Adaptive Changes and Genetic Mechanisms in Organisms Under Controlled Conditions: A Review
Abstract
1. Introduction
2. Adaptive Changes in Organisms Under Controlled Conditions
2.1. Adaptive Changes in Microorganisms
2.1.1. Changes in Morphological, Physiological, and Reproductive Traits
2.1.2. Changes in Biochemical Indicators
2.2. Adaptive Changes in Animals
2.2.1. Changes in Reproductive and Fertility-Related Phenotypes
2.2.2. Changes in Physiological and Lifespan-Related Phenotypes
2.2.3. Changes in Behavioral and Life History Traits
2.2.4. Changes in Morphological Traits
2.3. Adaptive Changes in Plants
2.3.1. Changes in Plant Morphology
2.3.2. Changes in Biochemical Parameters
3. Genetic Mechanisms and Their Role in Adaptive Changes
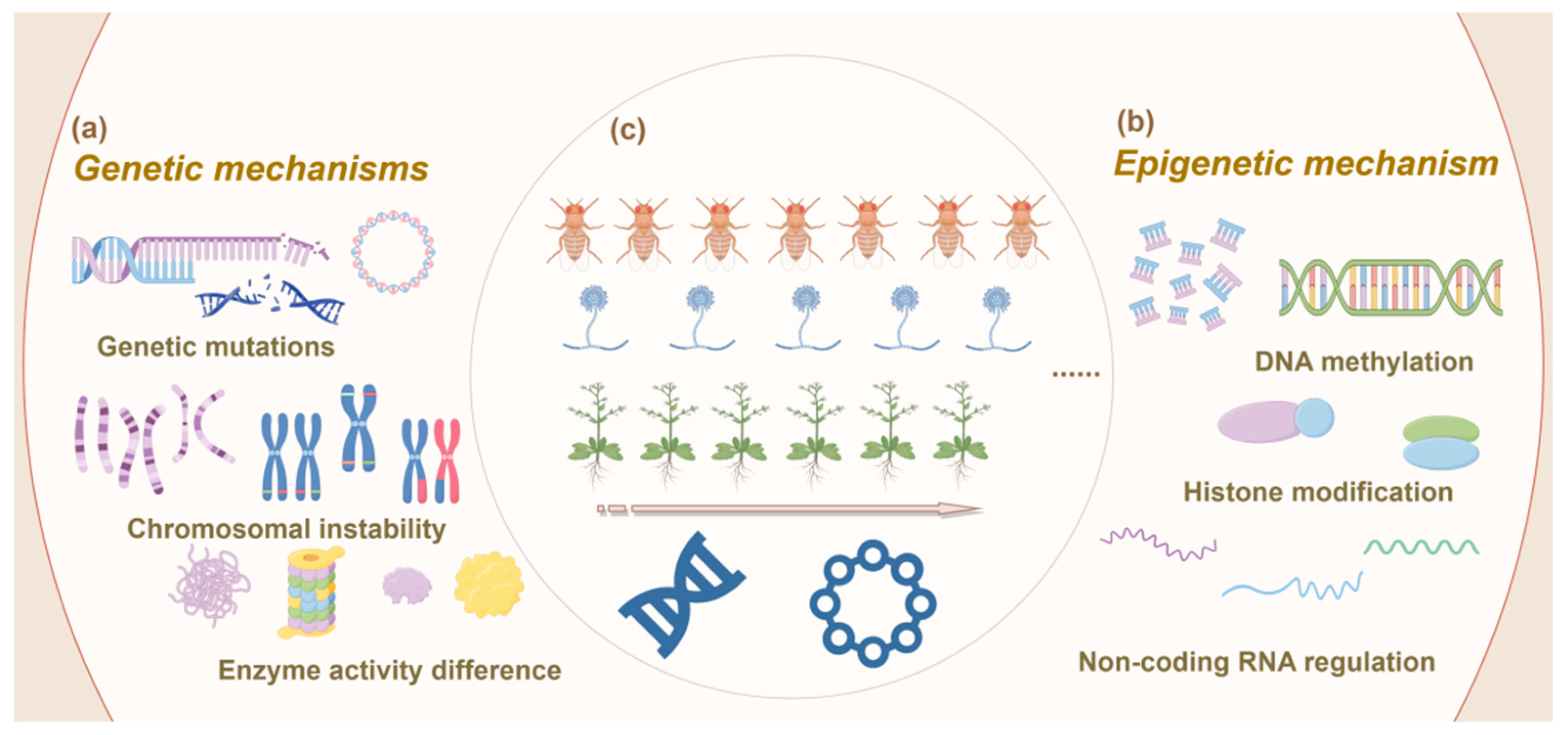
3.1. Genetic Mechanisms
3.1.1. Classical Genetic Mechanisms
3.1.2. Epigenetic Mechanisms
3.2. The Role of Genetic Mechanisms in Adaptation
3.2.1. Adaptation in Microorganisms
3.2.2. Adaptation in Animals
3.2.3. Adaptation in Plants
4. Discussion
4.1. Environmental Conditions and Adaptive Changes
4.2. Mechanisms Underlying Adaptive Changes
4.3. Variation in Adaptive Rates Across Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parsons, P.A. The metabolic cost of multiple environmental stresses: Implications for climatic change and conservation. Trends Ecol. Evol. 1990, 5, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Seaborn, T.; Griffith, D.; Kliskey, A.; Caudill, C.C. Building a bridge between adaptive capacity and adaptive potential to understand responses to environmental change. Glob. Chang. Biol. 2021, 27, 2656–2668. [Google Scholar] [CrossRef] [PubMed]
- Kingsolver, J.G.; Buckley, L.B. Evolution of plasticity and adaptive responses to climate change along climate gradients. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170386. [Google Scholar] [CrossRef]
- Grant, P.R. Evolution, climate change, and extreme events. Science 2017, 357, 451–452. [Google Scholar] [CrossRef]
- Zheng, J.; Payne, J.L.; Wagner, A. Cryptic genetic variation accelerates evolution by opening access to diverse adaptive peaks. Science 2019, 365, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, T.; Morrissey, M.B.; de Villemereuil, P.; Alberts, S.C.; Arcese, P.; Bailey, L.D.; Boutin, S.; Brekke, P.; Brent, L.J.N.; Camenisch, G. Genetic variance in fitness indicates rapid contemporary adaptive evolution in wild animals. Science 2022, 376, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Ghalambor, C.K.; Hoke, K.L.; Ruell, E.W.; Fischer, E.K.; Reznick, D.N.; Hughes, K.A. Erratum: Non-adaptive plasticity potentiates rapid adaptive evolution of gene expression in nature. Nature 2018, 555, 688. [Google Scholar] [CrossRef]
- Lustenhouwer, N.; Wilschut, R.A.; Williams, J.L.; van der Putten, W.H.; Levine, J.M. Rapid evolution of phenology during range expansion with recent climate change. Glob. Chang. Biol. 2018, 24, e534–e544. [Google Scholar] [CrossRef] [PubMed]
- Ashe, A.; Sapetschnig, A.; Weick, E.-M.; Mitchell, J.; Bagijn, M.P.; Cording, A.C.; Doebley, A.-L.; Goldstein, L.D.; Lehrbach, N.J.; Le Pen, J. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell 2012, 150, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.M.; Hellmann, J.K. An integrative framework for understanding the mechanisms and multigenerational consequences of transgenerational plasticity. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 97–118. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Cui, J.; Wang, L.; Teng, N.; Zhang, S.; Lam, H.-M.; Zhu, Y.; Xiao, S.; Ke, W.; Lin, J. Genome-wide DNA mutations in Arabidopsis plants after multigenerational exposure to high temperatures. Genome Biol. 2021, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.Q.; Cho, M.; Zhu, H.; Puppala, H.L.; Escalera, G.; Zhong, W.; Colvin, V.L. Toxicity of quantum dots and cadmium salt to Caenorhabditis elegans after multigenerational exposure. Environ. Sci. Technol. 2013, 47, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kwak, J.I.; An, Y.-J. Multigenerational study of gold nanoparticles in Caenorhabditis elegans: Transgenerational effect of maternal exposure. Environ. Sci. Technol. 2013, 47, 5393–5399. [Google Scholar] [CrossRef]
- Byrne, M.; Foo, S.A.; Ross, P.M.; Putnam, H.M. Limitations of cross-and multigenerational plasticity for marine invertebrates faced with global climate change. Glob. Chang. Biol. 2020, 26, 80–102. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ye, Q.; Dai, W.; Zheng, J.; Li, Y.; Wang, C.; Luo, Z.; Yang, J.; Zhuo, W.; Wan, Q.-L. Cadmium exposure induces multigenerational inheritance of germ cell apoptosis and fertility suppression in Caenorhabditis elegans. Environ. Int. 2024, 191, 108952. [Google Scholar] [CrossRef]
- Ramanan, D.; Sefik, E.; Galván-Peña, S.; Wu, M.; Yang, L.; Yang, Z.; Kostic, A.; Golovkina, T.V.; Kasper, D.L.; Mathis, D. An immunologic mode of multigenerational transmission governs a gut Treg setpoint. Cell 2020, 181, 1276–1290.e13. [Google Scholar] [CrossRef]
- Kwak, J.I.; Kim, L.; An, Y.J. Microplastics promote the accumulation of negative fungal groups and cause multigenerational effects in springtails. J. Hazard. Mater. 2024, 466, 133574. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Jander, G. Molecular ecology of plant volatiles in interactions with insect herbivores. J. Exp. Bot. 2022, 73, 449–462. [Google Scholar] [CrossRef]
- Sakauchi, K.; Otaki, J.M. Soil microbes and plant-associated microbes in response to radioactive pollution may indirectly affect plants and insect herbivores: Evidence for indirect field effects from chernobyl and fukushima. Microorganisms 2024, 12, 364. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Bao, Y.; Zhang, M.; Liu, S.; Yu, C.; Dai, J.; Wu, C.; Tang, D.; Fang, W. An inactivating mutation in the vacuolar arginine exporter gene Vae results in culture degeneration in the fungus Metarhizium robertsii. Environ. Microbiol. 2022, 24, 2924–2937. [Google Scholar] [CrossRef]
- Pichersky, E.; Lewinsohn, E. Convergent evolution in plant specialized metabolism. Annu. Rev. Plant Biol. 2011, 62, 549–566. [Google Scholar] [CrossRef]
- Ono, E.; Murata, J. Exploring the evolvability of plant specialized metabolism: Uniqueness out of uniformity and uniqueness behind uniformity. Plant Cell Physiol. 2023, 64, 1449–1465. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Liu, X.; Cui, B.; Miao, W.; Cheng, W.; Zhao, F. Characteristics analysis reveals the progress of Volvariella volvacea mycelium subculture degeneration. Front. Microbiol. 2019, 10, 2045. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, Q.; An, X.; Tan, Q.; Yun, J.; Zhang, Y. Oxidative damage from repeated tissue isolation for subculturing causes degeneration in Volvariella volvacea. Front. Microbiol. 2023, 14, 1210496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Liu, X.; Chen, C.; Cheng, Z.; Wang, W.; Yun, J. Successive mycelial subculturing decreased lignocellulase activity and increased ROS accumulation in Volvariella volvacea. Front. Microbiol. 2022, 13, 997485. [Google Scholar] [CrossRef]
- Chen, A.; Wang, Y.; Shao, Y.; Huang, B. A novel technique for rejuvenation of degenerated caterpillar medicinal mushroom, Cordyceps militaris (Ascomycetes), a valued traditional Chinese medicine. Int. J. Med. Mushrooms 2017, 19, 87–91. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.S.A.; Hassan, W.H.B.; Sweilam, S.H.; Alqarni, M.H.S.; El Sayed, Z.I.; Abdel-Aal, M.M.; Abdelsalam, E.; Abdelaziz, S. Production, bioprocessing and anti-proliferative activity of camptothecin from Penicillium chrysogenum, “an endozoic of marine sponge, Cliona sp.”, as a metabolically stable camptothecin producing isolate. Molecules 2022, 27, 3033. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.S.A.; Mohamed, N.Z.; Safan, S.; Yassin, M.A.; Shaban, L.; Shindia, A.A.; Shad Ali, G.; Sitohy, M.Z. Restoring the Taxol biosynthetic machinery of Aspergillus terreus by Podocarpus gracilior Pilger microbiome, with retrieving the ribosome biogenesis proteins of WD40 superfamily. Sci. Rep. 2019, 9, 11534. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, B.; Song, J.; Pang, S.; Song, T.; Gao, S.; Zhang, Y.; Huang, H.; Qi, T. A molecular framework for signaling crosstalk between jasmonate and ethylene in anthocyanin biosynthesis, trichome development, and defenses against insect herbivores in Arabidopsis. J. Integr. Plant Biol. 2022, 64, 1770–1788. [Google Scholar] [CrossRef] [PubMed]
- Wellham, P.A.D.; Hafeez, A.; Gregori, A.; Brock, M.; Kim, D.-H.; Chandler, D.; de Moor, C.H. Culture degeneration reduces sex-related gene expression, alters metabolite production and reduces insect pathogenic response in Cordyceps militaris. Microorganisms 2021, 9, 1559. [Google Scholar] [CrossRef] [PubMed]
- Sgrò, C.M.; Terblanche, J.S.; Hoffmann, A.A. What can plasticity contribute to insect responses to climate change? Annu. Rev. Entomol. 2016, 61, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Rudman, S.M.; Greenblum, S.I.; Rajpurohit, S.; Betancourt, N.J.; Hanna, J.; Tilk, S.; Yokoyama, T.; Petrov, D.A.; Schmidt, P. Direct observation of adaptive tracking on ecological time scales in Drosophila. Science 2022, 375, eabj7484. [Google Scholar] [CrossRef] [PubMed]
- Levell, S.T.; Bedgood, S.A.; Travis, J. Plastic maternal effects of social density on reproduction and fitness in the least killifish, Heterandria formosa. Ecol. Evol. 2023, 13, e10074. [Google Scholar] [CrossRef] [PubMed]
- Castano-Sanz, V.; Gomez-Mestre, I.; Garcia-Gonzalez, F. Evolutionary consequences of pesticide exposure include transgenerational plasticity and potential terminal investment transgenerational effects. Evolution 2022, 76, 2649–2668. [Google Scholar] [CrossRef] [PubMed]
- Gillis, M.K.; Walsh, M.R. Individual variation in plasticity dulls transgenerational responses to stress. Funct. Ecol. 2019, 33, 1993–2002. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, F.; Sun, S. Transgenerational effects and temperature variation alter life history traits of the moon jellyfish. Front. Mar. Sci. 2022, 9, 913654. [Google Scholar] [CrossRef]
- Webster, A.K.; Jordan, J.M.; Hibshman, J.D.; Chitrakar, R.; Baugh, L.R. Transgenerational effects of extended dauer diapause on starvation survival and gene expression plasticity in Caenorhabditis elegans. Genetics 2018, 210, 263–274. [Google Scholar] [CrossRef]
- Castano-Sanz, V.; Gomez-Mestre, I.; Rodriguez-Exposito, E.; Garcia-Gonzalez, F. Pesticide exposure triggers sex-specific inter-and transgenerational effects conditioned by past sexual selection. Proc. R. Soc. B Biol. Sci. 2024, 291, 20241037. [Google Scholar] [CrossRef] [PubMed]
- Beaty, L.E.; Wormington, J.D.; Kensinger, B.J.; Bayley, K.N.; Goeppner, S.R.; Gustafson, K.D.; Luttbeg, B. Shaped by the past, acting in the present: Transgenerational plasticity of anti-predatory traits. Oikos 2016, 125, 1570–1576. [Google Scholar] [CrossRef]
- Graeve, A.; Janßen, M.; Villalba de la Pena, M.; Tollrian, R.; Weiss, L.C. Higher, faster, better: Maternal effects shorten time lags and increase morphological defenses in Daphnia lumholtzi offspring generations. Front. Ecol. Evol. 2021, 9, 637421. [Google Scholar] [CrossRef]
- Sha, Y.; Hansson, L.-A. Ancestral environment determines the current reaction to ultraviolet radiation in Daphnia magna. Evolution 2022, 76, 1821–1835. [Google Scholar] [CrossRef]
- Sentis, A.; Bertram, R.; Dardenne, N.; Ramon-Portugal, F.; Espinasse, G.; Louit, I.; Negri, L.; Haeler, E.; Ashkar, T.; Pannetier, T. Evolution without standing genetic variation: Change in transgenerational plastic response under persistent predation pressure. Heredity 2018, 121, 266–281. [Google Scholar] [CrossRef]
- Smithson, M.; Thorson, J.L.M.; Sadler-Riggleman, I.; Beck, D.; Skinner, M.K.; Dybdahl, M. Between-generation phenotypic and epigenetic stability in a clonal snail. Genome Biol. Evol. 2020, 12, 1604–1615. [Google Scholar] [CrossRef]
- Nelson, A.C.; Cauceglia, J.W.; Merkley, S.D.; Youngson, N.A.; Oler, A.J.; Nelson, R.J.; Cairns, B.R.; Whitelaw, E.; Potts, W.K. Reintroducing domesticated wild mice to sociality induces adaptive transgenerational effects on MUP expression. Proc. Natl. Acad. Sci. USA 2013, 110, 19848–19853. [Google Scholar] [CrossRef]
- Gervasi, D.D.L.; Schiestl, F.P. Real-time divergent evolution in plants driven by pollinators. Nat. Commun. 2017, 8, 14691. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.E.; Schiestl, F.P. Rapid plant evolution driven by the interaction of pollination and herbivory. Science 2019, 364, 193–196. [Google Scholar] [CrossRef]
- Yu, X.; Lu, M.; Zhou, M.; Wang, H.; Feng, J.; Wen, Y. Reduction of pectin may decrease the embryogenicity of grapevine (Vitis vinifera) pro-embryonic masses after 10 years of in vitro culture. Sci. Hortic. 2023, 309, 111690. [Google Scholar] [CrossRef]
- Lin, X.; Yin, J.; Wang, Y.; Yao, J.; Li, Q.Q.; Latzel, V.; Bossdorf, O.; Zhang, Y.-Y. Environment-induced heritable variations are common in Arabidopsis thaliana. Nat. Commun. 2024, 15, 4615. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.; Becker, C.; Marconi, G.; Durr, J.; Price, J.; Hagmann, J.; Papareddy, R.; Putra, H.; Kageyama, J.; Becker, J. Correction: Hyperosmotic stress memory in Arabidopsis is mediated by distinct epigenetically labile sites in the genome and is restricted in the male germline by DNA glycosylase activity. eLife 2018, 7, e44302. [Google Scholar] [CrossRef]
- Smit, S.J.; Lichman, B.R. Plant biosynthetic gene clusters in the context of metabolic evolution. Nat. Prod. Rep. 2022, 39, 1465–1482. [Google Scholar] [CrossRef]
- Zhan, C.; Shen, S.; Yang, C.; Liu, Z.; Fernie, A.R.; Graham, I.A.; Luo, J. Plant metabolic gene clusters in the multi-omics era. Trends Plant Sci. 2022, 27, 981–1001. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Z. Unlocking plant metabolic diversity: A (pan)-genomic view. Plant Commun. 2022, 3, 100300. [Google Scholar] [CrossRef] [PubMed]
- Kominek, J.; Doering, D.T.; Opulente, D.A.; Shen, X.; Zhou, X.; DeVirgilio, J.; Hulfachor, A.B.; Groenewald, M.; McGee, M.A.; Karlen, S.D. Eukaryotic acquisition of a bacterial operon. Cell 2019, 176, 1356–1366.e10. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Guo, Z.; Yang, Z.; Han, H.; Wang, S.; Xu, H.; Yang, X.; Yang, F.; Wu, Q.; Xie, W. Whitefly hijacks a plant detoxification gene that neutralizes plant toxins. Cell 2021, 184, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- Nanney, D.L. Epigenetic control systems. Proc. Natl. Acad. Sci. USA 1958, 44, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Portela, A.; Esteller, M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010, 28, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Biswas, I. Genetic tools for manipulating Acinetobacter baumannii genome: An overview. J. Med. Microbiol. 2015, 64, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Forche, A.; Cromie, G.; Gerstein, A.C.; Solis, N.V.; Pisithkul, T.; Srifa, W.; Jeffery, E.; Abbey, D.; Filler, S.G.; Dudley, A.M. Rapid phenotypic and genotypic diversification after exposure to the oral host niche in Candida albicans. Genetics 2018, 209, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Danner, C.; Mach, R.L.; Mach-Aigner, A.R. The phenomenon of strain degeneration in biotechnologically relevant fungi. Microbiol. Biotechnol. 2023, 107, 4745–4758. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Lu, X.; Xing, L.; Ho, S.W.A.; Kwan, H.S. Genomic and transcriptomic comparison of Aspergillus oryzae strains: A case study in soy sauce koji fermentation. J. Ind. Microbiol. Biotechnol. 2018, 45, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.H.; Henriksen, C.M.; Nielsen, J.; Villadsen, J.; Egel-Mitani, M. Continuous cultivation of Penicillium chrysogenum. Growth on glucose and penicillin production. J. Biotechnol. 1995, 42, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Barredo, J.L.; Díez, B.; Alvarez, E.; Martín, J.F. Large amplification of a 35-kb DNA fragment carrying two penicillin biosynthetic genes in high penicillin producing strains of Penicillium chrysogenum. Curr. Genet. 1989, 16, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Fierro, F.; Barredo, J.L.; Díez, B.; Gutierrez, S.; Fernández, F.J.; Martin, J.F. The penicillin gene cluster is amplified in tandem repeats linked by conserved hexanucleotide sequences. Proc. Natl. Acad. Sci. USA 1995, 92, 6200–6204. [Google Scholar] [CrossRef]
- Newbert, R.W.; Barton, B.; Greaves, P.; Harper, J.; Turner, G. Analysis of a commercially improved Penicillium chrysogenum strain series: Involvement of recombinogenic regions in amplification and deletion of the penicillin biosynthesis gene cluster. J. Ind. Microbiol. Biotechnol. 1997, 19, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Douma, R.D.; Batista, J.M.; Touw, K.M.; Kiel, J.A.K.W.; Krikken, A.M.; Zhao, Z.; Veiga, T.; Klaassen, P.; Bovenberg, R.A.L.; Daran, J.-M. Degeneration of penicillin production in ethanol-limited chemostat cultivations of Penicillium chrysogenum: A systems biology approach. BMC Syst. Biol. 2011, 5, 132. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Lin, J.; Guo, L.; Wang, X.; Tian, S.; Liu, C.; Zhao, Y.; Zhao, R. Advances in research on Cordyceps militaris degeneration. Microbiol. Biotechnol. 2019, 103, 7835–7841. [Google Scholar] [CrossRef]
- Xie, H.; Ma, Q.; Wei, D.; Wang, F. Transcriptomic analysis of Aspergillus niger strains reveals the mechanism underlying high citric acid productivity. Bioresour. Bioprocess. 2018, 5, 21. [Google Scholar] [CrossRef]
- Li, X.; Huang, L.; Pan, L.; Wang, B.; Pan, L. CRISPR/dCas9-mediated epigenetic modification reveals differential regulation of histone acetylation on Aspergillus niger secondary metabolite. Microbiol. Res. 2021, 245, 126694. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, M.; Cai, X.; Han, Z.; Si, J.; Chen, D. Jasmonate signaling pathway modulates plant defense, growth, and their trade-offs. Int. J. Mol. Sci. 2022, 23, 3945. [Google Scholar] [CrossRef]
- Pierron, F.; Daffe, G.; Daramy, F.; Heroin, D.; Barré, A.; Bouchez, O.; Clérendeau, C.; Romero-Ramirez, A.; Nikolski, M. Transgenerational endocrine disruptor effects of cadmium in zebrafish and contribution of standing epigenetic variation to adaptation. J. Hazard. Mater. 2023, 455, 131579. [Google Scholar] [CrossRef] [PubMed]
- Maamar, M.B.; Nilsson, E.; Sadler-Riggleman, I.; Beck, D.; McCarrey, J.R.; Skinner, M.K. Developmental origins of transgenerational sperm DNA methylation epimutations following ancestral DDT exposure. Dev. Biol. 2019, 445, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Sgrò, C.M.; Weeks, A.R. Chromosomal inversion polymorphisms and adaptation. Trends Ecol. Evol. 2004, 19, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Rando, O.J.; Verstrepen, K.J. Timescales of genetic and epigenetic inheritance. Cell 2007, 128, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Tenaillon, O.; Rodríguez-Verdugo, A.; Gaut, R.L.; McDonald, P.; Bennett, A.F.; Long, A.D.; Gaut, B.S. The molecular diversity of adaptive convergence. Science 2012, 335, 457–461. [Google Scholar] [CrossRef]
- Shor, E.; Fox, C.A.; Broach, J.R. The yeast environmental stress response regulates mutagenesis induced by proteotoxic stress. PLoS Genet. 2013, 9, e1003680. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Mithani, A.; Belfield, E.J.; Mott, R.; Hurst, L.D.; Harberd, N.P. Environmentally responsive genome-wide accumulation of de novo Arabidopsis thaliana mutations and epimutations. Genome Res. 2014, 24, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Gorter, F.A.; Derks, M.F.L.; van den Heuvel, J.; Aarts, M.G.M.; Zwaan, B.J.; de Ridder, D.; de Visser, J.A.G.M. Genomics of adaptation depends on the rate of environmental change in experimental yeast populations. Mol. Biol. Evol. 2017, 34, 2613–2626. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Lu, M.Y.; Chang, Y.W.; Li, W.H. Experimental evolution of yeast for high-temperature tolerance. Mol. Biol. Evol. 2018, 35, 1823–1839. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.E.; Tittes, S.; Franks, S.J. Rapid, nonparallel genomic evolution of Brassica rapa (field mustard) under experimental drought. J. Evol. Biol. 2023, 36, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Zhu, X.; Xie, S.; Lang, Z.; Zhu, J.-K. Transgenerational increases in DNA methylation in Arabidopsis plants defective in active DNA demethylation. Proc. Natl. Acad. Sci. USA 2024, 121, e2320468121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tian, C.; Zhu, C.; Lai, Z.; Lin, Y.; Guo, Y. Hidden players in the regulation of secondary metabolism in tea plant: Focus on non-coding RNAs. Beverage Plant Res. 2022, 2, 19. [Google Scholar] [CrossRef]
- Yang, J.; Gu, D.; Wu, S.; Zhou, X.; Chen, J.; Liao, Y.; Zeng, L.; Yang, Z. Feasible strategies for studying the involvement of DNA methylation and histone acetylation in the stress-induced formation of quality-related metabolites in tea (Camellia sinensis). Hortic. Res. 2021, 8, 253. [Google Scholar] [CrossRef]
- Jia, H.; Jia, H.; Lu, S.; Zhang, Z.; Su, Z.; Sadeghnezhad, E.; Li, T.; Xiao, X.; Wang, M.; Pervaiz, T. DNA and histone methylation regulates different types of fruit ripening by transcriptome and proteome analyses. J. Agric. Food Chem. 2022, 70, 3541–3556. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Duncan, S.; Li, Y.; Huang, S.; Luo, M. Decoding plant specialized metabolism: New mechanistic insights. Trends Plant Sci. 2024, 29, 535–545. [Google Scholar] [CrossRef]

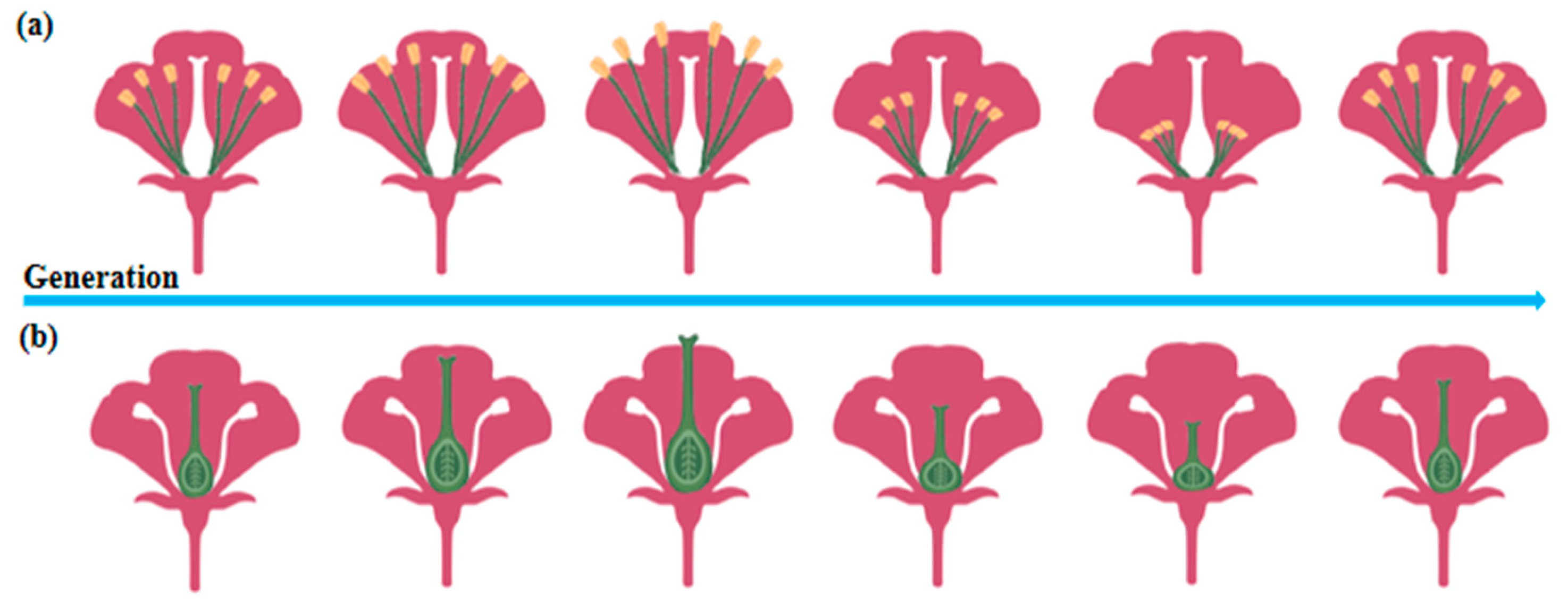

| Type of Change | Species | Generations | Phenotypes | Scope and Effectivity of Change | References |
|---|---|---|---|---|---|
| Reproductive and fertility-related phenotypes | Drosophila melanogaster | 10 | Number of eggs per female per day, mean egg size, starvation tolerance as time to death by starvation, and recovery time after chill coma | Quickly altered | [33] |
| Heterandria formosa | 2 | Reproductive rate | Decreased | [34] | |
| Offspring size | Increased | ||||
| Callosobruchus maculatus | 2 | Sex-specific and hormetic intergenerational and transgenerational effects on longevity and lifetime reproductive success | Great change | [35] | |
| Daphnia pulicaria | 2 | Fitness traits (delayed maturation, lower reproductive output, and increased clutch interval) | Decreased | [36] | |
| Aurelia coerulea | 10 | Polyps’ average budding reproduction rate | Decreased | [37] | |
| Physiological and lifespan-related phenotypes | Caenorhabditis elegans | 3 | Starvation resistance and lifespan | Increased | [38] |
| Insect model system | 80 | Fitness and longevity | Increased | [39] | |
| Freshwater snail Physa acuta | 2 | Shell size and crushing resistance | Increased | [40] | |
| Freshwater crustacean Daphnia lumholtzi | 2 | Lag phase | Decreased | [41] | |
| Behavioral and life history traits | Daphniamagna | 150 | Behavior and life history traits | Rapidly altered | [42] |
| Pea aphids | 27 | The persistence of the winged phenotype | Decreased | [43] | |
| The refractory phase duration | Increased | ||||
| Morphological traits | Freshwater snail (Potamopyrgus antipodarum) | 3 | Shell shape | Slowly altered | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.-W.; Liu, Y.; Huang, P.-C.; Rong, M.; Wei, W.; Xu, Y.-H.; Wei, J.-H. Adaptive Changes and Genetic Mechanisms in Organisms Under Controlled Conditions: A Review. Int. J. Mol. Sci. 2025, 26, 2130. https://doi.org/10.3390/ijms26052130
Guo Y-W, Liu Y, Huang P-C, Rong M, Wei W, Xu Y-H, Wei J-H. Adaptive Changes and Genetic Mechanisms in Organisms Under Controlled Conditions: A Review. International Journal of Molecular Sciences. 2025; 26(5):2130. https://doi.org/10.3390/ijms26052130
Chicago/Turabian StyleGuo, Yu-Wei, Yang Liu, Peng-Cheng Huang, Mei Rong, Wei Wei, Yan-Hong Xu, and Jian-He Wei. 2025. "Adaptive Changes and Genetic Mechanisms in Organisms Under Controlled Conditions: A Review" International Journal of Molecular Sciences 26, no. 5: 2130. https://doi.org/10.3390/ijms26052130
APA StyleGuo, Y.-W., Liu, Y., Huang, P.-C., Rong, M., Wei, W., Xu, Y.-H., & Wei, J.-H. (2025). Adaptive Changes and Genetic Mechanisms in Organisms Under Controlled Conditions: A Review. International Journal of Molecular Sciences, 26(5), 2130. https://doi.org/10.3390/ijms26052130





