Nephroprotective and Antioxidant Effects of Jatropha dioica Extract Against Ischemia–Reperfusion Injury in Wistar Rats
Abstract
1. Introduction
2. Results
2.1. Jd Nontoxicity Evaluation
2.2. Effects of Jd Extract on IR-Induced Renal Damage
2.3. Effects of Jd Extract on IR-Induced Oxidation
2.4. Effects of Jd Extract on IR-Histophatological Renal
3. Discussion
4. Materials and Methods
4.1. Plant and Extraction
4.2. Animals and Ethical Considerations
4.3. Experimental Study Groups
4.4. Laparotomy and IR Injury Induction
4.5. Biochemical and Oxidative Stress Marker Analysis
4.6. Histopathological Renal Evaluation
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chertow, G.M.; Burdick, E.; Honour, M.; Bonventre, J.V.; Bates, D.W. Acute kidney injury, mortality, length of stay and costs in hospitalised patients. J. Am. Soc. Nephrol. 2005, 16, 3365–3370. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Sanchez-Niño, M.D.; Martín-Cleary, C.; Ortiz, A.; Ramos, A.M. Progress in the development of animal models of acute kidney injury and its impact on drug discovery. Expert Opin. Drug Discov. 2013, 8, 879–895. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, M.; Faga, T.; Pisani, A.; Perticone, M.; Michael, A. The ischemic/nephrotoxic acute kidney injury and the use of renal biomarkers in clinical practice. Eur. J. Intern. Med. 2017, 39, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Endre, Z.H.; Pickering, J.W.; Walker, R.J.; Devarajan, P.; Edelstein, C.L.; Bonventre, J.V.; Frampton, C.M.; Bennett, M.R.; Ma, Q.; Sabbisetti, V.S.; et al. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int. 2011, 79, 1119–1130. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Meng, H.; Duan, H.; Liu, X.; Wu, J.; Gao, F.; Wang, S.; Tan, R.; Yuan, J. Ischemia-reperfusion injury: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 12. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Zhao, H.; Alam, A.; Soo, A.P.; George, A.J.T.; Ma, D. Ischemia-Reperfusion Injury Reduces Long Term Renal Graft Survival: Mechanism and Beyond. EBioMedicine 2018, 28, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Menke, J.; Sollinger, D.; Schamberger, B.; Heemann, U.; Lutz, J. The effect of ischemia/reperfusion on the kidney graft. Curr. Opin. Organ. Transplant. 2014, 19, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Rovcanin, B.; Medic, B.; Kocic, G.; Cebovic, T.; Ristic, M.; Prostran, M. Molecular Dissection of Renal Ischemia-Reperfusion: Oxidative Stress and Cellular Events. Curr. Med. Chem. 2016, 23, 1965–1980. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sun, Y.; Zhuang, Y.; Zhao, W.; Chen, Y.; Jiang, B.; Guo, C.; Zhang, Z.; Peng, H.; Chen, Y. Effects of kallistatin on oxidative stress and inflammation on renal ischemia-reperfusion injury in mice. Curr. Vasc. Pharmacol. 2015, 13, 265–273. [Google Scholar] [CrossRef]
- Trujillo-Rangel, W.Á.; García-Valdés, L.; Méndez-Del Villar, M.; Castañeda-Arellano, R.; Totsuka-Sutto, S.E.; García-Benavides, L. Therapeutic Targets for Regulating Oxidative Damage Induced by Ischemia-Reperfusion Injury: A Study from a Pharmacological Perspective. Oxidative Med. Cell. Longev. 2022, 2022, 8624318. [Google Scholar] [CrossRef] [PubMed]
- Welbourn, C.R.; Goldman, G.; Paterson, I.S.; Valeri, C.R.; Shepro, D.; Hechtman, H.B. Pathophysiology of ischaemia reperfusion injury: Central role of the neutrophil. Br. J. Surg. 1991, 78, 651–655. [Google Scholar] [CrossRef]
- Cowled, P.; Fitridge, R. Pathophysiology of Reperfusion Injury. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; p. 18. [Google Scholar]
- Dorweiler, B.; Pruefer, D.; Andrasi, T.B.; Maksan, S.M.; Schmiedt, W.; Neufang, A.; Vahl, C.F. Ischemia-Reperfusion Injury Pathophysiology and Clinical Implications. Eur. J. Trauma Emerg. Surg. 2007, 33, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Lasorsa, F.; Rutigliano, M.; Milella, M.; d’Amati, A.; Crocetto, F.; Pandolfo, S.D.; Barone, B.; Ferro, M.; Spilotros, M.; Battaglia, M.; et al. Ischemia–Reperfusion Injury in Kidney Transplantation: Mechanisms and Potential Therapeutic Targets. Int. J. Mol. Sci. 2024, 25, 4332. [Google Scholar] [CrossRef]
- Sabet Sarvestani, F.; Azarpira, N.; Al-Abdullah, I.H.; Tamaddon, A.M. MicroRNAs in liver and kidney ischemia reperfusion injury: Insight to improve transplantation outcome. Biomed. Pharmacother. 2021, 133, 110944. [Google Scholar] [CrossRef]
- LaGory, E.L.; Giaccia, A.J. The ever-expanding role of HIF in tumour and stromal biology. Nat. Cell Biol. 2016, 18, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wei, Q.; Liu, J.; Yi, M.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y.; Liu, F.; Venkatachalam, M.A.; et al. AKI on CKD: Heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017, 92, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Sureshbabu, A.; Ryter, S.W.; Choi, M.E. Oxidative stress and autophagy: Crucial modulators of kidney injury. Redox Biol. 2015, 4, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Aboutaleb, N.; Jamali, H.; Abolhasani, M.; Pazoki Toroudi, H. Lavender oil (Lavandula angustifolia) attenuates renal ischemia/reperfusion injury in rats through suppression of inflammation, oxidative stress and apoptosis. Biomed. Pharmacother. 2019, 110, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Turgut, F.; Bayrak, O.; Catal, F.; Bayrak, R.; Atmaca, A.F.; Koc, A.; Akbas, A.; Akcay, A.; Unal, D. Antioxidant and protective effects of silymarin on ischemia and reperfusion injury in the kidney tissues of rats. Int. Urol. Nephrol. 2008, 40, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, O.; Uz, E.; Bayrak, R.; Turgut, F.; Atmaca, A.F.; Sahin, S.; Yildirim, M.E.; Kaya, A.; Cimentepe, E.; Akcay, A. Curcumin protects against ischemia/reperfusion injury in rat kidneys. World J. Urol. 2008, 26, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Akinrinde, A.S.; Oduwole, O.; Akinrinmade, F.J.; Bolaji-Alabi, F.B. Nephroprotective effect of methanol extract of “Moringa oleifera” leaves on acute kidney injury induced by ischemia-reperfusion in rats. Afr. Health Sci. 2020, 20, 1382–1396. [Google Scholar] [CrossRef]
- Tienda-Vázquez, M.A.; Morreeuw, Z.P.; Sosa-Hernández, J.E.; Cardador-Martínez, A.; Sabath, E.; Melchor-Martínez, E.M.; Iqbal, H.M.N.; Parra-Saldívar, R. Nephroprotective Plants: A Review on the Use in Pre-Renal and Post-Renal Diseases. Plants 2022, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- Torres-González, L.; Cienfuegos-Pecina, E.; Perales-Quintana, M.M.; Alarcon-Galvan, G.; Muñoz-Espinosa, L.E.; Pérez-Rodríguez, E.; Cordero-Pérez, P. Nephroprotective Effect of Sonchus oleraceus Extract against Kidney Injury Induced by Ischemia-Reperfusion in Wistar Rats. Oxidative Med. Cell. Longev. 2018, 2018, 9572803. [Google Scholar] [CrossRef] [PubMed]
- Perez-Meseguer, J.; Torres-González, L.; Gutiérrez-González, J.A.; Alarcón-Galván, G.; Zapata-Chavira, H.; Waksman-de Torres, N.; Moreno-Peña, D.P.; Muñoz-Espinosa, L.E.; Cordero-Pérez, P. Anti-inflammatory and nephroprotective activity of Juglans mollis against renal ischemia-reperfusion damage in a Wistar rat model. BMC Complement. Altern. Med. 2019, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudzadeh, L.; Najafi, H.; Ashtiyani, S.C.; Yarijani, Z.M. Anti-inflammatory and protective effects of saffron extract in ischaemia/reperfusion-induced acute kidney injury. Nephrology 2017, 22, 748–754. [Google Scholar] [CrossRef]
- Najafi, H.; Mohamadi Yarijani, Z.; Changizi-Ashtiyani, S.; Mansouri, K.; Modarresi, M.; Madani, S.H.; Bastani, B. Protective effect of Malva sylvestris L. extract in ischemia-reperfusion induced acute kidney and remote liver injury. PLoS ONE 2017, 12, e0188270. [Google Scholar] [CrossRef]
- Cavalcante, N.B.; Diego da Conceição Santos, A.; Guedes da Silva Almeida, J.R. The genus Jatropha (Euphorbiaceae): A review on secondary chemical metabolites and biological aspects. Chem. Biol. Interact. 2020, 318, 108976. [Google Scholar] [CrossRef] [PubMed]
- Sabandar, C.W.; Ahmat, N.; Jaafar, F.M.; Sahidin, I. Medicinal property, phytochemistry and pharmacology of several Jatropha species (Euphorbiaceae): A review. Phytochemistry 2013, 85, 7–29. [Google Scholar] [CrossRef]
- Zhang, X.P.; Zhang, M.L.; Su, X.H.; Huo, C.H.; Gu, Y.C.; Shi, Q.W. Chemical constituents of the plants from genus Jatropha. Chem. Biodivers. 2009, 6, 2166–2183. [Google Scholar] [CrossRef] [PubMed]
- Devappa, R.K.; Makkar, H.P.; Becker, K. Nutritional, biochemical, and pharmaceutical potential of proteins and peptides from jatropha: Review. J. Agric. Food Chem. 2010, 58, 6543–6555. [Google Scholar] [CrossRef]
- Ramírez-Moreno, A.; Delgadillo-Guzmán, D.; Bautista-Robles, V.; Marszalek, J.E.; Keita, H.; Kourouma, A.; García, S.A.R.; Amado, J.R.R.; Tavares-Carvalho, J.C. Jatropha dioica, an Aztec plant with promising pharmacological properties: A systematic review. Afr. J. Pharm. Pharmacol. 2020, 14, 169–178. [Google Scholar] [CrossRef]
- Bautistaa, E.; Lozano-Gamboaa, S.; Fragoso-Serranob, M.; Rivera-Chávezc, J.; Salazar-Olivoa, L.A. Jatrophenediol, a pseudoguaiane sesquiterpenoid from Jatropha dioica rhizomes. Tetrahedron Lett. 2022, 104, 154040. [Google Scholar] [CrossRef]
- Valenzuela-Soto, R.; Jiménez-Villarreal, J.; García-Garza, R.; Betancourt-Martínez, N.D.; Lozoya-Martínez, R.; Almaraz-Celis, D.; Morán-Martínez, J. Evaluation of the Antioxidant Activity of Cnidoscolus chayamansa (Chaya), Euphorbia prostrata (Herb of the Swallow) and Jatropha dioica (Drago blood) in Wistar rats Induced to Hyperglycemia. Int. J. Morphol. 2019, 37, 36–42. [Google Scholar] [CrossRef]
- Martínez, N.; Almaguer, G.; Vázquez-Alvarado, P.; Figueroa, A.; Zúñiga, C.; Hernández-Ceruleos, A. Phytochemical analysis of Jatropha dioica and determination of its antioxidant and chemoprotective effects on the genotoxic potential of cyclophosphamide, daunorubicin, and methylmethanesulfonate, evaluated through the comet assay. BLACPMA 2014, 13, 437–457. [Google Scholar]
- Aguilera-Carbo, A.F.; Augur, C.; Prado-Barragan, L.A.; Aguilar, C.N.; Favela-Torres, E. Extraction and analysis of ellagic acid from novel complex sources. Chem. Pap. 2008, 62, 440–444. [Google Scholar] [CrossRef]
- Devappa, R.K.; Makkar, H.P.S.; Becker, K. Jatropha Diterpenes: A Review. J. Am. Oil Chem. Soc. 2011, 88, 301–322. [Google Scholar] [CrossRef]
- Ramírez-Moreno, A.; Serrano-Gallardo, L.B.; Barragán-Ledezma, L.E.; Quintanar- Escorza, M.A.; Arellano-Pérez-Vertti, R.D. Polyphenolic compounds determination in Jatropha dioica extracts and total antioxidant capacity. Rev. Mex. Cienc. Farm. 2016, 47, 42–48. [Google Scholar]
- Melchor-Martínez, E.M.; Silva-Mares, D.A.; Torres-López, E.; Waksman-Minsky, N.; Pauli, G.F.; Chen, S.N.; Niemitz, M.; Sánchez-Castellanos, M.; Toscano, A.; Cuevas, G.; et al. Stereochemistry of a Second Riolozane and Other Diterpenoids from Jatropha dioica. J. Nat. Prod. 2017, 80, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, A.M.; Dominguez, X.A.; Williams, H.J.; Scott, A.I.; Reibenspies, J. Citlalitrione, a new diterpene from Jatropha dioica var. sessiliflora. J. Nat. Prod. 1988, 51, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Silva-Belmares, Y.; Rivas-Morales, C.; Viveros-Valdez, E.; de la Cruz-Galicia, M.G.; Carranza-Rosales, P. Antimicrobial and cytotoxic activities from Jatropha dioica roots. Pak. J. Biol. Sci. 2014, 17, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Silva-Mares, D.; Torres-López, E.; Rivas-Estilla, A.M.; Cordero-Pérez, P.; Waksman-Minsky, N.; Rivas-Galindo, V.M. Plants from northeast Mexico with anti-HSV activity. Nat. Prod. Commun. 2013, 8, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Aguilara, F.J.; Roman-Ramos, R.; Perez-Gutierrez, S.; Aguilar-Contreras, A.; Contreras-Weber, C.C.; Flores-Saenz, J.L. Study of the anti-hyperglycemic effect of plants used as antidiabetics. J. Ethnopharmacol. 1998, 61, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Moreno, A.; García, R.; Pedroza, D.; Soto, A.; Flores-Loyola, E.; Castillo, I.; Keita, H.; Sharara, I.; Delgadillo, D. Antioxidant Effect of Jatropha dioica Extract on Immunoreactivity of Claudin 2 in the Kidney of Rats with Induced Diabetes. Nat. Prod. Commun. 2023, 18, 1934578X231157183. [Google Scholar] [CrossRef]
- Kobuchi, S.; Shintani, T.; Sugiura, T.; Tanaka, R.; Suzuki, R.; Tsutsui, H.; Fujii, T.; Ohkita, M.; Ayajiki, K.; Matsumura, Y. Renoprotective effects of gamma-aminobutyric acid on ischemia/reperfusion-induced renal injury in rats. Eur. J. Pharmacol. 2009, 623, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Wu, D.; Jiang, W.; Li, J.; Long, J.; Jia, C.; Zhou, T. Molecular Biomarkers in Drug-Induced Liver Injury: Challenges and Future Perspectives. Front. Pharmacol. 2020, 10, 1667. [Google Scholar] [CrossRef] [PubMed]
- Castro-Ríos, R.; Melchor-Martínez, E.M.; Solís-Cruz, G.Y.; Rivas-Galindo, V.M.; Silva-Mares, D.A.; Cavazos-Rocha, N.C. HPLC Method Validation for Jatropha dioica Extracts Analysis. J. Chromatogr. Sci. 2020, 58, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Al-Fatlawi, A.A.Y.; Al-Salih, A.R.H.; Reda-Yassen, M.A. β-sitosterol protects against cisplatin-induced nephrotoxicity through amelioration of oxidative stress in rats. Muthan Med. J. 2017, 4, 60–74. [Google Scholar] [CrossRef]
- Sharmila, R.; Sindhu, G.; Arockianathan, P.M. Nephroprotective effect of β-sitosterol on N-diethylnitrosamine initiated and ferric nitrilotriacetate promoted acute nephrotoxicity in Wistar rats. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Chander, V.; Singh, D.; Chopra, K. Catechin, a natural antioxidant protects against rhabdomyolysis-induced myoglobinuric acute renal failure. Pharmacol. Res. 2003, 48, 503–539. [Google Scholar] [CrossRef] [PubMed]
- Shabani, M.; Bayrami, D.; Moghadam, A.A.; Jamali, Z.; Salimi, A. Pretreatment of ellagic acid protects ifosfamide-induced acute nephrotoxicity in rat kidneys: A mitochondrial, histopathological and oxidative stress approaches. Toxicol. Rep. 2023, 10, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Kulkarni, V.H.; Chakraborty, M.; Habbu, P.V.; Ray, A. Ellagic acid restored lead-induced nephrotoxicity by anti-inflammatory, anti-apoptotic and free radical scavenging activities. Heliyon 2021, 7, e05921. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Wu, P.T.; Chyau, C.C.; Wu, P.H.; Lin, H.H. The Nephroprotective Effects of Hibiscus sabdariffa Leaf and Ellagic Acid In Vitro and In Vivo Models of Hyperuricemic Nephropathy. J. Agric. Food Chem. 2023, 71, 382–397. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Heibati, F.; Heidarian, E. Gallic acid exerts anti-inflammatory, anti-oxidative stress, and nephroprotective effects against paraquat-induced renal injury in male rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1–9. [Google Scholar] [CrossRef]
- Moradi, A.; Abolfathi, M.; Javadian, M.; Heidarian, E.; Roshanmehr, H.; Khaledi, M.; Nouri, A. Gallic Acid Exerts Nephroprotective, Anti-Oxidative Stress, and Anti-Inflammatory Effects Against Diclofenac-Induced Renal Injury in Malerats. Arch. Med. Res. 2021, 52, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Ahmadvand, H.; Nouryazdan, N.; Nasri, M.; Adibhesami, G.; Babaeenezhad, E. Renoprotective effects of Gallic acid against gentamicin nephrotoxicity through amelioration of oxidative stress in rats. Braz. Arch. Biol. Technol. 2020, 63, e20200131. [Google Scholar] [CrossRef]
- Pertino, M.; Schmeda-Hirschmann, G.; Rodríguez, J.A.; Theoduloz, C. Gastroprotective effect and cytotoxicity of semisynthetic jatropholone derivatives. Planta Medica 2007, 73, 1095–1100. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, E.J.; Park, W.; Ha, K.T.; Chung, H.S. Natural compounds as lactate dehydrogenase inhibitors: Potential therapeutics for lactate dehydrogenase inhibitors-related diseases. Front. Pharmacol. 2023, 14, 1275000. [Google Scholar] [CrossRef]
- Ouassou, H.; Bouhrim, M.; Daoudi, N.E.; Mekhfi, H.; Ziyyat, A.; Legssyer, A.; Aziz, M.; Bnouham, M. Evaluation of Hepatoprotective Activity of Caralluma europaea Stem Extract against CCl4-Induced Hepatic Damage in Wistar Rats. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 8883040. [Google Scholar] [CrossRef]
- Kuatsienu, L.E.; Ansah, C.; Adinortey, M.B. Toxicological evaluation and protective effect of ethanolic leaf extract of Launaea taraxacifolia on gentamicin induced rat kidney injury. Asian Pac. J. Trop. Biomed. 2017, 7, 640–646. [Google Scholar] [CrossRef]
- Iqbal, S.M.; Hussain, L.; Hussain, M.; Akram, H.; Asif, M.; Jamshed, A.; Saleem, A.; Siddique, R. Nephroprotective Potential of a Standardized Extract of Bambusa arundinacea: In Vitro and In Vivo Studies. ACS Omega 2022, 7, 18159–18167. [Google Scholar] [CrossRef] [PubMed]
- Mattew, L.P.; Gordon, M.B. Drugs and toxins that damage the kidney. Medicine 2007, 35, 399–403. [Google Scholar] [CrossRef]
- Hong, Y.A.; Park, C.W. Catalytic Antioxidants in the Kidney. Antioxidants 2021, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Gallardo, L.B.; Castillo-Maldonado, I.; Borjón-Ríos, C.G.; Rivera-Guillén, M.A.; Morán-Martínez, J.; Tellez-López, M.A.; García-Salcedo, J.J.; Pedroza-Escobar, D.; Vega-Menchaca, M.C. Antimicrobial activity and toxicity of plants from northern México. Indian J. Tradit. Knowl. 2017, 16, 203–207. [Google Scholar]
- Morales-Velazquez, G.; Lazalde-Ramos, B.P.; Gómez-Meda, B.C.; Zúñiga-González, G.M.; Ortiz-García, Y.M.; Gutiérrez-Hernández, R.; Guerrero-Velazquez, C.; Sánchez, S.V.; Zamora-Perez, A.L. Genome Damage in Rats after Transplacental Exposure to Jatropha dioica Root Extract. Evid. Based Complement. Altern. Med. 2019, 2019, 2962950. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef]
- Picard, F.; Auwerx, J. PPAR(gamma) and glucose homeostasis. Annu. Rev. Nutr. 2002, 22, 167–197. [Google Scholar] [CrossRef]
- Rau, O.; Wurglics, M.; Dingermann, T.; Abdel-Tawab, M.; Schubert-Zsilavecz, M. Screening of herbal extracts for activation of the human peroxisome proliferator-activated receptor. Die Pharm.-Int. J. Pharm. Sci. 2006, 61, 952–956. [Google Scholar]
- Karimi, G.; Ramezani, M.; Tahoonian, Z. Cisplatin nephrotoxicity and protection by milk thistle extract in rats. Evid. Based Complement. Altern. Med. 2005, 2, 383–386. [Google Scholar] [CrossRef]
- Cura-Esquivel, I.; Delgado-Chávez, E.N.; García-Narro, J.H.; Torres-González, L.; Alarcón-Galván, G.; Moreno-Peña, D.P.; Esquivel-Figueroa, D.; Cantú-Machuca, D.V.; Muñoz-Espinosa, L.E.; Garza-Ocañas, L.; et al. Attenuation of pro-inflammatory cytokines and oxidative stress by misoprostol in renal ischemia/reperfusion in rats. Die Pharm.-Int. J. Pharm. Sci. 2018, 73, 537–540. [Google Scholar] [CrossRef]
- Sánchez-Martínez, C.; Torres-González, L.; Alarcón-Galván, G.; Muñoz-Espinosa, L.E.; Zapata-Chavira, H.A.; Moreno-Peña, D.P.; Náñez-Terreros, H.; Pérez-Rodríguez, E.; Garza-Ocañas, L.; Guzmán-de la Garza, F.J.; et al. Anti-inflammatory and antioxidant activity of essential amino acid α-ketoacid analogues against renal ischemia-reperfusion damage in Wistar rats. Biomédica 2020, 40, 336–348. [Google Scholar] [CrossRef]
- Flemming, N.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grandjean, P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [CrossRef]
- Zhong, D.; Wang, H.; Liu, M.; Li, X.; Huang, M.; Zhou, H.; Lin, S.; Lin, Z.; Yang, B. Ganoderma lucidum polysaccharide peptide prevents renal ischemia reperfusion injury via counteracting oxidative stress. Sci. Rep. 2015, 5, 16910. [Google Scholar] [CrossRef]
- William, P.; López, H.; Britt, D.; Chan, C.; Erzin, A.; Hottendorf, R. Characterization of renal ischemia-reperfusion injury in rats. J. Pharmacol. Toxicol. Methods 1997, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari Godarzi, S.; Valizade Gorji, A.; Gholizadeh, B.; Mard, S.A.; Mansouri, E. Antioxidant effect of p-coumaric acid on interleukin 1-â and tumor necrosis factor-á in rats with renal ischemic reperfusion. Nefrología 2020, 40, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Diwani, G.; Rafie, S.E.; Hawash, S. Antioxidant activity of extracts obtained from residues of nodes leaves stem and root of Egyptian Jatropha curcas. Afr. J. Pharm. Pharmacol. 2009, 3, 521–530. [Google Scholar]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Norma Oficial Mexicana. (6 de Diciembre de 1999). Especificaciones Técnicas para la Producción, Cuidado y uso de los Animales de Laboratorio. [NOM-062-ZOO-1999]. Diario Oficial de la Federación, México. [Official Mexican Standard. Technical Specifications for the Production, Care and Use of Laboratory Animals. NOM-062-ZOO-1999]. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801 (accessed on 18 February 2025).
- Kruger, N.J. The Bradford method for protein quantitation. In The Protein Protocols Handbook; Walker, J.M., Ed.; Springer Protocols Handbooks; Human Press: Totowa, NJ, USA, 2009. [Google Scholar] [CrossRef]
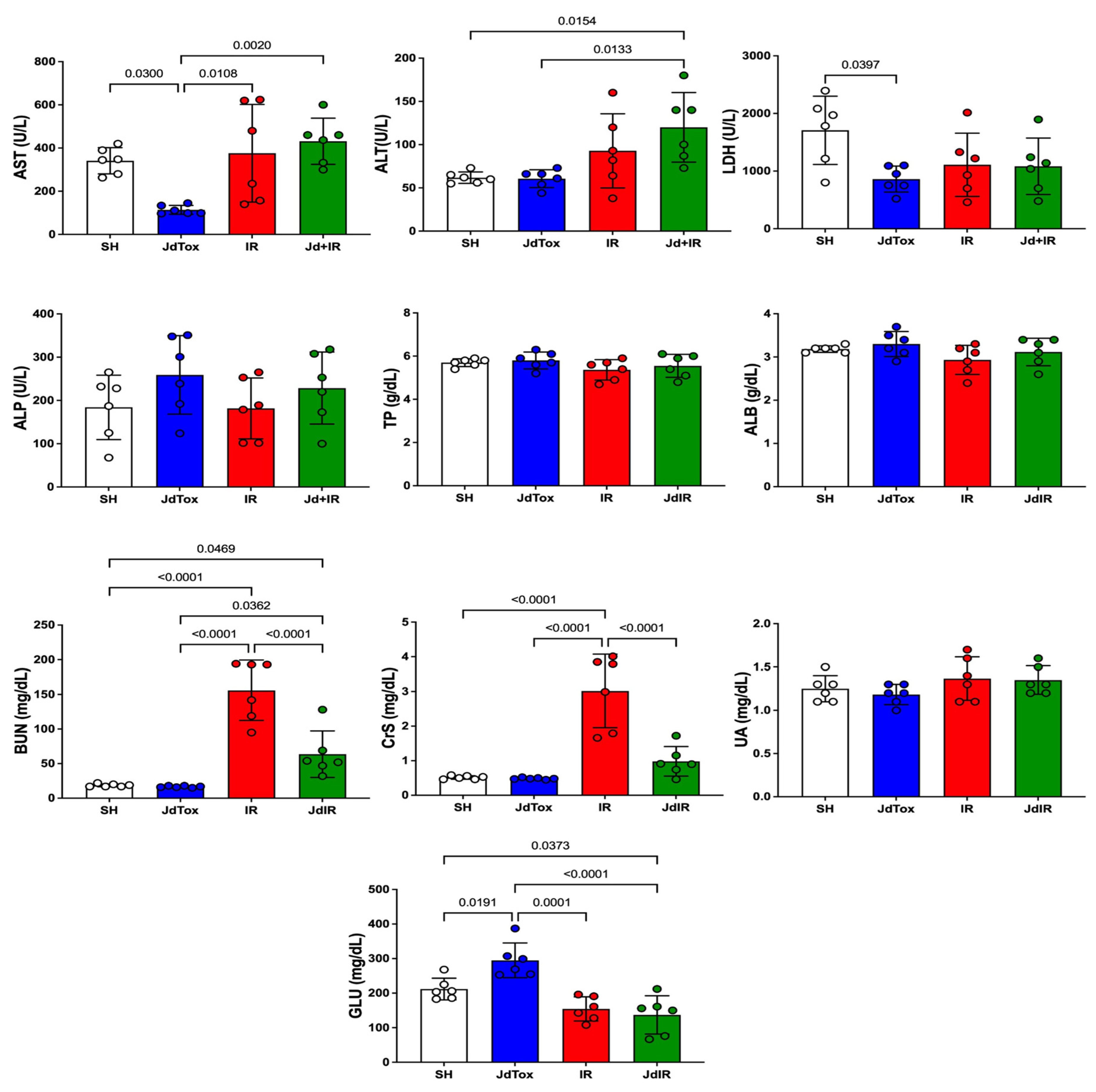
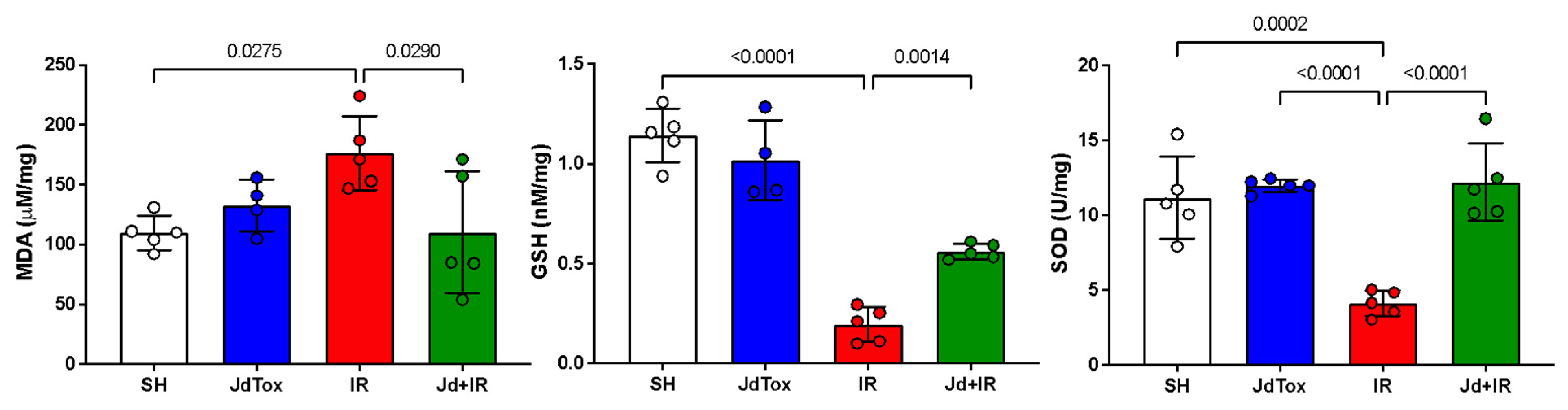
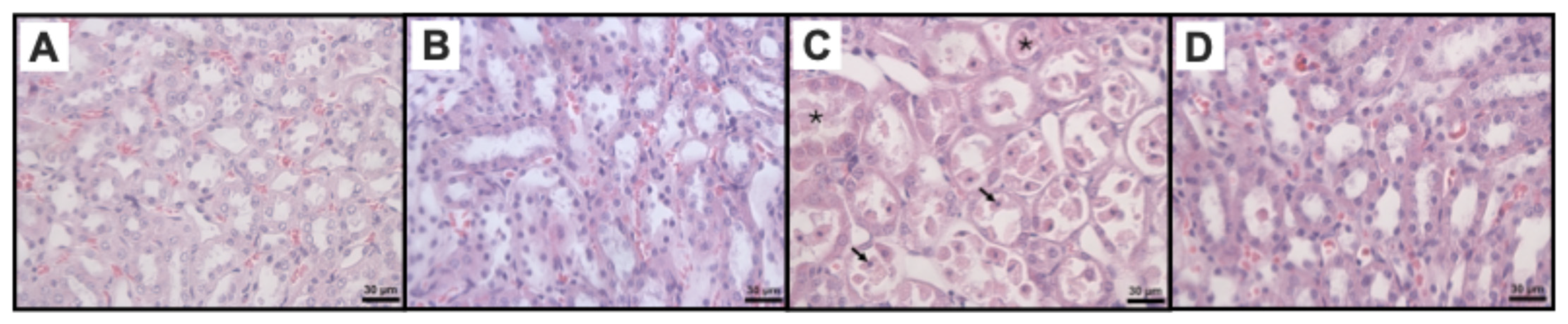
| Histological Markers of Renal Damage | Study Groups | p | |||
|---|---|---|---|---|---|
| SH | JdTox | IR | Jd+IR | ||
| Medullary tubular necrosis | 0 (0–0) a | 0 (0–0.25) a | 4 (3.75–4) | 0 (0–1) a | 0.0003 |
| Medullary protein casts | 0 (0–0.25) a | 0 (0–1) a | 4 (3–4) | 1.5 (1–2.25) | 0.0003 |
| Medullary vascular congestion | 0.5 (0–1) a | 0.5 (0–1) a | 4 (3.75–4) | 3 (2–3) | 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Rodríguez, D.R.; Mendoza-Hernández, O.H.; Cordero-Pérez, P.; Rivas-Galindo, V.M.; Moreno-Peña, D.P.; Tijerina-Márquez, R.; Garza-Villarreal, A.M.; Alarcón-Galván, G.; Muñoz-Espinosa, L.E.; Zapata-Chavira, H.A.; et al. Nephroprotective and Antioxidant Effects of Jatropha dioica Extract Against Ischemia–Reperfusion Injury in Wistar Rats. Int. J. Mol. Sci. 2025, 26, 1838. https://doi.org/10.3390/ijms26051838
Rodríguez-Rodríguez DR, Mendoza-Hernández OH, Cordero-Pérez P, Rivas-Galindo VM, Moreno-Peña DP, Tijerina-Márquez R, Garza-Villarreal AM, Alarcón-Galván G, Muñoz-Espinosa LE, Zapata-Chavira HA, et al. Nephroprotective and Antioxidant Effects of Jatropha dioica Extract Against Ischemia–Reperfusion Injury in Wistar Rats. International Journal of Molecular Sciences. 2025; 26(5):1838. https://doi.org/10.3390/ijms26051838
Chicago/Turabian StyleRodríguez-Rodríguez, Diana Raquel, Oscar Humberto Mendoza-Hernández, Paula Cordero-Pérez, Verónica Mayela Rivas-Galindo, Diana Patricia Moreno-Peña, Ramiro Tijerina-Márquez, Alondra Michelle Garza-Villarreal, Gabriela Alarcón-Galván, Linda Elsa Muñoz-Espinosa, Homero Arturo Zapata-Chavira, and et al. 2025. "Nephroprotective and Antioxidant Effects of Jatropha dioica Extract Against Ischemia–Reperfusion Injury in Wistar Rats" International Journal of Molecular Sciences 26, no. 5: 1838. https://doi.org/10.3390/ijms26051838
APA StyleRodríguez-Rodríguez, D. R., Mendoza-Hernández, O. H., Cordero-Pérez, P., Rivas-Galindo, V. M., Moreno-Peña, D. P., Tijerina-Márquez, R., Garza-Villarreal, A. M., Alarcón-Galván, G., Muñoz-Espinosa, L. E., Zapata-Chavira, H. A., Hernández-Guedea, M. A., Solis-Cruz, G. Y., & Torres-González, L. (2025). Nephroprotective and Antioxidant Effects of Jatropha dioica Extract Against Ischemia–Reperfusion Injury in Wistar Rats. International Journal of Molecular Sciences, 26(5), 1838. https://doi.org/10.3390/ijms26051838






