The Role of Adenogenesis Factors in the Pathogenesis of Endometriosis
Abstract
1. Introduction
2. Methods
3. Pathogenesis of Endometriosis: Mechanisms and Theories
| Theory | Proposed Mechanism | References |
|---|---|---|
| Retrograde menstruation | Retrograde menstrual flow allows the implantation of endometrial tissue in the peritoneal cavity | [19] |
| Coelomic metaplasia | Structures derived from the coelomic epithelium may undergo transformation into endometriotic tissue via metaplasia | [25] |
| Vascular dissemination | Endometrial cells spread through blood or lymphatic vessels, colonizing distant anatomical sites | [21] |
| Stem cell involvement | Endometrial or hematopoietic stem cells may differentiate into endometriotic lesions in ectopic locations | [28,29] |
| Embryonic remnants | Residual embryonic cells from Wolffian or Müllerian ducts may develop into endometriotic lesions under estrogen stimulation | [31,32,33,34,35] |
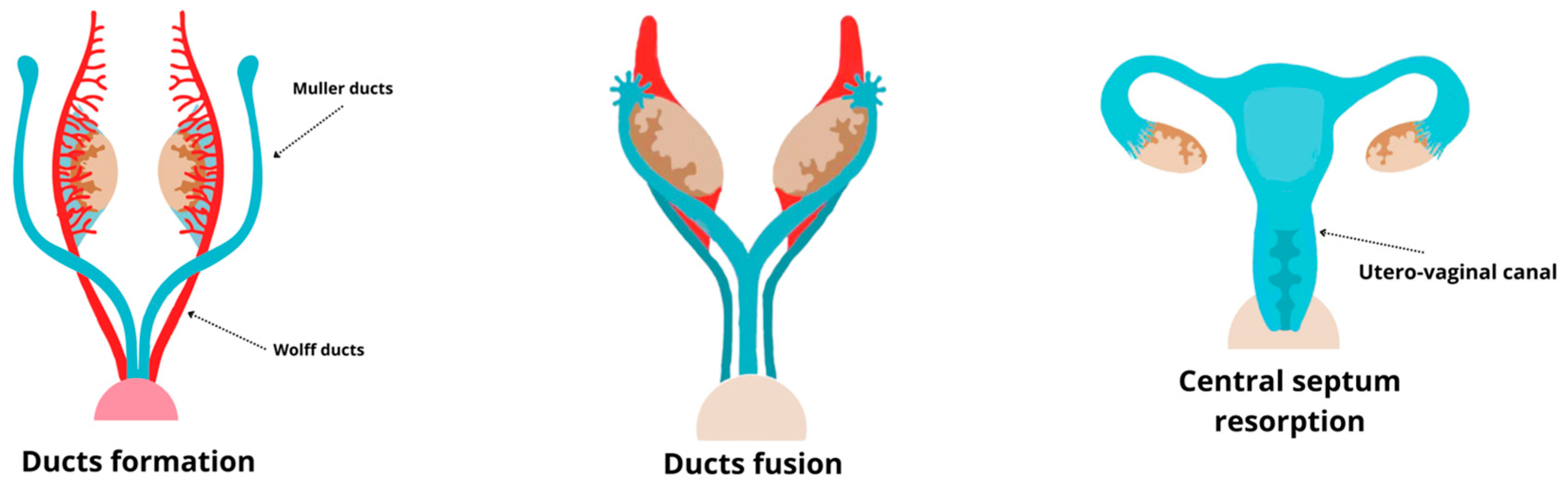
4. Development of the Female Reproductive System
5. Uterine Adenogenesis
6. Expression Patterns of Different Adenogenesis Factors in Endometriosis and Normal Endometrium
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, J.; Wang, Y.; Chen, G. Causal Relationship between Endometriosis, Female Infertility, and Primary Ovarian Failure through Bidirectional Mendelian Randomization. Int. J. Women’s Health 2024, 16, 2143–2155. [Google Scholar] [CrossRef] [PubMed]
- Signorile, P.G.; Cassano, M.; Viceconte, R.; Marcattilj, V.; Baldi, A. Endometriosis: A Retrospective Analysis of Clinical Data from a Cohort of 4,083 Patients, with Focus on Symptoms. In Vivo 2022, 36, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; Quagliuolo, L.; Verde, A.; La Porta, R.; Crispi, S.; Piccolo, M.T.; Vitiello, A.; Baldi, A.; Signorile, P.G. In Vitro Model of Stromal and Epithelial Immortalized Endometriotic Cells. J. Cell Biochem. 2012, 113, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Veth, V.B.; Keukens, A.; Reijs, A.; Bongers, M.Y.; Mijatovic, V.; Coppus, S.F.P.J.; Maas, J.W.M. Recurrence after Surgery for Endometrioma: A Systematic Review and Meta-Analysis. Fertil. Steril. 2024, 122, 1079–1093. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, S.; Barbieri, R.L. The Gold Standard for the Surgical Diagnosis of Endometriosis: Visual Findings or Biopsy Results? J. Gynecol. Technol. 1998, 4, 121–124. [Google Scholar]
- Signorile, P.G.; Baldi, A. Looking for an effective and non-invasive diagnostic test for endometriosis: Where are we? Ann. Transl. Med. 2018, 6 (Suppl. S2), S106. [Google Scholar] [CrossRef]
- Missmer, S.A.; Hankinson, S.E.; Spiegelman, D.; Barbieri, R.L.; Marshall, L.M.; Hunter, D.J. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am. J. Epidemiol. 2004, 160, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Alkatout, I.; Mettler, L.; Beteta, C.; Hedderich, J.; Jonat, W.; Schollmeyer, T.; Salmassi, A. Combined surgical and hormone therapy for endometriosis is the most effective treatment: Prospective, randomized, controlled trial. J. Minim. Invasive Gynecol. 2013, 20, 473–481. [Google Scholar] [CrossRef]
- Vercellini, P.; Vigano, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gou, Y.; Zhang, H.; Zuo, H.; Zhang, H.; Liu, Z.; Yao, D. Estradiol improves cardiovascular function through up-regulation of SOD2 on vascular wall. Redox Biol. 2014, 3, 88–99. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogenic control of mitochondrial function. Redox Biol. 2020, 31, 101435. [Google Scholar] [CrossRef] [PubMed]
- Su, E.J.; Lin, Z.H.; Zeine, R.; Yin, P.; Reierstad, S.; Innes, J.E.; Bulun, S.E. Estrogen receptor-beta mediates cyclooxygenase-2 expression and vascular prostanoid levels in human placental villous endothelial cells. Am. J. Obstet. Gynecol. 2009, 200, 427.e1–427.e8. [Google Scholar] [CrossRef] [PubMed]
- Hudelist, G.; Keckstein, J.; Czerwenka, K.; Lass, H.; Walter, I.; Auer, M.; Wieser, F.; Wenzl, R.; Kubista, E.; Singer, C.F. Estrogen receptor beta and matrix metalloproteinase 1 are coexpressed in uterine endometrium and endometriotic lesions of patients with endometriosis. Fertil. Steril. 2005, 84 (Suppl. S2), 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Signorile, P.G.; Baldi, A. New evidence in endometriosis. Int. J. Biochem. Cell Biol. 2015, 60, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Ballard, K.D.; Seaman, H.E.; De Vries, C.S.; Wright, J.T. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case–control study—Part 1. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1382–1391. [Google Scholar] [CrossRef]
- Pugsley, Z.; Ballard, K. Management of endometriosis in general practice: The pathway to diagnosis. Br. J. Gen. Pract. 2007, 57, 470–476. [Google Scholar]
- De Corte, P.; Klinghardt, M.; von Stockum, S.; Heinemann, K. Time to diagnose endometriosis: Current status, challenges and regional characteristics—A systematic literature review. BJOG Int. J. Obstet. Gynaecol. 2025, 132, 118–130. [Google Scholar] [CrossRef]
- Signorile, P.G.; Dominici, S.; Viceconte, R.; Baldi, A. Prototype salivary assay for quantification of two biomarkers for in vitro diagnosis of endometriosis. Crit. Rev. Eukaryot. Gene Exp. 2024, 34, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Signorile, P.G.; Cassano, M.; Viceconte, R.; Spyrou, M.; Marcattilj, V.; Baldi, A. Endometriosis: A retrospective analysis on diagnostic data in a cohort of 4401 patients. In Vivo 2022, 36, 430–438. [Google Scholar] [CrossRef]
- Robboy, S.J.; Bean, S.M. Pathogenesis of endometriosis. Reprod. Biomed. Online 2010, 21, 4–5. [Google Scholar] [CrossRef]
- Wang, Y.; Nicholes, K.; Shi, I.M. The origin and pathogenesis of endometriosis. Annu. Rev. Pathol. 2020, 15, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Dun, E.C.; Khom, K.A.; Morozov, V.V.; Kearney, S.; Zurawin, J.L.; Nezhat, C.H. Endometriosis in Adolescents. In Endometriosis in Adolescents; Nezhat, C.H., Ed.; Springer Nature: Berlin, Germany, 2020; pp. 129–141. [Google Scholar]
- Ricciardi, E.; Laganà, A.S.; Triolo, O.; Caserta, D. Epigenetic modifications of primordial reproductive tract: A common etiologic pathway for Mayer-Rokitansky-Kuster-Hauser Syndrome and endometriosis? Med. Hypotheses 2016, 90, 4–5. [Google Scholar]
- Gruenwald, P. Origin of endometriosis from the mesenchyme of the celomic walls. Am. J. Obstet. Gynecol. 1942, 44, 470–474. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Prime 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Salamonsen, L.A.; Hutchison, J.C.; Gargett, C.E. Cyclical endometrial repair and regeneration. Development 2021, 148, dev199577. [Google Scholar] [CrossRef]
- Parasar, P.; Sacha, C.R.; Ng, N.; McGuirk, E.R.; Chinthala, S.; Ozcan, P.; Lindsey, J.; Salas, S.; Laufer, M.R.; Missmer, S.A.; et al. Differentiating mouse embryonic stem cells express markers of human endometrium. Reprod. Biol. Endocrinol. 2017, 15, 52. [Google Scholar] [CrossRef]
- Figueira, P.G.; Abrao, M.S.; Krikun, G.; Taylor, H.S. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann. N. Y. Acad. Sci. 2011, 1221, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Klattig, J.; Englert, C. The mullerian duct: Recent insight into its development and regression. Sex. Dev. 2007, 1, 271–278. [Google Scholar] [CrossRef]
- Signorile, P.G.; Baldi, F.; Bussani, R.; D’Armiento, M.; De Falco, M.; Baldi, A. Ectopic endometrium in human foetuses is a common event and sustains the theory of müllerianosis in the pathogenesis of endometriosis, a disease that predisposes to cancer. J. Exp. Clin. Cancer Res. 2009, 28, 49. [Google Scholar] [CrossRef]
- Signorile, P.G.; Baldi, F.; Bussani, R.; D’Armiento, M.; De Falco, M.; Boccellino, M.; Quagliuolo, L.; Baldi, A. New evidence of the presence of endometriosis in the human fetus. Reprod. Biomed. Online 2010, 21, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Signorile, P.G.; Baldi, F.; Bussani, R.; Viceconte, R.; Bulzomi, P.; D’Armiento, M.; D’Avino, A.; Baldi, A. Embryologic origin of endometriosis: Analysis of 101 human female fetuses. J. Cell Physiol. 2012, 227, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Signorile, P.G.; Viceconte, R.; Baldi, A. New insights in pathogenesis of endometriosis. Front. Med. 2022, 9, 879015. [Google Scholar] [CrossRef] [PubMed]
- Bouquet de Jolinière, J.; Ayoubi, J.M.; Lesec, G.; Validire, P.; Goguin, A.; Gianaroli, L.; Dubuisson, J.B.; Feki, A.; Gogusev, J. Identification of displaced endometrial glands and embryonic duct remnants in female fetal reproductive tract: Possible pathogenetic role in endometriotic and pelvic neoplastic processes. Front. Physiol. 2012, 3, 35948. [Google Scholar] [CrossRef]
- Schuster, M.; Mackeen, D.A. Fetal endometriosis: A case report. Fertil. Steril. 2015, 103, 160–162. [Google Scholar] [CrossRef]
- Bhamidipaty-Pelosi, S.; Kyei-Barffour, I.; Volpert, M.; O’Neill, N.; Grimshaw, A.; Eriksson, L.; Vash-Margita, A.; Pelosi, E. Mullerian anomalies and endometriosis: Associations and phenotypic variations. Reprod. Biol. Endocrinol. 2024, 22, 157. [Google Scholar] [CrossRef]
- Pitot, M.A.; Bookwalter, C.A.; Dudiak, K.M. Müllerian duct anomalies coincident with endometriosis: A review. Abdom. Radiol. 2020, 45, 1723–1740. [Google Scholar] [CrossRef] [PubMed]
- Crispi, S.; Piccolo, M.T.; D’Avino, A.; Donizetti, A.; Viceconte, R.; Spyrou, M.; Calogero, R.A.; Baldi, A.; Signorile, P.G. Transcriptional profiling of endometriosis tissues identifies genes related to organogenesis defects. J. Cell Physiol. 2013, 228, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Signorile, P.G.; Severino, A.; Santoro, M.; Spyrou, M.; Viceconte, R.; Baldi, A. Methylation analysis of HOXA10 regulatory elements in patients with endometriosis. BMC Res. Notes 2018, 11, 722. [Google Scholar] [CrossRef]
- Newbold, R.R.; Jefferson, W.J.; Padilla-Banks, E. Prenatal exposure to bisphenol A at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ. Health Perspect. 2009, 117, 879–885. [Google Scholar] [CrossRef]
- Signorile, P.G.; Spugnini, E.P.; Mita, L.; Mellone, P.; D’Avino, A.; Bianco, M.; Diano, N.; Caputo, L.; Rea, F.; Viceconte, R.; et al. Prenatal exposure of mice to bisphenol A elicits an endometriosis-like phenotype in female offspring. Gen. Comp. Endocrinol. 2010, 168, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Signorile, P.G.; Spugnini, E.P.; Citro, G.; Viceconte, R.; Vincenzi, B.; Baldi, F.; Baldi, A. Endocrine disruptors in utero cause ovarian damages linked to endometriosis. Front. Biosci. 2012, 4, 1724–1730. [Google Scholar] [CrossRef][Green Version]
- Cunha, G.R.; Robboy, S.J.; Kurita, T.; Isaacson, D.; Shen, J.; Cao, M.; Baskin, L.S. Development of the human female reproductive tract. Differentiation 2018, 103, 46–65. [Google Scholar] [CrossRef]
- Chandler, T.M.; Machan, L.S.; Cooperberg, P.L.; Harris, A.C.; Chang, S.D. Mullerian duct anomalies: From diagnosis to intervention. Br. J. Radiol. 2009, 82, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Georgas, K.M.; Armstrong, J.; Keast, J.R.; Larkins, C.E.; McHugh, K.M.; Southard-Smith, E.M.; Cohn, M.J.; Batourina, E.; Dan, H.; Schneider, K.; et al. An illustrated anatomical ontology of the developing mouse lower urogenital tract. Development 2015, 142, 1893–1908. [Google Scholar] [CrossRef]
- Huang, C.-C.; Orvis, G.D.; Kwan, K.M.; Behringer, R.R. Lhx1 is required in Müllerian duct epithelium for uterine development. Dev. Biol. 2014, 389, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhou, J.; Li, R.; Dudley, E.A.; Ye, X. Novel function of LHFPL2 in female and male distal reproductive tract development. Sci. Rep. 2016, 6, 23037. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Bordoni, B. Embryology, Müllerian Ducts (Paramesonephric Ducts). StatPearls [Internet]. 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557727/ (accessed on 13 February 2025).
- Santana Gonzalez, L.; Rota, I.A.; Artibani, M.; Morotti, M.; Hu, Z.; Wietek, N.; Alsaadi, A.; Albukhari, A.; Sauka-Spengler, T.; Ahmed, A.A. Mechanistic Drivers of Müllerian Duct Development and Differentiation into the Oviduct. Front. Cell Dev. Biol. 2021, 9, 605301. [Google Scholar] [CrossRef] [PubMed]
- Venkata, V.D.; Jamaluddin, M.F.B.; Goad, J.; Drury, H.R.; Tadros, M.A.; Lim, R.; Karakoti, A.; O’Sullivan, R.; Ius, Y.; Jaaback, K.; et al. Development and characterization of human fetal female reproductive tract organoids to understand Müllerian duct anomalies. Proc. Natl. Acad. Sci. USA 2022, 119, e2118054119. [Google Scholar] [CrossRef] [PubMed]
- Robboy, S.J.; Kurita, T.; Baskin, L.; Cunha, G.R. New insights into human female reproductive tract development. Differentiation 2017, 97, 9–22. [Google Scholar] [CrossRef]
- Cunha, G.R.; Kurita, T.; Cao, M.; Shen, J.; Robboy, S.J.; Baskin, L. Response of xenografts of developing human female reproductive tracts to the synthetic estrogen, diethylstilbestrol. Differentiation 2017, 98, 35–54. [Google Scholar] [CrossRef]
- Acién, P.; Acién, M. Malformations of the female genital tract and embryological bases. Curr. Women’s Health Rev. 2007, 3, 248–288. [Google Scholar] [CrossRef]
- Greenfeld, H.; Lin, J.; Mullins, M.C. The BMP signaling gradient is interpreted through concentration thresholds in dorsal–ventral axial patterning. PLoS Biol. 2021, 19, e3001059. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.W.; Rizwan, M.; Yim, E.K.F. Emerging methods for enhancing pluripotent stem cell expansion. Front. Cell Dev. Biol. 2020, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Bier, E.; De Robertis, E.M. BMP gradients: A paradigm for morphogen-mediated developmental patterning. Science 2015, 348, aaa5838. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.C.O.; Zambuzzi, W.F.; Rossi, M.C.; Filho, R.A.; Sogayar, M.C.; Granjeiro, J.M. Bone Morphogenetic Proteins. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2015; pp. 293–322. [Google Scholar]
- Kossack, M.E.; High, S.K.; Hopton, R.E.; Yan, Y.L.; Postlethwait, J.H.; Draper, B.W. Female sex development and reproductive duct formation depend on Wnt4a in zebrafish. Genetics 2019, 211, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Méar, L.; Herr, M.; Fauconnier, A.; Pineau, C.; Vialard, F. Polymorphisms and endometriosis: A systematic review and meta-analyses. Hum. Reprod. Update 2020, 26, 73–102. [Google Scholar] [CrossRef]
- Edwards, T.L.; Giri, A.; Hellwege, J.N.; Hartmann, K.E.; Stewart, E.A.; Jeff, J.M.; Bray, M.J.; Pendergrass, S.A.; Torstenson, E.S.; Keaton, J.M.; et al. A trans-ethnic genome-wide association study of uterine fibroids. Front. Genet. 2019, 10, 511. [Google Scholar] [CrossRef]
- Gallagher, C.S.; Mäkinen, N.; Harris, H.R.; Rahmioglu, N.; Uimari, O.; Cook, J.P.; Shigesi, N.; Ferreira, T.; Velez-Edwards, D.R.; Edwards, T.L.; et al. Genome-wide association and epidemiological analyses reveal common genetic origins between uterine leiomyomata and endometriosis. Nat. Commun. 2019, 10, 4857. [Google Scholar] [CrossRef]
- Painter, J.N.; Anderson, C.A.; Nyholt, D.R.; Macgregor, S.; Lin, J.; Lee, S.H.; Lambert, A.; Zhao, Z.Z.; Roseman, F.; Guo, Q.; et al. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat. Genet. 2011, 43, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Feenstra, B.; Bacelis, J.; Liu, X.; Muglia, L.M.; Juodakis, J.; Miller, D.E.; Litterman, N.; Jiang, P.P.; Russell, L.; et al. Genetic associations with gestational duration and spontaneous preterm birth. N. Engl. J. Med. 2017, 377, 1156–1167. [Google Scholar] [CrossRef]
- Pitzer, L.M.; Moroney, M.R.; Nokoff, N.J.; Sikora, M.J. WNT4 balances development vs disease in gynecologic tissues and women’s health. Endocrinology 2021, 162, bqab093. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.A.; Taylor, K.M.; Ramsey, W.S.; Hill, J.R.; Bazer, F.W.; Bartol, F.F.; Spencer, T.E. Endometrial glands are required for preimplantation conceptus elongation and survival. Biol. Reprod. 2001, 64, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.A.; Burghardt, R.C.; Johnson, G.A.; Bazer, F.W.; Spencer, T.E. Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 2002, 124, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.E.; Dunlap, K.A.; Filant, J. Comparative developmental biology of the uterus: Insights into mechanisms and developmental disruption. Mol. Cell Endocrinol. 2012, 354, 34–53. [Google Scholar] [CrossRef]
- Cooke, P.S.; Spencer, T.E.; Bartol, F.F.; Hayashi, K. Uterine glands: Development, function and experimental model systems. Mol. Hum. Reprod. 2013, 19, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, P.; Fitzgerald, H.C.; Kelleher, A.M.; Liu, H.; Spencer, T.E. Uterine glands impact embryo survival and stromal cell decidualization in mice. FASEB J. 2021, 35, e21938. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, I.A.; Brosens, J.J. Decidualization of the human endometrium: Mechanisms, functions, and clinical perspectives. Semin. Reprod. Med. 2007, 25, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.L.; Kaspar, P.; Brunet, L.J.; Bhatt, H.; Gadi, I.; Köntgen, F.; Abbondanzo, S.J. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 1992, 359, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Salleh, N.; Giribabu, N. Leukemia inhibitory factor: Roles in embryo implantation and in nonhormonal contraception. Sci. World J. 2014, 2014, 201514. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, S.; Hiraoka, T.; Matsuo, M.; Fukui, Y.; Fujita, H.; Saito-Fujita, T.; Shimizu-Hirota, R.; Takeda, N.; Hiratsuka, D.; He, X.; et al. Spatiotemporal functions of leukemia inhibitory factor in embryo attachment and implantation chamber formation. Cell Death Discov. 2024, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Aghajanova, L. Leukemia inhibitory factor and human embryo implantation. Ann. N. Y. Acad. Sci. 2004, 1034, 176–183. [Google Scholar] [CrossRef]
- Kelleher, A.M.; Milano-Foster, J.; Behura, S.K.; Spencer, T.E. Uterine glands coordinate on-time embryo implantation and impact endometrial decidualization for pregnancy success. Nat. Commun. 2018, 9, 2435. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, A.M.; Peng, W.; Pru, J.K.; Pru, C.A.; DeMayo, F.J.; Spencer, T.E. Forkhead box a2 (FOXA2) is essential for uterine function and fertility. Proc. Natl. Acad. Sci. USA 2017, 114, E1018–E1026. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Kwak, I.; Lee, K.Y.; Kim, T.H.; Large, M.J.; Stewart, C.L.; Kaestner, K.H.; Lydon, J.P.; DeMayo, F.J. Foxa2 is essential for mouse endometrial gland development and fertility. Biol. Reprod. 2010, 83, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Dey, S.K. Hunting for Fox(A2): Dual roles in female fertility. Proc. Natl. Acad. Sci. USA 2017, 114, 1226–1228. [Google Scholar] [CrossRef]
- Franco, H.L.; Dai, D.; Lee, K.Y.; Rubel, C.A.; Roop, D.; Boerboom, D.; Jeong, J.W.; Lydon, J.P.; Bagchi, I.C.; Bagchi, M.K.; et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011, 25, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, K.A.; Filant, J.; Hayashi, K.; Rucker, E.B., 3rd; Song, G.; Deng, J.M.; Behringer, R.R.; DeMayo, F.J.; Lydon, J.; Jeong, J.W.; et al. Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol. Reprod. 2011, 85, 386–396. [Google Scholar] [CrossRef]
- Reardon, S.N.; King, M.L.; MacLean, J.A., II; Mann, J.L.; DeMayo, F.J.; Lydon, J.P.; Hayashi, K. Cdh1 is essential for endometrial differentiation, gland development, and adult function in the mouse uterus. Biol. Reprod. 2012, 86, 141. [Google Scholar] [CrossRef]
- Signorile, P.G.; Baldi, A.; Viceconte, R.; Vincenzi, B.; Montella, M. Adenogenesis factors FGF7, FGF10, FGF23, IFN-τ and HGF in endometriosis tissue respect to eutopic endometrium: An immunohistochemical study. Crit. Rev. Eukaryot. Gene Exp. 2023, 33, 85–94. [Google Scholar] [CrossRef]
- Zhou, W.; Hou, X.; Wang, X.; Li, D. Fibroblast growth factor 7 regulates proliferation and decidualization of human endometrial stromal cells via ERK and JNK pathway in an autocrine manner. Reprod. Sci. 2017, 24, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.; Gao, F.; Jegga, A.G.; Das, S.K. Estrogen-mediated epithelial proliferation in the uterus is directed by stromal Fgf10 and Bmp8a. Mol. Cell Endocrinol. 2015, 400, 48–60. [Google Scholar] [CrossRef]
- Yoshida, S.; Harada, T.; Mitsunari, M.; Iwabe, T.; Sakamoto, Y.; Tsukihara, S.; Iba, Y.; Horie, S.; Terakawa, N. Hepatocyte Growth Factor/Met System promotes endometrial and endometriotic stromal cell invasion via autocrine and paracrine pathways. J. Clin. Endocrinol. Metab. 2004, 89, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Heidari, S.; Kolahdouz-Mohammadi, R.; Khodaverdi, S.; Tajik, N.; Delbandi, A.A. Expression levels of MCP-1, HGF, and IGF-1 in endometriotic patients compared with non-endometriotic controls. BMC Womens Health 2021, 21, 422. [Google Scholar] [CrossRef]
- Park, Y.; Han, S.J. Interferon signaling in the endometrium and in endometriosis. Biomolecules 2022, 12, 1554. [Google Scholar] [CrossRef] [PubMed]
- Signorile, P.G.; Viceconte, R.; Vincenzi, B.; Baldi, A. Differential expression in endometriosis tissue versus endometrium of the uterine adenogenesis factors PRL-R, GH, IGF1, and IGF2. Crit. Rev. Eukaryot. Gene Exp. 2023, 33, 39–46. [Google Scholar] [CrossRef]
- Signorile, P.G.; Baldi, A.; Viceconte, R.; Carraturo, E.; Boccellino, M.; Fordellone, M.; Montella, M. Glycosaminoglycan adenogenesis factors: Immunohistochemical expression in endometriosis tissues compared with the endometrium. Crit. Rev. Eukaryot. Gene Exp. 2025, 35, 67–79. [Google Scholar] [CrossRef]
| Marker | Eutopic Epithelium/Stroma | Ectopic Epithelium/Stroma |
|---|---|---|
| FGF7 | High/High | Low/Low |
| FGF10 | High/High | Low/Low |
| FGF23 | High/Low | High/High |
| IFN-τ | High/Low | High/High |
| HGF | High/High | Low/Low |
| PRL-R | High/Low | Low/Low |
| GH | Low/Low | High/Low |
| IGF1 | High/High | Low/Low |
| IGF2 | High/High | Low/Low |
| CSPG4 | High/Low | High/Low |
| CS-56 | High/Low | High/Low |
| HEP | High/Low | High/Low |
| Keratan | Low/Low | Low/Low |
| Hyaluronic acid | Low/Low | Low/Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signorile, P.G.; Baldi, A.; Viceconte, R.; Boccellino, M. The Role of Adenogenesis Factors in the Pathogenesis of Endometriosis. Int. J. Mol. Sci. 2025, 26, 2076. https://doi.org/10.3390/ijms26052076
Signorile PG, Baldi A, Viceconte R, Boccellino M. The Role of Adenogenesis Factors in the Pathogenesis of Endometriosis. International Journal of Molecular Sciences. 2025; 26(5):2076. https://doi.org/10.3390/ijms26052076
Chicago/Turabian StyleSignorile, Pietro G., Alfonso Baldi, Rosa Viceconte, and Mariarosaria Boccellino. 2025. "The Role of Adenogenesis Factors in the Pathogenesis of Endometriosis" International Journal of Molecular Sciences 26, no. 5: 2076. https://doi.org/10.3390/ijms26052076
APA StyleSignorile, P. G., Baldi, A., Viceconte, R., & Boccellino, M. (2025). The Role of Adenogenesis Factors in the Pathogenesis of Endometriosis. International Journal of Molecular Sciences, 26(5), 2076. https://doi.org/10.3390/ijms26052076








