Circulating RNA Markers Associated with Adenoma–Carcinoma Sequence in Colorectal Cancer
Abstract
1. Introduction
2. Results
2.1. The Selection of Circulating Transcripts Associated with the ACS via RNA Sequencing
2.2. Circulating Transcripts in the Transition from HC to NDC
2.3. Transition from NDC to Non-Advanced Adenoma
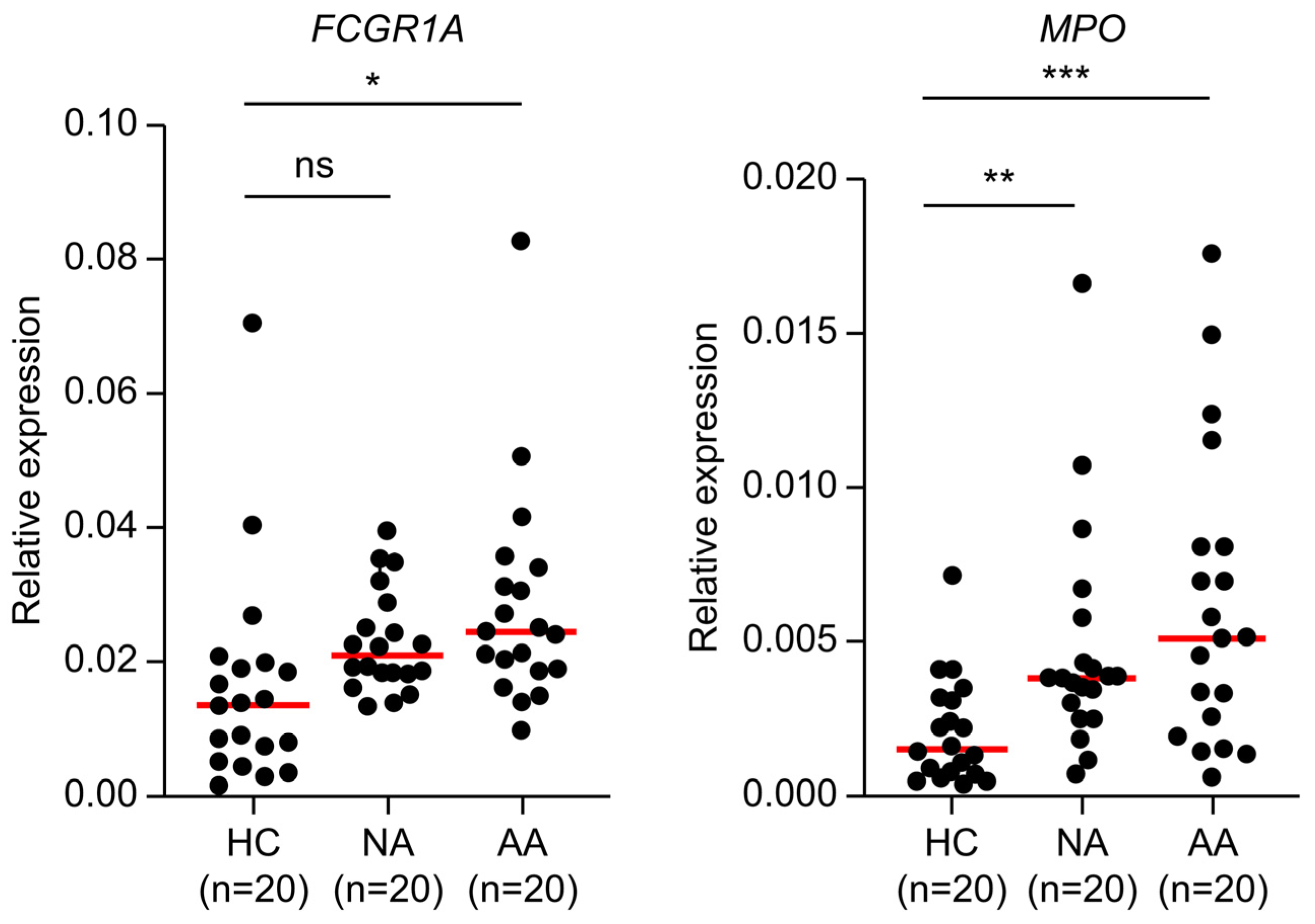
2.4. Transition from Non-Advanced Adenoma to Advanced Adenoma
2.5. Transition from Advanced Adenoma to Colorectal Cancer
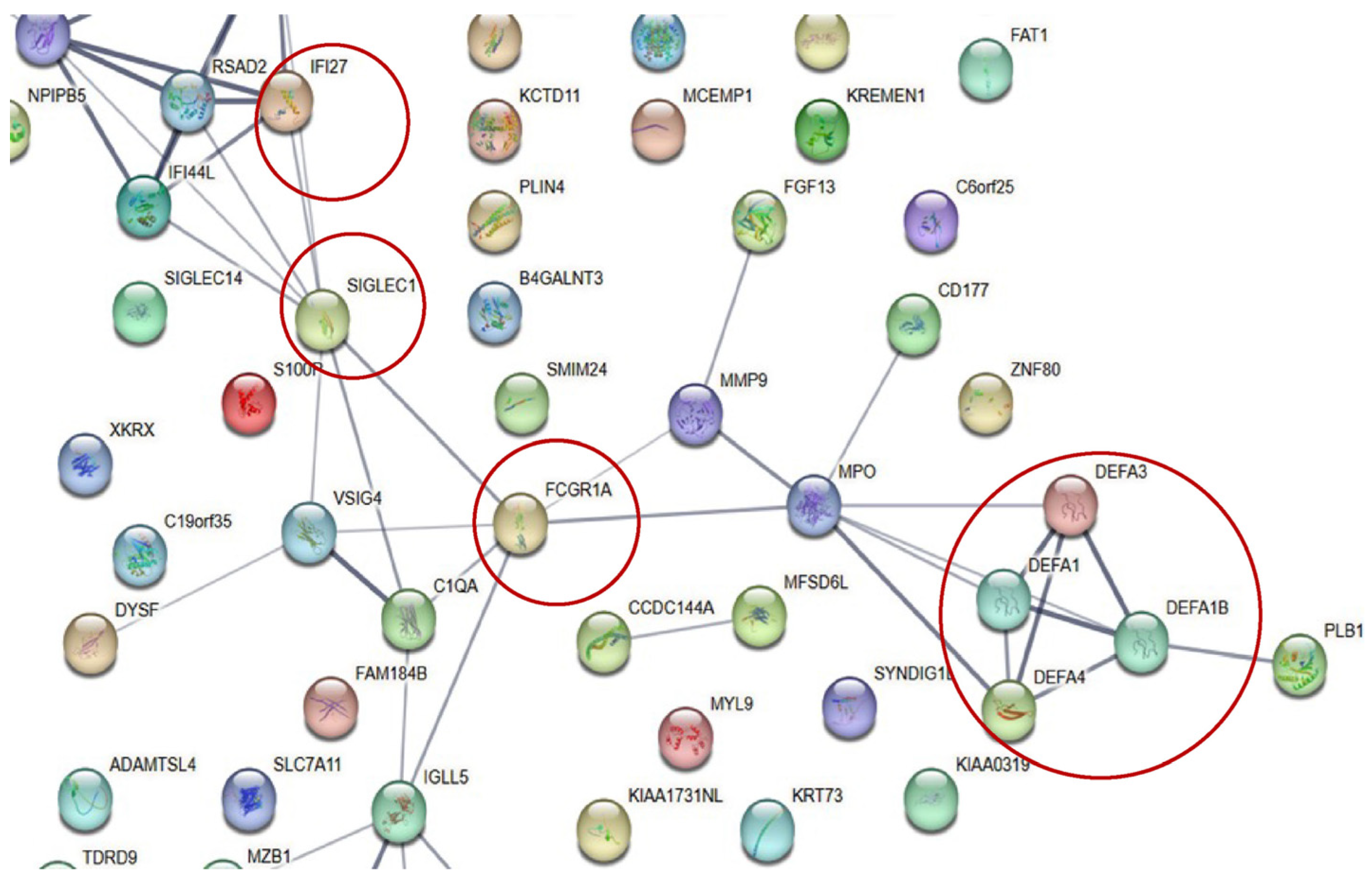
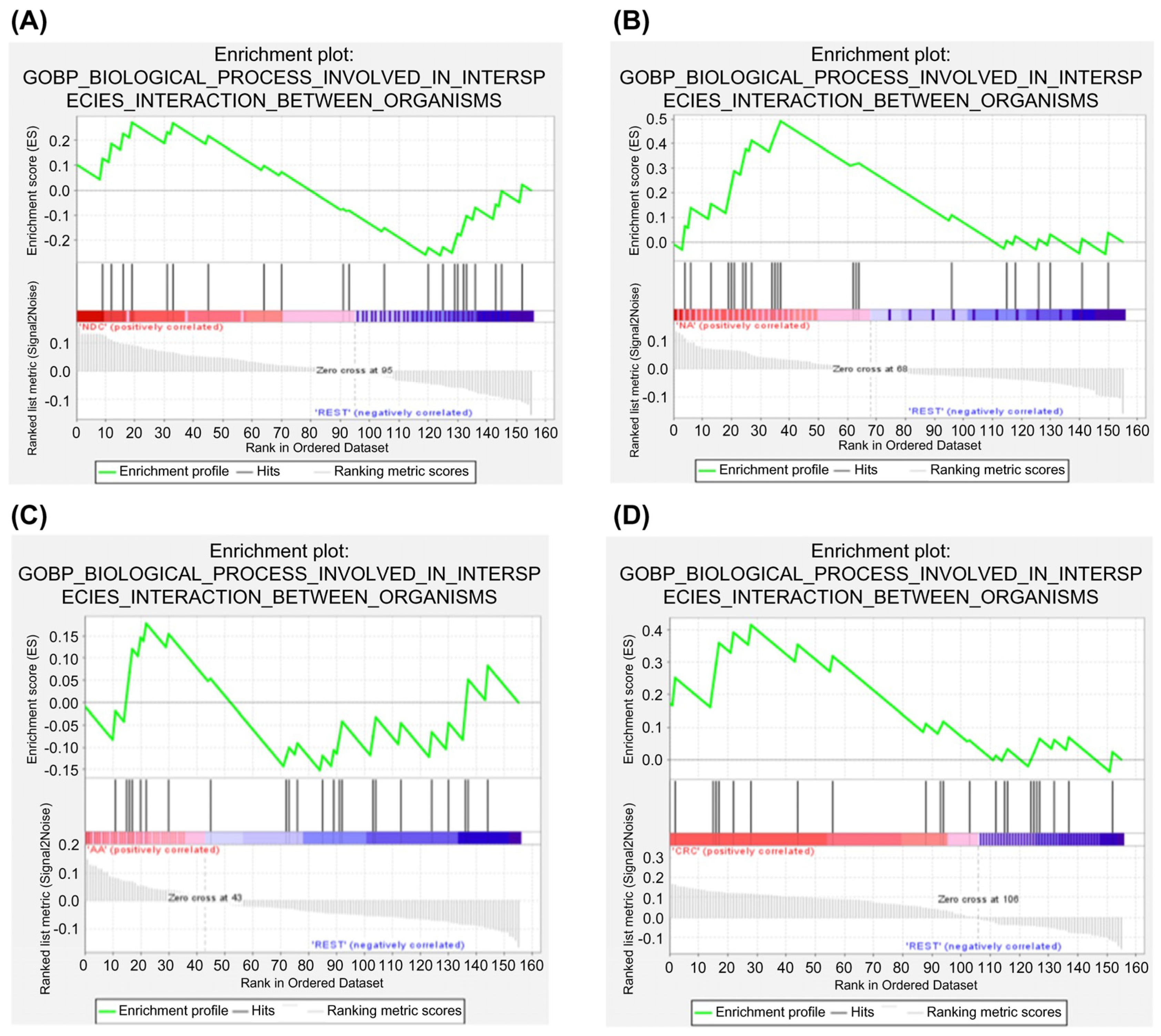
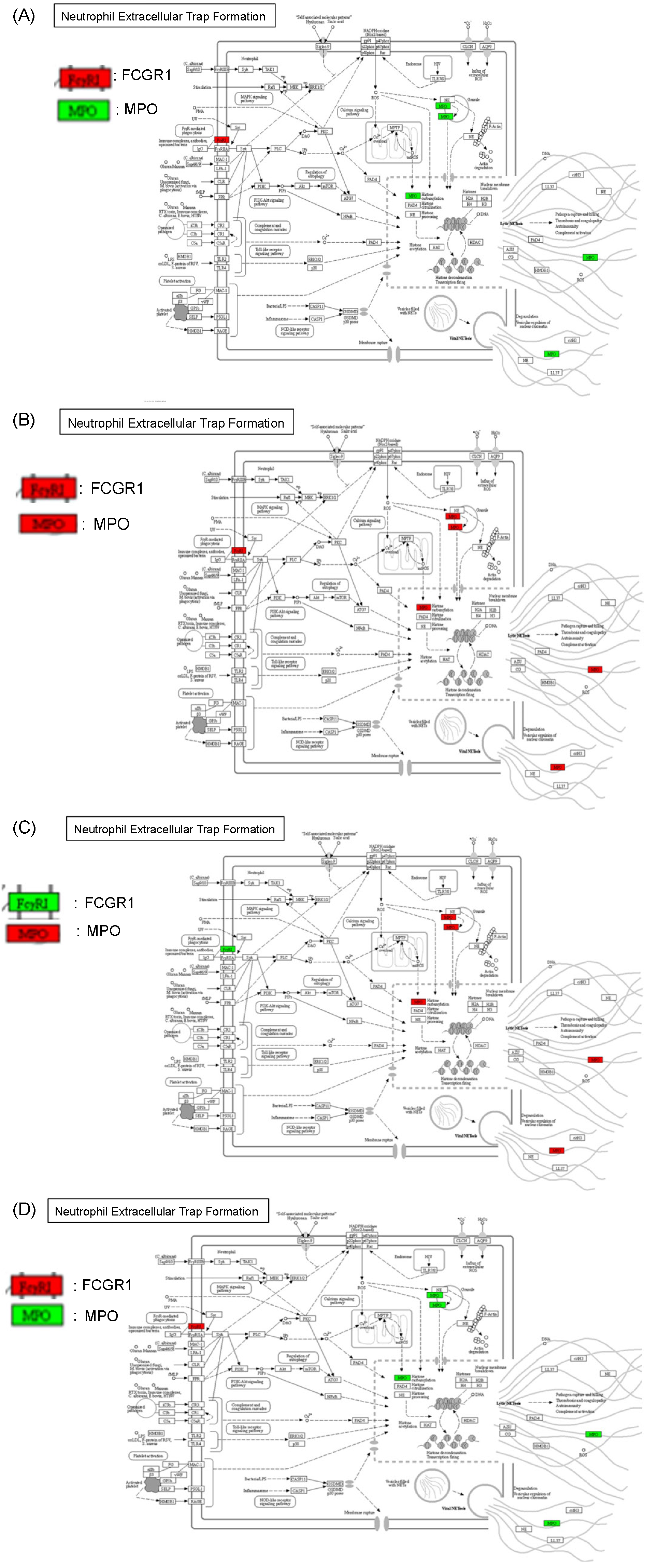
2.6. Protein–Protein Interaction and Pathway Analyses
2.7. Clinical Validation of Candidate Biomarkers
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Blood Collection and RNA Isolation
4.3. cDNA Synthesis
4.4. RNA Sequencing and Differential Gene Expression Analysis
4.5. Gene-Enrichment and Functional Annotation Analysis
4.6. Clustering Heatmap Analysis
4.7. Protein–Protein Interaction Network Analysis
4.8. GO Analysis I
4.9. GO Analysis II
4.10. Gene Pathway Analysis
4.11. Quantitative PCR Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Schmoll, H.J.; Van Cutsem, E.; Stein, A.; Valentini, V.; Glimelius, B.; Haustermans, K.; Nordlinger, B.; van de Velde, C.J.; Balmana, J.; Regula, J. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann. Oncol. 2012, 23, 2479–2516. [Google Scholar] [CrossRef] [PubMed]
- Bockelman, C.; Engelmann, B.E.; Kaprio, T.; Hansen, T.F.; Glimelius, B. Risk of recurrence in patients with colon cancer stage II and III: A systematic review and meta-analysis of recent literature. Acta Oncol. 2015, 54, 5–16. [Google Scholar] [CrossRef]
- Jungwirth, J.; Urbanova, M.; Boot, A.; Hosek, P.; Bendova, P.; Siskova, A.; Svec, J.; Kment, M.; Tumova, D.; Summerova, S.; et al. Author Correction: Mutational Analysis of Driver Genes Defines the Colorectal Adenoma: In Situ Carcinoma Transition. Sci. Rep. 2022, 12, 17701. [Google Scholar] [CrossRef]
- Markowitz, S.D.; Bertagnolli, M.M. Molecular Origins of Cancer: Molecular Basis of Colorectal Cancer. N. Engl. J. Med. 2009, 361, 2449–2460. [Google Scholar] [CrossRef]
- Atkin, W.S.; Edwards, R.; Kralj-Hans, I.; Wooldrage, K.; Hart, A.R.; Northover, J.M.; Parkin, D.M.; Wardle, J.; Duffy, S.W.; Cuzick, J. Once-Only Flexible Sigmoidoscopy Screening in Prevention of Colorectal Cancer: A Multicentre Randomised Controlled Trial. Lancet 2010, 375, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, T.F.; Ransohoff, D.F.; Itzkowitz, S.H.; Levin, T.R.; Lavin, P.; Lidgard, G.P.; Ahlquist, D.A.; Berger, B.M. Multitarget Stool DNA Testing for Colorectal-Cancer Screening. N. Engl. J. Med. 2014, 370, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, A.B.; Rutter, C.M.; Peterse, E.F.; Lietz, A.P.; Seguin, C.L.; Meester, R.G.; Perdue, L.A.; Lin, J.S.; Siegel, R.L.; Doria-Rose, V.P.; et al. Colorectal Cancer Screening: An Updated Modeling Study for the US Preventive Services Task Force. JAMA 2021, 325, 1998–2011. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.C.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid Biopsies Come of Age: Towards Implementation of Circulating Tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating Liquid Biopsies into the Management of Cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef]
- Turchinovich, A.; Weiz, L.; Langheinz, A.; Burwinkel, B. Characterization of Extracellular Circulating MicroRNA. Nucleic Acids Res. 2011, 39, 7223–7233. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Fang, J.; Qiu, Z.; Zhao, Y.; Zou, H. Methylated SEPT9 (mSEPT9): A Promising Blood-Based Biomarker for Colorectal Cancer Detection. Int. J. Mol. Sci. 2022, 23, 4567. [Google Scholar]
- Pantel, K.; Alix-Panabières, C. Circulating Tumor Cells for Early Detection of Clinically Relevant Cancer. Nat. Rev. Clin. Oncol. 2023, 20, 453–467. [Google Scholar]
- Choi, C.H.; Kim, Y.T.; Song, S.Y.; Kim, C.G.; Lee, J.H.; Park, S.W. MicroRNA Expression Signatures in Colorectal Adenoma and Carcinoma Progression. Clin. Epigenetics 2016, 8, 30. [Google Scholar]
- Kanaan, Z.; Rai, S.N.; Eichenberger, M.R.; Roberts, H.; Keskey, R.; Pan, J.; Yan, Y.; Jorden, J.; Denning, W.L.; Korkaya, H.; et al. Complex Patterns of Altered MicroRNA Expression During the Adenoma-Carcinoma Sequence in Colorectal Cancer. Clin. Cancer Res. 2011, 17, 7283–7293. [Google Scholar]
- Park, S.Y.; Kim, S.K.; Kim, J.; Park, S.; Kim, S.Y.; Kim, H. RNA-Based Blood Biomarkers for Early Breast Cancer Detection. Clin. Chem. 2020, 66, 1476–1485. [Google Scholar] [CrossRef]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and Localization of Surgically Resectable Cancers With a Multi-Analyte Blood Test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Giambra, V.; Pagliari, D.; Rio, P.; Totti, B.; Di Nunzio, C.; Bosi, A.; Giaroni, C.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Gut Microbiota, Inflammatory Bowel Disease, and Cancer: The Role of Guardians of Innate Immunity. Cells 2023, 12, 2654. [Google Scholar] [CrossRef]
- Yu, L.C.H. Microbiota Dysbiosis and Barrier Dysfunction in Inflammatory Bowel Disease and Colorectal Cancers: Exploring a Common Ground Hypothesis. J. Biomed. Sci. 2018, 25, 79. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Chowdhury, S.; Billah, M.M.; Islam, K.M.D.; Thorlacius, H.; Rahman, M. Neutrophil Extracellular Traps in Colorectal Cancer Progression and Metastasis. Int. J. Mol. Sci. 2021, 22, 7260. [Google Scholar] [CrossRef]
- Hidalgo, A.; Libby, P.; Soehnlein, O.; Aramburu, I.V.; Papayannopoulos, V.; Silvestre-Roig, C. Neutrophil extracellular traps: From physiology to pathology. Cardiovasc. Res. 2022, 118, 2737–2753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weng, M.; Yue, Y.; Wu, D.; Zhou, C.; Guo, M.; Sun, C.; Liao, Q.; Sun, M.; Zhou, D.; Miao, C. Increased MPO in Colorectal Cancer is Associated with High Peripheral Neutrophil Counts and a Poor Prognosis: A TCGA With Propensity Score-Matched Analysis. Front. Oncol. 2022, 12, 940706. [Google Scholar] [CrossRef] [PubMed]
- Malka, D.; Lièvre, A.; André, T.; Taïeb, J.; Ducreux, M.; Bibeau, F. Immune Scores in Colorectal Cancer: Where Are We? Eur. J. Cancer 2020, 140, 105–118. [Google Scholar] [CrossRef]
- Shetti, D.; Mallela, V.R.; Ye, W.; Sharif, M.; Ambrozkiewicz, F.; Trailin, A.; Liška, V.; Hemminki, K. Emerging Role of Circulating Cell-Free RNA as a Non-Invasive Biomarker for Hepatocellular Carcinoma. Crit. Rev. Oncol. Hematol. 2024, 200, 104391. [Google Scholar] [CrossRef] [PubMed]
- Click, B.; Pinsky, P.F.; Hickey, T.; Doroudi, M.; Schoen, R.E. Association of Colonoscopy Adenoma Findings with Long-term Colorectal Cancer Incidence. JAMA 2018, 319, 2021–2031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leslie, A.; Carey, F.A.; Pratt, N.R.; Steele, R.J. The colorectal adenoma-carcinoma sequence. Br. J. Surg. 2002, 89, 845–860. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Zhang, Z.Y.; Li, S.N.; Ma, J.Q.; Zhao, Z. Liquid Biopsy: Comprehensive Overview of Circulating Tumor DNA (Review). Oncol. Lett. 2024, 28, 548. [Google Scholar] [CrossRef] [PubMed]
- Bakarurraini, N.A.A.R.; Kamarudin, A.A.; Jamal, R.; Abu, N. Engineered T cells for Colorectal Cancer. Immunotherapy 2024, 16, 987–988. [Google Scholar] [CrossRef] [PubMed]
- Farber, D.L. Tissues, Not Blood, Are Where Immune Cells Function. Int. J. Mol. Sci. 2021, 593, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, Y.; Wang, X.; Zhang, C.; Hou, M.; Liu, Y. Integration of Single-Cell RNA Sequencing and Bulk RNA Transcriptome Sequencing Reveals a Heterogeneous Immune Landscape and Pivotal Cell Subpopulations Associated With Colorectal Cancer Prognosis. Front. Immunol. 2023, 14, 1184167. [Google Scholar] [CrossRef] [PubMed]
- Cabús, L.; Lagarde, J.; Curado, J.; Lizano, E.; Perez-Boza, J. Current Challenges and Best Practices for Cell-Free Long RNA Biomarker Discovery. Biomark. Res. 2022, 10, 62. [Google Scholar] [CrossRef]
- Nassar, S.I.; Suk, A.; Nguyen, S.A.; Adilbay, D.; Pang, J.; Nathan, C.A.O. The Role of ctDNA and Liquid Biopsy in the Diagnosis and Monitoring of Head and Neck Cancer: Towards Precision Medicine. Cancers 2024, 16, 3129. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Lin, H.; Zhu, Y.; Huang, D.; Lai, M.; Xi, X.; Huang, J.; Zhang, W.; Zhong, T. Research Progress of CTC, ctDNA, and EVs in Cancer Liquid Biopsy. Front. Oncol. 2024, 14, 1303335. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Yang, L.; Yin, F.T.; Sun, Y.; Li, X.H.; Li, J.; Wang, X.J. Multi-Omics Approaches in Colorectal Cancer Screening and Diagnosis, Recent Updates and Future Perspectives. Cancers 2022, 14, 5545. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Xiao, S.; Gao, Y.; Soung, Y.H. Recent Technologies Towards Diagnostic and Therapeutic Applications of Circulating Nucleic Acids in Colorectal Cancers. Int. J. Mol. Sci. 2024, 25, 8703. [Google Scholar] [CrossRef] [PubMed]
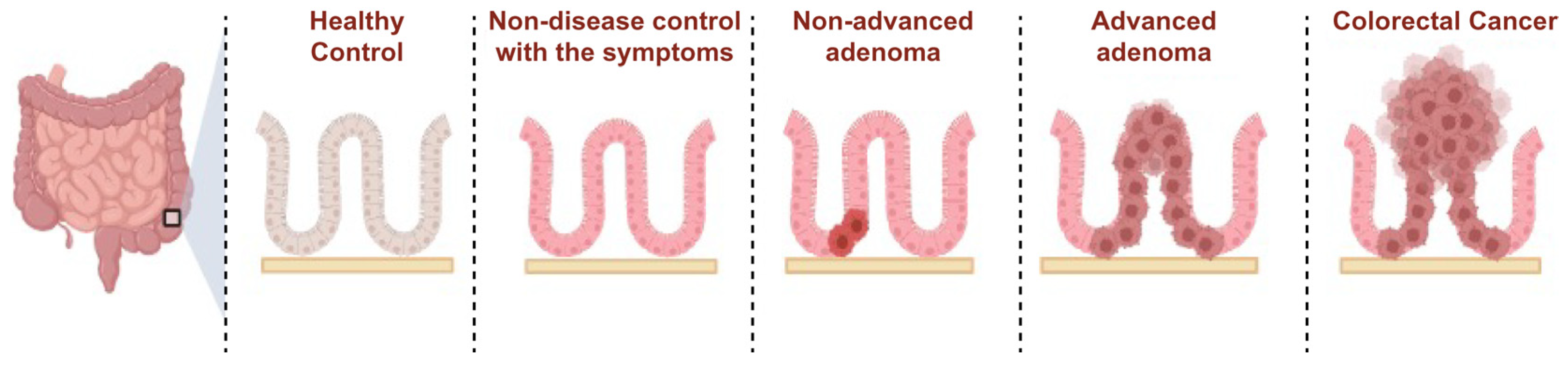


| Group | Description | No. of Samples | Collection Site |
|---|---|---|---|
| Colorectal cancer | Tissue damage and invasion; Cancer in colorectum; | 20 | (a), (b), and (c) |
| AA | Dysplasia: high grade. Villous component: more than 25%; Size: larger than 10 mm; Number: more than 3 in colorectum; | 40 | (a), (b), and (c) |
| NA | Dysplasia: low grade. Villous component: less than 25%; Size: smaller than 10 mm; Number: less than 3 in colorectum. | 40 | (a), (b), and (c) |
| NDC | No pathologic histological characteristics with symptoms such as stomach pain or abnormal bowel trouble. | 20 | (a), (b), and (c) |
| HC | No symptom; No colorectal disease. | 40 | (a), (b), (c), and (d) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, L.A.; Han, J.; Kim, T.I.; Park, J.J.; Lee, J.M.; Kim, J.K.; Park, S.; Lee, H. Circulating RNA Markers Associated with Adenoma–Carcinoma Sequence in Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 1518. https://doi.org/10.3390/ijms26041518
Kim LA, Han J, Kim TI, Park JJ, Lee JM, Kim JK, Park S, Lee H. Circulating RNA Markers Associated with Adenoma–Carcinoma Sequence in Colorectal Cancer. International Journal of Molecular Sciences. 2025; 26(4):1518. https://doi.org/10.3390/ijms26041518
Chicago/Turabian StyleKim, Li Ah, Jin Han, Tae Il Kim, Jae Jun Park, Jae Myun Lee, Jong Koo Kim, Sunyoung Park, and Hyeyoung Lee. 2025. "Circulating RNA Markers Associated with Adenoma–Carcinoma Sequence in Colorectal Cancer" International Journal of Molecular Sciences 26, no. 4: 1518. https://doi.org/10.3390/ijms26041518
APA StyleKim, L. A., Han, J., Kim, T. I., Park, J. J., Lee, J. M., Kim, J. K., Park, S., & Lee, H. (2025). Circulating RNA Markers Associated with Adenoma–Carcinoma Sequence in Colorectal Cancer. International Journal of Molecular Sciences, 26(4), 1518. https://doi.org/10.3390/ijms26041518







