CRISPR/Cas9 Genome Editing in the Diamondback Moth: Current Progress, Challenges, and Prospects
Abstract
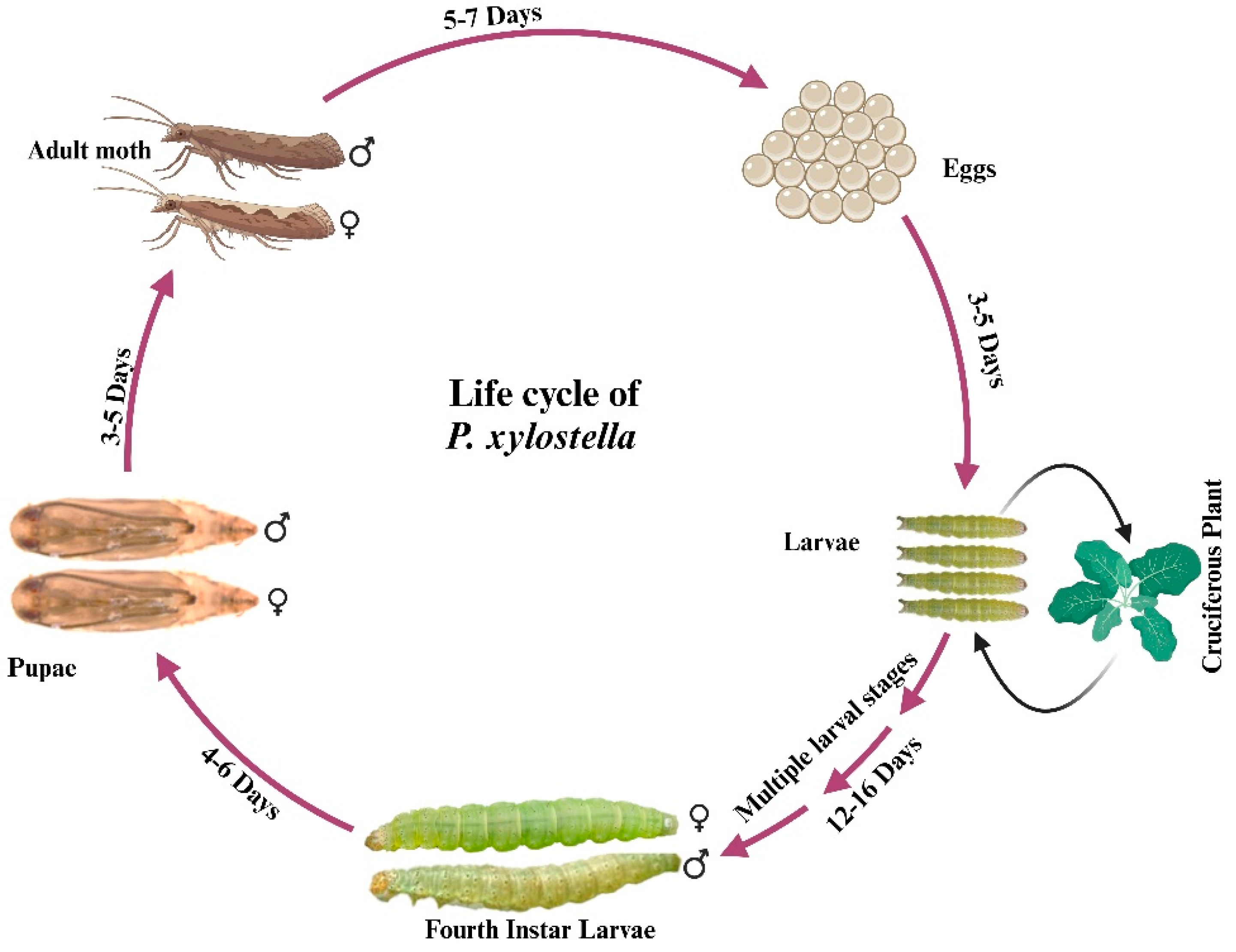
1. Introduction
2. Applications of CRISPR/Cas9-Based Gene Knockout System in Diamondback Moth
2.1. Development and Reproduction
2.2. Pigmentation
2.3. Sex Determination
2.4. Circadian Rhythms
2.5. Ecological Adaptability
2.6. Insecticide Resistance
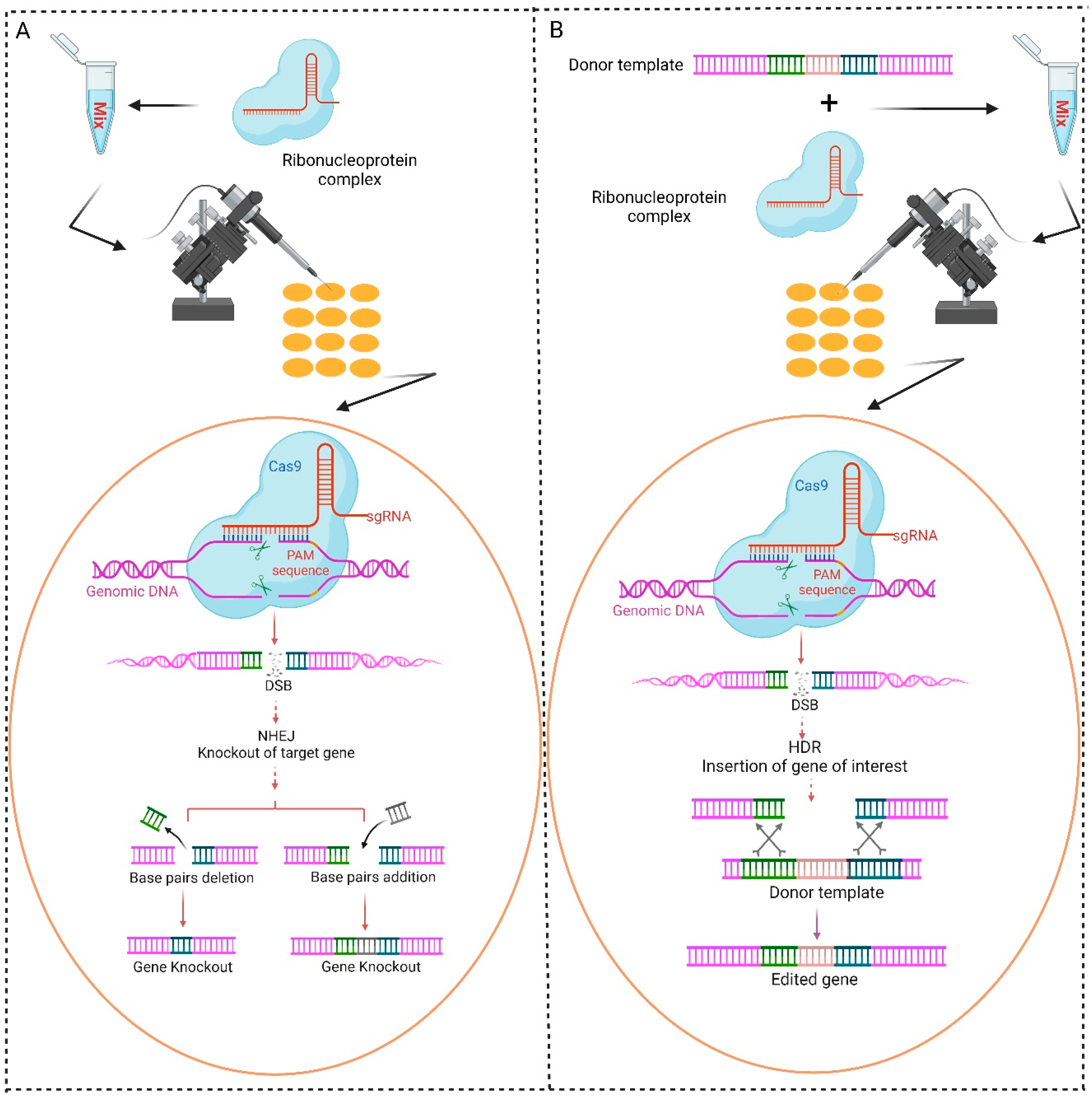
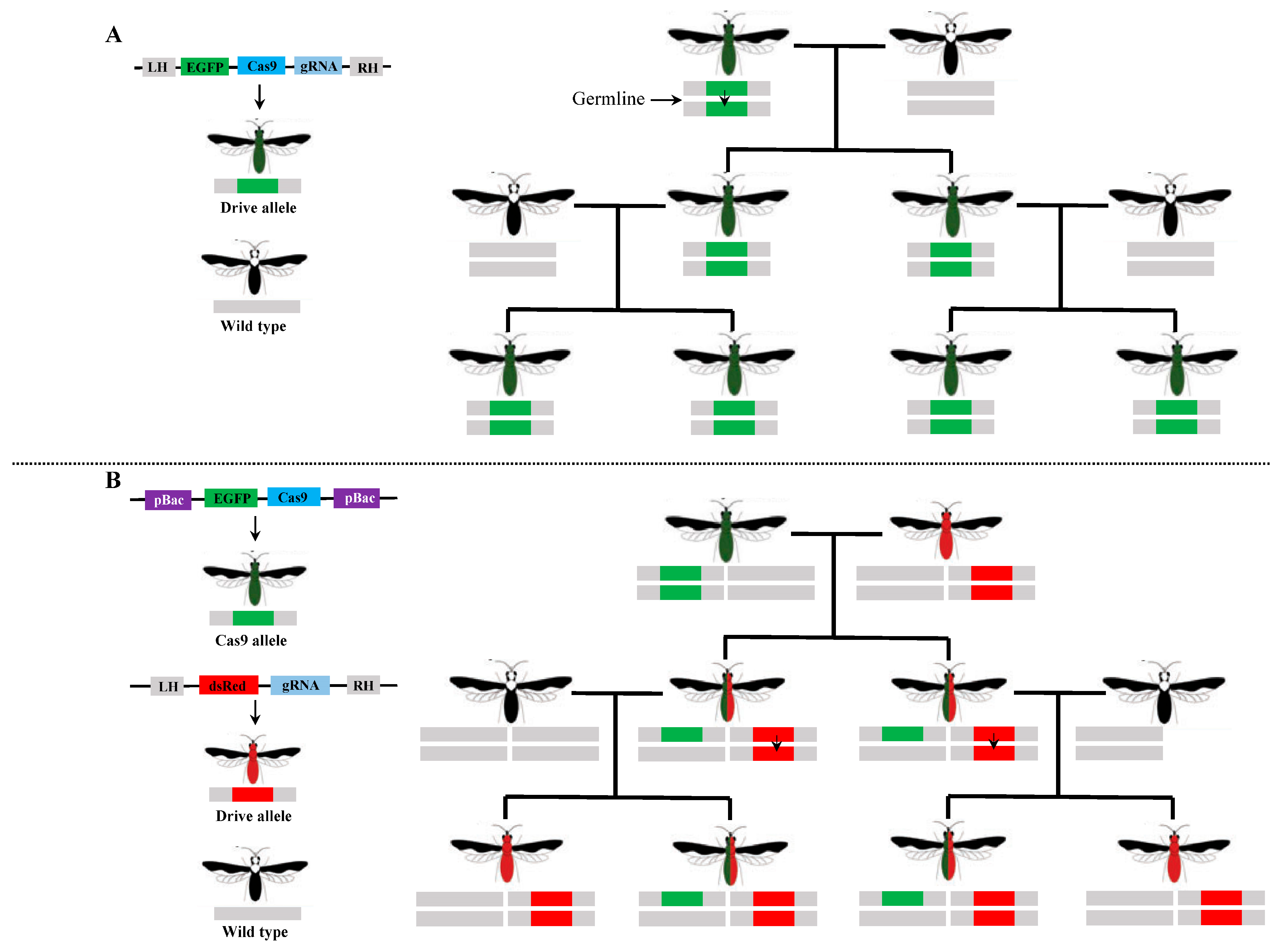
3. Application of CRISPR/Cas9 Knock-In and Gene Drive Systems in the Diamondback Moth
4. Challenges and Possible Solutions of CRISPR/Cas9 Gene-Editing in the Diamondback Moth
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, Z.; Feng, X.; Liu, S.-S.; You, M.; Furlong, M.J. Biology, ecology, and management of the diamondback moth in China. Annu. Rev. Entomol. 2016, 61, 277–296. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Ke, F.; You, S.; Wu, Z.; Liu, Q.; He, W.; Baxter, S.W.; Yuchi, Z.; Vasseur, L.; Gurr, G.M. Variation among 532 genomes unveils the origin and evolutionary history of a global insect herbivore. Nat. Commun. 2020, 11, 2321. [Google Scholar] [CrossRef] [PubMed]
- Dancau, T.; Mason, P.G.; Cappuccino, N. Elusively overwintering: A review of diamondback moth (Lepidoptera: Plutellidae) cold tolerance and overwintering strategy. Can. Entomol. 2018, 150, 156–173. [Google Scholar] [CrossRef]
- Sun, J.; Liang, P.; Gao, X. Cross-resistance patterns and fitness in fufenozide-resistant diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag. Sci. 2012, 68, 285–289. [Google Scholar] [CrossRef]
- Etebari, K.; Furlong, M.J.; Asgari, S. Genome wide discovery of long intergenic non-coding RNAs in Diamondback moth (Plutella xylostella) and their expression in insecticide resistant strains. Sci. Rep. 2015, 5, 14642. [Google Scholar] [CrossRef]
- Harvey-Samuel, T.; Ant, T.; Alphey, L. Towards the genetic control of invasive species. Biol. Invasions 2017, 19, 1683–1703. [Google Scholar] [CrossRef]
- Kyrou, K.; Hammond, A.M.; Galizi, R.; Kranjc, N.; Burt, A.; Beaghton, A.K.; Nolan, T.; Crisanti, A. A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat. Biotechnol. 2018, 36, 1062–1066. [Google Scholar] [CrossRef]
- Asad, M.; Liu, D.; Chen, J.; Yang, G. Applications of gene drive systems for population suppression of insect pests. Bull. Entomol. Res. 2022, 112, 724–733. [Google Scholar] [CrossRef]
- Kandul, N.P.; Liu, J.; Buchman, A.; Shriner, I.C.; Corder, R.M.; Warsinger-Pepe, N.; Yang, T.; Yadav, A.K.; Scott, M.J.; Marshall, J.M. Precision guided sterile males suppress populations of an invasive crop pest. GEN Biotechnol. 2022, 1, 372–385. [Google Scholar] [CrossRef]
- Li, M.; Yang, T.; Bui, M.; Gamez, S.; Wise, T.; Kandul, N.P.; Liu, J.; Alcantara, L.; Lee, H.; Edula, J.R.; et al. Suppressing mosquito populations with precision guided sterile males. Nat. Commun. 2021, 12, 5374. [Google Scholar] [CrossRef]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Embden, J.D.A.v.; Gaastra, W.; Schouls, L.M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 2002, 43, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Gostimskaya, I. CRISPR-Cas9: A history of its discovery and ethical considerations of its use in genome editing. Biochemistry 2022, 87, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Hwang, W.Y.; Fu, Y.; Reyon, D.; Maeder, M.L.; Tsai, S.Q.; Sander, J.D.; Peterson, R.T.; Yeh, J.R.J.; Joung, J.K. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 227–229. [Google Scholar] [CrossRef]
- Hille, F.; Charpentier, E. CRISPR-Cas: Biology, mechanisms and relevance. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150496. [Google Scholar] [CrossRef]
- Xue, C.; Greene, E.C. DNA repair pathway choices in CRISPR-Cas9-mediated genome editing. Trends Genet. 2021, 37, 639–656. [Google Scholar] [CrossRef]
- Wang, J.Y.; Doudna, J.A. CRISPR technology: A decade of genome editing is only the beginning. Science 2023, 379, eadd8643. [Google Scholar] [CrossRef]
- Hillary, V.E.; Ceasar, S.A. A Review on the Mechanism and Applications of CRISPR/Cas9/Cas12/Cas13/Cas14 Proteins Utilized for Genome Engineering. Mol. Biotechnol. 2023, 65, 311–325. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Asmamaw, M.; Zawdie, B. Mechanism and applications of CRISPR/Cas-9-mediated genome editing. Biol. Targets Ther. 2021, 15, 353–361. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Maharajan, T.; Hillary, V.E.; Krishna, T.P.A. Insights to improve the plant nutrient transport by CRISPR/Cas system. Biotechnol. Adv. 2022, 59, 107963. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, L.; Jiang, Y.; Zhou, Z.; Zou, G. Efficient genome editing in filamentous fungus Trichoderma reesei using the CRISPR/Cas9 system. Cell Discov. 2015, 1, 15007. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, D.E.; Kim, K. Genome editing methods in animal models. Anim. Cells Syst. 2020, 24, 8–16. [Google Scholar] [CrossRef]
- Singh, V.; Dhar, P.K. Genome Engineering via CRISPR-Cas9 System; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Hillary, V.E.; Ceasar, S.A. Genome engineering in insects for the control of vector borne diseases. Prog. Mol. Biol. Transl. Sci. 2021, 179, 197–223. [Google Scholar]
- Garza-Elizondo, M.A.; Rodríguez-Rodríguez, D.R.; Ramírez-Solís, R.; Garza-Rodríguez, M.D.L.; Barrera-Saldaña, H.A. Genome editing: A perspective on the application of CRISPR/Cas9 to study human diseases. Int. J. Mol. Med. 2019, 43, 1559–1574. [Google Scholar]
- Meccariello, A.; Monti, S.M.; Romanelli, A.; Colonna, R.; Primo, P.; Inghilterra, M.G.; Del Corsano, G.; Ramaglia, A.; Iazzetti, G.; Chiarore, A.; et al. Highly efficient DNA-free gene disruption in the agricultural pest Ceratitis capitata by CRISPR-Cas9 ribonucleoprotein complexes. Sci. Rep. 2017, 7, 10061. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Zeng, B.; Wang, Y.; James, A.A.; Gurr, G.M.; Yang, G.; Lin, X.; Huang, Y.; You, M. CRISPR/Cas9 mediated knockout of the abdominal-A homeotic gene in the global pest, diamondback moth (Plutella xylostella). Insect Biochem. Mol. Biol. 2016, 75, 98–106. [Google Scholar] [CrossRef]
- Zhao, Q.; Ma, D.; Huang, Y.; He, W.; Li, Y.; Vasseur, L.; You, M. Genome-wide investigation of transcription factors provides insights into transcriptional regulation in Plutella xylostella. Mol. Genet. Genomics 2017, 293, 435–449. [Google Scholar] [CrossRef]
- Zou, M.-M.; Wang, Q.; Chu, L.-N.; Vasseur, L.; Zhai, Y.-L.; Qin, Y.-D.; He, W.-Y.; Yang, G.; Zhou, Y.-Y.; Peng, L.; et al. CRISPR/Cas9-induced vitellogenin knockout lead to incomplete embryonic development in Plutella xylostella. Insect Biochem. Mol. Biol. 2020, 123, 103406. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, Q.; Zou, M.-M.; Qin, Y.-D.; Vasseur, L.; Chu, L.-N.; Zhai, Y.-L.; Dong, S.-J.; Liu, L.-L.; He, W.-Y.; et al. CRISPR/Cas9-mediated vitellogenin receptor knockout leads to functional deficiency in the reproductive development of Plutella xylostella. Front. Physiol. 2020, 10, 01585. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y.; Bi, H.; Xu, J.; Liu, Z.; Niu, C.; He, L.; James, A.A.; Li, K.; Huang, Y. Mutation of the seminal protease gene, serine protease 2, results in male sterility in diverse lepidopterans. Insect Biochem. Mol. Biol. 2020, 116, 103243. [Google Scholar] [CrossRef]
- Zhai, Y.-L.; Dong, S.-J.; Zou, M.-M.; Qin, Y.-D.; Liu, L.-L.; Cao, M.-H.; Huang, M.-Q.; Vasseur, L.; You, M.-S.; Peng, L. Vitelline membrane protein 26 mutagenesis, using CRISPR/Cas9, results in egg collapse in Plutella xylostella. Int. J. Mol. Sci. 2022, 23, 9538. [Google Scholar] [CrossRef]
- Liu, D.; Asad, M.; Liao, J.; Chen, J.; Li, J.; Chu, X.; Pang, S.; Tariq, M.; Abbas, A.N.; Yang, G. The potential role of the piwi gene in the development and reproduction of Plutella xylostella. Int. J. Mol. Sci. 2023, 24, 12321. [Google Scholar] [CrossRef]
- Zheng, W.; Ma, H.; Liu, Z.; Zhou, Y.; Zhu, H.; Liu, J.; Zhang, C.; Liu, Z.; Zhou, X. Knockout of tyramine receptor 1 results in a decrease of oviposition, mating, and sex pheromone biosynthesis in female Plutella xylostella. Pest Manag. Sci. 2023, 79, 3903–3912. [Google Scholar] [CrossRef]
- Asad, M.; Liao, J.; Chen, J.; Munir, F.; Pang, S.; Abbas, A.N.; Yang, G. Exploring the role of the ovary-serine protease gene in the female fertility of the diamondback moth using CRISPR/Cas9. Pest Manag. Sci. 2024, 80, 3194–3206. [Google Scholar] [CrossRef]
- Huang, P.; Yu, H.; Asad, M.; Liao, J.; Lin, S.; Pang, S.; Chu, X.; Yang, G. Functional characteristics of Dicer genes in Plutella xylostella. Pest Manag. Sci. 2024, 80, 2109–2119. [Google Scholar] [CrossRef]
- Xu, X.; Harvey-Samuel, T.; Yang, J.; Alphey, L.; You, M. Ommochrome pathway genes kynurenine 3-hydroxylase and cardinal participate in eye pigmentation in Plutella xylostella. BMC Mol. Cell Biol. 2020, 21, 63. [Google Scholar] [CrossRef]
- Xu, X.; Harvey-Samuel, T.; Yang, J.; You, M.; Alphey, L. CRISPR/Cas9-based functional characterization of the pigmentation gene ebony in Plutella xylostella. Insect Mol. Biol. 2021, 30, 615–623. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Xu, X.; Liu, Z.; Li, J.; Zhan, X.; Yang, G.; You, M.; You, S. CRISPR/Cas9-based functional analysis of yellow gene in the diamondback moth, Plutella xylostella. Insect Sci. 2020, 28, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.e.; Liu, Z.; Xu, J.; Li, X.; Bi, H.; Andongma, A.A.; Niu, C.; Huang, Y. Mutation of doublesex induces sex-specific sterility of the diamondback moth Plutella xylostella. Insect Biochem. Mol. Biol. 2019, 112, 103180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, X.; Chen, X.e.; Li, X.; Bi, H.; Xu, J.; Zhu, C.; Niu, C.; Huang, Y. Mutation of P-element somatic inhibitor induces male sterility in the diamondback moth, Plutella xylostella. Pest Manag. Sci. 2021, 77, 3588–3596. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.H.; Zou, M.M.; Liu, L.L.; Dong, S.J.; Huang, M.Q.; Zheng, J.H.; Li, R.N.; Cui, J.D.; Peng, L. Sast1-mediated manifold effects inhibit Plutella xylostella fertility. Pest Manag. Sci. 2024, 80, 2596–2609. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.P.; Wang, D.F.; Ma, W.F.; Lin, X.L.; Yang, G. Knockout of cryptochrome 1 disturbs the locomotor circadian rhythm and development of Plutella xylostella. Insect Sci. 2022, 30, 1035–1045. [Google Scholar] [CrossRef]
- Chen, S.-P.; Lin, X.-L.; Qiu, R.-Z.; Chi, M.-X.; Yang, G. An lw-opsin mutation changes the gene expression of the phototransduction pathway: A cryptochrome1 mutation enhances the phototaxis of male Plutella xylostella (lepidoptera: Plutellidae). Insects 2023, 14, 72. [Google Scholar] [CrossRef]
- Chen, W.; Wang, D.; Yu, L.; Zhong, W.; Yuan, Y.; Yang, G. Comparative analysis of locomotor behavior and head diurnal transcriptome regulation by PERIOD and CRY2 in the diamondback moth. Insect Sci. 2024, 31, 1697–1720. [Google Scholar] [CrossRef]
- Chen, W.; Dong, Y.; Saqib, H.S.A.; Vasseur, L.; Zhou, W.; Zheng, L.; Lai, Y.; Ma, X.; Lin, L.; Xu, X.; et al. Functions of duplicated glucosinolate sulfatases in the development and host adaptation of Plutella xylostella. Insect Biochem. Mol. Biol. 2020, 119, 103316. [Google Scholar] [CrossRef]
- Chen, W.; Dong, Y.; Zheng, L.; Lai, Y.; Li, F.; Zhou, L.; Wang, B.; You, M.; He, W. An inducible gene from glycoside hydrolase one family of Plutella xylostella decreases larval survival when feeding on host plant. Front. Physiol. 2022, 13, 1013092. [Google Scholar] [CrossRef]
- Liu, X.-L.; Zhang, J.; Yan, Q.; Miao, C.-L.; Han, W.-K.; Hou, W.; Yang, K.; Hansson, B.S.; Peng, Y.-C.; Guo, J.-M.; et al. The molecular basis of host selection in a crucifer-specialized moth. Curr. Biol. 2020, 30, 4476–4482.e4475. [Google Scholar] [CrossRef]
- Chen, W.; Saqib, H.S.A.; Xu, X.; Dong, Y.; Zheng, L.; Lai, Y.; Jing, X.; Lu, Z.; Sun, L.; You, M.; et al. Glucosinolate sulfatases–sulfatase-modifying factors system enables a crucifer-specialized moth to pre-detoxify defensive glucosinolate of the host plant. J. Agric. Food Chem. 2022, 70, 11179–11191. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-B.; Lai, Y.-F.; Li, F.-F.; Jiao, L.; Qiao, Q.-X.; Li, S.-Y.; Xiang, X.-J.; Liao, H.; You, M.-S.; He, W.-Y. Functional Characterization of Two RNA Methyltransferase Genes METTL3 and METTL14 Uncovers the Roles of m6A in Mediating Adaptation of Plutella xylostella to Host Plants. Int. J. Mol. Sci. 2022, 23, 10013. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Zhou, H.; Chen, Y.; Vasseur, L.; Gurr, G.M.; You, M.; You, S. A very long-chain fatty acid enzyme gene, PxHacd2 affects the temperature adaptability of a cosmopolitan insect by altering epidermal permeability. Sci. Total Environ. 2023, 891, 164372. [Google Scholar] [CrossRef]
- Zhou, H.; Lei, G.; Chen, Y.; You, M.; You, S. Pxtret1-like affects the temperature adaptability of a cosmopolitan pest by altering trehalose tissue distribution. Int. J. Mol. Sci. 2022, 23, 9019. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, S.; Cao, S.; Jacquin-Joly, E.; Zhou, Q.; Liu, Y.; Wang, G. An odorant receptor mediates the avoidance of Plutella xylostella against parasitoid. BMC Biol. 2024, 22, 61. [Google Scholar] [CrossRef]
- Lei, G.; Huang, J.; Zhou, H.; Chen, Y.; Song, J.; Xie, X.; Vasseur, L.; You, M.; You, S. Polygenic adaptation of a cosmopolitan pest to a novel thermal environment. Insect Mol. Biol. 2024, 33, 387–404. [Google Scholar] [CrossRef]
- Guest, M.; Goodchild, J.A.; Bristow, J.A.; Flemming, A.J. RDL A301S alone does not confer high levels of resistance to cyclodiene organochlorine or phenyl pyrazole insecticides in Plutella xylostella. Pestic. Biochem. Physiol. 2019, 158, 32–39. [Google Scholar] [CrossRef]
- Guo, Z.; Sun, D.; Kang, S.; Zhou, J.; Gong, L.; Qin, J.; Guo, L.; Zhu, L.; Bai, Y.; Luo, L.; et al. CRISPR/Cas9-mediated knockout of both the PxABCC2 and PxABCC3 genes confers high-level resistance to Bacillus thuringiensis Cry1Ac toxin in the diamondback moth, Plutella xylostella (L.). Insect Biochem. Mol. Biol. 2019, 107, 31–38. [Google Scholar] [CrossRef]
- Sun, D.; Zhu, L.; Guo, L.; Wang, S.; Wu, Q.; Crickmore, N.; Zhou, X.; Bravo, A.; Soberón, M.; Guo, Z.; et al. A versatile contribution of both aminopeptidases N and ABC transporters to Bt Cry1Ac toxicity in the diamondback moth. BMC Biol. 2022, 20, 33. [Google Scholar] [CrossRef]
- Xiong, L.; Liu, Z.; Li, J.; Yao, S.; Li, Z.; Chen, X.; Shen, L.; Zhang, Z.; Li, Y.; Hou, Q.; et al. Analysis of the effect of Plutella xylostella polycalin and ABCC2 transporter on Cry1Ac susceptibility by CRISPR/Cas9-mediated knockout. Toxins 2023, 15, 273. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, D.; Wang, F.; Yang, Y.; Tabashnik, B.E.; Wu, Y. Independent and synergistic effects of knocking out two ABC transporter genes on resistance to Bacillus thuringiensis toxins Cry1Ac and Cry1Fa in diamondback moth. Toxins 2020, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Y.; Wang, F.; Yang, Y.; Wu, S.; Wu, Y. Disruption of nicotinic acetylcholine receptor α6 mediated by CRISPR/Cas9 confers resistance to spinosyns in Plutella xylostella. Pest Manag. Sci. 2019, 76, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, X.; Jiang, D.; Yang, Y.; Wu, Y. CRISPR/Cas9 mediated ryanodine receptor I4790M knockin confers unequal resistance to diamides in Plutella xylostella. Insect Biochem. Mol. Biol. 2020, 125, 103453. [Google Scholar] [CrossRef]
- Ye, M.; Xiong, L.; Dong, Y.; Xie, C.; Zhang, Z.; Shen, L.; Li, Z.; Yue, Z.; Jiang, P.; Yuchi, Z.; et al. The potential role of the methionine aminopeptidase gene pxmetap1 in a cosmopolitan pest for Bacillus thuringiensis toxin tolerance. Int. J. Mol. Sci. 2022, 23, 13005. [Google Scholar] [CrossRef]
- Sun, X.; Hua, W.; Wang, K.; Song, J.; Zhu, B.; Gao, X.; Liang, P. A novel V263I mutation in the glutamate-gated chloride channel of Plutella xylostella (L.) confers a high level of resistance to abamectin. Int. J. Biol. Macromol. 2023, 230, 123389. [Google Scholar] [CrossRef]
- Jiang, D.; Yu, Z.; He, Y.; Wang, F.; Gu, Y.; Davies, T.G.E.; Fan, Z.; Wang, X.; Wu, Y. Key role of the ryanodine receptor I4790K mutation in mediating diamide resistance in Plutella xylostella. Insect Biochem. Mol. Biol. 2024, 168, 104107. [Google Scholar] [CrossRef]
- You, S.; Yao, S.; Chen, X.; Hou, Q.; Liu, Z.; Lei, G.; Xie, X.; Liang, Z.; Yuchi, Z.; You, M.; et al. CRISPR/Cas9-mediated knockout of the PxJHBP gene resulted in increased susceptibility to Bt Cry1Ac protoxin and reduced lifespan and spawning rates in Plutella xylostella. J. Agric. Food Chem. 2024, 72, 8180–8188. [Google Scholar] [CrossRef]
- Asad, M.; Liu, D.; Li, J.; Chen, J.; Yang, G. Development of CRISPR/Cas9-mediated gene-drive construct targeting the phenotypic gene in Putella xylostella. Front. Physiol. 2022, 13, 938621. [Google Scholar] [CrossRef]
- Xu, X.; Harvey-Samuel, T.; Siddiqui, H.A.; Ang, J.X.D.; Anderson, M.E.; Reitmayer, C.M.; Lovett, E.; Leftwich, P.T.; You, M.; Alphey, L. Toward a CRISPR-Cas9-based gene drive in the diamondback moth Plutella xylostella. CRISPR J. 2022, 5, 224–236. [Google Scholar] [CrossRef]
- Chaves, I.; Pokorny, R.; Byrdin, M.; Hoang, N.; Ritz, T.; Brettel, K.; Essen, L.O.; van der Horst, G.T.J.; Batschauer, A.; Ahmad, M. The cryptochromes: Blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 2011, 62, 335–364. [Google Scholar] [CrossRef]
- Young, M.W.; Kay, S.A. Time zones: A comparative genetics of circadian clocks. Nat. Rev. Genet. 2001, 2, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2002, 59, 237. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef]
- Chen, W.; Amir, M.B.; Liao, Y.; Yu, H.; He, W.; Lu, Z. New insights into the plutella xylostella detoxifying enzymes: Sequence evolution, structural similarity, functional diversity, and application prospects of glucosinolate sulfatases. J. Agric. Food Chem. 2023, 71, 10952–10969. [Google Scholar] [CrossRef] [PubMed]
- Li, B.J.; Wang, K.K.; Chen, D.P.; Yan, Y.; Cai, X.L.; Chen, H.M.; Dong, K.; Lin, F.; Xu, H.H. Distinct roles of two RDL GABA receptors in fipronil action in the diamondback moth (Plutella xylostella). Insect Sci. 2021, 28, 1721–1733. [Google Scholar] [CrossRef]
- Anderson, M.A.E.; Gonzalez, E.; Edgington, M.P.; Ang, J.X.D.; Purusothaman, D.-K.; Shackleford, L.; Nevard, K.; Verkuijl, S.A.N.; Harvey-Samuel, T.; Leftwich, P.T.; et al. A multiplexed, confinable CRISPR/Cas9 gene drive can propagate in caged Aedes aegypti populations. Nat. Commun. 2024, 15, 729. [Google Scholar] [CrossRef]
- Sinkins, S.P.; Gould, F. Gene drive systems for insect disease vectors. Nat. Rev. Genet. 2006, 7, 427–435. [Google Scholar] [CrossRef]
- Werren, J.H. Selfish genetic elements, genetic conflict, and evolutionary innovation. Proc. Nat. Acad. Sci. USA 2011, 108, 10863–10870. [Google Scholar] [CrossRef]
- Grunwald, H.A.; Gantz, V.M.; Poplawski, G.; Xu, X.-R.S.; Bier, E.; Cooper, K.L. Super-Mendelian inheritance mediated by CRISPR–Cas9 in the female mouse germline. Nature 2019, 566, 105–109. [Google Scholar] [CrossRef]
- Hammond, A.; Galizi, R.; Kyrou, K.; Simoni, A.; Siniscalchi, C.; Katsanos, D.; Gribble, M.; Baker, D.; Marois, E.; Russell, S.; et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 2016, 34, 78–83. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, L.; Xie, C.; Shen, L.; Chen, X.; Ye, M.; Sun, L.; Yang, X.; Yao, S.; Yue, Z.; et al. Optimization and application of CRISPR/Cas9 genome editing in a cosmopolitan pest, diamondback moth. Int. J. Mol. Sci. 2022, 23, 13042. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, G.; Wen, H.; Lu, H.; Yin, A.; Zheng, C.; Li, F.; Qiao, Q.; Jiao, L.; Lin, L.; et al. Knock-in of exogenous sequences based on CRISPR/Cas9 targeting autosomal genes and sex chromosomes in the diamondback moth, Plutella xylostella. J. Integr. Agric. 2024, 23, 3089–3103. [Google Scholar] [CrossRef]
- Zhang, X.H.; Tee, L.Y.; Wang, X.G.; Huang, Q.S.; Yang, S.H. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol. Ther. Nucleic Acids 2015, 4, e264. [Google Scholar] [CrossRef]
- Kaeuferle, T.; Stief, T.A.; Canzar, S.; Kutlu, N.N.; Willier, S.; Stenger, D.; Ferrada-Ernst, P.; Habjan, N.; Peters, A.E.; Busch, D.H.; et al. Genome-wide off-target analyses of CRISPR/Cas9-mediated T-cell receptor engineering in primary human T cells. Clin. Transl. Immun. 2022, 11, e1372. [Google Scholar] [CrossRef]
- Safari, F.; Zare, K.; Negahdaripour, M.; Barekati-Mowahed, M.; Ghasemi, Y. CRISPR Cpf1 proteins: Structure, function and implications for genome editing. Cell Biosci. 2019, 9, 36. [Google Scholar] [CrossRef]
- Kim, H.; Lee, W.-j.; Oh, Y.; Kang, S.-H.; Hur, J.K.; Lee, H.; Song, W.; Lim, K.-S.; Park, Y.-H.; Song, B.-S.; et al. Enhancement of target specificity of CRISPR–Cas12a by using a chimeric DNA–RNA guide. Nucleic Acids Res. 2020, 48, 8601–8616. [Google Scholar] [CrossRef]
- Swarts, D.C.; Jinek, M. Cas9 versus Cas12a/Cpf1: Structure–function comparisons and implications for genome editing. WIREs RNA 2018, 9, e1481. [Google Scholar] [CrossRef]
- Kordyś, M.; Sen, R.; Warkocki, Z. Applications of the versatile CRISPR-Cas13 RNA targeting system. WIREs RNA 2022, 13, e1694. [Google Scholar] [CrossRef]
- Yang, H.; Patel, D.J. Structures, mechanisms and applications of RNA-centric CRISPR–Cas13. Nat. Chem. Biol. 2024, 20, 673–688. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, J.; Li, D.; Cheng, Y. Strategies in the delivery of Cas9 ribonucleoprotein for CRISPR/Cas9 genome editing. Theranostics 2021, 11, 614–648. [Google Scholar] [CrossRef]
- Chaverra-Rodriguez, D.; Macias, V.M.; Hughes, G.L.; Pujhari, S.; Suzuki, Y.; Peterson, D.R.; Kim, D.; McKeand, S.; Rasgon, J.L. Targeted delivery of CRISPR-Cas9 ribonucleoprotein into arthropod ovaries for heritable germline gene editing. Nat. Commun. 2018, 9, 3008. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Dong, S.; Jiang, X.; Qiao, L.; Chen, J.; Li, T.; Pan, G.; Zhou, Z.; Li, C. Cas9-mediated gene editing using receptor-mediated ovary transduction of cargo (ReMOT) control in Bombyx mori. Insects 2023, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Riesenberg, S.; Maricic, T. Targeting repair pathways with small molecules increases precise genome editing in pluripotent stem cells. Nat. Commun. 2018, 9, 2164. [Google Scholar] [CrossRef] [PubMed]
- Tavecchio, M.; Munck, J.M.; Cano, C.; Newell, D.R.; Curtin, N.J. Further characterisation of the cellular activity of the DNA-PK inhibitor, NU7441, reveals potential cross-talk with homologous recombination. Cancer Chemother. Pharmacol. 2012, 69, 155–164. [Google Scholar] [CrossRef]
- Chu, V.T.; Weber, T.; Wefers, B.; Wurst, W.; Sander, S.; Rajewsky, K.; Kühn, R. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat. Biotechnol. 2015, 33, 543–548. [Google Scholar] [CrossRef]
- Li, G.; Liu, D.; Zhang, X.; Quan, R.; Zhong, C.; Mo, J.; Huang, Y.; Wang, H.; Ruan, X.; Xu, Z.; et al. Suppressing Ku70/Ku80 expression elevates homology-directed repair efficiency in primary fibroblasts. Int. J. Biochem. Cell Biol. 2018, 99, 154–160. [Google Scholar] [CrossRef]
- Hu, Z.; Shi, Z.; Guo, X.; Jiang, B.; Wang, G.; Luo, D.; Chen, Y.; Zhu, Y.-S. Ligase IV inhibitor SCR7 enhances gene editing directed by CRISPR–Cas9 and ssODN in human cancer cells. Cell Biosci. 2018, 8, 12. [Google Scholar] [CrossRef]
- Maruyama, T.; Dougan, S.K.; Truttmann, M.C.; Bilate, A.M.; Ingram, J.R.; Ploegh, H.L. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat. Biotechnol. 2015, 33, 538–542. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, M.L.; Wang, X.; Ying, W.Q.; Hu, X.D.; Dai, P.F.; Meng, F.L.; Shi, L.Y.; Sun, Y.; Yao, N.; et al. Tild-CRISPR Allows for Efficient and Precise Gene Knockin in Mouse and Human Cells. Dev. Cell 2018, 45, 526–536.e5. [Google Scholar] [CrossRef]
- Zhu, L.; Mon, H.; Xu, J.; Lee, J.M.; Kusakabe, T. CRISPR/Cas9-mediated knockout of factors in non-homologous end joining pathway enhances gene targeting in silkworm cells. Sci. Rep. 2015, 5, 18103. [Google Scholar] [CrossRef]
- Sartori, A.A.; Lukas, C.; Coates, J.; Mistrik, M.; Fu, S.; Bartek, J.; Baer, R.; Lukas, J.; Jackson, S.P. Human CtIP promotes DNA end resection. Nature 2007, 450, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Ranjha, L.; Cannavo, E.; Cejka, P. Phosphorylated CTIP functions as a co-factor of the mre11-rad50-nbs1 endonuclease in DNA end resection. Mol. Cell 2016, 64, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, M.; Khedher, A.H.Y.; Menoret, S.; Brion, A.; Lamribet, K.; Dardillac, E.; Boix, C.; Perrouault, L.; Tesson, L.; Geny, S.; et al. CtIP fusion to Cas9 enhances transgene integration by homology-dependent repair. Nat. Commun. 2018, 9, 1133. [Google Scholar] [CrossRef]
- Jimeno, S.; Fernández-Ávila, M.J.; Cruz-García, A.; Cepeda-García, C.; Gómez-Cabello, D.; Huertas, P. Neddylation inhibits CtIP-mediated resection and regulates DNA double strand break repair pathway choice. Nucleic Acids Res. 2015, 43, 987–999. [Google Scholar] [CrossRef]
- Yu, S.; Song, Z.; Luo, J.; Dai, Y.; Li, N. Over-expression of RAD51 or RAD54 but not RAD51/4 enhances extra-chromosomal homologous recombination in the human sarcoma (HT-1080) cell line. J. Biotechnol. 2011, 154, 21–24. [Google Scholar] [CrossRef]
- Cooper, S.; Iyer, G.; Tarquini, M.; Bissett, P. Nocodazole does not synchronize cells: Implications for cell-cycle control and whole-culture synchronization. Cell Tissue Res. 2006, 324, 237–242. [Google Scholar] [CrossRef]
- Hande, K.R.; Hagey, A.; Berlin, J.; Cai, Y.; Meek, K.; Kobayashi, H.; Lockhart, A.C.; Medina, D.; Sosman, J.; Gordon, G.B.; et al. The pharmacokinetics and safety of ABT-751, a novel, orally bioavailable sulfonamide antimitotic agent: Results of a phase 1 study. Clin. Cancer Res. 2006, 12, 2834–2840. [Google Scholar] [CrossRef]
- Rahman, S.H.; Bobis-Wozowicz, S.; Chatterjee, D.; Gellhaus, K.; Pars, K.; Heilbronn, R.; Jacobs, R.; Cathomen, T. The nontoxic cell cycle modulator indirubin augments transduction of adeno-associated viral vectors and zinc-finger nuclease-mediated gene targeting. Hum. Gene Ther. 2013, 24, 67–77. [Google Scholar] [CrossRef]
- Lin, S.; Staahl, B.T.; Alla, R.K.; Doudna, J.A. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. eLife 2014, 3, e04766. [Google Scholar] [CrossRef]
- Chen, P.J.; Liu, D.R. Prime editing for precise and highly versatile genome manipulation. Nat. Rev. Genet. 2023, 24, 161–177. [Google Scholar] [CrossRef]
- Zeng, H.; Daniel, T.C.; Lingineni, A.; Chee, K.; Talloo, K.; Gao, X. Recent advances in prime editing technologies and their promises for therapeutic applications. Curr. Opin. Biotechnol. 2024, 86, 103071. [Google Scholar] [CrossRef]
- Mikkelsen, N.S.; Bak, R.O. Enrichment strategies to enhance genome editing. J. Biomed. Sci. 2023, 30, 51. [Google Scholar] [CrossRef] [PubMed]
| Applications | Gene | Knockout/ Knock-In | CRISPR/Cas9 Component | Method of Delivery | Gene Function and Disruption Impact | References |
|---|---|---|---|---|---|---|
| Development and reproduction | PxHomeobox | Knockout | Cas9 mRNA | Embryo injections | Transcription factor; lethal to embryos | [31] |
| PxVg | Knockout | Ribonucleoprotein | Embryo injections | Egg maturation; reduced hatching rate | [32] | |
| PxVgR | Knockout | Cas9 mRNA | Embryo injections | Oocyte development; smaller eggs; low fertility | [33] | |
| PxSer2 | Knockout | Ribonucleoprotein | Embryo injections | Semen protein; male sterility | [34] | |
| PxVMP | Knockout | Ribonucleoprotein | Embryo injections | Egg structure formation; egg collapse and reduced hatching | [35] | |
| PxPiwi | Knockout | Ribonucleoprotein | Embryo injections | Germ cell development; delayed pupation; failed emergence | [36] | |
| PxTAR1 | Knockout | Ribonucleoprotein | Embryo injections | Germ cell development; delayed pupation,;failed emergence | [37] | |
| PxOsp | Knockout | Ribonucleoprotein | Embryo injections | Ovarian development; fewer and irregular eggs | [38] | |
| PxDcr-1 | Knockout | Ribonucleoprotein | Embryo injections | RNA processing; increased mortality; reduced reproduction | [39] | |
| PxDcr-2 | Knockout | Cas9 mRNA | Embryo injections | RNA processing; reduced RNAi efficiency | [39] | |
| Pigmentation | PxKMO | Knockout | Ribonucleoprotein | Embryo injections | Eye pigment gene; yellow/red eye color | [40] |
| PxCardinal | Knockout | Ribonucleoprotein | Embryo injections | Eye pigment gene; yellow eyes turning red with age | [40] | |
| PxEbony | Knockout | Ribonucleoprotein | Embryo injections | Dopamine metabolism; darker body color | [41] | |
| PxYellow | Knockout | Ribonucleoprotein | Embryo injections | Body pigmentation gene; change in body color | [42] | |
| Sex-determination | PxDsx | Knockout | Cas9 mRNA | Embryo injections | Sex differentiation; genital abnormalities | [43] |
| PxPSI | Knockout | Cas9 mRNA | Embryo injections | Male-specific splicing; genital defects | [44] | |
| PxSast1 | Knockout | Ribonucleoprotein | Embryo injections | Sex differentiation; male infertility; egg defects | [45] | |
| Circadian rhythms | PxCry1 | Knockout | Ribonucleoprotein | Embryo injections | Light-sensitive photoreceptor; altered activity rhythms | [46] |
| PxLW-opsin | Knockout | Ribonucleoprotein | Embryo injections | Phototaxis; impaired light response | [47] | |
| PxCry2 | Knockout | Ribonucleoprotein | Embryo injections | Circadian rhythm; disturb the rhythmic activities | [48] | |
| PxPer | Knockout | Ribonucleoprotein | Embryo injections | Circadian rhythm; activity disruption | [48] | |
| Ecological adaptability | PxGSS1 | Knockout | Ribonucleoprotein | Embryo injections | Glucosinolate metabolism; reduced host plant adaptation | [49,50] |
| PxGSS2 | Knockout | Ribonucleoprotein | Embryo injections | Glucosinolate metabolism; reduced host plant adaptation | [49,50] | |
| PxOr35 | Knockout | Ribonucleoprotein | Embryo injections | Olfactory receptor; oviposition preference reduced | [51] | |
| PXOr49 | Knockout | Ribonucleoprotein | Embryo injections | Olfactory receptor; oviposition preference reduced | [51] | |
| PxGSS3 | Knockout | Ribonucleoprotein | Embryo injections | Glucosinolate metabolism; host adaptability unchanged | [52] | |
| PxMETTL14 | Knockout | Ribonucleoprotein | Embryo injections | RNA methyltransferase; developmental defects | [53] | |
| Px008848(PxGH1) | Knockout | Ribonucleoprotein | Embryo injections | Insect–plant interaction; increased larval survival | [50] | |
| PxHacd2 | Knockout | Ribonucleoprotein | Embryo injections | Enzyme for VLCFAs synthesis; survival and fecundity decreased | [54] | |
| PxTret1-like | Knockout | Ribonucleoprotein | Embryo injections | Trehalose transport; reduced temperature resistance | [55] | |
| PxOr16 | Knockout | Ribonucleoprotein | Embryo injections | Odor receptor; reduced avoidance behavior to parasitoid | [56] | |
| PxGCC2 | Knockout | Ribonucleoprotein | Embryo injections | Peripheral gene for stress; reduced stress resistance | [57] | |
| PxKPNB1 | Knockout | Ribonucleoprotein | Embryo injections | Peripheral gene for stress; decreased environmental adaptation | [57] | |
| Insecticide resistance | PxGABAR-α1 (A282S) | Knock-in | Cas9 protein crRNA, tracRNA and ssODNA | Embryo injections | GABA-gated chloride channel; not involved in pesticide resistance | [58] |
| PxABCC2 | Knockout | Ribonucleoprotein/Cas9 mRNA | Embryo injections | Bt resistance; reduced Cry1Ac sensitivity | [51,59,60,61,62] | |
| PxABCC3 | Knockout | Ribonucleoprotein/Cas9 mRNA | Embryo injections | Bt resistance; reduced Cry1Ac sensitivity | [51,59,60,62] | |
| PxnAChRα6 | Knockout | Ribonucleoprotein | Embryo injections | Nicotinic acetylcholine receptor α6; increased pesticide resistance | [63] | |
| PxRyR (I4790M) | Knock-in | Ribonucleoprotein and ssODN | Embryo injections | Ryanodine receptor; increased diamine resistance” | [64] | |
| PxAPN1 | Knockout | Ribonucleoprotein | Embryo injections | Aminopeptidases increased Cry1Ac resistance | [60] | |
| PxAPN3a | Knockout | Ribonucleoprotein | Embryo injections | Aminopeptidases increased Cry1Ac resistance | [60] | |
| PxMetAP1 | Knockout | Ribonucleoprotein | Embryo injections | Methionine aminopeptidases; impaired peptide processing | [65] | |
| PxGluCl (V263I) | Knock-in | Cas9 proteinss/sgRNA/ssODN | Embryo injections | Glutamate-gated chloride chann; resistance to avermectin | [66] | |
| PxPolycalin | Knockout | Ribonucleoprotein | Embryo injections | Bt toxin receptor; decreased Cry1Ac sensitivity | [61] | |
| PxRyR (I4790K) | Knock-in | Ribonucleoprotein and ssODN | Embryo injections | Ryanodine receptor; chlorantraniliprole resistance | [67] | |
| PxJHBP | Knockout | Ribonucleoprotein | Embryo injections | JH signaling regulator; increased Cry1Ac susceptibility | [68] | |
| Gene drive | PxYellow | Knock-in | Ribonucleoprotein and plasmid | Embryo injections | Body pigmentation gene; change in body color | [69,70] |
| PxKmo | Knock-in | Ribonucleoprotein and plasmid | Embryo injections | Eye pigment gene; yellow/red eye color | [70] |
| Target Gene | No. of Eggs Injected | Survived Individuals | Survival Rate | Mutation Rate | Reference |
|---|---|---|---|---|---|
| PxABCC2 | 215 | 80 | 37.21% | 65.00% | [59] |
| PxABCC3 | 200 | 68 | 34.00% | 58.82% | [59] |
| PxDsxC | 489 | 151 | 30.87% | 62.91% | [43] |
| PxDsxF | 431 | 126 | 29.23% | 34.13% | [43] |
| PxDsxM | 518 | 107 | 20.66% | 42.99% | [43] |
| PxGSS1 | 205 | 52 | 25.36% | 3.85% | [52] |
| PxGSS2 | 205 | 52 | 25.36% | 5.77% | [52] |
| PxVgR | 258 | 122 | 47.29% | 63.11% | [33] |
| PxYellow | 676 | 480 | 71.01% | 56.66% | [42] |
| PxSer2 | 2000 | 490 | 36.5% | 1.02% | [34] |
| PxKmo | 222 | 94 | 42.34 | 17.02% | [40] |
| PxCardinal | 182 | 55 | 30.22% | 29.09% | [40] |
| PxVg | 135 | 99 | 73.33% | 4.04% | [32] |
| PxPSI | 638 | 324 | 50.78% | 23.15% | [44] |
| PxCry1 | 263 | 58 | 22.05% | 20.69% | [46] |
| PxMETTL14 | 256 | 30 | 11.72% | 10.00% | [53] |
| PxVMP | 52 | 10 | 19.23% | 22.22% | [35] |
| PxTret1-like | 100 | 62 | 62% | 11.29% | [55] |
| PxLW-opsin | 211 | 62 | 29.38% | 6.45% | [47] |
| PxHacd2 | 100 | 56 | 56.00% | 3.57% | [54] |
| PxPiwi | 212 | 98 | 46.23% | 55.10% | [36] |
| PxGluCl (V263I) | 300 | 11 | 3.67% | 9.09% | [66] |
| PxTAR1 | 154 | 68 | 44.16% | 13.24% | [37] |
| PxOsp | 180 | 123 | 68.33% | 19.25% | [38] |
| PxSast1 | 185 | 68 | 36.80% | 1.47% | [45] |
| PxPer | 210 | 72 | 34.29% | 4.17% | [48] |
| PxRyR (I4790K) | 1350 | 59 | 4.37% | 4.35% | [67] |
| PxGCC2 | 176 | 53 | 30.11% | 5.66% | [57] |
| PxKPNB1 | 154 | 42 | 27.27% | 9.52% | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asad, M.; Chang, Y.; Liao, J.; Yang, G. CRISPR/Cas9 Genome Editing in the Diamondback Moth: Current Progress, Challenges, and Prospects. Int. J. Mol. Sci. 2025, 26, 1515. https://doi.org/10.3390/ijms26041515
Asad M, Chang Y, Liao J, Yang G. CRISPR/Cas9 Genome Editing in the Diamondback Moth: Current Progress, Challenges, and Prospects. International Journal of Molecular Sciences. 2025; 26(4):1515. https://doi.org/10.3390/ijms26041515
Chicago/Turabian StyleAsad, Muhammad, Yanpeng Chang, Jianying Liao, and Guang Yang. 2025. "CRISPR/Cas9 Genome Editing in the Diamondback Moth: Current Progress, Challenges, and Prospects" International Journal of Molecular Sciences 26, no. 4: 1515. https://doi.org/10.3390/ijms26041515
APA StyleAsad, M., Chang, Y., Liao, J., & Yang, G. (2025). CRISPR/Cas9 Genome Editing in the Diamondback Moth: Current Progress, Challenges, and Prospects. International Journal of Molecular Sciences, 26(4), 1515. https://doi.org/10.3390/ijms26041515






