Effect of Vitamin E on Diabetic Nephropathy in Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
2. Results
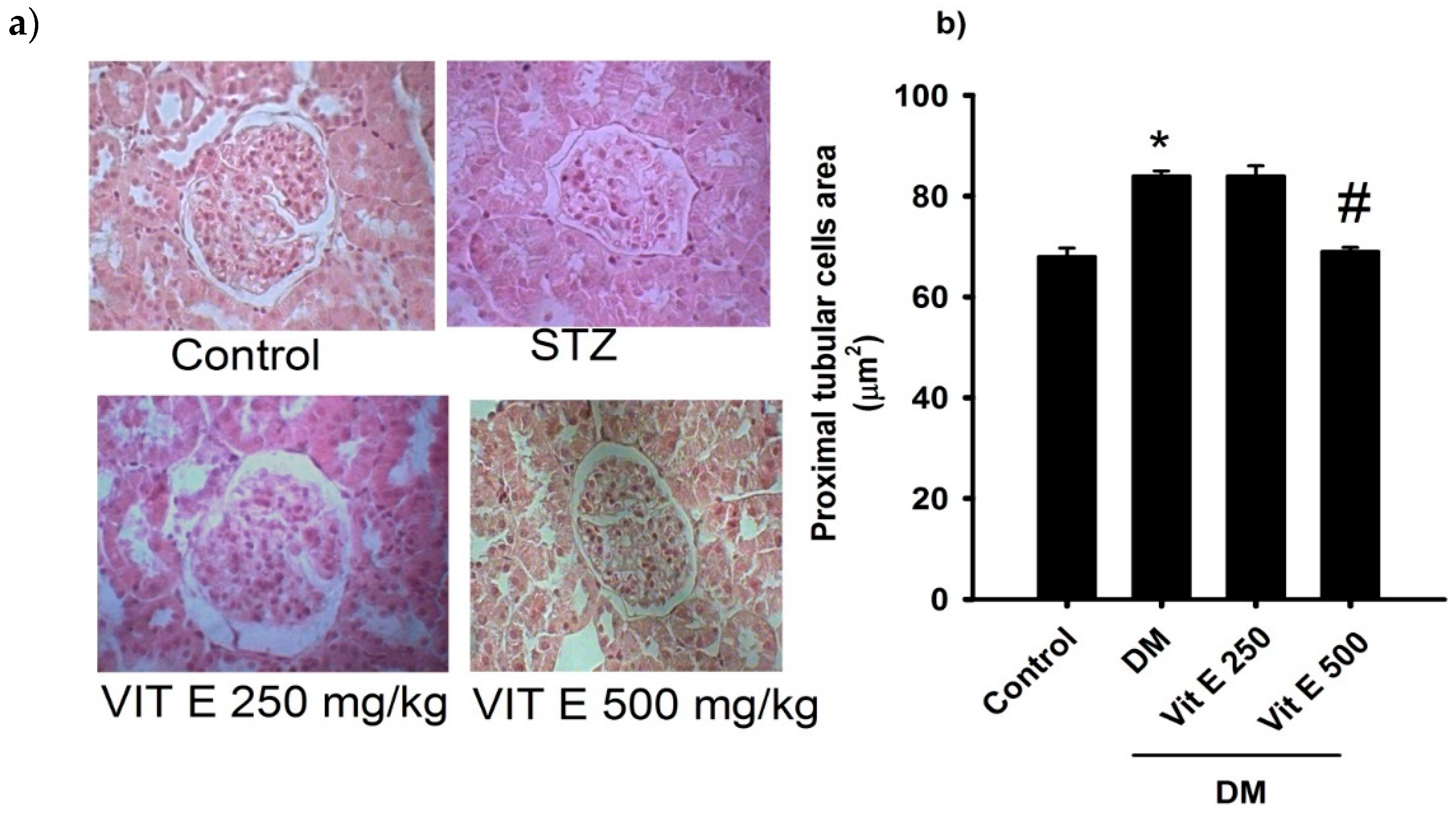
2.1. Effect of Vitamin E on the Cellular Area of the Proximal Tubules
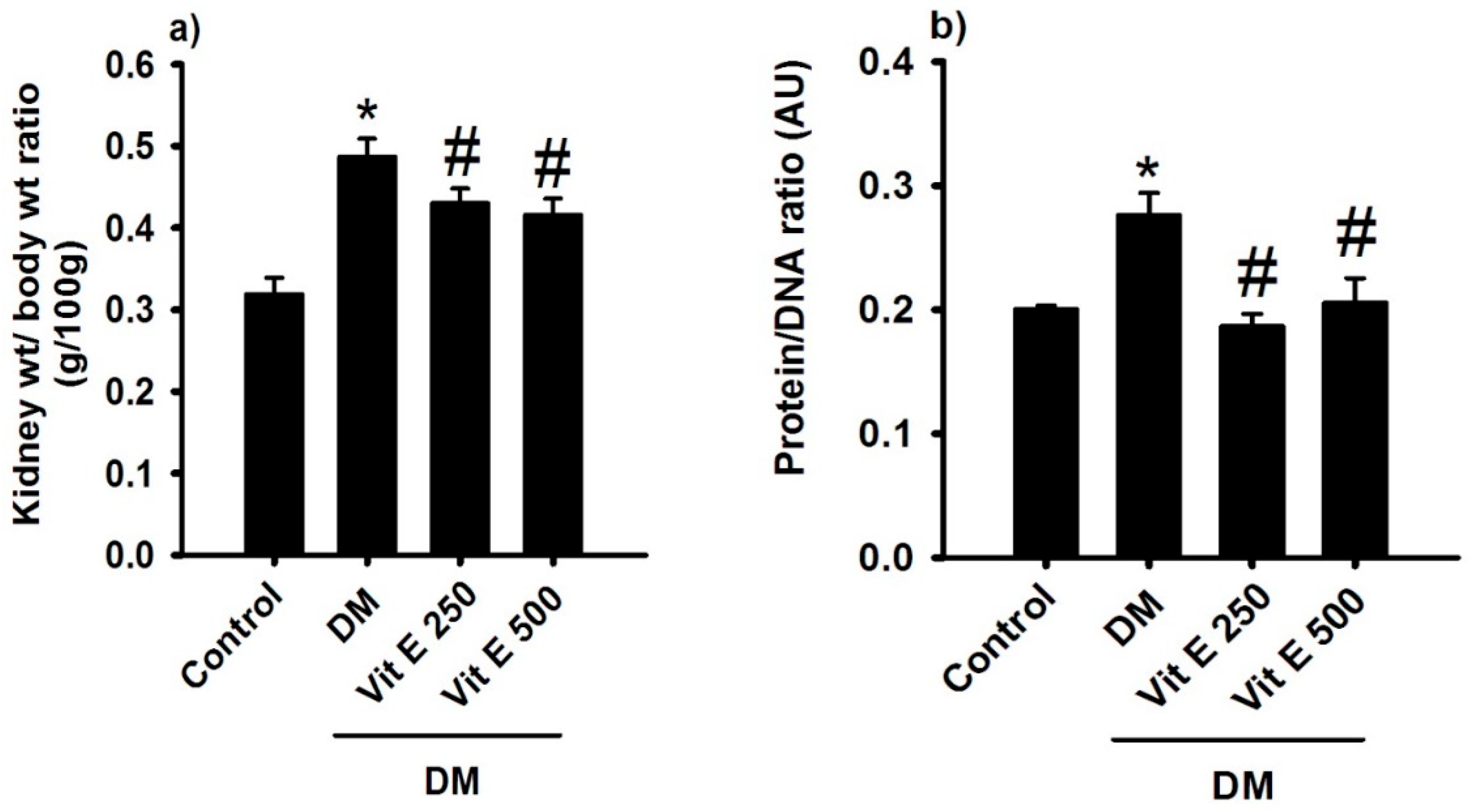
2.2. Effect of Vitamin E on Renal Hypertrophy and Protein/DNA Ratio
2.3. Effect of Vitamin E on Kidney Function
2.4. Effect of Vitamin E on the Activities of Antioxidant Enzymes
2.5. Effect of Vitamin E on AT1 and AT2 Receptor Protein Expression in the Renal Cortex
3. Discussion
4. Materials and Methods
4.1. Measurement of Renal Hypertrophy
4.2. Analytical Methods
4.3. Western Blotting
4.4. Evaluation of Oxidative Stress
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Martínez, R.; Barrientos-Gutiérrez, T.; Cuevas-Nasu, L.; Bautista-Arredondo, S.; Colchero, M.A.; Gaona-Pineda, E.B.; Martínez-Barnetche, J.; Alpuche-Aranda, C.M.; Gómez-Acosta, L.M.; Mendoza-Alvarado, L.R.; et al. Metodología de la Encuesta Nacional de Salud y Nutrición 2021. Salud Publica Mex 2021, 63, 813–818. [Google Scholar] [CrossRef]
- Hernandez Borquez, M.C.; Marquez Gonzalez, C.A.; Varela Brito, A.; Ramos Garcia, A.; Ramos Garcia, E.; Yocupicio Leyva, J.C. Estilos de vida de personas con diabetes mellitus tipo 2 que acuden a una unidad médica en Navojoa, Sonora. Rev. Investig. Acad. Front. Fac. Interdiscip. Cienc. Econ. Adm. Dep. Cienc.Econ. Adm. Campus Navojoa 2023. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed. Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.J.; Batlle, D. Revisiting the renin-angiotensin system. Mol. Cell. Endocrinol. 2021, 529, 111268. [Google Scholar] [CrossRef]
- Di Vincenzo, A.; Tana, C.; El Hadi, H.; Pagano, C.; Vettor, R.; Rossato, M. Antioxidant, Anti-Inflammatory, and Metabolic Properties of Tocopherols and Tocotrienols: Clinical Implications for Vitamin E Supplementation in Diabetic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 5101. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Reno-Bernstein, C.M.; Oxspring, M.; Bayles, J.; Huang, E.Y.; Holiday, I.; Fisher, S.J. Vitamin E treatment in insulin-deficient diabetic rats reduces cardiac arrhythmias and mortality during severe hypoglycemia. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E428–E434. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Barma, S.; Konwar, N.; Dewanjee, S.; Manna, P. Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: An update. Eur. J. Pharmacol. 2016, 791, 8–24. [Google Scholar] [CrossRef]
- Jin, Z.; Sun, J.; Zhang, W. Effect of Vitamin E on Diabetic Nephropathy: A Meta-Analysis. Altern. Ther. Health Med. 2024, 30, 344–349. [Google Scholar] [PubMed]
- Eleazu, C.O.; Eleazu, K.C.; Chukwuma, S. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J. Diabetes Metab. Disord. 2013, 12, 60. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, K.; Ni, Z.; He, J.C. Diabetic kidney disease: Challenges, advances, and opportunities. Kidney Dis. 2020, 6, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Amato, D.; Núñez-Ortiz, A.R.; del Carmen Benítez-Flores, J.; Segura-Cobos, D.; López-Sánchez, P.; Vázquez-Cruz, B. Role of Angiotensin-(1-7) on Renal Hypertrophy in Streptozotocin-Induced Diabetes Mellitus. Pharmacol. Pharmac. 2016, 7, 379–395. [Google Scholar] [CrossRef]
- Alqasim, A.A.; Nour, E.E.M.; Hammadi, S.H.; Esheba, G.E. Comparing the renoprotective effects of the antioxidant’s melatonin, vitamin D and vitamin E in diabetic rats. J. Taibah Univ. Med. Sci. 2020, 15, 351–357. [Google Scholar] [PubMed]
- Jozi, F.; Kheiripour, N.; Taheri, M.A.; Ardjmand, A.; Ghavipanjeh, G.; Nasehi, Z. L-lysine ameliorates diabetic nephropathy in rats with streptozotocin-induced diabetes mellitus. Biomed. Res. Int. 2022, 2022, 4547312. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, H.; Kaya, S.; Seker, U.; Nergiz, Y. Comparison of the anti-diabetic and nephroprotective activities of vitamin E, metformin, and Nigella sativa oil on kidney in experimental diabetic rats. Iran J. Basic Med. Sci. 2023, 26, 395–399. [Google Scholar] [PubMed]
- Daehn, I.S.; Ekperikpe, U.S.; Stadler, K. Redox regulation in diabetic kidney disease. Am. J. Physiol. Renal. Physiol. 2023, 325, F135–F149. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Kuo, M.; Huang, J.C.; Chang, W.A.; Wu, L.Y.; Huang, Y.C.; Chang, C.Y.; Lee, S.C.; Hsu, Y.L. Single-cell transcriptomic profiles in the pathophysiology within the microenvironment of early diabetic kidney disease. Cell Death Dis. 2023, 14, 442. [Google Scholar] [CrossRef] [PubMed]
- Dada, S.; Fabiyi-Edebor, T.; Akintoye, O.; Ezekpo, O.; Dada, O.; Bamikefa, T.; Sanya, J. α-Tocopherol Ameliorates Redox Equilibrium Disorders and Reduces Inflammatory Response Caused by Diclofenac-Induced Nephrotoxicity in Male Wistar Rats. Cureus 2023, 15, e50474. [Google Scholar] [CrossRef] [PubMed]
- Perez-Araluce, M.; Jüngst, T.; Sanmartin, C.; Prosper, F.; Plano, D.; Mazo, M.M. Biomaterials-Based Antioxidant Strategies for the Treatment of Oxidative Stress Diseases. Biomimetics 2024, 9, 23. [Google Scholar] [CrossRef]
- Elsayed, H.R.H.; Ali, E.M.T.; Rabei, M.R.; El Nashar, E.M.; Alghamdi, M.A.; Al-Zahrani, N.S.; Alshehri, S.H.; Aldahhan, R.A.; Morsy, A.I. Angiotensin II Type 1 receptor blockade attenuates the neuropathological changes in the spinal cords of diabetic rats with modulation of nuclear factor erythroid 2-related factor 2/heme oxygenase 1 system. Tissue Cell 2024, 88, 102420. [Google Scholar] [CrossRef] [PubMed]
- Hernández, E.A.G.; Ocaña, A.M.; Pedraza, O.O.; Aguilar, M.E.G.; Chávez, R.S.M.; Morales, M.P.; Cobos, D.S. Effect of Buddleja cordata Leaf Extract on Diabetic Nephropathy in Rats. Int. J. Mol. Sci. 2024, 25, 11432. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Control | DM | DM + Vit E 250 mg/kg | DM + Vit E 500 mg/kg |
|---|---|---|---|---|
| Glycemia (mg/dL) | 127 ± 7 | 406 ± 19 * | 405 ± 18 | 405 ± 23 |
| Body weight (g) | 341 ± 10 | 214 ± 24 * | 246 ± 14 * | 263 ± 8 * |
| Water ingestion (mL/24 h) | 81 ± 14 | 161 ± 2 * | 169 ± 10 * | 168 ± 10 * |
| Urinary volume (mL/24 h) | 10 ± 7 | 90 ± 14 * | 124 ± 14 * | 120 ± 10 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segura Cobos, D.; Díaz Salgado, E.E.; Amato, D.; Cardoso García, S.E.; Villamar Duque, T.E.; Antúnez, A.P.H.; del Valle Mondragón, L.; Magos Guerrero, G.A.; Guzmán Hernández, E.A. Effect of Vitamin E on Diabetic Nephropathy in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2025, 26, 1597. https://doi.org/10.3390/ijms26041597
Segura Cobos D, Díaz Salgado EE, Amato D, Cardoso García SE, Villamar Duque TE, Antúnez APH, del Valle Mondragón L, Magos Guerrero GA, Guzmán Hernández EA. Effect of Vitamin E on Diabetic Nephropathy in Streptozotocin-Induced Diabetic Rats. International Journal of Molecular Sciences. 2025; 26(4):1597. https://doi.org/10.3390/ijms26041597
Chicago/Turabian StyleSegura Cobos, David, Esperanza Enedina Díaz Salgado, Dante Amato, Sinaí Ernesto Cardoso García, Tomás Ernesto Villamar Duque, Anayantzin Paulina Heredia Antúnez, Leonardo del Valle Mondragón, Gil Alfonso Magos Guerrero, and Elizabeth Alejandrina Guzmán Hernández. 2025. "Effect of Vitamin E on Diabetic Nephropathy in Streptozotocin-Induced Diabetic Rats" International Journal of Molecular Sciences 26, no. 4: 1597. https://doi.org/10.3390/ijms26041597
APA StyleSegura Cobos, D., Díaz Salgado, E. E., Amato, D., Cardoso García, S. E., Villamar Duque, T. E., Antúnez, A. P. H., del Valle Mondragón, L., Magos Guerrero, G. A., & Guzmán Hernández, E. A. (2025). Effect of Vitamin E on Diabetic Nephropathy in Streptozotocin-Induced Diabetic Rats. International Journal of Molecular Sciences, 26(4), 1597. https://doi.org/10.3390/ijms26041597









