Phenolics Profile and Phenol-Related Enzyme Activities in Cucumber Plants Under Ni Stress
Abstract
:1. Introduction
2. Results
2.1. Growth, Ni Accumulation, and Relative Water Content (RWC)
2.2. Photosynthetic Pigments and Chlorophyll a Fluorescence
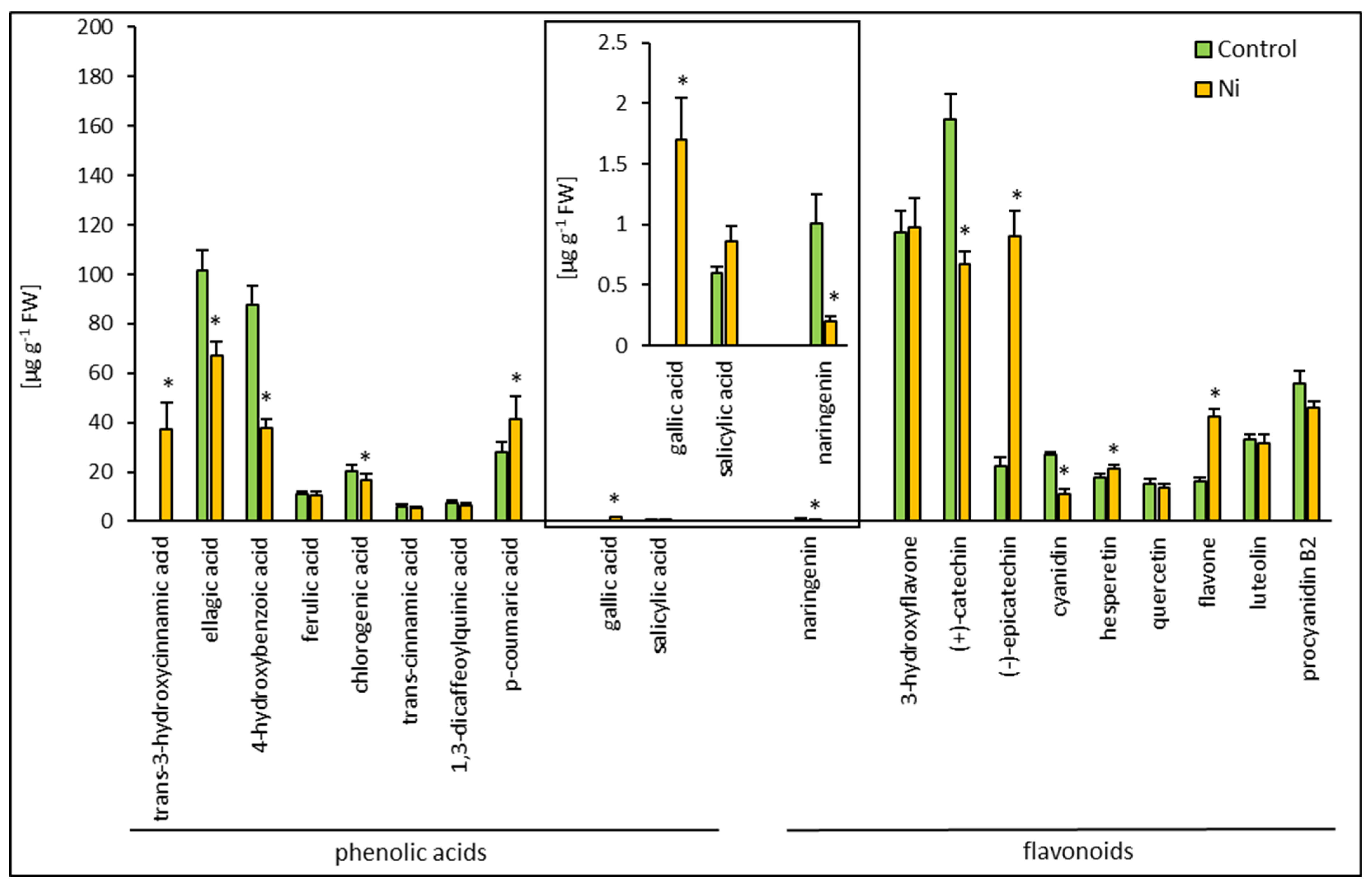
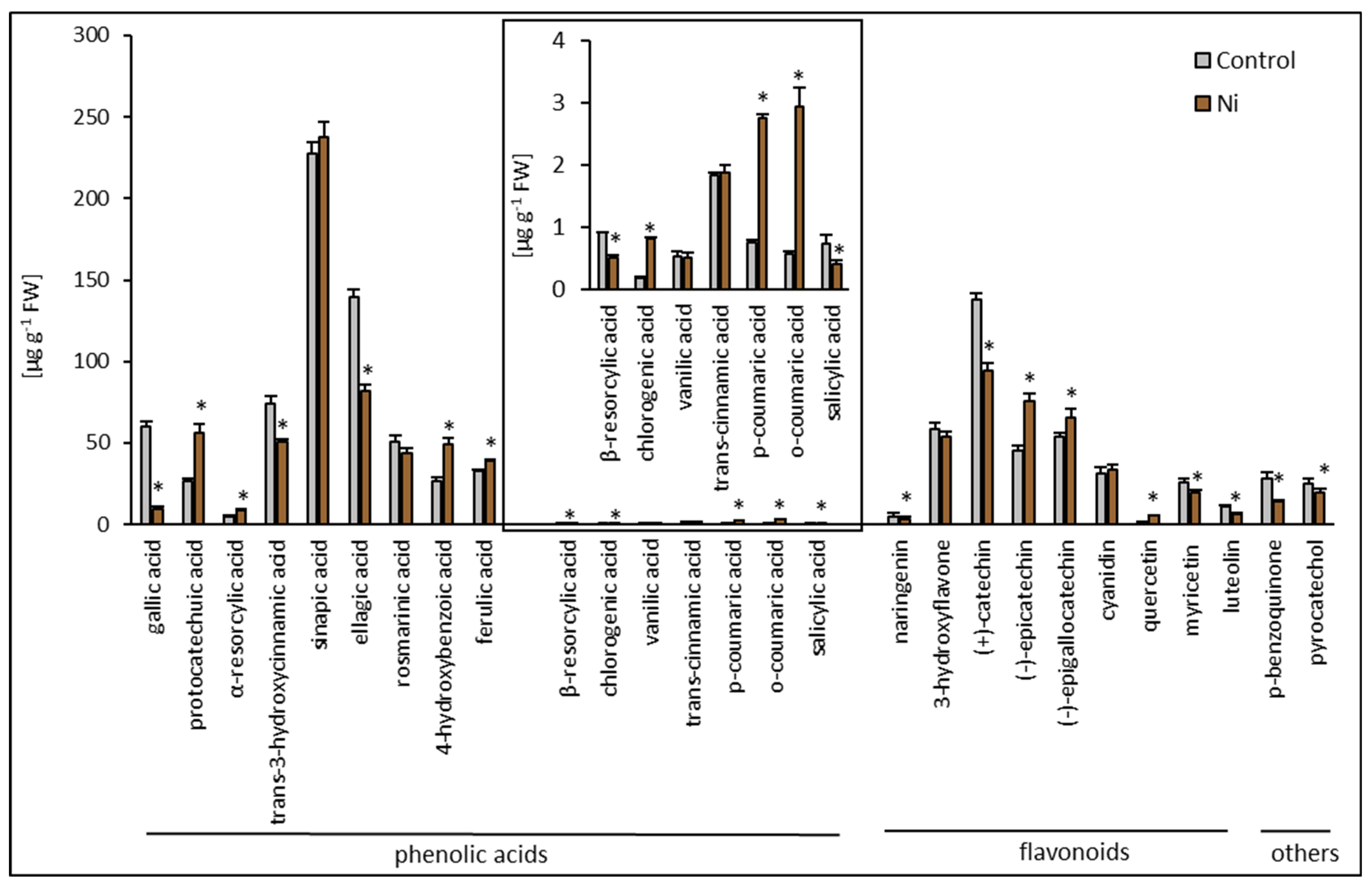
2.3. Phenolic Compound Composition Analysis
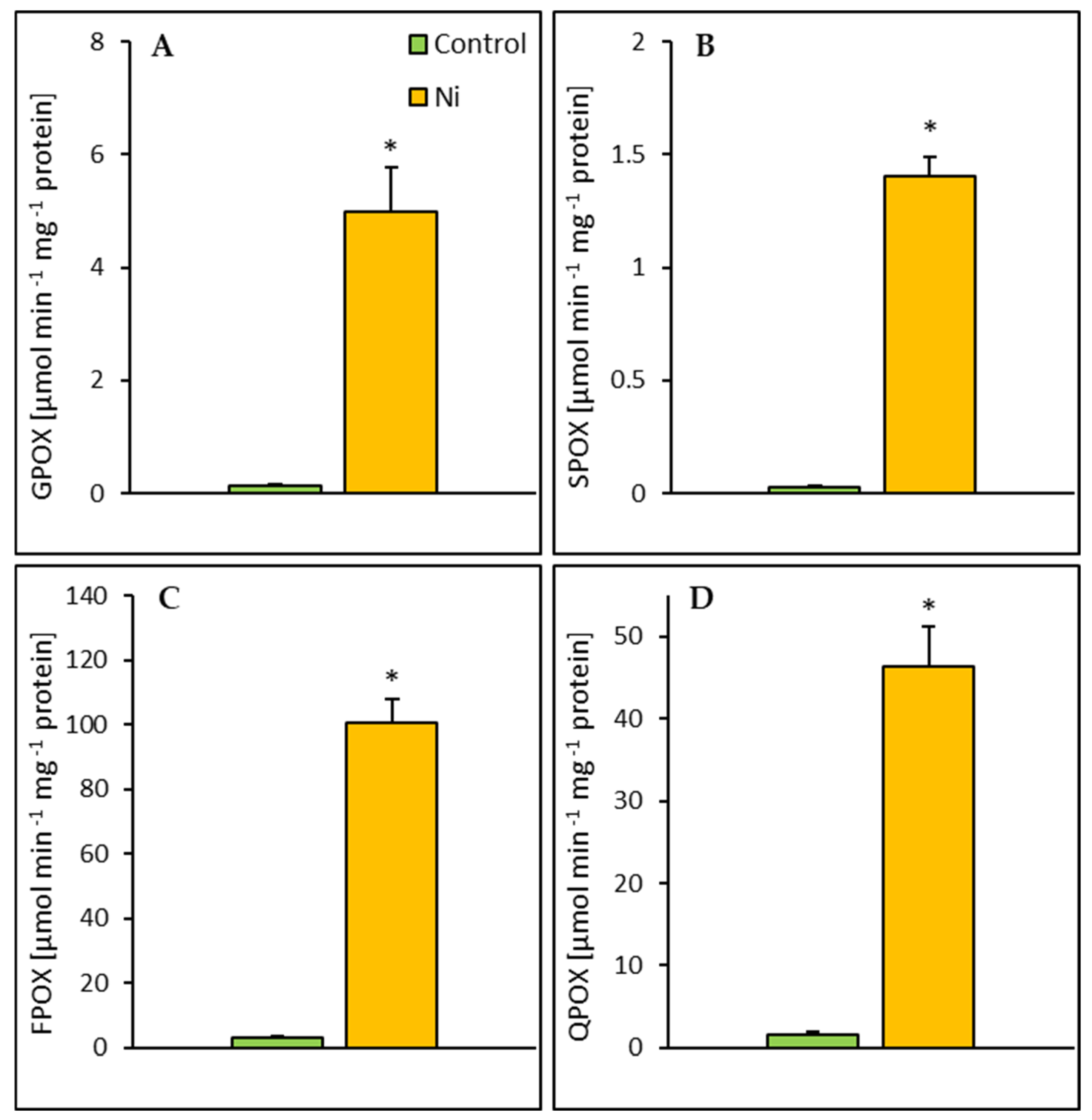
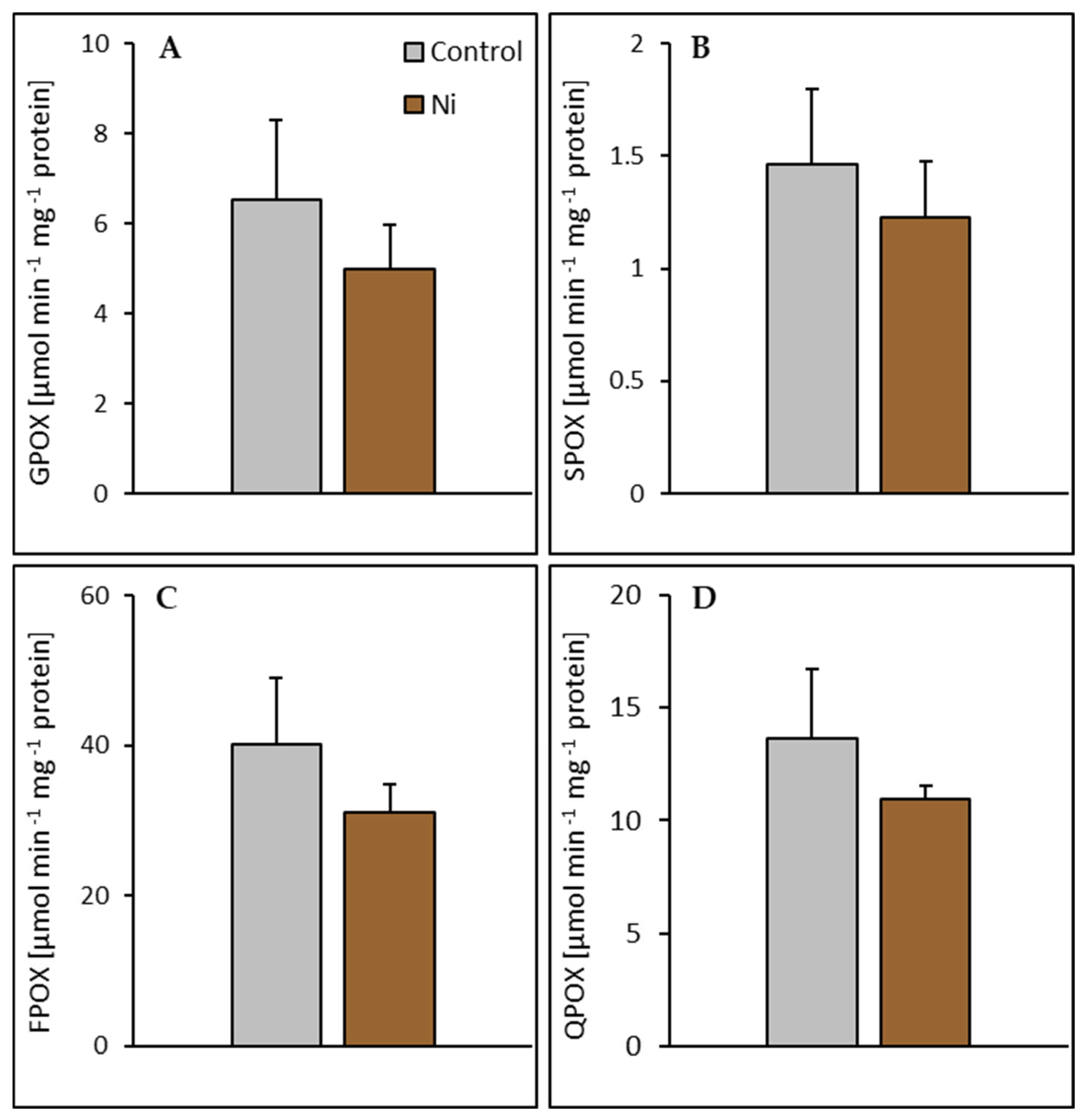
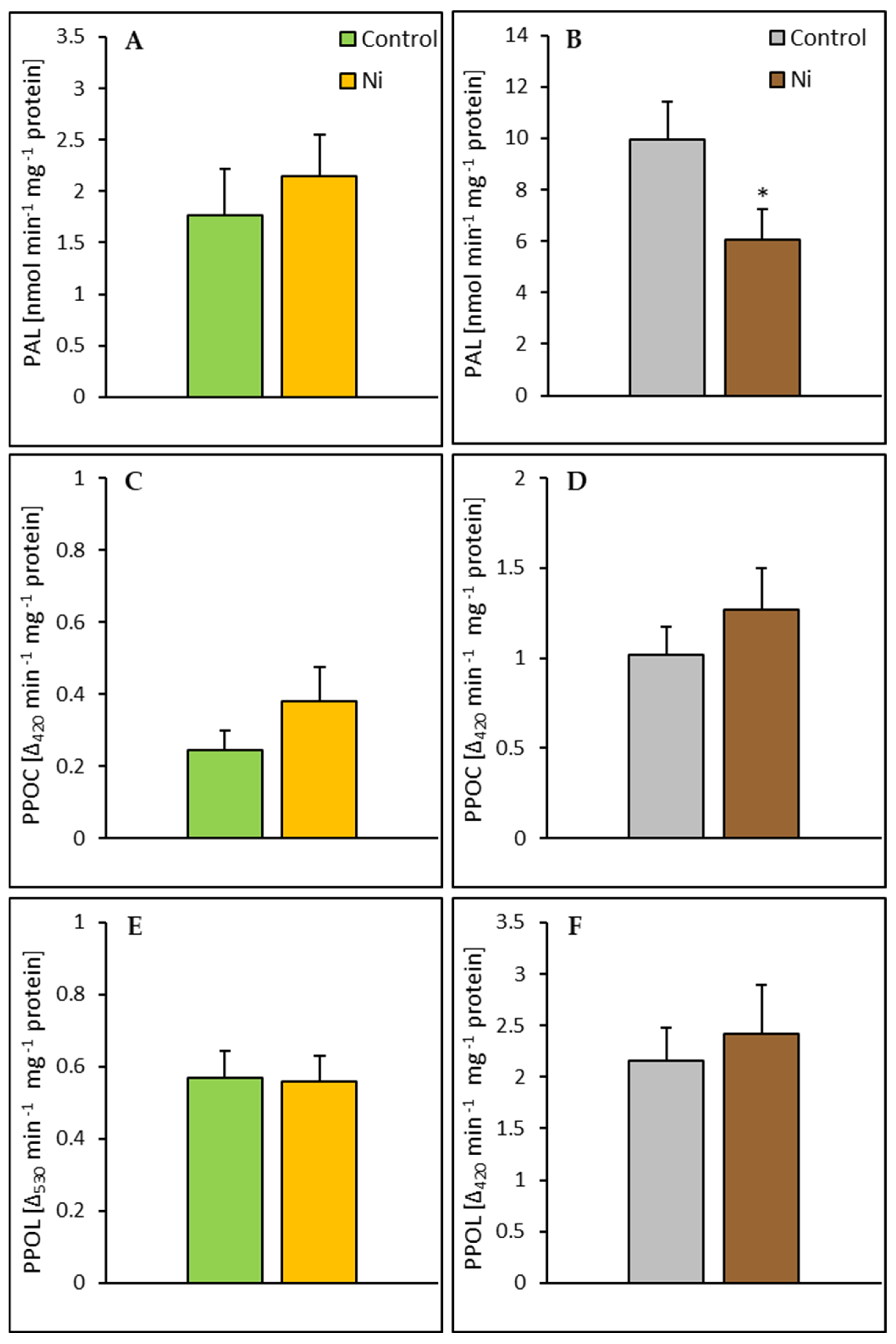
2.4. Enzyme Activities
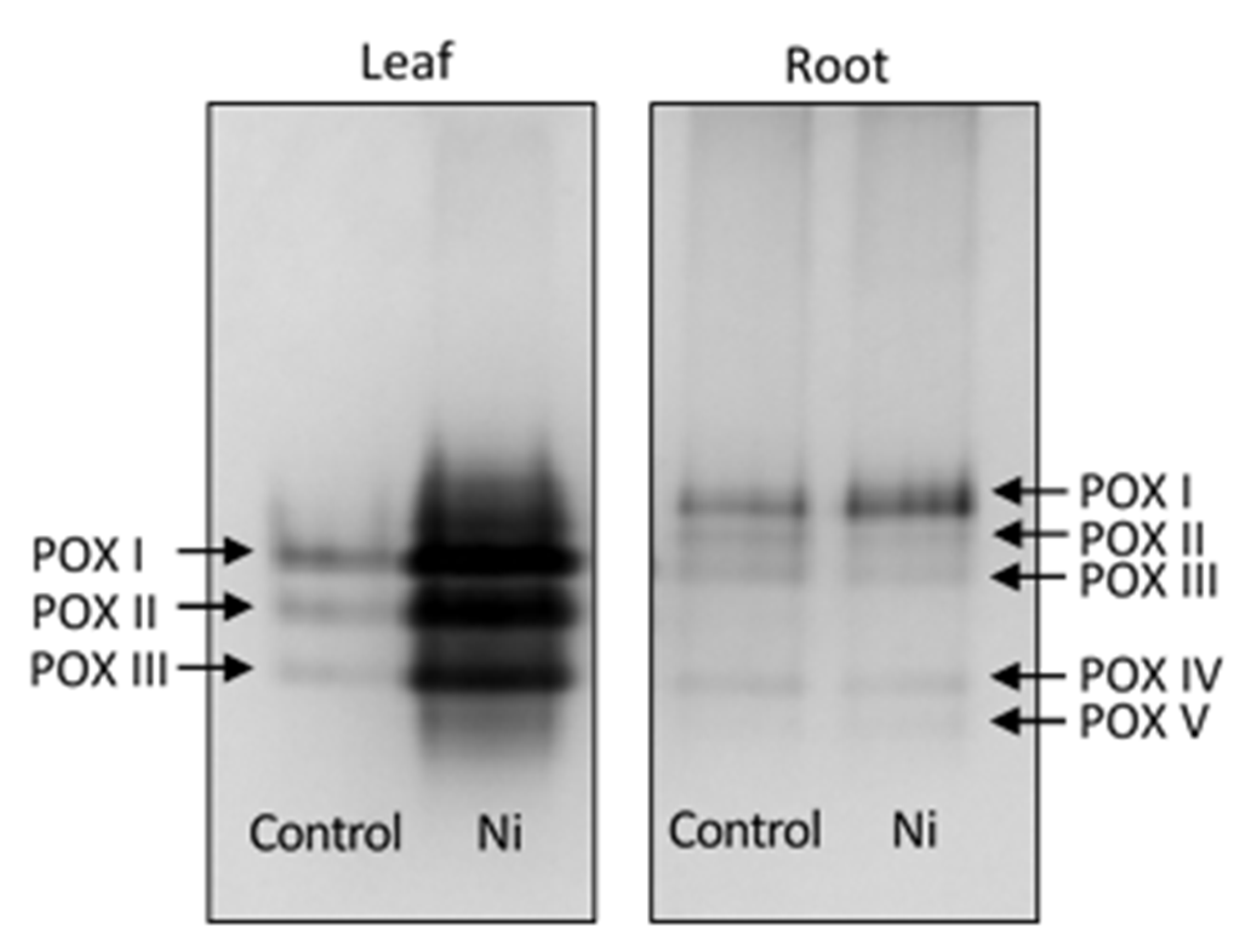
2.5. Native Polyacrylamide Gel Electrophoresis (PAGE)
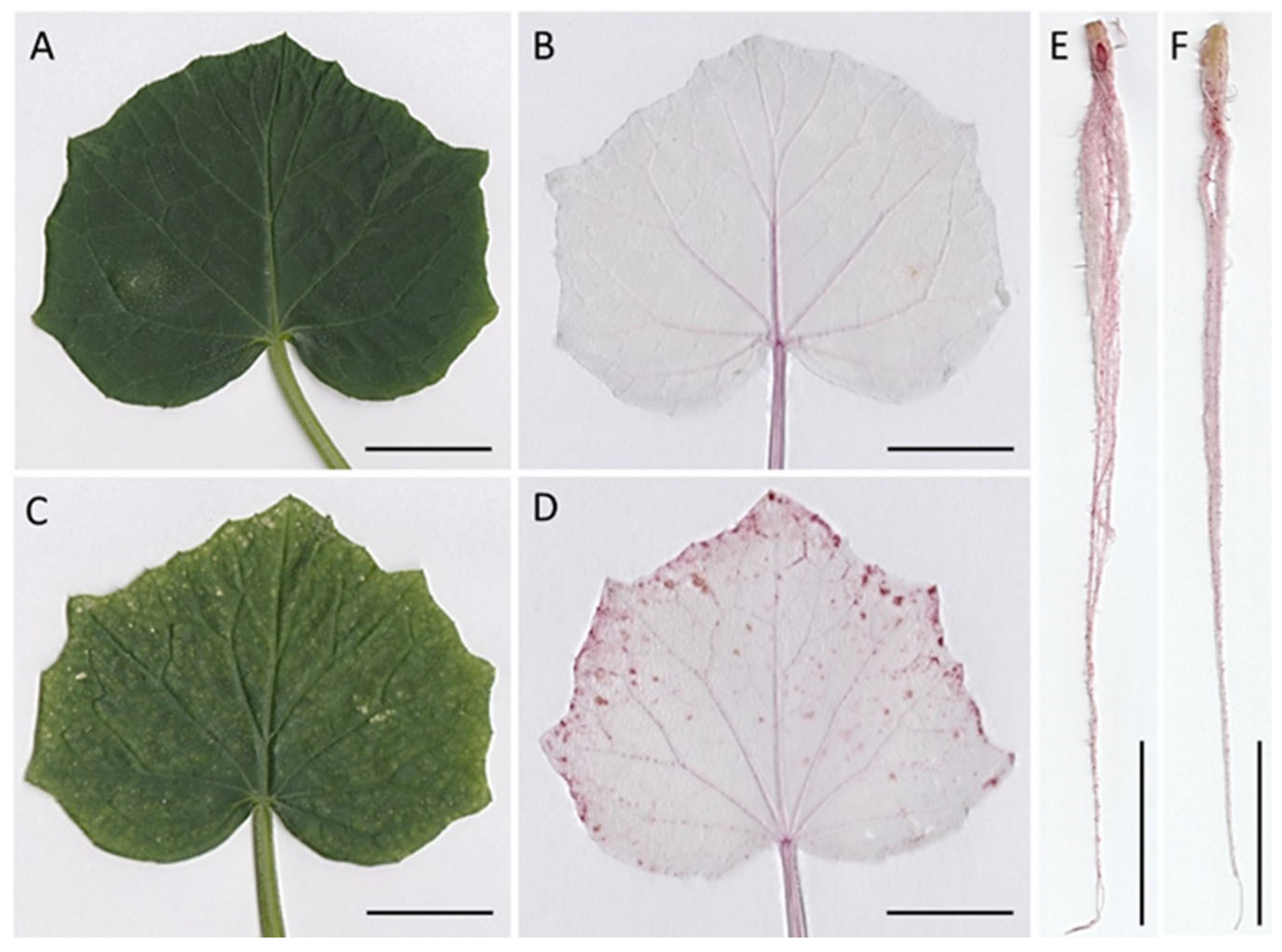
2.6. Histochemical Detection of Lignin
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Growth Parameters, Ni Contents, and Relative Water Content
4.3. Photosynthetic Pigment Concentrations and Chlorophyll a Fluorescence
4.4. HPLC Analysis
4.5. Enzyme Extraction and Assays
4.6. Native Polyacrylamide Gel Electrophoresis (PAGE)
4.7. Lignin Detection
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DW | Dry weight |
| FW | Fresh weight |
| FSW | Fully saturated weight |
| FPOX | Peroxidase assayed with ferulic acid |
| GPOX | Peroxidase assayed with guaiacol |
| Hydrogen peroxide | |
| NPQ | Non-photochemical quenching |
| PAL | Phenylalanine ammonia lyase |
| POX | Peroxidase |
| PPFD | Photosynthetic photon flux density |
| PPOC | Catecholase |
| PPOL | Laccase |
| PS II | Photosystem II |
| Rfd | Fluorescence decline ratio |
| ROS | Reactive oxygen species |
| RWC | Relative water content |
| SA | Salicylic acid |
| SPOX | Peroxidase assayed with syringaldazine |
| QPOX | Peroxidase assayed with quercetin |
| QY | Maximum photochemical efficiency of PS II |
References
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; Whether Toxic or Essential for Plants and Environment—A Review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef]
- Yan, R.; Gao, S.; Yang, W.; Cao, M.; Wang, S.; Chen, F. Nickel Toxicity Induced Antioxidant Enzyme and Phenylalanine Ammonia-Lyase Activities in Jatropha curcas L. Cotyledons. Plant Soil. Environ. 2008, 54, 294–300. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Bačkor, M. Phenolic Metabolism of Matricaria Chamomilla Plants Exposed to Nickel. J. Plant Physiol. 2009, 166, 1460–1464. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A. Heavy Metals Toxicity Phenolic Compounds and Their Antioxidant Activity in Plants Growing under Heavy Metal Stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Goncharuk, E.A.; Zagoskina, N.V. Heavy Metals, Their Phytotoxicity, and the Role of Phenolic Antioxidants in Plant Stress Responses with Focus on Cadmium: Review. Molecules 2023, 28, 3921. [Google Scholar] [CrossRef]
- Olszowy, M. What Is Responsible for Antioxidant Properties of Polyphenolic Compounds from Plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal Chelation of Polyphenols. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2001; Volume 335, pp. 190–203. [Google Scholar]
- Hiraga, S.; Sasaki, K.; Ito, H.; Ohashi, Y.; Matsui, H. A Large Family of Class III Plant Peroxidases. Plant Cell Physiol. 2001, 42, 462–468. [Google Scholar] [CrossRef]
- Passardi, F.; Penel, C.; Dunand, C. Performing the Paradoxical: How Plant Peroxidases Modify the Cell Wall. Trends Plant Sci. 2004, 9, 534–540. [Google Scholar] [CrossRef]
- Zelinová, V.; Mistrík, I.; Paľove-Balang, P.; Tamás, L. Peroxidase Activity against Guaiacol, NADH, Chlorogenic Acid, Ferulic Acid and Coniferyl Alcohol in Root Tips of Lotus Japonicus and L. Corniculatus Grown under Low PH and Aluminium Stress. Biologia 2010, 65, 279–283. [Google Scholar] [CrossRef]
- Quiroga, M.; Guerrero, C.; Botella, M.A.; Barceló, A.; Amaya, I.; Medina, M.I.; Alonso, F.J.; de Forchetti, S.M.; Tigier, H.; Valpuesta, V. A Tomato Peroxidase Involved in the Synthesis of Lignin and Suberin. Plant Physiol. 2000, 122, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Pena, M.J.; Revilla, G.; Zarra, I. Changes in Dehydrodiferulic Acids and Peroxidase Activity against Ferulic Acid Associated with Cell Walls during Growth of Pinus Pinaster Hypocotyl. Plant Physiol. 1996, 111, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Halušková, L.; Valentovičová, K.; Huttová, J.; Mistrík, I.; Tamás, L. Effect of Heavy Metals on Root Growth and Peroxidase Activity in Barley Root Tip. Acta Physiol. Plant 2010, 32, 59–65. [Google Scholar] [CrossRef]
- Awad, H.M.; Boersma, M.G.; Vervoort, J.; Rietjens, I.M.C.M. Peroxidase-Catalyzed Formation of Quercetin Quinone Methide–Glutathione Adducts. Arch. Biochem. Biophys. 2000, 378, 224–233. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Frei, M. Lignin: Characterization of a Multifaceted Crop Component. Sci. World J. 2013, 2013, 436517. [Google Scholar] [CrossRef]
- Gajewska, E.; Witusińska, A.; Bernat, P. Nickel-Induced Oxidative Stress and Phospholipid Remodeling in Cucumber Leaves. Plant Sci. 2024, 348, 112229. [Google Scholar] [CrossRef]
- Rahman, H.; Sabreen, S.; Alam, S.; Kawai, S. Effects of Nickel on Growth and Composition of Metal Micronutrients in Barley Plants Grown in Nutrient Solution. J. Plant Nutr. 2005, 28, 393–404. [Google Scholar] [CrossRef]
- Gajewska, E.; Bernat, P.; Dlugoński, J.; Sklodowska, M. Effect of Nickel on Membrane Integrity, Lipid Peroxidation and Fatty Acid Composition in Wheat Seedlings. J. Agron. Crop Sci. 2012, 198, 286–294. [Google Scholar] [CrossRef]
- Helaoui, S.; Boughattas, I.; Hattab, S.; Mkhinini, M.; Alphonse, V.; Livet, A.; Bousserrhine, N.; Banni, M. Physiological, Biochemical and Transcriptomic Responses of Medicago sativa to Nickel Exposure. Chemosphere 2020, 249, 126121. [Google Scholar] [CrossRef]
- Parlak, K.U. Effect of Nickel on Growth and Biochemical Characteristics of Wheat (Triticum aestivum L.) Seedlings. NJAS-Wagening. J. Life Sci. 2016, 76, 1–5. [Google Scholar] [CrossRef]
- Gopal, R. Excess Nickel Modulates Oxidative Stress Responsive Enzymes in Groundnut. J. Plant Nutr. 2014, 37, 1433–1440. [Google Scholar] [CrossRef]
- Baker, A.J.M. Accumulators and Excluders—Strategies in the Response of Plants to Heavy Metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Krämer, U.; Pickering, I.J.; Prince, R.C.; Raskin, I.; Salt, D.E. Subcellular Localization and Speciation of Nickel in Hyperaccumulator and Non-Accumulator Thlaspi Species. Plant Physiol. 2000, 122, 1343–1353. [Google Scholar] [CrossRef]
- Gajewska, E.; Skłodowska, M. Nickel-Induced Changes in Nitrogen Metabolism in Wheat Shoots. J. Plant Physiol. 2009, 166, 1034–1044. [Google Scholar] [CrossRef]
- Gajewska, E.; Niewiadomska, E.; Tokarz, K.; Słaba, M.; Skłodowska, M. Nickel-Induced Changes in Carbon Metabolism in Wheat Shoots. J. Plant Physiol. 2013, 170, 369–377. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Jagetiya, B.; Soni, A.; Yadav, S. Effect of Nickel on Plant Water Relations and Growth in Green Gram. Indian. J. Plant Physiol. 2013, 18, 372–376. [Google Scholar] [CrossRef]
- Fiala, R.; Fialová, I.; Vaculík, M.; Luxová, M. Effect of Silicon on the Young Maize Plants Exposed to Nickel Stress. Plant Physiol. Biochem. 2021, 166, 645–656. [Google Scholar] [CrossRef]
- Stobart, A.K.; Griffiths, W.T.; Ameen-Bukthari, I.; Sherwood, R.P. The Effect of Cd2+ on the Biosynthesis of Chlorophyll in Leaves of Barley. Physiol. Plant. 1985, 63, 293–298. [Google Scholar] [CrossRef]
- Somashekaraiah, B.V.; Somashekaraiah, P.; Padmaja, K.; Prasad, A.R.K. Phytotoxicity of Cadmium Ions on Germinating Seedlings of Mung Bean (Phaseolus vulgaris): Involvement of Lipid Peroxides in Chlorophyll Degradation. Physiol. Plant. 1992, 85, 85–89. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agrawal, M. Biological Effects of Heavy Metals: An Overview. J. Environ. Biol. 2005, 26, 301–313. [Google Scholar]
- Valivand, M.; Amooaghaie, R. Foliar Spray with Sodium Hydrosulfide and Calcium Chloride Advances Dynamic of Critical Elements and Efficiency of Nitrogen Metabolism in Cucurbita pepo L. under Nickel Stress. Sci. Hortic. 2021, 283, 110052. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Multi-Colour Fluorescence Imaging of Photosynthetic Activity and Plant Stress. Photosynthetica 2021, 59, 364–380. [Google Scholar] [CrossRef]
- Varela Hernandez, H.P. Effects of Heavy Metals Ions on Primary Photosynthetic Processes in Antarctic Filamentous Alga zygnema sp. Czech Polar Rep. 2016, 6, 180–185. [Google Scholar] [CrossRef]
- Gajewska, E.; Drobik, D.; Wielanek, M.; Sekulska-Nalewajko, J.; Gocławski, J.; Mazur, J.; Skłodowska, M. Alleviation of Nickel Toxicity in Wheat (Triticum aestivum L.) Seedlings by Selenium Supplementation. Biol. Lett. 2013, 50, 63–76. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin Alleviates Nickel Phytotoxicity by Improving Photosynthesis, Secondary Metabolism and Oxidative Stress Tolerance in Tomato Seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593. [Google Scholar] [CrossRef]
- Li, Y.; Shi, S.; Zhang, Y.; Zhang, A.; Wang, Z.; Yang, Y. Copper Stress-Induced Phytotoxicity Associated with Photosynthetic Characteristics and Lignin Metabolism in Wheat Seedlings. Ecotoxicol. Environ. Saf. 2023, 254, 114739. [Google Scholar] [CrossRef]
- Huihui, Z.; Xin, L.; Zisong, X.; Yue, W.; Zhiyuan, T.; Meijun, A.; Yuehui, Z.; Wenxu, Z.; Nan, X.; Guangyu, S. Toxic Effects of Heavy Metals Pb and Cd on Mulberry (Morus alba L.) Seedling Leaves: Photosynthetic Function and Reactive Oxygen Species (ROS) Metabolism Responses. Ecotoxicol. Environ. Saf. 2020, 195, 110469. [Google Scholar] [CrossRef]
- Pluciński, B.; Nowicka, B.; Waloszek, A.; Rutkowska, J.; Strzałka, K. The Role of Antioxidant Response and Nonphotochemical Quenching of Chlorophyll Fluorescence in Long-Term Adaptation to Cu-Induced Stress in Chlamydomonas Reinhardtii. Environ. Sci. Pollut. Res. 2023, 30, 67250–67262. [Google Scholar] [CrossRef]
- Chen, S.; Lin, R.; Lu, H.; Wang, Q.; Yang, J.; Liu, J.; Yan, C. Effects of Phenolic Acids on Free Radical Scavenging and Heavy Metal Bioavailability in Kandelia Obovata under Cadmium and Zinc Stress. Chemosphere 2020, 249, 126341. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Klejdus, B.; Hedbavny, J.; Zoń, J. Significance of Phenols in Cadmium and Nickel Uptake. J. Plant Physiol. 2011, 168, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Taulavuori, K.; Hyöky, V.; Oksanen, J.; Taulavuori, E.; Julkunen-Tiitto, R. Species-Specific Differences in Synthesis of Flavonoids and Phenolic Acids under Increasing Periods of Enhanced Blue Light. Environ. Exp. Bot. 2016, 121, 145–150. [Google Scholar] [CrossRef]
- Das, S.; Sultana, K.W.; Mondal, M.; Chandra, I.; Ndhlala, A.R. Unveiling the Dual Nature of Heavy Metals: Stressors and Promoters of Phenolic Compound Biosynthesis in Basilicum polystachyon (L.) Moench In Vitro. Plants 2023, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Meychik, N.; Nikolaeva, Y.; Kushunina, M.; Yermakov, I. Are the Carboxyl Groups of Pectin Polymers the Only Metal-Binding Sites in Plant Cell Walls? Plant Soil. 2014, 381, 25–34. [Google Scholar] [CrossRef]
- Le Roy, J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation Is a Major Regulator of Phenylpropanoid Availability and Biological Activity in Plants. Front. Plant Sci. 2016, 7, 735. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, M.; Yan, H.; Li, C. Effects of Salicylic Acid on Heavy Metal Resistance in Eukaryotic Algae and Its Mechanisms. Int. J. Environ. Res. Public. Health 2022, 19, 13415. [Google Scholar] [CrossRef]
- Parry, A.D.; Tiller, S.A.; Edwards, R. The Effects of Heavy Metals and Root Immersion on Isoflavonoid Metabolism in Alfalfa (Medicago sativa L.). Plant Physiol. 1994, 106, 195–202. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Yamasaki, H.; Sakihama, Y.; Ikehara, N. Flavonoid-Peroxidase Reaction as a Detoxification Mechanism of Plant Cells against H2O2. Plant Physiol. 1997, 115, 1405–1412. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic Acid-Induced Abiotic Stress Tolerance and Underlying Mechanisms in Plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The Role of Salicylic Acid in Plants Exposed to Heavy Metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.; Mohammad, F.; Wani, S.H.; Siddique, K.M.H. Salicylic Acid Enhances Nickel Stress Tolerance by Up-Regulating Antioxidant Defense and Glyoxalase Systems in Mustard Plants. Ecotoxicol. Environ. Saf. 2019, 180, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Pawlak-Sprada, S.; Arasimowicz-Jelonek, M.; Podgórska, M.; Deckert, J. Activation of Phenylpropanoid Pathway in Legume Plants Exposed to Heavy Metals. Part I. Effects of Cadmium and Lead on Phenylalanine Ammonia-Lyase Gene Expression, Enzyme Activity and Lignin Content. Acta Biochim. Pol. 2011, 58, 211–216. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B. Dynamics of Phenolic Acids and Lignin Accumulation in Metal-Treated Matricaria Chamomilla Roots. Plant Cell Rep. 2008, 27, 605–615. [Google Scholar] [CrossRef]
- Beshamgan, E.S.; Sharifi, M.; Zarinkamar, F. Crosstalk among Polyamines, Phytohormones, Hydrogen Peroxide, and Phenylethanoid Glycosides Responses in Scrophularia Striata to Cd Stress. Plant Physiol. Biochem. 2019, 143, 129–141. [Google Scholar] [CrossRef]
- Kapoor, D.; Kaur, S.; Bhardwaj, R. Physiological and Biochemical Changes in Brassica Juncea Plants under Cd-Induced Stress. Biomed. Res. Int. 2014, 2014, 726070. [Google Scholar] [CrossRef]
- Gajewska, E.; Skłodowska, M. Differential Effect of Equal Copper, Cadmium and Nickel Concentration on Biochemical Reactions in Wheat Seedlings. Ecotoxicol. Environ. Saf. 2010, 73, 996–1003. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Kao, C.-H. Nickel Toxicity of Rice Seedlings: Cell Wall Peroxidase, Lignin, and NiSO4-Inhibited Root Growth. Crop Environ. Bioinform. 2005, 2, 131–136. [Google Scholar]
- Abdel-Aty, A.M.; Elsayed, A.M.; Gad, A.A.M.; Barakat, A.Z.; Mohamed, S.A. Antioxidant System of Garden Cress Sprouts for Using in Bio-Monitor of Cadmium and Lead Contamination. Sci. Rep. 2023, 13, 10445. [Google Scholar] [CrossRef]
- Ma, Q.-H. Lignin Biosynthesis and Its Diversified Roles in Disease Resistance. Genes 2024, 15, 295. [Google Scholar] [CrossRef] [PubMed]
- Finger-Teixeira, A.; Ferrarese, M.D.L.L.; Soares, A.R.; da Silva, D.; Ferrarese-Filho, O. Cadmium-Induced Lignification Restricts Soybean Root Growth. Ecotoxicol. Environ. Saf. 2010, 73, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Smart, R.E.; Bingham, G.E. Rapid Estimates of Relative Water Content. Plant Physiol. 1974, 53, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Maehly, A.C.; Chance, B. The Assay of Catalases and Peroxidases. Methods Biochem. Anal. 1954, 1, 357–425. [Google Scholar] [CrossRef]
- Imberty, A.; Goldberg, R.; Catesson, A.M. Isolation and Characterization of Populus Isoperoxidases Involved in the Last Step of Lignin Formation. Planta 1985, 164, 221–226. [Google Scholar] [CrossRef]
- Takahama, U. Oxidation of Hydroxycinnamic Acid and Hydroxycinnamyl Alcohol Derivatives by Laccase and Peroxidase. Interactions among p-Hydroxyphenyl, Guaiacyl and Syringyl Groups during the Oxidation Reactions. Physiol. Plant 1995, 93, 61–68. [Google Scholar] [CrossRef]
- Takahama, U.; Egashira, T. Peroxidases in Vacuoles of Vicia Faba Leaves. Phytochemistry 1991, 30, 73–77. [Google Scholar] [CrossRef]
- Takahama, U.; Hirota, S. Deglucosidation of Quercetin Glucosides to the Aglycone and Formation of Antifungal Agents by Peroxidase-Dependent Oxidation of Quercetin on Browning of Onion Scales. Plant Cell Physiol. 2000, 41, 1021–1029. [Google Scholar] [CrossRef]
- Kolupaev, Y.E.; Horielova, E.I.; Yastreb, T.O.; Popov, Y.V.; Ryabchun, N.I. Phenylalanine Ammonia-Lyase Activity and Content of Flavonoid Compounds in Wheat Seedlings at the Action of Hypothermia and Hydrogen Sulfide Donor. Ukr. Biochem. J. 2018, 90, 12–20. [Google Scholar] [CrossRef]
- Dörnenburg, H.; Knorr, D. Evaluation of Elicitor-and High-Pressure-Induced Enzymatic Browning Utilizing Potato (Solanum tuberosum) Suspension Cultures as a Model System for Plant Tissues. J. Agric. Food Chem. 1997, 45, 4173–4177. [Google Scholar] [CrossRef]
- Pradhan Mitra, P.; Loqué, D. Histochemical Staining of Arabidopsis Thaliana Secondary Cell Wall Elements. J. Vis. Exp. 2014, 51381. [Google Scholar] [CrossRef]
- Mittal, A.; Balasubramanian, R.; Cao, J.; Singh, P.; Subramanian, S.; Hicks, G.; Nothnagel, E.A.; Abidi, N.; Janda, J.; Galbraith, D.W.; et al. TOPOISOMERASE 6B Is Involved in Chromatin Remodelling Associated with Control of Carbon Partitioning into Secondary Metabolites and Cell Walls, and Epidermal Morphogenesis in Arabidopsis. J. Exp. Bot. 2014, 65, 4217–4239. [Google Scholar] [CrossRef] [PubMed]
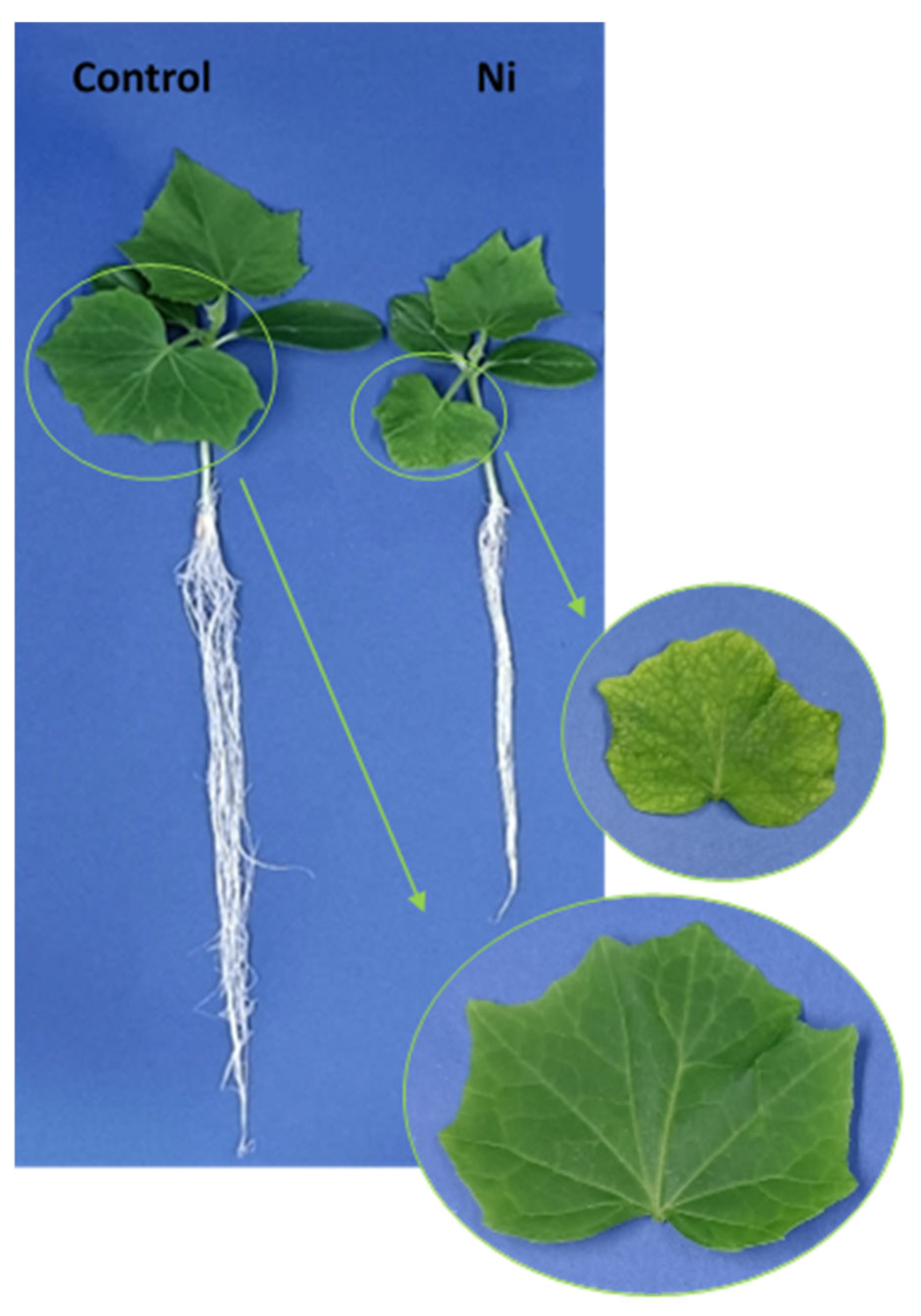
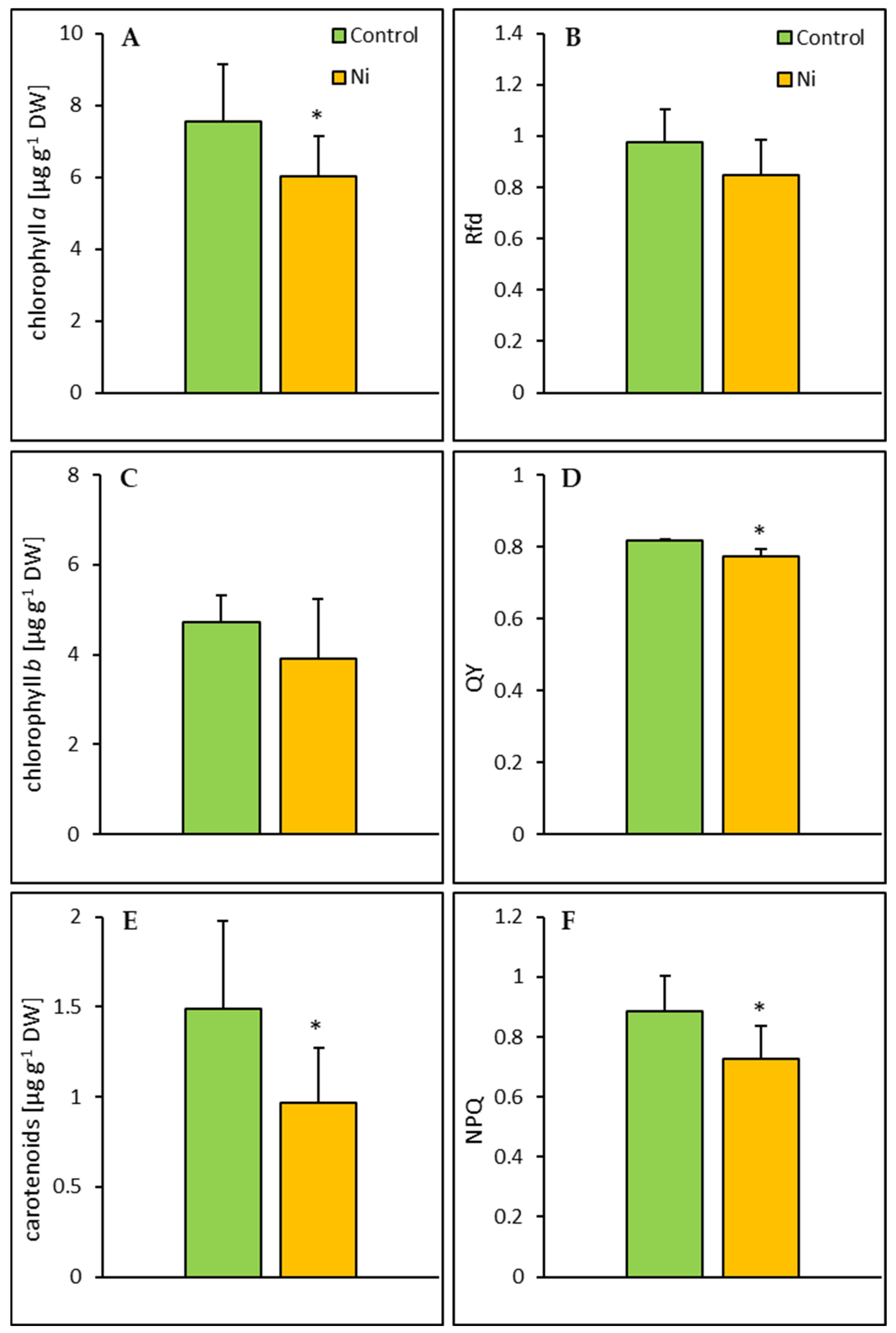
| Treatment | Fresh weight [mg] | Ni content [μg g−1 DW] | RWC [%] |
|---|---|---|---|
| Control leaf | 284.3 ± 58.9 | 1.98 ± 0.75 | 89.84 ± 0.22 |
| Ni leaf | 133.6 ± 43.3 * | 292.85 ± 65.81 * | 87.62 ± 1.33 |
| Control root | 533.47 ± 104.87 | 4.38 ± 0.42 | 95.16 ± 0.40 |
| Ni root | 221.24 ± 58.77 * | 592.89 ± 82.12 * | 94.97 ± 0.48 |
 /
/ coloration indicate increases/decreases compared to the control.
coloration indicate increases/decreases compared to the control.
 /
/ coloration indicate increases/decreases compared to the control.
coloration indicate increases/decreases compared to the control.| Metabolite [µg g−1 FW] | Free | Conjugated | Free/Total [%] | ||||
|---|---|---|---|---|---|---|---|
| Control | Ni | Control | Ni | Control | Ni | ||
| Phenolic acids | trans-3-hydroxycinnamic acid | 0 | 33.75 ± 8.72 | 0 | 4.52 ± 1.25 | - | 91.0 ± 8.65 |
| ellagic acid | 37.06 ± 2.97 | 36.34 ± 4.8 | 64.49 ± 5.57 | 30.75 ± 2.27 * | 36.5 ± 1.44 | 54.2 ± 3.47 * | |
| 4-hydroxybenzoic acid | 13.91 ± 1.41 | 12.52 ± 1.07 | 73.63 ± 6.72 | 25.15 ± 2.76 * | 15.9 ± 0.55 | 33.2 ± 0.97 * | |
| ferulic acid | 4.81 ± 0.41 | 4.82 ± 0.67 | 6.43 ± 0.47 | 5.81 ± 1.06 | 42.8 ± 0.62 | 45.3 ± 4.82 | |
| chlorogenic acid | 16.64 ± 2.2 | 13.88 ± 1.74 | 3.43 ± 0.89 | 2.96 ± 0.90 | 82.9 ± 2.69 | 82.4 ± 3.55 | |
| trans-cinnamic acid | 4.64 ± 0.49 | 4.67 ± 0.47 | 1.04 ± 0.53 | 0.69 ± 0.28 | 81.7 ± 9.36 | 89.8 ± 4.23 * | |
| p-coumaric acid | 18.62 ± 2.42 | 24.6 ± 2.65 * | 9.32 ± 1.67 | 16.67 ± 3.42 * | 66.6 ± 1.86 | 59.6 ± 7.91 | |
| 1,3-dicaffeoylquinic acid | 6.68 ± 1.15 | 5.42 ± 0.65 | 1.25 ± 0.32 | 0.97 ± 0.30 | 87.2 ± 2.99 | 84.8 ± 3.55 | |
| gallic acid | 0 | 0.27 ± 0.05 | 0 | 1.43 ± 0.31 | - | 15.9 ± 1.48 | |
| salicylic acid | 0.15 ± 0.02 | 0.29 ± 0.09 | 0.44 ± 0.05 | 0.57 ± 0.08 | 25.5 ± 3.16 | 33.3 ± 7.52 | |
| Total | 102.51 | 136.56 (133%) | 160.02 | 89.5 (56%) | 39.0 | 60.8 (156%) | |
| Flavonoids | naringenin | 0.89 ± 0.22 | 0.17 ± 0.04 * | 0.12 ± 0.06 | 0.03 ± 0.008 * | 88.1 ± 5.47 | 85.0 ± 7.14 |
| 3-hydroxyflavone | 56.26 ± 4.58 | 56.28 ± 4.35 | 60.79 ± 4.35 | 62.59 ± 8.32 * | 48.1 ± 0.70 | 47.3 ± 2.24 | |
| (+)-catechin | 59.93 ± 5.76 | 33.41 ± 2.65 * | 102.59 ± 4.85 | 70.45 ± 2.89 * | 36.9 ± 1.21 | 32.2 ± 1.05 | |
| (−)-epicatechin | 21.39 ± 3.08 | 36.92 ± 4.66 * | 3.48 ± 1.44 | 78.42 ± 5.99 * | 96.5 ± 4.82 | 32.0 ± 1.45 * | |
| cyanidin | 22.54 ± 4.66 | 9.75 ± 1.37 * | 0.65 ± 0.16 | 1.88 ± 0.56 * | 83.8 ± 5.02 | 86.7 ± 6.93 | |
| quercetin | 0 | 0 | 15.19 ± 1.99 | 13.44 ± 1.91 | - | - | |
| luteolin | 0 | 0 | 33.37 ± 1.63 | 31.65 ± 3.34 | - | - | |
| hesperetin | 2.39 ± 0.48 | 1.93 ± 0.36 | 15.23 ± 1.41 | 19.56 ± 1.17 | 13.6 ± 2.11 | 9.0 ± 1.21 * | |
| flavone | 6.99 ± 0.94 | 19.17 ± 1.71 * | 9.00 ± 1.02 | 23.02 ± 1.65 * | 43.7 ± 3.33 | 45.4 ± 1.26 | |
| procyanidin B2 | 19.35 ± 3.65 | 43.05 ± 2.35 * | 36.48 ± 3.00 | 0.77 ± 0.93 * | 34.7 ± 4.31 | 93.3 ± 3.59 * | |
| Total | 189.74 | 200.68 (106%) | 276.91 | 301.81 (109%) | 40.6 | 39.8 (98%) | |
 /
/ coloration indicate increases/decreases compared to the control.
coloration indicate increases/decreases compared to the control.
 /
/ coloration indicate increases/decreases compared to the control.
coloration indicate increases/decreases compared to the control.| Metabolite [µg g−1 FW] | Free | Conjugated | Free/Total [%] | ||||
|---|---|---|---|---|---|---|---|
| Control | Ni | Control | Ni | Control | Ni | ||
| Phenolic acids | gallic acid | 59.9 ± 3.84 | 6.36 ± 1.17 * | 1.05 ± 0.55 | 3.47 ± 0.31 * | 99.1 ± 7.32 | 64.7 ± 5.76 * |
| protocatechuic acid | 21.92 ± 2.99 | 54.81 ± 4.99 * | 4.32 ± 1.59 | 1.60 ± 0.48 * | 83.5 ± 6.55 | 97.0 ± 0.69 * | |
| α-resorcylic acid | 4.56 ± 0.47 | 8.07± 0.84 * | 0.45 ± 0.08 | 0.61 ± 0.51 | 91.0 ± 1.62 | 93.0 ± 0.51 | |
| trans-3-hydroxycinnamic acid | 51.02 ± 2.6 | 22.73 ± 0.33 * | 23.40 ± 2.42 | 28.19 ± 1.54 * | 68.6 ± 1.78 | 44.6 ± 1.68 * | |
| sinapic acid | 147.37 ± 2.98 | 189.9 ± 4.36 * | 80.49 ± 5.23 | 48.03 ± 4.87 * | 64.7 ± 1.52 | 79.8 ± 1.31 * | |
| ellagic acid | 15.67 ± 0.86 | 21.18 ± 2.11 * | 124.15 ± 5.36 | 60.88 ± 2.07 * | 11.2 ± 0.99 | 25.8 ± 1.78 * | |
| rosmarinic acid | 24.91 ± 1.93 | 24.84 ± 1.13 | 25.89 ± 3.18 | 18.52 ± 3.85 | 49.0 ± 4.02 | 57.3 ± 5.77 | |
| 4-hydroxybenzoic acid | 5.46 ± 1.11 | 6.7 ± 1.06 | 21.45 ± 0.8 | 42.21 ± 2.73 * | 20.3 ± 2.88 | 13.7 ± 1.12 * | |
| ferulic acid | 1.15 ± 0.17 | 11.52 ± 0.54 * | 31.51 ± 0.91 | 27.47 ± 0.56 * | 3.5 ± 0.41 | 29.5 ± 0.64 * | |
| β-resorcylic acid | 0.1 ± 0.01 | 0.07 ± 0.004 * | 0.81 ± 0.01 | 0.43 ± 0.04 * | 11.0 ± 1.09 | 13.7 ± 1.68 | |
| chlorogenic acid | 0.18 ± 0.03 | 0.75 ± 0.04 * | 0.01 ± 0.001 | 0.08 ± 0.05 * | 94.7 ± 2.54 | 91.5 ± 2.28 | |
| vanillic acid | 0.44 ± 0.01 | 0.43 ± 0.12 | 0.09 ± 0.08 | 0.07 ± 0.05 | 83.0 ± 13.13 | 86.0 ± 12.09 | |
| trans-cinnamic acid | 0.32 ± 0.02 | 0.88 ± 0.06 * | 1.51 ± 0.03 | 1.00 ± 0.06 | 17.5 ± 0.92 | 46.8 ± 1.46 * | |
| p-coumaric acid | 0.27 ± 0.05 | 2.14 ± 0.17 * | 0.48 ± 0.05 | 0.62 ± 0.12 | 36.0 ± 5.70 | 77.5 ± 4.73 * | |
| o-coumaric acid | 0.5 ± 0.02 | 1.29 ± 0.11 * | 0.06 ± 0.01 | 1.65 ± 0.28 * | 89.3 ± 1.88 | 43.9 ± 3.75 * | |
| salicylic acid | 0.22 ± 0.08 | 0.16 ± 0.05 | 0.50 ± 0.12 | 0.25 ± 0.02 | 30.8 ± 9.56 | 39.0 ± 6.84 | |
| Total | 333.99 | 351.83 (105%) | 316.17 | 235.08 (74%) | 51.4 | 59.9 (117%) | |
| Flavonoids | naringenin | 2.86 ± 0.08 | 1.43 ± 0.11 * | 2.12 ± 0.18 | 1.71 ± 0.16 * | 57.4 ± 2.66 | 45.5 ± 0.44 * |
| 3-hydroxyflavone | 25.26 ± 2.25 | 22.95 ± 2.22 | 32.90 ± 2.59 | 30.58 ± 1.82 | 43.4 ± 1.93 | 42.9 ± 2.10 | |
| (+)-catechin | 40.21 ± 3.07 | 45.65 ± 4.18 | 98.03 ± 5.81 | 48.36 ± 6.14 * | 29.1 ± 2.72 | 48.6 ± 4.84 * | |
| (−)-epicatechin | 24.74 ± 1.59 | 20.06 ± 2.71 | 20.70 ± 1.17 | 55.26 ± 5.84 * | 54.4 ± 2.0 | 26.6 ± 3.73 * | |
| (−)-epigallocatechin | 53.08 ± 3.57 | 15.75 ± 1.6 * | 0.70 ± 0.28 | 49.45 ± 3.5 * | 98.7 ± 0.53 | 24.2 ± 2.65 * | |
| cyanidin | 5.75 ± 0.38 | 12.36 ± 1.89 * | 25.52 ± 0.61 | 21.06 ± 3.03 | 18.4 ± 1.28 | 37.1 ± 3.33 * | |
| quercetin | 0 | 0 | 1.94 ± 0.1 | 5.24 ± 0.15 * | - | - | |
| myricetin | 21.48 ± 2.39 | 17.03 ± 2.32 | 4.32 ± 0.39 | 2.49 ± 0.33 * | 83.2 ± 9.58 | 87.3 ± 7.91 | |
| luteolin | 1.59 ± 0.31 | 0 | 9.22 ± 0.86 | 6.38 ± 0.93 | 14.7 ± 2.49 | - | |
| Total | 174.97 | 135.23 (77%) | 195.45 | 220.53 (113%) | 47.2 | 38.0 (81%) | |
| Other | p-benzoquinone | 4.8 ± 0.24 | 4.91 ± 0.68 | 22.99 ± 3.9 | 9.04 ± 0.89 * | 17.3 ± 1.94 | 35.2 ± 3.88 * |
| pyrocatechol | 4.39 ± 0.55 | 9.25 ± 0.72 * | 20.96 ± 2.5 | 10.28 ± 2.33 * | 17.3 ± 2.38 | 47.3 ± 6.65 * | |
| Total | 9.19 | 14.16 (154%) | 43.95 | 19.32 (44%) | 17.3 | 42.3 (244%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajewska, E.; Witusińska, A.; Kornaś, A.; Wielanek, M. Phenolics Profile and Phenol-Related Enzyme Activities in Cucumber Plants Under Ni Stress. Int. J. Mol. Sci. 2025, 26, 1237. https://doi.org/10.3390/ijms26031237
Gajewska E, Witusińska A, Kornaś A, Wielanek M. Phenolics Profile and Phenol-Related Enzyme Activities in Cucumber Plants Under Ni Stress. International Journal of Molecular Sciences. 2025; 26(3):1237. https://doi.org/10.3390/ijms26031237
Chicago/Turabian StyleGajewska, Ewa, Aleksandra Witusińska, Andrzej Kornaś, and Marzena Wielanek. 2025. "Phenolics Profile and Phenol-Related Enzyme Activities in Cucumber Plants Under Ni Stress" International Journal of Molecular Sciences 26, no. 3: 1237. https://doi.org/10.3390/ijms26031237
APA StyleGajewska, E., Witusińska, A., Kornaś, A., & Wielanek, M. (2025). Phenolics Profile and Phenol-Related Enzyme Activities in Cucumber Plants Under Ni Stress. International Journal of Molecular Sciences, 26(3), 1237. https://doi.org/10.3390/ijms26031237





