AgNP-Containing Niosomes Functionalized with Fucoidan Potentiated the Intracellular Killing of Mycobacterium abscessus in Macrophages
Abstract
1. Introduction
2. Results and Discussion
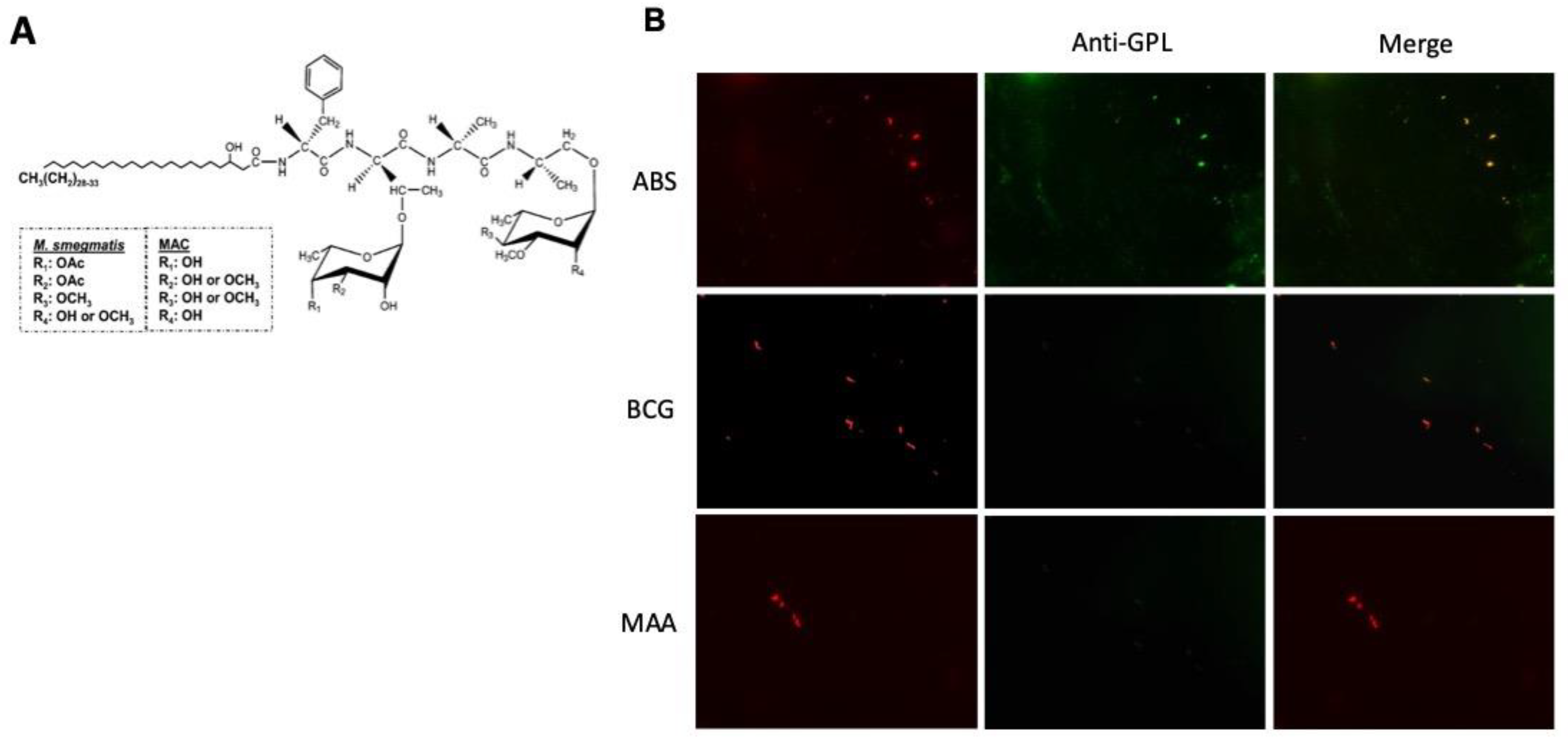
2.1. ScFv-51 Identified GPL-M. abscessus
2.2. Characterization of the Niosomes
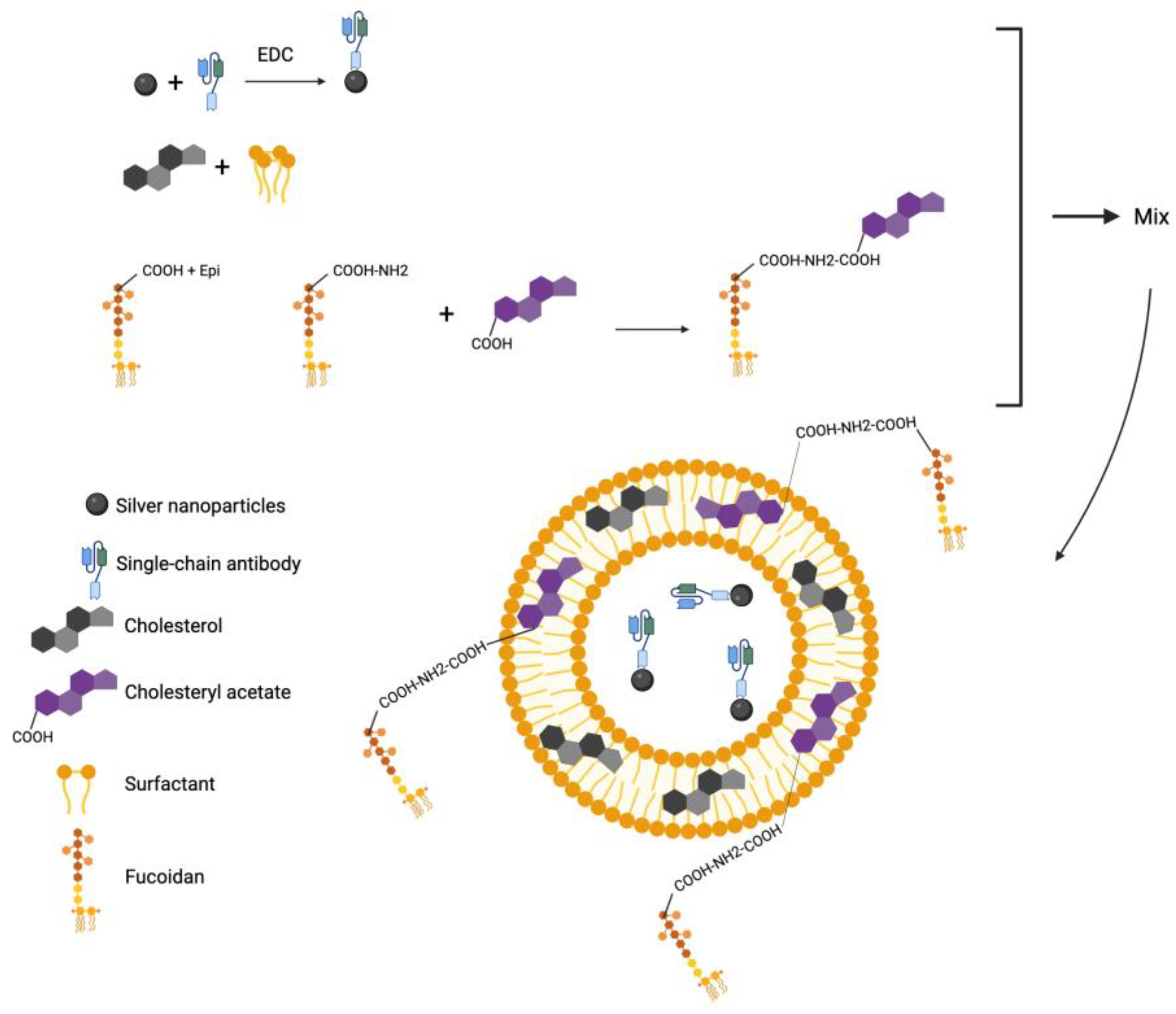
2.3. Characterization of Niosomes Encapsulated with AgNP-scFv-51 and Aminated Fucoidan
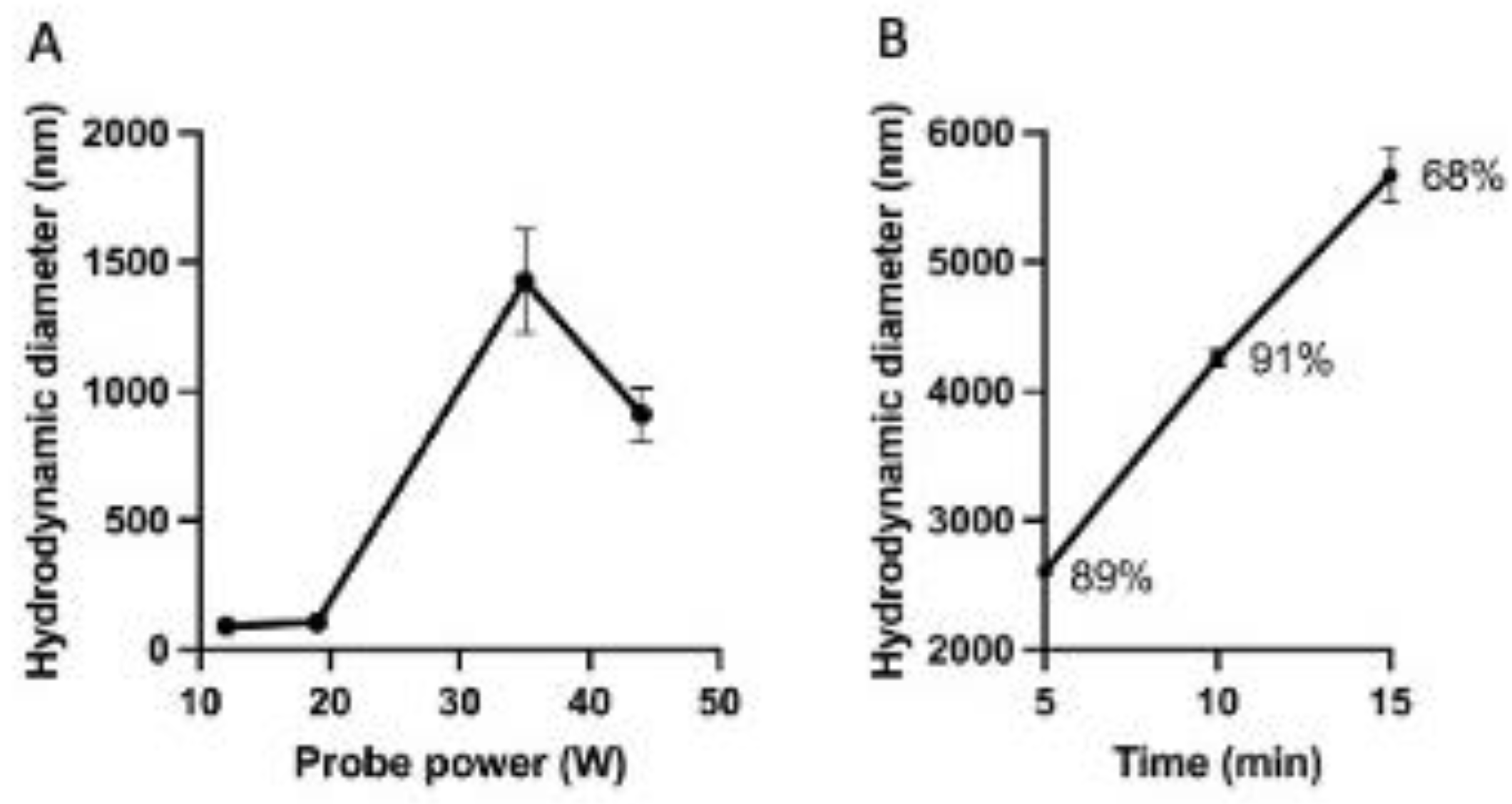
2.4. Stability of Niosomes
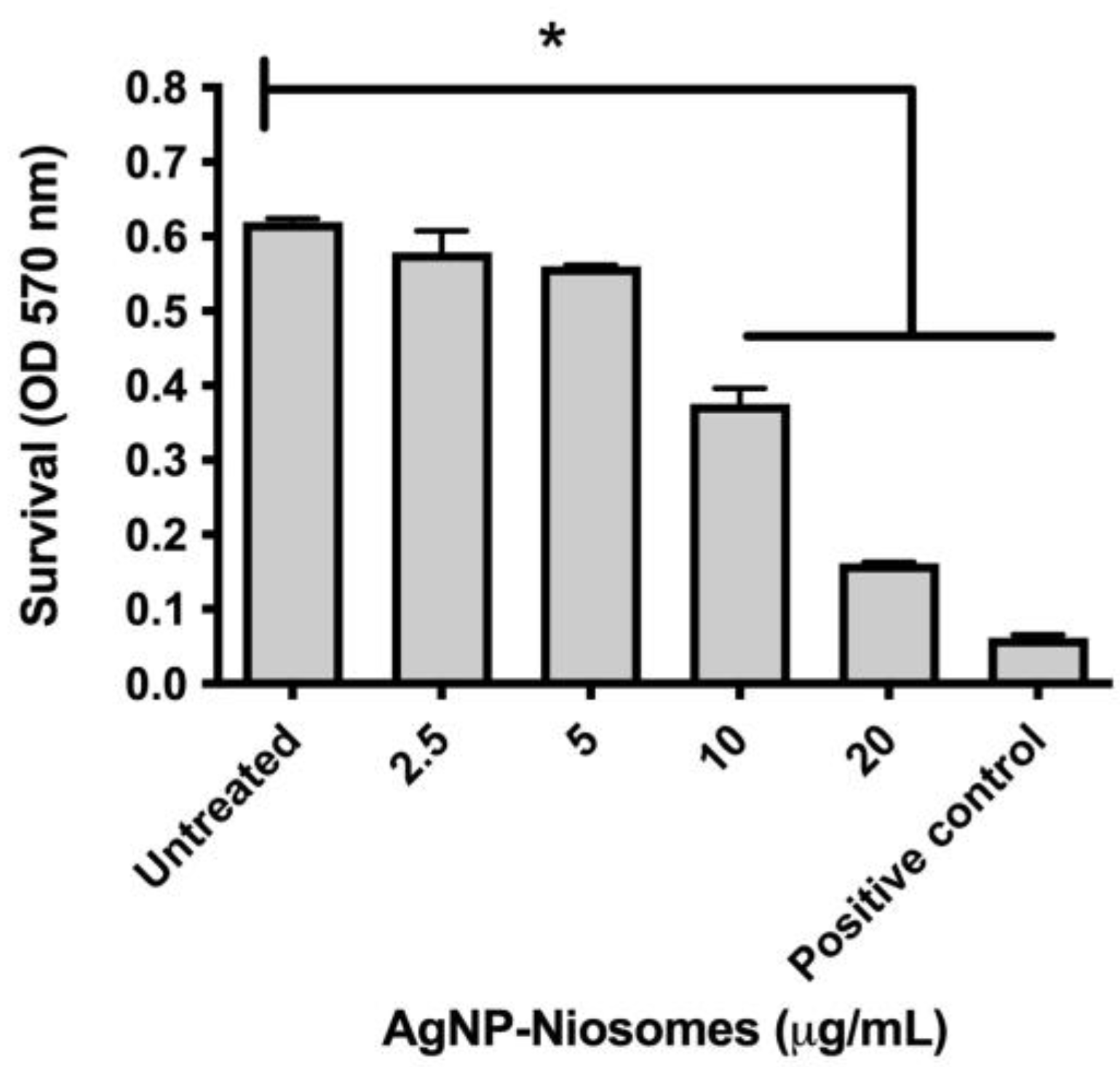
2.5. Cytotoxicity of the Niosomes
2.6. Survival of M. abscessus in Treated Niosomes
3. Materials and Methods
3.1. ScFv-51 Validation Against GPL-M. abscessus
3.2. Synthesis and Characterization of AgNPs
3.3. Conjugation of AgNPs and scFv-51
3.4. Synthesis and Characterization of Niosomes
3.5. Functionalization of Fucoidan and Conjugation with Cholesterol
3.6. Synthesis of Niosomes Using Cholesteryl Acetate
3.7. Synthesis of the Functionalized Niosome
3.8. Encapsulation Efficiency of the AgNP-scFv-51 and the Complex AgNP-scFv-51 with Aminated Fucoidan
3.9. Cytotoxicity of Niosomes
3.10. Infection of Macrophages
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pieters, J. Entry and Survival of Pathogenic Mycobacteria in Macrophages. Microbes Infect. 2001, 3, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Dahl, V.N.; Mølhave, M.; Fløe, A.; van Ingen, J.; Schön, T.; Lillebaek, T.; Andersen, A.B.; Wejse, C. Global Trends of Pulmonary Infections with Nontuberculous Mycobacteria: A Systematic Review. Int. J. Infect. Dis. 2022, 125, 120–131. [Google Scholar] [CrossRef]
- Ahmed, I.; Tiberi, S.; Farooqi, J.; Jabeen, K.; Yeboah-Manu, D.; Migliori, G.B.; Hasan, R. Non-Tuberculous Mycobacterial Infections—A Neglected and Emerging Problem. Int. J. Infect. Dis. 2020, 92, S46–S50. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-R.; Sheng, W.-H.; Hung, C.-C.; Yu, C.-J.; Lee, L.-N.; Hsueh, P.-R. Mycobacterium Abscessus Complex Infections in Humans. Emerg. Infect. Dis. 2015, 21, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.V.; Viljoen, A.; Ghigo, E.; Herrmann, J.-L.; Kremer, L. Glycopeptidolipids, a Double-Edged Sword of the Mycobacterium Abscessus Complex. Front. Microbiol. 2018, 9, 1145. [Google Scholar] [CrossRef]
- Parmar, S.; Tocheva, E.I. The Cell Envelope of Mycobacterium Abscessus and Its Role in Pathogenesis. PLoS Pathog. 2023, 19, e1011318. [Google Scholar] [CrossRef]
- Musumeci, T.; Bonaccorso, A.; Carbone, C. Chapter 2—Basic Concepts of Liposomes: Components, Structures, Properties and Classification. In Liposomes in Drug Delivery; Antimisiaris, S.G., Ed.; Academic Press: Cambridge, MA, USA, 2024; pp. 19–48. ISBN 978-0-443-15491-1. [Google Scholar]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Immordino, L.M.; Dosio, F.; Cattel, L. Stealth Liposomes: Review of the Basic Science, Rationale, and Clinical Applications, Existing and Potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar] [CrossRef]
- Wen, J.; Al Gailani, M.; Yin, N.; Rashidinejad, A. Liposomes and Niosomes. In Emulsion-Based Systems for Delivery of Food Active Compounds; John Wiley and Son Ltd.: West Sussex, UK, 2018; pp. 263–292. [Google Scholar]
- Osman, N.; Omolo, C.A.; Gafar, M.A.; Devnarain, N.; Rambharose, S.; Ibrahim, U.H.; Fasiku, V.O.; Govender, T. Niosomes Modified with a Novel pH-Responsive Coating (mPEG-OA) Enhance the Antibacterial and Anti-Biofilm Activity of Vancomycin against Methicillin-Resistant Staphylococcus Aureus. Nano Ex. 2024, 5, 015008. [Google Scholar] [CrossRef]
- Hemmati, J.; Chiani, M.; Asghari, B.; Roshanaei, G.; Soleimani Asl, S.; Shafiei, M.; Arabestani, M.R. Antibacterial and Antibiofilm Potentials of Vancomycin-Loaded Niosomal Drug Delivery System against Methicillin-Resistant Staphylococcus Aureus (MRSA) Infections. BMC Biotechnol. 2024, 24, 47. [Google Scholar] [CrossRef]
- Maurizi, L.; Forte, J.; Ammendolia, M.G.; Hanieh, P.N.; Conte, A.L.; Relucenti, M.; Donfrancesco, O.; Ricci, C.; Rinaldi, F.; Marianecci, C.; et al. Effect of Ciprofloxacin-Loaded Niosomes on Escherichia Coli and Staphylococcus Aureus Biofilm Formation. Pharmaceutics 2022, 14, 2662. [Google Scholar] [CrossRef] [PubMed]
- Haddadian, A.; Robattorki, F.F.; Dibah, H.; Soheili, A.; Ghanbarzadeh, E.; Sartipnia, N.; Hajrasouliha, S.; Pasban, K.; Andalibi, R.; Ch, M.H.; et al. Niosomes-Loaded Selenium Nanoparticles as a New Approach for Enhanced Antibacterial, Anti-Biofilm, and Anticancer Activities. Sci. Rep. 2022, 12, 21938. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Ivanova, K.; Tang, W.; Tzanov, T.; Li, S.; Bach, H. Targeting Intracellular Mycobacteria Using Nanosized Niosomes Loaded with Antibacterial Agents. Nanomaterials 2021, 11, 1984. [Google Scholar] [CrossRef] [PubMed]
- Deshayes, C.; Bach, H.; Euphrasie, D.; Attarian, R.; Coureuil, M.; Sougakoff, W.; Laval, F.; Av-Gay, Y.; Daffé, M.; Etienne, G.; et al. MmpS4 Promotes Glycopeptidolipids Biosynthesis and Export in Mycobacterium Smegmatis. Mol. Microbiol. 2010, 78, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Suzuki, H.; Wada, Y.; Kodama, T.; Doi, T. Fucoidan Induces Nitric Oxide Production via P38 Mitogen-Activated Protein Kinase and NF-κB-Dependent Signaling Pathways through Macrophage Scavenger Receptors. Biochem. Biophys. Res. Commun. 2006, 343, 286–294. [Google Scholar] [CrossRef]
- Griffin, W. Classification of Surface-Active Agents by “HLB”. J. Soc. Cosmet. Chem. 1949, 1, 311. [Google Scholar]
- Griffin, W. Calculation of HLB Values of Non-Ionic Surfactants. J. Soc. Cosmet. Chem. 1954, 5, 249–256. [Google Scholar]
- Ruckmani, K.; Sankar, V. Formulation and Optimization of Zidovudine Niosomes. AAPS PharmSciTech 2010, 11, 1119–1127. [Google Scholar] [CrossRef]
- Taymouri, S.; Varshosaz, J. Effect of Different Types of Surfactants on the Physical Properties and Stability of Carvedilol Nano-Niosomes. Adv. Biomed. Res. 2016, 5, 48. [Google Scholar] [CrossRef]
- Mehta, S.K.; Jindal, N. Formulation of Tyloxapol Niosomes for Encapsulation, Stabilization and Dissolution of Anti-Tubercular Drugs. Colloids Surf. B Biointerfaces 2013, 101, 434–441. [Google Scholar] [CrossRef]
- Pando, D.; Gutiérrez, G.; Coca, J.; Pazos, C. Preparation and Characterization of Niosomes Containing Resveratrol. J. Food Eng. 2013, 117, 227–234. [Google Scholar] [CrossRef]
- Nadzir, M.M.; Fen, T.W.; Mohamed, A.R.; Hisham, S.F. Size and Stability of Curcumin Niosomes from Combinations of Tween 80 and Span 80. Sains Malays. 2017, 46, 2455–2460. [Google Scholar] [CrossRef]
- Khan, D.H.; Bashir, S.; Figueiredo, P.; Santos, H.A.; Khan, M.I.; Peltonen, L. Process Optimization of Ecological Probe Sonication Technique for Production of Rifampicin Loaded Niosomes. J. Drug Deliv. Sci. Technol. 2019, 50, 27–33. [Google Scholar] [CrossRef]
- Krieger, M. The Other Side of Scavenger Receptors: Pattern Recognition for Host Defense. Curr. Opin. Lipidol. 1997, 8, 275. [Google Scholar] [CrossRef]
- Akbari, V.; Abedi, D.; Pardakhty, A.; Sadeghi-Aliabadi, H. Ciprofloxacin Nano-Niosomes for Targeting Intracellular Infections: An in Vitro Evaluation. J. Nanopart. Res. 2013, 15, 1556. [Google Scholar] [CrossRef]
- Wong, J.P.; Yang, H.; Blasetti, K.L.; Schnell, G.; Conley, J.; Schofield, L.N. Liposome Delivery of Ciprofloxacin against Intracellular Francisella Tularensis Infection. J. Control. Release 2003, 92, 265–273. [Google Scholar] [CrossRef]
- Slavin, Y.; Ivanova, K.; Hoyo, J.; Perelshtein, I.; Owen, G.; Haegert, A.; Lin, Y.; LeBihan, S.; Gedanken, A.; Häfeli, U.; et al. Novel Lignin-Capped Silver Nanoparticles against Multidrug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2021, 5, 48. [Google Scholar] [CrossRef]
- Thabet, Y.; Elsabahy, M.; Eissa, N.G. Methods for Preparation of Niosomes: A Focus on Thin-Film Hydration Method. Methods 2022, 199, 9–15. [Google Scholar] [CrossRef]

| Surfactant | Sample | Molar Ratio (Span:Tween:Chol) | DH ± SD (nm) | PDI (%) | ζ Potential (mV) |
|---|---|---|---|---|---|
| Span-80 + Tween-80 | NA1 | 3:0:1 | 712 ± 79.6 | 28.72 | −38.8 |
| NA2 | 2.4:0.6:1 | 340 ± 168 | 26.43 | −17.9 | |
| NA3 | 1.8:1.2:1 | 367 ± 534 | 27.13 | −10.2 | |
| NA4 | 1.5:1.5:1 | 91 ± 79.9 | 25.14 | −5.80 | |
| NA5 | 1.2:1.8:1 | 245 ± 218 | 25.26 | −1.11 | |
| NA6 | 0.6:2.4:1 | 504 ± 315 | 27.47 | −0.26 | |
| NA7 | 0:3:1 | 200 ± 151 | 29.29 | +0.32 | |
| Span-60 + Tween-60 | NB1 | 0.5:0:1 | 251 ± 75 | 14.17 | −23.1 |
| NB2 | 0.4:0.1:1 | 289 ± 155 | 25.05 | −25.4 | |
| NB3 | 0.3:0.2:1 | 429 ± 349 | 23.46 | −20.7 | |
| NB4 | 0.25:0.25:1 | 262 ± 156 | 24.70 | −21.2 | |
| NB5 | 0.2:0.3:1 | 200 ± 115 | 19.44 | −26.4 | |
| NB6 | 0.1:0.4:1 | 276 ± 137 | 24.42 | −12.3 | |
| NB7 | 0:0.5:1 | 120 ± 102 | 24.93 | −23.3 | |
| Span-40 + Tween-40 | NC1 | 2:0:1 | 181 ± 65 | 17.79 | −33.9 |
| NC2 | 1.6:0.4:1 | 740 ± 23 | 35.12 | −39.5 | |
| NC3 | 1.2:0.8:1 | 134 ± 13 | 28.52 | −13.2 | |
| NC4 | 1:1:1 | 275 ± 189 | 33.07 | −27.9 | |
| NC5 | 0.8:1.2:1 | 120 ± 76 | 27.84 | −9.74 | |
| NC6 | 0.4:1.6:1 | 354 ± 91 | 38.31 | −8.37 | |
| NC7 | 0:2:1 | 17 ± 4 | 25.98 | −10.0 | |
| Span-20 + Tween-20 | ND1 | 1:0:1 | 170 ± 54 | 20.84 | −44.3 |
| ND2 | 0.8:0.2:1 | 246 ± 80 | 24.28 | −25.3 | |
| ND3 | 0.6:0.4:1 | 170 ± 287 | 26.52 | −17.1 | |
| ND4 | 0.5:0.5:1 | 224 ± 228 | 30.67 | −26.0 | |
| ND5 | 0.4:0.6:1 | 244 ± 81 | 29.13 | −18.1 | |
| ND6 | 0.2:0.8:1 | 333 ± 287 | 25.75 | −19.7 | |
| ND7 | 0:1:1 | 430 ± 228 | 27.71 | +5.72 |
| Sample | Molar Ratio (Span:Tween:Chol) | DH ± SD (nm) | PDI (%) | ζ Potential (mV) |
|---|---|---|---|---|
| CXN | 0.2:0.3:1 | 195 ± 64 | 26.99 | −11.5 |
| CXN50 | 0.2:0.3:1 | 470 ± 366 | 26.19 | −12.2 |
| CXN100 | 0.2:0.3:1 | 149 ± 137 | 30.87 | −7.85 |
| Sample | Molar Ratio (Span:Tween:Chol) | DH ± SD (nm) | PDI (%) | ζ Potential (mV) | EE (%) |
|---|---|---|---|---|---|
| Ag | N/A | 23.4 ± 1.8 | 23.13 | −42.8 | N/A |
| AgNP-scFv-51 | N/A | 4406 ± 1046 | 5.58 | −6.62 | N/A |
| NA4 AgNP-scFv-51 | 1.5:1.5:1 | 93 ± 91 | 28.31 | −1.06 | 43.5 |
| NB5 AgNP-scFv-51 | 0.2:0.3:1 | 309 ± 342 | 28.14 | −1.24 | 71.3 |
| NC3 AgNP-scFv-51 | 1.2:0.8:1 | 120 ± 365 | 25.87 | −2.42 | 77.6 |
| ND3 AgNP-scFv-51 | 0.6:0.4:1 | 864 ± 589 | 26.60 | −12.7 | 63.0 |
| NB5 AgNP-scFv-51 | 0.2:0.3:1 | 372 ± 249 | 28.95 | −1.14 | NM |
| NB5 AgNP-scFv-51 + 20% CA:fucoidan | 0.2:0.3:1 | 244 ± 83 | 27.12 | +0.65 | NM |
| NB5 AgNP-scFv-51 + 10% CA:fucoidan | 0.2:0.3:1 | 149 ± 86 | 26.74 | −1.32 | NM |
| Series | Time = 0 | Time = 60 Days |
|---|---|---|
| NA | ||
| NA1 | ||
| DH (nm) | 712.9 | 823 |
| PDI (%) | 28.72 | 28.93 |
| Peak analysis intensity * | 1, 551.6 (100%) | 2, 1329 (74.71%); 188.2 (29.29%) |
| ζ potential (mV) | −38.85 | −26.59 |
| NA2 | ||
| DH (nm) | 340.8 | 315.1 |
| PDI (%) | 26.43 | 21.76 |
| Peak analysis intensity * | 2, 391.7 (94.03%); 43.9 (5.97%) | 1, 344.8 (100%) |
| ζ potential (mV) | −17.96 | −20.49 |
| NA3 | ||
| DH (nm) | 367.3 | 241.3 |
| PDI (%) | 27.13 | 19.87 |
| Peak analysis intensity * | 2, 466.4 (98.23%); 21.85 (1.77%) | 2, 252.7 (96.87%); 15.05 (3.13%) |
| ζ potential (mV) | −10.21 | −7.45 |
| NA4 | ||
| DH (nm) | 91.7 | 265.4 |
| PDI (%) | 25.14 | 30.08 |
| Peak analysis intensity * | 1, 112.8 (100%) | 2, 307.1 (90.75%); 36.7 (9.25%) |
| ζ potential (mV) | −5.8 | −5.37 |
| NA5 | ||
| DH (nm) | 245.7 | 192.4 |
| PDI (%) | 25.26 | 30.86 |
| Peak analysis intensity * | 2, 282.7 (95.73%); 22.05 (4.27%) | 2, 241.4 (92.34%); 24.23 (7.66%) |
| ζ potential (mV) | −1.11 | −0.23 |
| NA6 | ||
| DH (nm) | 504.5 | 280.5 |
| PDI (%) | 27.47 | 29 |
| Peak analysis intensity * | 3, 804.3 (66.66%); 137.7 (29.09%); | 2, 367.9, (89.71%); 27.3 (10.29%) |
| ζ potential (mV) | −0.26 | −0.27 |
| NA7 | ||
| DH (nm) | 200.7 | 111.7 |
| PDI (%) | 29.29 | 29.44 |
| Peak analysis intensity * | 2, 317.3 (79.62%); 29.63 (20.38%) | 2, 188.0 (78.6%); 26.51 (21.35%) |
| ζ potential (mV) | +0.32 mV | −0.31 |
| NB | ||
| NB1 | ||
| DH (nm) | 251.5 | 247.2 |
| PDI (%) | 14.17 | 14.05 |
| Peak analysis intensity * | 1, 255.8 (100%) | 1, 223.9 (100%) |
| ζ potential (mV) | −23.1 | −19.9 |
| NB2 | ||
| DH (nm) | 289.8 | 286.8 |
| PDI (%) | 25.05 | 19.16 |
| Peak analysis intensity * | 1, 310.6, (100%) | 1, 321.9, (100%) |
| ζ potential (mV) | −25.46 | −26.4 |
| NB3 | ||
| DH (nm) | 429.5 | 446.9 |
| PDI (%) | 23.46 | 30.03 |
| Peak analysis intensity * | 2, 799.3 (52.31%); 173.6 (49.69%) | 2, 555.1 (85.69%); 81.8 (14.31%) |
| ζ potential (mV) | −20.77 | −34.6 |
| NB4 | ||
| DH (nm) | 262.5 | 351.8 |
| PDI (%) | 24.7 | 24.54 |
| Peak analysis intensity * | 1, 392.4 (100%) | 2, 714.5 (50.51%); 168.9 (49.5%) |
| ζ potential (mV) | −21.2 | −32.5 |
| NB5 | ||
| DH (nm) | 200.8 | 185.9 |
| PDI (%) | 19.44 | 23.43 |
| Peak analysis intensity * | 1, 244.1 (100%) | 1, 223.2 (100%) |
| ζ potential (mV) | −26.4 | −27.4 |
| NB6 | ||
| D DH (nm) | 276.7 | 261.3 |
| PDI (%) | 24.42 | 26.61 |
| Peak analysis intensity * | 2, 305.5 (98.10%); 29.46 (1.90%) | 1, 304.6 (100%) |
| ζ potential (mV) | −12.36 | −23 |
| NB7 | ||
| DH (nm) | 120.1 | 107.4 |
| PDI (%) | 24.93 | 23.54 |
| Peak analysis intensity * | 2, 140.7 (94.93%); 13.94 (5.07%) | 2, 129.3 (96.88%); 14.15 (3.12%) |
| ζ potential (mV) | −23.37 | −13.2 |
| NC | ||
| NC1 | ||
| DH (nm) | 181 | 166.6 |
| PDI (%) | 17.79 | 14.14 |
| Peak analysis intensity * | 1, 186.9 (100%) | 1, 162.1 (100%) |
| ζ potential (mV) | −33.9 | −38.5 |
| NC2 | ||
| DH (nm) | 740.9 | 225.7 |
| PDI (%) | 35.12 | 23.51 |
| Peak analysis intensity * | 2, 919.4 (83.01%); 121.2 (16.99%) | 1, 222.3 (100%) |
| ζ potential (mV) | −39.53 | −41.45 |
| NC3 | ||
| DH (nm) | 134.9 | 136.2 |
| PDI (%) | 28.52 | 28.63 |
| Peak analysis intensity * | 1, 211.4 (100%) | 1, 185.1 (100%) |
| ζ potential (mV) | −13.2 | −9.69 |
| NC4 | ||
| DH (nm) | 275 | 251.7 |
| PDI (%) | 33.07 | 33.85 |
| Peak analysis intensity * | 2, 654.6 (61.61%); 57.13 (35.19%); | 3, 545.3 (63.87%); 55.66 (33.23 %); 10.93 (2.89%) |
| ζ potential (mV) | −27.9 | |
| NC5 | ||
| DH (nm) | 120.2 | 103.2 |
| PDI (%) | 27.84 | 28.52 |
| Peak analysis intensity * | 2, 95.16 (73.91%); 621.2 (26.09%) | 2, 117 (88.99%); 1033 (11.01%) |
| ζ potential (mV) | −9.74 | −9.74 |
| NC6 | ||
| DH (nm) | 354.5 | 48.58 |
| PDI (%) | 38.31 | 32.64 |
| Peak analysis intensity * | 3, 1303 (46.40%); 560.4 (28.73%); | 3, 499.2 (32.53%); 5402 (28.46%); 24.9 (39.02%) |
| ζ potential (mV) | −8.37 | |
| NC7 | ||
| DH (nm) | 17.19 | 15.09 |
| PDI (%) | 25.98 | 23.55 |
| Peak analysis intensity * | 2, 13.7 (76.90%); 727.2 (23.10%) | 3, 13.2 (77.78%); 2166 (14.04%); |
| ζ potential (mV) | −10.02 | −10.02 |
| ND | ||
| ND1 | ||
| DH (nm) | 170.4 | 197.7 |
| PDI (%) | 20.84 | 13.22 |
| Peak analysis intensity * | 1, 178.3 (100 %) | 1, 202.5 (100%) |
| ζ potential (mV) | −44.38 | −35.17 |
| ND2 | ||
| DH (nm) | 246.9 | 175 |
| PDI (%) | 24.28 | 26.93 |
| Peak analysis intensity * | 2, 263.8 (95.13%); 29.04 (4.87%) | 2, 223.5 (97.36%); 21.9 (2.64%) |
| ζ potential (mV) | −25.31 | −23.03 |
| ND3 | ||
| DH (nm) | 170.1 | 212.2 |
| PDI (%) | 26.52 | 29.27 |
| Peak analysis intensity * | 2, 218.0 (92.48%); 36.55 (7.52%) | 2, 262.0 (88.80%); 34.1 (11.20%) |
| ζ potential (mV) | −17.13 | −18.63 |
| ND4 | ||
| DH (nm) | 224.8 | 127 |
| PDI (%) | 30.67 | 26.82 |
| Peak analysis intensity * | 3, 249.8 (84.58%); 1455 (14.42%); | 1, 157.2 (100%) |
| ζ potential (mV) | −26 | −25.38 |
| ND5 | ||
| DH (nm) | 244.5 | 136.1 |
| PDI (%) | 29.13 | 27.25 |
| Peak analysis intensity * | 2, 270.3 (92.22 %); 33.65 (7.78%) | 2, 179.6 (91.78%); 26.1 (8.22%) |
| ζ potential (mV) | −18.15 | −20.74 |
| ND6 | ||
| DH (nm) | 333.8 | 195.1 |
| PDI (%) | 25.75 | 24.83 |
| Peak analysis intensity * | 3, 824.5 (42.67%); 138.8 (55.78%); | 2, 251.7 (95.48%); 20.0 (4.52%) |
| ζ potential (mV) | −19.78 | −25.5 |
| ND7 | ||
| DH (nm) | 430.9 | 502.7 |
| PDI (%) | 27.71 | 31.78 |
| Peak analysis intensity * | 2, 496.8 (93.65%); 67.0 (6.35%) | 3, 1121 (66.21%); 181.4 (31.14%); 23.11 (2.65%) |
| ζ potential (mV) | 5.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niño-Martínez, N.; Audreyartha, K.; Cheung, K.; Parra, S.M.; Martínez-Castañón, G.; Bach, H. AgNP-Containing Niosomes Functionalized with Fucoidan Potentiated the Intracellular Killing of Mycobacterium abscessus in Macrophages. Int. J. Mol. Sci. 2025, 26, 1366. https://doi.org/10.3390/ijms26031366
Niño-Martínez N, Audreyartha K, Cheung K, Parra SM, Martínez-Castañón G, Bach H. AgNP-Containing Niosomes Functionalized with Fucoidan Potentiated the Intracellular Killing of Mycobacterium abscessus in Macrophages. International Journal of Molecular Sciences. 2025; 26(3):1366. https://doi.org/10.3390/ijms26031366
Chicago/Turabian StyleNiño-Martínez, Nereyda, Kayla Audreyartha, Kaitlyn Cheung, Sol Melchor Parra, Gabriel Martínez-Castañón, and Horacio Bach. 2025. "AgNP-Containing Niosomes Functionalized with Fucoidan Potentiated the Intracellular Killing of Mycobacterium abscessus in Macrophages" International Journal of Molecular Sciences 26, no. 3: 1366. https://doi.org/10.3390/ijms26031366
APA StyleNiño-Martínez, N., Audreyartha, K., Cheung, K., Parra, S. M., Martínez-Castañón, G., & Bach, H. (2025). AgNP-Containing Niosomes Functionalized with Fucoidan Potentiated the Intracellular Killing of Mycobacterium abscessus in Macrophages. International Journal of Molecular Sciences, 26(3), 1366. https://doi.org/10.3390/ijms26031366







