Association of Genes TRH, PRL and PRLR with Milk Performance, Reproductive Traits and Heat Stress Response in Dairy Cattle
Abstract
:1. Introduction
2. Results
2.1. Polymorphism Screening
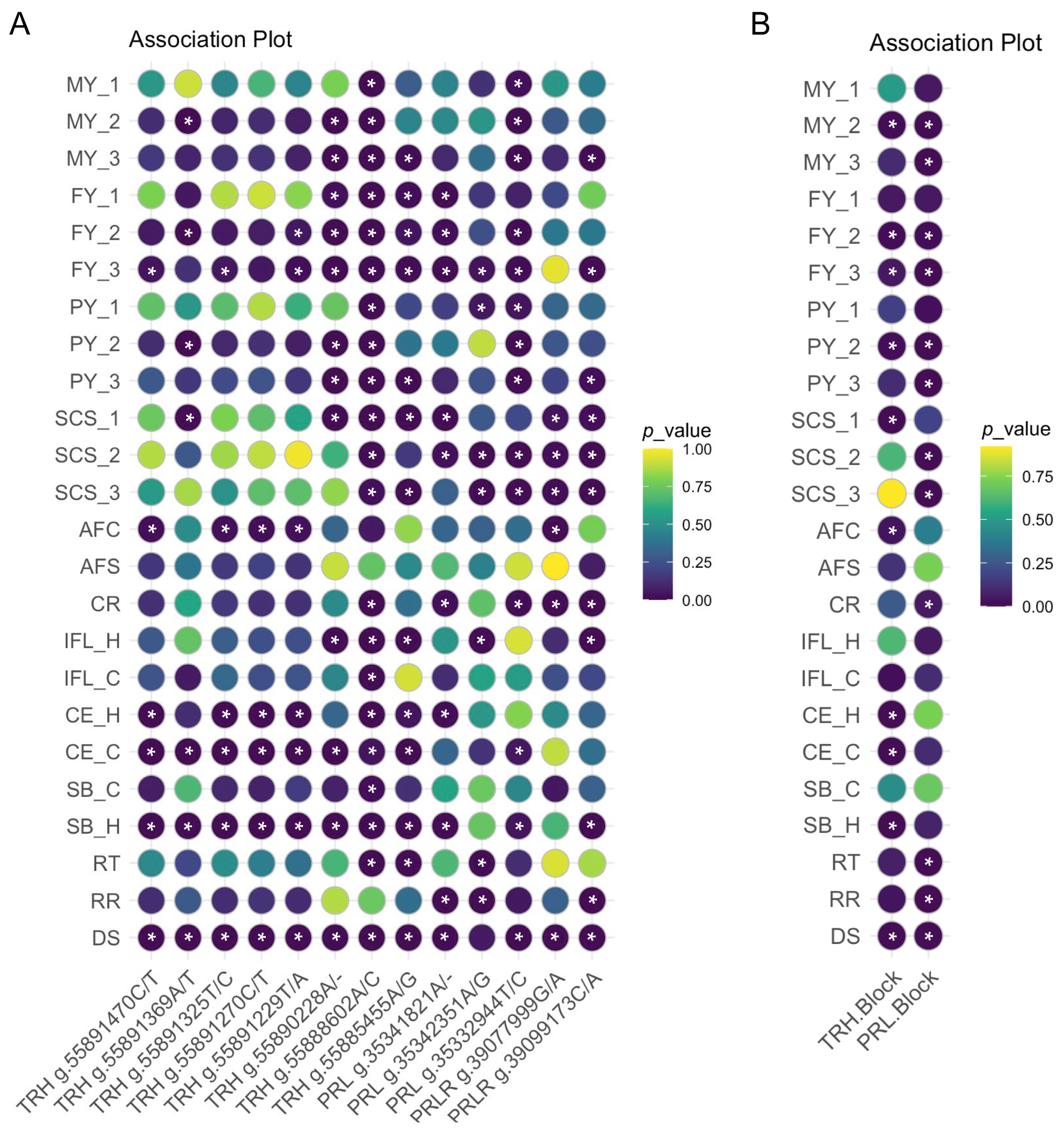
2.2. Association Analyses of SNP and Haplotype Blocks with Milk Performance
2.3. Association Analyses of SNPs with Reproductive Traits
2.4. Association Analyses of SNPs with Heat Stress Response Traits
2.5. TRH, PRL, and PRLR Expressed in Different Tissues and Associated with Multiple Traits
3. Discussion
4. Materials and Methods
4.1. SNP Identification in 70 Bulls
4.2. Genotyping and Phenotyping in 1152 Cows
4.3. Construct Haplotypes Based on LD Structures
4.4. Association Analysis
4.5. Functional Annotation Analysis and Visualization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRL | Prolactin |
| TRH | Thyrotropin releasing hormone |
| PRLR | Prolactin receptor |
| PCR | Polymerase chain reaction |
| KASP | Kompetitive allele-specific PCR |
| LD | Linkage disequilibrium |
| QTL | Quantitative trait locus |
| SNP | Single-nucleotide polymorphism |
| GEBVs | Genomic estimated breeding values |
| MY | Milk yield |
| FY | Fat yield |
| PY | Protein yield |
| SCS | Somatic cells score |
| AFS | Age at the first service |
| AFC | Age at first calving |
| CR | Conception rate |
| IFL_H | Interval from first to last insemination in heifers |
| IFL_C | Interval from first to last insemination in cows |
| SB_H | Stillbirth in heifers |
| SB_C | Stillbirth in cows |
| CE_H | Calving ease in heifers |
| CE_C | Calving ease in cows |
| RT | Rectal temperature |
| RR | Respiratory rate |
| DS | Drooling score |
References
- Walsh, S.W.; Williams, E.J.; Evans, A.C. A review of the causes of poor fertility in high milk producing dairy cows. Anim. Reprod. Sci. 2011, 123, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Polsky, L.; von-Keyserlingk, M.A.G. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef] [PubMed]
- Sammad, A.; Umer, S.; Shi, R.; Zhu, H.B.; Zhao, X.M.; Wang, Y.C. Dairy cow reproduction under the influence of heat stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Schüller, L.K.; Burfeind, O.; Heuwieser, W. Impact of heat stress on conception rate of dairy cows in the moderate climate considering different temperature-humidity index thresholds periods relative to breeding and heat load indices. Theriogenology 2014, 81, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Hagiya, K.; Hayasaka, K.; Yamazaki, T.; Shirai, T.; Osawa, T.; Terawaki, Y.; Nagamine, Y.; Masuda, Y.; Suzuki, M. Effects of heat stress on production, somatic cell score and conception rate in Holsteins. Anim. Sci. J. 2017, 88, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Georges, M.; Nielsen, D.; Mackinnon, M.; Mishra, A.; Okimoto, R.; Pasquino, A.T.; Sargeant, L.S.; Sorensen, A.; Steele, M.R.; Zhao, X. Mapping quantitative trait loci controlling milk production in dairy cattle by exploiting progeny testing. Genetics 1995, 139, 907–920. [Google Scholar] [CrossRef]
- Andersson, L. Genome-wide association analysis in domestic animals: A powerful approach for genetic dissection of trait loci. Genetica 2009, 136, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Van-Eenennaam, A.L.; Weigel, K.A.; Young, A.E.; Cleveland, M.A.; Dekkers, J.C. Applied animal genomics: Results from the field. Annu. Rev. Anim. Biosci. 2014, 2, 105–139. [Google Scholar] [CrossRef] [PubMed]
- de Roos, A.P.; Schrooten, C.; Mullaart, E.; Calus, M.P.; Veerkamp, R.F. Breeding value estimation for fat percentage using dense markers on Bos taurus autosome 14. J. Dairy Sci. 2007, 90, 4821–4829. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [CrossRef]
- Ilie, D.E.; Mizeranschi, A.E.; Mihali, C.V.; Neamț, R.I.; Cziszter, L.T.; Carabaș, M.; Grădinaru, A.C. Polymorphism of the Prolactin (PRL) Gene and Its Effect on Milk Production Traits in Romanian Cattle Breeds. Vet. Sci. 2023, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, A.; Binart, N. Reproductive role of prolactin. Reproduction 2007, 133, 361–369. [Google Scholar] [CrossRef] [PubMed]
- do Amaral, B.C.; Connor, E.E.; Tao, S.; Hayen, M.J.; Bubolz, J.W.; Dahl, G.E. Heat stress abatement during the dry period influences metabolic gene expression and improves immune status in the transition period of dairy cows. J. Dairy Sci. 2011, 94, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.H.; Song, X.M.; Zhang, L.; Jiang, J.F.; Zhou, J.P.; Jiang, Y.Q. New insights into the prolactin-RsaI (PRL-RsaI) locus in Chinese Holstein cows and its effect on milk performance traits. Genet. Mol. Res. 2013, 12, 5766–5773. [Google Scholar] [CrossRef]
- Borba, V.V.; Zandman-Goddard, G.; Shoenfeld, Y. Prolactin and Autoimmunity. Front. Immunol. 2018, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.R.; Kang, L.; Wang, S.H.; Li, W.; Yan, X.Y.; Luo, H.P.; Dong, G.H.; Wang, X.Y.; Wang, Y.C.; Xu, Q. Effects of Cold and Heat Stress on Milk Production Traits and Blood Biochemical Parameters of Holstein Cows in Beijing Area. Sci. Agric. Sin. 2018, 51, 3791–3799. [Google Scholar] [CrossRef]
- Mehmannavaz, Y.; Amirinia, C.; Bonyadi, M.; Torshizi, R.V. Effects of bovine prolactin gene polymorphism within exon 4 on milk-related traits and genetic trends in Iranian Holstein bulls. Afr. J. Biotechnol. 2009, 8, 4797–4801. [Google Scholar]
- Cunningham, F.; Achuthan, P.; Akanni, W.; Allen, J.; Amode, M.R.; Armean, I.M.; Bennett, R.; Bhai, J.; Billis, K.; Boddu, S.; et al. Ensembl 2019. Nucleic Acids Res. 2019, 47, D745–D751. [Google Scholar] [CrossRef] [PubMed]
- Grădinaru, A.C.; Ilie, D.E. Molecular insights into PRL and PRLR genes’ influence on milk obtaining–a review. Anim. Biol. Anim. Husb. 2022, 14, 12–17. [Google Scholar]
- Boleckova, J.; Matejickova, J.; Stipkova, M.; Kyselova, J.; Barton, L. The association of five polymorphisms with milk production traits in Czech Fleckvieh cattle. Czech J. Anim. Sci. 2012, 57, 45–53. [Google Scholar] [CrossRef]
- Li, J.; Liang, A.; Li, Z.; Du, C.; Hua, G.; Salzano, A.; Campanile, G.; Gasparrini, B.; Yang, L. An association analysis between PRL genotype and milk production traits in Italian Mediterranean river buffalo. J. Dairy Res. 2017, 84, 430–433. [Google Scholar] [CrossRef]
- Landa, M.S.; García, S.I.; Schuman, M.L.; Peres Diaz, L.S.; Aisicovich, M.; Pirola, C.J. Cardiovascular and body weight regulation changes in transgenic mice overexpressing thyrotropin-releasing hormone (TRH). J. Physiol. Biochem. 2020, 76, 599–608. [Google Scholar] [CrossRef] [PubMed]
- El-Magd, M.A.; Fathy, A.; Kahilo, K.A.; Saleh, A.A.; El-Sheikh, A.I.; Al-Shami, S.; El-Komy, S.M. Polymorphisms of the PRLR Gene and Their Association with Milk Production Traits in Egyptian Buffaloes. Animals 2021, 11, 1237. [Google Scholar] [CrossRef] [PubMed]
- Huber, E.; Notaro, U.S.; Recce, S.; Rodríguez, F.M.; Ortega, H.H.; Salvetti, N.R.; Rey, F. Fetal programming in dairy cows: Effect of heat stress on progeny fertility and associations with the hypothalamic-pituitary-adrenal axis functions. Anim. Reprod. Sci. 2020, 216, 106348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.K.; Zheng, Y.; Guo, H.X.; Wang, H.Q.; Ji, Z.H.; Wang, T.; Yu, S.; Zhang, J.B.; Yuan, B.; Ren, W.Z. TRH Regulates the Synthesis and Secretion of Prolactin in Rats with Adenohypophysis through the Differential Expression of miR-126a-5p. Int. J. Mol. Sci. 2022, 23, 15914. [Google Scholar] [CrossRef] [PubMed]
- Cabell, S.B.; Esbenshade, K.L. Effect of feeding thyrotropin-releasing hormone to lactating sows. J. Anim. Sci. 1990, 68, 4292–4302. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Wang, P.S.; Chuang, J.; Fan, L.J.; Won, S.J. Cold stress or a pyrogenic substance elevates thyrotropin-releasing hormone levels in the rat hypothalamus and induces thermogenic reactions. Neuroendocrinology 1989, 50, 177–181. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Petricoin, E.F., 3rd; Igarashi, K.; Feldman, G.M.; Finbloom, D.S.; Larner, A.C. Prolactin activates the interferon-regulated p91 transcription factor and the Jak2 kinase by tyrosine phosphorylation. Proc. Natl. Acad. Sci. USA 1994, 91, 7174–7178. [Google Scholar] [CrossRef] [PubMed]
- Steger, R.W.; Chandrashekar, V.; Zhao, W.; Bartke, A.; Horseman, N.D. Neuroendocrine and reproductive functions in male mice with targeted disruption of the prolactin gene. Endocrinology 1998, 139, 3691–3695. [Google Scholar] [CrossRef]
- Auchtung, T.L.; Rius, A.G.; Kendall, P.E.; McFadden, T.B.; Dahl, G.E. Effects of photoperiod during the dry period on prolactin, prolactin receptor, and milk production of dairy cows. J. Dairy Sci. 2005, 88, 121–127. [Google Scholar] [CrossRef]
- Viitala, S.; Szyda, J.; Blott, S.; Schulman, N.; Lidauer, M.; Mäki-Tanila, A.; Georges, M.; Vilkki, J. The role of the bovine growth hormone receptor and prolactin receptor genes in milk, fat and protein production in Finnish Ayrshire dairy cattle. Genetics. 2006, 173, 2151–2164. [Google Scholar] [CrossRef] [PubMed]
- Lü, A.; Hu, X.; Chen, H.; Dong, Y.; Pang, Y. Single nucleotide polymorphisms of the prolactin receptor (PRLR) gene and its association with growth traits in Chinese cattle. Mol. Biol. Rep. 2011, 38, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Fattah, E.M.; Behour, T.S.; Ashour, A.F.; Amin, A.M.S. Association analysis of prolactin and prolactin receptor genes with selected productive and reproductive traits in Egyptian buffalo. Anim. Biotechnol. 2023, 34, 1397–1405. [Google Scholar] [CrossRef]
- Cosenza, G.; Iannaccone, M.; Auzino, B.; Macciotta, N.P.P.; Kovitvadhi, A.; Nicolae, I.; Pauciullo, A. Remarkable genetic diversity detected at river buffalo prolactin receptor (PRLR) gene and association studies with milk fatty acid composition. Anim. Genet. 2018, 49, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, S.; Sargolzaei, M.; Abo-Ismail, M.K.; Miller, S.; Schenkel, F.; Moore, S.S.; Stothard, P. Genome-wide association study for lactation persistency, female fertility, longevity, and lifetime profit index traits in Holstein dairy cattle. J. Dairy Sci. 2017, 100, 1246–1258. [Google Scholar] [CrossRef]
- Luo, H.; Hu, L.; Brito, L.F.; Dou, J.; Sammad, A.; Chang, Y.; Ma, L.; Guo, G.; Liu, L.; Zhai, L.; et al. Weighted single-step GWAS and RNA sequencing reveals key candidate genes associated with physiological indicators of heat stress in Holstein cattle. J. Anim. Sci. Biotechnol. 2022, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Lund, M.S.; Wang, Y.; Guo, G.; Dong, G.; Madsen, P.; Su, G. Variance components and correlations of female fertility traits in Chinese Holstein population. J Anim Sci Biotechnol. 2017, 8, 56. [Google Scholar] [CrossRef]
- Chen, Z.; Brito, L.F.; Luo, H.; Shi, R.; Chang, Y.; Liu, L.; Guo, G.; Wang, Y. Genetic and Genomic Analyses of Service Sire Effect on Female Reproductive Traits in Holstein Cattle. Front Genet. 2021, 12, 713575. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Li, T.; Liu, D.; Wang, S.; Wang, S.; Wang, Q.; Pan, Y.; Zan, L.; Ma, P. Estimation of genetic parameters for fertility traits in Chinese Holstein of south China. Front Genet. 2024, 14, 1288375. [Google Scholar] [CrossRef]
- Luo, H.; Brito, L.F.; Li, X.; Su, G.; Dou, J.; Xu, W.; Yan, X.; Zhang, H.; Guo, G.; Liu, L.; et al. Genetic parameters for rectal temperature, respiration rate, and drooling score in Holstein cattle and their relationships with various fertility, production, body conformation, and health traits. J Dairy Sci. 2021, 104, 4390–4403. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Hill, W.G.; Mackay, T.F.D.S. Falconer and Introduction to quantitative genetics. Genetics 2004, 167, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.K.; Jain, M. NGS QC Toolkit: A toolkit for quality control of next generation sequencing data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
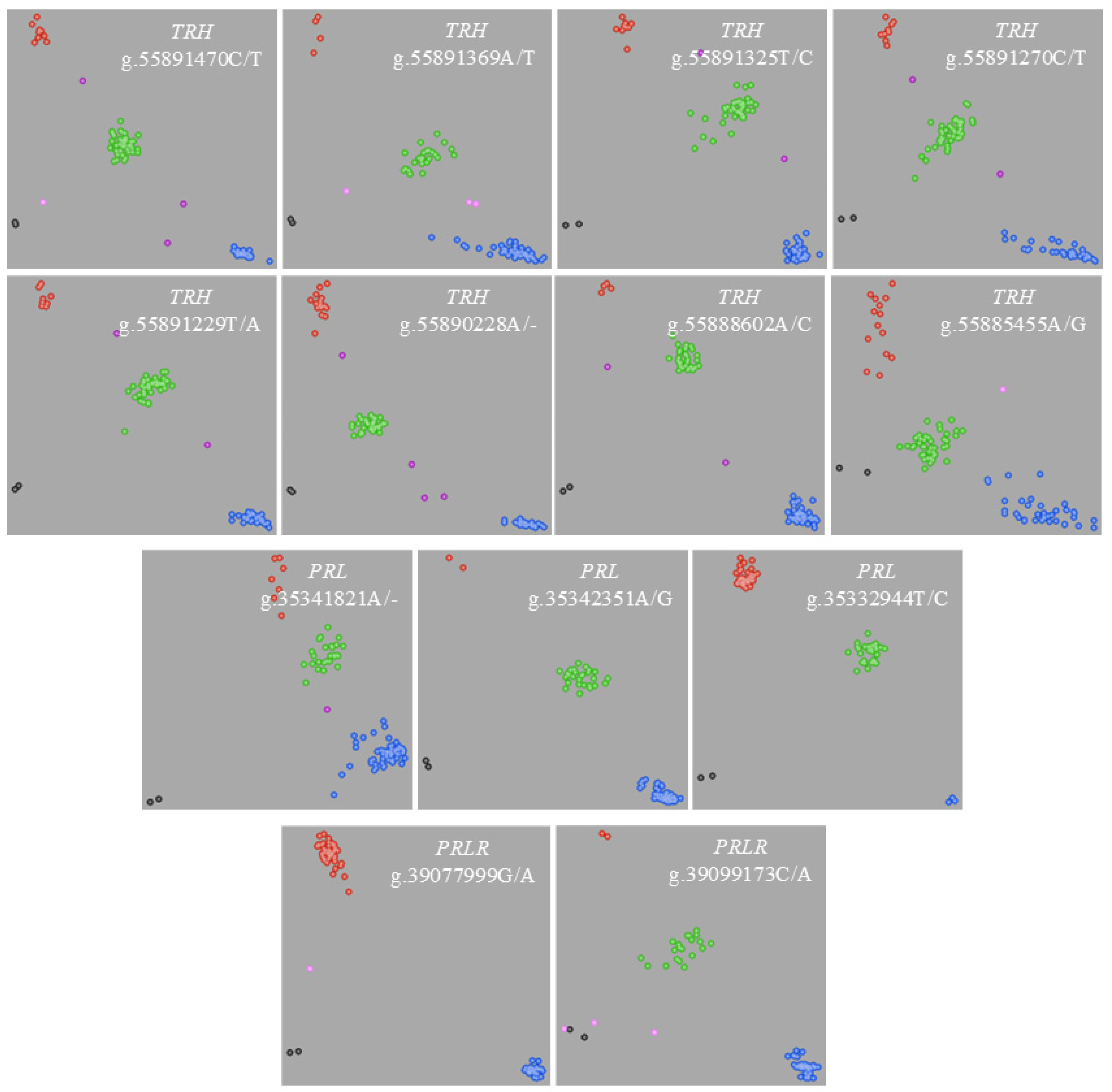
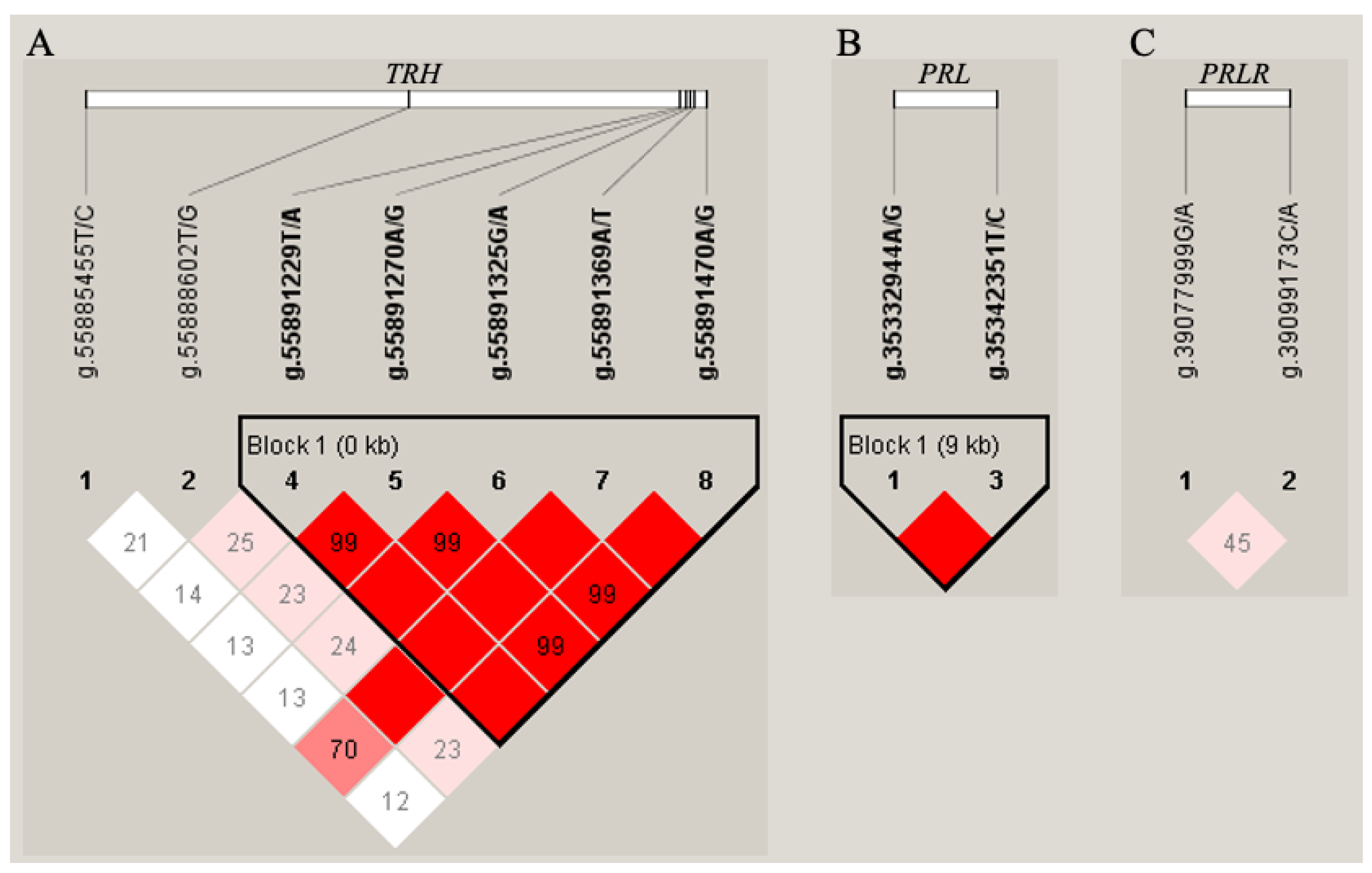


| Gene | SNP | Genotype | Number of Individuals | Genotypic Frequency | Allele | Allelic Frequency | p Value, (df = 2) 1,2 |
|---|---|---|---|---|---|---|---|
| TRH | g.55891470C/T | C:C | 174 | 0.154 | C | 0.383 | 0.615 |
| C:T | 519 | 0.459 | T | 0.617 | |||
| T:T | 438 | 0.387 | |||||
| TRH | g.55891369A/T | T:T | 785 | 0.703 | T | 0.843 | 0.161 |
| A:T | 312 | 0.280 | A | 0.157 | |||
| A:A | 19 | 0.017 | |||||
| TRH | g.55891325T/C | T:T | 172 | 0.153 | T | 0.381 | 0.576 |
| T:C | 515 | 0.457 | C | 0.619 | |||
| C:C | 440 | 0.390 | |||||
| TRH | g.55891270C/T | C:C | 172 | 0.153 | C | 0.382 | 0.664 |
| C:T | 518 | 0.460 | T | 0.618 | |||
| T:T | 437 | 0.388 | |||||
| TRH | g.55891229T/A | T:T | 170 | 0.151 | T | 0.381 | 0.720 |
| T:A | 518 | 0.460 | A | 0.619 | |||
| A:A | 437 | 0.388 | |||||
| TRH | g.55890228A/- | A:A | 118 | 0.108 | A | 0.327 | 0.972 |
| A:- | 476 | 0.437 | - | 0.673 | |||
| -:- | 496 | 0.455 | |||||
| TRH | g.55888602A/C | A:A | 753 | 0.662 | A | 0.811 | 0.680 |
| C:A | 339 | 0.298 | C | 0.189 | |||
| C:C | 45 | 0.040 | |||||
| TRH | g.55885455A/G | A:A | 424 | 0.378 | A | 0.621 | 0.540 |
| G:A | 545 | 0.486 | G | 0.379 | |||
| G:G | 152 | 0.136 | |||||
| PRL | g.35341821A/- | A:A | 103 | 0.092 | A | 0.255 | 0.000 |
| A:- | 367 | 0.327 | - | 0.745 | |||
| -:- | 653 | 0.581 | |||||
| PRL | g.35342351A/G | A:A | 900 | 0.790 | A | 0.888 | 0.739 |
| G:A | 222 | 0.195 | G | 0.112 | |||
| G:G | 17 | 0.015 | |||||
| PRL | g.35332944T/C | C:C | 673 | 0.591 | C | 0.772 | 0.760 |
| C:T | 410 | 0.360 | T | 0.228 | |||
| T:T | 55 | 0.048 | |||||
| PRLR | g.39077999G/A | A:A | 725 | 0.635 | A | 0.644 | 0.000 |
| A:G | 22 | 0.019 | G | 0.356 | |||
| G:G | 395 | 0.346 | |||||
| PRLR | g.39099173C/A | A:A | 35 | 0.031 | A | 0.145 | 0.036 |
| A:C | 262 | 0.230 | C | 0.855 | |||
| C:C | 844 | 0.740 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Q.; Zhang, H.; Gao, Q.; Hu, L.; Zhang, F.; Xu, Q.; Wang, Y. Association of Genes TRH, PRL and PRLR with Milk Performance, Reproductive Traits and Heat Stress Response in Dairy Cattle. Int. J. Mol. Sci. 2025, 26, 1963. https://doi.org/10.3390/ijms26051963
Fang Q, Zhang H, Gao Q, Hu L, Zhang F, Xu Q, Wang Y. Association of Genes TRH, PRL and PRLR with Milk Performance, Reproductive Traits and Heat Stress Response in Dairy Cattle. International Journal of Molecular Sciences. 2025; 26(5):1963. https://doi.org/10.3390/ijms26051963
Chicago/Turabian StyleFang, Qianhai, Hailiang Zhang, Qing Gao, Lirong Hu, Fan Zhang, Qing Xu, and Yachun Wang. 2025. "Association of Genes TRH, PRL and PRLR with Milk Performance, Reproductive Traits and Heat Stress Response in Dairy Cattle" International Journal of Molecular Sciences 26, no. 5: 1963. https://doi.org/10.3390/ijms26051963
APA StyleFang, Q., Zhang, H., Gao, Q., Hu, L., Zhang, F., Xu, Q., & Wang, Y. (2025). Association of Genes TRH, PRL and PRLR with Milk Performance, Reproductive Traits and Heat Stress Response in Dairy Cattle. International Journal of Molecular Sciences, 26(5), 1963. https://doi.org/10.3390/ijms26051963








