Role of Mitochondrial Dynamics in Skin Homeostasis: An Update
Abstract
1. Introduction
2. Skin Structure
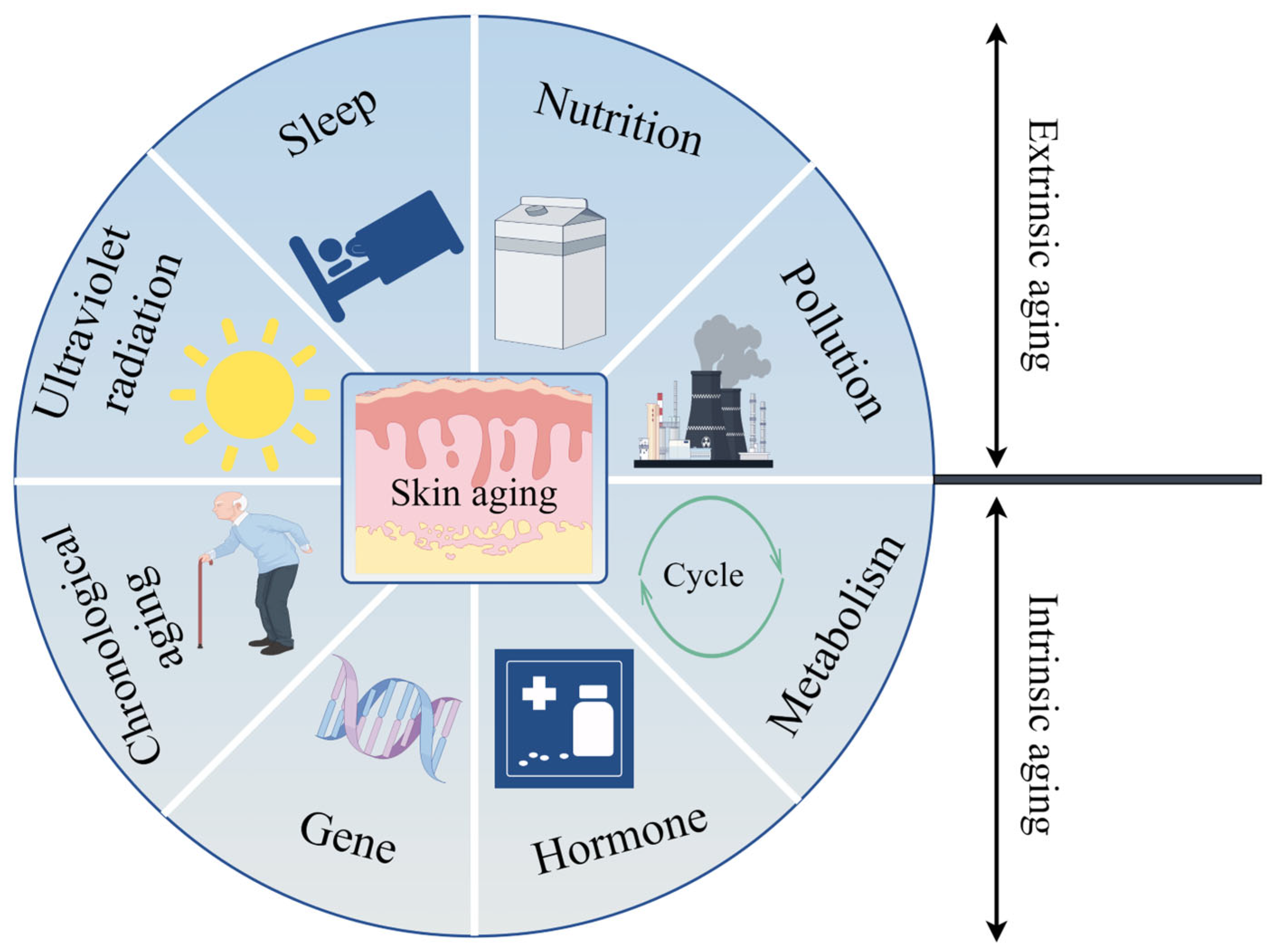
3. Skin Aging
4. Mitochondria and Skin
4.1. Effects of Mitochondria on the Epidermis
4.2. Effects of Mitochondria on the Dermis
4.3. Effects of Mitochondria on the Skin Accessories
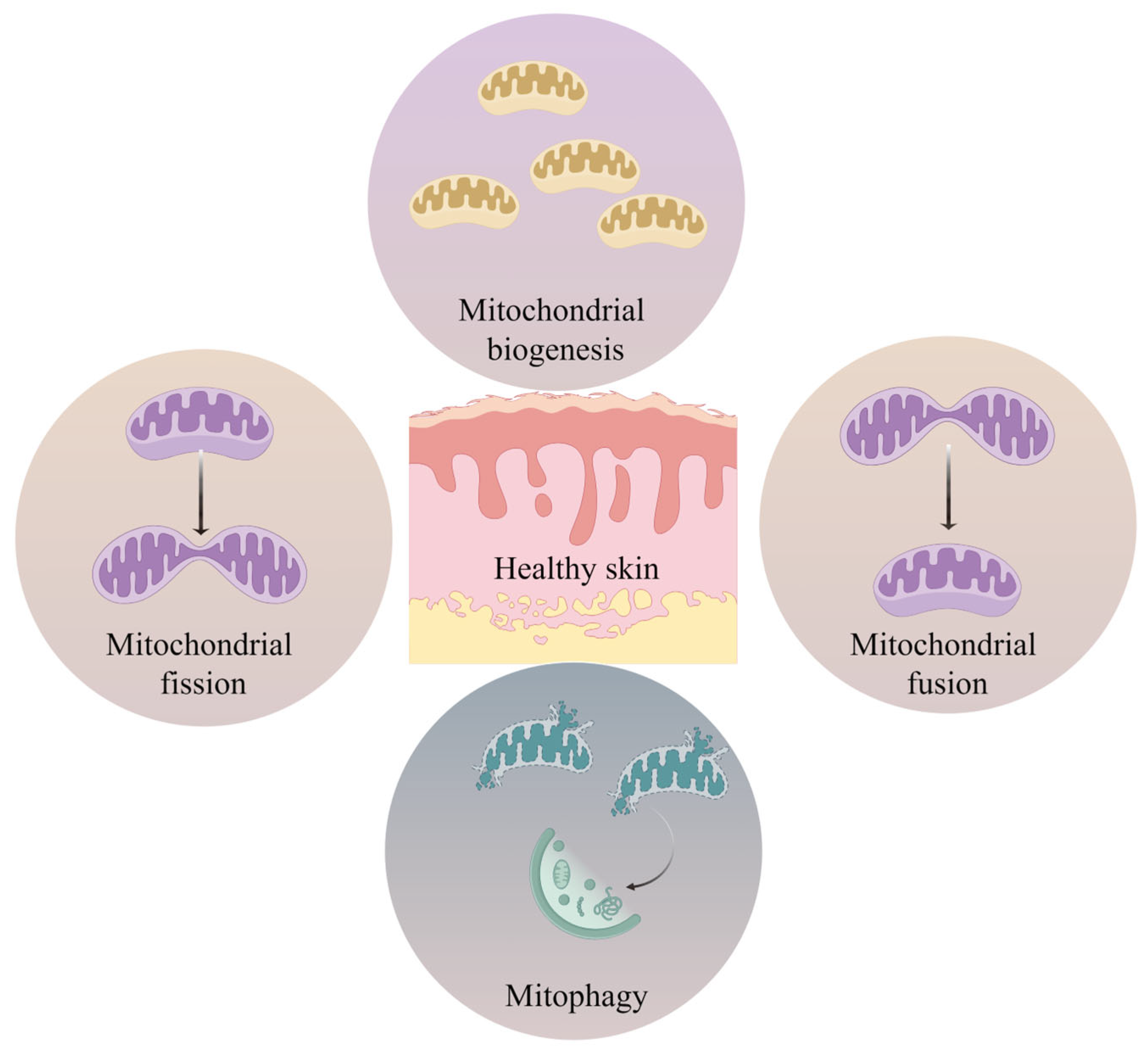
5. Structure and Function of Mitochondria
5.1. Mitophagy
5.2. Mitochondrial Dynamics
5.3. Mitochondrial Biogenesis
6. Mitochondrial Dysfunction and Aging
6.1. The Regulatory Roles of Mitochondrial Biogenesis in Aging
6.2. The Regulatory Roles of Mitophagy in Aging
6.3. The Regulatory Roles of Mitochondrial Dynamics in Aging
7. Mitochondrial Dysfunction and Skin Aging
7.1. Mitochondrial Dysfunction and Epidermal Aging
7.2. Mitochondrial Dysfunction and Dermal Aging
7.3. Mitochondrial Dysfunction and Adipose Tissue Aging
8. Mechanisms of Mitochondrial Dysfunction Leading to Skin Aging
8.1. Mitophagy and Skin Aging
8.2. Mitochondrial Dynamics and Skin Aging
8.3. Mitochondrial Biogenesis and Skin Aging
9. Therapeutic Targeting of Skin Mitochondria
10. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ogrodnik, M. Cellular aging beyond cellular senescence: Markers of senescence prior to cell cycle arrest in vitro and in vivo. Aging Cell 2021, 20, e13338. [Google Scholar] [CrossRef] [PubMed]
- Summer, R.; Shaghaghi, H.; Schriner, D.; Roque, W.; Sales, D.; Cuevas-Mora, K.; Desai, V.; Bhushan, A.; Ramirez, M.I.; Romero, F. Activation of the mTORC1/PGC-1 axis promotes mitochondrial biogenesis and induces cellular senescence in the lung epithelium. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L1049–L1060. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin senescence: Mechanisms and impact on whole-body aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef]
- Hu, S.C.-S.; Lin, C.-L.; Yu, H.-S. Dermoscopic assessment of xerosis severity, pigmentation pattern and vascular morphology in subjects with physiological aging and photoaging. Eur. J. Dermatol. 2019, 29, 274–280. [Google Scholar] [CrossRef]
- D’Uva, G.; Baci, D.; Albini, A.; Noonan, D.M. Cancer chemoprevention revisited: Cytochrome P450 family 1B1 as a target in the tumor and the microenvironment. Cancer Treat. Rev. 2018, 63, 1–18. [Google Scholar] [CrossRef]
- Banach, K.; Kowalska, J.; Rzepka, Z.; Beberok, A.; Rok, J.; Wrze’sniok, D. The role of UVA radiation in ketoprofen-mediated BRAF-mutant amelanotic melanoma cells death—A study at the cellular and molecular level. Toxicol. In Vitro 2021, 72, 105108. [Google Scholar] [CrossRef]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020, 11, 444. [Google Scholar] [CrossRef]
- Popov, L.D. Mitochondrial biogenesis: An update. J. Cell. Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef]
- Martic, I.; Papaccio, F.; Bellei, B.; Cavinato, M. Mitochondrial dynamics and metabolism across skin cells: Implications for skin homeostasis and aging. Front. Physiol. 2023, 14, 1284410. [Google Scholar] [CrossRef]
- Zhang, Z.; Michniak-Kohn, B.B. Tissue engineered human skin equivalents. Pharmaceutics 2012, 4, 26–41. [Google Scholar] [CrossRef]
- Hill, D.S.; Robinson, N.D.; Caley, M.P.; Chen, M.; O’Toole, E.A.; Armstrong, J.L.; Przyborski, S.; Lovat, P.E. A novel fully humanized 3D skin equivalent to model early melanoma invasion. Mol. Cancer Ther. 2015, 14, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, B.; Hui, Q.; Lin, F.; Tao, K. CO2 lattice laser reverses skin aging caused by UVB. Aging 2020, 12, 7056–7065. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jeong, H.D.; Song, M.J.; Lee, D.H.; Chung, J.H.; Lee, S.T. SOD3 suppresses the expression of MMP-1 and increases the integrity of extracellular matrix in fibroblasts. Antioxidants 2022, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.; Schwarz, A.; Fink, J.; Kamolz, L.P.; Kotzbeck, P. Modelling the complexity of human skin in vitro. Biomedicines 2023, 11, 794. [Google Scholar] [CrossRef]
- Abdallah, F.; Mijouin, L.; Pichon, C. Skin immune landscape: Inside and outside the organism. Mediat. Inflamm. 2017, 2017, 5095293. [Google Scholar] [CrossRef]
- Trevor, L.V.; Riches-Suman, K.; Mahajan, A.L.; Thornton, M.J. Adipose tissue: A source of stem cells with potential for regenerative therapies for wound healing. J. Clin. Med. 2020, 9, 2161. [Google Scholar] [CrossRef]
- Mancuso, P.; Bouchard, B. The impact of aging on adipose function and adipokine synthesis. Front. Endocrinol. 2019, 10, 137. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef]
- Naidoo, K.; Hanna, R.; Birch-Machin, M.A. What is the role of mitochondrial dysfunction in skin photoaging? Exp. Dermatol. 2018, 27, 124–128. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Yaar, M.; Eller, M.S.; Gilchrest, B.A. Fifty years of skin aging. J. Investig. Dermatol. Symp. Proc. 2002, 7, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Iwasaki, A.; Chien, A.L.; Kang, S. UVB-mediated DNA damage induces matrix metalloproteinases to promote photoaging in an AhR- and SP1-dependent manner. JCI Insight 2022, 7, e156344. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, J.; Song, M.J.; Park, C.H.; Lee, D.H.; Lee, S.H.; Chung, J.H. Inhibition of matrix metalloproteinase expression by selective clearing of senescent dermal fibroblasts attenuates ultraviolet-induced photoaging. Biomed. Pharmacother. 2022, 150, 113034. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, M.; Moriyama, H.; Uda, J.; Kubo, H.; Nakajima, Y.; Goto, A.; Morita, T.; Hayakawa, T. BNIP3 upregulation via stimulation of ERK and JNK activity is required for the protection of keratinocytes from UVB-induced apoptosis. Cell Death Dis. 2017, 8, e2576. [Google Scholar] [CrossRef]
- Brand, R.M.; Wipf, P.; Durham, A.; Epperly, M.W.; Greenberger, J.S.; Falo, L.D., Jr. Targeting mitochondrial oxidative stress to mitigate UV-induced skin damage. Front. Pharmacol. 2018, 9, 920. [Google Scholar] [CrossRef]
- Valejo Coelho, M.M.; Matos, T.R.; Apetato, M. The dark side of the light: Mechanisms of photocarcinogenesis. Clin. Dermatol. 2016, 34, 563–570. [Google Scholar] [CrossRef]
- Kauppila, T.E.S.; Kauppila, J.H.K.; Larsson, N.G. Mammalian mitochondria and aging: An update. Cell Metab. 2017, 25, 57–71. [Google Scholar] [CrossRef]
- Nicholls, D.G. Mitochondria and calcium signaling. Cell Calcium. 2005, 38, 311–731. [Google Scholar] [CrossRef]
- Liu, Z.; Butow, R.A. Mitochondrial retrograde signaling. Annu. Rev. Genet. 2006, 40, 159–185. [Google Scholar] [CrossRef]
- Nilsson, R.; Schultz, I.J.; Pierce, E.L.; Soltis, K.A.; Naranuntarat, A.; Ward, D.M.; Baughman, J.M.; Paradkar, P.N.; Kingsley, P.D.; Culotta, V.C.; et al. Discovery of genes essential for heme biosynthesis through large-scale gene expression analysis. Cell Metab. 2009, 10, 119–130. [Google Scholar] [CrossRef]
- Wallace, D.C.; Fan, W.; Procaccio, V. Mitochondrial energetics and therapeutics. Annu. Rev. Pathol. 2010, 5, 297–348. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S. Emerging therapeutic roles for NAD+ metabolism in mitochondrial and age-related disorders. Clin. Transl. Med. 2016, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Butow, R.A.; Avadhani, N.G. Mitochondrial signaling: The retrograde response. Mol. Cell 2004, 14, 1–15. [Google Scholar] [CrossRef]
- Sun, N.; Youle, R.J.; Finkel, T. The mitochondrial basis of aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef]
- Petersen, K.F.; Befroy, D.; Dufour, S.; Dziura, J.; Ariyan, C.; Rothman, D.L.; DiPietro, L.; Cline, G.W.; Shulman, G.I. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science 2003, 300, 1140–1142. [Google Scholar] [CrossRef]
- Lowell, B.B.; Shulman, G.I. Mitochondrial dysfunction and type 2 diabetes. Science 2005, 307, 384–387. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef]
- Lane, R.K.; Hilsabeck, T.; Rea, S.L. The role of mitochondrial dysfunction in age-related diseases. Biochim. Biophys. Acta 2015, 1847, 1387–1400. [Google Scholar] [CrossRef]
- Tocchi, A.; Quarles, E.K.; Basisty, N.; Gitari, L.; Rabinovitch, P.S. Mitochondrial dysfunction in cardiac aging. Biochim. Biophys. Acta 2015, 1847, 1424–1433. [Google Scholar] [CrossRef]
- Li, Q.O.Y.; Soro-Arnaiz, I.; Aragonés, J. Age-dependent obesity and mitochondrial dysfunction. Adipocyte 2017, 6, 161–166. [Google Scholar] [CrossRef]
- Ahn, C.S.M.; Etallo, C.M. Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab. 2015, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.; Birch-Machin, M. Mitochondria’s role in skin ageing. Biology 2019, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Kaeberlein, M.; Andalis, A.A.; Sturtz, L.A.; Defossez, P.A.; Culotta, V.C.; Fink, G.R.; Guarente, L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 2002, 418, 344–348. [Google Scholar] [CrossRef]
- Kirkinezos, I.G.; Moraes, C.T. Reactive oxygen species and mitochondrial diseases. Semin. Cell Dev. Biol. 2001, 12, 449–457. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial metabolism as a regulator of keratinocyte differentiation. Cell Logist. 2013, 3, e25456. [Google Scholar] [CrossRef]
- Trompette, A.; Pernot, J.; Perdijk, O.; Alqahtani, R.A.A.; Domingo, J.S.; Camacho-Muñoz, D.; Wong, N.C.; Kendall, A.C.; Wiederkehr, A.; Nicod, L.P.; et al. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal. Immunol. 2022, 15, 908–926. [Google Scholar] [CrossRef]
- Yaar, M.; Gilchrest, B.A. Ageing and photoageing of keratinocytes and melanocytes. Clin. Exp. Dermatol. 2001, 26, 583–591. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Characteristics of the aging skin. Adv. Wound Care 2013, 2, 5–10. [Google Scholar] [CrossRef]
- Khalid, K.A.; Nawi, A.F.M.; Zulkifli, N.; Barkat, M.A.; Hadi, H. Aging and wound healing of the skin: A review of clinical and pathophysiological hallmarks. Life 2022, 12, 2142. [Google Scholar] [CrossRef]
- Mellem, D.; Sattler, M.; Pagel-Wolff, S.; Jaspers, S.; Wenck, H.; Rübhausen, M.A.; Fischer, F. Fragmentation of the mitochondrial network in skin in vivo. PLoS ONE 2017, 12, e0174469. [Google Scholar] [CrossRef]
- Chevalier, F.P.; Rorteau, J.; Ferraro, S.; Martin, L.S.; Gonzalez-Torres, A.; Berthier, A.; El Kholti, N.; Lamartine, J. MiR-30a-5p alters epidermal terminal differentiation during aging by regulating BNIP3L/NIX-dependent mitophagy. Cells 2022, 11, 836. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schroeder, P. Role of mitochondria in photoaging of human skin: The defective powerhouse model. J. Investig. Dermatol. Symp. Proc. 2009, 14, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, Y.; Yamawaki, Y.; Sato, Y.; Muraoka, S.; Yoshida, M.; Okano, Y.; Masaki, H. Decreased mitochondrial function in UVA-irradiated dermal fibroblasts causes the insufficient formation of type I collagen and fibrillin-1 fibers. J. Dermatol. Sci. 2022, 108, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Yanes, B.; Rainero, E. The interplay between cell-extracellular matrix interaction and mitochondria dynamics in cancer. Cancers 2022, 14, 1433. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Foote, J.B.; Singh, K.K. Impaired mitochondria promote aging-associated sebaceous gland dysfunction and pathology. Am. J. Pathol. 2022, 192, 1546–1558. [Google Scholar] [CrossRef]
- Lovászi, M.; Szegedi, A.; Zouboulis, C.C.; Törőcsik, D. Sebaceous-immunobiology is orchestrated by sebum lipids. Derm.-Endocrinol. 2017, 9, e1375636. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Nicu, C.; Gherardini, J.; Mello, A.C.G.C.V.; Chéret, J.; Paus, R. Mitochondrially localized MPZL3 functions as a negative regulator of sebaceous gland size and sebocyte proliferation. J. Investig. Dermatol. 2022, 142, 2524–2527.e7. [Google Scholar] [CrossRef]
- Schneider, M.R.; Paus, R. Sebocytes, multifaceted epithelial cells: Lipid production and holocrine secretion. Int. J. Biochem. Cell Biol. 2010, 42, 181–185. [Google Scholar] [CrossRef]
- Yu, R.; Lendahl, U.; Nistér, M.; Zhao, J. Regulation of mammalian mitochondrial dynamics: Opportunities and challenges. Front. Endocrinol. 2020, 11, 374. [Google Scholar] [CrossRef]
- Kim, E.S.; Park, S.J.; Goh, M.J.; Na, Y.J.; Jo, D.S.; Jo, Y.K.; Shin, J.H.; Choi, E.S.; Lee, H.K.; Kim, J.Y.; et al. Mitochondrial dynamics regulate melanogenesis through proteasomal degradation of MITF via ROS-ERK activation. Pigment Cell Melanoma Res. 2014, 27, 1051–1062. [Google Scholar] [CrossRef]
- Vasileiou, P.V.S.; Evangelou, K.; Vlasis, K.; Fildisis, G.; Panayiotidis, M.I.; Chronopoulos, E.; Passias, P.G.; Kouloukoussa, M.; Gorgoulis, V.G.; Havaki, S. Mitochondrial homeostasis and cellular senescence. Cells 2019, 8, 686. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kang, Y.C.; Kim, Y.; Kim, S.; Yu, S.H.; Park, J.H.; Kim, I.H.; Kim, H.Y.; Han, K.; Lee, H.K.; et al. Preferred migration of mitochondria toward cells and tissues with mitochondrial damage. Int. J. Mol. Sci. 2022, 23, 15734. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, H.; Zheng, L.; Xia, J.; Yang, X.; Men, S.; Yuan, Y.; Fan, Y. Ginsenoside CK improves skeletal muscle insulin resistance by activating DRP1/PINK1-mediated mitophagy. Food Funct. 2023, 14, 1024–1036. [Google Scholar] [CrossRef]
- Ma, F.; Li, H.; Huo, H.; Han, Q.; Liao, J.; Zhang, H.; Li, Y.; Pan, J.; Hu, L.; Guo, J.; et al. N-acetyl-L-cysteine alleviates FUNDC1-mediated mitophagy by regulating mitochondrial dynamics in type 1 diabetic nephropathy canine. Life Sci. 2023, 313, 121278. [Google Scholar] [CrossRef]
- Al-Bari, M.A.A.; Xu, P. Molecular regulation of autophagy machinery by mTOR-dependent and -independent pathways. Ann. N. Y. Acad. Sci. 2020, 1467, 3–20. [Google Scholar] [CrossRef]
- Shirihai, O.S.; Song, M.; Dorn, G.W., 2nd. How mitochondrial dynamism orchestrates mitophagy. Circ. Res. 2015, 116, 1835–1849. [Google Scholar] [CrossRef]
- Shi, R.; Guberman, M.; Kirshenbaum, L.A. Mitochondrial quality control: The role of mitophagy in aging. Trends Cardiovasc. Med. 2018, 28, 246–260. [Google Scholar] [CrossRef]
- Zhu, N.; Xu, M.H.; Li, Y. Bioactive oligopeptides from ginseng (Panax ginseng Meyer) suppress oxidative stress-induced senescence in fibroblasts via NAD+/SIRT1/PGC-1α signaling pathway. Nutrients 2022, 14, 5289. [Google Scholar] [CrossRef]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef]
- Matsuda, N.; Sato, S.; Shiba, K.; Okatsu, K.; Saisho, K.; Gautier, C.A.; Sou, Y.S.; Saiki, S.; Kawajiri, S.; Sato, F.; et al. PINK1 stabilized by mitochondrial depolarization recruits parkin to damaged mitochondria and activates latent parkin for mitophagy. J. Cell Biol. 2010, 189, 211–221. [Google Scholar] [CrossRef]
- Geisler, S.; Holmström, K.M.; Skujat, D.; Fiesel, F.C.; Rothfuss, O.C.; Kahle, P.J.; Springer, W. PINK1/parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, I.G.; Kwak, M.K. Regulatory crosstalk between the oxidative stress-related transcription factor Nfe2l2/Nrf2 and mitochondria. Toxicol. Appl. Pharmacol. 2018, 359, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Chen, J.; Xia, T.; Li, P.; Zhou, G.; Xu, C.; Zhao, Q.; Dong, L.; Zhang, S.; Wang, A. Excessive ER stress and the resulting autophagic flux dysfunction contribute to fluoride-induced neurotoxicity. Environ. Pollut. 2018, 233, 889–899. [Google Scholar] [CrossRef]
- Meyer, J.N.; Leuthner, T.C.; Luz, A.L. Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 2017, 391, 42–53. [Google Scholar] [CrossRef]
- Morton, H.; Kshirsagar, S.; Orlov, E.; Bunquin, L.E.; Sawant, N.; Boleng, L.; George, M.; Basu, T.; Ramasubramanian, B.; Pradeepkiran, J.A.; et al. Defective mitophagy and synaptic degeneration in Alzheimer’s disease: Focus on aging, mitochondria and synapse. Free Radic. Biol. Med. 2021, 172, 652–667. [Google Scholar] [CrossRef]
- Jugé, R.; Breugnot, J.; Da Silva, C.; Bordes, S.; Closs, B.; Aouacheria, A. Quantification and characterization of UVB-induced mitochondrial fragmentation in normal primary human keratinocytes. Sci. Rep. 2016, 6, 35065. [Google Scholar] [CrossRef]
- Pagliuso, A.; Cossart, P.; Stavru, F. The ever-growing complexity of the mitochondrial fission machinery. Cell. Mol. Life Sci. 2018, 75, 355–374. [Google Scholar] [CrossRef]
- Gupta, D.; Abdullah, T.S. Regulation of mitochondrial dynamics in skin: Role in pathophysiology. Int. J. Dermatol. 2022, 61, 541–547. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondrial dynamics and its involvement in disease. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 235–259. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, X.; Li, M.; Yu, X.; Huang, F.; Wang, Y.; Yan, Y.; Zhang, H.; Shi, Y.; He, X. The role of mitochondrial quality surveillance in skin aging: Focus on mitochondrial dynamics, biogenesis and mitophagy. Ageing Res. Rev. 2023, 87, 101917. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-1alpha. Cardiovasc. Res. 2008, 79, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [PubMed]
- Hock, M.B.; Kralli, A. Transcriptional control of mitochondrial biogenesis and function. Annu. Rev. Physiol. 2009, 71, 177–203. [Google Scholar] [CrossRef] [PubMed]
- Virbasius, J.V.; Scarpulla, R.C. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: A potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 1309–1313. [Google Scholar] [CrossRef]
- Reznick, R.M.; Shulman, G.I. The role of AMP-activated protein kinase in mitochondrial biogenesis. J. Physiol. 2006, 574, 33–39. [Google Scholar] [CrossRef]
- Ren, J.; Pulakat, L.; Whaley-Connell, A.; Sowers, J.R. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J. Mol. Med. 2010, 88, 993–1001. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef]
- Chapman, J.; Fielder, E.; Passos, J.F. Mitochondrial dysfunction and cell senescence: Deciphering a complex relationship. FEBS Lett. 2019, 593, 1566–1579. [Google Scholar] [CrossRef]
- Ghosh-Choudhary, S.K.; Liu, J.; Finkel, T. The role of mitochondria in cellular senescence. FASEB J. 2021, 35, e21991. [Google Scholar] [CrossRef]
- Shigenaga, M.K.; Hagen, T.M.; Ames, B.N. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. USA 1994, 91, 10771–10778. [Google Scholar] [CrossRef]
- Liesa, M.; Shirihai, O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013, 17, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yu, X.; Zhang, C.; Wang, Y.; Sun, Y.; Sun, H.; Zhang, H.; Shi, Y.; He, X. Telomeres and mitochondrial metabolism: Implications for cellular senescence and age-related diseases. Stem Cell Rev. Rep. 2022, 18, 2315–2327. [Google Scholar] [CrossRef]
- Zorov, D.B.; Vorobjev, I.A.; Popkov, V.A.; Babenko, V.A.; Zorova, L.D.; Pevzner, I.B.; Silachev, D.N.; Zorov, S.D.; Andrianova, N.V.; Plotnikov, E.Y. Lessons from the discovery of mitochondrial fragmentation (fission): A review and update. Cells 2019, 8, 175. [Google Scholar] [CrossRef]
- Cavinato, M.; Madreiter-Sokolowski, C.T.; Büttner, S.; Schosserer, M.; Zwerschke, W.; Wedel, S.; Grillari, J.; Graier, W.F.; Jansen-Dürr, P. Targeting cellular senescence based on interorganelle communication, multilevel proteostasis, and metabolic control. FEBS J. 2021, 288, 3834–3854. [Google Scholar] [CrossRef]
- Jing, R.; Guo, K.; Zhong, Y.; Wang, L.; Zhao, J.; Gao, B.; Ye, Z.; Chen, Y.; Li, X.; Xu, N.; et al. Protective effects of fucoidan purified from Undaria pinnatifida against UV-irradiated skin photoaging. Ann. Transl. Med. 2021, 9, 1185. [Google Scholar] [CrossRef]
- Guo, K.; Liu, R.; Jing, R.; Wang, L.; Li, X.; Zhang, K.; Fu, M.; Ye, J.; Hu, Z.; Zhao, W.; et al. Cryptotanshinone protects skin cells from ultraviolet radiation-induced photoaging via its antioxidant effect and by reducing mitochondrial dysfunction and inhibiting apoptosis. Front. Pharmacol. 2022, 13, 1036013. [Google Scholar] [CrossRef]
- Park, J.E.; Woo, S.W.; Kim, M.B.; Kim, C.; Hwang, J.K. Standardized kaempferia parviflora extract inhibits intrinsic aging process in human dermal fibroblasts and hairless mice by inhibiting cellular senescence and mitochondrial dysfunction. Evid. Based Complement. Alternat. Med. 2017, 2017, 6861085. [Google Scholar] [CrossRef]
- Palikaras, K.; Daskalaki, I.; Markaki, M.; Tavernarakis, N. Mitophagy and age-related pathologies: Development of new therapeutics by targeting mitochondrial turnover. Pharmacol. Ther. 2017, 178, 157–174. [Google Scholar] [CrossRef]
- Mengus, C.; Neutzner, M.; Bento, A.C.P.F.; Bippes, C.C.; Kohler, C.; Decembrini, S.; Häusel, J.; Hemion, C.; Sironi, L.; Frank, S.; et al. VCP/p97 cofactor UBXN1/SAKS1 regulates mitophagy by modulating MFN2 removal from mitochondria. Autophagy 2022, 18, 171–190. [Google Scholar] [CrossRef]
- Ko, H.J.; Tsai, C.Y.; Chiou, S.J.; Lai, Y.L.; Wang, C.H.; Cheng, J.T.; Chuang, T.H.; Huang, C.F.; Kwan, A.L.; Loh, J.K.; et al. The phosphorylation status of Drp1-Ser637 by PKA in mitochondrial fission modulates mitophagy via PINK1/Parkin to exert multipolar spindles assembly during mitosis. Biomolecules 2021, 11, 424. [Google Scholar] [CrossRef]
- Barazzuol, L.; Giamogante, F.; Brini, M.; Calì, T. PINK1/Parkin mediated mitophagy, Ca2+ signalling, and ER-mitochondria contacts in Parkinson’s disease. Int. J. Mol. Sci. 2020, 21, 1772. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, S.A.; Sideris, D.P.; Giagtzoglou, N.; Ni, L.; Kankel, M.W.; Sen, A.; Bochicchio, L.E.; Huang, C.H.; Nussenzweig, S.C.; Worley, S.H.; et al. PINK1/Parkin influences cell cycle by sequestering TBK1 at damaged mitochondria, inhibiting mitosis. Cell Rep. 2019, 29, 225–235.e5. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. Mitochondrial aging and age-related dysfunction of mitochondria. BioMed Res. Int. 2014, 2014, 238463. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Thanan, R.; Oikawa, S.; Hiraku, Y.; Ohnishi, S.; Ma, N.; Pinlaor, S.; Yongvanit, P.; Kawanishi, S.; Murata, M. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 2015, 16, 193–217. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Harman, D. The biologic clock: The mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef]
- Alexeyev, M.F. Is there more to aging than mitochondrial DNA and reactive oxygen species? FEBS J. 2009, 276, 5768–5787. [Google Scholar] [CrossRef]
- Francisco, A.; Engel, D.F.; Figueira, T.R.; Rogério, F.; de Bem, A.F.; Castilho, R.F. Mitochondrial NAD(P)+ transhydrogenase is unevenly distributed in different brain regions, and its loss causes depressive-like behavior and motor dysfunction in mice. Neuroscience 2020, 440, 210–229. [Google Scholar] [CrossRef]
- Navarro, C.D.C.; Francisco, A.; Costa, E.F.D.; Dalla Costa, A.P.; Sartori, M.R.; Bizerra, P.F.V.; Salgado, A.R.; Figueira, T.R.; Vercesi, A.E.; Castilho, R.F. Aging-dependent mitochondrial bioenergetic impairment in the skeletal muscle of NNT-deficient mice. Exp. Gerontol. 2024, 193, 112465. [Google Scholar] [CrossRef] [PubMed]
- Kadoguchi, T.; Takada, S.; Yokota, T.; Furihata, T.; Matsumoto, J.; Tsuda, M.; Mizushima, W.; Fukushima, A.; Okita, K.; Kinugawa, S. Deletion of NAD(P)H oxidase 2 prevents angiotensin II-induced skeletal muscle atrophy. BioMed Res. Int. 2018, 2018, 3194917. [Google Scholar] [CrossRef] [PubMed]
- Figueira, T.R.; Francisco, A.; Ronchi, J.A.; Dos Santos, G.R.R.M.; Santos, W.D.; Treberg, J.R.; Castilho, R.F. NADPH supply and the contribution of NAD(P)+ transhydrogenase (NNT) to H2O2 balance in skeletal muscle mitochondria. Arch. Biochem. Biophys. 2021, 707, 108934. [Google Scholar] [CrossRef]
- Bokov, A.; Chaudhuri, A.; Richardson, A. The role of oxidative damage and stress in aging. Mech. Ageing Dev. 2004, 125, 811–826. [Google Scholar] [CrossRef]
- Bernard, J.J.; Cowing-Zitron, C.; Nakatsuji, T.; Muehleisen, B.; Muto, J.; Borkowski, A.W.; Martinez, L.; Greidinger, E.L.; Yu, B.D.; Gallo, R.L. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat. Med. 2012, 18, 1286–1290. [Google Scholar] [CrossRef]
- Borkowski, A.W.; Gallo, R.L. UVB radiation illuminates the role of TLR3 in the epidermis. J. Investig. Dermatol. 2014, 134, 2315–2320. [Google Scholar] [CrossRef]
- Li, C.; Liu, W.; Wang, F.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ikejima, T. DNA damage-triggered activation of cGAS-STING pathway induces apoptosis in human keratinocyte HaCaT cells. Mol. Immunol. 2021, 131, 180–190. [Google Scholar] [CrossRef]
- Kelly, J.; Murphy, J.E. Mitochondrial tolerance to single and repeat exposure to simulated sunlight in human epidermal and dermal skin cells. J. Photochem. Photobiol. B 2016, 165, 298–304. [Google Scholar] [CrossRef]
- Bellei, B.; Picardo, M. Premature cell senescence in human skin: Dual face in chronic acquired pigmentary disorders. Ageing Res. Rev. 2020, 57, 100981. [Google Scholar] [CrossRef]
- Dumas, M.; Maftah, A.; Bonte, F.; Ratinaud, M.H.; Meybeck, A.; Julien, R. Flow cytometric analysis of human epidermal cell ageing using two fluorescent mitochondrial probes. Comptes Rendus L’academie Sci. Ser. III 1995, 318, 191–197. [Google Scholar]
- Tan, C.L.; Chin, T.; Tan, C.Y.R.; Rovito, H.A.; Quek, L.S.; Oblong, J.E.; Bellanger, S. Nicotinamide metabolism modulates the proliferation/differentiation balance and senescence of human primary keratinocytes. J. Investig. Dermatol. 2019, 139, 1638–1647.e3. [Google Scholar] [CrossRef] [PubMed]
- Folmes, C.D.; Dzeja, P.P.; Nelson, T.J.; Terzic, A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell 2012, 11, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Dreesen, O. Faces of cellular senescence in skin aging. Mech. Ageing Dev. 2021, 198, 111525. [Google Scholar] [CrossRef]
- Rorteau, J.; Chevalier, F.P.; Bonnet, S.; Barthélemy, T.; Lopez-Gaydon, A.; Martin, L.S.; Bechetoille, N.; Lamartine, J. Maintenance of chronological aging features in culture of normal human dermal fibroblasts from old donors. Cells 2022, 11, 858. [Google Scholar] [CrossRef] [PubMed]
- Bowman, A.; Birch-Machin, M.A. Age-dependent decrease of mitochondrial complex II activity in human skin fibroblasts. J. Investig. Dermatol. 2016, 136, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Schniertshauer, D.; Gebhard, D.; Bergemann, J. Age-dependent loss of mitochondrial function in epithelial tissue can be reversed by coenzyme Q10. J. Aging Res. 2018, 2018, 6354680. [Google Scholar] [CrossRef]
- Wedel, S.; Martic, I.; Guerrero Navarro, L.; Ploner, C.; Pierer, G.; Jansen-Dürr, P.; Cavinato, M. Depletion of growth differentiation factor 15 (GDF15) leads to mitochondrial dysfunction and premature senescence in human dermal fibroblasts. Aging Cell 2023, 22, e13752. [Google Scholar] [CrossRef]
- Sgarbi, G.; Matarrese, P.; Pinti, M.; Lanzarini, C.; Ascione, B.; Gibellini, L.; Dika, E.; Patrizi, A.; Tommasino, C.; Capri, M.; et al. Mitochondria hyperfusion and elevated autophagic activity are key mechanisms for cellular bioenergetic preservation in centenarians. Aging 2014, 6, 296–310. [Google Scholar] [CrossRef]
- Woo, C.Y.; Jang, J.E.; Lee, S.E.; Koh, E.H.; Lee, K.U. Mitochondrial dysfunction in adipocytes as a primary cause of adipose tissue inflammation. Diabetes Metab. J. 2019, 43, 247–256. [Google Scholar] [CrossRef]
- Ou, M.Y.; Zhang, H.; Tan, P.C.; Zhou, S.B.; Li, Q.F. Adipose tissue aging: Mechanisms and therapeutic implications. Cell Death Dis. 2022, 13, 300. [Google Scholar] [CrossRef]
- Krstic, J.; Reinisch, I.; Schupp, M.; Schulz, T.J.; Prokesch, A. p53 functions in adipose tissue metabolism and homeostasis. Int. J. Mol. Sci. 2018, 19, 2622. [Google Scholar] [CrossRef] [PubMed]
- de Lange, P.; Lombardi, A.; Silvestri, E.; Cioffi, F.; Giacco, A.; Iervolino, S.; Petito, G.; Senese, R.; Lanni, A.; Moreno, M. Physiological approaches targeting cellular and mitochondrial pathways underlying adipose organ senescence. Int. J. Mol. Sci. 2023, 24, 11676. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yan, F.; Xu, Z.; Chen, Q.; Ren, J.; Wang, Q.; Chen, L.; Ying, J.; Liu, Z.; Zhao, J.; et al. Urolithin A protects human dermal fibroblasts from UVA-induced photoaging through NRF2 activation and mitophagy. J. Photochem. Photobiol. B. 2022, 232, 112462. [Google Scholar] [CrossRef]
- Chen, L.; Qin, Y.; Liu, B.; Gao, M.; Li, A.; Li, X.; Gong, G. PGC-1α-mediated mitochondrial quality control: Molecular mechanisms and implications for heart failure. Front. Cell Dev. Biol. 2022, 10, 871357. [Google Scholar] [CrossRef]
- Guo, Z.; Fan, D.; Liu, F.Y.; Ma, S.Q.; An, P.; Yang, D.; Wang, M.Y.; Yang, Z.; Tang, Q.Z. NEU1 Regulates Mitochondrial Energy Metabolism and Oxidative Stress Post-myocardial Infarction in Mice via the SIRT1/PGC-1 Alpha Axis. Front. Cardiovasc. Med. 2022, 9, 821317. [Google Scholar] [CrossRef]
- Ledrhem, M.; Nakamura, M.; Obitsu, M.; Hirae, K.; Kameyama, J.; Bouamama, H.; Gadhi, C.; Katakura, Y. Essential oils derived from cistus species activate mitochondria by inducing SIRT1 expression in human keratinocytes, leading to senescence inhibition. Molecules 2022, 27, 2053. [Google Scholar] [CrossRef]
- Soares, C.D.; de Lima Morais, T.M.; Mariano, F.V.; Altemani, A.; Corrêa, M.B.; Reis, R.R.D.D.; Amorim, L.S.; Ferreira, S.M.S.; de Almeida, O.P.; Carlos, R.; et al. Expression of mitochondrial dynamics markers during melanoma progression: Comparative study of head and neck cutaneous and mucosal melanomas. J. Oral Pathol. Med. 2019, 48, 373–381. [Google Scholar] [CrossRef]
- Chen, G.; Kroemer, G.; Kepp, O. Mitophagy: An emerging role in aging and age-associated diseases. Front. Cell Dev. Biol. 2020, 8, 200. [Google Scholar] [CrossRef]
- Russell, O.M.; Gorman, G.S.; Lightowlers, N.; Turnbull, D.M. Mitochondrial diseases: Hope for the future. Cell 2020, 181, 168–188. [Google Scholar] [CrossRef]
- Ayunin, Q.; Miatmoko, A.; Soeratri, W.; Erawati, T.; Susanto, J.; Legowo, D. Improving the anti-ageing activity of coenzyme Q10 through protransfersome-loaded emulgel. Sci. Rep. 2022, 12, 906. [Google Scholar] [CrossRef]
- Wu, H.; Zhong, Z.; Lin, S.; Qiu, C.; Xie, P.; Lv, S.; Cui, L.; Wu, T. Coenzyme Q10 sunscreen prevents progression of ultraviolet-induced skin damage in mice. BioMed Res. Int. 2020, 2020, 9039843. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Mariani, P.; Spinozzi, F.; Benedusi, M.; Cervellati, F.; Cortesi, R.; Drechsler, M.; Prieux, R.; Valacchi, G.; Esposito, E. Ethosomes for Coenzyme Q10 cutaneous administration: From design to 3D skin tissue evaluation. Antioxidants 2020, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, U.; Bergemann, J.; Diembeck, W.; Ennen, J.; Gohla, S.; Harris, I.; Jacob, J.; Kielholz, J.; Mei, W.; Pollet, D.; et al. Coenzyme Q10, a cutaneous antioxidant and energizer. Biofactors 1999, 9, 371–378. [Google Scholar] [CrossRef]
- Knott, A.; Achterberg, V.; Smuda, C.; Mielke, H.; Sperling, G.; Dunckelmann, K.; Vogelsang, A.; Krüger, A.; Schwengler, H.; Behtash, M.; et al. Topical treatment with coenzyme Q10-containing formulas improves skin’s Q10 level and provides antioxidative effects. Biofactors 2015, 41, 383–390. [Google Scholar] [CrossRef]
- Schniertshauer, D.; Müller, S.; Mayr, T.; Sonntag, T.; Gebhard, D.; Bergemann, J. Accelerated Regeneration of ATP Level after Irradiation in Human Skin Fibroblasts by Coenzyme Q10. Photochem. Photobiol. 2016, 92, 488–494. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.; Sung, G.Y. Coenzyme Q10 efficacy test for human skin equivalents using a pumpless skin-on-a-chip system. Int. J. Mol. Sci. 2020, 21, 8475. [Google Scholar] [CrossRef]
- Jung, H.J.; Kim, S.M.; Kim, D.H.; Bang, E.; Kang, D.; Lee, S.; Chun, P.; Moon, H.R.; Chung, H.Y. 2,4-Dihydroxyphenyl-benzo[d] thiazole (MHY553), a synthetic PPARα agonist, decreases age-associated inflammatory responses through PPARα activation and RS scavenging in the skin. Exp. Gerontol. 2021, 143, 111153. [Google Scholar] [CrossRef]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted antioxidants. FASEB J. 2015, 29, 4766–4771. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, T.; Li, R.; Gao, T. Role of Mitochondrial Dynamics in Skin Homeostasis: An Update. Int. J. Mol. Sci. 2025, 26, 1803. https://doi.org/10.3390/ijms26051803
Quan T, Li R, Gao T. Role of Mitochondrial Dynamics in Skin Homeostasis: An Update. International Journal of Molecular Sciences. 2025; 26(5):1803. https://doi.org/10.3390/ijms26051803
Chicago/Turabian StyleQuan, Tao, Ran Li, and Ting Gao. 2025. "Role of Mitochondrial Dynamics in Skin Homeostasis: An Update" International Journal of Molecular Sciences 26, no. 5: 1803. https://doi.org/10.3390/ijms26051803
APA StyleQuan, T., Li, R., & Gao, T. (2025). Role of Mitochondrial Dynamics in Skin Homeostasis: An Update. International Journal of Molecular Sciences, 26(5), 1803. https://doi.org/10.3390/ijms26051803




