The Expanding Role of Aquaporin-1, Aquaporin-3 and Aquaporin-5 as Transceptors: Involvement in Cancer Development and Potential Druggability
Abstract
1. Introduction
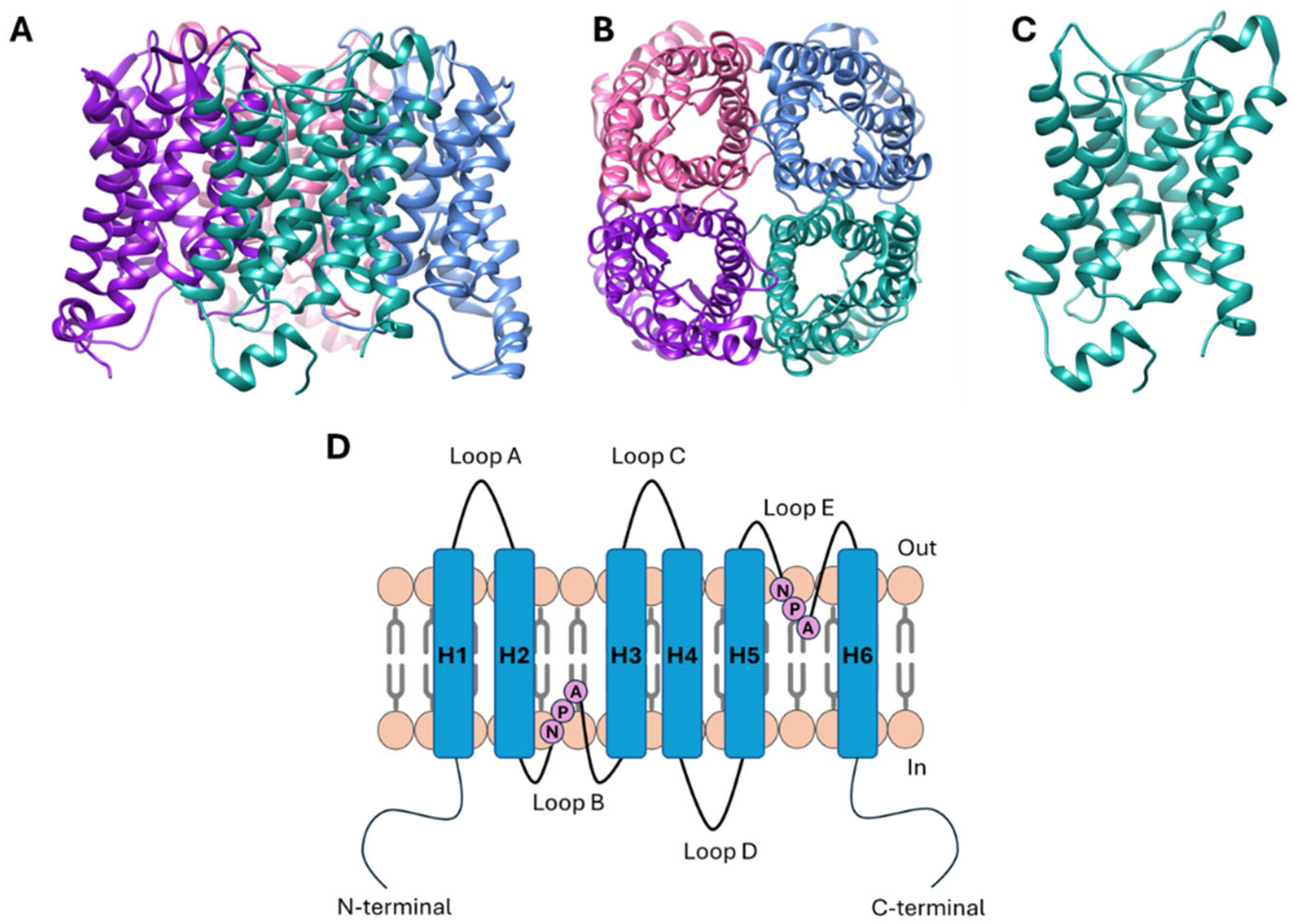
2. Physiological Roles of AQPs and Their Implication in Cancer
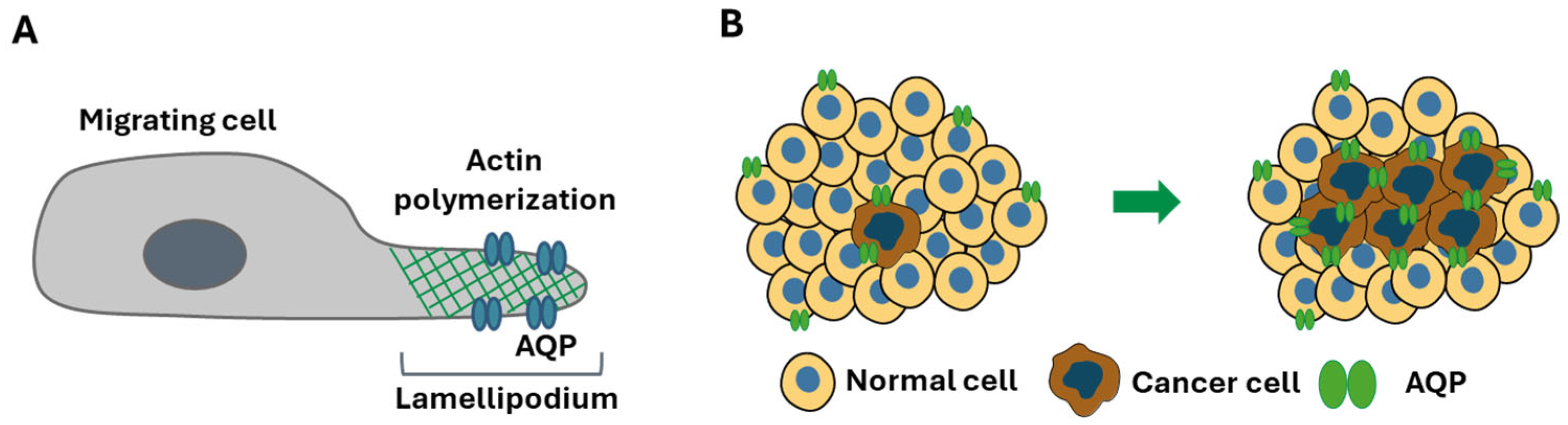
2.1. AQP1, AQP3 and AQP5: Key Players in Cancer Progression
2.2. Peroxiporin Activity of AQP1, AQP3 and AQP5
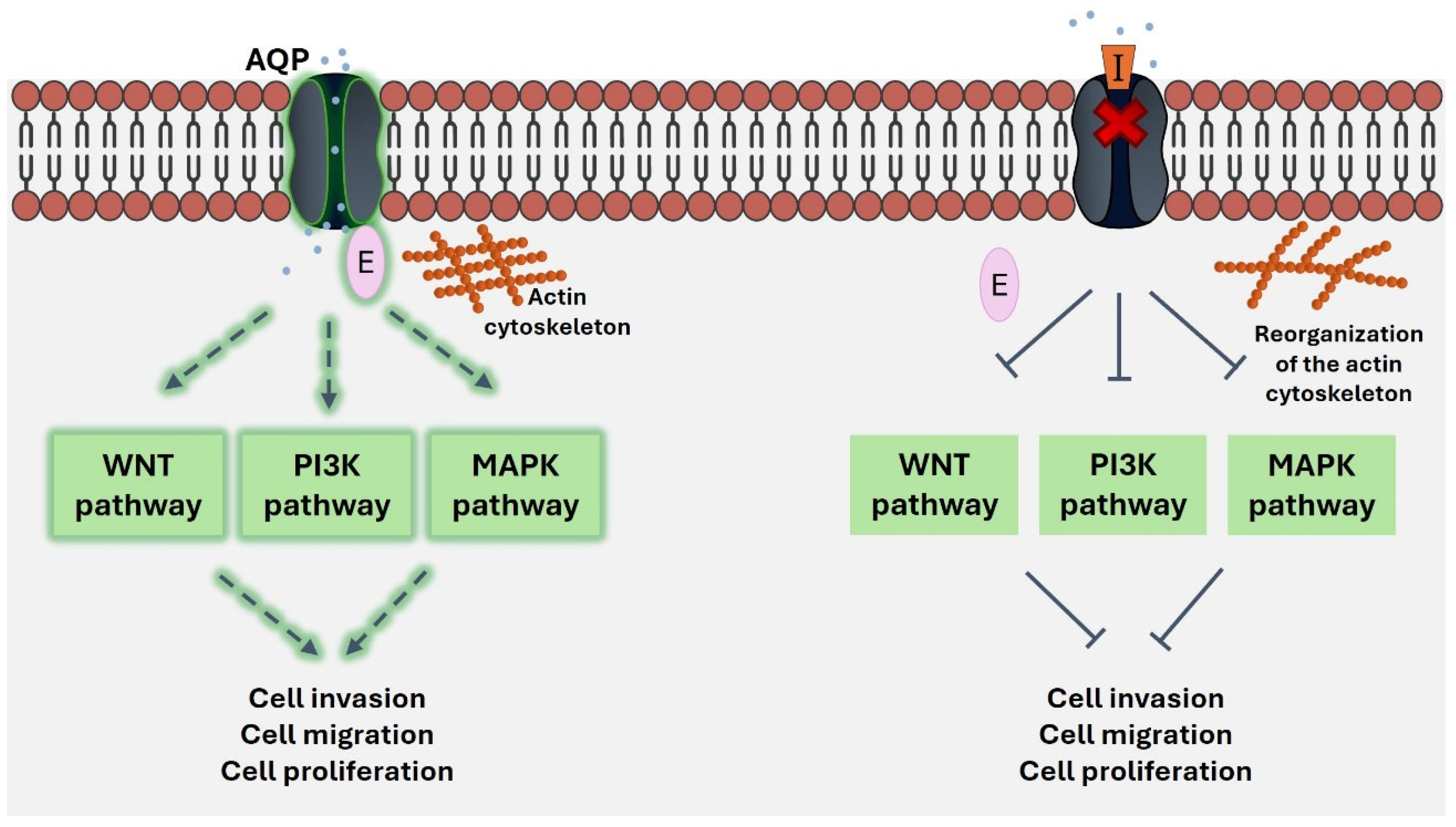
3. Role of AQP1, AQP3 and AQP5 as Transceptors in Cancer
4. AQP1, AQP3 and AQP5 as Druggable Targets for Pharmacological Modulators
| AQP | Modulator | Effect on AQP | Targeted Disease | References |
|---|---|---|---|---|
| AQP1 | HgCl2 | Inhibition | - | [116] |
| Silver nitrate | Inhibition | - | [122] | |
| Silver sulfadiazine | Inhibition | - | [122] | |
| AqB011 | Inhibition | Colon cancer | [139] | |
| Bacopaside II | Inhibition | Cardiac hypertrophy | [152] | |
| Estrogen | Upregulation | - | [164,165,166] | |
| Progesterone | Upregulation | - | [164,165,166] | |
| Arachidonic acid | Upregulation | - | [164,165,166] | |
| Gonadotropins | Upregulation | - | [169] | |
| Prolactin | Upregulation | - | [169] | |
| Growth hormone | Upregulation | - | [169] | |
| Testosterone | Upregulation | - | [171] | |
| miR-29a | Downregulation | IBS-D | [189] | |
| miR-126-5p | Downregulation | Acute lung injury | [192] | |
| miR-133a-3p | Downregulation | Sepsis | [191] | |
| miR-144-3p | Downregulation | Acute lung injury | [195] | |
| miR-320 | Downregulation | Breast cancer | [176,177] | |
| miR-495 | Downregulation | - | [193] | |
| miR-874 | Downregulation | Sepsis | [179] | |
| AQP3 | HgCl2 | Inhibition | Prostate cancer | [117,118,119] |
| Auphen | Inhibition | Epidermoid carcinoma; hepatocellular carcinoma; inflammation; melanoma; triple-negative breast cancer | [57,89,123,124,125,128,130] | |
| Au(III) CNHN | Inhibition | Melanoma | [57] | |
| Au(III) CCON | Inhibition | Melanoma | [57] | |
| ST004 | Inhibition | Melanoma | [131] | |
| Cuphen | Inhibition | Melanoma; colon cancer | [132,133,134] | |
| P2W18 | Inhibition | Melanoma | [135] | |
| Ciglitazone | Upregulation | - | [143] | |
| SAHA | Upregulation | - | [144] | |
| MMF | Upregulation | Psoriasis | [145] | |
| Bisacodyl | Downregulation | Constipation | [147] | |
| DFP00173 | Inhibition | Multiple myeloma | [148,149] | |
| atRA | Upregulation | Skin photoaging | [153] | |
| Chrysin | Upregulation | Skin photoaging | [154] | |
| Glycolic acid | Upregulation | Skin photoaging | [155] | |
| Resveratrol | Downregulation | - | [156] | |
| 18β-glycyrrhetinic acid | Upregulation | - | [157] | |
| Curcumin | Downregulation | Ovarian cancer | [158] | |
| Daiokanzoto | Downregulation | Constipation | [159] | |
| RFP | Downregulation | Constipation | [160] | |
| Naringenin | Upregulation | Constipation | [161] | |
| β-patchoulene | Downregulation | Intestinal mucositis | [162] | |
| Rottlerin | Inhibition | - | [163] | |
| Estrogen | Upregulation | ER-positive breast cancer; ovarian cancer | [97,167] | |
| Testosterone | Upregulation | - | [170] | |
| Uroguanylin | Upregulation | - | [172] | |
| Leptin | Downregulation | Obesity; NAFLD | [173] | |
| Erythropoietin | Upregulation | Acute renal failure | [174] | |
| Dexamethasone | Upregulation | - | [175] | |
| Ambroxol | Upregulation | - | [175] | |
| miR-29a | Downregulation | IBS-D | [189] | |
| miR-124 | Downregulation | Hepatocellular carcinoma | [60] | |
| miR-185 | Downregulation | Squamous cell carcinoma | [184] | |
| miR-874 | Downregulation | Gastric cancer; pancreatic ductal adenocarcinoma; NSCLC; intestinal barrier dysfunction | [28,180,181,182,183] | |
| miR-877 | Downregulation | Gastric cancer | [186] | |
| Anti-AQP3 monoclonal antibody | Inhibition | Liver injury; colorectal cancer; multiple myeloma | [88,149,194] | |
| AQP5 | HgCl2 | Inhibition | Pancreatic ductal adenocarcinoma | [78] |
| SAHA | Upregulation | - | [146] | |
| Niclosamide | Upregulation | - | [150] | |
| Methazolamide | Downregulation | Sepsis | [151] | |
| Estrogen | Upregulation | - | [164,165,166] | |
| Progesterone | Upregulation | - | [164,165,166] | |
| Arachidonic acid | Upregulation | - | [164,165,166] | |
| Testosterone | Upregulation | - | [171] | |
| Dexamethasone | Upregulation | - | [175] | |
| Ambroxol | Upregulation | - | [175] | |
| miR-19a-3p | Downregulation | Breast cancer | [178] | |
| miR-19b-3p | Downregulation | Breast cancer | [178] | |
| miR-21 | Downregulation | Gallbladder carcinoma | [188] | |
| miR-96 | Downregulation | Disseminated intravascular coagulation | [190] | |
| miR-185 | Downregulation | Colorectal cancer | [185] | |
| miR-330 | Downregulation | Disseminated intravascular coagulation | [190] | |
| miR-1226-3p | Downregulation | Breast cancer | [178] | |
| miR-1271-5p | Downregulation | Hepatitis B-virus-mediated liver cancer | [187] |
5. Final Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Zardoya, R. Phylogeny and evolution of the major intrinsic protein family. Biol. Cell 2005, 97, 397–414. [Google Scholar] [CrossRef]
- Soveral, G.; Nielsen, S.; Casini, A. Aquaporins in Health and Disease; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Ishibashi, K.; Tanaka, Y.; Morishita, Y. The role of mammalian superaquaporins inside the cell. Biochim. Biophys. Acta 2014, 1840, 1507–1512. [Google Scholar] [CrossRef]
- Ishibashi, K.; Tanaka, Y.; Morishita, Y. The role of mammalian superaquaporins inside the cell: An update. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183617. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Fernandez-Veledo, S.; Camps, M.; Zorzano, A.; Moura, T.F.; Ceperuelo-Mallafre, V.; Vendrell, J.; Soveral, G. Human aquaporin-11 is a water and glycerol channel and localizes in the vicinity of lipid droplets in human adipocytes. Obesity 2014, 22, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Jahn, T.P.; Moller, A.L.; Zeuthen, T.; Holm, L.M.; Klaerke, D.A.; Mohsin, B.; Kuhlbrandt, W.; Schjoerring, J.K. Aquaporin homologues in plants and mammals transport ammonia. FEBS Lett. 2004, 574, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.R.; Musa-Aziz, R.; Qin, X.; Boron, W.F. Relative CO2/NH3 selectivities of mammalian aquaporins 0–9. Am. J. Physiol. Cell Physiol. 2013, 304, C985–C994. [Google Scholar] [CrossRef]
- Zwiazek, J.J.; Xu, H.; Tan, X.; Navarro-Rodenas, A.; Morte, A. Significance of oxygen transport through aquaporins. Sci. Rep. 2017, 7, 40411. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Hong, N.J.; Garvin, J.L. Aquaporin-1 transports NO across cell membranes. Hypertension 2006, 48, 157–164. [Google Scholar] [CrossRef]
- Prata, C.; Hrelia, S.; Fiorentini, D. Peroxiporins in Cancer. Int. J. Mol. Sci. 2019, 20, 1371. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.V.; Mlinaric, M.; Lourenco, A.R.; Perez-Garcia, O.; Cipak Gasparovic, A.; Soveral, G. Peroxiporins and Oxidative Stress: Promising Targets to Tackle Inflammation and Cancer. Int. J. Mol. Sci. 2024, 25, 8381. [Google Scholar] [CrossRef]
- King, L.S.; Kozono, D.; Agre, P. From structure to disease: The evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 2004, 5, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Mitra, A.K. Structure and function of aquaporin water channels. Am. J. Physiol. Ren. Physiol. 2000, 278, F13–F28. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Mitsuoka, K.; Hirai, T.; Walz, T.; Agre, P.; Heymann, J.B.; Engel, A.; Fujiyoshi, Y. Structural determinants of water permeation through aquaporin-1. Nature 2000, 407, 599–605. [Google Scholar] [CrossRef] [PubMed]
- de Groot, B.L.; Frigato, T.; Helms, V.; Grubmuller, H. The mechanism of proton exclusion in the aquaporin-1 water channel. J. Mol. Biol. 2003, 333, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Steinbronn, C.; Alsterfjord, M.; Zeuthen, T.; Beitz, E. Concerted action of two cation filters in the aquaporin water channel. EMBO J. 2009, 28, 2188–2194. [Google Scholar] [CrossRef] [PubMed]
- Wree, D.; Wu, B.; Zeuthen, T.; Beitz, E. Requirement for asparagine in the aquaporin NPA sequence signature motifs for cation exclusion. FEBS J. 2011, 278, 740–748. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.V.; Rodrigues, J.S.; Rebelo, I.; Miranda, J.P.G.; Soveral, G. Revisiting the metabolic syndrome: The emerging role of aquaglyceroporins. Cell Mol. Life Sci. 2018, 75, 1973–1988. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.V.; Soveral, G. Aquaporins in Immune Cells and Inflammation: New Targets for Drug Development. Int. J. Mol. Sci. 2021, 22, 1845. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Yasui, M. Aquaporin-4 in Neuromyelitis Optica Spectrum Disorders: A Target of Autoimmunity in the Central Nervous System. Biomolecules 2022, 12, 591. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Jana, A.; Bhattacharjee, S.; Mitra, S.; De, S.; Alghamdi, B.S.; Alam, M.Z.; Mahmoud, A.B.; Al Shareef, Z.; Abdel-Rahman, W.M.; et al. The role of Aquaporins in tumorigenesis: Implications for therapeutic development. Cell Commun. Signal. 2024, 22, 106. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Saadoun, S. Key roles of aquaporins in tumor biology. Biochim. Biophys. Acta 2015, 1848, 2576–2583. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.V.; Silva, A.G.; Pimpao, C.; Soveral, G. Skin aquaporins as druggable targets: Promoting health by addressing the disease. Biochimie 2021, 188, 35–44. [Google Scholar] [CrossRef]
- Pimpao, C.; Wragg, D.; da Silva, I.V.; Casini, A.; Soveral, G. Aquaglyceroporin Modulators as Emergent Pharmacological Molecules for Human Diseases. Front. Mol. Biosci. 2022, 9, 845237. [Google Scholar] [CrossRef]
- Wei, X.; Dong, J. Aquaporin 1 promotes the proliferation and migration of lung cancer cell in vitro. Oncol. Rep. 2015, 34, 1440–1448. [Google Scholar] [CrossRef]
- Oishi, M.; Munesue, S.; Harashima, A.; Nakada, M.; Yamamoto, Y.; Hayashi, Y. Aquaporin 1 elicits cell motility and coordinates vascular bed formation by downregulating thrombospondin type-1 domain-containing 7A in glioblastoma. Cancer Med. 2020, 9, 3904–3917. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, L.; Shao, M. Aquaporin 3 facilitates tumor growth in pancreatic cancer by modulating mTOR signaling. Biochem. Biophys. Res. Commun. 2017, 486, 1097–1102. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Song, Y.; Zhang, P.; Hu, J.; Bai, C. Expression of aquaporin 5 increases proliferation and metastasis potential of lung cancer. J. Pathol. 2010, 221, 210–220. [Google Scholar] [CrossRef]
- Yoshida, T.; Hojo, S.; Sekine, S.; Sawada, S.; Okumura, T.; Nagata, T.; Shimada, Y.; Tsukada, K. Expression of aquaporin-1 is a poor prognostic factor for stage II and III colon cancer. Mol. Clin. Oncol. 2013, 1, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sun, T.; Yang, M.; Li, Z.; Li, Z.; Gao, Y. Prognostic value of combined aquaporin 3 and aquaporin 5 overexpression in hepatocellular carcinoma. Biomed. Res. Int. 2013, 2013, 206525. [Google Scholar] [CrossRef] [PubMed]
- Direito, I.; Paulino, J.; Vigia, E.; Brito, M.A.; Soveral, G. Differential expression of aquaporin-3 and aquaporin-5 in pancreatic ductal adenocarcinoma. J. Surg. Oncol. 2017, 115, 980–996. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Ma, Y.; Li, W.; Liu, X.; Ying, G.; Fu, L.; Gu, F. Role of aquaporin-4 in the regulation of migration and invasion of human glioma cells. Int. J. Oncol. 2011, 38, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Shi, L.; Su, Y. Aquaporin-4 as a New Potential Molecular Biomarker for Prognosis of Low-Grade Glioma: Comprehensive Analysis Based on Online Platforms. World Neurosurg. 2023, 175, e713–e722. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Peng, L.; Xiao, Y.; Zhou, Q.; Wang, Z.; Tang, L.; Xiao, H.; Yang, K.; Liu, H.; Li, L. Single-cell RNA sequencing reveals changes in glioma-associated macrophage polarization and cellular states of malignant gliomas with high AQP4 expression. Cancer Gene Ther. 2023, 30, 716–726. [Google Scholar] [CrossRef]
- Jing, J.; Sun, J.; Wu, Y.; Zhang, N.; Liu, C.; Chen, S.; Li, W.; Hong, C.; Xu, B.; Chen, M. AQP9 Is a Prognostic Factor for Kidney Cancer and a Promising Indicator for M2 TAM Polarization and CD8+ T-Cell Recruitment. Front. Oncol. 2021, 11, 770565. [Google Scholar] [CrossRef]
- Gao, C.; Shen, J.; Yao, L.; Xia, Z.; Liang, X.; Zhu, R.; Chen, Z. Low expression of AQP9 and its value in hepatocellular carcinoma. Transl. Cancer Res. 2021, 10, 1826–1841. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yasui, M.; Hara-Chikuma, M. AQP9 transports lactate in tumor-associated macrophages to stimulate an M2-like polarization that promotes colon cancer progression. Biochem. Biophys. Rep. 2022, 31, 101317. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.B.; Zhang, R.J.; Tan, Y.J.; Ding, G.L.; Shi, S.; Zhang, D.; He, R.H.; Liu, A.X.; Wang, T.T.; Leung, P.C.; et al. Identification of estrogen response element in the aquaporin-2 gene that mediates estrogen-induced cell migration and invasion in human endometrial carcinoma. J. Clin. Endocrinol. Metab. 2011, 96, E1399–E1408. [Google Scholar] [CrossRef] [PubMed]
- Pellavio, G.; Martinotti, S.; Patrone, M.; Ranzato, E.; Laforenza, U. Aquaporin-6 May Increase the Resistance to Oxidative Stress of Malignant Pleural Mesothelioma Cells. Cells 2022, 11, 1892. [Google Scholar] [CrossRef]
- Dai, C.; Charlestin, V.; Wang, M.; Walker, Z.T.; Miranda-Vergara, M.C.; Facchine, B.A.; Wu, J.; Kaliney, W.J.; Dovichi, N.J.; Li, J.; et al. Aquaporin-7 Regulates the Response to Cellular Stress in Breast Cancer. Cancer Res. 2020, 80, 4071–4086. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, Z.F.; Wang, K.J.; Feng, X.Y.; Lv, Z.J.; Li, Y.; Jian, Z.X. AQP8 inhibits colorectal cancer growth and metastasis by down-regulating PI3K/AKT signaling and PCDH7 expression. Am. J. Cancer Res. 2018, 8, 266–279. [Google Scholar]
- Hao, Z.; Huajun, S.; Zhen, G.; Yu, X.; Qian, L.; Ziling, C.; Zihao, S.; Qingqian, X.; Shujuan, Z. AQP8 promotes glioma proliferation and growth, possibly through the ROS/PTEN/AKT signaling pathway. BMC Cancer 2023, 23, 516. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Song, Y.; Pan, C.; Yu, J.; Zhang, J.; Zhu, X. Aquaporin-8 is a novel marker for progression of human cervical cancer cells. Cancer Biomark. 2021, 32, 391–400. [Google Scholar] [CrossRef]
- Saadoun, S.; Papadopoulos, M.C.; Hara-Chikuma, M.; Verkman, A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 2005, 434, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Saadoun, S.; Verkman, A.S. Aquaporins and cell migration. Pflug. Arch. 2008, 456, 693–700. [Google Scholar] [CrossRef]
- Nicchia, G.P.; Stigliano, C.; Sparaneo, A.; Rossi, A.; Frigeri, A.; Svelto, M. Inhibition of aquaporin-1 dependent angiogenesis impairs tumour growth in a mouse model of melanoma. J. Mol. Med. 2012, 91, 613–623. [Google Scholar] [CrossRef]
- Esteva-Font, C.; Jin, B.J.; Verkman, A.S. Aquaporin-1 gene deletion reduces breast tumor growth and lung metastasis in tumor-producing MMTV-PyVT mice. FASEB J. 2013, 28, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Liao, X.; Jiang, Y.; Wei, W.; Yang, H. Aquaporin 1 knockdown inhibits triple-negative breast cancer cell proliferation and invasion in vitro and in vivo. Oncol. Lett. 2021, 21, 437. [Google Scholar] [CrossRef]
- Jiang, Y. Aquaporin-1 activity of plasma membrane affects HT20 colon cancer cell migration. IUBMB Life 2009, 61, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Sun, C.C.; Zhou, C.Y.; Huang, H.F. Expression of aquaporin-1 in normal, hyperplasic, and carcinomatous endometria. Int. J. Gynaecol. Obs. 2008, 101, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.S.; Moon, D.; Kang, S.K. Aquaporins in Cancer Biology. Front. Oncol. 2022, 12, 782829. [Google Scholar] [CrossRef]
- Kaneko, K.; Yagui, K.; Tanaka, A.; Yoshihara, K.; Ishikawa, K.; Takahashi, K.; Bujo, H.; Sakurai, K.; Saito, Y. Aquaporin 1 is required for hypoxia-inducible angiogenesis in human retinal vascular endothelial cells. Microvasc. Res. 2008, 75, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Tie, L.; Lu, N.; Pan, X.Y.; Pan, Y.; An, Y.; Gao, J.W.; Lin, Y.H.; Yu, H.M.; Li, X.J. Hypoxia-induced up-regulation of aquaporin-1 protein in prostate cancer cells in a p38-dependent manner. Cell Physiol. Biochem. 2012, 29, 269–280. [Google Scholar] [CrossRef]
- Yun, S.; Sun, P.L.; Jin, Y.; Kim, H.; Park, E.; Park, S.Y.; Lee, K.; Lee, K.; Chung, J.H. Aquaporin 1 Is an Independent Marker of Poor Prognosis in Lung Adenocarcinoma. J. Pathol. Transl. Med. 2016, 50, 251–257. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Verkman, A.S. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol. Cell Biol. 2008, 28, 326–332. [Google Scholar] [CrossRef]
- da Silva, I.V.; Pimpao, C.; Paccetti-Alves, I.; Thomas, S.R.; Barateiro, A.; Casini, A.; Soveral, G. Blockage of aquaporin-3 peroxiporin activity by organogold compounds affects melanoma cell adhesion, proliferation and migration. J. Physiol. 2024, 602, 3111–3129. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Matsuzaki, T.; Nakazawa, T.; Murata, S.; Nakamura, N.; Kondo, T.; Iwashina, M.; Mochizuki, K.; Yamane, T.; Takata, K.; et al. Expression of aquaporin 3 (AQP3) in normal and neoplastic lung tissues. Hum. Pathol. 2007, 38, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, T.; Zhu, J.; Tuo, B.; Liu, X. Physiological and pathophysiological role of ion channels and transporters in the colorectum and colorectal cancer. J. Cell Mol. Med. 2020, 24, 9486–9494. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shi, Y.; Liu, M.; Sun, J. circHIPK3 regulates cell proliferation and migration by sponging miR-124 and regulating AQP3 expression in hepatocellular carcinoma. Cell Death Dis. 2018, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- De Ieso, M.L.; Yool, A.J. Mechanisms of Aquaporin-Facilitated Cancer Invasion and Metastasis. Front. Chem. 2018, 6, 135. [Google Scholar] [CrossRef]
- Li, A.; Lu, D.; Zhang, Y.; Li, J.; Fang, Y.; Li, F.; Sun, J. Critical role of aquaporin-3 in epidermal growth factor-induced migration of colorectal carcinoma cells and its clinical significance. Oncol. Rep. 2013, 29, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, T.; Zhou, Y.C.; Gao, F.; Zhang, Z.H.; Xu, H.; Wang, S.L.; Shen, L.Z. Aquaporin 3 promotes epithelial-mesenchymal transition in gastric cancer. J. Exp. Clin. Cancer Res. 2014, 33, 38. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Wen, J.; Zhao, H.; Dong, X.; Zhang, Z.; Wang, S.; Shen, L. Aquaporin 3 promotes the stem-like properties of gastric cancer cells via Wnt/GSK-3beta/beta-catenin pathway. Oncotarget 2016, 7, 16529–16541. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Z.; Xu, D.; Liu, Y.; Gao, Y. Aquaporin 3 promotes prostate cancer cell motility and invasion via extracellular signal-regulated kinase 1/2-mediated matrix metalloproteinase-3 secretion. Mol. Med. Rep. 2015, 11, 2882–2888. [Google Scholar] [CrossRef] [PubMed]
- Kusayama, M.; Wada, K.; Nagata, M.; Ishimoto, S.; Takahashi, H.; Yoneda, M.; Nakajima, A.; Okura, M.; Kogo, M.; Kamisaki, Y. Critical role of aquaporin 3 on growth of human esophageal and oral squamous cell carcinoma. Cancer Sci. 2011, 102, 1128–1136. [Google Scholar] [CrossRef]
- Direito, I.; Madeira, A.; Brito, M.A.; Soveral, G. Aquaporin-5: From structure to function and dysfunction in cancer. Cell Mol. Life Sci. 2016, 73, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Chae, Y.K.; Woo, J.; Kim, M.S.; Park, J.C.; Lee, J.; Soria, J.C.; Jang, S.J.; Sidransky, D.; Moon, C. Role of human aquaporin 5 in colorectal carcinogenesis. Am. J. Pathol. 2008, 173, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Lee, J.; Kim, M.S.; Jang, S.J.; Sidransky, D.; Moon, C. The effect of aquaporin 5 overexpression on the Ras signaling pathway. Biochem. Biophys. Res. Commun. 2008, 367, 291–298. [Google Scholar] [CrossRef]
- Chen, C.; Ma, T.; Zhang, C.; Zhang, H.; Bai, L.; Kong, L.; Luo, J. Down-regulation of aquaporin 5-mediated epithelial-mesenchymal transition and anti-metastatic effect by natural product Cairicoside E in colorectal cancer. Mol. Carcinog. 2017, 56, 2692–2705. [Google Scholar] [CrossRef]
- Wang, W.; Li, Q.; Yang, T.; Li, D.; Ding, F.; Sun, H.; Bai, G. Anti-cancer effect of Aquaporin 5 silencing in colorectal cancer cells in association with inhibition of Wnt/beta-catenin pathway. Cytotechnology 2018, 70, 615–624. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Dong, W.; Hu, J.; Ren, X. AQP5 promotes hepatocellular carcinoma metastasis via NF-kappaB-regulated epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2017, 490, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Qiu, G.; Liu, H.; Gao, F.; Liu, X.; Chen, Y.; Yang, M. Hypotonic induction of aquaporin5 expression in rat astrocytes through p38 MAPK pathway. Anat. Histol. Embryol. 2022, 51, 769–780. [Google Scholar] [CrossRef]
- Moon, C.; Soria, J.C.; Jang, S.J.; Lee, J.; Obaidul Hoque, M.; Sibony, M.; Trink, B.; Chang, Y.S.; Sidransky, D.; Mao, L. Involvement of aquaporins in colorectal carcinogenesis. Oncogene 2003, 22, 6699–6703. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiao, L.; Li, T.; Wang, H.; Wei, W.; Qian, H. Expression of AQP3 and AQP5 as a prognostic marker in triple-negative breast cancer. Oncol. Lett. 2018, 16, 2661–2667. [Google Scholar] [CrossRef]
- Edamana, S.; Login, F.H.; Riishede, A.; Dam, V.S.; Tramm, T.; Nejsum, L.N. The cell polarity protein Scribble is downregulated by the water channel aquaporin-5 in breast cancer cells. Am. J. Physiol. Cell Physiol. 2023, 324, C307–C319. [Google Scholar] [CrossRef] [PubMed]
- Edamana, S.; Login, F.H.; Riishede, A.; Dam, V.S.; Kirkegaard, T.; Nejsum, L.N. The water channels aquaporin-1 and aquaporin-3 interact with and affect the cell polarity protein Scribble in 3D in vitro models of breast cancer. Am. J. Physiol. Cell Physiol. 2024, 327, C1323–C1334. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Pimpao, C.; Mosca, A.F.; Coxixo, A.S.; Lopes, D.; da Silva, I.V.; Pedersen, P.A.; Antunes, F.; Soveral, G. Human Aquaporin-5 Facilitates Hydrogen Peroxide Permeation Affecting Adaption to Oxidative Stress and Cancer Cell Migration. Cancers 2019, 11, 932. [Google Scholar] [CrossRef]
- Silva, P.M.; da Silva, I.V.; Sarmento, M.J.; Silva, I.C.; Carvalho, F.A.; Soveral, G.; Santos, N.C. Aquaporin-3 and Aquaporin-5 Facilitate Migration and Cell-Cell Adhesion in Pancreatic Cancer by Modulating Cell Biomechanical Properties. Cells 2022, 11, 1308. [Google Scholar] [CrossRef]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Rahn, J.; Lichtenfels, R.; Wessjohann, L.A.; Seliger, B. Hydrogen peroxide—Production, fate and role in redox signaling of tumor cells. Cell Commun. Signal 2015, 13, 39. [Google Scholar] [CrossRef]
- Acharya, A.; Das, I.; Chandhok, D.; Saha, T. Redox regulation in cancer: A double-edged sword with therapeutic potential. Oxid. Med. Cell Longev. 2010, 3, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The Role of Hydrogen Peroxide in Redox-Dependent Signaling: Homeostatic and Pathological Responses in Mammalian Cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Milković, L.; Čipak Gašparović, A. AQP3 and AQP5—Potential Regulators of Redox Status in Breast Cancer. Molecules 2021, 26, 2613. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Watanabe, S.; Satooka, H. Involvement of aquaporin-3 in epidermal growth factor receptor signaling via hydrogen peroxide transport in cancer cells. Biochem. Biophys. Res. Commun. 2016, 471, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lin, B.; Wei, H.; Wang, X.; Nie, X.; Shi, Y. AQP3 Promotes the Invasion and Metastasis in Cervical Cancer by Regulating NOX4-derived H2O2 Activation of Syk/PI3K/Akt Signaling Axis. J. Cancer 2024, 15, 1124–1137. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Tanaka, M.; Verkman, A.S.; Yasui, M. Inhibition of aquaporin-3 in macrophages by a monoclonal antibody as potential therapy for liver injury. Nat. Commun. 2020, 11, 5666. [Google Scholar] [CrossRef]
- da Silva, I.V.; Cardoso, C.; Martínez-Banaclocha, H.; Casini, A.; Pelegrín, P.; Soveral, G. Aquaporin-3 is involved in NLRP3-inflammasome activation contributing to the setting of inflammatory response. Cell. Mol. Life Sci. 2020, 78, 3073–3085. [Google Scholar] [CrossRef]
- Chae, Y.K.; Woo, J.; Kim, M.J.; Kang, S.K.; Kim, M.S.; Lee, J.; Lee, S.K.; Gong, G.; Kim, Y.H.; Soria, J.C.; et al. Expression of aquaporin 5 (AQP5) promotes tumor invasion in human non small cell lung cancer. PLoS ONE 2008, 3, e2162. [Google Scholar] [CrossRef]
- Cipak Gasparovic, A.; Milkovic, L.; Rodrigues, C.; Mlinaric, M.; Soveral, G. Peroxiporins Are Induced upon Oxidative Stress Insult and Are Associated with Oxidative Stress Resistance in Colon Cancer Cell Lines. Antioxidants 2021, 10, 1856. [Google Scholar] [CrossRef]
- Rodrigues, C.; Milkovic, L.; Bujak, I.T.; Tomljanovic, M.; Soveral, G.; Cipak Gasparovic, A. Lipid Profile and Aquaporin Expression under Oxidative Stress in Breast Cancer Cells of Different Malignancies. Oxid. Med. Cell Longev. 2019, 2019, 2061830. [Google Scholar] [CrossRef]
- Brown, D. Aquaporin Function: Seek and You Shall Find! Function 2021, 2, zqaa041. [Google Scholar] [CrossRef] [PubMed]
- McLennan, R.; McKinney, M.C.; Teddy, J.M.; Morrison, J.A.; Kasemeier-Kulesa, J.C.; Ridenour, D.A.; Manthe, C.A.; Giniunaite, R.; Robinson, M.; Baker, R.E.; et al. Neural crest cells bulldoze through the microenvironment using Aquaporin 1 to stabilize filopodia. Development 2020, 147, dev185231. [Google Scholar] [CrossRef]
- Meng, F.; Rui, Y.; Xu, L.; Wan, C.; Jiang, X.; Li, G. Aqp1 enhances migration of bone marrow mesenchymal stem cells through regulation of FAK and beta-catenin. Stem Cells Dev. 2014, 23, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Monzani, E.; Bazzotti, R.; Perego, C.; La Porta, C.A. AQP1 is not only a water channel: It contributes to cell migration through Lin7/beta-catenin. PLoS ONE 2009, 4, e6167. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Zhou, J.; Shi, S.; Xu, H.Y.; Qu, F.; Zhang, D.; Chen, Y.D.; Yang, J.; Huang, H.F.; Sheng, J.Z. Identification of Estrogen Response Element in Aquaporin-3 Gene that Mediates Estrogen-induced Cell Migration and Invasion in Estrogen Receptor-positive Breast Cancer. Sci. Rep. 2015, 5, 12484. [Google Scholar] [CrossRef]
- Cui, D.; Sui, L.; Han, X.; Zhang, M.; Guo, Z.; Chen, W.; Yu, X.; Sun, Q.; Dong, M.; Ma, T.; et al. Aquaporin-3 mediates ovarian steroid hormone-induced motility of endometrial epithelial cells. Hum. Reprod. 2018, 33, 2060–2073. [Google Scholar] [CrossRef] [PubMed]
- Chivasso, C.; Hagstromer, C.J.; Rose, K.L.; Lhotellerie, F.; Leblanc, L.; Wang, Z.; Moscato, S.; Chevalier, C.; Zindy, E.; Martin, M.; et al. Ezrin Is a Novel Protein Partner of Aquaporin-5 in Human Salivary Glands and Shows Altered Expression and Cellular Localization in Sjogren’s Syndrome. Int. J. Mol. Sci. 2021, 22, 9213. [Google Scholar] [CrossRef] [PubMed]
- Tada, J.; Sawa, T.; Yamanaka, N.; Shono, M.; Akamatsu, T.; Tsumura, K.; Parvin, M.N.; Kanamori, N.; Hosoi, K. Involvement of vesicle-cytoskeleton interaction in AQP5 trafficking in AQP5-gene-transfected HSG cells. Biochem. Biophys. Res. Commun. 1999, 266, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Muroi, S.I.; Isohama, Y. Ezrin Regulates Ca2+ Ionophore-Induced Plasma Membrane Translocation of Aquaporin-5. Int. J. Mol. Sci. 2021, 22, 13505. [Google Scholar] [CrossRef]
- Tietz, P.S.; McNiven, M.A.; Splinter, P.L.; Huang, B.Q.; Larusso, N.F. Cytoskeletal and motor proteins facilitate trafficking of AQP1-containing vesicles in cholangiocytes. Biol. Cell 2006, 98, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Sidhaye, V.K.; Chau, E.; Srivastava, V.; Sirimalle, S.; Balabhadrapatruni, C.; Aggarwal, N.R.; D’Alessio, F.R.; Robinson, D.N.; King, L.S. A novel role for aquaporin-5 in enhancing microtubule organization and stability. PLoS ONE 2012, 7, e38717. [Google Scholar] [CrossRef]
- Huysseune, S.; Kienlen-Campard, P.; Hebert, S.; Tasiaux, B.; Leroy, K.; Devuyst, O.; Brion, J.P.; De Strooper, B.; Octave, J.N. Epigenetic control of aquaporin 1 expression by the amyloid precursor protein. FASEB J. 2009, 23, 4158–4167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, H.; Liu, E.; Guang, Y.; Yang, L.; Mao, J.; Zhu, L.; Chen, L.; Wang, L. The AQP-3 water channel and the ClC-3 chloride channel coordinate the hypotonicity-induced swelling volume in nasopharyngeal carcinoma cells. Int. J. Biochem. Cell Biol. 2014, 57, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, Z.; Yang, L.; Luo, H.; Liu, S.; Li, Y.; Wei, Y.; Peng, S.; Zhu, L.; Wang, L.; et al. The AQP-3 water channel is a pivotal modulator of glycerol-induced chloride channel activation in nasopharyngeal carcinoma cells. Int. J. Biochem. Cell Biol. 2016, 72, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Bollinger Bollag, W. Aquaporin 3 colocates with phospholipase d2 in caveolin-rich membrane microdomains and is downregulated upon keratinocyte differentiation. J. Investig. Dermatol. 2003, 121, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Reppetti, J.; Reca, A.; Seyahian, E.A.; Medina, Y.; Martinez, N.; Szpilbarg, N.; Damiano, A.E. Intact caveolae are required for proper extravillous trophoblast migration and differentiation. J. Cell Physiol. 2020, 235, 3382–3392. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Hansen, J.S.; Saba, K.H.; Bergman, A.; Negoita, F.; Gourdon, P.; Hagstrom-Andersson, A.; Lindkvist-Petersson, K. Aquaglyceroporins and orthodox aquaporins in human adipocytes. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183795. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Kang, J.Y.; Kim, M.J.; Shin, D.M.; Hong, J.H. Carbonic anhydrase 12 mutation modulates membrane stability and volume regulation of aquaporin 5. J. Enzym. Inhib. Med. Chem. 2019, 34, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Tsuzaka, K.; Takeuchi, T.; Sasaki, Y.; Tsubota, K. Altered distribution of aquaporin 5 and its C-terminal binding protein in the lacrimal glands of a mouse model for Sjogren’s syndrome. Curr. Eye Res. 2008, 33, 621–629. [Google Scholar] [CrossRef]
- Chivasso, C.; Nesverova, V.; Jarva, M.; Blanchard, A.; Rose, K.L.; Oberg, F.K.; Wang, Z.; Martin, M.; Lhotellerie, F.; Zindy, E.; et al. Unraveling Human AQP5-PIP Molecular Interaction and Effect on AQP5 Salivary Glands Localization in SS Patients. Cells 2021, 10, 2108. [Google Scholar] [CrossRef] [PubMed]
- Chin, W.-C.; Bhattacharya, D.; Yu, L.; Wang, M. Expression patterns of conjunctival mucin 5AC and aquaporin 5 in response to acute dry eye stress. PLoS ONE 2017, 12, e0187188. [Google Scholar] [CrossRef]
- Login, F.H.; Palmfeldt, J.; Cheah, J.S.; Yamada, S.; Nejsum, L.N. Aquaporin-5 regulation of cell-cell adhesion proteins: An elusive “tail” story. Am. J. Physiol. Cell Physiol. 2021, 320, C282–C292. [Google Scholar] [CrossRef] [PubMed]
- Tradtrantip, L.; Jin, B.J.; Yao, X.; Anderson, M.O.; Verkman, A.S. Aquaporin-Targeted Therapeutics: State-of-the-Field. Adv. Exp. Med. Biol. 2017, 969, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; van Hoek, A.N.; Biwersi, J.; Verkman, A.S. A point mutation at cysteine 189 blocks the water permeability of rat kidney water channel CHIP28k. Biochemistry 1993, 32, 2938–2941. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, M.; Gu, Y.; Ishibashi, K.; Marumo, F.; Sasaki, S. Mercury-sensitive residues and pore site in AQP3 water channel. Biochemistry 1997, 36, 13973–13978. [Google Scholar] [CrossRef]
- Muller-Lucks, A.; Gena, P.; Frascaria, D.; Altamura, N.; Svelto, M.; Beitz, E.; Calamita, G. Preparative scale production and functional reconstitution of a human aquaglyceroporin (AQP3) using a cell free expression system. N. Biotechnol. 2013, 30, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Bokaee, S.; Morgan, R.; Davies, J.; Harrington, K.J.; Pandha, H. Inhibition of the aquaporin 3 water channel increases the sensitivity of prostate cancer cells to cryotherapy. Br. J. Cancer 2009, 100, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Castle, N.A. Aquaporins as targets for drug discovery. Drug Discov. Today 2005, 10, 485–493. [Google Scholar] [CrossRef]
- Rubenwolf, P.C.; Georgopoulos, N.T.; Kirkwood, L.A.; Baker, S.C.; Southgate, J. Aquaporin expression contributes to human transurothelial permeability in vitro and is modulated by NaCl. PLoS ONE 2012, 7, e45339. [Google Scholar] [CrossRef][Green Version]
- Niemietz, C.M.; Tyerman, S.D. New potent inhibitors of aquaporins: Silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Lett. 2002, 531, 443–447. [Google Scholar] [CrossRef]
- Martins, A.P.; Marrone, A.; Ciancetta, A.; Galan Cobo, A.; Echevarria, M.; Moura, T.F.; Re, N.; Casini, A.; Soveral, G. Targeting aquaporin function: Potent inhibition of aquaglyceroporin-3 by a gold-based compound. PLoS ONE 2012, 7, e37435. [Google Scholar] [CrossRef]
- Serna, A.; Galan-Cobo, A.; Rodrigues, C.; Sanchez-Gomar, I.; Toledo-Aral, J.J.; Moura, T.F.; Casini, A.; Soveral, G.; Echevarria, M. Functional inhibition of aquaporin-3 with a gold-based compound induces blockage of cell proliferation. J. Cell Physiol. 2014, 229, 1787–1801. [Google Scholar] [CrossRef]
- Martins, A.P.; Ciancetta, A.; de Almeida, A.; Marrone, A.; Re, N.; Soveral, G.; Casini, A. Aquaporin inhibition by gold(III) compounds: New insights. ChemMedChem 2013, 8, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Camps, M.; Zorzano, A.; Moura, T.F.; Soveral, G. Biophysical assessment of human aquaporin-7 as a water and glycerol channel in 3T3-L1 adipocytes. PLoS ONE 2013, 8, e83442. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; de Almeida, A.; de Graaf, C.; Camps, M.; Zorzano, A.; Moura, T.F.; Casini, A.; Soveral, G. A gold coordination compound as a chemical probe to unravel aquaporin-7 function. Chembiochem 2014, 15, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Zhao, G.X.; Li, J.; Zhang, Y.; Shen, X.Z.; Wang, J.Y.; Sun, J.Y. Auphen and dibutyryl cAMP suppress growth of hepatocellular carcinoma by regulating expression of aquaporins 3 and 9 in vivo. World J. Gastroenterol. 2016, 22, 3341–3354. [Google Scholar] [CrossRef]
- Pimpao, C.; Wragg, D.; Bonsignore, R.; Aikman, B.; Pedersen, P.A.; Leoni, S.; Soveral, G.; Casini, A. Mechanisms of irreversible aquaporin-10 inhibition by organogold compounds studied by combined biophysical methods and atomistic simulations. Metallomics 2021, 13, mfab053. [Google Scholar] [CrossRef] [PubMed]
- Babak, M.V.; Chong, K.R.; Rapta, P.; Zannikou, M.; Tang, H.M.; Reichert, L.; Chang, M.R.; Kushnarev, V.; Heffeter, P.; Meier-Menches, S.M.; et al. Interfering with Metabolic Profile of Triple-Negative Breast Cancers Using Rationally Designed Metformin Prodrugs. Angew. Chem. Int. Ed. 2021, 60, 13405–13413. [Google Scholar] [CrossRef] [PubMed]
- Pinho, J.O.; Coelho, M.; Pimpão, C.; Konwar, J.; Godinho-Santos, A.; Noiva, R.M.; Thomas, S.R.; Casini, A.; Soveral, G.; Gaspar, M.M. Liposomal Formulation of an Organogold Complex Enhancing Its Activity as Antimelanoma Agent—In Vitro and In Vivo Studies. Pharmaceutics 2024, 16, 1566. [Google Scholar] [CrossRef] [PubMed]
- Nave, M.; Castro, R.E.; Rodrigues, C.M.; Casini, A.; Soveral, G.; Gaspar, M.M. Nanoformulations of a potent copper-based aquaporin inhibitor with cytotoxic effect against cancer cells. Nanomedicine 2016, 11, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Pinho, J.O.; Amaral, J.D.; Castro, R.E.; Rodrigues, C.M.; Casini, A.; Soveral, G.; Gaspar, M.M. Copper complex nanoformulations featuring highly promising therapeutic potential in murine melanoma models. Nanomedicine 2019, 14, 835–850. [Google Scholar] [CrossRef]
- Pinho, J.O.; da Silva, I.V.; Amaral, J.D.; Rodrigues, C.M.P.; Casini, A.; Soveral, G.; Gaspar, M.M. Therapeutic potential of a copper complex loaded in pH-sensitive long circulating liposomes for colon cancer management. Int. J. Pharm. 2021, 599, 120463. [Google Scholar] [CrossRef]
- Pimpao, C.; da Silva, I.V.; Mosca, A.F.; Pinho, J.O.; Gaspar, M.M.; Gumerova, N.I.; Rompel, A.; Aureliano, M.; Soveral, G. The Aquaporin-3-Inhibiting Potential of Polyoxotungstates. Int. J. Mol. Sci. 2020, 21, 2467. [Google Scholar] [CrossRef]
- Detmers, F.J.; de Groot, B.L.; Muller, E.M.; Hinton, A.; Konings, I.B.; Sze, M.; Flitsch, S.L.; Grubmuller, H.; Deen, P.M. Quaternary ammonium compounds as water channel blockers. Specificity, potency, and site of action. J. Biol. Chem. 2006, 281, 14207–14214. [Google Scholar] [CrossRef]
- Seeliger, D.; Zapater, C.; Krenc, D.; Haddoub, R.; Flitsch, S.; Beitz, E.; Cerda, J.; de Groot, B.L. Discovery of novel human aquaporin-1 blockers. ACS Chem. Biol. 2013, 8, 249–256. [Google Scholar] [CrossRef]
- Migliati, E.; Meurice, N.; DuBois, P.; Fang, J.S.; Somasekharan, S.; Beckett, E.; Flynn, G.; Yool, A.J. Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site. Mol. Pharmacol. 2009, 76, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kourghi, M.; Pei, J.V.; De Ieso, M.L.; Flynn, G.; Yool, A.J. Bumetanide Derivatives AqB007 and AqB011 Selectively Block the Aquaporin-1 Ion Channel Conductance and Slow Cancer Cell Migration. Mol. Pharmacol. 2016, 89, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Yool, A.J.; Morelle, J.; Cnops, Y.; Verbavatz, J.M.; Campbell, E.M.; Beckett, E.A.; Booker, G.W.; Flynn, G.; Devuyst, O. AqF026 is a pharmacologic agonist of the water channel aquaporin-1. J. Am. Soc. Nephrol. 2013, 24, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Kim, J.K.; Verkman, A.S. Comparative efficacy of HgCl2 with candidate aquaporin-1 inhibitors DMSO, gold, TEA+ and acetazolamide. FEBS Lett. 2006, 580, 6679–6684. [Google Scholar] [CrossRef] [PubMed]
- Esteva-Font, C.; Jin, B.J.; Lee, S.; Phuan, P.W.; Anderson, M.O.; Verkman, A.S. Experimental Evaluation of Proposed Small-Molecule Inhibitors of Water Channel Aquaporin-1. Mol. Pharmacol. 2016, 89, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.J.; Kim, P.; Lu, Y.F.; Feingold, K.R. PPARgamma activators stimulate aquaporin 3 expression in keratinocytes/epidermis. Exp. Dermatol. 2011, 20, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Olala, L.O.; Kagha, K.; Pan, Z.Q.; Chen, X.; Yang, R.; Cline, A.; Helwa, I.; Marshall, L.; Kaddour-Djebbar, I.; et al. Regulation of the Glycerol Transporter, Aquaporin-3, by Histone Deacetylase-3 and p53 in Keratinocytes. J. Investig. Dermatol. 2017, 137, 1935–1944. [Google Scholar] [CrossRef]
- Helwa, I.; Choudhary, V.; Chen, X.; Kaddour-Djebbar, I.; Bollag, W.B. Anti-Psoriatic Drug Monomethylfumarate Increases Nuclear Factor Erythroid 2-Related Factor 2 Levels and Induces Aquaporin-3 mRNA and Protein Expression. J. Pharmacol. Exp. Ther. 2017, 362, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Flodby, P.; Li, C.; Liu, Y.; Wang, H.; Rieger, M.E.; Minoo, P.; Crandall, E.D.; Ann, D.K.; Borok, Z.; Zhou, B. Cell-specific expression of aquaporin-5 (Aqp5) in alveolar epithelium is directed by GATA6/Sp1 via histone acetylation. Sci. Rep. 2017, 7, 3473. [Google Scholar] [CrossRef]
- Ikarashi, N.; Baba, K.; Ushiki, T.; Kon, R.; Mimura, A.; Toda, T.; Ishii, M.; Ochiai, W.; Sugiyama, K. The laxative effect of bisacodyl is attributable to decreased aquaporin-3 expression in the colon induced by increased PGE2 secretion from macrophages. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G887–G895. [Google Scholar] [CrossRef]
- Sonntag, Y.; Gena, P.; Maggio, A.; Singh, T.; Artner, I.; Oklinski, M.K.; Johanson, U.; Kjellbom, P.; Nieland, J.D.; Nielsen, S.; et al. Identification and characterization of potent and selective aquaporin-3 and aquaporin-7 inhibitors. J. Biol. Chem. 2019, 294, 7377–7387. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Yasui, M.; Hara-Chikuma, M. Aquaporin 3 inhibition suppresses the mitochondrial respiration rate and viability of multiple myeloma cells. Biochem. Biophys. Res. Commun. 2023, 676, 158–164. [Google Scholar] [CrossRef]
- Villandre, J.; White, V.; Lear, T.B.; Chen, Y.; Tuncer, F.; Vaiz, E.; Tuncer, B.; Lockwood, K.; Camarco, D.; Liu, Y.; et al. A Repurposed Drug Screen for Compounds Regulating Aquaporin 5 Stability in Lung Epithelial Cells. Front. Pharmacol. 2022, 13, 828643. [Google Scholar] [CrossRef] [PubMed]
- Rump, K.; Koos, B.; Ziehe, D.; Thon, P.; Rahmel, T.; Palmowski, L.; Marko, B.; Wolf, A.; Witowski, A.; Bazzi, Z.; et al. Methazolamide Reduces the AQP5 mRNA Expression and Immune Cell Migration-A New Potential Drug in Sepsis Therapy? Int. J. Mol. Sci. 2024, 25, 610. [Google Scholar] [CrossRef]
- Montiel, V.; Bella, R.; Michel, L.Y.M.; Esfahani, H.; De Mulder, D.; Robinson, E.L.; Deglasse, J.-P.; Tiburcy, M.; Chow, P.H.; Jonas, J.-C.; et al. Inhibition of aquaporin-1 prevents myocardial remodeling by blocking the transmembrane transport of hydrogen peroxide. Sci. Transl. Med. 2020, 12, eaay2176. [Google Scholar] [CrossRef]
- Cao, C.; Wan, S.; Jiang, Q.; Amaral, A.; Lu, S.; Hu, G.; Bi, Z.; Kouttab, N.; Chu, W.; Wan, Y. All-trans retinoic acid attenuates ultraviolet radiation-induced down-regulation of aquaporin-3 and water permeability in human keratinocytes. J. Cell Physiol. 2008, 215, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.L.; Fang, J.Y.; Chen, M.; Wu, C.J.; Huang, C.C.; Hung, C.F. Chrysin protects epidermal keratinocytes from UVA- and UVB-induced damage. J. Agric. Food Chem. 2011, 59, 8391–8400. [Google Scholar] [CrossRef]
- Tang, S.C.; Tang, L.C.; Liu, C.H.; Liao, P.Y.; Lai, J.C.; Yang, J.H. Glycolic acid attenuates UVB-induced aquaporin-3, matrix metalloproteinase-9 expression, and collagen degradation in keratinocytes and mouse skin. Biochem. J. 2019, 476, 1387–1400. [Google Scholar] [CrossRef]
- Wu, Z.; Uchi, H.; Morino-Koga, S.; Shi, W.; Furue, M. Resveratrol inhibition of human keratinocyte proliferation via SIRT1/ARNT/ERK dependent downregulation of aquaporin 3. J. Dermatol. Sci. 2014, 75, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.F.; Hsiao, C.Y.; Hsieh, W.H.; Li, H.J.; Tsai, Y.J.; Lin, C.N.; Chang, H.H.; Wu, N.L. 18ss-glycyrrhetinic acid derivative promotes proliferation, migration and aquaporin-3 expression in human dermal fibroblasts. PLoS ONE 2017, 12, e0182981. [Google Scholar] [CrossRef]
- Ji, C.; Cao, C.; Lu, S.; Kivlin, R.; Amaral, A.; Kouttab, N.; Yang, H.; Chu, W.; Bi, Z.; Di, W.; et al. Curcumin attenuates EGF-induced AQP3 up-regulation and cell migration in human ovarian cancer cells. Cancer Chemother. Pharmacol. 2008, 62, 857–865. [Google Scholar] [CrossRef]
- Kon, R.; Yamamura, M.; Matsunaga, Y.; Kimura, H.; Minami, M.; Kato, S.; Ikarashi, N.; Sugiyama, K. Laxative effect of repeated Daiokanzoto is attributable to decrease in aquaporin-3 expression in the colon. J. Nat. Med. 2018, 72, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Chen, C.; Bai, L.; Kong, L.; Luo, J. Downregulation of Aquaporin 3 Mediated the Laxative Effect in the Rat Colon by a Purified Resin Glycoside Fraction from Pharbitis Semen. Evid. Based Complement. Altern. Med. 2019, 2019, 9406342. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Liang, Y.; Wang, D.; Yan, Z.; Yin, H.; Wu, D.; Su, Q. Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int. J. Mol. Med. 2018, 41, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gan, Y.; Luo, H.; Xu, N.; Chen, L.; Li, M.; Guan, F.; Su, Z.; Lin, Z.; Xie, J.; et al. beta-Patchoulene Ameliorates Water Transport and the Mucus Barrier in 5-Fluorouracil-Induced Intestinal Mucositis Rats via the cAMP/PKA/CREB Signaling Pathway. Front. Pharmacol. 2021, 12, 689491. [Google Scholar] [CrossRef]
- Paccetti-Alves, I.; Batista, M.S.P.; Pimpao, C.; Victor, B.L.; Soveral, G. Unraveling the Aquaporin-3 Inhibitory Effect of Rottlerin by Experimental and Computational Approaches. Int. J. Mol. Sci. 2023, 24, 6004. [Google Scholar] [CrossRef] [PubMed]
- Skowronska, A.; Mlotkowska, P.; Nielsen, S.; Skowronski, M.T. Difference in expression between AQP1 and AQP5 in porcine endometrium and myometrium in response to steroid hormones, oxytocin, arachidonic acid, forskolin and cAMP during the mid-luteal phase of the estrous cycle and luteolysis. Reprod. Biol. Endocrinol. 2015, 13, 131. [Google Scholar] [CrossRef]
- Skowronska, A.; Mlotkowska, P.; Wojciechowicz, B.; Okrasa, S.; Nielsen, S.; Skowronski, M.T. Progesterone, estradiol, arachidonic acid, oxytocin, forskolin and cAMP influence on aquaporin 1 and 5 expression in porcine uterine explants during the mid-luteal phase of the estrous cycle and luteolysis: An in vitro study. Reprod. Biol. Endocrinol. 2015, 13, 7. [Google Scholar] [CrossRef]
- Tanski, D.; Skowronska, A.; Tanska, M.; Lepiarczyk, E.; Skowronski, M.T. The In Vitro Effect of Steroid Hormones, Arachidonic Acid, and Kinases Inhibitors on Aquaporin 1, 2, 5, and 7 Gene Expression in the Porcine Uterine Luminal Epithelial Cells during the Estrous Cycle. Cells 2021, 10, 832. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lim, W.; Bae, H.; Song, G. Aquaporin 3 is regulated by estrogen in the chicken oviduct and is involved in progression of epithelial cell-derived ovarian carcinomas. Domest. Anim. Endocrinol. 2016, 55, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Takahashi, E.; Miyagawa, S.; Watanabe, H.; Iguchi, T. Chromatin immunoprecipitation-mediated target identification proved aquaporin 5 is regulated directly by estrogen in the uterus. Genes. Cells 2006, 11, 1133–1143. [Google Scholar] [CrossRef]
- Skowronski, M.; Mlotkowska, P.; Tanski, D.; Lepiarczyk, E.; Oklinski, M.; Nielsen, S.; Skowronska, A. Pituitary Gonadotropins, Prolactin and Growth Hormone Differentially Regulate AQP1 Expression in the Porcine Ovarian Follicular Cells. Int. J. Mol. Sci. 2017, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Hermo, L.; Krzeczunowicz, D.; Ruz, R. Cell Specificity of Aquaporins 0, 3, and 10 Expressed in the Testis, Efferent Ducts, and Epididymis of Adult Rats. J. Androl. 2013, 25, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Salleh, N.; Mokhtar, H.M.; Kassim, N.M.; Giribabu, N. Testosterone Induces Increase in Aquaporin (AQP)-1, 5, and 7 Expressions in the Uteri of Ovariectomized Rats. J. Membr. Biol. 2015, 248, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Gómez-Ambrosi, J.; Catalán, V.; Ezquerro, S.; Méndez-Giménez, L.; Becerril, S.; Ibáñez, P.; Vila, N.; Margall, M.A.; Moncada, R.; et al. Guanylin and uroguanylin stimulate lipolysis in human visceral adipocytes. Int. J. Obes. 2016, 40, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Moreno, N.R.; Balaguer, I.; Mendez-Gimenez, L.; Becerril, S.; Catalan, V.; Gomez-Ambrosi, J.; Portincasa, P.; Calamita, G.; Soveral, G.; et al. Leptin administration restores the altered adipose and hepatic expression of aquaglyceroporins improving the non-alcoholic fatty liver of ob/ob mice. Sci. Rep. 2015, 5, 12067. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Wang, W.; Kwon, T.H.; Jonassen, T.; Li, C.; Ring, T.; Froki, A.J.; Nielsen, S. EPO and alpha-MSH prevent ischemia/reperfusion-induced down-regulation of AQPs and sodium transporters in rat kidney. Kidney Int. 2004, 66, 683–695. [Google Scholar] [CrossRef]
- Ben, Y.; Chen, J.; Zhu, R.; Gao, L.; Bai, C. Upregulation of AQP3 and AQP5 induced by dexamethasone and ambroxol in A549 cells. Respir. Physiol. Neurobiol. 2008, 161, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.; Zhang, H.; Guo, Z.; Yang, L.; Shao, Y.; Liu, X.; Zhao, Y.; Wang, Z.; Zhang, M.; Guo, C.; et al. Aquaporin 1 promotes sensitivity of anthracycline chemotherapy in breast cancer by inhibiting beta-catenin degradation to enhance TopoIIalpha activity. Cell Death Differ. 2021, 28, 382–400. [Google Scholar] [CrossRef]
- Luo, L.; Yang, R.; Zhao, S.; Chen, Y.; Hong, S.; Wang, K.; Wang, T.; Cheng, J.; Zhang, T.; Chen, D. Decreased miR-320 expression is associated with breast cancer progression, cell migration, and invasiveness via targeting Aquaporin 1. Acta Biochim. Et. Biophys. Sin. 2018, 50, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Jung, H.J.; Choi, H.J.; Jang, H.J.; Park, H.J.; Nejsum, L.N.; Kwon, T.H. Exosomes co-expressing AQP5-targeting miRNAs and IL-4 receptor-binding peptide inhibit the migration of human breast cancer cells. FASEB J. 2020, 34, 3379–3398. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Hu, J.; Wang, Z.; Zong, H.; Zhang, L.; Zhang, R.; Sun, L. LncRNA H19 functions as an Aquaporin 1 competitive endogenous RNA to regulate microRNA-874 expression in LPS sepsis. Biomed. Pharmacother. 2018, 105, 1183–1191. [Google Scholar] [CrossRef]
- Jiang, B.; Li, Z.; Zhang, W.; Wang, H.; Zhi, X.; Feng, J.; Chen, Z.; Zhu, Y.; Yang, L.; Xu, H.; et al. miR-874 inhibits cell proliferation, migration and invasion through targeting aquaporin-3 in gastric cancer. J. Gastroenterol. 2014, 49, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, Y.; Yang, S.; Liu, X.; Lu, Y.; Liu, F.; Li, G.; Tian, G. miR-874 directly targets AQP3 to inhibit cell proliferation, mobility and EMT in non-small cell lung cancer. Thorac. Cancer 2020, 11, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Tao, J.; Li, Z.; Jiang, B.; Feng, J.; Yang, L.; Xu, H.; Xu, Z. MiR-874 promotes intestinal barrier dysfunction through targeting AQP3 following intestinal ischemic injury. FEBS Lett. 2014, 588, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Zhi, X.; Zhang, Q.; Yang, L.; Xu, H.; Xu, Z. LncRNA H19 functions as a competing endogenous RNA to regulate AQP3 expression by sponging miR-874 in the intestinal barrier. FEBS Lett. 2016, 590, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Ratovitski, E.A. Phospho-DeltaNp63alpha regulates AQP3, ALOX12B, CASP14 and CLDN1 expression through transcription and microRNA modulation. FEBS Lett. 2013, 587, 3581–3586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Kong, W.; Ju, T.; Xie, Q.; Zhai, L. MiR-185-3p mimic promotes the chemosensitivity of CRC cells via AQP5. Cancer Biol. Ther. 2020, 21, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wu, Y.; Kang, M.; Zhang, B. MiR-877 suppresses gastric cancer progression by downregulating AQP3. J. Int. Med. Res. 2020, 48, 0300060520903661. [Google Scholar] [CrossRef]
- Li, Z.; Ma, L.; Di, L.; Lin, X. MicroRNA-1271-5p alleviates the malignant development of hepatitis B virus-mediated liver cancer via binding to AQP5. Mol. Med. Rep. 2021, 23, 386. [Google Scholar] [CrossRef]
- Sekine, S.; Shimada, Y.; Nagata, T.; Sawada, S.; Yoshioka, I.; Matsui, K.; Moriyama, M.; Omura, T.; Osawa, S.; Shibuya, K.; et al. Role of aquaporin-5 in gallbladder carcinoma. Eur. Surg. Res. 2013, 51, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Chao, G.; Wang, Y.; Zhang, S.; Yang, W.; Ni, Z.; Zheng, X. Correction: MicroRNA-29a increased the intestinal membrane permeability of colonic epithelial cells in irritable bowel syndrome rats. Oncotarget 2018, 9, 15816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, M.; Zhang, Y.; Peng, P.; Li, J.; Xin, X. miR-96 and miR-330 overexpressed and targeted AQP5 in lipopolysaccharide-induced rat lung damage of disseminated intravascular coagulation. Blood Coagul. Fibrinolysis 2014, 25, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Wang, X.; Yu, N.; Li, Y.; Kan, J. Long non-coding RNA FGD5-AS1/microRNA-133a-3p upregulates aquaporin 1 to decrease the inflammatory response in LPS-induced sepsis. Mol. Med. Rep. 2021, 24, 784. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Pei, L.; Bai, T.; Wang, J. Down-regulation of microRNA-126-5p contributes to overexpression of VEGFA in lipopolysaccharide-induced acute lung injury. Biotechnol. Lett. 2016, 38, 1277–1284. [Google Scholar] [CrossRef]
- Zhu, L.; Lin, Z.W.; Wang, G.; Zhang, H.; Liu, B.; Xu, Q.J. MicroRNA-495 downregulates AQP1 and facilitates proliferation and differentiation of osteoblasts in mice with tibial fracture through activation of p38 MAPK signaling pathway. Sci. Rep. 2019, 9, 16171. [Google Scholar] [CrossRef]
- Tanaka, M.; Ito, A.; Shiozawa, S.; Hara-Chikuma, M. Anti-tumor effect of aquaporin 3 monoclonal antibody on syngeneic mouse tumor model. Transl. Oncol. 2022, 24, 101498. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, H.; Gao, M.; Ma, N.; Sun, R. Long non-coding RNA CASC2 improved acute lung injury by regulating miR-144-3p/AQP1 axis to reduce lung epithelial cell apoptosis. Cell Biosci. 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimpão, C.; da Silva, I.V.; Soveral, G. The Expanding Role of Aquaporin-1, Aquaporin-3 and Aquaporin-5 as Transceptors: Involvement in Cancer Development and Potential Druggability. Int. J. Mol. Sci. 2025, 26, 1330. https://doi.org/10.3390/ijms26031330
Pimpão C, da Silva IV, Soveral G. The Expanding Role of Aquaporin-1, Aquaporin-3 and Aquaporin-5 as Transceptors: Involvement in Cancer Development and Potential Druggability. International Journal of Molecular Sciences. 2025; 26(3):1330. https://doi.org/10.3390/ijms26031330
Chicago/Turabian StylePimpão, Catarina, Inês V. da Silva, and Graça Soveral. 2025. "The Expanding Role of Aquaporin-1, Aquaporin-3 and Aquaporin-5 as Transceptors: Involvement in Cancer Development and Potential Druggability" International Journal of Molecular Sciences 26, no. 3: 1330. https://doi.org/10.3390/ijms26031330
APA StylePimpão, C., da Silva, I. V., & Soveral, G. (2025). The Expanding Role of Aquaporin-1, Aquaporin-3 and Aquaporin-5 as Transceptors: Involvement in Cancer Development and Potential Druggability. International Journal of Molecular Sciences, 26(3), 1330. https://doi.org/10.3390/ijms26031330








