TOR Mediates Stress Responses Through Global Regulation of Metabolome in Plants
Abstract
:1. Introduction
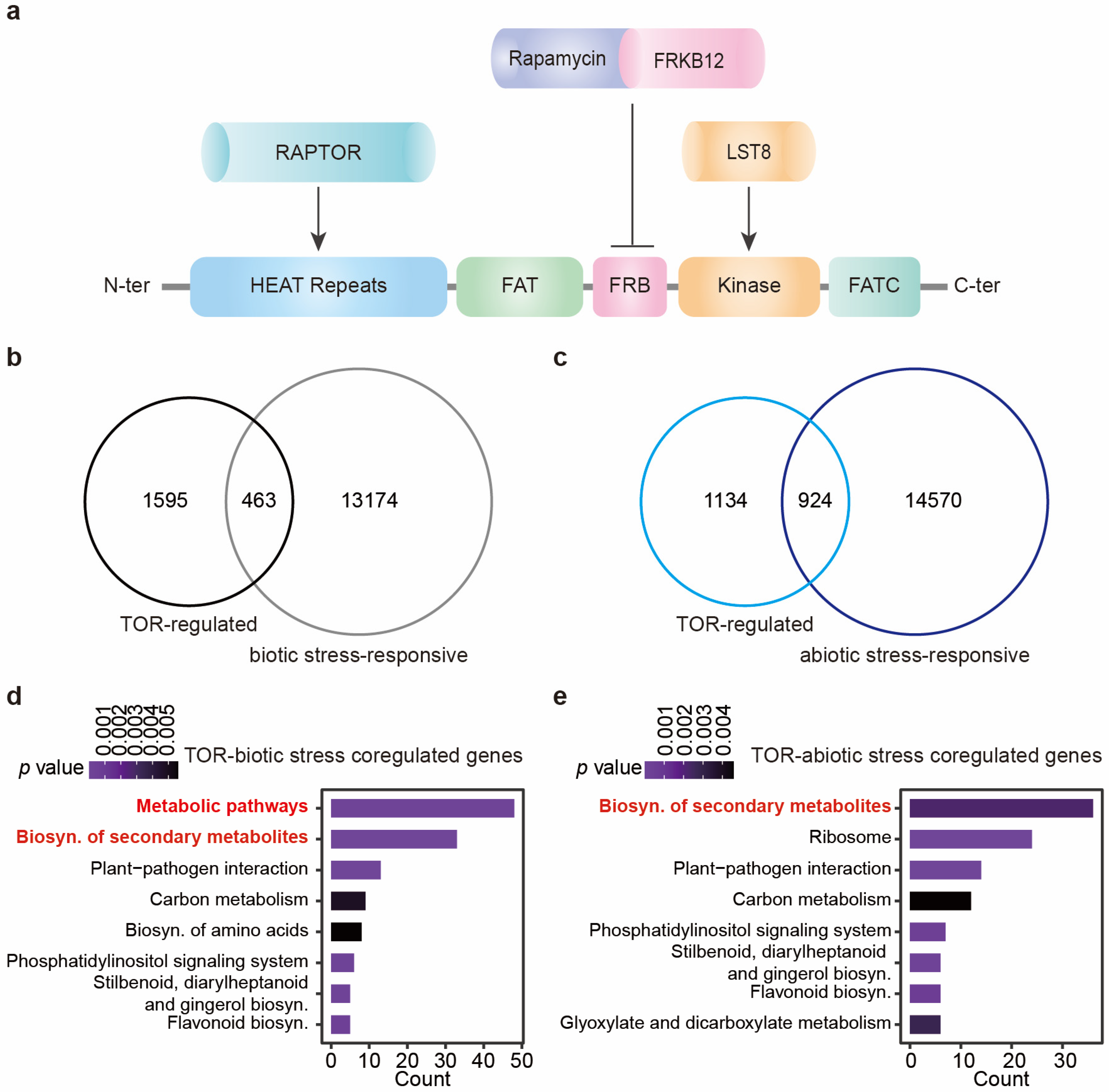
2. Insight on TOR-Mediated Stress Responses via Metabolome Reprogramming
2.1. TOR Is Widely Involved in Plants Responses to Various Environmental Stresses
2.2. TOR-Regulated Plant Responses to Abiotic Stress
2.3. TOR-Regulated Plant Responses to Biotic Stress
2.4. TOR-Modulated Metabolic Reprogramming Contributes to Plant Stress Responses
3. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Staskawicz, B.J.; Dangl, J.L. The plant immune system: From discovery to deployment. Cell 2024, 187, 2095–2116. [Google Scholar] [CrossRef] [PubMed]
- Monson, R.K.; Trowbridge, A.M.; Lindroth, R.L.; Lerdau, M.T. Coordinated resource allocation to plant growth-defense tradeoffs. New Phytol. 2022, 233, 1051–1066. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiong, Y. Plant target of rapamycin signaling network: Complexes, conservations, and specificities. J. Integr. Plant Biol. 2022, 64, 342–370. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, N.; Li, J.; Shen, X.; Sheen, J.; Xiong, Y. TOR kinase, a GPS in the complex nutrient and hormonal signaling networks to guide plant growth and development. J. Exp. Bot. 2022, 73, 7041–7054. [Google Scholar] [CrossRef]
- Heitman, J.; Movva, N.R.; Hall, M.N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 1991, 253, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Collins, S. Regulation of mTOR signaling: Emerging role of cyclic nucleotide-dependent protein kinases and implications for cardiometabolic disease. Int. J. Mol. Sci. 2023, 24, 11497. [Google Scholar] [CrossRef]
- Emmerstorfer-Augustin, A.; Thorner, J. Regulation of TORC2 function and localization in yeast. Annu. Rev. Cell Dev. Biol. 2023, 39, 363–389. [Google Scholar] [CrossRef]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wu, Y.; Sheen, J. TOR signaling in plants: Conservation and innovation. Development 2018, 145, dev160887. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Kim, J.; Guan, K.L. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 381–400. [Google Scholar] [CrossRef]
- Burkart, G.M.; Brandizzi, F. A tour of TOR complex signaling in plants. Trends Biochem. Sci. 2021, 46, 417–428. [Google Scholar] [CrossRef]
- Saha, S.; Fang, X.; Green, C.D.; Das, A. mTORC1 and SGLT2 Inhibitors—A therapeutic perspective for diabetic cardiomyopathy. Int. J. Mol. Sci. 2023, 24, 15078. [Google Scholar] [CrossRef]
- He, L.; Cho, S.; Blenis, J. mTORC1, the maestro of cell metabolism and growth. Genes. Dev. 2025, 39, 109–131. [Google Scholar] [CrossRef]
- Ragupathi, A.; Kim, C.; Jacinto, E. The mTORC2 signaling network: Targets and cross-talks. Biochem. J. 2024, 481, 45–91. [Google Scholar] [CrossRef]
- Foltman, M.; Sanchez-Diaz, A. TOR complex 1: Orchestrating nutrient signaling and cell cycle progression. Int. J. Mol. Sci. 2023, 24, 15745. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Sheen, J. Rapamycin and glucose-target of rapamycin (TOR) protein signaling in plants. J. Biol. Chem. 2012, 287, 2836–2842. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; McCormack, M.; Li, L.; Hall, Q.; Xiang, C.; Sheen, J. Glucose–TOR signalling reprograms the transcriptome and activates meristems. Nature 2013, 496, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Sheen, J. The role of target of rapamycin signaling networks in plant growth and metabolism. Plant Physiol. 2014, 164, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, P.; Xiong, Y. Target of rapamycin signaling in plant stress responses. Plant Physiol. 2020, 182, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Sheen, J. Moving beyond translation: Glucose-TOR signaling in the transcriptional control of cell cycle. Cell Cycle 2013, 12, 1989–1990. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Liu, Y.; Qin, G.; Wu, P.; Zi, H.; Xu, Z.; Zhao, X.; Wang, Y.; Li, Y.; Yang, S.; et al. The TOR-EIN2 axis mediates nuclear signalling to modulate plant growth. Nature 2021, 591, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, L.; Wu, Y.; Zhang, R.; Yu, S.; Fu, L. TOR balances plant growth and cold tolerance by orchestrating amino acid-derived metabolism in tomato. Hortic. Res. 2024, 11, uhae253. [Google Scholar] [CrossRef]
- Hu, C.; Wei, C.; Ma, Q.; Dong, H.; Shi, K.; Zhou, Y.; Foyer, C.H.; Yu, J. Ethylene response factors 15 and 16 trigger jasmonate biosynthesis in tomato during herbivore resistance. Plant Physiol. 2021, 185, 1182–1197. [Google Scholar] [CrossRef]
- Naveed, Z.A.; Ali, G.S. Comparative transcriptome analysis between a resistant and a susceptible wild tomato accession in response to Phytophthora parasitica. Int. J. Mol. Sci. 2018, 19, 3735. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, Q.; Foyer, C.H.; Lei, C.; Choi, H.W.; Zheng, C.; Li, J.; Zuo, J.; Mao, Z.; Mei, Y.; et al. High CO2- and pathogen-driven expression of the carbonic anhydrase βCA3 confers basal immunity in tomato. New Phytol. 2021, 229, 2827–2843. [Google Scholar] [CrossRef] [PubMed]
- Ikuyinminu, E.; Goñi, O.; Łangowski, Ł.; O’Connell, S. Transcriptome, biochemical and phenotypic analysis of the effects of a precision engineered biostimulant for inducing salinity stress tolerance in tomato. Int. J. Mol. Sci. 2023, 24, 6988. [Google Scholar] [CrossRef]
- Huang, Y.; An, J.; Sircar, S.; Bergis, C.; Lopes, C.D.; He, X.; Da Costa, B.; Tan, F.Q.; Bazin, J.; Antunez-Sanchez, J.; et al. HSFA1a modulates plant heat stress responses and alters the 3D chromatin organization of enhancer-promoter interactions. Nat. Commun. 2023, 14, 469. [Google Scholar] [CrossRef]
- Liu, M.; Yu, H.; Zhao, G.; Huang, Q.; Lu, Y.; Ouyang, B. Profiling of drought-responsive microRNA and mRNA in tomato using high-throughput sequencing. BMC Genom. 2017, 18, 481. [Google Scholar] [CrossRef]
- Lin, R.; Song, J.; Tang, M.; Wang, L.; Yu, J.; Zhou, Y. CALMODULIN6 negatively regulates cold tolerance by attenuating ICE1-dependent stress responses in tomato. Plant Physiol. 2023, 193, 2105–2121. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Regulatory networks underlying plant responses and adaptation to cold stress. Annu. Rev. Genet. 2024, 58, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Mu, X.R.; Gao, J.; Lin, H.X.; Lin, Y. The molecular basis of heat stress responses in plants. Mol. Plant 2023, 16, 1612–1634. [Google Scholar] [CrossRef]
- Pereyra, C.M.; Aznar, N.R.; Rodriguez, M.S.; Salerno, G.L.; Martínez-Noël, G.M. Target of rapamycin signaling is tightly and differently regulated in the plant response under distinct abiotic stresses. Planta 2019, 251, 21. [Google Scholar] [CrossRef]
- Pacheco, J.M.; Song, L.; Kuběnová, L.; Ovečka, M.; Gabarain, V.B.; Peralta, J.M.; Lehuedé, T.U.; Ibeas, M.A.; Ricardi, M.M.; Zhu, S.; et al. Cell surface receptor kinase FERONIA linked to nutrient sensor TORC signaling controls root hair growth at low temperature linked to low nitrate in Arabidopsis thaliana. New Phytol. 2023, 238, 169–185. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Zhao, C.; Li, S.; Kong, L.; Wu, W.; Kong, W.; Liu, Y.; Wei, Y.; Zhu, J.K.; et al. The inhibition of protein translation mediated by AtGCN1 is essential for cold tolerance in Arabidopsis thaliana. Plant Cell Environ. 2017, 40, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Teleman, A.A.; Jedmowski, C.; Wirtz, M.; Hell, R. The Arabidopsis THADA homologue modulates TOR activity and cold acclimation. Plant Biol. 2019, 21 (Suppl. S1), 77–83. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Banday, Z.Z.; Shukla, B.N.; Laxmi, A. Glucose-regulated HLP1 acts as a key molecule in governing thermomemory. Plant Physiol. 2019, 180, 1081–1100. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Jamsheer, K.M.; Shukla, B.N.; Sharma, M.; Awasthi, P.; Mahtha, S.K.; Yadav, G.; Laxmi, A. Arabidopsis target of rapamycin coordinates with transcriptional and epigenetic machinery to regulate thermotolerance. Front. Plant Sci. 2021, 12, 741965. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, M.; Jamsheer, K.M.; Laxmi, A. A glucose-target of rapamycin signaling axis integrates environmental history of heat stress through maintenance of transcription-associated epigenetic memory in Arabidopsis. J. Exp. Bot. 2022, 73, 7083–7102. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Han, G.L.; Yang, Z.R.; Li, Y.X.; Wang, B.S. Plant salinity sensors: Current understanding and future directions. Front. Plant Sci. 2022, 13, 859224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Deprost, D.; Yao, L.; Sormani, R.; Moreau, M.; Leterreux, G.; Nicolaï, M.; Bedu, M.; Robaglia, C.; Meyer, C. The Arabidopsis TOR kinase links plant growth, yield, stress resistance and mRNA translation. EMBO Rep. 2007, 8, 864–870. [Google Scholar] [CrossRef]
- Bakshi, A.; Moin, M.; Madhav, M.S.; Datla, R.; Kirti, P.B. Target of Rapamycin (TOR) negatively regulates chlorophyll degradation and lipid peroxidation and controls responses under abiotic stress in Arabidopsis thaliana. Plant Stress. 2021, 2, 100020. [Google Scholar] [CrossRef]
- Kim, D.; Ntui, V.O.; Xiong, L. Arabidopsis YAK1 regulates abscisic acid response and drought resistance. FEBS Lett. 2016, 590, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Forzani, C.; Duarte, G.T.; Van Leene, J.; Clément, G.; Huguet, S.; Paysant-Le-Roux, C.; Mercier, R.; De Jaeger, G.; Leprince, A.S.; Meyer, C. Mutations of the AtYAK1 kinase suppress TOR deficiency in Arabidopsis. Cell Rep. 2019, 27, 3696–3708.e5. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Tiozon, R.N.; Fernie, A.R.; Sreenivasulu, N. Abscisic acid and its role in the modulation of plant growth, development, and yield stability. Trends Plant Sci. 2022, 27, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Fu, L.; Xiong, Y.; Wang, P. How long should a kiss last between a kinase and its substrate? J. Integr. Plant Biol. 2022, 64, 789–791. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Y.; Li, Z.; Hsu, C.C.; Liu, X.; Fu, L.; Hou, Y.J.; Du, Y.; Xie, S.; Zhang, C.; et al. Reciprocal regulation of the TOR kinase and ABA receptor balances plant growth and stress response. Mol. Cell 2018, 69, 100–112.e6. [Google Scholar] [CrossRef]
- Jamsheer, K.M.; Awasthi, P.; Laxmi, A. The social network of target of rapamycin complex 1 in plants. J. Exp. Bot. 2022, 73, 7026–7040. [Google Scholar] [CrossRef] [PubMed]
- Mugume, Y.; Kazibwe, Z.; Bassham, D.C. Target of rapamycin in control of autophagy: Puppet master and signal integrator. Int. J. Mol. Sci. 2020, 21, 8259. [Google Scholar] [CrossRef]
- Soto-Burgos, J.; Bassham, D.C. SnRK1 activates autophagy via the TOR signaling pathway in Arabidopsis thaliana. PLoS ONE 2017, 12, e0182591. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Soto-Burgos, J.; Bassham, D.C. Regulation of autophagy through SnRK1 and TOR signaling pathways. Plant Signal Behav. 2017, 12, e1395128. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Luo, X.; Bassham, D.C. TOR-dependent and -independent pathways regulate autophagy in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1204. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bassham, D.C. Autophagy: Pathways for self-eating in plant cells. Annu. Rev. Plant Biol. 2012, 63, 215–237. [Google Scholar] [CrossRef]
- Van Leene, J.; Han, C.; Gadeyne, A.; Eeckhout, D.; Matthijs, C.; Cannoot, B.; De Winne, N.; Persiau, G.; Van De Slijke, E.; Van de Cotte, B.; et al. Capturing the phosphorylation and protein interaction landscape of the plant TOR kinase. Nat. Plants 2019, 5, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Jhu, M.Y.; Sinha, N.R. Parasitic plants: An overview of mechanisms by which plants perceive and respond to parasites. Annu. Rev. Plant Biol. 2022, 73, 433–455. [Google Scholar] [CrossRef] [PubMed]
- Shelake, R.M.; Wagh, S.G.; Patil, A.M.; Červený, J.; Waghunde, R.R.; Kim, J.Y. Heat stress and plant–biotic interactions: Advances and perspectives. Plants 2024, 13, 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xing, H.; Wang, H.; Yu, L.; Yang, Z.; Meng, X.; Hu, P.; Fan, H.; Yu, Y.; Cui, N. SlMYC2 interacted with the SlTOR promoter and mediated JA signaling to regulate growth and fruit quality in tomato. Front. Plant Sci. 2022, 13, 1013445. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Ayyaz, A.; Zou, H.X.; Zhou, W.; Hannan, F.; Yan, X. Jasmonic acid mediates Ca2+ dependent signal transduction and plant immunity. Plant Sci. 2024, 348, 112239. [Google Scholar] [CrossRef]
- Meteignier, L.V.; El Oirdi, M.; Cohen, M.; Barff, T.; Matteau, D.; Lucier, J.F.; Rodrigue, S.; Jacques, P.E.; Yoshioka, K.; Moffett, P. Translatome analysis of an NB-LRR immune response identifies important contributors to plant immunity in Arabidopsis. J. Exp. Bot. 2017, 68, 2333–2344. [Google Scholar] [CrossRef]
- Popa, C.; Li, L.; Gil, S.; Tatjer, L.; Hashii, K.; Tabuchi, M.; Coll, N.S.; Ariño, J.; Valls, M. The effector AWR5 from the plant pathogen Ralstonia solanacearum is an inhibitor of the TOR signalling pathway. Sci. Rep. 2016, 6, 27058. [Google Scholar] [CrossRef] [PubMed]
- Marash, I.; Leibman-Markus, M.; Gupta, R.; Avni, A.; Bar, M. TOR inhibition primes immunity and pathogen resistance in tomato in a salicylic acid-dependent manner. Mol. Plant Pathol. 2022, 23, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Marash, I.; Gupta, R.; Anand, G.; Leibman-Markus, M.; Lindner, N.; Israeli, A.; Nir, D.; Avni, A.; Bar, M. TOR coordinates cytokinin and gibberellin signals mediating development and defense. Plant Cell Environ. 2024, 47, 629–650. [Google Scholar] [CrossRef] [PubMed]
- De Vleesschauwer, D.; Filipe, O.; Hoffman, G.; Seifi, H.S.; Haeck, A.; Canlas, P.; Van Bockhaven, J.; De Waele, E.; Demeestere, K.; Ronald, P.; et al. Target of rapamycin signaling orchestrates growth-defense trade-offs in plants. New Phytol. 2018, 217, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, F.; Song, W.; Yang, Z.; Li, L.; Ma, Q.; Tan, X.; Wei, Z.; Li, Y.; Li, J.; et al. Different viral effectors suppress hormone-mediated antiviral immunity of rice coordinated by OsNPR1. Nat. Commun. 2023, 14, 3011. [Google Scholar] [CrossRef] [PubMed]
- Aznar, N.R.; Consolo, V.F.; Salerno, G.L.; Martínez-Noël, G.M. TOR signaling downregulation increases resistance to the cereal killer Fusarium graminearum. Plant Signal Behav. 2018, 13, e1414120. [Google Scholar] [CrossRef] [PubMed]
- Schepetilnikov, M.; Kobayashi, K.; Geldreich, A.; Caranta, C.; Robaglia, C.; Keller, M.; Ryabova, L.A. Viral factor TAV recruits TOR/S6K1 signalling to activate reinitiation after long ORF translation. Embo J. 2011, 30, 1343–1356. [Google Scholar] [CrossRef]
- Ouibrahim, L.; Rubio, A.G.; Moretti, A.; Montané, M.H.; Menand, B.; Meyer, C.; Robaglia, C.; Caranta, C. Potyviruses differ in their requirement for TOR signalling. J. Gen. Virol. 2015, 96, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Szwed, A.; Kim, E.; Jacinto, E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol. Rev. 2021, 101, 1371–1426. [Google Scholar] [CrossRef]
- Li, L.; Zhu, T.; Song, Y.; Luo, X.; Datla, R.; Ren, M. Target of rapamycin controls hyphal growth and pathogenicity through FoTIP4 in Fusarium oxysporum. Mol. Plant Pathol. 2021, 22, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Calderan-Rodrigues, M.J.; Luzarowski, M.; Monte-Bello, C.C.; Minen, R.I.; Zühlke, B.M.; Nikoloski, Z.; Skirycz, A.; Caldana, C. Proteogenic dipeptides are characterized by diel fluctuations and target of rapamycin complex-signaling dependency in the model plant Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 758933. [Google Scholar] [CrossRef]
- da Silva, V.C.H.; Martins, M.C.; Calderan-Rodrigues, M.J.; Artins, A.; Monte Bello, C.C.; Gupta, S.; Sobreira, T.J.; Riaño-Pachón, D.M.; Mafra, V.; Caldana, C. Shedding light on the dynamic role of the “Target of Rapamycin” kinase in the fast-growing C4 species Setaria viridis, a suitable model for biomass crops. Front. Plant Sci. 2021, 12, 637508. [Google Scholar] [CrossRef]
- Busche, M.; Scarpin, M.R.; Hnasko, R.; Brunkard, J.O. TOR coordinates nucleotide availability with ribosome biogenesis in plants. Plant Cell 2021, 33, 1615–1632. [Google Scholar] [CrossRef]
- Mubeen, U.; Giavalisco, P.; Caldana, C. TOR inhibition interrupts the metabolic homeostasis by shifting the carbon-nitrogen balance in Chlamydomonas reinhardtii. Plant Signal Behav. 2019, 14, 1670595. [Google Scholar] [CrossRef]
- Scarpin, M.R.; Leiboff, S.; Brunkard, J.O. Parallel global profiling of plant TOR dynamics reveals a conserved role for LARP1 in translation. Elife 2020, 9, e58795. [Google Scholar] [CrossRef] [PubMed]
- Roustan, V.; Weckwerth, W. Quantitative phosphoproteomic and system-level analysis of TOR inhibition unravel distinct organellar acclimation in Chlamydomonas reinhardtii. Front. Plant Sci. 2018, 9, 1590. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Hua, W.; Li, J.; Qiao, Y.; Yao, L.; Hao, W.; Li, R.; Fan, M.; De Jaeger, G.; Yang, W.; et al. TOR promotes guard cell starch degradation by regulating the activity of β-AMYLASE1 in Arabidopsis. Plant Cell 2022, 34, 1038–1053. [Google Scholar] [CrossRef]
- Moreau, M.; Azzopardi, M.; Clément, G.; Dobrenel, T.; Marchive, C.; Renne, C.; Martin-Magniette, M.-L.; Taconnat, L.; Renou, J.-P.; Robaglia, C.; et al. Mutations in the Arabidopsis homolog of LST8/GβL, a partner of the target of Rapamycin kinase, impair plant growth, flowering, and metabolic adaptation to long days. Plant Cell 2012, 24, 463–481. [Google Scholar] [CrossRef]
- Li, D.; Ding, Y.; Cheng, L.; Zhang, X.; Cheng, S.; Ye, Y.; Gao, Y.; Qin, Y.; Liu, Z.; Li, C.; et al. Target of rapamycin (TOR) regulates the response to low nitrogen stress via autophagy and hormone pathways in Malus hupehensis. Hortic. Res. 2022, 9, uhac143. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Venglat, P.; Qiu, S.; Feng, L.; Cao, Y.; Wang, E.; Xiang, D.; Wang, J.; Alexander, D.; Chalivendra, S.; et al. Target of rapamycin signaling regulates metabolism, growth, and life span in Arabidopsis. Plant Cell 2012, 24, 4850–4874. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, T.; Song, Y.; Luo, X.; Feng, L.; Zhuo, F.; Li, F.; Ren, M. Functional characterization of target of rapamycin signaling in Verticillium dahliae. Front. Microbiol. 2019, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xu, G.; Li, T.; Zhou, H.; Lin, Q.; Chen, J.; Wang, L.; Wu, D.; Li, X.; Wang, L.; et al. The RALF1-FERONIA complex interacts with and activates TOR signaling in response to low nutrients. Mol. Plant 2022, 15, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, U.; Jüppner, J.; Alpers, J.; Hincha, D.K.; Giavalisco, P. Target of rapamycin inhibition in Chlamydomonas reinhardtii triggers de Novo amino acid synthesis by enhancing nitrogen assimilation. Plant Cell 2018, 30, 2240–2254. [Google Scholar] [CrossRef]
- Caldana, C.; Li, Y.; Leisse, A.; Zhang, Y.; Bartholomaeus, L.; Fernie, A.R.; Willmitzer, L.; Giavalisco, P. Systemic analysis of inducible target of rapamycin mutants reveal a general metabolic switch controlling growth in Arabidopsis thaliana. Plant J. 2013, 73, 897–909. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.M.; Oh, G.G.K.; Lee, C.P.; Millar, A.H. Metabolite regulatory interactions control plant respiratory metabolism via target of rapamycin (TOR) kinase activation. Plant Cell 2020, 32, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Artins, A.; Martins, M.C.M.; Meyer, C.; Fernie, A.R.; Caldana, C. Sensing and regulation of C and N metabolism—Novel features and mechanisms of the TOR and SnRK1 signaling pathways. Plant J. 2024, 118, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yang, S. Surviving and thriving: How plants perceive and respond to temperature stress. Dev. Cell 2022, 57, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Jancewicz, A.L.; Gibbs, N.M.; Masson, P.H. Cadaverine’s functional role in plant development and environmental response. Front. Plant Sci. 2016, 7, 870. [Google Scholar] [CrossRef]
- Ozmen, S.; Tabur, S.; Oney-Birol, S. Alleviation role of exogenous cadaverine on cell cycle, endogenous polyamines amounts and biochemical enzyme changes in barley seedlings under drought stress. Sci. Rep. 2023, 13, 17488. [Google Scholar] [CrossRef]
- You, J.; Zhang, Y.; Liu, A.; Li, D.; Wang, X.; Dossa, K.; Zhou, R.; Yu, J.; Zhang, Y.; Wang, L.; et al. Transcriptomic and metabolomic profiling of drought-tolerant and susceptible sesame genotypes in response to drought stress. BMC Plant Biol. 2019, 19, 267. [Google Scholar] [CrossRef]
- Barqawi, A.A.; Abulfaraj, A.A. Salt stress-related mechanisms in leaves of the wild barley Hordeum spontaneum generated from RNA-Seq datasets. Life 2023, 13, 1454. [Google Scholar] [CrossRef]
- Brocker, C.; Cantore, M.; Failli, P.; Vasiliou, V. Aldehyde dehydrogenase 7A1 (ALDH7A1) attenuates reactive aldehyde and oxidative stress induced cytotoxicity. Chem. Biol. Interact. 2011, 191, 269–277. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Clément, G.; Anne, P.; Routaboul, J.M.; Guiboileau, A.; Soulay, F.; Shirasu, K.; Yoshimoto, K. Stitching together the multiple dimensions of autophagy using metabolomics and transcriptomics reveals impacts on metabolism, development, and plant responses to the environment in Arabidopsis. Plant Cell 2014, 26, 1857–1877. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.U.; Lee, S.B.; Kim, H.H.; Paek, K.H. ATAF2, a NAC transcription factor, binds to the promoter and regulates NIT2 gene expression involved in auxin biosynthesis. Mol. Cells 2012, 34, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Lee, D.Y.; Rakwal, R.; Baek, S.B.; Lee, J.H.; Kwak, Y.S.; Seo, J.S.; Chung, W.S.; Bae, D.W.; Kim, S.G. Proteomic analyses of the interaction between the plant-growth promoting rhizobacterium Paenibacillus polymyxa E681 and Arabidopsis thaliana. Proteomics 2016, 16, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Rocheleau, H.; Qi, P.F.; Zheng, Y.L.; Zhao, H.Y.; Ouellet, T. Indole-3-acetic acid in Fusarium graminearum: Identification of biosynthetic pathways and characterization of physiological effects. Fungal Biol. 2016, 120, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Alsherif, E.A.; Almaghrabi, O.; Elazzazy, A.M.; Abdel-Mawgoud, M.; Beemster, G.T.S.; Sobrinho, R.L.; AbdElgawad, H. How carbon nanoparticles, arbuscular mycorrhiza, and compost mitigate drought stress in maize plant: A growth and biochemical study. Plants 2022, 11, 3324. [Google Scholar] [CrossRef]
- Hijaz, F.; Killiny, N. Exogenous GABA is quickly metabolized to succinic acid and fed into the plant TCA cycle. Plant Signal Behav. 2019, 14, e1573096. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, F.; Jiang, W.; Lu, X.; Zhang, R.; Huang, A.; Wu, K.; Deng, P.; Wang, Y.; Zhao, H.; et al. Potassium indole-3-butyric acid affects rice’s adaptability to salt stress by regulating carbon metabolism, transcription factor genes expression, and biosynthesis of secondary metabolites. Front. Plant Sci. 2024, 15, 1416936. [Google Scholar] [CrossRef]
- Yang, L.; Wu, L.; Yao, X.; Zhao, S.; Wang, J.; Li, S.; Ding, W. Hydroxycoumarins: New, effective plant-derived compounds reduce Ralstonia pseudosolanacearum populations and control tobacco bacterial wilt. Microbiol. Res. 2018, 215, 15–21. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, C.; Li, Y.; Meng, F.; Du, Y.; Zhang, S.; Jiang, W.; Feng, N.; Zhao, L.; Zheng, D. 5-ALA, DTA-6, and nitrogen mitigate NaCl stress by promoting photosynthesis and carbon metabolism in rice seedlings. Metabolites 2024, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.S.; Imran, S.; Karim, M.M.; Chakrobortty, J.; Mahamud, M.A.; Sarker, P.; Tahjib-Ul-Arif, M.; Robin, A.H.K.; Ye, W.; Murata, Y.; et al. 5-aminolevulinic acid-mediated plant adaptive responses to abiotic stress. Plant Cell Rep. 2021, 40, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Cao, J.; Xia, X.; Li, Z. Advances in 5-aminolevulinic acid priming to enhance plant tolerance to abiotic stress. Int. J. Mol. Sci. 2022, 23, 702. [Google Scholar] [CrossRef] [PubMed]
- El-Shora, H.M.; Massoud, G.F.; El-Sherbeny, G.A.; Alrdahe, S.S.; Darwish, D.B. Alleviation of lead stress on sage plant by 5-aminolevulinic acid (ALA). Plants 2021, 10, 1969. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, X.; Chen, M.; Wang, L.; Xia, J.; Wang, Z.; Fang, J.; Tran, L.S.P.; Shangguan, L. Copper stress in grapevine: Consequences, responses, and a novel mitigation strategy using 5-aminolevulinic acid. Environ. Pollut. 2022, 307, 119561. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, J.; Fang, X.; Jia, H.; Wang, X.; Lin, Y.; Liu, S.; Ge, M.; Pu, Y.; Fang, J.; et al. Drought stress in ’Shine Muscat’ grapevine: Consequences and a novel mitigation strategy-5-aminolevulinic acid. Front. Plant Sci. 2023, 14, 1129114. [Google Scholar] [CrossRef] [PubMed]
- Helaly, M.N.; El-Hoseiny, H.M.; Elsheery, N.I.; Kalaji, H.M.; Santos-Villalobos, S.d.L.; Wróbel, J.; Hassan, I.F.; Gaballah, M.S.; Abdelrhman, L.A.; Mira, A.M.; et al. 5-aminolevulinic acid and 24-epibrassinolide improve the drought stress resilience and productivity of banana plants. Plants 2022, 11, 743. [Google Scholar] [CrossRef]
- Yu, J.; Li, R.; Fan, N.; Yang, Z.; Huang, B. Metabolic pathways involved in carbon dioxide enhanced heat tolerance in bermudagrass. Front. Plant Sci. 2017, 8, 1506. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Rossi, S.; Yang, Z.; Yu, J.; Huang, B. Metabolic regulation of 5-oxoproline for enhanced heat tolerance in perennial ryegrass. Stress Biol. 2024, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, X.; Xu, Q.; Mei, X.; Yuan, H.; Jiabu, D.; Sang, Z.; Nyima, T. Metabolite profiling in two contrasting Tibetan hulless barley cultivars revealed the core salt-responsive metabolome and key salt-tolerance biomarkers. AoB Plants 2019, 11, plz021. [Google Scholar] [CrossRef] [PubMed]
- Bruňáková, K.; Bálintová, M.; Petijová, L.; Čellárová, E. Does phenotyping of Hypericum secondary metabolism reveal a tolerance to biotic/abiotic stressors? Front. Plant Sci. 2022, 13, 1042375. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Meng, R.; Feng, W.; Wongsnansilp, T.; Li, Z.; Lu, X.; Wang, X. Study of dandelion (Taraxacum mongolicum Hand.-Mazz.) salt response and caffeic acid metabolism under saline stress by transcriptome analysis. Genes 2024, 15, 220. [Google Scholar] [CrossRef]
- Ramzan, M.; Haider, S.T.A.; Hussain, M.B.; Ehsan, A.; Datta, R.; Alahmadi, T.A.; Ansari, M.J.; Alharbi, S.A. Potential of kaempferol and caffeic acid to mitigate salinity stress and improving potato growth. Sci. Rep. 2024, 14, 21657. [Google Scholar] [CrossRef]
- Li, J.; Ren, J.; Zhang, Q.; Lei, X.; Feng, Z.; Tang, L.; Bai, J.; Gong, C. Strigolactone enhances tea plant adaptation to drought and Phyllosticta theicola petch by regulating caffeine content via CsbHLH80. Plant Physiol. Biochem. 2024, 216, 109161. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.; Yoo, Y.H.; Lee, D.Y.; Jung, K.H.; Lee, S.W.; Park, J.C. Caffeine produced in rice plants provides tolerance to water-deficit stress. Antioxidants 2023, 12, 1984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lariviere, A.; Tonsor, S.J.; Traw, M.B. Constitutive camalexin production and environmental stress response variation in Arabidopsis populations from the Iberian Peninsula. Plant Sci. 2014, 225, 77–85. [Google Scholar] [CrossRef]
- Xiao, P.; Qu, J.; Wang, Y.; Fang, T.; Xiao, W.; Wang, Y.; Zhang, Y.; Khan, M.; Chen, Q.; Xu, X.; et al. Transcriptome and metabolome atlas reveals contributions of sphingosine and chlorogenic acid to cold tolerance in Citrus. Plant Physiol. 2024, 196, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, A.; Sawikowska, A.; Kuczyńska, A.; Ogrodowicz, P.; Mikołajczak, K.; Krajewski, P.; Kachlicki, P. Phenolic metabolites from barley in contribution to phenome in soil moisture deficit. Int. J. Mol. Sci. 2020, 21, 6032. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.; Figueiredo, C.; Santos, C.; Silva, A.M. Phenolic and lipophilic metabolite adjustments in Olea europaea (olive) trees during drought stress and recovery. Phytochemistry 2021, 185, 112695. [Google Scholar] [CrossRef] [PubMed]
- Lacrampe, N.; Colombié, S.; Dumont, D.; Nicot, P.; Lecompte, F.; Lugan, R. Nitrogen-mediated metabolic patterns of susceptibility to Botrytis cinerea infection in tomato (Solanum lycopersicum) stems. Planta 2023, 257, 41. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, S.; Logeswari, P.; Vangnai, A.S.; Pérez, R.; Kamaraj, B.; Cornejo, P. Co-application of citric acid and Nocardiopsis sp. strain RA07 enhances phytoremediation potentiality of Sorghum bicolor L. Environ. Sci. Pollut. Res. 2023, 30, 86244–86254. [Google Scholar] [CrossRef]
- Menhas, S.; Hayat, K.; Lin, D.; Shahid, M.; Bundschuh, J.; Zhu, S.; Hayat, S.; Liu, W. Citric acid-driven cadmium uptake and growth promotion mechanisms in Brassica napus. Chemosphere 2024, 368, 143716. [Google Scholar] [CrossRef]
- Cay, S. Assessment of tea saponin and citric acid-assisted phytoextraction of Pb-contaminated soil by Salvia virgata Jacq. Environ. Sci. Pollut. Res. 2023, 30, 49771–49778. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Rinklebe, J.; Ahmad, P. Citric acid and hydrogen sulfide cooperate to mitigate chromium stress in tomato plants by modulating the ascorbate-glutathione cycle, chromium sequestration, and subcellular allocation of chromium. Environ. Pollut. 2023, 335, 122292. [Google Scholar] [CrossRef] [PubMed]
- Tahjib-Ul-Arif, M.; Zahan, M.I.; Karim, M.M.; Imran, S.; Hunter, C.T.; Islam, M.S.; Mia, M.A.; Hannan, M.A.; Rhaman, M.S.; Hossain, M.A.; et al. Citric acid-mediated abiotic stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 7235. [Google Scholar] [CrossRef] [PubMed]
- Święcicka, M.; Dmochowska-Boguta, M.; Orczyk, W.; Grądzielewska, A.; Stochmal, A.; Kowalczyk, M.; Bolibok, L.; Rakoczy-Trojanowska, M. Changes in benzoxazinoid contents and the expression of the associated genes in rye (Secale cereale L.) due to brown rust and the inoculation procedure. PLoS ONE 2020, 15, e0233807. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, T.; Wang, Q.; Guo, Y.; Zhang, P.; Xie, H.; Liu, J.; Li, L.; Zhang, C.; Qin, P. Mechanisms of resistance to spot blotch in Yunnan iron shell wheat based on metabolome and transcriptomics. Int. J. Mol. Sci. 2022, 23, 5184. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Feng, B.; Ahmad, M.H.; Qamar, M.T.U.; Aslam, M.Z.; Khalid, M.F.; Hussain, S.; Zhong, R.; Ali, Q.; Xu, Q.; et al. LC-MS/MS-based metabolomics approach identified novel antioxidant flavonoids associated with drought tolerance in citrus species. Front. Plant Sci. 2023, 14, 1150854. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Bian, X.; Lee, C.J.; Park, S.U.; Lim, Y.H.; Kim, B.H.; Park, W.S.; Ahn, M.J.; Ji, C.Y.; Yu, Y.; et al. Overexpression of 4-hydroxyphenylpyruvate dioxygenase (IbHPPD) increases abiotic stress tolerance in transgenic sweetpotato plants. Plant Physiol. Biochem. 2021, 167, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Mata-Pérez, C.; Begara-Morales, J.C.; Chaki, M.; Sánchez-Calvo, B.; Valderrama, R.; Padilla, M.N.; Corpas, F.J.; Barroso, J.B. Protein tyrosine nitration during development and abiotic stress response in plants. Front. Plant Sci. 2016, 7, 1699. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Sharma, A.; Srivastava, A.K. Ascorbic acid: A metabolite switch for designing stress-smart crops. Crit. Rev. Biotechnol. 2024, 44, 1350–1366. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Biswas, A.K.; De, B. Influence of sodium chloride on growth and metabolic reprogramming in nonprimed and haloprimed seedlings of blackgram (Vigna mungo L.). Protoplasma 2020, 257, 1559–1583. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Hu, W.; Yin, B.; Liang, B.; Li, Z.; Zhang, X.; Xu, J.; Zhou, S. Integrative physiological, metabolomic, and transcriptomic analysis reveals the drought responses of two apple rootstock cultivars. BMC Plant Biol. 2024, 24, 219. [Google Scholar] [CrossRef]
- Zeier, J. New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ. 2013, 36, 2085–2103. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.-N.; Qin, Q.-Y.; Ma, W.-Y.; Zhou, L.-J.; Wu, Q.-S.; Xu, Y.-J.; Kuča, K.; Hashem, A.; Al-Arjani, A.-B.F.; Almutairi, K.F.; et al. Metabolomics reveals arbuscular mycorrhizal fungi-mediated tolerance of walnut to soil drought. BMC Plant Biol. 2023, 23, 118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Z.; Wang, Q.; Xie, J.; Yu, L. Transcriptomics and metabolomics revealed that phosphate improves the cold tolerance of alfalfa. Front. Plant Sci. 2023, 14, 1100601. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vázquez, L.; Lozano, M.V.; Rodríguez-Robledo, V.; González-Fuentes, J.; Marcos, P.; Villaseca, N.; Arroyo-Jiménez, M.M.; Santander-Ortega, M.J. Pressurized extraction as an opportunity to recover antioxidants from orange peels: Heat treatment and nanoemulsion design for modulating oxidative stress. Molecules 2021, 26, 5928. [Google Scholar] [CrossRef] [PubMed]
- Lwalaba, J.L.W.; Zvobgo, G.; Gai, Y.; Issaka, J.H.; Mwamba, T.M.; Louis, L.T.; Fu, L.; Nazir, M.M.; Kirika, B.A.; Tshibangu, A.K.; et al. Transcriptome analysis reveals the tolerant mechanisms to cobalt and copper in barley. Ecotoxicol. Environ. Saf. 2021, 209, 111761. [Google Scholar] [CrossRef]
- Jin, S.; Yoshida, M.; Nakajima, T.; Murai, A. Accumulation of hydroxycinnamic acid amides in winter wheat under snow. Biosci. Biotechnol. Biochem. 2003, 67, 1245–1249. [Google Scholar] [CrossRef]
- Neamțu, A.A.; Maghiar, T.A.; Turcuș, V.; Maghiar, P.B.; Căpraru, A.M.; Lazar, B.A.; Dehelean, C.A.; Pop, O.L.; Neamțu, C.; Totolici, B.D.; et al. A comprehensive view on the impact of chlorogenic acids on colorectal cancer. Curr. Issues Mol. Biol. 2024, 46, 6783–6804. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Wang, C.; Huang, Y.; Kong, W.; Wang, X. Effect of S-Allyl-L-Cysteine on nitric oxide and Cadmium processes in Rice (Oryza sativa L. sp. Zhongzao35) seedlings. Toxics 2024, 12, 805. [Google Scholar] [CrossRef]
- Cheng, L.L.; Huang, Y.C.; Wang, C.R.; Liu, Z.Q.; Huang, Y.Z.; Zhang, C.B.; Wagn, X.L. Mechanism of S-allyl-L-cysteine alleviating Cadmium stress in seedling roots and buds of rice seedlings. Huan Jing Ke Xue 2021, 42, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Owusu, A.G.; Lv, Y.P.; Liu, M.; Wu, Y.; Li, C.L.; Guo, N.; Li, D.H.; Gao, J.S. Transcriptomic and metabolomic analyses reveal the potential mechanism of waterlogging resistance in cotton (Gossypium hirsutum L.). Front. Plant Sci. 2023, 14, 1088537. [Google Scholar] [CrossRef]
- Zhuang, Q.; Chen, S.; Jua, Z.; Yao, Y. Joint transcriptomic and metabolomic analysis reveals the mechanism of low-temperature tolerance in Hosta ventricosa. PLoS ONE 2021, 16, e0259455. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, J.; Miao, P.; Dong, Q.; Lin, Y.; Li, D.; Pan, C. Novel finding on how melatonin and nanoselenium alleviate 2,4-D butylate stress in wheat plants. J. Agric. Food Chem. 2023, 71, 12943–12957. [Google Scholar] [CrossRef]
- Dong, X.; Han, B.; Chen, J.; Luo, D.; Zhou, Q.; Liu, Z. Multiomics analyses reveal MsC3H29 positively regulates flavonoid biosynthesis to improve drought resistance of autotetraploid cultivated alfalfa (Medicago sativa L.). J. Agric. Food Chem. 2024, 72, 14448–14465. [Google Scholar] [CrossRef] [PubMed]
- Kasthuri, T.; Barath, S.; Nandhakumar, M.; Karutha Pandian, S. Proteomic profiling spotlights the molecular targets and the impact of the natural antivirulent umbelliferone on stress response, virulence factors, and the quorum sensing network of Pseudomonas aeruginosa. Front. Cell Infect. Microbiol. 2022, 12, 998540. [Google Scholar] [CrossRef] [PubMed]
- Beesley, A.; Beyer, S.F.; Wanders, V.; Levecque, S.; Bredenbruch, S.; Habash, S.S.; Schleker, A.S.S.; Gätgens, J.; Oldiges, M.; Schultheiss, H.; et al. Engineered coumarin accumulation reduces mycotoxin-induced oxidative stress and disease susceptibility. Plant Biotechnol. J. 2023, 21, 2490–2506. [Google Scholar] [CrossRef]
- Zhou, Y.; Bai, Y.H.; Han, F.X.; Chen, X.; Wu, F.S.; Liu, Q.; Ma, W.Z.; Zhang, Y.Q. Transcriptome sequencing and metabolome analysis reveal the molecular mechanism of Salvia miltiorrhiza in response to drought stress. BMC Plant Biol. 2024, 24, 446. [Google Scholar] [CrossRef]
| Compounds | Class | Up- or Down-Regulated in tor-es | Function | References |
|---|---|---|---|---|
| 1,5-Diaminopentane | Phenolamides | up | Drought, oxidative stress, heavy metal stress | [89,90] |
| 2-Aminoadipic acid (L-Homoglutamic acid) | Amino acids and derivatives | up | Oxidative stress, drought, salt | [91,92,93,94] |
| 3-Indoleacetonitrile | Indole derivatives | down | Pathogen attack | [95,96,97] |
| 3-Methylmalic acid | Organic acids and derivatives | up | Salt, drought, biotic stress | [98,99,100] |
| 4-Hydroxycoumarin | Phenylpropanoids | down | Pathogen attack | [101] |
| 5-Aminolevulinate | Organic acids and derivatives | up | Drought, salt, heavy metal | [102,103,104,105,106,107,108] |
| 5-Oxoproline | Amino acids and derivatives | up | Heat stress | [109,110] |
| 6-Aminocaproic acid | Organic acids and derivatives | up | Salt stress | [111] |
| Amentoflavone | Flavone | up | Temperature, light, drought, biotic stress | [112] |
| Caffeic acid | Phenylpropanoids | up | Salt stress | [113,114] |
| Caffeine | Alkaloids | down | Drought, biotic stress | [115,116] |
| Camalexin | Alkaloids | up | Pathogen attack | [117] |
| Chlorogenic acid (3-O-Caffeoylquinic acid) | Organic acids and derivatives | down | Cold stress | [118] |
| Chrysoeriol | Flavone | up | Oxidative stress | [119,120] |
| Citramalate | Organic acids and derivatives | up | Pathogen attack | [121] |
| Citric acid monohydrate | Organic acids and derivatives | up | Heavy metal | [122,123,124,125,126] |
| Citric acid | Organic acids and derivatives | up | Heavy metal | [122,123,124,125,126] |
| DIMBOA glucoside | Others | up | Biotic stress | [127,128] |
| Genistein 7-O-Glucoside (Genistin) | Isoflavone | up | Drought | [129] |
| Homogentisic acid | Organic acids and derivatives | up | Abiotic stress, ABA signaling | [130] |
| L-(-)-Tyrosine | Amino acids and derivatives | up | Abiotic stress | [131] |
| L-Ascorbate | Vitamins and derivatives | up | Abiotic stress | [132] |
| L-Homoserine | Amino acids and derivatives | up | Drought, salt stress | [133,134] |
| L-Pipecolic acid | Amino acids and derivatives | up | Biotic stress | [135] |
| N-Acetyl-L-phenylalanine | Amino acids and derivatives | up | Cold, drought | [136,137] |
| Narirutin | Flavone | up | Heavy metal stress, oxidative stress | [138,139] |
| N-p-Coumaroyl agmatine | Phenolamides | up | Biotic stress | [140] |
| Quinic acid | Organic acids and derivatives | up | Abiotic stress | [141] |
| S-Allyl-L-cysteine | Amino acids and derivatives | up | Heavy metal stress | [142,143] |
| Sinapyl alcohol | Phenylpropanoids | up | Flooding stress, cold stress | [144,145] |
| Tricin | Flavone | down | Cold, drought, salt stress | [146,147] |
| Umbelliferone | Phenylpropanoids | down | Pathogen attack | [148,149] |
| Xanthohumol | Flavanone | up | Drought stress | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhang, R.; Zhang, H.; Yang, Y.; Fu, L. TOR Mediates Stress Responses Through Global Regulation of Metabolome in Plants. Int. J. Mol. Sci. 2025, 26, 2095. https://doi.org/10.3390/ijms26052095
Yang L, Zhang R, Zhang H, Yang Y, Fu L. TOR Mediates Stress Responses Through Global Regulation of Metabolome in Plants. International Journal of Molecular Sciences. 2025; 26(5):2095. https://doi.org/10.3390/ijms26052095
Chicago/Turabian StyleYang, Lin, Ran Zhang, Huan Zhang, Yingyu Yang, and Liwen Fu. 2025. "TOR Mediates Stress Responses Through Global Regulation of Metabolome in Plants" International Journal of Molecular Sciences 26, no. 5: 2095. https://doi.org/10.3390/ijms26052095
APA StyleYang, L., Zhang, R., Zhang, H., Yang, Y., & Fu, L. (2025). TOR Mediates Stress Responses Through Global Regulation of Metabolome in Plants. International Journal of Molecular Sciences, 26(5), 2095. https://doi.org/10.3390/ijms26052095





