The Silencing of the StPAM16-1 Gene Enhanced the Resistance of Potato Plants to the Phytotoxin Thaxtomin A
Abstract
1. Introduction
2. Results
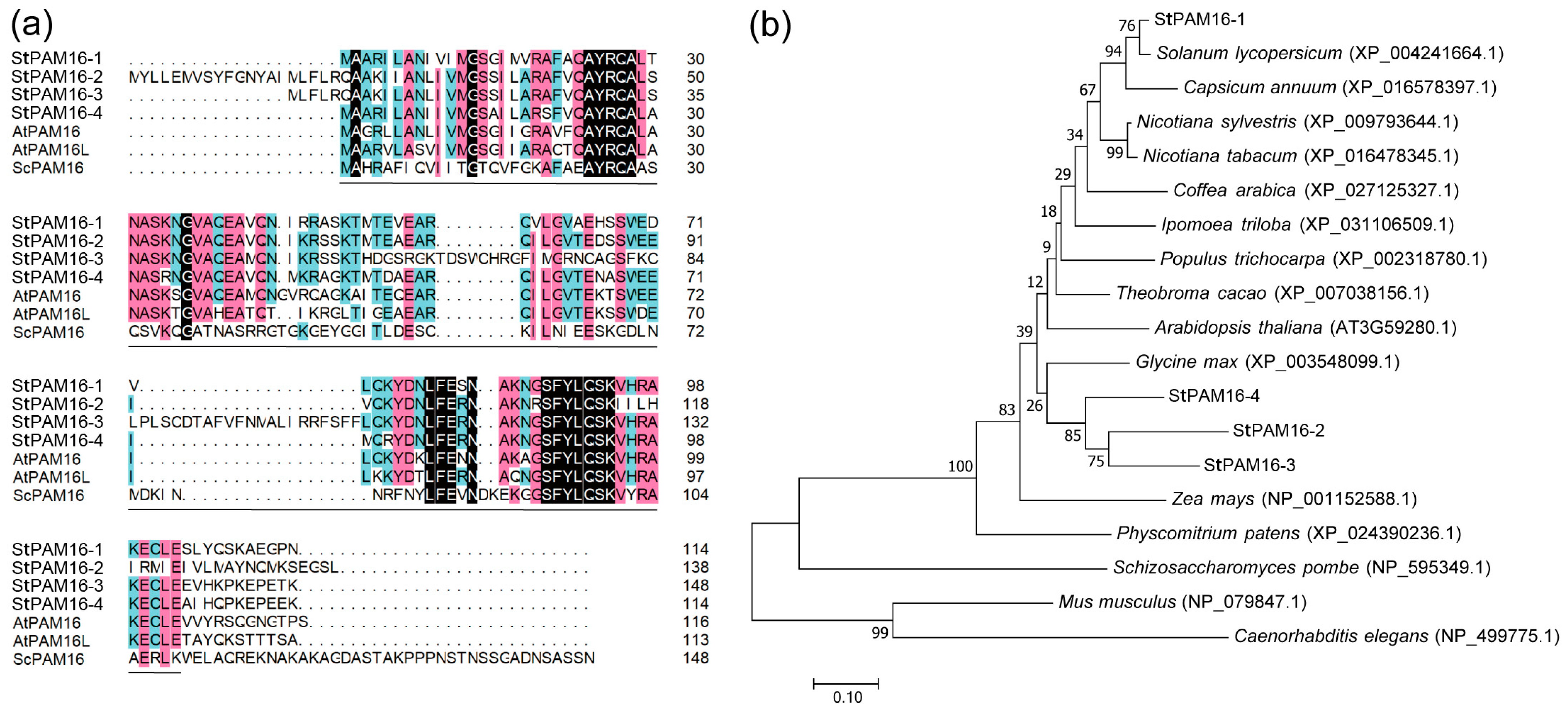
2.1. Identification of StPAM16-1
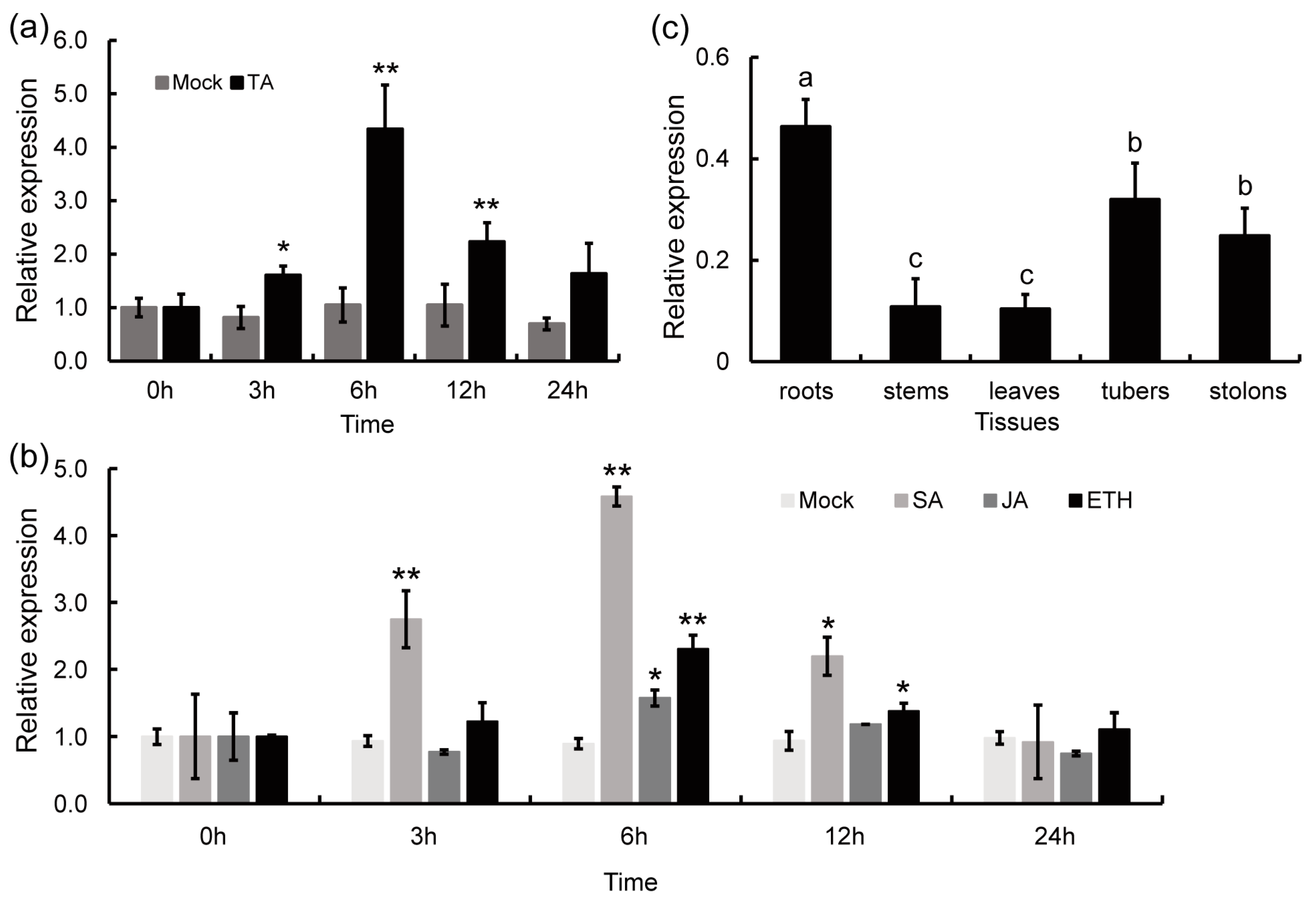
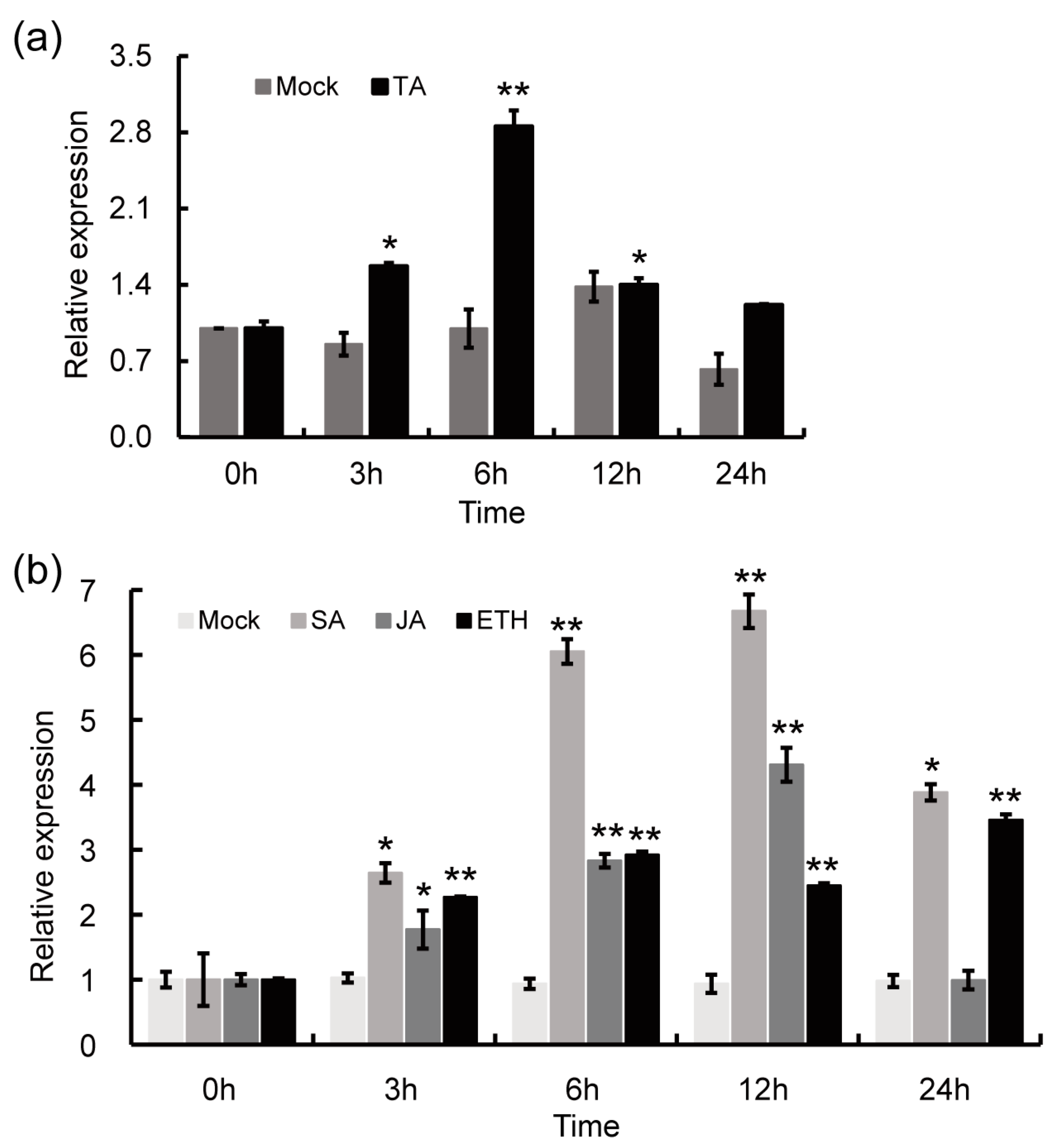
2.2. StPAM16-1 Expression Is Induced by TA and Defense-Related Plant Hormones
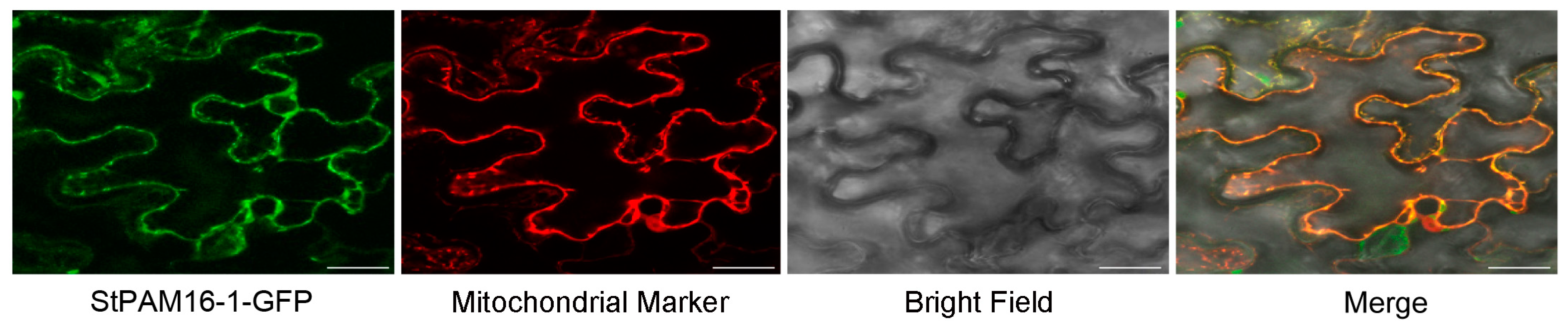
2.3. StPAM16-1 Localizes to Mitochondria
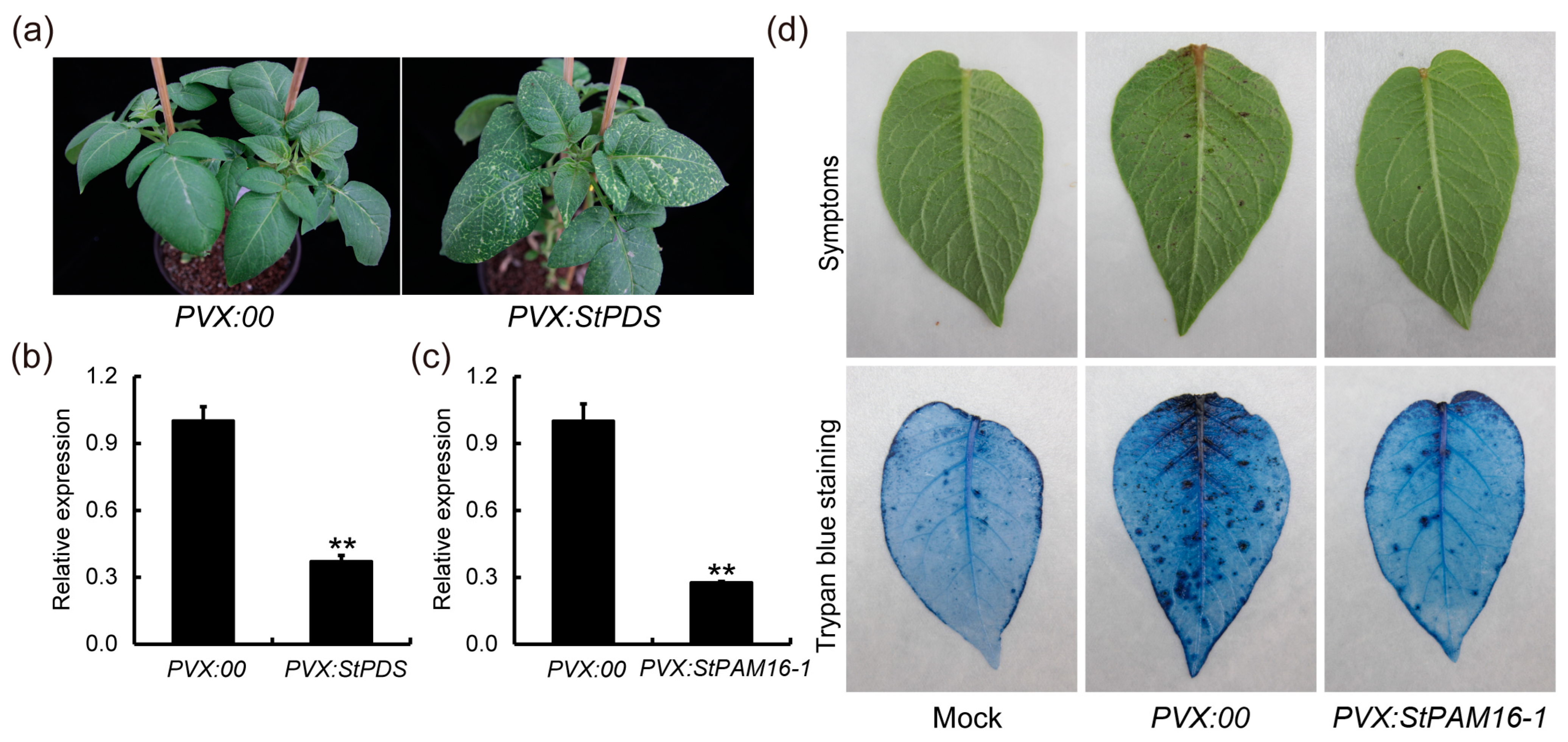
2.4. Suppression of StPAM16-1 Expression Improves Tolerance of Potato Plants to Thaxtomin A and S. scabiei
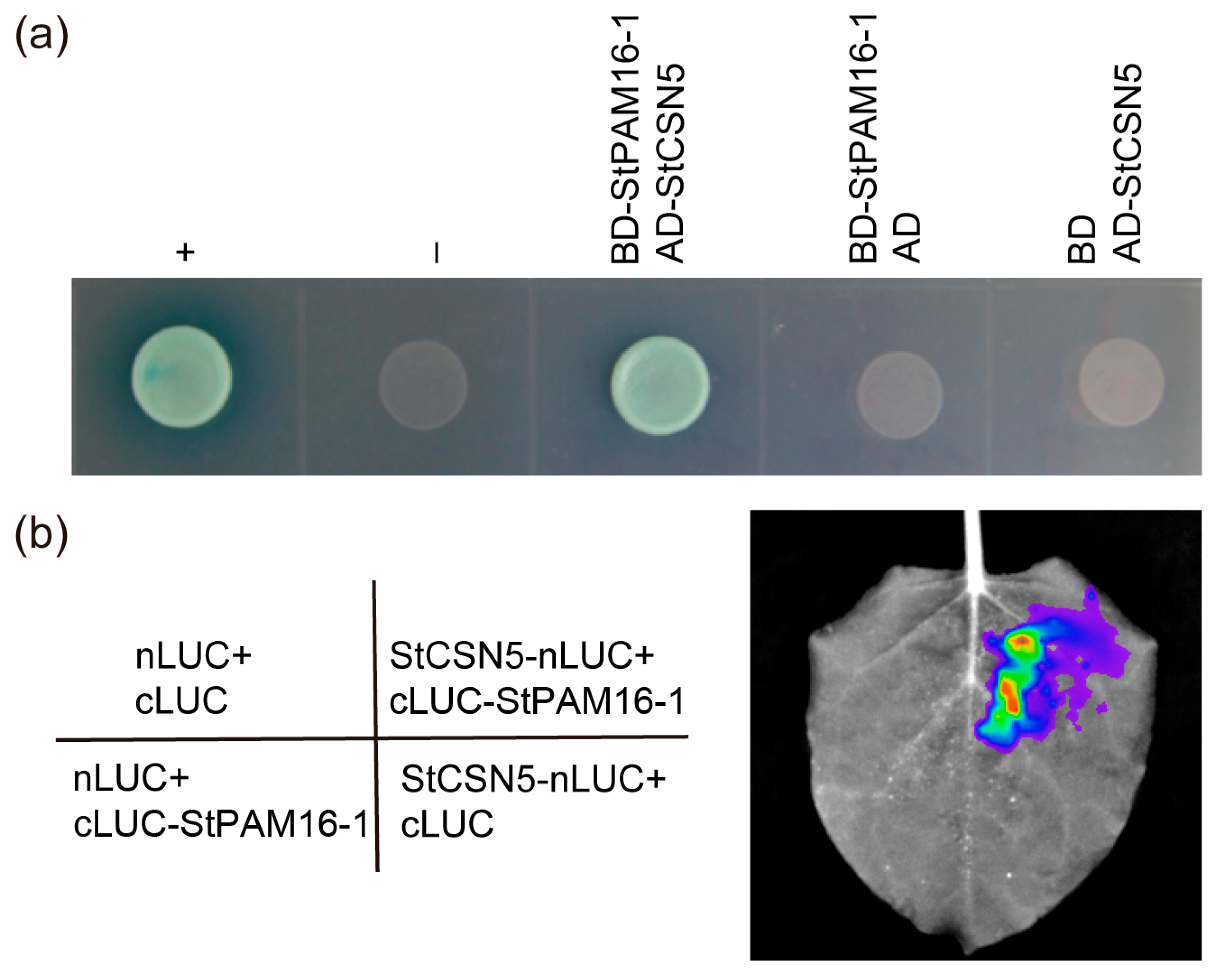
2.5. StPAM16-1 Interacts with StCSN5
2.6. Expression of StCSN5 Is Significantly Induced by TA and Several Defense-Related Plant Hormones
3. Discussion
4. Materials and Methods
4.1. Plant Growth and Treatments
4.2. Pathogen Cultivation and Inoculation
4.3. Subcellular Localization of StPAM16-1
4.4. Virus-Induced Gene Silencing
4.5. Vector Construction and Potato Transformation
4.6. RNA Extraction and RT-qPCR Analysis
4.7. Y2H Assay
4.8. Split Luciferase Complementation Assays
4.9. Trypan Blue Staining
4.10. Phylogenetic Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lapaz, M.I.; Huguet-Tapia, J.C.; Siri, M.I.; Verdier, E.; Loria, R.; Pianzzola, M.J. Genotypic and Phenotypic Characterization of Streptomyces Species Causing Potato Common Scab in Uruguay. Plant Dis. 2017, 101, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Loria, R.; Bukhalid, R.A.; Fry, B.A.; King, R.R. Plant pathogenicity in the genus Streptomyces. Plant Dis. 1997, 81, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Loria, R.; Kers, J.; Joshi, M. Evolution of plant pathogenicity in Streptomyces. Annu. Rev. Phytopathol. 2006, 44, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, V.; Cookson, S.J.; Wu, S.; Scheible, W.R. Thaxtomin A affects CESA-complex density, expression of cell wall genes, cell wall composition, and causes ectopic lignification in Arabidopsis thaliana seedlings. J. Exp. Bot. 2009, 60, 955–965. [Google Scholar] [CrossRef]
- Scheible, W.R.; Fry, B.; Kochevenko, A.; Schindelasch, D.; Zimmerli, L.; Somerville, S.; Loria, R.; Somerville, C.R. An Arabidopsis mutant resistant to thaxtomin A, a cellulose synthesis inhibitor from Streptomyces species. Plant Cell 2003, 15, 1781–1794. [Google Scholar] [CrossRef]
- Leiner, R.H.; Fry, B.A.; Carling, D.E.; Loria, R. Probable involvement of thaxtomin A in pathogenicity of Streptomyces scabies on seedlings. Phytopathology 1996, 86, 709–713. [Google Scholar] [CrossRef]
- Tegg, R.S.; Melian, L.; Wilson, C.R.; Shabala, S. Plant cell growth and ion flux responses to the streptomycete phytotoxin thaxtomin A: Calcium and hydrogen flux patterns revealed by the non-invasive MIFE technique. Plant Cell Physiol. 2005, 46, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Errakhi, R.; Dauphin, A.; Meimoun, P.; Lehner, A.; Reboutier, D.; Vatsa, P.; Briand, J.; Madiona, K.; Rona, J.P.; Barakate, M.; et al. An early Ca2+ influx is a prerequisite to thaxtomin A-induced cell death in Arabidopsis thaliana cells. J. Exp. Bot. 2008, 59, 4259–4270. [Google Scholar] [CrossRef]
- Duval, I.; Brochu, V.; Simard, M.; Beaulieu, C.; Beaudoin, N. Thaxtomin A induces programmed cell death in Arabidopsis thaliana suspension-cultured cells. Planta 2005, 222, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Dees, M.W.; Wanner, L.A. In Search of Better Management of Potato Common Scab. Potato Res. 2012, 55, 249–268. [Google Scholar] [CrossRef]
- Murphy, A.M.; De Jong, H.; Tai, G.C.C. Transmission of resistance to common scab from the diploid to the tetraploid level via 4x-2x crosses in potatoes. Euphytica 1995, 82, 227–233. [Google Scholar] [CrossRef]
- Alam, Z. Inheritance of Scab Resistance in 24-Chromosome Potatoes. Ph.D. Thesis, University of Wisconsin-Madison, Madison, WI, USA, 1972. [Google Scholar]
- Tang, D.; Jia, Y.; Zhang, J.; Li, H.; Cheng, L.; Wang, P.; Bao, Z.; Liu, Z.; Feng, S.; Zhu, X.; et al. Genome evolution and diversity of wild and cultivated potatoes. Nature 2022, 606, 535–541. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Bao, Z.; Li, H.; Lyu, Y.; Zan, Y.; Wu, Y.; Cheng, L.; Fang, Y.; Wu, K.; et al. Graph pangenome captures missing heritability and empowers tomato breeding. Nature 2022, 606, 527–534. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, N.; Bao, Z.; Zhou, Q.; Guarracino, A.; Yang, Y.; Wang, P.; Zhang, Z.; Tang, D.; Zhang, P.; et al. Leveraging a phased pangenome for haplotype design of hybrid potato. Nature 2025. [Google Scholar] [CrossRef]
- Frazier, A.E.; Dudek, J.; Guiard, B.; Voos, W.; Li, Y.; Lind, M.; Meisinger, C.; Geissler, A.; Sickmann, A.; Meyer, H.E.; et al. Pam16 has an essential role in the mitochondrial protein import motor. Nat. Struct. Mol. Biol. 2004, 11, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, X.; Liu, Y.; Roth, C.; Copeland, C.; McFarlane, H.E.; Huang, S.; Lipka, V.; Wiermer, M.; Li, X. Mitochondrial AtPAM16 is required for plant survival and the negative regulation of plant immunity. Nat. Commun. 2013, 4, 2558. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.; Casey, C.; Köcher, T.; Champion, C.; Jandrasits, K.; Mosiolek, M.; Bonnot, C.; Dolan, L. Reduced coenzyme Q synthesis confers non-target site resistance to the herbicide thaxtomin A. PLoS Genet. 2023, 19, e1010423. [Google Scholar] [CrossRef]
- Waingankar, T.P.; D’Silva, P. Multiple variants of the human presequence translocase motor subunit Magmas govern the mitochondrial import. J. Biol. Chem. 2021, 297, 101349. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, D.; Saha, P.P.; Marthala, H.; D’Silva, P. Magmas functions as a ROS regulator and provides cytoprotection against oxidative stress-mediated damages. Cell Death Dis. 2014, 5, e1394. [Google Scholar] [CrossRef]
- Qin, N.; Xu, D.; Li, J.; Deng, X.W. COP9 signalosome: Discovery, conservation, activity, and function. J. Integr. Plant Biol. 2020, 62, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Deng, X.W. The COP9 signalosome. Annu. Rev. Cell Dev. Biol. 2003, 19, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Mikus, P.; Zundel, W. COPing with hypoxia. Semin. Cell Dev. Biol. 2005, 16, 462–473. [Google Scholar] [CrossRef]
- Stratmann, J.W.; Gusmaroli, G. Many jobs for one good cop–The COP9 signalosome guards development and defense. Plant Sci. 2012, 185–186, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Deng, X.W. The COP9 signalosome: Regulating plant development through the control of proteolysis. Annu. Rev. Plant Biol. 2003, 54, 165–182. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Serino, G.; Callis, J.; Crosby, W.L.; Lyapina, S.; Deshaies, R.J.; Gray, W.M.; Estelle, M.; Deng, X.W. Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIR1 in mediating auxin response. Science 2001, 292, 1379–1382. [Google Scholar] [CrossRef]
- Feng, S.; Ma, L.; Wang, X.; Xie, D.; Dinesh-Kumar, S.P.; Wei, N.; Deng, X.W. The COP9 signalosome interacts physically with SCF COI1 and modulates jasmonate responses. Plant Cell 2003, 15, 1083–1094. [Google Scholar] [CrossRef]
- Bai, X.; Huang, X.; Tian, S.; Peng, H.; Zhan, G.; Goher, F.; Guo, J.; Kang, Z.; Guo, J. RNAi-mediated stable silencing of TaCSN5 confers broad-spectrum resistance to Puccinia striiformis f. sp. tritici. Mol. Plant Pathol. 2021, 22, 410–421. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, K.; Sun, S.; Zhou, J.; Yu, J.Q. COP9 Signalosome CSN4 and CSN5 Subunits Are Involved in Jasmonate-Dependent Defense Against Root-Knot Nematode in Tomato. Front. Plant Sci. 2019, 10, 1223. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Y.; Zhang, Z.; Quan, R.; Zhang, H.; Ma, L.; Deng, X.W.; Huang, R. Arabidopsis CSN5B interacts with VTC1 and modulates ascorbic acid synthesis. Plant Cell 2013, 25, 625–636. [Google Scholar] [CrossRef]
- Wei, N.; Tsuge, T.; Serino, G.; Dohmae, N.; Takio, K.; Matsui, M.; Deng, X.W. The COP9 complex is conserved between plants and mammals and is related to the 26S proteasome regulatory complex. Current Biol. 1998, 8, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Poupon, V.; Blondeau, F.; McPherson, P.S. The DnaJ-domain protein RME-8 functions in endosomal trafficking. J. Biol. Chem. 2005, 280, 40135–40143. [Google Scholar] [CrossRef]
- Schwechheimer, C. The COP9 signalosome (CSN): An evolutionary conserved proteolysis regulator in eukaryotic development. Biochim. Biophys. Acta 2004, 1695, 45–54. [Google Scholar] [CrossRef]
- Braun, S.; Gevens, A.; Charkowski, A.; Allen, C.; Jansky, S. Potato Common Scab: A Review of the Causal Pathogens, Management Practices, Varietal Resistance Screening Methods, and Host Resistance. Am. J. Potato Res. 2017, 94, 283–296. [Google Scholar] [CrossRef]
- Liu, L.; Hao, L.; Liu, N.; Zhao, Y.; Zhong, N.; Zhao, P. iTRAQ-Based Proteomics Analysis of Response to Solanum tuberosum Leaves Treated with the Plant Phytotoxin Thaxtomin, A. Int. J. Mol. Sci. 2021, 22, 12036. [Google Scholar] [CrossRef]
- Lapin, D.; Van den Ackerveken, G. Susceptibility to plant disease: More than a failure of host immunity. Trends Plant Sci. 2013, 18, 546–554. [Google Scholar] [CrossRef]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.M.; He, S.Y.; Xin, X.F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Loo, E.P. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef]
- Zhou, J.M.; Zhang, Y. Plant Immunity: Danger Perception and Signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef]
- Nazarian-Firouzabadi, F.; Joshi, S.; Xue, H.; Kushalappa, A.C. Genome-wide in silico identification of LysM-RLK genes in potato (Solanum tuberosum L.). Mol. Biol. Rep. 2019, 46, 5005–5017. [Google Scholar] [CrossRef]
- Tai, H.H.; Goyer, C.; Murphy, A.M. Potato MYB and bHLH transcription factors associated with anthocyanin intensity and common scab resistance. Botany 2013, 91, 722–730. [Google Scholar] [CrossRef]
- Kozany, C.; Mokranjac, D.; Sichting, M.; Neupert, W.; Hell, K. The J domain-related cochaperone Tim16 is a constituent of the mitochondrial TIM23 preprotein translocase. Nat. Struct. Mol. Biol. 2004, 11, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dudek, J.; Guiard, B.; Pfanner, N.; Rehling, P.; Voos, W. The presequence translocase-associated protein import motor of mitochondria: Pam16 functions in an antagonistic manner to Pam18. J. Biol. Chem. 2004, 279, 38047–38054. [Google Scholar] [CrossRef] [PubMed]
- Chacinska, A.; van der Laan, M.; Mehnert, C.S.; Guiard, B.; Mick, D.U.; Hutu, D.P.; Truscott, K.N.; Wiedemann, N.; Meisinger, C.; Pfanner, N.; et al. Distinct forms of mitochondrial TOM-TIM supercomplexes define signal-dependent states of preprotein sorting. Mol. Cell Biol. 2010, 30, 307–318. [Google Scholar] [CrossRef]
- Johnson, E.G.; Krasnoff, S.B.; Bignell, D.R.; Chung, W.C.; Tao, T.; Parry, R.J.; Loria, R.; Gibson, D.M. 4-Nitrotryptophan is a substrate for the non-ribosomal peptide synthetase TxtB in the thaxtomin A biosynthetic pathway. Mol. Microbiol. 2009, 73, 409–418. [Google Scholar] [CrossRef]
- King, R.R.; Calhoun, L.A. The thaxtomin phytotoxins: Sources, synthesis, biosynthesis, biotransformation and biological activity. Phytochemistry 2009, 70, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shah, S.V.; Xiang, X.; Wang, J.; Deng, Z.B.; Liu, C.; Zhang, L.; Wu, J.; Edmonds, T.; Jambor, C.; et al. COP9-associated CSN5 regulates exosomal protein deubiquitination and sorting. Am. J. Pathol. 2009, 174, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Serino, G.; Deng, X.W. Multiple ubiquitin ligase-mediated processes require COP9 signalosome and AXR1 function. Plant Cell 2002, 14, 2553–2563. [Google Scholar] [CrossRef]
- Cui, K.-C.; Liu, M.; Ke, G.-H.; Zhang, X.-Y.; Mu, B.; Zhou, M.; Hu, Y.; Wen, Y.-Q. Transient silencing of VvCSN5 enhances powdery mildew resistance in grapevine (Vitis vinifera). Plant Cell Tiss. Org. Cult. 2021, 146, 621–633. [Google Scholar] [CrossRef]
- Hind, S.R.; Pulliam, S.E.; Veronese, P.; Shantharaj, D.; Nazir, A.; Jacobs, N.S.; Stratmann, J.W. The COP9 signalosome controls jasmonic acid synthesis and plant responses to herbivory and pathogens. Plant J. 2011, 65, 480–491. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Giroux, M.J.; Huang, L. A wheat COP9 subunit 5-like gene is negatively involved in host response to leaf rust. Mol. Plant Pathol. 2017, 18, 125–133. [Google Scholar] [CrossRef]
- Gray, J.; Rustgi, S.; von Wettstein, D.; Reinbothe, C.; Reinbothe, S. Common functions of the chloroplast and mitochondrial co-chaperones cpDnaJL (CDF1) and mtDnaJ (PAM16) in protein import and ROS scavenging in Arabidopsis thaliana. Commun. Integr. Biol. 2015, 9, e1119343. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, L.; Zhao, P.; Tong, J.; Zhong, N.; Zhang, H.; Liu, N. Genome-Wide Identification, Expression Profile and Evolution Analysis of Karyopherin beta Gene Family in Solanum tuberosum Group Phureja DM1-3 Reveals Its Roles in Abiotic Stresses. Int. J. Mol. Sci. 2020, 21, 931. [Google Scholar] [CrossRef]

| Number | Protein Description | NCBI Database Accession No. |
|---|---|---|
| 1 | PREDICTED: COP9 signalosome complex subunit 5b-like protein | XP_006351153.1 |
| 2 | PREDICTED: anthocyanidin 3-O-glucosyltransferase 2-like protein | XP_006353729.1 |
| 3 | fructose-bisphosphate aldolase-like protein | NP_001275379.1 |
| 4 | PREDICTED: uncharacterized protein LOC102596406 | XP_006344642.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Song, S.; Liu, N.; Wang, Z.; Zhao, Y.; Zhong, N.; Zhao, P.; Wang, H. The Silencing of the StPAM16-1 Gene Enhanced the Resistance of Potato Plants to the Phytotoxin Thaxtomin A. Int. J. Mol. Sci. 2025, 26, 1361. https://doi.org/10.3390/ijms26031361
Liu L, Song S, Liu N, Wang Z, Zhao Y, Zhong N, Zhao P, Wang H. The Silencing of the StPAM16-1 Gene Enhanced the Resistance of Potato Plants to the Phytotoxin Thaxtomin A. International Journal of Molecular Sciences. 2025; 26(3):1361. https://doi.org/10.3390/ijms26031361
Chicago/Turabian StyleLiu, Lu, Shuangwei Song, Ning Liu, Zhiqin Wang, Yonglong Zhao, Naiqin Zhong, Pan Zhao, and Haiyun Wang. 2025. "The Silencing of the StPAM16-1 Gene Enhanced the Resistance of Potato Plants to the Phytotoxin Thaxtomin A" International Journal of Molecular Sciences 26, no. 3: 1361. https://doi.org/10.3390/ijms26031361
APA StyleLiu, L., Song, S., Liu, N., Wang, Z., Zhao, Y., Zhong, N., Zhao, P., & Wang, H. (2025). The Silencing of the StPAM16-1 Gene Enhanced the Resistance of Potato Plants to the Phytotoxin Thaxtomin A. International Journal of Molecular Sciences, 26(3), 1361. https://doi.org/10.3390/ijms26031361






