Abstract
Dietary polyphenols are garnering attention in the scientific community due to their potential health-beneficial properties and preventative effects against chronic diseases, viz. cardiovascular diseases, diabetes, obesity, and neurodegenerative diseases. Polyphenols are antioxidants that change microbial composition by suppressing pathogenic bacteria and stimulating beneficial bacteria. The interaction of polyphenols with dietary fibers affects their bioaccessibility in the upper and lower parts of the digestive tract. Dietary fibers, polyphenols, their conjugates, and their metabolites modulate microbiome population and diversity. Consuming polyphenol-rich dietary fibers such as pomegranate, cranberry, berries, and tea improves gut health. A complex relationship exists between polyphenol-rich diets and gut microbiota for functioning in human health. In this review, we provide an overview of the interactions of dietary polyphenols, fibers, and gut microbiota, improving the understanding of the functional properties of dietary polyphenols.
1. Introduction
Polyphenols are secondary metabolites primarily sourced from fruits, vegetables, cereals, tea, coffee, wine, and beverages. They comprise several classes of phenolic compounds, including flavonoids, phenolic acids, stilbenes, and lignans [1,2]. Polyphenolic compounds possess several health beneficial properties such as anticancer, antioxidant, antimicrobial, and anti-inflammatory activities and play a significant role in the prevention of chronic diseases such as cardiovascular diseases, diabetes, obesity, and neurodegenerative diseases [1,2,3].
Distinctive guidelines and dietary habits among geographically diverse individuals worldwide result in significant variations in polyphenol intake [4,5]. Moreover, polyphenolic content greatly varies with the types of food matrices and their preparation methods [5,6]. In plants, fruits, and vegetables, polyphenols exist in free and bound forms. Most of the analytical, nutritional, and clinical studies of food polyphenols consider phenolic compounds extracted with aqueous organic solvents [7]. These polyphenols are referred to as extractable polyphenols (EPPs). Notably, potentially bioactive polyphenols remain bound in residues comprising macromolecules such as cellulose, protein, and lignin [8]. These polyphenols are referred to as non-extractable polyphenols (NEPPs) and are usually ignored during analysis [8]. Several studies have indicated the presence of abundant NEPPs in specific foods and vegetables such as berries, brown rice, carrots, onions, spinach, apples, and oranges [8,9,10]. Peels, pomace, and seeds also have abundant NEPPs. NEPPs comprise compounds with high-molecular-weight polymers and polyphenols related to cell wall macromolecules (proteins and polysaccharides) or entrapped inside the food matrix [9,10,11]. Major NEPPs constitute condensed tannins, proanthocyanidins, hydrolyzable polyphenols (including hydrolyzable tannins), or polyphenols [8,9,10]. These bioactive compounds exhibit high antioxidant, anti-inflammatory, antidiabetic, and other biological activities [12].
Bioaccessibility is a crucial factor affecting the bioavailability, physiological effects, and biochemical activities of polyphenols. Polyphenols are usually released from food matrices by interacting with digestive enzymes and bacterial microflora in the small and large intestines, respectively, and potentially become bioavailable for absorption [13,14]. Significant differences are observed in the bioavailability of polyphenols, influenced by the type of food matrix, molecular mass, conformations, and physicochemical activity, such as digestibility [14,15]. Consequently, not all phenolic compounds are equally available to exert their functions in the human body after the consumption of phenolic-rich foods. NEPPs, which are not released or absorbed into the gastrointestinal (GI) tract, pass to the lower GI tract, where they are fermented by bacterial flora [10,12]. Upon reaching the lower intestine, polyphenols are biotransformed into their metabolites by gut microbiota and exert various functional and biological effects [11,12,13]. Moreover, these polyphenols and their metabolites modulate the bacterial composition in the colon and influence the gut health of the host [10,11,12]. Several studies have indicated that the interactions of dietary fibers and polyphenols may affect their functional relationship, as well as the integrity and diversity of gut microbiota [9,12,13]. However, there is a complex relationship between polyphenol-rich diets and gut microbiota and their effects on human health, which is yet to be elucidated. In this review, we provide a systematic overview of the effects of dietary polyphenols on the human gut microbiota and their impact on gut integrity and human health. This information will improve the understanding of the function of NEPPs in modulating the gut microbiota composition and reducing health risks.
2. Gut Microbiota
The human microbiota comprises 10–100 trillion microorganisms (mostly bacteria, but also viruses, fungi, and protozoa) primarily in the gut [16,17,18,19], similar to the number of human cells [20]. The microbiota abundance gradually increases along the GI tract, with lesser proportion in the stomach and a higher density in the colon (1010–1012/g) and at least 400 bacterial species [19,21]. The GI tract has a large surface area between 250 and 400 m2. During a human’s life, an average of 60 tons of food passes through the intestines, along with abundant microorganisms from the environment [22].
A human intestinal microbial gene catalog of 3.3 million genes in the human gut microbiome has been reported, compared with approximately 22,000 genes in the entire human genome [16,19,20,22,23,24]. The microbiota comprises harmless bacteria [16]. Studies have indicated 2172 species in humans, classified into 12 divisions, of which 93.5% belong to phyla Proteobacteria, Firmicutes, and Bacteroidetes [21,25]. In humans, 386 of the identified species are strictly anaerobic and occupy areas such as the oral cavity and the GI tract [25]. Less than 1% of gut bacteria belong to the phyla Actinobacteria, Verrumicrobia, Acidobacteria, or Fusobacteria. The small intestine and the colon are dominated by Bacteroidetes and Firmicutes [21].
Intestinal bacteria provide several benefits in the human intestinal tract. Gut microbes aid in maintaining the intestinal mucus barrier, protecting against pathogens, and developing the immune system. However, these mechanisms may be disrupted by an altered microbial composition, known as dysbiosis, resulting in metabolic diseases such as obesity and type 2 diabetes [26].
Gut bacteria primarily generate energy through fermentation by converting sugars to [16,19,20,22,23,24] short-chain fatty acids (SCFAs) [27]. Gut microbiota synthesizes essential vitamins, including vitamin K, riboflavin, biotin, nicotinic acid, pantothenic acid, pyridoxine, thiamine, B12, and folate. Lactic acid bacteria are significant for vitamin B12 production, which is essential for the normal function of the brain and nervous system and DNA synthesis. Bifidobacteria primarily generate folate, a vitamin responsible for human metabolic processes such as DNA synthesis and repair [27,28,29].
The gut microbiota is shaped early in life in the human intestine and undergoes marked changes before reaching equilibrium in adulthood [19,30,31,32,33,34,35]. In the early developmental stages, microbiota is predominantly composed of Actinobacteria and Proteobacteria. Over the age of 65, the microbial community changes and is abundant with Bacteroidetes phyla and Clostridium species [26]. Many factors affect microbiome composition throughout a human’s life. These include microbiome inheritance from the mother, infant delivery mode (vaginal or cesarean), diet, and age-related changes in adults [30]. Everyone has a unique microbiome that develops early in childhood and is stable throughout the lifespan [19,30,31]. The intestinal microbiota reaches a steady state between the ages of 2 and 5 years [19,26]. The gut microbiota is relatively stable in adulthood; however, it exhibits a higher individual variation in older people than in younger adults [36,37,38]. This difference is also affected by genetics, personal hygiene, infection, medication, and diet [30,39,40]. Health in older people is related to gut microbiota modifications. These are associated with changes in the GI tract and diets and a decline in cognitive and immune functions. The gut microbiota’s response to dietary interventions varies among individuals [37,38].
Diet, lifestyle, antibiotics, other drugs, sanitation, and the genetics and immune system of humans may cause changes in microbiota composition [19,30,34,39,40]. Antibiotic treatment significantly disrupts the microbial equilibrium, reducing the abundance and diversity of the community [26]. Dysbiosis occurs when the specific microbial group of the gut microbiota and the abundance and functions of the intestinal microorganisms are altered [19,30,34]. Consequently, illnesses and diseases occur, ranging from cardiovascular, neurological, respiratory, and metabolic illnesses to cancer, inflammatory bowel and skin diseases, Alzheimer’s disease, autism, multiple sclerosis, and allergies [16,19,22,26,30,34]. Similarly, there is an increased risk of metabolic syndromes, including type 2 diabetes mellitus and obesity [34].
Gut microbiota is influenced by geographical locations and differs between developed and developing countries, as well as rural and industrialized regions [33]. Stability is a crucial attribute of good microbial composition. Gut bacteria resist changes induced by ecological stress and return to a balanced state after a stress-related event [34].
Diet accounts for over 50% of the variabilities influencing the microbiota composition over time [34,41,42]. Food availability in developed countries has caused the demand for safer and longer-lasting foods, prompting the industry to produce pre-cooked and ready-to-eat food products containing high amounts of cost-effective, artificial ingredients such as preservatives, colorants, fats, sugar, and salt. The dietary model involving a high consumption of saturated fats and sucrose and a low intake of fiber is called the “Western diet”. A poor diet alters the microbiota and causes disease. Diet markedly influences chronic inflammation, and Western diets are at risk of becoming health hazards that promote high incidences of metabolic diseases and inflammation [34].
3. Effect of Diet on Microbiota
Nutrition significantly influences the structure and diversity of the gut microbiota [36,37,43]. Specific foods and diets influence the abundance of gut bacteria and human health [44]. Each individual has a well-defined combination of gut microbes [28]. Differences in the gut microbiota community translate into a distinct ability to process dietary components, resulting in different propensities to diseases [45]. A change in diet exerts different effects on individuals owing to the unique nature of their gut microbiota [41]. Diet amount and content are critical in shaping the microbiota composition and function. Nutrient and microorganism interactions determine beneficial or detrimental effects on health [36].
Dietary additives such as artificial sweeteners and emulsifiers may affect the gut microbiota and modify its composition [36,44]. Artificial sweeteners used as sugar alternatives, which are sweeter than sugars and have fewer calories, may disturb and harm the gut microbiota. Sucralose, aspartame, and saccharin are sugar substitutes that perturb the balance and diversity of the gut microbiota, contributing to metabolic diseases [46]. Similarly, common emulsifiers in processed foods affect the gut microbiota, causing reduced microbial diversity and possibly resulting in metabolic syndromes and inflammatory diseases [47].
Furthermore, food preference causes individual variations in the human microbiota [37]. Obese individuals have more diverse microbial compositions than non-obese individuals do. The proportion of Bacteroidetes is lower in obese individuals than in non-obese individuals [43]. Consuming a high-caloric meal with a stable carbohydrate, protein, and fat ratio increases Firmicutes and decreases Bacteroidetes abundance. This results in reduced diversity of gut microbiota [45,48]. However, a restricted diet with reduced calorie intake increases the diversity [48].
In contrast to other carbohydrates, polysaccharides, such as resistant starch, oligosaccharides, and non-starch polysaccharides, are dietary fibers only accessible to the microbiota of the large intestine; they are not digested in the small intestine [36,43,44,48]. Some dietary fiber sources are fermentable, and bacterial species digest different types of fibers [44]. Gut microbiota, Bifidobacterium, Bacteroides, Faecalibacterium, Lactobacillus, and Roseburia ferment short-chain fructooligosaccharides to generate SCFAs, primarily butyrate, acetate, and propionate, in the large intestine [44,48]. These SCFAs account for approximately 10% of human energy requirements, leading to reduced calorie needs from the diet [48]. Increased SCFA production correlates with reduced obesity and insulin resistance [44,48]. Abundant fibers in a diet cause a healthy gut microbiome [48].
Some studies showed that the short-term consumption of a diet composed entirely of animal or plant products alters the microbial community and suppresses individual differences in microbial gene expression [45,48,49]. The animal-based diet comprises dairy, meat, and cheeses, while the plant-based diet is rich in grains, legumes, fruits, and vegetables. Fiber consumption is increased through plant diets. Fat and protein intake increases and decreases with animal and plant diets, respectively. The diversity within the sample species remains unchanged under each diet. An animal-based diet has a greater impact on the gut microbiota than a plant-based diet. An animal-based diet increases the number of bile-tolerant microorganisms Alistipes, Bilophila, and Bacteroides but decreases the levels of Firmicutes (Roseburia, Eubacterium rectale, and Ruminococcus bromii) that break down plant polysaccharides [48,49]. Both diets have food-borne microbes, including bacteria, fungi, and viruses, that colonize the gut. These imply that the gut microbiome quickly reacts to a modified diet [49]. A diet over 24 h with high fat/low fiber or low fat/high fiber influences the microbiota moderately, with the microbial species being affected differently among individuals. This slight diet modification has certain consequences on human GI health [50].
A “Western diet” prompts significant changes in the microbiota compared with the effect of a low-fat diet and decreases the diversity of the gut microbiota [36,43]. Individuals consuming this diet take in an abundance of animal protein, total and saturated fats, and simple sugars and low quantities of fruits, vegetables, and other plants. Similarly, this diet has low quantities of dietary fiber, non-starch polysaccharides, and resistant starch [45]. It is correlated with a decreased beneficial bacteria abundance of Bifidobacteria and Lactobacilli and increased organisms belonging to the phyla Firmicutes and Proteobacteria. This modified microbial community may affect brain function and structure [43]. Its SCFA content is low, and it is associated with a high risk of developing inflammatory bowel disorder, obesity, and type 2 diabetes. This “Western diet” does not benefit humans or the microbiome [43]. Conversely, the “Mediterranean diet”, which is considered the healthier of the two diets, is rich in plant-based proteins. The diet involves the consumption of polyunsaturated fats, fruits, vegetables, bread, olives and olive oil, dairy products, and fish with lower intakes of potatoes, red meat, and sweets [43]. Polyphenol metabolites metabolized by gut bacteria in the colon modulate the gut microbial composition considerably by increasing the abundance of intestinal bacteria like Bifidobacteria and Lactobacilli and reducing the abundance of Firmicutes and Proteobacteria organisms. These plant-based diets are correlated with improved cardiovascular health and reduced risk of inflammation. This diet is also associated with enhanced cognitive function [43].
In humans, nutrient digestion and absorption usually occur in the small intestine and stomach. Subsequently, the residual food passes to the colon, where most gut microbes reside [36,43]. Most vitamins are obtained from food and are absorbed in the intestine; only a few are produced by intestinal microorganisms [36]. The microbiome absorbs and produces energy and micronutrients, including essential vitamins, such as vitamin K and many of the water-soluble B vitamins. These are vital for body functions and brain development and cannot be produced by other means [36,43]. Vitamin deficiencies result from perturbed intestinal absorption or inadequate vitamin consumption. Insufficient levels of these vitamins cause cognitive disruption and neurodegenerative deterioration [36]. Moreover, zinc and iron are significant minerals regulated by the microorganisms in the microbiome [43,48]. Iron is essential for oxygen transport and energy production, neurotransmitters, and DNA synthesis. Iron deficiency decreases the abundance of the bacteria that synthesize butyrate in the gut microbiota, resulting in decreased butyrate levels. However, excess iron causes an increased abundance of propionate-producing Bacteriodaceae and fewer butyrate-producing Lachnospiraceae, leading to increased propionate levels and decreased butyrate levels [43,48]. Zinc is crucial for functions related to gene expression and replication and participates in the regulation and differentiation of neurogenesis [43,48]. Zinc deficiency may be caused by malnutrition, diarrhea, malabsorptive or inflammatory bowel disease (IBD), consumption of antibiotics, metal-chelating agents, or anticonvulsants [48]. This deficiency may alter the gut composition and decrease microbiota diversity and species [43].
The diet of urban industrialized populations has a lower microbial richness than the diet of rural individuals. Bacteroides is predominant in gut microbiomes among individuals in an urban-industrial setting. This diet comprises fiber, fat, protein, polyphenols, and micronutrients, influences the shape of the gut microbiota, and is metabolized by general or specific microorganisms alone or through cross-feeding [36,44,48]. Owing to the decline in dietary fiber intake, microbiota diversity is reduced in individuals of industrialized societies, accompanied by increased food-associated chronic diseases, such as obesity and metabolic syndrome, malnutrition and eating disorders, intestinal inflammatory diseases, or colorectal diseases [51]. Many diseases in industrialized and developing countries are associated with diet. Diet–microbiota interactions have beneficial or detrimental consequences on human health [36].
4. Microbiota Correlation with Diseases
The gut microbial population is believed to markedly influence human physiology, considering its contribution to the growth and development of human intestinal cells, supporting its equilibrium [52]. The primary functions of the microbiota are the fermentation of indigestible food components, the synthesis of amino acids, the production of SCFAs, the metabolism of toxins and carcinogens, and the conversion of cholesterol and bile acids [16,27,28,29,39,40]. Gut microbes are critical for humans because they take in energy from food, and the disruption of the gut microbiome may cause obesity or IBD [41,52,53].
Obesity is related to changes in the relative abundances of the two dominant bacterial divisions, the Bacteroidetes and Firmicutes [54,55]. Firmicutes digest long-chain carbohydrates, providing the microbiota with more nutrients, leading to greater energy intake and weight gain [21]. The microbiota of an obese individual readily obtains energy from the diet [54,55].
IBD, a class of inflammatory diseases that include Crohn’s disease and ulcerative colitis, may affect the GI tract due to intestinal dysbiosis. The microbiota of individuals suffering from IBD indicates decreased biodiversity and reduced abundances of Firmicutes, Bacteroidetes, Lactobacillus, Eubacterium, and butyrate-producing species. Butyrate is a fatty acid that modulates homeostatic equilibrium within the microbial community and reduces inflammation [56]. While a robust, healthy microbiota protects from dysbiosis-related diseases, such as IBD or metabolic disorders, a resistant, dysbiotic microbiota may cause diseases [57].
The microbiota plays a role in basic biological processes and the development and progression of conditions such as GI cancers and cardiovascular, infectious, liver, metabolic, respiratory, mental or psychological, and autoimmune diseases [41,58,59,60,61,62,63,64,65,66]. The disturbance of the intestinal flora affects the generation of immune mediators and provokes chronic inflammation and metabolic dysfunction [41,63,64,65,66]. Although other diseases may not be directly associated with the microbiota, various mechanisms that include immune, hormonal, and neural pathways may participate in the pathway of diseases such as asthma, food allergies, autism, and major depressive disorder [66,67].
5. Interactions of Phenolics, Fibers, and Gut Microbiota
Phenolics and dietary fibers are major concomitant ingredients in plants and food matrices. The association of dietary fibers and polyphenols in food matrices plays a critical role in the bioaccessibility, bioavailability, and biochemical activities of polyphenols [68]. Dietary fibers act as carriers of antioxidants and phytochemicals such as phenolics and deliver these compounds for interaction with the gut microbiota [68]. The composition of polysaccharides and their interaction with polyphenols in food matrices result in the variation in the adsorption and retention properties of polyphenols in the digestion tract [11,68,69]. These interactions could be covalent, non-covalent (hydrogen bonding, ionic bonding, electrostatic interaction, and van der Waals), or hydrophobic [69]. Various factors such as molecular size, polymerization degree in procyanidins, branching degree, surface porosity of polysaccharides, glycosylation percentage, the stereochemistry of flavan-3-ol subunits in proanthocyanidins, gallolylation percentage, and steric hindrance affect the interactions between polyphenols and polysaccharides [70,71,72,73,74]. Simple phenolic acids such as ferulic acid, p-hydroxyphenyl, syringyl, and p-coumaroyl acids covalently associate with plant cell wall arabinogalactans and arabinoxylanes in sugar beet and wheat, bamboo, maize, and spinach. Proanthocyanidins bind to the cell wall via non-covalent interactions like hydrogen bonding and van der Waals forces [12]. Flavonols such as rutin, isoquercetin, and quercitrin were reportedly parts of NEPPs in fruits or leaves, tomato peel, and tropical and subtropical fruits. However, the exact interaction of flavonoids and the cell wall is partially understood. Hu et al. (2023) reported that specific non-covalent interactions occur in pectin-polyphenols via hydrophobic interactions and hydrogen bonding [75]. Watrelot et al. (2013) reported that aromatic groups in polymeric procyanidin led to a hydrophobic interaction with pectin polysaccharides [76]. Fernandes et al. (2020) reported that branched arabinan-pectic polysaccharides hinder the potential interactions between polyphenols and polysaccharides, where linear arabinans exhibited 2–8-fold higher retention for phenolic compounds such as phloridzin and chlorogenic acid than the branched arabinans [77].
Polyphenol–polysaccharide interaction significantly influences polyphenol extractability and potential nutritional biochemical properties [78,79,80]. While most polyphenols are extracted using a conventional water–organic solvent solution, the optimum extractability depends on the polyphenol–polysaccharide ratio, pH, ionic strength of the extracting solvents including the time, temperature, and atmospheric conditions [9,10,13]. As NEPPs are extracted through acidic, alkaline, or enzymatic hydrolysis, these factors also influence the extraction rate of bound phenolics from the food matrix [79].
The polyphenol–polysaccharide interaction properties strongly influence the rate of polyphenol release at various stages of the digestive system involving the upper and lower GI tracts and colon [68]. A fraction of polyphenols is partially released in the small intestine owing to the weakening of the ester-phenolic carbohydrate bonds and the perturbation of phenolic polysaccharide interactions. An abundant fraction of NEPPs reaches the lower intestinal tract, where it undergoes fermentation by the microflora in the colon and produces diverse metabolites, including phenolic acids such as ferulic acid, valeric acid, SCFA, and phenolic-SCFA conjugates [68,80,81]. In the colon, some hydrolytic enzymes, including carbohydrolases and protease, are secreted by microbiomes such as Bifidobacterium spp., Clostridium spp., and Lactobacillus spp. and weaken the phenolic–polysaccharide interaction by hydrolyzing the covalent bonds between phenols and carbohydrates and the glycosidic linkages in polyphenol compounds [68,79]. In some cases, the metabolites are biologically more active than the native compounds [68]. Figure 1 represents the fates of the bioaccessibility of free and bound phenolic compounds during the entire digestive process.
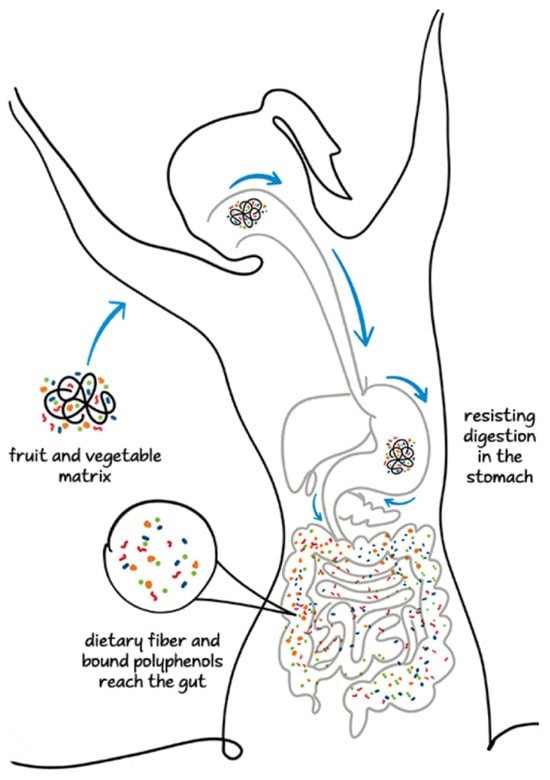
Figure 1.
Fate of inaccessibility of polyphenols from food matrices in digestive system.
In a preclinical study, ferulic acid-bound wheat bran produced more bioavailable phenolic compounds than did the free ferulic acid extract alone [82]. Vitaglione et al. (2015) found that whole grain fiber enriched with polyphenols acted as a carrier of bioactive phenolic compounds to the lower gut, while bound phenolics were not bioaccessible in the small intestine, making them bioavailable for the colon [83].
Sugarcane contains fiber-bound phenolics such as phenolic acids (ferulic acid, coumaric acid, hydroxybenzoic acid, vanillic acid, syringic acid), flavones (apigenin, luteolin, and tricin), flavanone (5,7-dihydroxyflavanone), flavanol (catechin), and flavonol (6,8-dihydroxykaempferol) [84,85]. The fibers in sugarcane act as carriers of these phenolic and polyphenolic compounds via the small intestine to the colon for fermentation [84,85]. Apple fiber contains significant amounts of non-extractable polyphenolic compounds that originate from the interactions of dietary fiber and polyphenols and affect gut microbial diversity [86]. The procyanidin–polysaccharide interactions within the apple matrices decrease procyanidin degradation by the human gut microbiota [87].
In addition to the beneficial effects as carriers of phenolics to the colon, fibers and polyphenols act synergistically with potential biochemical properties. Previous studies have reported that the individual physiological effects of NEPPs or dietary fibers are less pronounced than the combined effects of both. They provide a synergistic effect, with enhanced fermentation of NEPPs and bioavailability of metabolites for their various physiological functions. During the entire fermentation process, fibers or polysaccharides could act as prebiotics for colonic microflora. For example, Saura-Calixto et al. (2010) demonstrated that in vitro fermentation with dietary fiber-enriched NEPPs and fiber alone showed 53% and 23% fermentability, respectively [80]. The association of a fiber–polyphenol matrix could affect the microbiota community. In a randomized control study, Vitaglione et al. (2015) found that whole wheat grain intake increased Bacteroidetes and Firmicutes proportions but decreased Clostridium abundance, accompanied by reduced inflammatory markers, while refined wheat grain lowered Bifidobacteriales abundance and increased Bacteroidetes abundance [83].
A reciprocal relationship between polyphenols and the gut microbial community could occur during the digestion processes of food matrices. After reaching the polyphenols to the colon, the gut microbiota transforms polyphenols into varieties of bioactive metabolites, and then those metabolites influence the diversity of the microbiome, including the Firmicutes/Bacteroidetes F/B ratio. In most cases, phytochemicals are transformed into low-molecular-weight metabolites [88,89]. Most food polyphenolic compounds are present as monosaccharides, organic acid, and their conjugates. During the digestive process, these derivatives undergo microbial enzymatic catabolism via O-deglycosylations and ester hydrolysis, C-ring cleavage, delactonization, demethylation, dehydroxylation, and the reduction of double-bond compounds [89]. Various microbial enzymes transform polymeric NEPPs into low-molecular-weight metabolites [90]. During this process, they are further depolymerized and catabolized through microbial enzyme-induced delactonization and decarboxylation and finally form phenolic acids such as phenylacetic acid, phenyl propionic acid, phenylvaleric acid, cinnamic acid, benzoic acid, and hippuric acid [91,92]. Finally, these metabolites are delivered to the liver after passing through colonocytes, followed by systemic circulation and subsequent absorption by different tissues or elimination through the urine [93]. During the microbial transformation of hydrolyzable tannins, the gallotannins are hydrolyzed to gallic acid and glucose and converted to pyrogallol and phloroglucinol and, subsequently, to their final products such as acetate and butyrate. Ellagitannins are biotransformed by the cleavage of the lactone ring, decarboxylation, and dehydroxylation [89].
Furthermore, polyphenols and gut microbiota are interrelated. The effects of phenolic compounds on the abundance and diversity of gut microbiota have been studied extensively. The incubation of flavanols such as catechin and epicatechin from tea, with human fecal bacteria reduced the growth of certain pathogens such as Clostridium difficile, Clostridium perfringens, Streptococcus pyogenes, and Streptococcus pneumoniae [94]. Another study by Tzounis et al. (2008) on the batch-culture incubation of human fecal bacteria with (+)-catechin observed that catechin exerted a strong prebiotic effect by enhancing the growth of the Erec group, Bifidobacterium spp., and E. coli. significantly [95]. Catechin also inhibited pathogenic Clostridium histolyticum growth. The growth of Lactobacillus spp., Bifidobacterium spp., and butyrate-producing Erec were enhanced in a batch-culture fermentation with anthocyanins such as malvidin-3-glucoside [96]. Moreover, when a mixture of anthocyanins with malvidin-3-glucoside, delphinidin-3-glucoside, petunidin-3-glucoside, peonidin-3-glucoside, and cyanidin-3-glucoside was fermented, an increase in the abundances of Lactobacillus spp. and Bifidobacterium spp. occurred compared with malvidin-3-glucoside alone [96]. In vitro (batch fermentation of human fecal bacteria) and in vivo (rat) studies of quercetin and rutin demonstrated that they enhanced the growth of Bifidobacterium spp. (at 10 ug/mL) and reduced the Firmicutes/Bacteroidetes F/B ratio [97,98]. Naringenin inhibited the growth of E. coli, S. aureus, S. typhimurium, L. rhamnosus, B. galactorunicus, Lactobacillus sp., E. caccae, B. catenulatum, and R. gauvreauii. Isoflavones such as daidzein and genistein showed antibacterial activities against E. coli, S. typhimurium, and S. rhamnosus [99]. Phenolic acids such as hydroxycinnamic acid inhibited the growth of E. coli, S. aureus, S. typhimurium, and L. rhamnosus. Caffeic acid and chlorogenic acid reduced the Firmicutes/Bacteroidetes F/B ratio of the fecal bacterial community [98]. Gallic and caffeic acids inhibited the growth of pathogenic bacteria C. perfringens, C. difficile, Listeria monocytogenes, and S. aureus [99].
6. Interactions of Phenol-Rich Sources and Gut Microbiota
6.1. Pomegranate
Pomegranate is a rich phenol source, comprising extractable and non-extractable phenolic compounds. Pomegranate has abundant antioxidants and anti-inflammatory bioactive compounds, primarily ellagitannins and anthocyanins, which are concentrated in the peel, membranes, and piths [100]. Sun et al. (2021) identified ten NEPPs in pomegranate peel, among which they separated six [101]. These were recognized as β-sitosterol-3-O-glycoside, β-sitosterol, ursolic acid, corosolic acid, asiatic acid, and arjunolic acid. Ursolic and asiatic acids demonstrated antimicrobial activity against various pathogens [101]. Pomegranate peel extract hindered the growth of C. perfringens, C. ramosum, S. aureus, and C. clostridioforme and markedly increased Bifidobacterium breve and Bifidobacterium infantis [102]. Pomegranate by-product increased the growth of total bacteria, Bifidobacterium spp., and Lactobacillus–Enterococcus and SCFA production [103]. This implies that consuming pomegranate peel extract improves health through the modulation of gut microbiota [100].
Pomegranate juice is considered a significant source of phenolic compounds, with anthocyanins being the most crucial [104], along with lignans [105], gallagyl-type tannins, ellagic acid derivatives, and other hydrolyzable tannins, which enhance the antioxidant activity of the juice [106,107]. Pomegranate juice is superior to other juices, as it is enriched with polyphenols and has a higher antioxidant activity than do common juices or extracts [108]. The antioxidant activity of pomegranate juice reported by Gil et al. (2000) was thrice higher than that of red wine and green tea [106]. Similarly, pomegranate juice has displayed notable anti-atherosclerotic (prevention of oxidative stress and inflammation in the arterial wall), anti-hypertensive, and anti-inflammatory effects in humans. The gut microbiota composition is not significantly altered by the consumption of pomegranate juice [109].
6.2. Berries
Berries contain an abundance of polyphenolic compounds, such as phenolic acids, flavonols, and anthocyanins. Their polyphenol profile varies from one species to another. Most of the berry polyphenols reach the gut and metabolize smaller phenolic compounds there through the action of microorganisms [110]. Polyphenols and their metabolites can also modulate microbial populations, which can lead to an increase in the healthy bacteria Bifidobacterium, Lactobacillus, and Akkermansia. The consumption of berries has led to beneficial effects, primarily anti-inflammatory activity and the promotion of apoptosis [110].
Cranberries are among the richest sources of polyphenols, comprising flavonols, particularly quercetin, anthocyanins, and oligomeric A-type proanthocyanidins [111]. Cranberry polyphenols (CPs) have several biological effects, such as antioxidant, antibacterial, antifungal, urinary tract protection, cardioprotective, and anticancer activities [111]. Certain CPs, particularly proanthocyanidins, are poorly absorbed in the small intestine. These polyphenols, therefore, are bioaccessible only in the large intestine, where they may interact with the microbiota and modify gut microbial composition to maintain the metabolic health of humans [112]. A cranberry-rich diet may be beneficial in preventing metabolic syndromes associated with chronic diseases and improving obesity-induced dysbiosis. The consumption of cranberry extracts rich in polyphenols, such as proanthocyanidin polymers, promotes the growth of bacteria, such as Barnesiella, Akkermansia muciniphila, Lactobacillus, and Coriobacteriales, while repressing the growth of others, such as Oscillibacter, Romboutsia, Ruminiclostridium, and Roseburia. CPs could inhibit nonbeneficial bacteria in the human microbiota and facilitate the growth of probiotic bacteria [112]. Cranberries comprise anthocyanins, the major polyphenols in the EPP fractions, while phenolic acids are abundant in the NEPP fraction [113]. Han et al. (2019) showed that the oxygen radical absorbance capacity of the NEPPs is significantly higher than that of the EPPs. The EPP and NEPP fractions from cranberries are bioactive, and the NEPPs showed potentially stronger anti-inflammatory and anti-colon cancer activity than the EPs [113].
The most important polyphenols found in blueberries are anthocyanins, and the content varies depending on the blueberry variety. For example, Giacalone et al. (2015) observed a wild type of blueberries contained 340 mg/100 g of fresh fruit, which is more than 50% of the total polyphenol content in the pulp and skin of the blueberries. Polyphenol content increases as the fresh fruit matures [114]. The primary anthocyanins contained in blueberry are pelargonidin-3-D-galactoside, delphinidin-3-glucoside, and malvin-3-O-glucose. Pelargonidin-3-D-galactoside may prevent and control illnesses associated with high cholesterol. Delphinidin-3-glucoside can prevent the spread of breast and colon cancer [115]. The consumption of blueberries, which are rich in anthocyanins, increases antioxidant activity. Blueberry polyphenols are good neuroprotective agents that can decelerate aging and treat neurological disorders [114]. Moreover, blueberry polyphenols regulate the composition of gut microbiota. The diversity of gut microbes improves with an increasing amount of Akkermansia and a decreased amount of Proteobacteria, suggesting that blueberries may correct gut dysbiosis. The consumption of blueberries increases the abundance of Bifidobacterium, Faecalibaculum, Lactobacillus, Parabacteroides, and Roseburia organisms. Bifidobacterium and Lactobacillus have beneficial effects such as reduction in low-grade inflammation and better gut barrier function [116]. The other three bacteria produce SCFAs with Roseburia, especially yielding butyrate. Gut microbiota ferment the fiber in blueberries, which can change the activity of bacteria responsible for the breakdown of polyphenols. Moreover, the metabolism of polyphenols can be reduced by binding fibers with proanthocyanidins, decreasing the production of bioactive anti-inflammatory metabolites [116].
Elderberry is abundant in bioactive compounds, such as polyphenolic compounds and terpenoid compounds. The polyphenolic compounds include phenolic acids, flavanones, flavonols, and anthocyanins in elderberry leaves and fruits [117]. Liu et al. (2022) determined that the primary phenolic acids in elderberry fruit were gallic acid and gentisic acid, while the main flavonoids were rutin and quercetin [117]. The primary phenolic acids in elderberry flower extracts were chlorogenic acid, 5-p-coumaroylquinic acid, and dicaffeoylquinic acid, whereas rutin and naringenin were the main flavonols and flavanones detected in the extracts. The major anthocyanins in elderberry fruits were cyanidin 3-O-sambubioside and cyanidin 3-O-glucoside. Additionally, 13 polyphenolic compounds, mainly anthocyanins, were detected in both elderberry fruits and branches [117]. Overall, the elderberry flowers are more abundant in polyphenolic compounds than the fruits. Moreover, the number of polyphenols was higher in fruits and flowers than in the other parts of the elderberry plant, its branches, leaves, and floral stems. The primary triterpenoids detected in elderberry fruits were ursolic and oleanolic acids [117]. Elderberry fruits exhibit various health benefits such as antioxidant, anti-inflammatory, anticancer, anti-influenza, antimicrobial, antidiabetic, cardiovascular-protective, and neuroprotective functions [117]. Teets et al. (2024) observed that the consumption of elderberry juice significantly increased the abundance of Firmicutes and Actinobacteria and decreased the Bacteroidetes phyla [118]. Drinking elderberry juice increased Faecalibacterium, Ruminococcaceae, and Bifidobacterium and decreased Bacteroides and lactic acid-producing bacteria [118].
6.3. Tea
Tea (from the plant Camellia sinensis) contains substantial flavonoids, with catechins being the major class, including epicatechin, epigallocatechin, epicatechin-3-gallate, and epigallocatechin-3-gallate. Additionally, tea contains flavanols, such as quercetin, kaempferol, myricetin, and their glycosides [100]. The NEPP content in green tea is four times lower than that of EPP. The antioxidant activity of NEPPs is lower than that of EPPs but higher than that of vitamin C. NEPPs also display an α-glucosidase inhibitory effect. Gallocatechin, epigallocatechin, catechin, epicatechin, and caffeine were found to be the most abundant compounds in green tea [119]. Phenolic compounds in green tea reduce the abundance of C. perfringens and other Clostridium species [100]. A study of 10 participants showed that the proportion of Bifidobacteria increased after drinking green tea and decreased after stopping tea consumption. However, the makeup of the Bifidobacterium species showed no significant changes in each sample. This indicates that green tea may act as a prebiotic and enhance the colon microbiota by increasing the proportion of the Bifidobacterium species [120].
6.4. Cocoa
Cocoa and its products are enriched with polyphenols, such as flavan-3-ols, in the form of epicatechin and catechin, as well as type-B proanthocyanidins [98,121]. These polyphenols may help alleviate conditions such as hypertension, oxidative stress, cancer, atherosclerosis, diabetes, and diverse central nervous system disorders related to gut microbiota [100]. Many cocoa polyphenols reach the colon, where they are degraded into smaller metabolites by gut microbiota. Cocoa metabolites improve gut health by exerting anti-inflammatory and antioxidant effects, positively affecting immunity and reducing the risk of various diseases [121]. Cocoa polyphenols exert prebiotic effects, as they promote the growth of beneficial bacteria, such as Lactobacillus and Bifidobacterium, but reduce the abundance of harmful bacteria, such as certain Clostridium species [121]. Cocoa intake significantly reduces the abundance of the Bacteroides, Staphylococcus, and Clostridium genera. Lactobacillus spp. and Bifidobacterium spp. abundances were increased significantly with the intake of high-cocoa flavanol, while the C. histolyticum group, including the C. perfringens pathogen, which contributes to human disease, showed a decreased abundance [100].
6.5. Red Wine
Wine has abundant phenolic compounds, comprising a mixture of flavonoids (flavan-3-ols and anthocyanins), non-flavonoids (resveratrol, cinnamates, and gallic acid), oligomeric and polymeric proanthocyanins, catechins, and phenolic acid [122,123]. Dolara et al. (2005) reported that the wine polyphenolic extract contained 4.4% anthocyanins, 0.8% flavanols, 2.0% phenolic acids, 1.4% catechin, 1.0% epicatechin, and 28% proanthocyanidin [124]. Drinking red wine is associated with multiple health benefits, ranging from a reduction in cardiovascular disease risk factors, metabolic syndrome, and depression to improved cognition and positive effects related to gut microbiota diversity [123,124,125,126,127]. Polyphenols reduce oxidative stress, exhibit anti-inflammatory activity, and promote beneficial gut bacteria [124]. Polyphenol-treated rats showed higher Bacteroides, Lactobacillus, and Bifidobacterium spp. proportions than did the controls. However, Bacteroides, Clostridium, and Propionibacterium spp. showed higher abundances in the control rat feces. These imply that wine polyphenols simulate the favorable effects of fibers and prebiotics on the colon bacteria [124]. Queipo-Ortuno et al. (2012) detected an increase in four major bacteria phyla (Proteobacteria, Fusobacteria, Firmicutes, and Bacteroidetes) and significant increases in Bifidobacterium and Prevotella abundance after drinking red wine. No changes were observed in the Actinobacteria phyla; however, significant decreases were detected in the Clostridium genera and Clostridium histolyticum group following red wine consumption [127]. This was associated with decreased blood pressure, triglycerides, total cholesterol, and C-reactive protein [125,126,127].
6.6. Grapes
Grapes (Vitis vinifera) are rich in bioactive compounds, particularly polyphenols, which primarily comprise proanthocyanidins, anthocyanins, flavonols, phenolic acids, and stilbenes [128,129]. The polyphenol contents depend on the grape parts. Most of the polyphenols, primarily proanthocyanidins, are in the grape seed [130,131]. The major flavonoids in grapes include flavan-3-ols, flavonols, and anthocyanins, whereas non-flavonoids primarily comprise phenolic acids and stilbenes. Flavan-3-ols are the richest flavonoids in grapes, stored primarily in the seeds but are rarely detected in the skin [132]. Grape skin contains relatively substantial amounts of anthocyanins, whereas almost no anthocyanins are in grape seed [132,133]. Grape polyphenols have diverse health benefits, such as antioxidant, anti-inflammatory, gut-microbiota-modulating, anti-obesity, cardioprotective, hepatoprotective, antidiabetic, and anticancer effects [134]. Han et al. (2020) reported that grape extract restored the dysbiosis of gut microbiota produced by a high-fat diet by increasing the B/F ratio and the abundances of Bifidobacteria, Clostridia, and Akkermansia [135].
Table 1 presents the polyphenol dietary sources, their human health benefits, and beneficial bacteria, where available in the literature. Polyphenols reduce the concentration of pathogenic bacteria and increase the population of those that are beneficial. Consequently, polyphenols change the microbiota composition to a more beneficial profile, favoring human health.

Table 1.
Polyphenol dietary sources, their human health benefits, and beneficial bacteria.
7. Conclusions
This review summarizes the basic relationship between fibers, polyphenols, and gut microbiota and their modulatory role on human gut health. Also, this review offers fundamental insights for researchers, clinicians, and food scientists aiming to harness nutrition to support gut microbiome diversity and health. Gut microbiota influences the biotransformation of polyphenols to bioactive metabolites that control the diversity and quality of microbiomes. Fiber–polyphenol interaction significantly influences the efficient release of polyphenols at various stages of the digestive process and their bioaccessibility in the colon, where they are fermented by microflora to produce many metabolites with enhanced bioavailability and bioactivities. Moreover, the composition and structure of dietary fibers affect the interaction level and influence the diversity and activity of the gut microbiota. A complex interactive relationship exists between food matrices containing fibers and polyphenols and their role in modulating the gut microbiota. The formulation of new products with the desired fibers and polyphenols is promising in improving human gut health by modulating the microbiota. Future studies exploring a) how the types of dietary fibers and polyphenols influence microbial diversity and metabolite production and b) the optimal combinations of dietary fibers and polyphenols that maximize bioaccessibility and bioactivities in the colon would be valuable for developing cost-effective and consumer-friendly new functional food products.
Author Contributions
Conceptualization, B.V.N., F.A.-T. and D.K.; writing—original draft preparation, A.Y.Y., Y.I.Y., B.V.N., D.K. and F.A.-T.; review and editing, B.V.N., F.A.-T. and D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Authors Boris Nemzer, Fadwa Al-Taher and Diganta Kalita were employed by the VDF FutureCeuticals, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Han, X.; Shen, T.; Lou, H. Dieatry polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Tufarelli, V.; Casalino, E.; D’Alessandro, A.G.; Laudadio, V. Dietary Phenolic Compounds: Biochemistry, Metabolism and Significance in Animal and Human Health. Curr. Drug Metab. 2017, 18, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions or future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Bo’, C.D.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic review on polyphenol intake and health outcomes: Is there sufficient evidence to define a health-promoting polyphenol-rich dietary pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Burkholder-Cooley, N.; Rajaram, S.; Haddad, E.; Fraser, G.E.; Jaceldo-Siegl, K. Comparison of polyphenol intakes according to distinct dietary patterns and food sources in the adventist health study-2 cohort. Br. J. Nutr. 2016, 115, 2162–2169. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary plant polyphenols: Effects of food processing on their content and bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Torres, J.L. Analysis of nonextractable phenolic compounds in foods: The current state of the art. J. Agric. Food Chem. 2011, 59, 12713–12724. [Google Scholar] [CrossRef]
- Arranz, S.; Silva’n, J.M.; Saura-Calixto, F. Nonextractable polyphenols, usually ignored, are the major part of dietary polyphenols: A study on the Spanish diet. Mol. Nutr. Food Res. 2010, 54, 1646–1658. [Google Scholar] [CrossRef]
- Pe’rez-Jime’nez, J.; Dı’az-Rubio, M.E.; Saura-Calixto, F. Non-extractable polyphenols, a major dietary antioxidant: Occurrence, metabolic fate and health effects. Nutr. Res. Rev. 2013, 26, 118–129. [Google Scholar] [CrossRef]
- Ding, Y.; Morozova, K.; Scamicchio, M.; Ferrentino, G. Non-extractable polyphenols from food by-products: Current knowledge on recovery, characterization, and potential applications. Processes 2020, 8, 925. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber-polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Fernandes, A.; Mateus, N.; de Freitas, V. Polyphenol-dietary fiber conjugates from fruits and vegetables: Nature and biological fate in a food and nutrition perspective. Foods 2023, 12, 1052. [Google Scholar] [CrossRef] [PubMed]
- Bié, J.; Spodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in health and disease: Gut microbiota, bioaccessibility, and bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Lorenzo, C.D.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, 538–544. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef]
- Illiano, P.; Brambilla, R.; Parolini, C. The mutual interplay of gut microbiota, diet, and human disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the gastrointestinal tract. Med. Sci. 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Bengmark, S. Ecological control of the gastrointestinal tract. The role of probiotic flora. Gut 1998, 42, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbassociated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, X.; Li, L. Human gut microbiome–the second genome of human body. Protein Cell 2010, 1, 718–725. [Google Scholar] [CrossRef]
- Hugon, P.; Dufour, J.-C.; Colson, P.; Fournier, P.E.; Sallah, K.; Raoult, D. A comprehensive repertoire of prokaryotic species identified in human beings. Lancet Infect. Dis. 2015, 15, 1211–1219. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Hill, M.J. Intestinal flora and endogenous vitamin synthesis. Eur. J. Cancer Prev. 1997, 6, S43–S45. [Google Scholar] [CrossRef]
- Martens, J.H.; Barg, H.; Warren, M.; Jahn, D. Microbial production of vitamin B-12. Appl. Microbiol. Biotechnol. 2002, 58, 275–285. [Google Scholar] [CrossRef]
- Pompei, A.; Cordisco, L.; Amaretti, A.; Zanoni, S.; Matteuzzi, D.; Rossi, M. Folate production by bifidobacterial as a potential probiotic property. Appl. Environ. Microbiol. 2007, 73, 179–185. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Agostoni, C.; Kim, K.S. Nutrition and the microbiome. Pediatr. Res. 2015, 77, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.A.; Worobey, M. Geographical variation of human gut microbial composition. Biol. Lett. 2014, 10, 20131037. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Moles, L.; Otaegui, D. The impact of diet on microbiota evolution and human health. Is diet an adequate tool for microbiota modulation? Review. Nutrients 2020, 12, 1654. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Healey, G.R.; Murphy, R.; Brough, L.; Butts, C.; Coad, J. Interindividual variability in gut microbiota and host response to dietary interventions. Nutr. Rev. 2017, 75, 1059–1080. [Google Scholar] [CrossRef]
- Salazar, N.; Valdés, L.; González, S.; Gueimonde, M.; De Los Reyes-Gavilán., C.G. Nutrition and the microbiome in the elderly. Nutrition and the gut microbiome in the elderly. Gut Microbes 2017, 8, 82–97. [Google Scholar] [CrossRef]
- Sommer, F.; Backhed, F. The gut microbiota- Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–763. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet-microbiota interaction as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Espín, J.C.; Tomás-Barberán, F.A. Non-extractable polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical-scavenging effects. Trends Food Sci. Technol. 2017, 69, 281–288. [Google Scholar] [CrossRef]
- Tengeler, A.C.; Kozicz, T.; Kiliaan, A.J. Relationship between diet, the gut microbiota and brain function. Nutr. Rev. 2018, 76, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef]
- Nettleton, J.E.; Reimer, R.A.; Shearer, J. Reshaping the gut microbiota: Impact of low calorie sweeteners and the link to insulin resistance? Physiol. Behav. 2016, 164 Pt B, 488–493. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.; Poole, A.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Frame, L.A.; Costa, E.; Jackson, S.A. Current explorations of nutrition and the gut microbiome: A comprehensive evaluation of the review literature. Nutr. Rev. 2020, 78, 798–812. [Google Scholar] [CrossRef]
- David, L.A.; Mourice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Moschen, A.; Wieser, V.; Tilg, H. Dietary factors: Major regulators of the gut’s microbiota. Gut Liver 2012, 6, 411–416. [Google Scholar] [CrossRef]
- Requena, T.; Martinez-Cuesta, M.C.; Pelaez, C. Diet and microbiota linked in health and disease. Food Funct. 2018, 9, 688–704. [Google Scholar] [CrossRef] [PubMed]
- Haller, D. The Gut Microbiome in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Di Baise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittman, B.E.; et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. Nutrition, IBD and gut microbiota: A review. Nutrients 2020, 12, 944. [Google Scholar] [CrossRef]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosentiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, W.D.; Wang, Y.D. Gut microbiota–an integral moderator in health and disease. Front. Microbiol. 2018, 9, 151. [Google Scholar] [CrossRef]
- Gao, Z.; Guo, B.; Gao, R.; Zhu, Q.; Qin, H. Microbiota disbiosis is associated with colorectal cancer. Front. Microbiol. 2015, 6, 20. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiota in health and disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Wong, J.M.W.; Esfahani, A.; Singh, N.; Villa, C.R.; Mirrahimi, A.; Jenkins, D.J.A.; Kendall, C.W.C. Gut microbiota, diet, and heart disease. J. AOAC Int. 2012, 95, 24–30. [Google Scholar] [CrossRef]
- Tang, W.H.; Hazen, S.L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Investig. 2014, 124, 4204–4211. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Gallenzzi, L.; Viand, S.; Vétizou, M.; Daillère, R.; Merad, M.; Kroemer, G. Cancer and the gut microbiota—An unexpected link. Sci. Transl. Med. 2015, 7, 271. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, M.; Ferro, A.; Gruden, G. Gastrointestinal microbiota and Type 1 diabetes mellitus: The state of art. J. Clin. Med. 2019, 8, 1843. [Google Scholar] [CrossRef]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The human microbiota in health and disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Wypych, T.P.; Marsland, B.J. Diet hypothesis in light of the microbiota revolution-new perspectives. Nutrients 2017, 7, 537. [Google Scholar] [CrossRef]
- Rocchetti, G.; Gregorio, R.P.; Lorenzo, J.M.; Barba, F.J.; Oliveira, P.G.; Prieto, M.A.; Simal-Gandara, J.; Mosele, J.I.; Motilva, M.J.; Tomas, M.; et al. Functional implications of bound phenolic compounds and phenolics–food interaction: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 811–842. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Serrano, J.; Goni, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef]
- Wong, X.; Carrasco-Pozo, C.; Escobar, E.; Navarrete, P.; Blachier, F.; Andriamihaja, M.; Lan, A.; Tom’e, D.; Cires, M.J.; Pastene, E.; et al. Deleterious effect of p-Cresol on human colonic epithelial cells prevented by proanthocyanidin- containing polyphenol extracts from fruits and proanthocyanidin bacterial metabolites. J. Agric. Food Chem. 2016, 64, 3574–3583. [Google Scholar] [CrossRef]
- Carboni Martins, C.; Rodrigues, R.C.; Domeneghini Mercali, G.; Rodrigues, E. New insights into non-extractable phenolic compounds analysis. Food Res. Int. 2022, 157, 111487. [Google Scholar] [CrossRef]
- Chirug, L.; Okun, Z.; Ramon, O.; Shpigelman, A. Iron ions as mediators in pectin-flavonols interactions. Food Hydrocoll. 2018, 84, 441–449. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Cano-Lechuga, M.; Ruiz-García, Y.; Gómez-Plaza, E. Interactions between grape skin cell wall material and commercial enological tannins. Practical implications. Food Chem. 2014, 152, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Le Bourvellec, C.; Renard, C.M.G.C. Interactions between cell wall polysaccharides and polyphenols: Effect of molecular internal structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Bi, J.; Li, X.; Wu, X.; Wang, W.; Yu, Q. Understanding the impact of pectin on browning of polyphenol oxidation system in thermal and storage processing. Carbohydr. Polym. 2023, 307, 120641. [Google Scholar] [CrossRef] [PubMed]
- Watrelot, A.A.; Le Bourvellec, C.; Imberty, A.; Renard, C.M.G.C. Interactions between pectic compounds and procyanidins are influenced by methylation degree and chain length. Biomacromolecules 2013, 14, 709–718. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Le Bourvellec, C.; Renard, C.M.G.C.; Wessel, D.F.; Cardoso, S.M.; Coimbra, M.A. Interactions of arabinan-rich pectic polysaccharides with polyphenols. Carbohydr. Polym. 2020, 230, 115644. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Interactions between apple (Malus x domestica Borkh.) polyphenols and cell walls modulate the extractability of polysaccharides. Carbohydr. Polym. 2009, 75, 251–261. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Strategies for the extraction and analysis of non-extractable polyphenols from plants. J. Chromatogr. A 2017, 1514, 1–15. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Pérez-Jiménez, J.; Touriño, S.; Serrano, J.; Fuguet, E.; Torres, J.L.; Goñi, I. Proanthocyanidin metabolites associated with dietary fibre from in vitro colonic fermentation and proanthocyanidin metabolites in human plasma. Mol. Nutr. Food Res. 2010, 54, 939–946. [Google Scholar] [CrossRef]
- Das, T.; Chatterjee, N.; Capanoglu, E.; Lorenzo, M.; Das, A.K.; Dhar, P. The synergistic ramification of insoluble dietary fiber and associated non-extractable polyphenols on gut microbial population escorting alleviation of lifestyle diseases. Food Chem. 2023, 18, 100697. [Google Scholar] [CrossRef]
- Rondini, L.; Peyrat-Maillard, M.-N.; Marsset-Baglieri, A.; Fromentin, G.; Durand, P.; Tom’e, D.; Prost, M.; Berset, C. Bound ferulic acid from bran is more bioavailable than the free compound in Rat. J. Agric. Food Chem. 2004, 52, 4338–4343. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Mennella, I.; Ferracane, R.; Rivellese, A.A.; Giacco, R.; Ercolini, D.; Gibbons, S.M.; La Storia, A.; Gilbert, J.A.; Jonnalagadda, S.; et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: Role of polyphenols bound to cereal dietary fiber 2–4. Am. J. Clin. Nutr. 2015, 101, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F. Concept and health-related properties of nonextractable polyphenols: The missing dietary polyphenols. J. Agric. Food Chem. 2012, 60, 11195–11200. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.; Gómez-Caravaca, A.M.; Di Nunzio, M.; Danesi, F.; Caboni, M.F.; Bordoni, A. Sugar cane and sugar beet molasses, antioxidant-rich alternatives to refined sugar. J. Agric. Food Chem. 2012, 60, 12508–12515. [Google Scholar] [CrossRef]
- Koutsos, A.; Tuohy, K.; Lovegrove, J. Apples and cardiovascular health--is the gut microbiota a core consideration? Nutrients 2015, 26, 3959–3998. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Bagano Vilas Boas, P.; Lepercq, P.; Comtet-Marre, S.; Auffret, P.; Ruiz, P.; Chatel, J.-M. Procyanidin—Cell wall interactions within apple matrices decrease the metabolization of procyanidins by the human gut microbiota and the anti-inflammatory effect of the resulting microbial metabolome in vitro. Nutrients 2019, 11, 664. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Hu, T.; Zhao, H. Metabolic fate of tea polyphenols and their crosstalk with gut microbiota. Food Sci. Human Wellness 2022, 11, 455–466. [Google Scholar] [CrossRef]
- Tom’as-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Vincken, J.P.; Aura, A.M.; Hollman, P.C.H.; Gruppen, H. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-γ- valerolactone as the major metabolites. J. Agric. Food Chem. 2009, 57, 1084–1092. [Google Scholar] [CrossRef]
- Li, H.; Christman, L.M.; Li, R.; Gu, L. Synergic interactions between polyphenols and gut microbiota in mitigating inflammatory bowel diseases. Food Funct. 2020, 11, 4878–4891. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Barroso, E.; Van De Wiele, T.; Jiménez-Girón, A.; Martín- Alvarez, P.J.; Moreno-Arribas, M.V.; Martínez-Cuesta, M.C.; Peláez, C.; Requena, T.; Bartolomé, B. Comparative in vitro fermentations of cranberry and grape seed polyphenols with colonic microbiota. Food Chem. 2015, 183, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Margalef, M.; Pons, Z.; Bravo, F.I.; Muguerza, B.; Arola-Arnal, A. Plasma kinetics and microbial biotransformation of grape seed flavanols in rats. J. Funct. Foods. 2015, 12, 478–488. [Google Scholar] [CrossRef]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.; George, T.; Leonczak, J.; Gibson, G.R.; Spencer, J.P. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boque, N.; Macarulla, M.; Portillo, M.; Martinez, J.; Milagro, F. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high -at sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef]
- Etxeberria, U.; Fernandes-Quintela, A.; Milargo, F.T.; Leixuri Aguirre, J.; Martínez, A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef]
- Sun, S.; Huang, S.; Shi, Y.; Qiu, J.; Sedjoah, R.-C.A.-A.; Yan, Z.; Ding, L.; Zou, D.; Xin, Z. Extraction, isolation, characterization and antimicrobial activities of non-extractable polyphenols from pomegranate peel. Food Chem. 2021, 351, 129232. [Google Scholar] [CrossRef]
- Bialonska, D.; Kasimsetty, S.G.; Schrader, K.K.; Ferreira, D. The effect of pomegranate (Punica granatum L.) byproducts and ellagitannins on the growth of human gut bacteria. J. Agric. Food Chem. 2009, 57, 8344–8349. [Google Scholar] [CrossRef] [PubMed]
- Bialonska, D.; Ramnani, P.; Kasimsetty, S.G.; Muntha, K.R.; Gibson, G.R.; Ferreira, D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010, 140, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Miguel, G.; Fontes, C.; Martins, D.; Neves, A.; Antunes, D. Evolution of anthocyanins in pomegranate juice (Punica granatum L.) of two cultivars “Mollar” and “Assaria” during cold storage. Sci. Aliment. 2007, 27, 431–438. [Google Scholar] [CrossRef]
- Bonzanini, F.; Bruni, R.; Palla, G.; Serlataite, N.; Caligiani, A. Identification and distribution of lignans in Punica granatum L. fruit endocarp, pulp, seeds, wood knots and commercial juices by GC-MS. Food Chem. 2009, 117, 745–749. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Heber, D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett. 2008, 269, 262–268. [Google Scholar] [CrossRef]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef]
- Basu, A.; Penugonda, K. Pomegranate juice: A heart-healthy fruit juice. Nutr. Rev. 2009, 67, 49–56. [Google Scholar] [CrossRef]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef]
- Jurikova, T.; Skrovankova, S.; Mlcek, J.; Balla, S.; Snopek, L. Bioactive compounds, antioxidant activity, and biological effects of European cranberry(Vacciniumoxycoccos). Molecules 2018, 24, 24. [Google Scholar] [CrossRef]
- Prasain, J.; Barnes, S. Cranberry polyphenols-gut microbiota interactions and potential health benefits: An updated review. Food Front. 2020, 1, 459–464. [Google Scholar] [CrossRef]
- Han, Y.; Huang, M.; Li, L.; Cai, X.; Gao, Z.; Li, F.; Rakariyatham, K.; Song, M.; Tomé, S.F.; Xiao, H. Non-extractable polyphenols from cranberries: Potential anti-inflammation and anti-colon cancer agents. Food Funct. 2019, 10, 7714–7723. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, M.; Di Sacco, F.; Traupe, I.; Pagnucci, N.; Forfori, F.; Giunta, F. Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease. In Blueberry Polyphenols and Neuroprotection; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Pisa, Italy, 2015; pp. 17–28. [Google Scholar]
- Yang, S.; Wang, C.; Li, X.; Wu, C.; Liu, C.; Xue, Z.; Kou, X. Investigation on the biological activity of anthocyanins and polyphenols in blueberry. J. Food Sci. 2021, 86, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Della Lucia, C.M.; Oliviera, L.A.; Dias, K.A.; Santana Pereira, S.M.; da Conceicao, A.R.; Anandh Babu, P.V. Scientific evidence for the beneficial effects of dietary blueberries on gut health: A systematic review. Mol. Nutr. Food Res. 2023, 67, c2300096. [Google Scholar] [CrossRef]
- Liu, D.; He, X.-Q.; Wu, D.-T.; Li, H.-B.; Feng, Y.-B.; Zou, L.; Gan, R.-Y. Elderberry (Sambucus nigra L.): Bioactive compounds, health functions, and applications. J. Agric. Food Chem. 2022, 70, 4202–4220. [Google Scholar] [CrossRef]
- Teets, C.; Ghanem, N.; Ma, G.; Minj, J.; Perkins-Veazie, P.; Johnson, S.A.; Etter, A.J.; Carbonero, F.G.; Solverson, P.M. A one-week elderberry juice intervention augments the fecal microbiota and suggests improvement in glucose tolerance and fat oxidation in a randomized controlled trial. Nutrients 2024, 16, 3555. [Google Scholar] [CrossRef]
- Yan, S.; Shao, H.; Zhou, Z.; Wang, Q.; Zhao, L.; Yang, X. Non-extractable polyphenols of green tea and their antioxidant, anti-α-glucosidase capacity, and release during in vitro digestion. J. Funct. Food. 2018, 42, 129–136. [Google Scholar] [CrossRef]
- Jin, J.S.; Tougama, M.; Hisada, T.; Benno, Y. Effects of green tea consumption on human fecal microbiota with special reference to Bifidobacterium species. Microbiol. Immunol. 2012, 56, 725–739. [Google Scholar] [CrossRef]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef]
- Kemperman, R.A.; Gross, G.; Mondot, S.; Possemiers, S.; Marzorati, M.; Van de Wiele, T.; Doré, J.; Vaughan, E.E. Impact of polyphenols from black tea and red wine/grape juice on a gut model microbiome. Food Res. Int. 2013, 53, 659–669. [Google Scholar] [CrossRef]
- Nash, V.; Ranadheera, C.S.; Georgousopoulou, E.N.; Mellor, D.D.; Demosthenes, B.; Panagiotakos, D.B.; McKune, A.J.; Kellett, J.; Naumovski, N. The effects of grape and red wine polyphenols on gut microbiota—A Systematic review. Food Res. Int. 2018, 113, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Dolara, P.; Luceri, C.; De Filippo, C.; Femia, A.P.; Giovannellis, L.; Caderni, G.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Cresci, A. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucose in F344 rats. Mutat. Res. 2005, 591, 237–246. [Google Scholar] [CrossRef]
- Pavlidou, E.; Mantzorou, M.; Fasoulas, A.; Tryfonos, C.; Petridis, D.; Giaginis, C. Wine: An aspiring agent in promoting longevity and preventing chronic diseases. Diseases 2018, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Sanchez-Alcoholado, L.; Perez-Martinez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuno, M.I.; Boto-Ordonez, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andres-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, Y.-Y. Grape phytochemicals and associated health benefits. Crit. Rev. Food Sci. Nutr. 2013, 53, 1202–1225. [Google Scholar] [CrossRef]
- Zhu, J.X.; Du, C.G. Could grape-Based food supplements prevent the development of chronic kidney disease? Crit. Rev. Food Sci. Nutr. 2020, 60, 3054–3062. [Google Scholar] [CrossRef]
- Benbouguerra, N.; Richard, T.; Saucier, C.; Garcia, F. Voltammetric behavior, flavanol and anthocyanin contents, and antioxidant capacity of grape skins and seeds during ripening (Vitis vinifera var. Merlot, tannat, and Syrah). Antioxidants 2020, 9, 800. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Simon, J.E.; Wu, Q.L. A critical review on grape polyphenols for neuroprotection: Strategies to enhance bioefficacy. Crit. Rev. Food Sci. Nutr. 2020, 60, 597–625. [Google Scholar] [CrossRef]
- de Oliveira, J.B.; Egipto, R.; Laureano, O.; de Castro, R.; Pereira, G.E.; Ricardo-da-Silva, J.M. Chemical characteristics of grapes cv. Syrah (Vitis vinifera L.) grown in the tropical semiarid region of Brazil (pernambuco state): Influence of rootstock and harvest season. J. Sci. Food Agric. 2019, 99, 5050–5063. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-D.; Li, J.; Xiong, R.-G.; Saimaiti, A.; Huang, S.Y.; Wu, S.-X.; Yang, Z.-J.; Shang, A.; Zhao, C.N.; Gan, R.-Y.; et al. Bioactive compounds, health benefits and food applications of grape. Foods 2022, 11, 2755. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Guo, J.L.; Yin, M.W.; Liu, Y.W.; You, Y.L.; Zhan, J.C.; Huang, W.D. Grape extract activates brown adipose tissue through pathway involving the regulation of gut microbiota and bile acid. Mol. Nutr. Food Res. 2020, 64, 2000149. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).