Anas barbariae 200K Modulates Cell Stiffness and Oxidative Stress in Microglial Cells In Vitro
Abstract
1. Introduction
2. Results
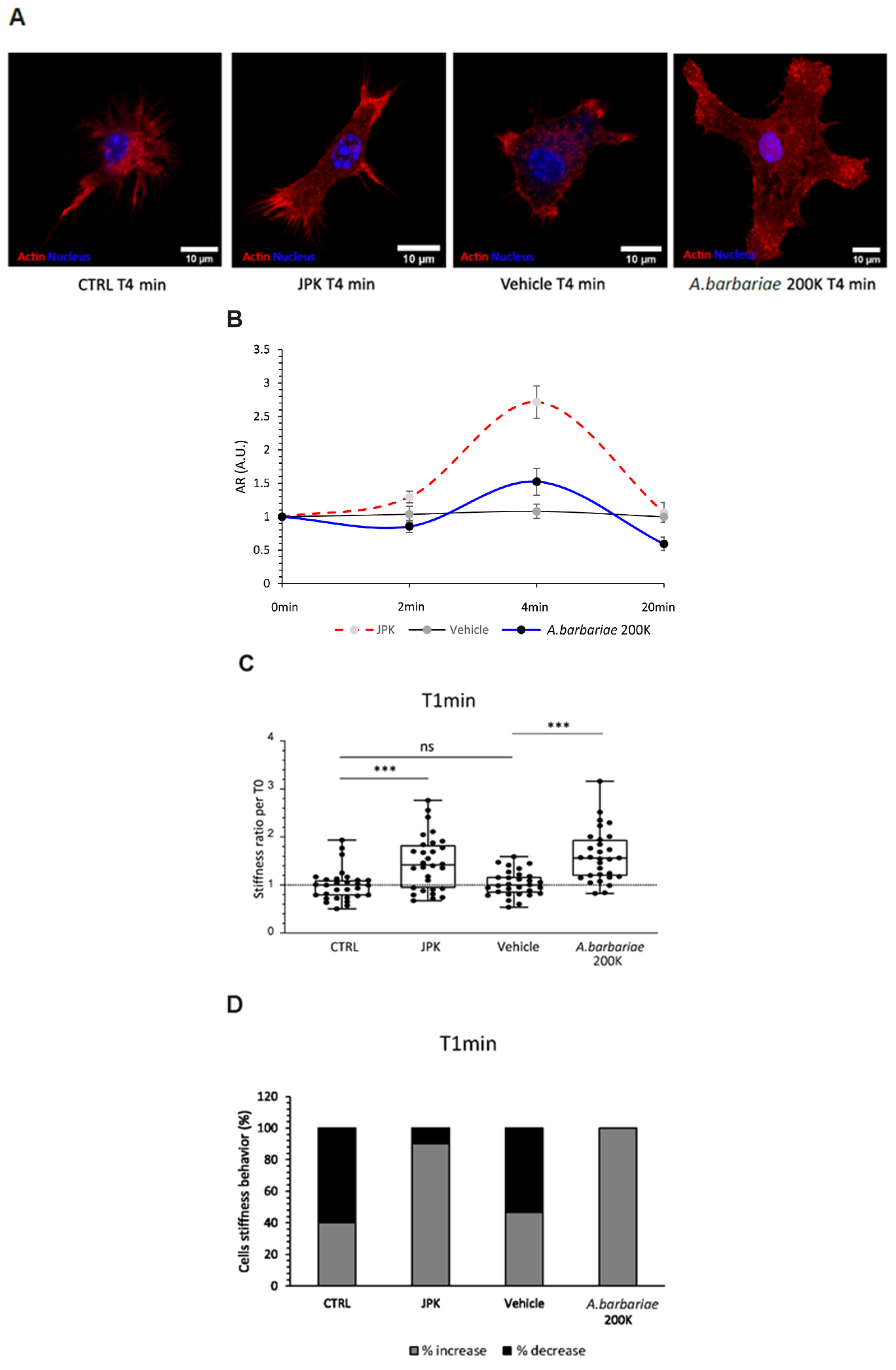
2.1. Treatments Induce Time-Dependent Changes in Morphology
2.2. Increase in Stiffness Following Short-Term Treatments
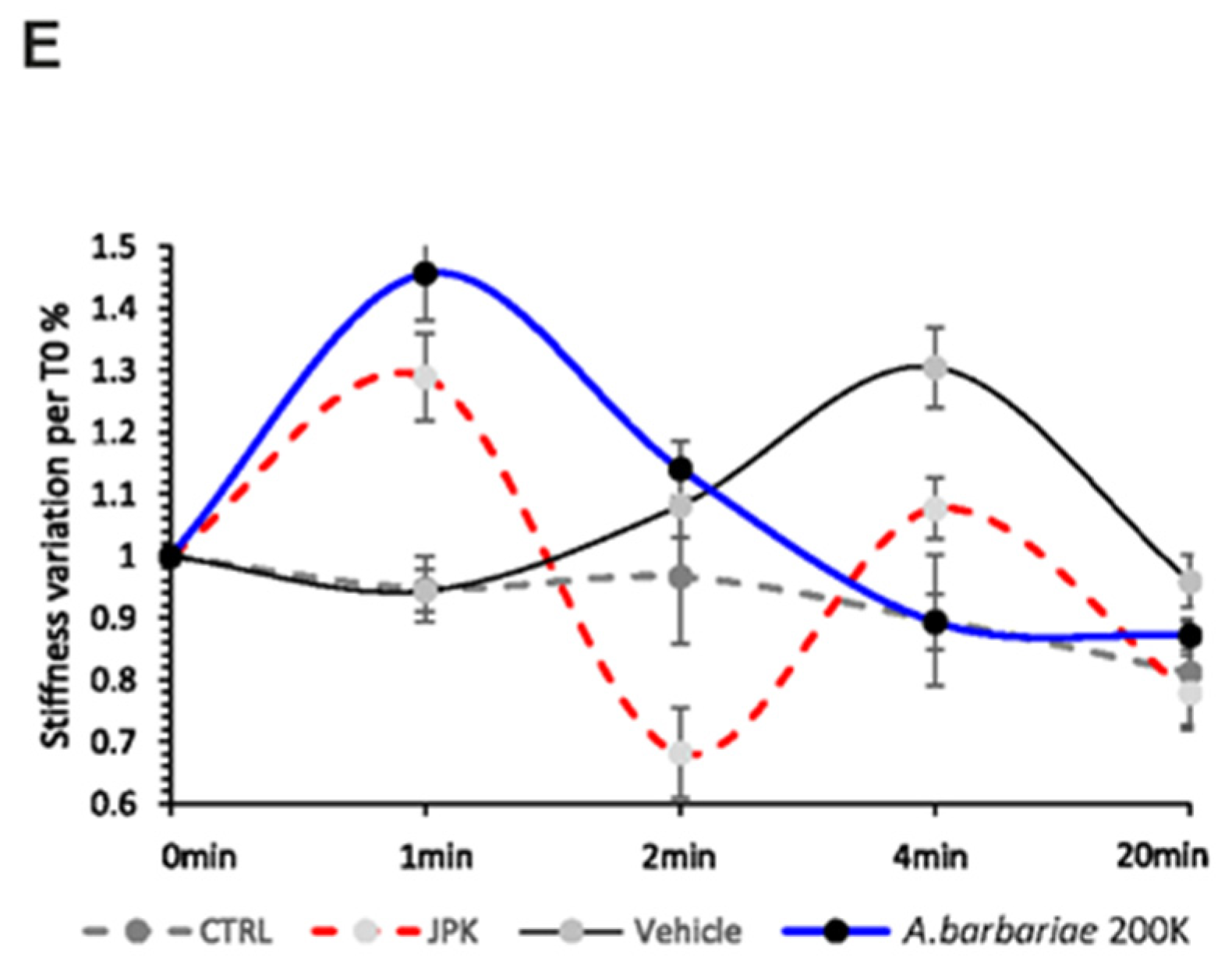
2.3. Temporal Dynamics of the Cellular Response
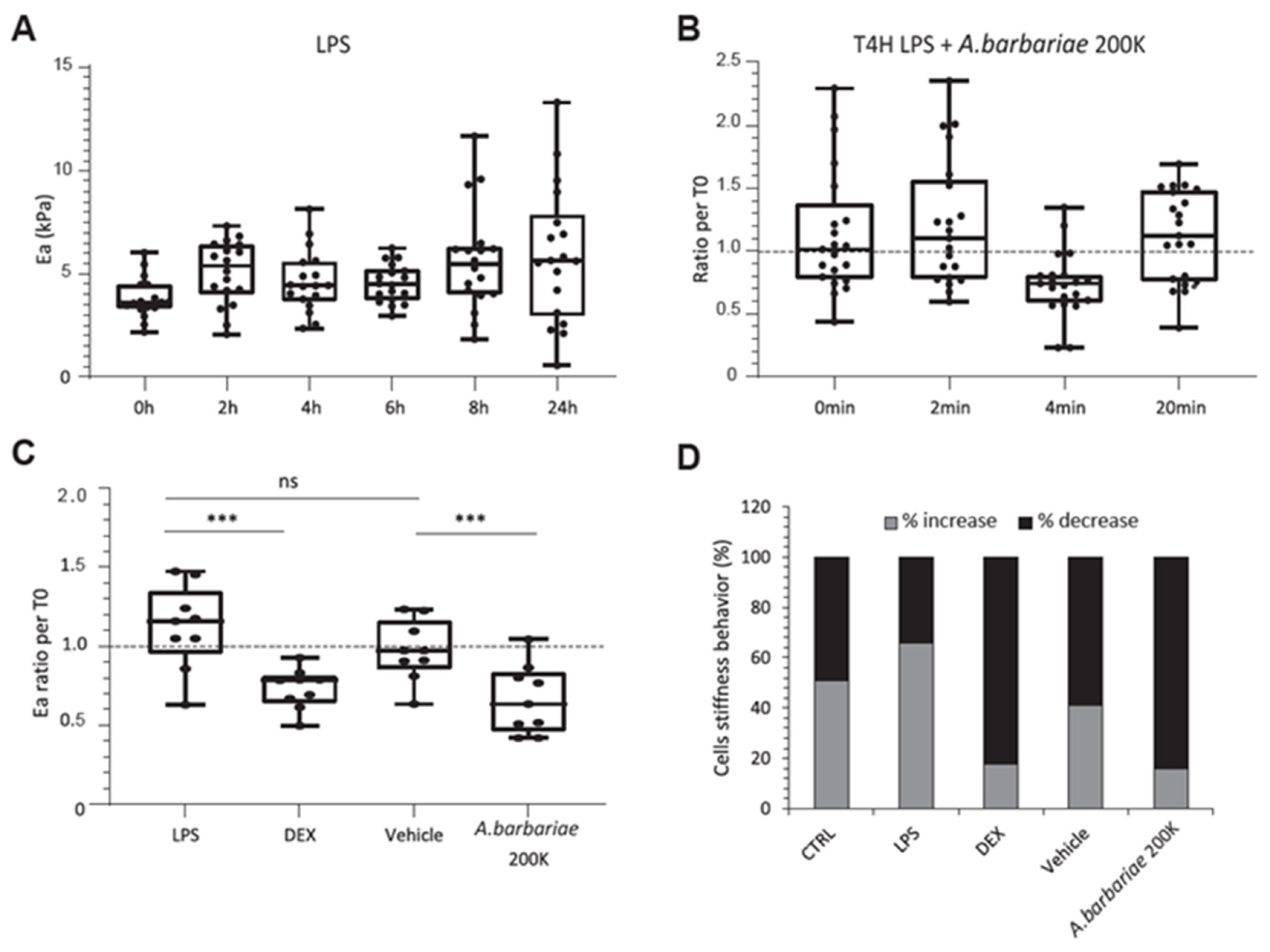
2.4. A. barbariae 200K Induces Stiffness Reduction in Inflamed Cells
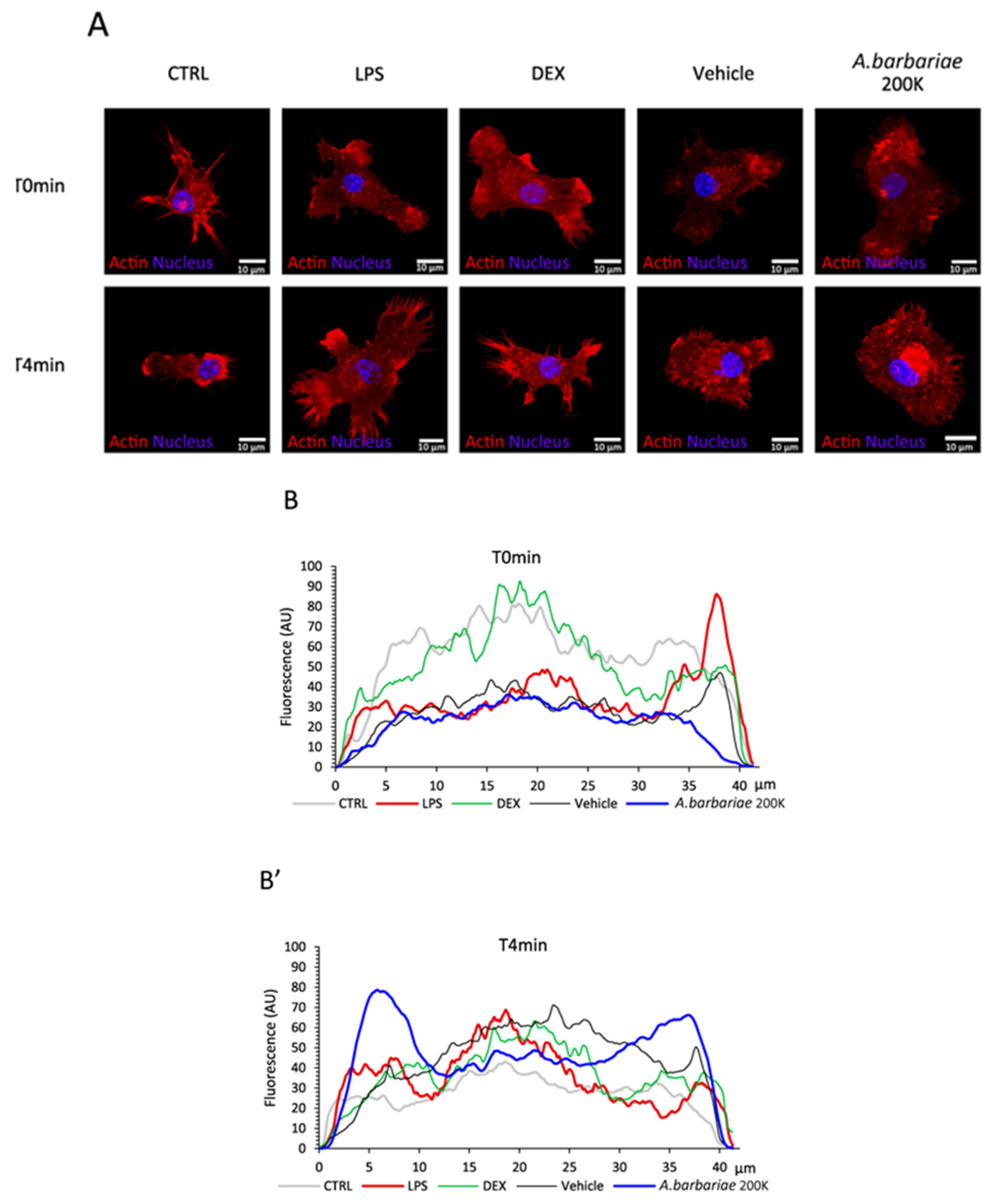
2.5. A. barbariae 200K Enables Actin Filament Re-Localisation
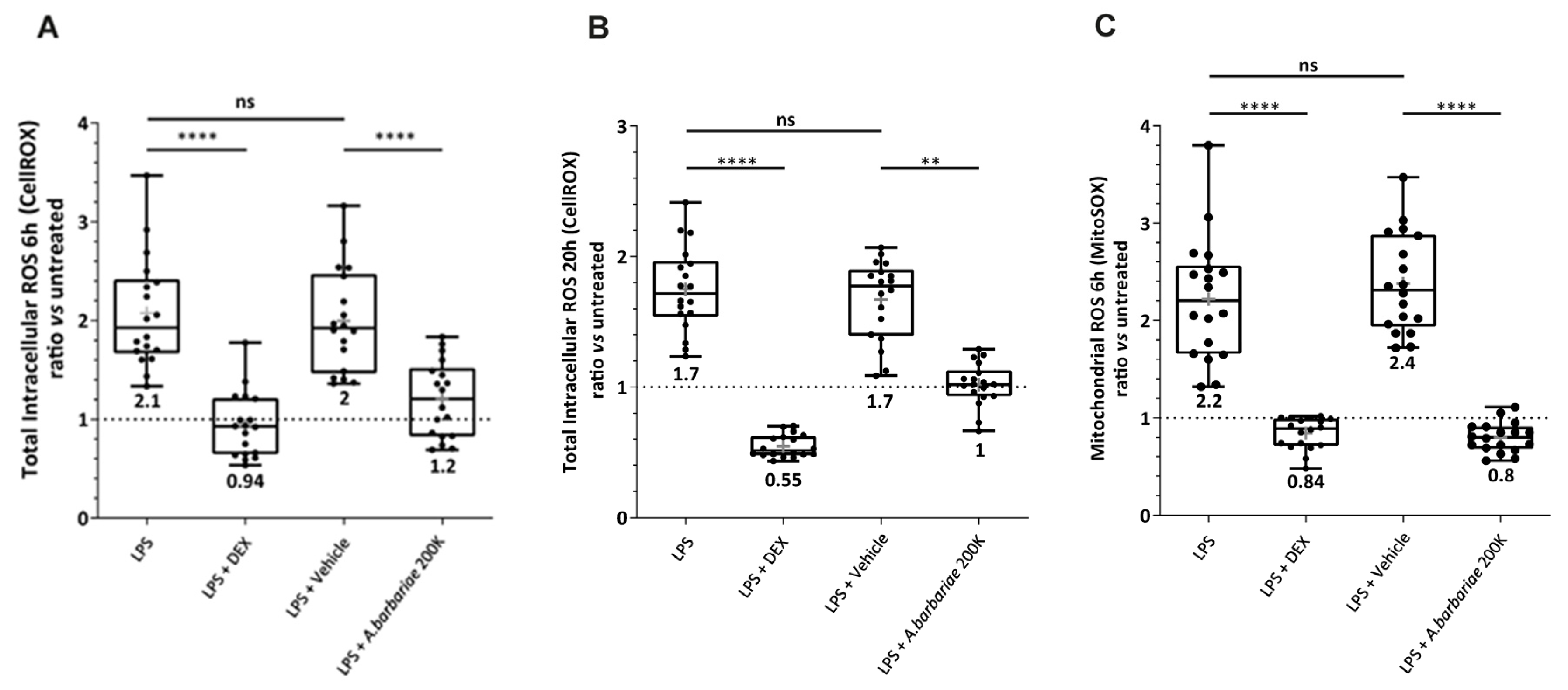
2.6. A. barbariae 200K Reduces Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Homeopathic Solutions
4.2. Primary Murine Microglial Cells
4.3. Atomic Force Microscopy (AFM) Sample Preparations
4.4. Atomic Force Microscopy (AFM) Analysis
4.5. Confocal Microscopy
4.6. Viability Assay
4.7. Measurement of the Total and Mitochondria-Derived ROS Level
4.8. Statistical Analysis
4.8.1. ROS
4.8.2. AFM
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clausen, J.; van Wijk, R.; Albrecht, H. Infection Models in Basic Research on Homeopathy. Homeopathy 2010, 99, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Manna, A.; Ghosh, S.; Mundle, M.; Saha, M.; Gourav, K.; Maiti, S.; Chattopadhyay, B. Prespecified Homeopathic Medicines in the Prevention of Confirmed and Suspected Cases of COVID-19: A Community-Based, Double-Blind, Randomized, Placebo-Controlled Prophylaxis Trial. Complement. Med. Res. 2024, 31, 140–148. [Google Scholar] [CrossRef]
- Nayak, D.; Devarajan, K.; Pal, P.P.; Ponnam, H.B.; Jain, N.; Shastri, V.; Bawaskar, R.; Chinta, R.; Khurana, A. Efficacy of Arsenicum Album 30C in the Prevention of COVID-19 in Individuals Residing in Containment Areas—A Prospective, Multicentre, Cluster-Randomized, Parallel Arm, Community Based, Open-Label Study. Complement. Med. Res. 2022, 30, 375–385. [Google Scholar] [CrossRef]
- Varanasi, R.; Nayak, D. Homoeopathy in the Management of Infectious Diseases: Different Facets of Its Use and Implications for the Future. Indian J. Res. Homoeopath. 2020, 14, 110–121. [Google Scholar] [CrossRef]
- Ferley, J.P.; Zmirou, D.; D’Adhemar, D.; Balducci, F. A Controlled Evaluation of a Homoeopathic Preparation in the Treatment of Influenza-like Syndromes. Br. J. Clin. Pharmacol. 1989, 27, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Papp, R.; Schuback, G.; Beck, E.; Burkard, G.; Bengel, J.; Lehrl, S.; Belon, P. OscillococcinumR in Patients with Influenza-like Syndromes: A Placebo-Controlled Double-Blind Evaluation. Br. Homoeopath. J. 1998, 87, 69–76. [Google Scholar] [CrossRef]
- Aouina, H.; Bamri, A.; Vesin, A.; Danno, K.; Aubry, E.; Faure, C.; Boujedaini, N. Oscillococcinum® for Upper Respiratory Tract Infections and Exacerbations in COPD: An Observational, Prospective Study (OXITUNIS). Drugs Context 2021, 10, 2021-4-2. [Google Scholar] [CrossRef]
- Conde Diez, S.; Viejo Casas, A.; Garcia Rivero, J.L.; Lopez Caro, J.C.; Ortiz Portal, F.; Diaz Saez, G. Impact of a Homeopathic Medication on Upper Respiratory Tract Infections in COPD Patients: Results of an Observational, Prospective Study (EPOXILO). Respir. Med. 2019, 146, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, Y.; Yang, Z. The Cytokine Storm of Severe Influenza and Development of Immunomodulatory Therapy. Cell Mol. Immunol. 2016, 13, 3–10. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef] [PubMed]
- Guck, J.; Schinkinger, S.; Lincoln, B.; Wottawah, F.; Ebert, S.; Romeyke, M.; Lenz, D.; Erickson, H.M.; Ananthakrishnan, R.; Mitchell, D.; et al. Optical Deformability as an Inherent Cell Marker for Testing Malignant Transformation and Metastatic Competence. Biophys. J. 2005, 88, 3689–3698. [Google Scholar] [CrossRef]
- Gardel, M.L.; Schneider, I.C.; Aratyn-Schaus, Y.; Waterman, C.M. Mechanical Integration of Actin and Adhesion Dynamics in Cell Migration. Annu. Rev. Cell Dev. Biol. 2010, 26, 315–333. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.; Biber, K.; Michelucci, A. Cellular and Molecular Characterization of Microglia: A Unique Immune Cell Population. Front. Immunol. 2017, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Valin, K.L.; Dixon, M.L.; Leavenworth, J.W. The Role of Microglia and Macrophages in CNS Homeostasis, Autoimmunity, and Cancer. J. Immunol. Res. 2017, 2017, 5150678. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Gottlieb, A.; Toledano-Furman, N.; Prabhakara, K.S.; Kumar, A.; Caplan, H.W.; Bedi, S.; Cox, C.S.; Olson, S.D. Time Dependent Analysis of Rat Microglial Surface Markers in Traumatic Brain Injury Reveals Dynamics of Distinct Cell Subpopulations. Sci. Rep. 2022, 12, 6289. [Google Scholar] [CrossRef] [PubMed]
- Vijaya, A.K.; Iešmantaitė, M.; Mela, V.; Baltriukienė, D.; Burokas, A. Microglia Isolation from Aging Mice for Cell Culture: A Beginner’s Guide. Front. Cell Neurosci. 2023, 17, 1082180. [Google Scholar] [CrossRef] [PubMed]
- Runel, G.; Paumier, A.; Verre, J.; Catte, A.; Tribolo, S.; Chlasta, J.; Boujedaini, N. Action of Ultra-Low Dose Medicine on Oxidative Stress and Cell Stiffness of Microglial Cells In Vitro with Actin Filaments Reorganization. Int. J. Pharm. Res. 2022, 14, 171–181. [Google Scholar] [CrossRef]
- Block, M.L.; Hong, J.-S. Microglia and Inflammation-Mediated Neurodegeneration: Multiple Triggers with a Common Mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Bollmann, L.; Koser, D.E.; Shahapure, R.; Gautier, H.O.B.; Holzapfel, G.A.; Scarcelli, G.; Gather, M.C.; Ulbricht, E.; Franze, K. Microglia Mechanics: Immune Activation Alters Traction Forces and Durotaxis. Front. Cell Neurosci. 2015, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Cai, H.; Yang, F.; Jin, H.; Liu, J.; Yang, P.; Cai, J. Atomic Force Microscopy Based Investigations of Anti-Inflammatory Effects in Lipopolysaccharide-Stimulated Macrophages. Anal. Bioanal. Chem. 2016, 408, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Fuselier, C.; Dufay, E.; Berquand, A.; Terryn, C.; Bonnomet, A.; Molinari, M.; Martiny, L.; Schneider, C. Dynamized Ultra-Low Dilution of Ruta Graveolens Disrupts Plasma Membrane Organization and Decreases Migration of Melanoma Cancer Cell. Cell Adh Migr. 2023, 17, 1–13. [Google Scholar] [CrossRef]
- Paumier, A.; Verre, J.; Tribolo, S.; Boujedaini, N. Anti-Oxidant Effect of High Dilutions of Arnica Montana, Arsenicum Album, and Lachesis Mutus in Microglial Cells in Vitro. Dose Response 2022, 20, 1–7. [Google Scholar] [CrossRef]
- Verre, J.; Boisson, M.; Paumier, A.; Tribolo, S.; Boujedaini, N. Anti-Inflammatory Effects of Arnica Montana (Mother Tincture and Homeopathic Dilutions) in Various Cell Models. J. Ethnopharmacol. 2024, 318, 117064. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Diaz, J.E.; Ouidja, M.O.; Socias, S.B.; Hamadat, S.; Guerreiro, S.; Raisman-Vozari, R.; Michel, P.P. A Simplified Approach for Efficient Isolation of Functional Microglial Cells: Application for Modeling Neuroinflammatory Responses in Vitro. Glia 2016, 64, 1912–1924. [Google Scholar] [CrossRef]
- dos Santos Pereira, M.; Maitan Santos, B.; Gimenez, R.; Guimarães, F.S.; Raisman-Vozari, R.; Del Bel, E.; Michel, P.P. The Two Synthetic Cannabinoid Compounds 4′-F-CBD and HU-910 Efficiently Restrain Inflammatory Responses of Brain Microglia and Astrocytes. Glia 2024, 72, 529–545. [Google Scholar] [CrossRef]
- dos-Santos-Pereira, M.; Guimarães, F.S.; Del-Bel, E.; Raisman-Vozari, R.; Michel, P.P. Cannabidiol Prevents LPS-induced Microglial Inflammation by Inhibiting ROS/NF-κB-dependent Signaling and Glucose Consumption. Glia 2020, 68, 561–573. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, Oxidants, and Aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Huff, L.P.; Fujii, M.; Griendling, K.K. Redox Regulation of the Actin Cytoskeleton and Its Role in the Vascular System. Free Radic. Biol. Med. 2017, 109, 84–107. [Google Scholar] [CrossRef] [PubMed]
- Bodega, G.; Alique, M.; Puebla, L.; Carracedo, J.; Ramírez, R.M. Microvesicles: ROS Scavengers and ROS Producers. J. Extracell. Vesicles 2019, 8, 1626654. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Wang, Y.; Fa, S.; Yuan, C.; Yang, L. Extracellular Vesicles as Natural Delivery Carriers Regulate Oxidative Stress Under Pathological Conditions. Front. Bioeng. Biotechnol. 2021, 9, 752019. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, R.; Chen, Y.; Wang, M.; Du, J. Crosstalk between Oxidative Stress and Exosomes. Oxid. Med. Cell Longev. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Socodato, R.; Relvas, J.B. A Cytoskeleton Symphony: Actin and Microtubules in Microglia Dynamics and Aging. Progress Neurobiol. 2024, 234, 102586. [Google Scholar] [CrossRef] [PubMed]
- Dupré-Crochet, S.; Erard, M.; Nüβe, O. ROS Production in Phagocytes: Why, When, and Where? J. Leukoc. Biol. 2013, 94, 657–670. [Google Scholar] [CrossRef]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef]
- Olioso, D.; Marzotto, M.; Bonafini, C.; Brizzi, M.; Bellavite, P. Arnica Montana Effects on Gene Expression in a Human Macrophage Cell Line. Evaluation by Quantitative Real-Time PCR. Homeopathy 2016, 105, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Lejri, I.; Grimm, A.; Trempat, P.; Boujedaini, N.; Eckert, A. Gelsemium Low Doses Increases Bioenergetics and Neurite Outgrowth. AJBIO 2022, 10, 51. [Google Scholar] [CrossRef]
- Lejri, I.; Grimm, A.; Trempat, P.; Boujedaini, N.; Eckert, A. Gelsemium Low Doses Protect against Serum Deprivation-Induced Stress on Mitochondria in Neuronal Cells. J. Ethnopharmacol. 2025, 336, 118714. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.-C.; Marrelli, M.; Puticiu, M.; Conforti, F.; Statti, G. Effects of Arnica Phytotherapeutic and Homeopathic Formulations on Traumatic Injuries and Inflammatory Conditions: A Systematic Review. Plants 2024, 13, 3112. [Google Scholar] [CrossRef] [PubMed]
- Monograph 2371 (Methods of Preparation of Homoeopathic Stocks and Potentisation). In European Pharmacopoeia; EDQM Council of Europe Editions: Strasbourg, France, 2024.
- Monograph 1038 (Homeopathic Preparations). In European Pharmacopoeia; EDQM Council of Europe Editions: Strasbourg, France, 2024.
- Schillers, H.; Rianna, C.; Schäpe, J.; Luque, T.; Doschke, H.; Wälte, M.; Uriarte, J.J.; Campillo, N.; Michanetzis, G.P.A.; Bobrowska, J.; et al. Standardized Nanomechanical Atomic Force Microscopy Procedure (SNAP) for Measuring Soft and Biological Samples. Sci. Rep. 2017, 7, 5117. [Google Scholar] [CrossRef] [PubMed]
- Oommen, B.; Van Vliet, K.J. Effects of Nanoscale Thickness and Elastic Nonlinearity on Measured Mechanical Properties of Polymeric Films. Thin Solid. Films 2006, 513, 235–242. [Google Scholar] [CrossRef]
- Santos, J.A.C.; Rebêlo, L.M.; Araujo, A.C.; Barros, E.B.; de Sousa, J.S. Thickness-Corrected Model for Nanoindentation of Thin Films with Conical Indenters. Soft Matter 2012, 8, 4441. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paumier, A.; Verre, J.; Runel, G.; Chlasta, J.; Tribolo, S.; Chanut, S. Anas barbariae 200K Modulates Cell Stiffness and Oxidative Stress in Microglial Cells In Vitro. Int. J. Mol. Sci. 2025, 26, 1451. https://doi.org/10.3390/ijms26041451
Paumier A, Verre J, Runel G, Chlasta J, Tribolo S, Chanut S. Anas barbariae 200K Modulates Cell Stiffness and Oxidative Stress in Microglial Cells In Vitro. International Journal of Molecular Sciences. 2025; 26(4):1451. https://doi.org/10.3390/ijms26041451
Chicago/Turabian StylePaumier, Anne, Justine Verre, Gaël Runel, Julien Chlasta, Sandra Tribolo, and Stéphanie Chanut. 2025. "Anas barbariae 200K Modulates Cell Stiffness and Oxidative Stress in Microglial Cells In Vitro" International Journal of Molecular Sciences 26, no. 4: 1451. https://doi.org/10.3390/ijms26041451
APA StylePaumier, A., Verre, J., Runel, G., Chlasta, J., Tribolo, S., & Chanut, S. (2025). Anas barbariae 200K Modulates Cell Stiffness and Oxidative Stress in Microglial Cells In Vitro. International Journal of Molecular Sciences, 26(4), 1451. https://doi.org/10.3390/ijms26041451





