on Gut Function in Mice Using Neurotransmitter Analyses, 16S rRNA Sequencing and Targeted Screening. Molecules 2022, 27, 2235. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Zheng, J.; Jiang, N.; Sun, G.; Bao, X.; Lin, A.; Liu, H. Chitosan oligosaccharides attenuate loperamide-induced constipation through regulation of gut microbiota in mice. Carbohydr. Polym. 2021, 253, 117218. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, S.; Yan, Q.; Li, Y.; Jiang, Z. Effect of Konjac mannan oligosaccharides on diphenoxylate-induced constipation in mice. J. Funct. Food. 2019, 57, 399–407. [Google Scholar] [CrossRef]
- Pan, R.; Wang, L.; Xu, X.; Chen, Y.; Wang, H.; Wang, G.; Zhao, J.; Chen, W. Crosstalk between the Gut Microbiome and Colonic Motility in Chronic Constipation: Potential Mechanisms and Microbiota Modulation. Nutrients 2022, 14, 3704. [Google Scholar] [CrossRef]
- Shin, A.; Preidis, G.A.; Shulman, R.; Kashyap, P.C. The Gut Microbiome in Adult and Pediatric Functional Gastrointestinal Disorders. Clin. Gastroenterol. Hepatol. 2019, 17, 256–274. [Google Scholar] [CrossRef]
- Feng, C.; Gao, G.; Wu, K.; Weng, X. Causal relationship between gut microbiota and constipation: A bidirectional Mendelian randomization study. Front. Microbiol. 2024, 15, 1438778. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Sheng, K.; Li, G.; Zhang, S. The causal relationship between gut microbiota and constipation: A two-sample Mendelian randomization study. BMC Gastroenterol. 2024, 24, 271. [Google Scholar] [CrossRef]
- Lai, H.; Li, Y.; He, Y.; Chen, F.; Mi, B.; Li, J.; Xie, J.; Ma, G.; Yang, J.; Xu, K.; et al. Effects of dietary fibers or probiotics on functional constipation symptoms and roles of gut microbiota: A double-blinded randomized placebo trial. Gut Microbes 2023, 15, 2197837. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Pi, Y.; Ye, H.; Wu, Y.; Bai, Y.; Lian, S.; Han, D.; Ni, D.; Zou, X.; Zhao, J.; et al. Consumption of Dietary Fiber with Different Physicochemical Properties during Late Pregnancy Alters the Gut Microbiota and Relieves Constipation in Sow Model. Nutrients 2022, 14, 2511. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, Z.; Liu, X.; Hu, W.; Wu, W. Gastrointestinal Fermentable Polysaccharide Is Beneficial in Alleviating Loperamide-Induced Constipation in Mice. Nutrients 2023, 15, 4364. [Google Scholar] [CrossRef]
- Bai, J.; Ren, Y.; Li, Y.; Fan, M.; Qian, H.; Wang, L.; Wu, G.; Zhang, H.; Qi, X.; Xu, M.; et al. Physiological functionalities and mechanisms of β-glucans. Trends Food Sci. Technol. 2019, 88, 57–66. [Google Scholar] [CrossRef]
- Do, C.M.; Walker, J.C.; Novello, D.; Caselato, V.M.; Sgarbieri, V.C.; Ouwehand, A.C.; Andreollo, N.A.; Hiane, P.A.; Dos, S.E. Polydextrose: Physiological Function, and Effects on Health. Nutrients 2016, 8, 553. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Saeidy, S.; Petera, B.; Pierre, G.; Fenoradosoa, T.A.; Djomdi, D.; Michaud, P.; Delattre, C. Plants arabinogalactans: From structures to physico-chemical and biological properties. Biotechnol. Adv. 2021, 53, 107771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, B.; Liu, X.; Hu, W.; Zhang, C.; Guo, Y.; Wu, W. Effects of Steaming on Sweet Potato Soluble Dietary Fiber: Content, Structure, and Lactobacillus Proliferation In Vitro. Foods 2023, 12, 1620. [Google Scholar] [CrossRef]
- Wang, L.; Cen, S.; Wang, G.; Lee, Y.; Zhao, J.; Zhang, H.; Chen, W. Acetic acid and butyric acid released in large intestine play different roles in the alleviation of constipation. J. Funct. Food. 2020, 69, 103953. [Google Scholar] [CrossRef]
- Serra, J.; Mascort-Roca, J.; Marzo-Castillejo, M.; Delgado Aros, S.; Ferrándiz Santos, J.; Diaz Rubio, E.R.; Mearin Manrique, F. Clinical practice guidelines for the management of constipation in adults. Part 2: Diagnosis and treatment. Gastroenterol. Hepatol. (Engl. Ed.) 2017, 40, 303–316. [Google Scholar] [CrossRef]
- Paquette, I.M.; Varma, M.; Ternent, C.; Melton-Meaux, G.; Rafferty, J.F.; Feingold, D.; Steele, S.R. The American Society of Colon and Rectal Surgeons’ Clinical Practice Guideline for the Evaluation and Management of Constipation. Dis. Colon Rectum 2016, 59, 479–492. [Google Scholar] [CrossRef]
- National Library of Medicine. Constipation in Children and Young People: Diagnosis and Management; (NICE Clinical Guidelines, No. 99); National Institute for Health and Care Excellence (NICE): London, UK, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554924/ (accessed on 24 January 2025).
- Gill, S.; Chater, P.I.; Wilcox, M.D.; Pearson, J.P.; Brownlee, I.A. The impact of dietary fibres on the physiological processes of the large intestine. Bioact. Carbohydr. Diet. Fibre 2018, 16, 62–74. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Chen, J.; Chen, X.; Chia, N.; O’Connor, H.M.; Wolf, P.G.; Gaskins, H.R.; Bharucha, A.E. Relationship Between Microbiota of the Colonic Mucosa vs Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients With Chronic Constipation. Gastroenterology 2016, 150, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- La Reau, A.J.; Suen, G. The Ruminococci: Key symbionts of the gut ecosystem. J. Microbiol. 2018, 56, 199–208. [Google Scholar] [CrossRef]
- Ren, J.; Ren, Y.; Mu, Y.; Zhang, L.; Chen, B.; Li, S.; Fang, Q.; Zhang, Z.; Zhang, K.; Li, S.; et al. Microbial imbalance in Chinese children with diarrhea or constipation. Sci. Rep. 2024, 14, 13516. [Google Scholar] [CrossRef] [PubMed]
- Gophna, U.; Konikoff, T.; Nielsen, H.B. Oscillospira and related bacteria—From metagenomic species to metabolic features. Environ. Microbiol. 2017, 19, 835–841. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira—A candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef] [PubMed]
- Robertson, B.R.; O’Rourke, J.L.; Neilan, B.A.; Vandamme, P.; On, S.; Fox, J.G.; Lee, A. Mucispirillum schaedleri gen. nov., sp. nov., a spiral-shaped bacterium colonizing the mucus layer of the gastrointestinal tract of laboratory rodents. Int. J. Syst. Evol. Microbiol. 2005, 55, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.P.N.; Ritari, J.; Boeren, S.; de Waard, P.; Plugge, C.M.; de Vos, W.M. Production of butyrate from lysine and the Amadori product fructoselysine by a human gut commensal. Nat. Commun. 2015, 6, 10062. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.P.N.; Troise, A.D.; Nijsse, B.; Roviello, G.N.; Fogliano, V.; de Vos, W.M. Intestinimonas-like bacteria are important butyrate producers that utilize Nε-fructosyllysine and lysine in formula-fed infants and adults. J. Funct. Food. 2020, 70, 103974. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Zhou, J.; Yuan, X.; Liu, Y. The gut microbiota-constipation connection: Insights from a two sample bidirectional Mendelian randomization study. Microb. Pathog. 2024, 192, 106667. [Google Scholar] [CrossRef]
- Geirnaert, A.; Calatayud, M.; Grootaert, C.; Laukens, D.; Devriese, S.; Smagghe, G.; De Vos, M.; Boon, N.; Van de Wiele, T. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci. Rep. 2017, 7, 11450. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Chen, X.; Tang, X. Short-chain fatty acid, acylation and cardiovascular diseases. Clin. Sci. 2020, 134, 657–676. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; DiBaise, J.K.; Ilhan, Z.E.; Crowell, M.D.; Rideout, J.R.; Caporaso, J.G.; Rittmann, B.E.; Krajmalnik-Brown, R. Gut microbial and short-chain fatty acid profiles in adults with chronic constipation before and after treatment with lubiprostone. Anaerobe 2015, 33, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, Q.; Huang, Y.; Ni, L.; Liu, J.; Jiang, J.; Li, N. Function and clinical implications of short-chain fatty acids in patients with mixed refractory constipation. Color. Dis. 2016, 18, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Soret, R.; Chevalier, J.; De Coppet, P.; Poupeau, G.; Derkinderen, P.; Segain, J.P.; Neunlist, M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 2010, 138, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Shang, W.; Ma, Q.; Strappe, P.; Zhou, Z. Abundance of Probiotics and Butyrate-Production Microbiome Manages Constipation via Short-Chain Fatty Acids Production and Hormones Secretion. Mol. Nutr. Food Res. 2019, 63, e1801187. [Google Scholar] [CrossRef] [PubMed]
- Vogt, J.A.; Wolever, T.M. Fecal acetate is inversely related to acetate absorption from the human rectum and distal colon. J. Nutr. 2003, 133, 3145–3148. [Google Scholar] [CrossRef]
- Jensen, N.; Maldonado-Gomez, M.; Krishnakumar, N.; Weng, C.; Castillo, J.; Razi, D.; Kalanetra, K.; German, J.B.; Lebrilla, C.B.; Mills, D.A.; et al. Dietary fiber monosaccharide content alters gut microbiome composition and fermentation. Appl. Environ. Microbiol. 2024, 90, e96424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Ma, Q.; Wang, D.; Jiang, Q.; Wang, P.; Ge, Z.; Wang, J.; Qin, P.; Zhao, X. Different microbiota modulation and metabolites generation of five dietary glycans during in vitro gut fermentation are determined by their monosaccharide profiles. Food Res. Int. 2024, 196, 115011. [Google Scholar] [CrossRef] [PubMed]
- Zeybek, N.; Rastall, R.A.; Buyukkileci, A.O. Utilization of xylan-type polysaccharides in co-culture fermentations of Bifidobacterium and Bacteroides species. Carbohydr. Polym. 2020, 236, 116076. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M.; Handa, O.; Fukushima, S.; Matsumoto, H.; Umegaki, E.; Inoue, R.; Naito, Y.; Shiotani, A. Reduced abundance of butyric acid-producing bacteria in the ileal mucosa-associated microbiota of ulcerative colitis patients. J. Clin. Biochem. Nutr. 2023, 73, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Ahmadi, S.; Shen, S.; Wu, D.; Xiao, H.; Ding, T.; Liu, D.; Ye, X.; Chen, S. Structure and fermentation characteristics of five polysaccharides sequentially extracted from sugar beet pulp by different methods. Food Hydrocoll. 2022, 126, 107462. [Google Scholar] [CrossRef]
- Hernot, D.C.; Boileau, T.W.; Bauer, L.L.; Middelbos, I.S.; Murphy, M.R.; Swanson, K.S.; Fahey, G.J. In vitro fermentation profiles, gas production rates, and microbiota modulation as affected by certain fructans, galactooligosaccharides, and polydextrose. J. Agric. Food Chem. 2009, 57, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, W.; Pi, X. In Vitro Effects of Stachyose on the Human Gut Microbiota. Starch-Stärke 2021, 73, 2100029. [Google Scholar] [CrossRef]




| Items | SDF-S | OB | PD | IN | AG |
|---|---|---|---|---|---|
| Molecular weight (kDa) | 5.30 | 101.24 | 3.01 | 3.35 | 48.07 |
| Monosaccharide composition (mg/g) | |||||
| Guluronic acid | 23.94 | 0.62 | 1.20 | 0.47 | 0.34 |
| Mannose | 4.00 | 6.05 | 0.57 | 0.73 | 2.41 |
| Ribose | 0.87 | 0.15 | 0.30 | 0.05 | 0.18 |
| Rhamnose | 5.65 | 0.53 | 0.71 | N.D. | 0.74 |
| Glucuronic acid | 1.44 | 1.98 | 0.06 | N.D. | 5.03 |
| Galacturonic acid | 25.48 | 1.22 | 1.43 | 1.24 | 0.06 |
| Glucose | 846.85 | 826.24 | 909.06 | 278.73 | 2.16 |
| Galactose | 30.03 | 11.68 | 1.37 | 6.09 | 864.58 |
| Xylose | 1.75 | 40.04 | 0.96 | N.D. | N.D. |
| Arabinose | 12.39 | 48.59 | 0.65 | 0.91 | 99.20 |
| Fucose | 1.13 | 1.17 | N.D. | 0.92 | 1.42 |
| Fructose * | / | / | / | 675.83 | / |
| Groups | 6 h Stool Weight (g) | Stool Moisture (%) | GI Transit Rate (%) |
|---|---|---|---|
| NC | 0.60 ± 0.31 bc | 68.86 ± 2.51 a | 88.35 ± 5.17 a |
| MC | 0.40 ± 0.09 c | 30.42 ± 3.98 d | 33.32 ± 7.32 d |
| PT | 0.83 ± 0.12 ab | 45.56 ± 5.37 bc | 63.23 ± 7.67 b |
| SDF-S | 0.83 ± 0.12 ab | 49.53 ± 2.31 bc | 58.92 ± 7.78 b |
| OB | 0.77 ± 0.29 ab | 46.50 ± 4.50 bc | 59.67 ± 10.62 b |
| PD | 0.76 ± 0.08 ab | 50.35 ± 3.64 b | 37.82 ± 3.95 d |
| AG | 1.12 ± 0.21 a | 46.15 ± 3.36 bc | 68.10 ±4.88 b |
| IN | 0.99 ± 0.20 a | 42.68 ± 2.04 c | 49.36 ± 7.84 c |
| Groups | MLT (ng/L) | GAS (ng/L) | SP (ng/mL) | VIP (ng/L) | SS (ng/L) |
|---|---|---|---|---|---|
| NC | 170.07 ± 38.22 | 254.21 ± 24.48 bc | 1.24 ± 0.17 a | 337.00 ± 55.65 d | 174.34 ± 13.32 c |
| MC | 163.09 ± 26.92 | 205.23 ± 54.06 c | 0.43 ± 0.09 d | 613.64 ± 57.77 a | 255.73 ± 15.85 a |
| PT | 182.51 ± 29.77 | 351.85 ± 33.14 a | 1.29 ± 0.05 a | 482.35 ± 53.87 bc | 168.34 ± 12.21 c |
| SDF-S | 138.27 ± 30.12 | 356.13 ± 52.96 a | 0.87 ± 0.16 b | 482.09 ± 83.19 bc | 172.51 ± 19.32 c |
| OB | 146.04 ± 33.76 | 293.47 ± 48.30 ab | 0.75 ± 0.14 bc | 330.75 ± 43.21 d | 172.12 ± 9.26 c |
| PD | 158.82 ± 13.85 | 263.93 ± 20.68 bc | 0.77 ± 0.02 bc | 380.90 ± 74.65 cd | 193.99 ± 15.77 bc |
| AG | 176.77 ± 50.81 | 262.16 ± 43.13 bc | 0.66 ± 0.16 bc | 527.47 ± 47.90 ab | 184.34 ± 18.84 bc |
| IN | 201.40 ± 31.86 | 279.57 ± 24.70 b | 0.57 ± 0.19 cd | 477.51 ± 61.48 bc | 201.19 ± 20.40 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Liu, B.; Liu, W.; Liu, X.; Zhang, C.; Hu, W.; Wu, W. Different Efficacy of Five Soluble Dietary Fibers on Alleviating Loperamide-Induced Constipation in Mice: Influences of Different Structural Features. Int. J. Mol. Sci. 2025, 26, 1236. https://doi.org/10.3390/ijms26031236
Zhang Z, Liu B, Liu W, Liu X, Zhang C, Hu W, Wu W. Different Efficacy of Five Soluble Dietary Fibers on Alleviating Loperamide-Induced Constipation in Mice: Influences of Different Structural Features. International Journal of Molecular Sciences. 2025; 26(3):1236. https://doi.org/10.3390/ijms26031236
Chicago/Turabian StyleZhang, Zhiguo, Buyu Liu, Wei Liu, Xingquan Liu, Chengcheng Zhang, Weiwei Hu, and Weicheng Wu. 2025. "Different Efficacy of Five Soluble Dietary Fibers on Alleviating Loperamide-Induced Constipation in Mice: Influences of Different Structural Features" International Journal of Molecular Sciences 26, no. 3: 1236. https://doi.org/10.3390/ijms26031236
APA StyleZhang, Z., Liu, B., Liu, W., Liu, X., Zhang, C., Hu, W., & Wu, W. (2025). Different Efficacy of Five Soluble Dietary Fibers on Alleviating Loperamide-Induced Constipation in Mice: Influences of Different Structural Features. International Journal of Molecular Sciences, 26(3), 1236. https://doi.org/10.3390/ijms26031236







