Improved Adhesion and Biocompatibility of Chitosan-Coated Super-Hydrophilic PVC Polymer Substrates for Urothelial Catheters
Abstract
:1. Introduction
2. Results and Discussion
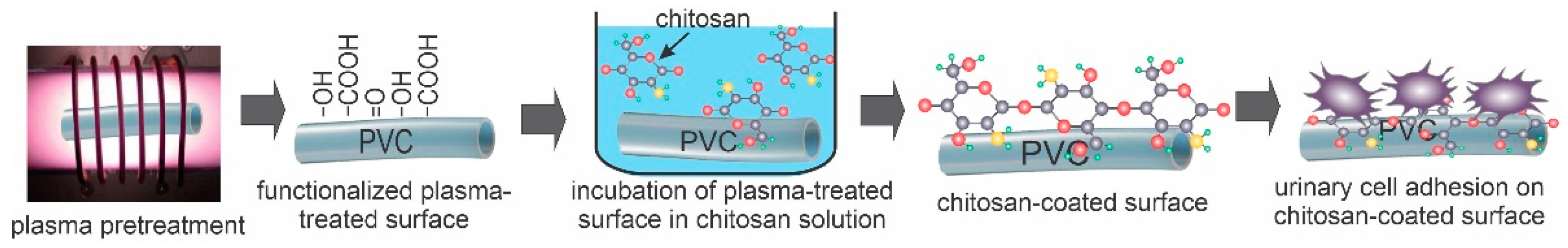
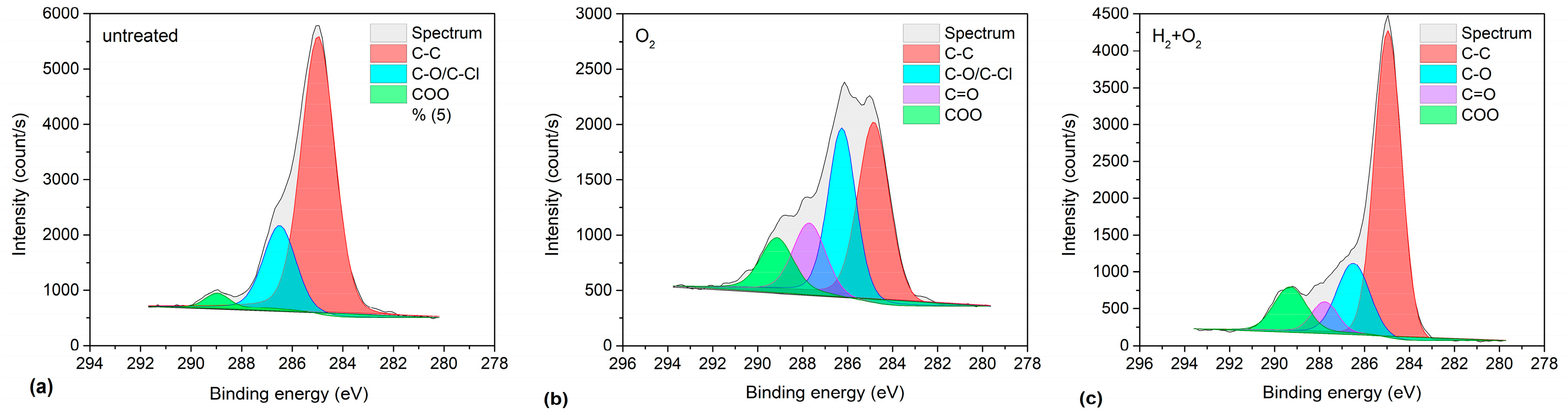
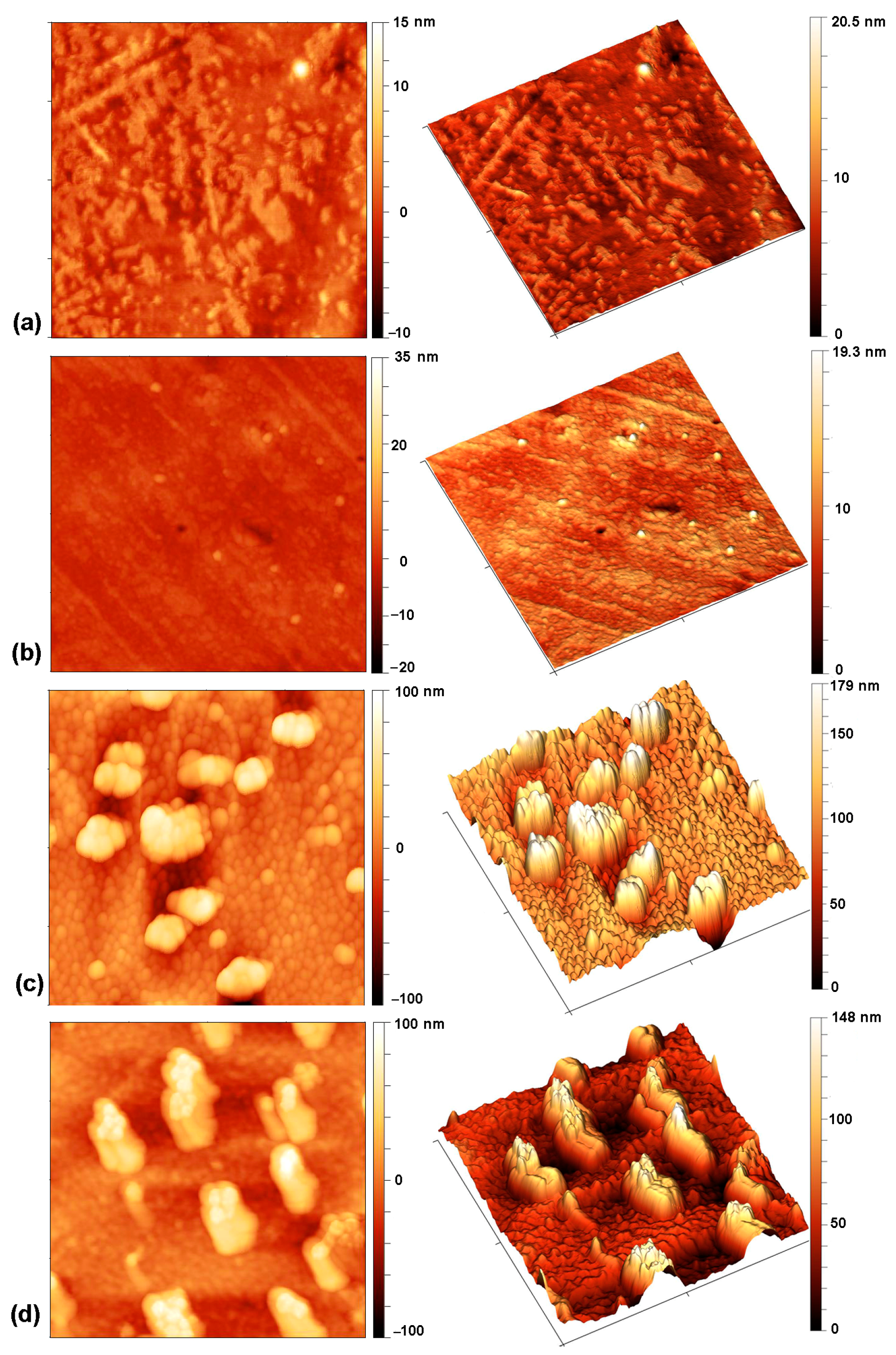
2.1. Plasma Treatment of PVC Substrates
2.2. Deposition of Chitosan on Plasma-Treated PVC Substrates
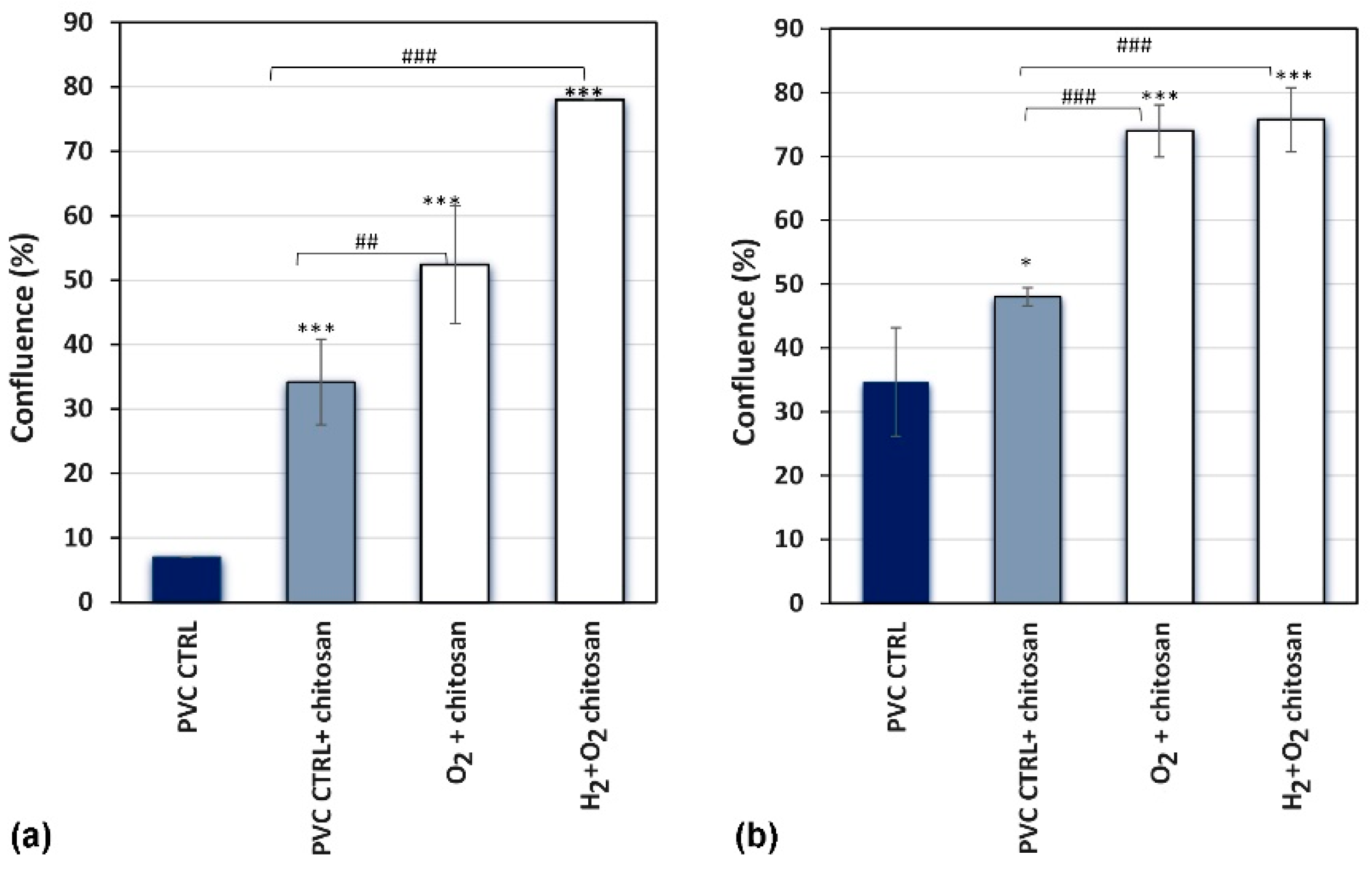
2.3. Adhesion and Proliferation of Urothelial Cells on Chitosan-Coated PVC Substrates
2.3.1. Metabolic Activity
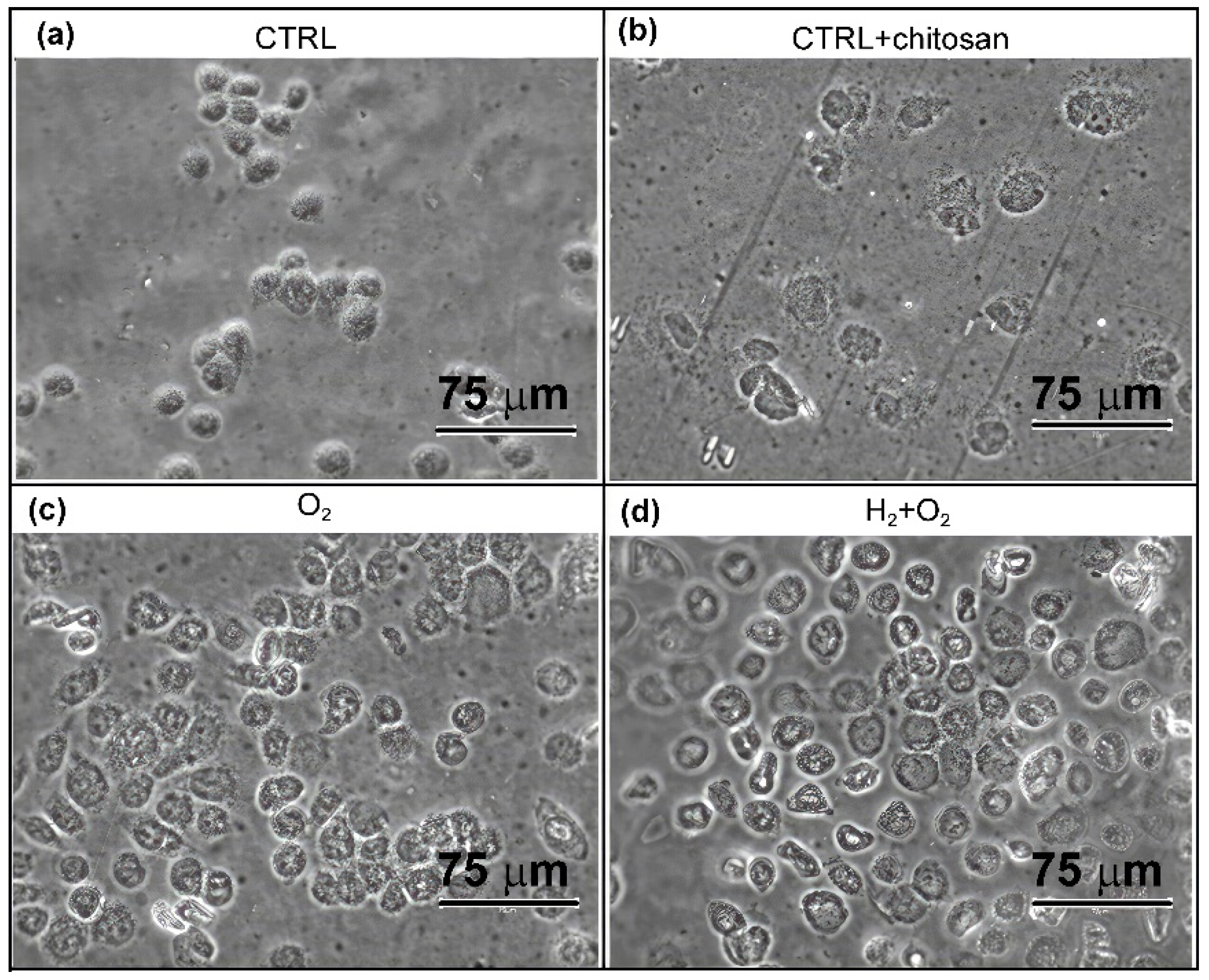
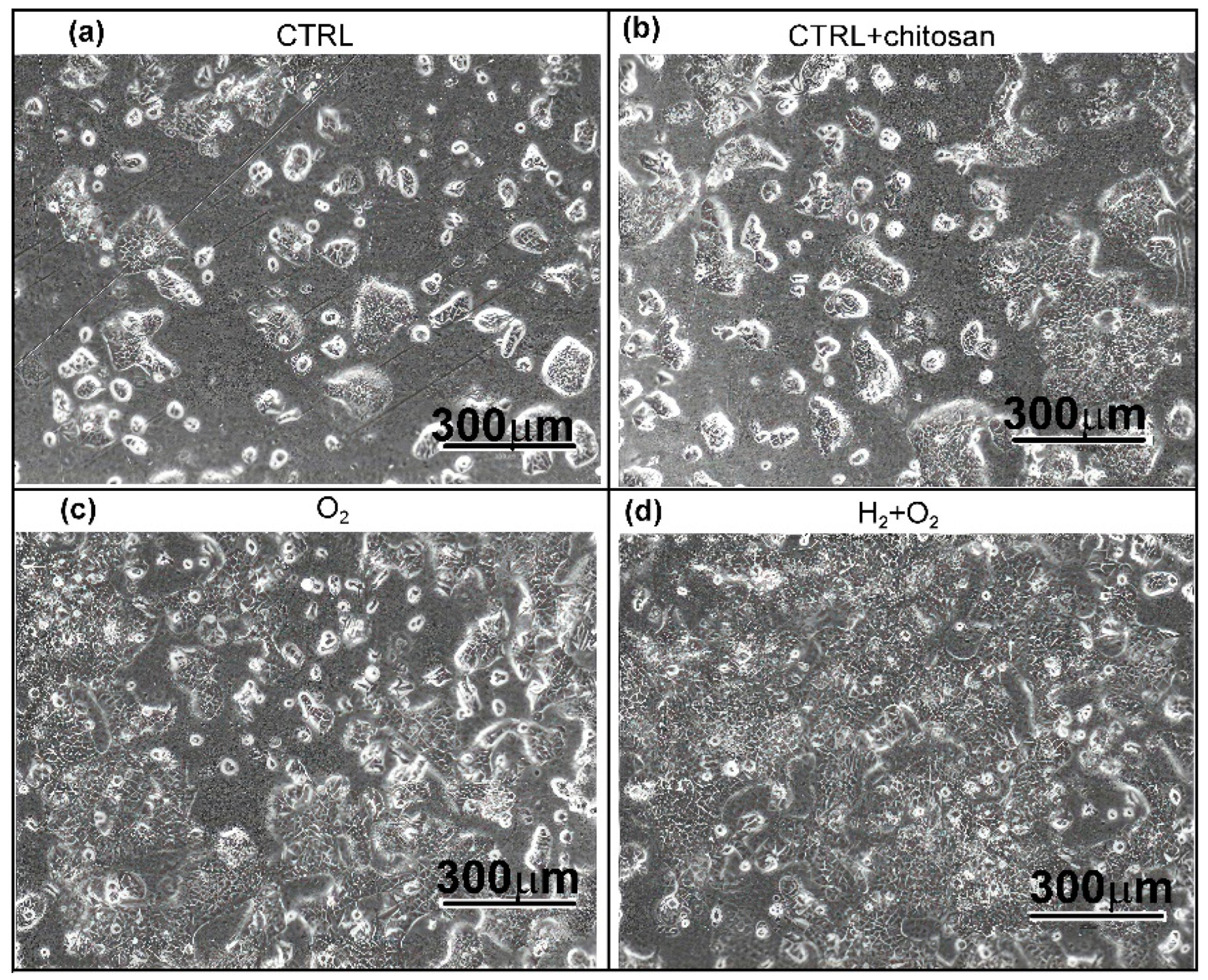
2.3.2. Visualisation and Confluence
3. Materials and Methods
3.1. Plasma Treatment Procedure
3.2. Surface Wettability
3.3. Deposition of Chitosan
3.4. Surface Morphology
3.5. Chemical Composition
3.6. Resazurin Reduction-Based Cell Viability Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PVC | Polyvinyl chloride |
| WCA | Water contact angle |
| VUV | Vacuum ultraviolet radiation |
| AFM | Atomic force microscopy |
| SEM | Scanning electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
| HRES | High-resolution XPS spectra |
| LMWOM | Low-molecular weight-oxidized material |
| CTRL | Control sample (untreated PVC) |
| RT4 | Human urinary bladder cell line |
| FBS | Fetal bovine serum |
| PBS | Phosphate-buffered saline |
| DMEM | Dulbecco’s Modified Eagle Medium |
| RF | Radiofrequency |
References
- Lucas, E.J.; Baxter, C.; Singh, C.; Mohamed, A.Z.; Li, B.; Zhang, J.; Jayanthi, V.R.; Koff, S.A.; VanderBrink, B.; Justice, S.S. Comparison of the microbiological milieu of patients randomized to either hydrophilic or conventional PVC catheters for clean intermittent catheterization. J. Pediatr. Urol. 2016, 12, 172.e1–172.e8. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Eljezi, T.; Clauson, H.; Lambert, C.; Bouattour, Y.; Chennell, P.; Pereira, B.; Sautou, V. ARMED Study Group, Effects of flow rate on the migration of different plasticizers from PVC infusion medical devices. PLoS ONE 2018, 13, e0192369. [Google Scholar] [CrossRef]
- Li, S.; Duan, W.; Lei, Y.; Wang, Z.; Fu, C.; He, L.; Shen, Z.; Li, M.; Chen, Y.; Huang, Y. Effects of lipid emulsions on the formation of Escherichia coli–Candida albicans mixed-species biofilms on PVC. Sci. Rep. 2021, 11, 16929. [Google Scholar] [CrossRef] [PubMed]
- Feit, C.G.; Chug, M.K.; Brisbois, E.J. Development of S-nitroso-N-acetylpenicillamine impregnated medical grade Polyvinyl Chloride for antimicrobial medical device interfaces. ACS Appl. Bio Mater. 2019, 2, 4335–4345. [Google Scholar] [CrossRef]
- Dadi, N.C.T.; Radochová, B.; Vargová, J.; Bujdáková, H. Impact of healthcare-associated infections connected to medical devices-an update. Microorganisms 2021, 9, 2332. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Weinstein, R.A.; Darouiche, R.O. Device-associated infections: A macroproblem that starts with microadherence. Clin. Infect. Dis. 2001, 33, 1567–1572. [Google Scholar] [CrossRef]
- Shafer, C.W.; Allison, J.R.; Hogue, A.L.; Huntington, M.K. Infectious disease: Health care-associated infections. FP Essent. 2019, 476, 30–42. [Google Scholar]
- Forrester, J.D.; Maggio, P.M.; Tennakoon, L. Cost of health care-associated infections in the United States. J. Patient. Saf. 2022, 18, e477–e479. [Google Scholar] [CrossRef] [PubMed]
- Gahlot, R.; Nigam, C.; Kumar, V.; Yadav, G.; Anupurba, S. Catheter-related bloodstream infections. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Atkins, L.; Sallis, A.; Chadborn, T.; Shaw, K.; Schneider, A.; Hopkins, S.; Bunten, A.; Michie, S.; Lorencatto, F. Reducing catheter-associated urinary tract infections: A systematic review of barriers and facilitators and strategic behavioural analysis of interventions. Implement. Sci. 2020, 15, 44. [Google Scholar] [CrossRef]
- Allegranzi, B.; Bischoff, P.; de Jonge, S.; Kubilay, N.Z.; Zayed, B.; Gomes, S.M.; Abbas, M.; Atema, J.J.; Gans, S.; WHO Guidelines Development Group; et al. New WHO recommendations on preoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e276–e287. [Google Scholar] [CrossRef] [PubMed]
- Shin, E. Antimicrobials and antimicrobial resistant superbacteria. Ewha Med. J. 2017, 40, 99–103. [Google Scholar] [CrossRef]
- Lunkov, A.P.; Ilyina, A.V.; Varlamov, V.P. Antioxidant, antimicrobial, and fungicidal properties of chitosan based films (Review). Appl. Biochem. Microbiol. 2018, 54, 449–458. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial chitosan and chitosan derivatives: A review of the structure–activity relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.P.; Taffs, R.; Davison, W.M.; Stewart, P.S. Anti-biofilm properties of chitosan-coated surfaces. J. Biomater. Sci. Polym. Ed. 2008, 19, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Erişen, D.E.; Yang, K.; Zhang, B.; Shen, M.; Zou, J.; Qi, X.; Chen, S.; Xu, X. Antibacterial and anti-inflammatory activities of chitosan/copper complex coating on medical catheters: In vitro and in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food. Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-L.; Wang, J.-L.; Li, D.-D.; Ren, K.-F.; Ji, J. Chitosan/poly (vinyl pyrollidone) coatings improve the antibacterial properties of poly(ethylene terephthalate). Appl. Surf. Sci. 2012, 258, 7801–7808. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Lima, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial coatings based on chitosan to prevent implant-associated infections: A systematic review. iScience 2021, 24, 103480. [Google Scholar] [CrossRef] [PubMed]
- Asadinezhad, A.; Novák, I.; Lehocký, M.; Bílek, F.; Vesel, A.; Junkar, I.; Sáha, P.; Popelka, A. Polysaccharides coatings on medical-grade PVC: A probe into surface characteristics and the extent of bacterial adhesion. Molecules 2010, 15, 1007–1027. [Google Scholar] [CrossRef]
- Niemczyk, A.; El Fray, M.; Franklin, S.E. Friction behaviour of hydrophilic lubricious coatings for medical device applications. Tribol. Int. 2015, 89, 54–61. [Google Scholar] [CrossRef]
- López-Valverde, N.; Aragoneses, J.; López-Valverde, A.; Rodríguez, C.; de Sousa, B.M.; Aragoneses, J.M. Role of chitosan in titanium coatings. trends and new generations of coatings. Front. Bioeng. Biotechnol. 2022, 10, 907589. [Google Scholar] [CrossRef]
- Paradowska-Stolarz, A.; Mikulewicz, M.; Laskowska, J.; Karolewicz, B.; Owczarek, A. The importance of chitosan coatings in dentistry. Mar. Drugs 2023, 21, 613. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, M.; Attik, N.; Amalric, J.; Brunon, C.; Renaud, F.; Abouelleil, H.; Toury, B.; Grosgogeat, B. Chitosan coating as an antibacterial surface for biomedical applications. PLoS ONE 2017, 12, e0189537. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.H.; Bumgardner, J.D.; Yang, Y.; Moseley, J.; Haggard, W.O. Chitosan-coated stainless steel screws for fixation in contaminated fractures. Clin. Orthop. Relat. Res. 2008, 466, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.-W.; Niu, G.C.-C.; Chang, S.J.; Yao, C.-H.; Kuo, S.M. Chitosan in applications of biomedical devices. Biomed. Eng. Appl. Basis Commun. 2011, 23, 51–62. [Google Scholar] [CrossRef]
- Ji, M.; Li, J.; Wang, Y.; Li, F.; Man, J.; Li, J.; Zhang, C.; Peng, S.; Wang, S. Advances in chitosan-based wound dressings: Modifications, fabrications, applications and prospects. Carbohydr. Polym. 2022, 297, 120058. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Huo, Q.; Lin, X.; Chu, X.; Deng, Z.; Guo, H.; Peng, Y.; Lu, S.; Zhou, X.; Wang, X.; et al. Drug-free contact lens based on quaternized chitosan and tannic acid for bacterial keratitis therapy and corneal repair. Carbohydr. Polym. 2022, 286, 119314. [Google Scholar] [CrossRef] [PubMed]
- Ashtiani, M.K.; Zandi, M.; Shokrollahi, P.; Ehsani, M.; Baharvand, H. Chitosan surface modified hydrogel as a therapeutic contact lens. Polym. Adv. Technol. 2020, 31, 741–748. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Niu, W.; Dong, Y.; Fu, Z.; Lv, J.; Wang, L.; Zhang, Z.; Huo, J.; Ju, J. Effects of molecular weight of chitosan on anti-inflammatory activity and modulation of intestinal microflora in an ulcerative colitis model. Int. J. Biol. Macromol. 2021, 193, 1927–1936. [Google Scholar] [CrossRef]
- Feneley, R.C.L.; Hopley, I.B.; Wells, P.N.T. Urinary catheters: History, current status, adverse events and research agenda. J. Med. Eng. Technol. 2015, 39, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface modification of polymers: Methods and applications. Adv. Mater. Interfaces 2018, 5, 1801247. [Google Scholar] [CrossRef]
- Tu, C.-Y.; Liu, Y.-L.; Lee, K.-R.; Lai, J.-Y. Surface grafting polymerization and modification on poly(tetrafluoroethylene) films by means of ozone treatment. Polymer 2005, 46, 6976–6985. [Google Scholar] [CrossRef]
- Popelka, A.; Novák, I.; Al-Maadeed, M.A.S.A.; Ouederni, M.; Krupa, I. Effect of corona treatment on adhesion enhancement of LLDPE. Surf. Coat. Technol. 2018, 335, 118–125. [Google Scholar] [CrossRef]
- Zaporojtchenko, V.; Zekonyte, J.; Faupel, F. Effects of ion beam treatment on atomic and macroscopic adhesion of copper to different polymer materials. Nucl. Instrum. Methods Phys. Res. B 2007, 265, 139–145. [Google Scholar] [CrossRef]
- Kurose, K.; Okuda, T.; Nakai, S.; Tsai, T.-Y.; Nishijima, W.; Okada, M. Hydrophilization of polyvinyl chloride surface by ozonation. Surf. Rev. Lett. 2008, 15, 711–715. [Google Scholar] [CrossRef]
- Gabriel, M.; Strand, D.; Vahl, C.F. Cell adhesive and antifouling polyvinyl chloride surfaces via wet chemical modification. Artif. Organs 2012, 36, 839–844. [Google Scholar] [CrossRef]
- Zhao, E.; Wang, L.; Yan, L.; Torimoto, Y.; Li, Q. Surface modification of medical poly(vinyl chloride) with O− water. J. Appl. Polym. Sci. 2008, 110, 39–48. [Google Scholar] [CrossRef]
- Herrero, M.; Quéméner, E.; Ulvé, S.; Reinecke, H.; Mijangos, C.; Grohens, Y. Bacterial adhesion to poly(vinyl chloride) films: Effect of chemical modification and water induced surface reconstruction. J. Adhes. Sci. Technol. 2006, 20, 183–195. [Google Scholar] [CrossRef]
- Ru, L.; Jierong, C. Surface modification of poly (vinyl chloride) by long-distance and direct argon RF plasma. Chin. Sci. Bull. 2006, 51, 615–619. [Google Scholar] [CrossRef]
- Ghoranneviss, M.; Shahidi, S.; Wiener, J. Surface modification of poly vinyl chloride (PVC) using low pressure argon and oxygen plasma. Plasma Sci. Technol. 2010, 12, 204. [Google Scholar] [CrossRef]
- Xiao-jing, L.; Guan-jun, Q.; Jie-rong, C. The effect of surface modification by nitrogen plasma on photocatalytic degradation of polyvinyl chloride films. Appl. Surf. Sci. 2008, 254, 6568–6574. [Google Scholar] [CrossRef]
- Chicea, D.; Nicolae-Maranciuc, A. A review of chitosan-based materials for biomedical, food, and water treatment applications. Materials 2024, 17, 5770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H.; Liu, C.G. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Arkhangelskiy, A.; Quaranta, A.; Motta, A.; Yang, Y.; Yadavalli, V.K.; Maniglio, D. Atmospheric plasma-assisted deposition and patterning of natural polymers. Adv. Mater. Interfaces 2022, 9, 2200454. [Google Scholar] [CrossRef]
- Bertin, M.; Leitao, E.M.; Bickerton, S.; Verbeek, C.J.R. A review of polymer surface modification by cold plasmas toward bulk functionalization. Plasma Process. Polym. 2024, 21, 2300208. [Google Scholar] [CrossRef]
- Lojen, D.; Zaplotnik, R.; Primc, G.; Mozetič, M.; Vesel, A. Optimization of surface wettability of polytetrafluoroethylene (PTFE) by precise dosing of oxygen atoms. Appl. Surf. Sci. 2022, 598, 153817. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Mozetič, M.; Recek, N. Advanced method for efficient functionalization of polymers by intermediate free-radical formation with vacuum-ultraviolet radiation and producing superhydrophilic surfaces. J. Photochem. Photobiol. A Chem. 2023, 443, 114876. [Google Scholar] [CrossRef]
- Komppula, J.; Tarvainen, O. VUV diagnostics of electron impact processes in low temperature molecular hydrogen plasma. Plasma Sources Sci. Technol. 2015, 24, 045008. [Google Scholar] [CrossRef]
- Fantz, U.; Briefi, S.; Rauner, D.; Wünderlich, D. Quantification of the VUV radiation in low pressure hydrogen and nitrogen plasmas. Plasma Sources Sci. Technol. 2016, 25, 045006. [Google Scholar] [CrossRef]
- Wertheimer, M.R.; Fozza, A.C.; Holländer, A. Industrial processing of polymers by low-pressure plasmas: The role of VUV radiation. Nucl. Instrum. Methods Phys. Res. B 1999, 151, 65–75. [Google Scholar] [CrossRef]
- Zhang, Y.; Ishikawa, K.; Mozetič, M.; Tsutsumi, T.; Kondo, H.; Sekine, M.; Hori, M. Polyethylene terephthalate (PET) surface modification by VUV and neutral active species in remote oxygen or hydrogen plasmas. Plasma Process. Polym. 2019, 16, 1800175. [Google Scholar] [CrossRef]
- Asadinezhad, A.; Lehocký, M.; Sáha, P.; Mozetič, M. Recent progress in surface modification of polyvinyl chloride. Materials 2012, 5, 2937–2959. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; John Wiley & Sons: Chichester, UK, 1992. [Google Scholar]
- Bond Strength and Energy, Text Chemistry Libraries, LibreTexts: CA, USA, 2008, LibreTexts. Available online: https://chem.libretexts.org/Courses/Bellarmine_University/BU%3A_Chem_103_(Christianson)/Phase_3%3A_Atoms_and_Molecules_-_the_Underlying_Reality/9%3A_Chemical_Bonding/9.4%3A_Bond_Strength_and_Energy#:~:text=When%20one%20atom%20bonds%20to%20various%20atoms%20in,is%20330%20kJ%2Fmol%2C%20and%20C%E2%80%93Br%20is%20275%20kJ%2Fmol (accessed on 20 August 2024).
- Yousif, E.; Hasan, A. Photostabilization of poly(vinyl chloride)—Still on the run. J. Taibah Univ. Sci. 2015, 9, 421–448. [Google Scholar] [CrossRef]
- Dorai, R.; Kushner, M.J. A model for plasma modification of polypropylene using atmospheric pressure discharges. J. Phys. D Appl. Phys. 2003, 36, 666. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Primc, G.; Mozetič, M. Kinetics of surface wettability of aromatic polymers (PET, PS, PEEK, and PPS) upon treatment with neutral oxygen atoms from non-equilibrium oxygen plasma. Polymers 2024, 16, 1381. [Google Scholar] [CrossRef] [PubMed]
- Drelich, J.W. Contact angles: From past mistakes to new developments through liquid-solid adhesion measurements. Adv. Colloid Interface Sci. 2019, 267, 1–14. [Google Scholar] [CrossRef]
- Extrand, C.W. Contact Angles and Their Hysteresis as a Measure of Liquid−Solid Adhesion. Langmuir 2004, 20, 4017–4021. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, R.; Das, R.; Gulec, S.; Liu, J.; N’guessan, H.E.; Shah, M.; Wasnik, P.S.; Yadav, S.B. Solid–Liquid Work of Adhesion. Langmuir 2017, 33, 3594–3600. [Google Scholar] [CrossRef]
- Marmur, A.; Della Volpe, C.; Siboni, S.; Amirfazli, A.; Drelich, J.W. Contact angles and wettability: Towards common and accurate terminology. Surf. Innov. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- O’Hare, L.-A.; Leadley, S.; Parbhoo, B. Surface physicochemistry of corona-discharge-treated polypropylene film. Surf. Interface Anal. 2002, 33, 335–342. [Google Scholar] [CrossRef]
- Mix, R.; Friedrich, J.; Rau, A. Polymer Surface Modification by Aerosol Based DBD Treatment of Foils. Plasma Process. Polym. 2009, 6, 566–574. [Google Scholar] [CrossRef]
- Vida, J.; Ilčíková, M.; Přibyl, R.; Homola, T. Rapid Atmospheric Pressure Ambient Air Plasma Functionalization of Poly(styrene) and Poly(ethersulfone) Foils. Plasma Chem. Plasma Process. 2021, 41, 841–854. [Google Scholar] [CrossRef]
- Strobel, M.; Jones, V.; Lyons, C.S.; Ulsh, M.; Kushner, M.J.; Dorai, R.; Branch, M.C. A Comparison of Corona-Treated and Flame-Treated Polypropylene Films. Plasmas Polym. 2003, 8, 61–95. [Google Scholar] [CrossRef]
- Rochefeuille, S.; Berjoan, R.; Seta, P.; Jimenez, C.; Desfours, J.-P. XPS and AFM characterisation of selective monolayers for cationic detection: Application to field effect chemical micro-sensors. Chem. Phys. Lett. 2003, 376, 274–281. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. A step-by-step guide to perform x-ray photoelectron spectroscopy. J. Appl. Phys. 2022, 132, 011101. [Google Scholar] [CrossRef]
- Gouzman, I.; Dubey, M.; Carolus, M.D.; Schwartz, J.; Bernasek, S.L. Monolayer vs. multilayer self-assembled alkylphosphonate films: X-ray photoelectron spectroscopy studies. Surf. Sci. 2006, 600, 773–781. [Google Scholar] [CrossRef]
- Afanas’ev, V.P.; Selyakov, D.N.; Ridzel, O.Y.; Semenov-Shefov, M.A.; Strukov, A.N. Investigation of monolayer and submonolayer films using X-ray photoelectron spectroscopy. J. Phys. Conf. Ser. 2020, 1713, 012002. [Google Scholar] [CrossRef]
- Vesel, A.; Recek, N.; Zaplotnik, R.; Kurinčič, A.; Kuzmič, K.; Zemljič, L.F. A method for the immobilization of chitosan onto urinary catheters. Int. J. Mol. Sci. 2022, 23, 15075. [Google Scholar] [CrossRef] [PubMed]
- Vesel, A. Deposition of chitosan on plasma-treated polymers—A review. Polymers 2023, 15, 1109. [Google Scholar] [CrossRef]
- Renoud, P.; Toury, B.; Benayoun, S.; Attik, G.; Grosgogeat, B. Functionalization of titanium with chitosan via silanation: Evaluation of biological and mechanical performances. PLoS ONE 2012, 7, e39367. [Google Scholar] [CrossRef] [PubMed]
- Pusic, T.; Kaurin, T.; Liplin, M.; Budimir, A.; Curlin, M.; Grgic, K.; Sutlovic, A.; Valh, J.V. The stability of the chitosan coating on polyester fabric in the washing process. Tekstilec 2023, 66, 85–104. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Z.; Zhang, H.; Yao, C. A review on chitosan-based biomaterial as carrier in tissue engineering and medical applications. Eur. Polym. J. 2023, 191, 112059. [Google Scholar] [CrossRef]
- Di Lisa, D.; Muzzi, L.; Pepe, S.; Dellacasa, E.; Frega, M.; Fassio, A.; Martinoia, S.; Pastorino, L. On the way back from 3D to 2D: Chitosan promotes adhesion and development of neuronal networks onto culture supports. Carbohydr. Polym. 2022, 297, 120049. [Google Scholar] [CrossRef]
- Veronesi, F.; Brogini, S.; De Luca, A.; Bellini, D.; Casagranda, V.; Fini, M.; Giavaresi, G. Cell adhesion and initial bone matrix deposition on titanium-based implants with chitosan–collagen coatings: An in vitro study. Int. J. Mol. Sci. 2023, 24, 4810. [Google Scholar] [CrossRef]
- Qiu, P.; Feng, L.; Fu, Q.; Dai, T.; Liu, M.; Wang, P.; Lan, Y. Dual-functional polyetheretherketone surface with an enhanced osteogenic capability and an antibacterial adhesion property in vitro by chitosan modification. Langmuir 2022, 38, 14712–14724. [Google Scholar] [CrossRef]
- Kafi, M.A.; Aktar, K.; Todo, M.; Dahiya, R. Engineered chitosan for improved 3D tissue growth through paxillin-FAK-ERK activation. Regen. Biomater. 2020, 7, 141–151. [Google Scholar] [CrossRef]
- Law, K.-Y.; Zhao, H. Contact angle measurements and surface characterization techniques. In Surface Wetting: Characterization, Contact Angle, and Fundamentals; Law, K.-Y., Zhao, H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; p. 7. [Google Scholar] [CrossRef]
- Zemljič, L.F.; Tkavc, T.; Vesel, A.; Šauperl, O. Chitosan coatings onto polyethylene terephthalate for the development of potential active packaging material. Appl. Surf. Sci. 2013, 265, 697–703. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M. Analysis of chemical composition of biopolymers and biomaterials: An XPS study. In Functional Biomaterials; Mohan, T., Kleinschek, K.S., Eds.; John Wiley & Sons: Weinheim, Germany, 2023; p. 45. [Google Scholar] [CrossRef]
- Petiti, J.; Revel, L.; Divieto, C. Standard operating procedure to optimize resazurin-based viability assays. Biosensors 2024, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Tymetska, S.; Shymborska, Y.; Stetsyshyn, Y.; Budkowski, A.; Bernasik, A.; Awsiuk, K.; Donchak, V.; Raczkowska, J. Thermoresponsive smart copolymer coatings based on p(NIPAM-co-HEMA) and p(OEGMA-co-HEMA) brushes for regenerative medicine. ACS Biomater. Sci. Eng. 2023, 9, 6256–6272. [Google Scholar] [CrossRef] [PubMed]
- Ślusarczyk, K.; Flejszar, M.; Spilarewicz, K.; Wytrwal, M.; Awsiuk, K.; Wolski, K.; Raczkowska, J.; Janiszewska, N.; Chmielarz, P. On the way to increase osseointegration potential: Sequential SI-ATRP as promising tool for PEEK-based implant nano-engineering. Eur. Polym. J. 2024, 210, 112953. [Google Scholar] [CrossRef]
- Tymetska, S.; Lalik, S.; Rysz, J.; Bernasik, A.; Brzychczy-Włoch, M.; Gosiewski, T.; Drożdż, K.; Marzec, M.; Xi, Z.; Struczyńska, M.; et al. Poly(vinyl pyridine) coatings cross-linked with transition metal complexes as active layers for biosensors sensitive to protein adsorption and cell adhesion. Appl. Surf. Sci. 2024, 670, 160639. [Google Scholar] [CrossRef]
- Macior, A.; Zaborniak, I.; Wolski, K.; Spilarewicz, K.; Raczkowska, J.; Janiszewska, N.; Awsiuk, K.; Chmielarz, P. Synthesis of hydrophobic and antifouling wood-polymer materials through SI-ATRP: Exploring a versatile pathway for wood functionalization. ACS Appl. Polym. Mat. 2024, 6, 11427–11443. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.-D. The kINPen—A review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef]
- Drozdz, K.; Golda-Cepa, M.; Brzychczy-Wloch, M. Polyurethanes as biomaterials in medicine: Advanced applications, infection challenges, and innovative surface modification methods. Adv. Microbiol. 2024, 63, 223–238. [Google Scholar] [CrossRef]
- Mozetič, M. Plasma-stimulated super-hydrophilic surface finish of polymers. Polymers 2020, 12, 2498. [Google Scholar] [CrossRef]
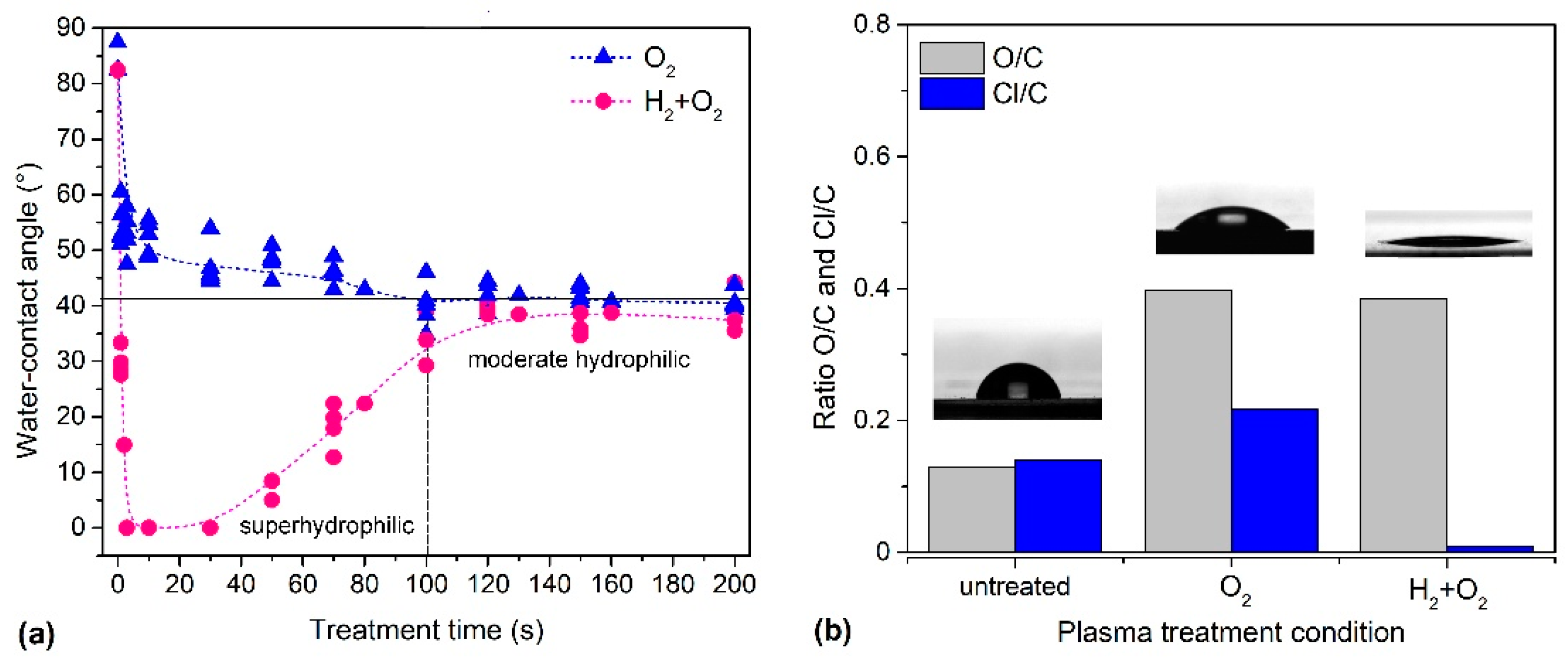

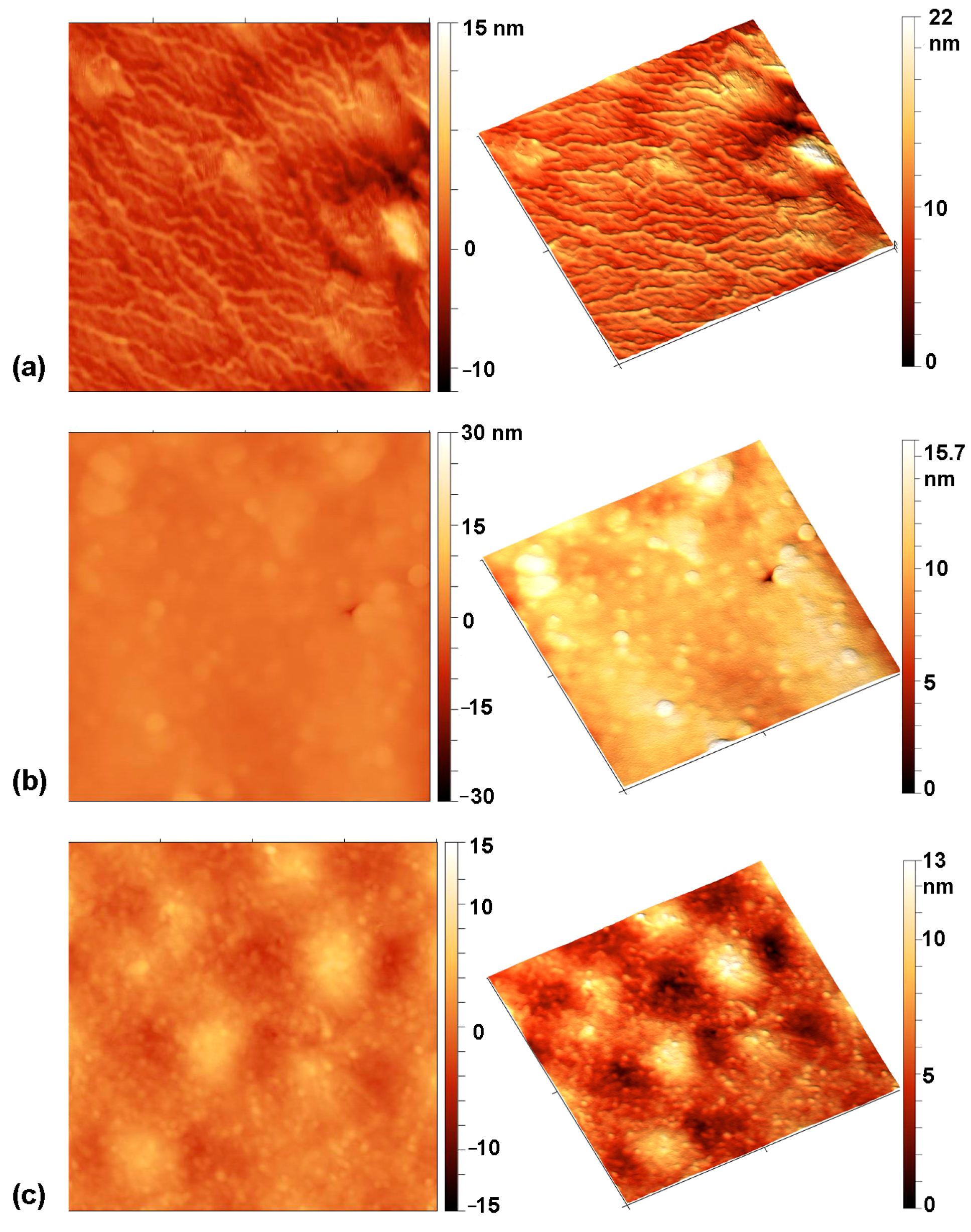
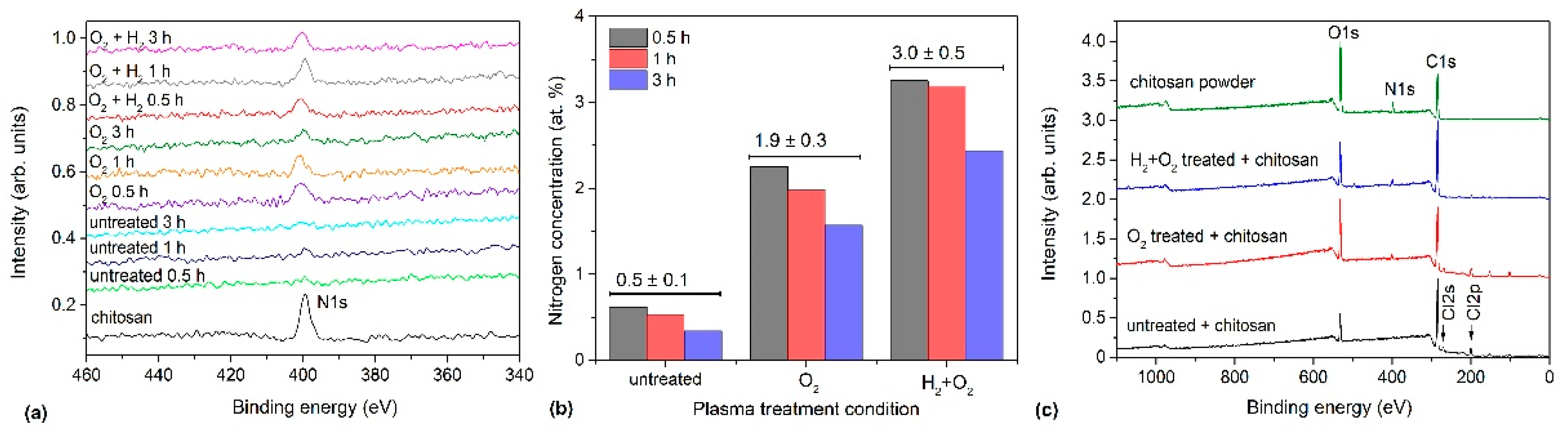
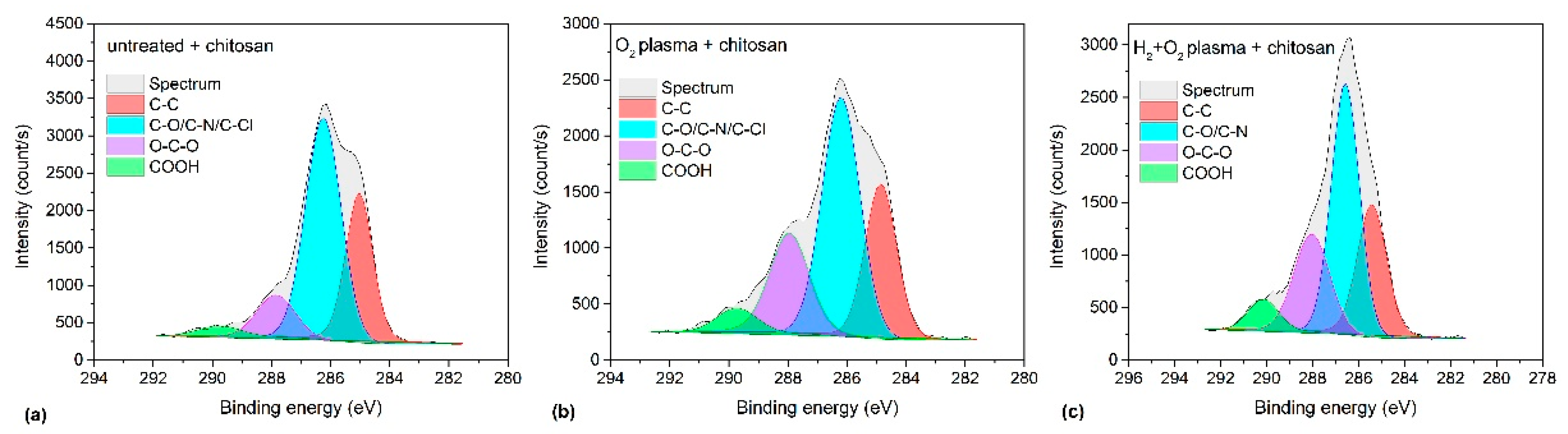
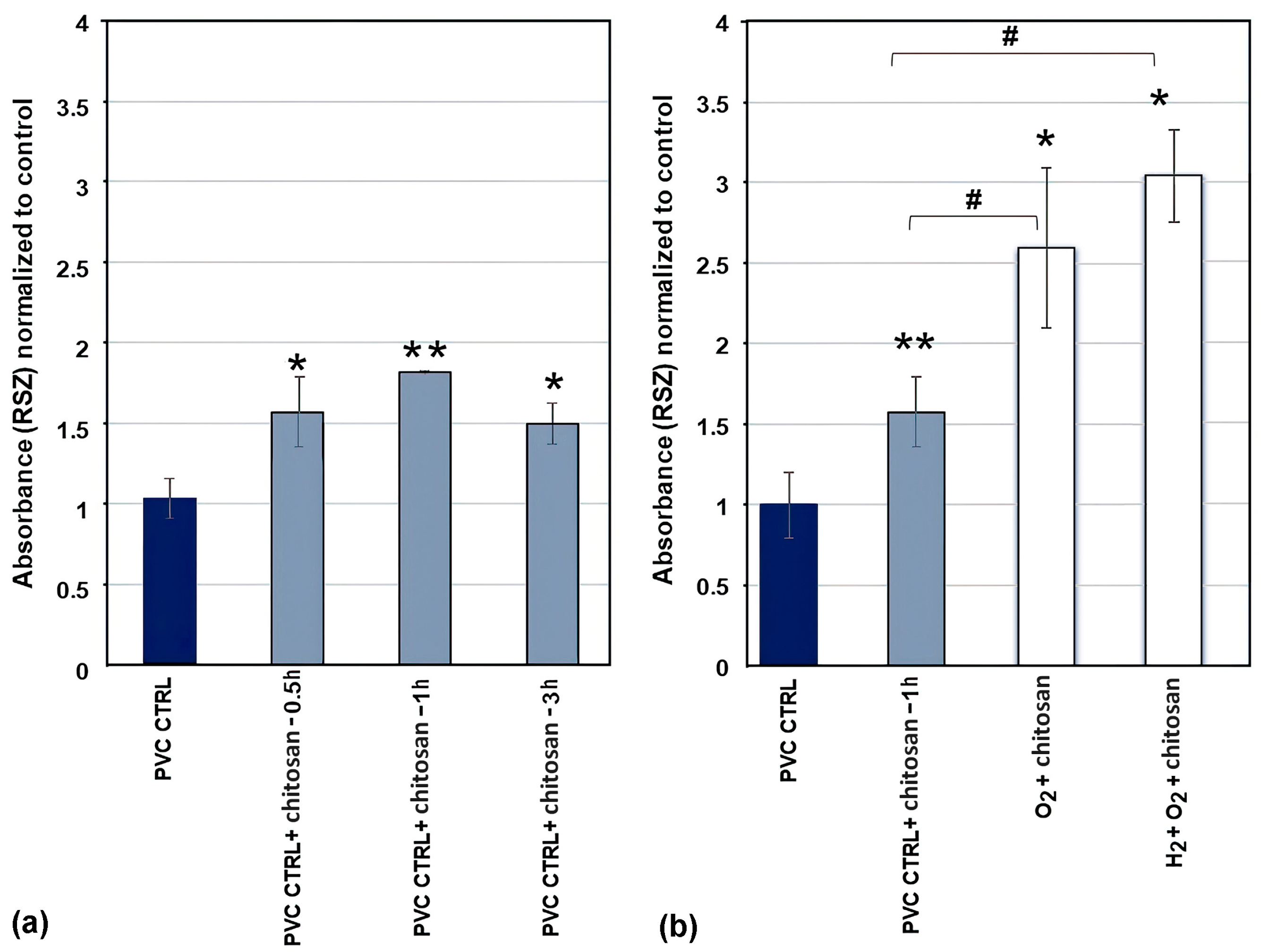

| Pre-Treatment | Treatment | C (at.%) | O (at.%) | Cl (at.%) | O/C | Cl/C | WCA (°) |
|---|---|---|---|---|---|---|---|
| none | untreated | 78.8 | 10.2 | 11.0 | 0.13 | 0.14 | 82.0 |
| none | O2 (100 s) | 60.9 | 24.3 | 14.8 | 0.40 | 0.24 | 38.0 |
| H2 (1 s) | O2 (3 s) | 71.6 | 28.0 | 0.4 | 0.39 | 0.01 | <2 |
| Pre-Treatment | Treatment | C–C (%) | C–O/C–Cl (%) | C=O (%) | COO(H) (%) |
|---|---|---|---|---|---|
| none | none | 79.5 | 17.8 | 2.7 | |
| none | O2 (100 s) | 42.2 | 36.8 | 11.0 | 10.0 |
| H2 plasma (1 s) | O2 (3 s) | 65.0 | 18.8 | 6.9 | 9.3 |
| Pre-Treatment | Treatment | (°) | (°) | (°) | (−) (°) | Ra (nm) | |
|---|---|---|---|---|---|---|---|
| none | untreated | 82.0 | 89.9 | 66.6 | 23.3 | 0.26 | 1.5 |
| none | O2 (100 s) | 38.0 | 42.3 | 17.5 | 24.8 | 0.59 | 13.9 |
| H2 (1 s) | O2 (3 s) | <5 | 8.4 | 4.5 | 3.9 | 0.46 | 1.2 |
| Pre-Treatment | Treatment | C (at.%) | N (at.%) | O (at.%) | Cl (at.%) |
|---|---|---|---|---|---|
| none | none | 80.8 | 0.6 | 14.4 | 4.2 |
| none | O2 | 72.6 | 2.2 | 21.4 | 3.8 |
| H2 plasma | O2 | 75.1 | 3.2 | 20.8 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesel, A.; Motaln, H.; Mozetič, M.; Lojen, D.; Recek, N. Improved Adhesion and Biocompatibility of Chitosan-Coated Super-Hydrophilic PVC Polymer Substrates for Urothelial Catheters. Int. J. Mol. Sci. 2025, 26, 2128. https://doi.org/10.3390/ijms26052128
Vesel A, Motaln H, Mozetič M, Lojen D, Recek N. Improved Adhesion and Biocompatibility of Chitosan-Coated Super-Hydrophilic PVC Polymer Substrates for Urothelial Catheters. International Journal of Molecular Sciences. 2025; 26(5):2128. https://doi.org/10.3390/ijms26052128
Chicago/Turabian StyleVesel, Alenka, Helena Motaln, Miran Mozetič, Dane Lojen, and Nina Recek. 2025. "Improved Adhesion and Biocompatibility of Chitosan-Coated Super-Hydrophilic PVC Polymer Substrates for Urothelial Catheters" International Journal of Molecular Sciences 26, no. 5: 2128. https://doi.org/10.3390/ijms26052128
APA StyleVesel, A., Motaln, H., Mozetič, M., Lojen, D., & Recek, N. (2025). Improved Adhesion and Biocompatibility of Chitosan-Coated Super-Hydrophilic PVC Polymer Substrates for Urothelial Catheters. International Journal of Molecular Sciences, 26(5), 2128. https://doi.org/10.3390/ijms26052128








