Abstract
Adaptive changes encompass physiological, morphological, or behavioral modifications occurring in organisms in response to specific environmental conditions. These modifications may become established within a population through natural selection. While adaptive changes can influence individuals or populations over short timeframes, evolution involves the inheritance and accumulation of these changes over extended periods under environmental pressures through natural selection. At present, addressing climate change, emerging infectious diseases, and food security are the main challenges faced by scientists. A comprehensive and profound understanding of the mechanisms of adaptive evolution is of great significance for solving these problems. The genetic basis of these adaptations can be examined through classical genetics, which includes stochastic gene mutations and chromosomal instability, as well as epigenetics, which involves DNA methylation and histone modifications. These mechanisms not only govern the rate and magnitude of adaptive changes but also affect the transmission of adaptive traits to subsequent generations. In the study of adaptive changes under controlled conditions, short-term controlled experiments are commonly utilized in microbial and animal research to investigate long-term evolutionary trends. However, the application of this approach in plant research remains limited. This review systematically compiles the findings on adaptive changes and their genetic foundations in organisms within controlled environments. It aims to provide valuable insights into fundamental evolutionary processes, offering novel theoretical frameworks and research methodologies for future experimental designs, particularly in the field of plant studies.
1. Introduction
In natural environments, organisms encounter diverse environmental pressures, including abiotic stressors such as temperature fluctuations, resource limitations, drought, and high salinity, as well as biotic factors like predation and pathogens [1]. These pressures drive adaptive modifications that enhance survival and reproductive success. The duration required for such adaptations varies, depending on the species, environmental conditions, and the nature of the change, ranging from a few generations to millions of years [2]. Several factors influence the rate of evolution, including the intensity of environmental fluctuations [3], survival constraints [4], genetic diversity [5], and genetic drift [6].
Investigating adaptive changes often necessitates controlled cultivation environments to precisely assess the impact of specific environmental variables on organisms. Previous research has identified notable adaptive modifications in various species through multigenerational cultivation under controlled conditions. For instance, in microorganisms, adaptive changes may involve increased resistance to environmental stressors like high temperatures or toxins. In animals, these changes can manifest as alterations in reproductive traits or stress responses. In plants, adaptive changes often include adjustments in leaf morphology or root structure in response to water availability or light conditions. These modifications manifest in physiological, biochemical, morphological, and behavioral traits [7]. Consequently, continuous monitoring of dynamic trait changes through multigenerational cultivation has emerged as a valuable approach for understanding evolutionary mechanisms within a relatively short timespan [8]. This method offers novel opportunities for studying biological evolution [9,10,11].
Research on adaptive modifications under controlled conditions has primarily focused on animals [12,13,14,15] and microorganisms [16,17], while studies on plants remain limited. In plants, biochemical traits, particularly specialized metabolites, are essential for defense against pathogens and herbivores, environmental adaptation, signal transduction, and interspecies interactions [18,19,20,21]. These metabolites are crucial for plant survival and reproduction, and have significant applications in agriculture and medicine [22,23].
Currently, a substantial knowledge gap exists regarding how specialized metabolites change under controlled conditions, the mechanisms governing these changes, and potential regulatory pathways. Multigenerational plant cultivation under controlled conditions, similar to research in microorganisms (e.g., Saccharomyces cerevisiae) and animals (e.g., Drosophila melanogaster), could facilitate a deeper understanding of the evolutionary mechanisms regulating specialized metabolite production. Saccharomyces cerevisiae is widely used as a model organism in evolutionary biology due to its short generation time and well-characterized metabolic pathways. Drosophila melanogaster, selected for its extensively characterized genetic background and the ease with which multigenerational populations can be maintained, provides an invaluable model for studying genetic adaptation. In contrast, Arabidopsis thaliana has been selected for its genetic tractability and significance in plant biology. However, further research is required to gain a deeper understanding of its specialized metabolite production under controlled conditions (figure in Section 3.1.1). A comprehensive summary of adaptive changes in organisms under controlled environments would provide a foundation for elucidating biodiversity and adaptability, advancing knowledge of organism–environment interactions, and contributing to agricultural and medical advancements while uncovering evolutionary pressures and selection mechanisms.
2. Adaptive Changes in Organisms Under Controlled Conditions
2.1. Adaptive Changes in Microorganisms
In microbiology, multigenerational cultivation of fungi under controlled conditions results in substantial modifications in morphological traits, physiological characteristics, reproductive attributes, and biochemical indicators.
2.1.1. Changes in Morphological, Physiological, and Reproductive Traits
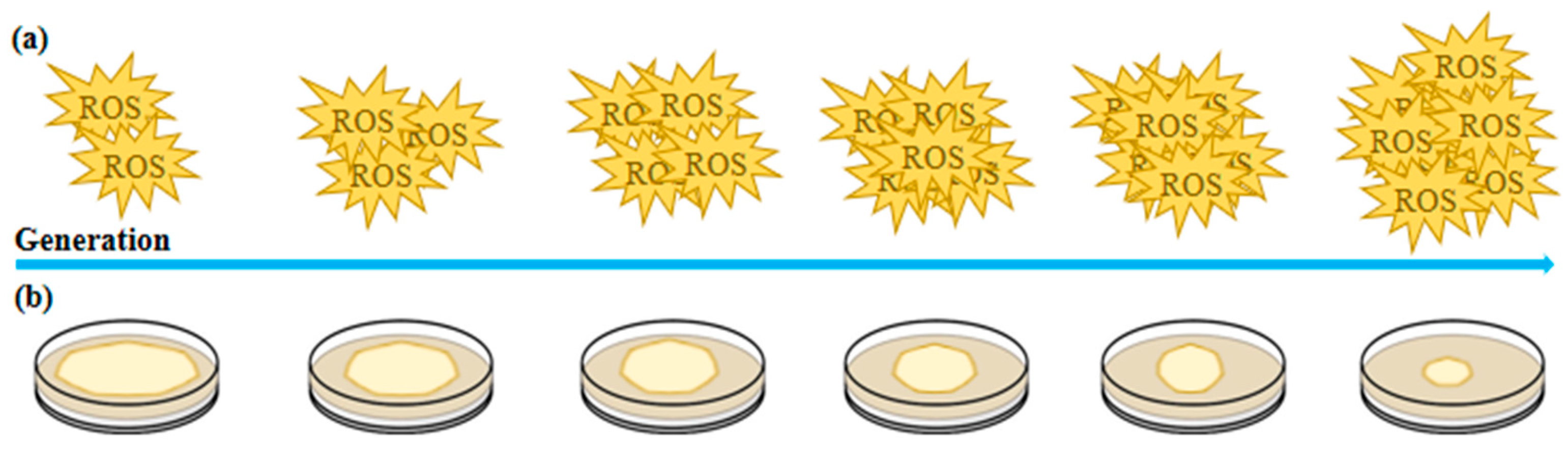
After 12 consecutive subcultures of fungi, significant signs of strain degradation were observed, including reduced antioxidant enzyme activity and increased reactive oxygen species (ROS) levels (Figure 1). With each successive passage, additional indicators of cellular stress emerged, such as a decline in nuclear number, decreased mitochondrial membrane potential, and alterations in mitochondrial morphology. These findings suggest that ROS accumulation, combined with repeated tissue isolation, exacerbates oxidative damage in inherited strains, ultimately contributing to cellular senescence and degeneration in Volvariella volvacea strains [24,25]. Continuous subculturing of Volvariella volvacea strains over 20 months, with subcultures performed every three days, resulted in a progressive decline in growth rate, mycelial biomass, fruiting body count, and bioefficiency as the number of subcultures increased. Simultaneously, the production cycle and primordium formation time were progressively extended. Strains subcultured for 13 to 20 months (S13–S20) failed to produce fruiting bodies during cultivation experiments. Additionally, ROS content measurements and enzyme activity analyses indicated a decline in lignocellulase activity and excessive ROS accumulation, both of which were associated with subculture-induced degeneration. Reverse transcription polymerase chain reaction (RT-PCR) analysis of gene expression related to lignocellulase and antioxidant enzymes in the subcultured strains demonstrated consistency with the observed enzymatic activity changes [26].
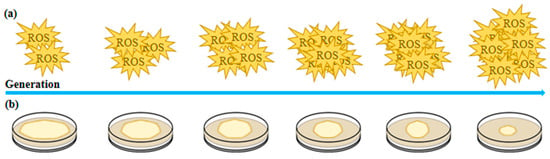
Figure 1.
Trends in the adaptive changes in microbial traits under controlled conditions. (a) Multigenerational cultivation gradually increases reactive oxygen species (ROS) levels in microorganisms. (b) Multigenerational cultivation leads to a progressive decline in microbial colony size and biomass.
2.1.2. Changes in Biochemical Indicators
In microbiology, biochemical indicators undergo significant changes following multigenerational cultivation under controlled conditions, which typically involve maintaining a constant temperature, pH, light exposure, and nutrient supply. These conditions ensure that the organisms are exposed to minimal external variability, allowing for the observation of genetic and phenotypic changes over multiple generations. A notable trend is the decline in specialized metabolite content with an increasing number of subcultures. However, certain experiments have demonstrated that mimicking environmental stimuli can restore strains’ productivity, allowing for the renewed production of specialized metabolites. The loss of productivity for compounds such as paclitaxel, camptothecin, and cordycepin during laboratory storage or successive subculturing has been linked to the absence of host-related stimuli. Chen et al., (2017) conducted 10 consecutive subcultures of a wild-type Cordyceps strain, resulting in strain degeneration. By hybridizing four monospore isolates derived from the degenerated strain, fruiting body production was restored to levels comparable with the wild-type strain. The revitalized strain not only exhibited well-developed fruiting bodies but also accumulated higher concentrations of cordycepin and adenosine than both the original wild-type and degenerated strains. These newly acquired traits remained stable after four consecutive transfers [27]. Similarly, nutrient optimization in Penicillium chrysogenum was shown to enhance camptothecin production by 1.8 times. However, after 8 months of storage at 4 °C, camptothecin productivity declined by 40% compared with the initial culture. The application of a dichloromethane extract from Cliona sp. was found to fully restore the ability of P. chrysogenum to produce camptothecin [28]. Successive subcultures of Aspergillus terreus have been reported to cause a substantial decline in paclitaxel production. By the 10th subculture, the paclitaxel yield (78.2 μg/L) was reduced to one-fourth of that observed in the original culture (268 μg/L). Notably, supplementation with the plant microbiome fully restored both paclitaxel biosynthesis and cellular acetyl-CoA levels, indicating that the introduction of the plant microbiome can reactivate the molecular mechanisms responsible for paclitaxel biosynthesis [29]. After continuous subculture, a reduction in the production of specialized metabolites, such as cordycepin, has been noted. During subculturing on solid media, filamentous fungal colonies frequently undergo degeneration, which is characterized by the loss of conidia, pigment deposition, and a marked decline or a complete loss of specialized metabolite production [30]. Wellham et al., (2021) reported that repeated subculturing of Cordyceps militaris under laboratory conditions led to strain degeneration, evidenced by a reduction in cordycepin production [31]. Similarly, Chen et al. (2017) also observed that multigenerational cultivation of Cordyceps resulted in decreased synthesis of specialized metabolites, including cordycepin [27].
2.2. Adaptive Changes in Animals
In animal studies, multiple species have demonstrated notable adaptive modifications following multigenerational cultivation under controlled environments, such as stable temperature, humidity, and light conditions, along with a controlled food supply and selective pressure (e.g., temperature stress, limited resources). These modifications encompass changes in reproductive and fertility-related phenotypes, physiological and lifespan-associated traits, behavioral and life history characteristics, as well as morphological traits [32]. A comprehensive summary of all the observed phenotypic changes and the extent of transgenerational cultivation in the studied animal species is presented in Table 1.

Table 1.
Phenotypic changes and scope of transgenerational cultivation in animals.
2.2.1. Changes in Reproductive and Fertility-Related Phenotypes
Reproductive and fertility-related phenotypic modifications have been observed under controlled cultivation conditions across multiple generations. For example, rapid phenotypic adaptations have been documented in traits such as mean fecundity (number of eggs per female per day), egg size, starvation tolerance (measured by time to death due to starvation), and recovery time following chill coma. The extent of these adaptations was considerable, with at least 165 independent genomic regions identified as being under selection during the experiment. Allele frequencies at more than 60% of variant sites across the genome were partially influenced by selection, indicating significant genetic adaptation to the controlled environment [33]. A multigenerational laboratory study using females from two populations of Heterandria formosa investigated transgenerational plasticity in reproductive traits in response to social density variations and their impact on maternal fitness. The findings indicated that increased social density resulted in a lower reproductive rate and larger offspring size in females from both populations during the first and second generations [34]. Additionally, parental exposure to a common pesticide was shown to induce intra-, inter-, and transgenerational responses in life history traits such as fecundity, longevity, and lifetime reproductive success, as demonstrated in studies using Callosobruchus maculatus as an insect model. The results revealed sex-specific and hormetic intergenerational and transgenerational effects on longevity and reproductive success, with both maternal and paternal contributions to these effects [35]. Research on Daphnia pulicaria has shown that the first generation exhibited reduced fitness traits, including delayed maturation, lower reproductive output, and increased clutch interval when reared on cyanobacteria compared with high-quality food. However, maternal stress had no significant impact on the offspring’s fitness traits, as the second generation displayed similar mean trait values regardless of maternal diet [36]. In Aurelia coerulea polyps, transgenerational effects on traits such as budding reproduction and strobilation were examined over 10 asexual generations. The results indicated a 32.82% decline in the average budding reproduction rate across asexual generations within the experimental period [37]. Studies on wild-derived mice after eight generations under laboratory conditions have demonstrated rapid adaptation to reintroduced sociality and promiscuity within two generations. Males from the promiscuous lineage exhibited reduced viability but gained a considerable advantage in mate attraction through increased expression of major urinary protein (MUP) pheromones, which were inherited transgenerationally. This enhanced MUP expression was associated with reduced DNA methylation in the promoter region, leading to heightened female attraction [45].
2.2.2. Changes in Physiological and Lifespan-Related Phenotypes
Physiological and lifespan-related phenotypic modifications have been observed following an extended diapause in Caenorhabditis elegans. Initially, post-diapause individuals exhibit reduced reproductive success and greater interindividual variation. However, the F3 progeny of these individuals demonstrate enhanced starvation resistance and increased lifespan, suggesting potentially adaptive transgenerational effects [38]. Experimental evolution studies on an insect model system have investigated the role of sexual selection history in shaping transgenerational plasticity in response to rapid environmental changes, such as pesticide exposure. In a study with Callosobruchus maculatus, it was demonstrated that deltamethrin, a neurotoxic pyrethroid pesticide (applied at 2 g/L for 24 h), induces both transgenerational plasticity and hormetic responses to sublethal exposure. The results revealed an extension of lifespan in F2 descendants, alongside hormetic responses modulated by the intensity of sexual selection history. After varying the intensity of sexual selection over more than 80 generations before pesticide exposure, the findings indicated that sexual selection history constrained adaptation to rapid environmental change. Additionally, inter- and transgenerational effects of pesticide exposure were observed, leading to increased fitness and longevity in subsequent generations [39]. The transgenerational and within-generation plasticity of anti-predator traits have been examined in the freshwater snail Physa acuta. Across two generations, most morphological traits, including shell size and crushing resistance, exhibited transgenerational plasticity, with the offspring of predator-exposed parents being larger and more resistant to crushing. However, shell shape exhibited plasticity only within a single generation [40]. In the freshwater crustacean Daphnia lumholtzi, exposure to chemical cues from fish predators has been shown to induce the development of elongated head and tail spines. In the F0 generation, these defensive traits exhibited a prolonged lag phase, emerging only by the third instar. However, in the F1 and F2 generations, this lag phase was reduced, with defensive traits appearing immediately upon birth, indicating a shift in phenotypic expression across generations [41].
2.2.3. Changes in Behavioral and Life History Traits
Behavioral and life history trait modifications have been observed in response to transgenerational exposure to ultraviolet radiation (UVR). A reciprocal split-brood experiment conducted over 150 generations demonstrated that transgenerational responses to UVR varied depending on ancestral exposure. Descendants from unexposed ancestor lineages exhibited enhanced phototactic avoidance and delayed reproduction upon initial exposure to ultraviolet radiation (UVR), whereas descendants originating from UVR-exposed populations demonstrated reduced stress responses and accelerated larval development under low-UVR conditions. These patterns reveal a fundamental mechanism by which ancestral environmental conditions shape adaptive plasticity: Populations with no prior UVR exposure prioritize immediate survival through rapid phenotypic adjustments, while those adapted to historical UVR exposure maintain energy-conserving behaviors as the threat diminishes. This divergence in phenotypic strategies across generations highlights the dynamic interplay between evolutionary history and the environmental context in mediating organismal responses to fluctuating stressors [42]. Transgenerational phenotypic responses of pea aphids to ladybird predators have been examined over 27 generations. The frequency of winged aphids initially increased rapidly and remained stable for 25 generations. After predator removal, the winged phenotype persisted for one generation before entering a refractory phase, during which its frequency dropped below control levels for at least two generations before recovering. With prolonged predator exposure, the persistence of the winged phenotype declined, and the refractory phase was extended. Additionally, aphids exposed to predators for 22 generations exhibited a reduced plastic response, which conferred a fitness advantage in the presence of predators [43].
2.2.4. Changes in Morphological Traits
Examples of morphological trait modifications include a study by Mark Smithson, which investigated the stability of an adaptive phenotype (shell shape) and DNA methylation across generations in a clonally reproducing freshwater snail (Potamopyrgus antipodarum). Three generations of snails, originally adapted to river currents, were reared in a laboratory environment without a current. The results indicated that the habitat-specific adaptive shell shape remained relatively stable across three generations but exhibited a slight shift in the second and third generations toward a no-current lake phenotype [44].
2.3. Adaptive Changes in Plants
In plant studies, multigenerational cultivation under controlled conditions results in notable modifications in morphological traits and biochemical indicators.
2.3.1. Changes in Plant Morphology
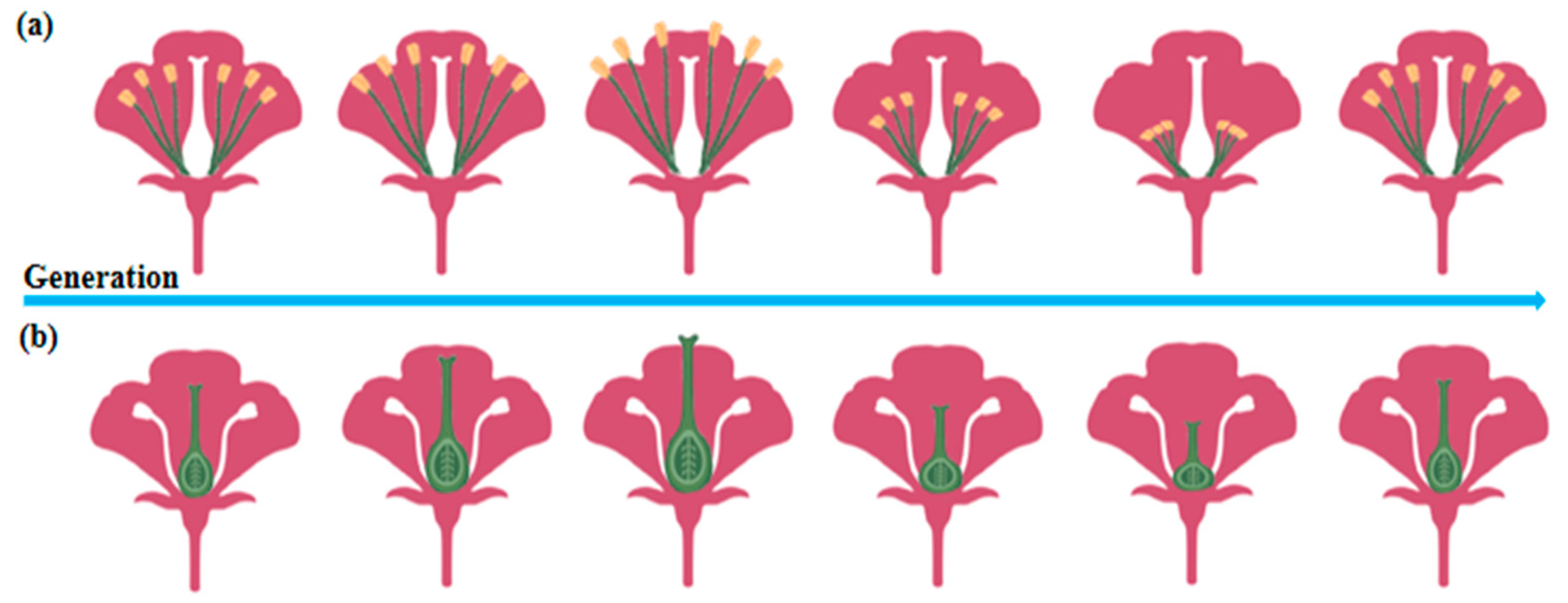
In plant biology, multigenerational cultivation under controlled conditions results in significant morphological modifications. After 11 consecutive generations of co-cultivation with pollinators, bumblebee-pollinated plant species exhibited a notable increase in plant height. Additionally, pollination efficiency, measured by the number of seeds per fruit, showed a steady improvement throughout the experimental period [46]. Real-time evolutionary tracking of plant traits over six generations using rapidly cycling Brassica plants revealed substantial changes in reproductive structures. The lengths of both the pistil and long stamens initially shortened before exhibiting a subsequent elongation trend (Figure 2), highlighting dynamic trait adjustments influenced by the presence or absence of pollinators [47]. Similarly, somatic embryos serve as optimal material for woody plant propagation, germplasm conservation, and genetic transformation. A comparison of grape proembryonic masses (PEMs; early-stage plant tissues undergoing embryogenesis during in vitro culture) that underwent continuous tissue culture for 10 years under controlled environmental conditions (e.g., a constant temperature, light cycles, and nutrient supply) with newly induced PEMs revealed a significant reduction in somatic embryo induction rates in long-term PEMs, with an abnormal embryo ratio reaching 92.2%. Additionally, the classical cellular cluster structure nearly disappeared in repeatedly subcultured PEMs, along with an increase in the space between adjacent cells [48]. Exposure of 14 Arabidopsis thaliana genotypes to 10 distinct controlled environmental stresses over four consecutive generations led to pronounced phenotypic alterations in most genotypes. Under stable nutrient stress, aboveground biomass consistently increased with each successive generation. However, fruit yield displayed variable responses, showing a marked increase under jasmonic acid treatment but a significant decrease under high cadmium stress, which was also associated with reduced plant stature [49]. To assess the extent of stress-induced transgenerational adaptation, Arabidopsis thaliana (including Col-0) was subjected to two distinct hyperosmotic conditions for more than five generations. The direct progeny (P1) of stressed plants from generations G2 to G5 exhibited higher germination and survival rates, along with more vigorous vegetative growth when cultivated in high-salinity (150 mM) media [50].
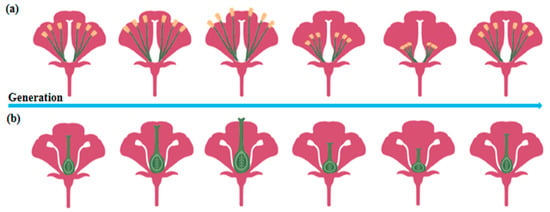
Figure 2.
Trends in plant trait adaptation under controlled conditions. (a) Multigenerational cultivation initially increases stamen length, followed by a subsequent decrease in plants. (b) Multigenerational cultivation causes an initial rise in pistil length, followed by a gradual decline in plants.
2.3.2. Changes in Biochemical Parameters
Multigenerational cultivation of plants has resulted in significant alterations in biochemical indicators. After 11 consecutive generations of co-cultivation with pollinators, notable shifts in floral volatile profiles were observed. Nearly half of the plants pollinated by flies had ceased anisaldehyde emission by the 11th generation. In contrast, plants pollinated by bumblebees exhibited a substantial increase in indole and glutaraldehyde content, along with a decline in anisaldehyde levels, particularly in response to aphid herbivory [46]. Real-time evolutionary tracking of plant traits over six generations using rapidly cycling Brassica plants revealed a significant rise in the emission of three aromatic floral volatiles—benzaldehyde, benzonitrile, and p-anisaldehyde—in plants exposed to bee-pollinated conditions. These findings emphasize the role of pollinators in shaping floral volatile composition across successive generations [47]. Plant in vitro culture, a crucial technique in plant research, is significantly affected by multigenerational cultivation. In grape proembryonic masses (PEMs), repeated subculturing led to a considerable decline in cell wall pectin content [48].
3. Genetic Mechanisms and Their Role in Adaptive Changes
Genetic mechanisms, encompassing both classical and epigenetic processes, play a central role in driving adaptive changes across diverse organisms. The interplay between classical and epigenetic mechanisms is particularly evident in their roles across different taxa. In microorganisms, rapid mutation rates and horizontal gene transfer drive the evolution of antibiotic resistance and metabolic versatility. In animals, genetic and epigenetic changes underpin adaptations to climatic shifts, dietary changes, and pathogen pressures. In plants, epigenetic modifications enable responses to abiotic stresses, such as drought or salinity, while genetic diversity supports long-term evolutionary resilience. Together, these mechanisms form an integrated framework that shapes adaptive changes at both the organismal and population levels, highlighting their fundamental importance in evolutionary biology.
3.1. Genetic Mechanisms
3.1.1. Classical Genetic Mechanisms
Classical genetic mechanisms encompass a range of processes, including random gene mutations, chromosomal instability, and genetic variations. Random genetic mutations, which occur spontaneously at low frequencies, represent a fundamental driver of evolutionary change. Chromosomal instability has also been recognized as a significant contributor to the loss of biosynthetic genes, influencing genomic architecture and functional diversity. Furthermore, genetic variation serves as a critical determinant in shaping the structural diversity of specialized metabolites across populations, thereby underpinning adaptive potential and ecological interactions.
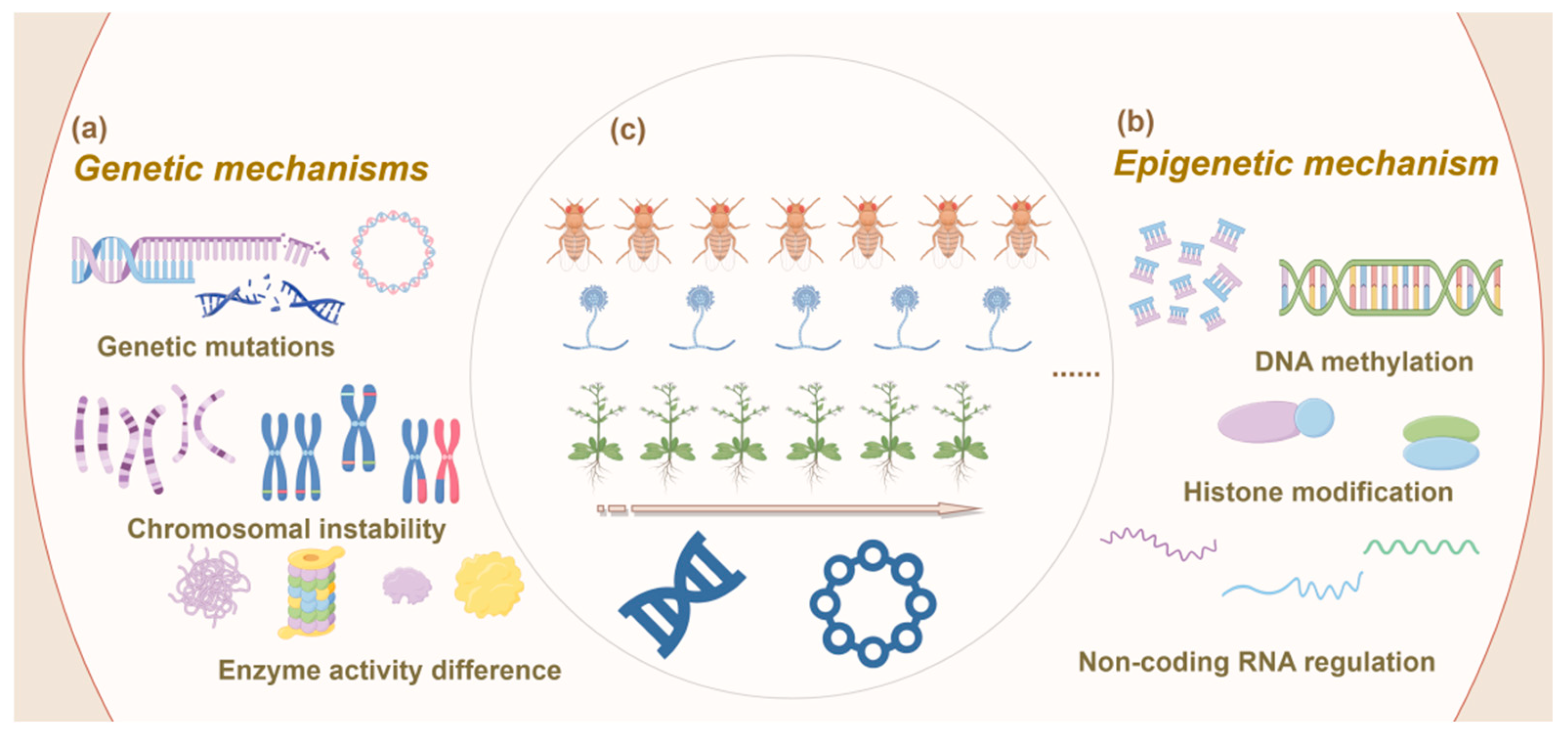
Dynamic genetic modifications are fundamental drivers of adaptive variations in organisms. Processes such as random genetic mutations, gene and whole-genome duplications, gene neofunctionalization, chromosomal rearrangements, horizontal gene transfer, and chromatin remodeling contribute to evolutionary changes [51,52,53] (Figure 3a). As genetic variations emerge, adaptive traits in organisms are modified, leading to adjustments in specialized metabolic pathways that influence both the types and quantities of metabolites produced. In plants, most specialized metabolic pathways are likely established through successive duplications and neofunctionalization of endogenous genes, whereas in fungi, some metabolic pathway genes may also evolve through horizontal gene transfer [54,55]. This complex interplay provides a strong genetic foundation for biological adaptation.
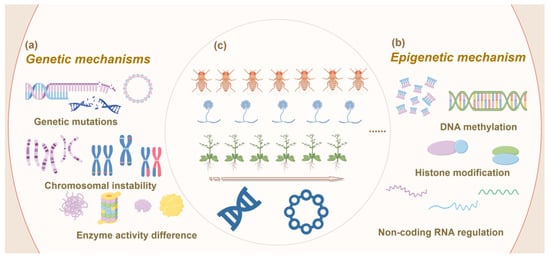
Figure 3.
Schematic representation of genetic mechanisms driving adaptive changes in organisms under controlled conditions. (a) Genetic mechanisms: This panel illustrates the key genetic processes involved in adaptive changes, including gene mutations, which introduce new genetic variations; chromosomal instability, which involves structural alterations of chromosomes, such as inversions or translocations; and enzyme activity differences, which influence metabolic pathways and can drive phenotypic changes. (b) Epigenetic mechanisms: This panel depicts various epigenetic processes that regulate gene expression without altering the underlying DNA sequence. These include DNA methylation, which typically suppresses gene expression; histone modifications, such as acetylation or methylation, which alter chromatin structure and affect gene accessibility; and non-coding RNA regulation, which involves RNA molecules that can modulate gene expression post-transcriptionally. (c) Multigenerational cultivation across different organisms (microorganisms, animals and plants): This panel represents the application of controlled multigenerational cultivation to study adaptive changes in different organisms. At the top are animals, represented by Drosophila melanogaster (fruit flies), known for their genetic tractability and rapid generation time; in the middle are microorganisms, represented by fungi, particularly species like Saccharomyces cerevisiae, which are commonly used in evolutionary studies due to their short life cycle and high mutation rates; at the bottom are plants, represented by Arabidopsis thaliana, a model organism in plant biology due to its well-characterized genetics and significance in plant research. The figure highlights how different organisms are used to explore the genetic and epigenetic mechanisms that drive adaptation under controlled experimental conditions.
3.1.2. Epigenetic Mechanisms
In 1958, Nanney introduced the concept of “epigenetics” to differentiate whether observed variations in cellular characteristics resulted from changes in the “original genetic material” or other cellular components. This concept underscored the dependence of the epigenetic system on the genetic system and its significance during development [56]. Epigenetics refers to heritable modifications in gene expression that occur without alterations in the DNA sequence, primarily mediated by mechanisms such as DNA methylation, histone modifications, and regulation by non-coding RNAs, including miRNAs (Figure 3b). These epigenetic changes often arise in response to environmental stimuli and interact to modulate gene expression, ultimately shaping cellular phenotypes [57].
Epigenetic mechanisms comprise three major regulatory modalities: DNA methylation, histone modifications, and non-coding RNA-mediated regulation. Among these, DNA methylation serves as a fundamental epigenetic mark, playing a pivotal role in gene silencing, genomic imprinting, and chromatin remodeling. Histone modifications, including acetylation, methylation, and phosphorylation, constitute another critical layer of epigenetic regulation by dynamically altering the chromatin structure and modulating gene expression. Additionally, non-coding RNAs, such as microRNAs and long non-coding RNAs, have emerged as essential regulators of epigenetic processes, fine-tuning gene expression through transcriptional and post-transcriptional mechanisms. Collectively, these interconnected mechanisms form a sophisticated regulatory network that governs cellular function and phenotypic plasticity. Epigenetic modifications play a crucial role in the intricate mechanisms that facilitate adaptive changes in organisms.
3.2. The Role of Genetic Mechanisms in Adaptation
3.2.1. Adaptation in Microorganisms
After 12 successive subcultures, Volvariella volvacea strains exhibited pronounced degenerative phenotypes, marked by a significant decline in antioxidant enzyme activity and a concomitant increase in reactive oxygen species (ROS) accumulation (Figure 1). These degenerative changes are mechanistically linked to oxidative stress-induced chromosomal instability, evidenced by increased chromosomal fragmentation and aneuploidy, and genetic variation, characterized by the accumulation of point mutations and structural rearrangements. These alterations progressively disrupt genomic integrity and gene regulatory networks during repeated subculturing, ultimately driving cellular dysfunction and senescence in fungal strains [24,25].
Continuous subculturing of Volvariella volvacea strains over a period of 20 months resulted in a progressive decline in growth rate, mycelial biomass, and fruiting body production, along with prolonged production cycles and delayed primordium formation. Strains subcultured for 13 to 20 months (S13–S20) completely lost the ability to form fruiting bodies. The mechanistic basis for this degeneration involves two interconnected processes: Genetic variation and non-coding RNA dysregulation. Genetic variation, driven by the accumulation of point mutations and structural rearrangements, led to the downregulation of several key genes, including LAC-1, LAC-4, EG-B, CBH, BGL, Xyl, Mn-SOD, CAT-1, CAT-2, GPX, and GR. Among these, LAC-1 and LAC-4 exhibited the most significant decline in expression, decreasing by 77.36% and 72.67%, respectively, at S20 compared with S0. This reduction directly impaired lignin degradation and fruiting body morphogenesis. Simultaneously, excessive reactive oxygen species (ROS) accumulation disrupted non-coding RNA regulatory networks, which further suppressed the expression of genes involved in lignocellulase production and antioxidant enzyme activity. This dual mechanism of genetic instability and epigenetic dysregulation progressively compromised cellular function, nutrient metabolism, and stress response, ultimately leading to the loss of fruiting body formation and reduced bioefficiency [26].
In fungi, high mutation rates are often associated with transposable element (TE) activity or alterations in chromosomal ploidy [58,59]. Although mutations originate from low frequencies, their prevalence can increase due to positive selection, where novel phenotypes providing a selective advantage, gradually replacing the wild-type [60]. High-frequency mutations resulting from transposable element activity have been documented in multiple cases of fungal degeneration during biotechnological applications. For example, Zhong et al., (2018) reported a decline in hydrolase productivity [61], while Christensen et al., (1995) observed reduced penicillin production in P. chrysogenum, both illustrating this phenomenon [62].
Research indicates that the degeneration of penicillin production capability is linked to high-producing strains of penicillin-producing fungi, which contain an increased copy number of biosynthetic gene clusters arranged in tandem repeats [63,64,65]. These amplifications are believed to result from misaligned chromatids and recombination events occurring within recombinogenic regions. The same mechanisms may also lead to the deletion of genes involved in penicillin biosynthesis [65]. Similarly, in fungi, chromosomal instability may drive the expansion or contraction of gene clusters through mechanisms like non-homologous end joining (NHEJ) or homologous recombination, thereby modulating metabolic pathways in response to selective pressures [66]. The presence of multiple copies of penicillin biosynthetic cluster genes, especially during extended cultivation under conditions that are unfavorable for penicillin production, may impose a metabolic burden on fungal cells. Under such conditions, cells with fewer or no copies of penicillin cluster genes may experience a selective advantage [66]. These findings suggest that the regulation of gene copy number and genetic architecture, influenced by both chromosomal instability and environmental factors, plays a key role in maintaining the long-term stability and productivity of industrial microbial strains.
It has been recognized as a key factor contributing to the degradation of Cordyceps militaris strains. Variations in gene expression levels and enzymatic activities among different genotypes lead to significant alterations in specialized metabolite profiles, highlighting the genetic basis of strain degeneration [67]. In citric acid-producing strains, degeneration is characterized by a substantial downregulation of the genes involved in its biosynthesis, including pathways associated with the tricarboxylic acid (TCA) cycle, glycolysis, starch and sucrose metabolism, and pyruvate metabolism. These disruptions illustrate the molecular mechanisms underlying strain degradation [68].
The application of the epigenome-targeting CRISPR/dCas9 system to regulate specialized metabolism genes in Aspergillus niger marked a significant advancement in this field. By fusing HosA-dCas9 with the pigment gene fwnA, it was demonstrated that the histone deacetylase HosA activated fwnA expression, leading to an acceleration of melanin synthesis. This approach underscores the potential of epigenetic manipulation in enhancing metabolic pathways in fungi, providing opportunities for targeted improvements in specialized metabolite production [69]. In Penicillium sp. KM18029, an endophytic fungus isolated from Aconitum brachypodum Diels, meroterpenoid biosynthesis was observed. Li et al., (2022) utilized histone deacetylase inhibitors as epigenetic modifiers, leading to the isolation of four novel compounds from Penicillium sp. KM18029. This study demonstrated that epigenetic modifications can serve as an effective strategy for activating terpenoid biosynthetic gene clusters in Penicillium sp. [70].
3.2.2. Adaptation in Animals
In animal research, Pierron et al., (2023) utilized copy number variations (CNVs) to investigate the potential role of gene mutations in transgenerational processes. While CNVs were relatively rare in the F1 generation, their frequency significantly increased in the sperm of the F3 generation, suggesting that environmentally induced epigenetic modifications may contribute to genomic instability and facilitate the emergence of CNV mutations in offspring. Cadmium (Cd) exposure has been shown to induce epigenetic effects across four generations in zebrafish, primarily through DNA methylation changes in the estrogen receptor alpha gene (esr1). These methylation modifications were sex-dependent and inherited transgenerationally. Cd-induced methylation changes were linked to single nucleotide polymorphisms (SNPs), which facilitated genetic selection and influenced gene transcription. While Cd exposure contributed to transgenerational disease susceptibility, it also promoted rapid transgenerational adaptation to Cd. These findings provide insights into the mechanisms of rapid adaptation and highlight the crucial role of genetic diversity in enhancing species’ resilience to global environmental changes [71]. Exposure to the pesticide DDT significantly influenced trait expression in male rats across multiple generations, primarily through the transgenerational inheritance of differential DNA methylation regions (DMRs) in the sperm. From the F3 generation onward, DDT exposure induced specific DMR alterations during sperm development, affecting key stages such as embryonic prospermatogonia, juvenile spermatogonia, adult spermatocytes, round spermatids, and caudal epididymal sperm. Notably, DMR changes originating during the spermatogonia stage in the testes were inherited by offspring through epigenetic mechanisms, leading to alterations in gene expression patterns across generations [72].
Chromosomal aberrations, including inversions and translocations, are prevalent among eukaryotic organisms and have been widely implicated in adaptive evolution. A prominent example is the role of heterozygous inversions in Drosophila melanogaster, a model organism extensively utilized in cytogenetic research. These inversions have been demonstrated to generate genetic variation that is subject to rapid selection under environmental stress. Notably, the In(3R)Payne inversion in D. melanogaster has been shown to play a pivotal role in shaping adaptive clines for traits such as body size and thermal tolerance. For instance, In(3R)Payne has been strongly associated with latitudinal variation in body size, accounting for over 70% of clinal size differences observed along the east coast of Australia and in parallel South American clines. This inversion likely facilitates adaptation by suppressing recombination, thereby preserving co-adapted gene complexes that enhance fitness under specific environmental conditions, such as colder climates, where larger body size confers a selective advantage. Furthermore, In(3R)Payne has been linked to thermal resistance, potentially mediated by genes within the inversion, such as hsr-omega, which exhibit pronounced clinal patterns. These findings underscore the significance of inversions in promoting rapid adaptation by maintaining adaptive allele combinations and responding to environmental gradients, such as temperature and latitude [73].
3.2.3. Adaptation in Plants
In plant biology, a study by Lu et al., (2021) investigated 22 generations of Arabidopsis thaliana exposed to heat stress. The research demonstrated that prolonged exposure of mutation accumulation (MA) lines and MA populations to extreme heat and moderate warming resulted in a significant increase in the mutation rate of single nucleotide variants (SNVs) and small insertions and deletions [11]. These findings highlight that environmental changes can accelerate the accumulation of mutations throughout the genome, altering the spectrum of novel mutations within an organism. This process may play a crucial role in long-term evolutionary adaptation, enabling species to better withstand shifting environmental conditions [74,75,76,77,78,79].
The diversity of specialized metabolites, driven by genetic variation, plays a critical role in enhancing organismal adaptability. Genome-wide gene expression analyses conducted across multiple generations of Arabidopsis thaliana exposed to control and ten specific environmental treatments (jasmonic acid addition (JA), the control for JA, nutrient addition, nutrient depletion, leaf removal, drought, low cadmium (50 μmol/L CdCl2), high cadmium (1500 μmol/L CdCl2), low salinity (50 mM NaCl), and high salinity (150 mM NaCl)) have demonstrated that such treatments consistently induce heritable phenotypic and gene expression changes. These findings challenge the conventional view of the environment solely as a selective force for genetic variation, instead highlighting its active role in generating genetic diversity [49]. Genetic evolution is closely linked to adaptability, enabling organisms to modify their genotypes in response to environmental challenges. This process involves the regulation of secondary metabolic pathways, allowing the synthesis of specialized metabolites tailored to specific environmental conditions. Studies have shown that nearly all genetic changes within experimental populations are unique, indicating that evolutionary trajectories are highly divergent. Even genetically similar replicates derived from the same ancestral population can exhibit distinct evolutionary paths under controlled and consistent selection pressures, underscoring the complexity of evolutionary processes [80].
In plant research, a study investigating the transgenerational effects of salt stress on Arabidopsis thaliana identified substantial changes in DNA methylation levels over 10 generations under both normal and salt stress conditions. A comparison between two genotypes, namely wild-type and ros1 mutants, revealed that ros1 mutants exhibited more than twice the number of differentially methylated cytosines (DMCs) and a higher CG epimutation rate in the 10th generation compared with the wild-type. In wild-type plants, about 50% of transgenerational DMCs showed increased methylation, whereas this proportion was significantly higher in ros1 mutants. These results indicate that active DNA demethylation plays a critical role in regulating transgenerational increases in DNA methylation, thereby contributing to epigenomic stability [81]. Exposure of Arabidopsis plants to two distinct hyperosmotic conditions over five generations identified specific genomic regions susceptible to DNA (de)methylation in response to hyperosmotic stress. These stress-induced epigenetic modifications were linked to conditionally heritable adaptive phenotypic responses, suggesting that hyperosmotic stress can drive epigenetic changes that enhance a plant’s ability to adapt to environmental stressors across generations [50].
Recent studies have emphasized the regulatory roles of non-coding RNAs (ncRNAs) in the biosynthesis of specialized metabolites in plants. miRNAs, lncRNAs, and circRNAs have been shown to interact with coding genes, influencing their expression and subsequently affecting metabolic pathways. These ncRNAs, which do not encode proteins, constitute a substantial portion of the plant transcriptome and play essential roles in modulating the biosynthesis of specialized metabolites by regulating key synthesis-related genes. This expanding body of research highlights the significance of ncRNAs in the intricate regulatory networks governing plant metabolism [82]. Although often examined separately, DNA methylation, histone modifications, and transcription factor activity can integrate to regulate gene expression. Under various stress conditions, significant variations in the expression of genes associated with DNA methylation and histone acetylation were detected in tea leaves. This study demonstrated that both DNA methylation and histone modifications contribute to the regulation of specialized metabolism in tea. Specifically, the transcription of DNA methyltransferase, DNA demethylase, histone acetyltransferase (HAT), and histone deacetylase (HDAC) genes exhibited notable alterations under stress. For instance, the inhibition of HDACs (e.g., with trichostatin A treatment) reduces histone deacetylation, leading to elevated histone acetylation levels at the promoters of target genes, such as CsTSB2, CsLOX1, and CsNES. These genes are crucial for the synthesis of stress-responsive specialized metabolites, including indole and (E)-nerolidol. These findings suggest that epigenetic mechanisms, including DNA methylation and histone modification, play a pivotal role in the metabolic responses of tea plants to environmental stressors [83]. Changes in DNA methylation levels have been shown to suppress carotenoid and anthocyanin production, with modifications in aroma components observed following 5-azacytidine treatment. DNA hypomethylation led to the upregulation of genes involved in photosynthesis, flavonoid biosynthesis, and hormone signaling pathways. However, this increase in gene expression was accompanied by a corresponding decline in protein levels. These findings highlight the crucial role of DNA methylation in regulating key metabolic pathways, impacting both pigment production and specialized metabolism in plants [84]. Although these findings do not directly pertain to the regulation of specialized metabolism genes, it is reasonable to hypothesize that similar epigenetic mechanisms may govern gene expression in these contexts [85].
4. Discussion
4.1. Environmental Conditions and Adaptive Changes
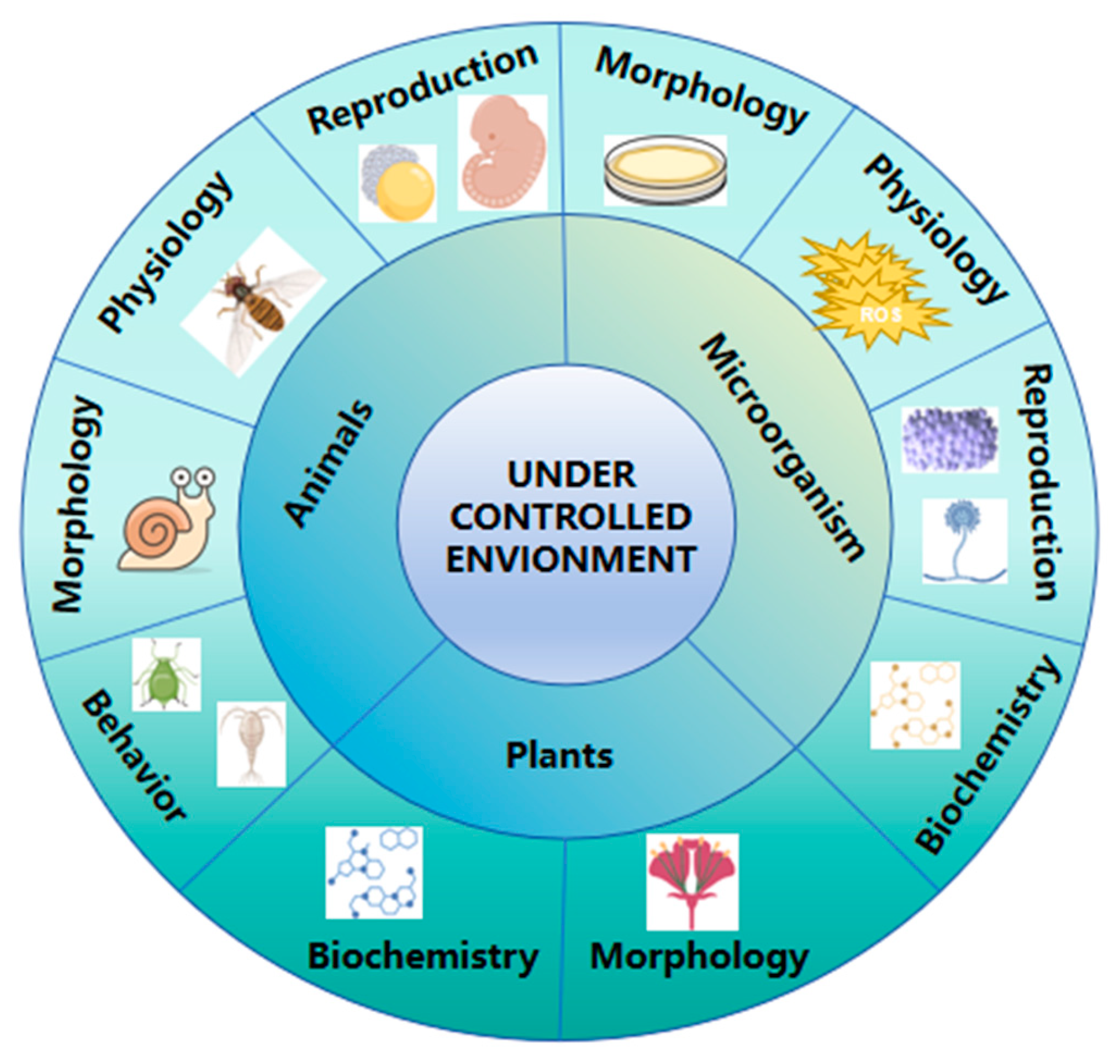
The type and intensity of environmental conditions play a pivotal role in determining the direction and magnitude of adaptive changes. For instance, microorganisms subjected to nutrient-limited environments often exhibit rapid morphological and physiological adaptations, such as biofilm formation or metabolic reprogramming, to enhance survival. In contrast, animals exposed to controlled stressors like temperature fluctuations or dietary restrictions may develop adaptive changes in reproductive strategies, lifespan, or behavior (Figure 4). These adaptations have been extensively studied, with well-documented examples such as the rapid evolution of antibiotic resistance in bacteria and the phenotypic plasticity of reproductive traits in rodents under dietary stress.
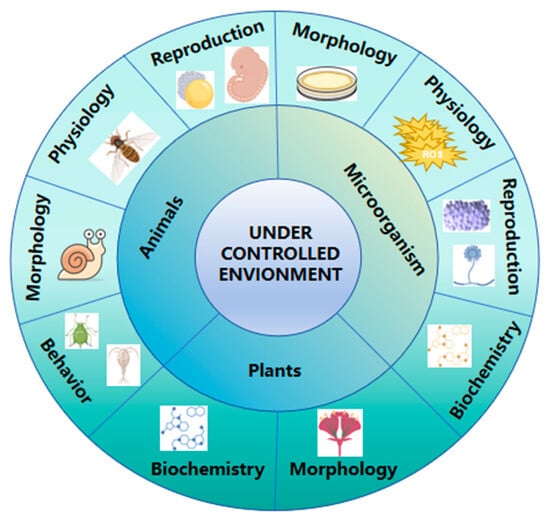
Figure 4.
Types of adaptive traits exhibited by different organisms under controlled conditions. In animals, adaptive changes include reproductive, physiological, morphological, and behavioral traits. In microorganisms, variations occur in morphological, physiological, reproductive, and biochemical traits. In plants, adaptations involve morphological and biochemical traits.
In plants, however, research on adaptive changes under controlled conditions remains relatively limited, particularly in comparison with the breadth of studies on microorganisms and animals. While some studies have explored morphological and biochemical adjustments, such as changes in stamen and pistil length or stress-induced metabolite accumulation in response to abiotic stresses like drought or salinity, fewer investigations have focused on reproductive traits. For example, the role of seed-related traits—such as seed size, germination rate, and dormancy—in plant adaptations to controlled environments remains underexplored. Given the critical importance of seeds in plant survival and dispersal, future research could leverage insights from microbial and animal studies to investigate how environmental pressures shape seed-related adaptations. By examining these traits, researchers may uncover novel mechanisms of plant adaptation and provide a more comprehensive understanding of evolutionary processes across taxa.
4.2. Mechanisms Underlying Adaptive Changes
The diversity of adaptive changes is underpinned by a combination of classical genetic and epigenetic mechanisms. In microorganisms, stochastic gene mutations and chromosomal instability are primary drivers of rapid adaptation, enabling swift responses to environmental challenges. For instance, fungi subjected to successive subculturing often exhibit significant degenerative changes, such as reduced fruiting body production and metabolic activity, which have been linked to chromosomal instability and genetic variation. In animals, adaptive changes in reproductive and physiological traits are often mediated by genetic variation and epigenetic modifications, such as DNA methylation and histone modifications, which fine-tune gene expression in response to environmental cues. A notable example is the role of DNA methylation in regulating stress-responsive genes in mammals exposed to fluctuating temperatures.
In plants, current research has primarily focused on epigenetic mechanisms, with DNA methylation and non-coding RNA regulation being the most extensively studied. For example, DNA methylation has been demonstrated to play a critical role in mediating stress responses, such as drought tolerance in Arabidopsis thaliana, by modulating the expression of stress-related genes. Similarly, non-coding RNAs, including microRNAs and long non-coding RNAs, have been implicated in regulating developmental plasticity and stress adaptation in plants. However, chromosomal instability, a well-documented driver of adaptation in microorganisms and animals, has not been widely reported or summarized as a major factor in plant adaptation. This discrepancy may reflect a true biological difference or a gap in current research. Exploring the potential role of chromosomal instability in plant adaptations, particularly under extreme environmental stressors, could uncover novel genetic and epigenetic mechanisms and provide a more comprehensive understanding of evolutionary processes across taxa.
4.3. Variation in Adaptive Rates Across Species
The rate of adaptive changes varies significantly among microorganisms, animals, and plants, reflecting differences in their life history traits and genetic architectures. Microorganisms, with their short generation times and high mutation rates, exhibit the fastest adaptive responses, often observable within days or weeks. For example, fungi subjected to successive subculturing over just 20 months have been shown to undergo significant degenerative changes, including reduced fruiting body production, metabolic activity, and stress tolerance, likely due to chromosomal instability and genetic variation. In animals, adaptive rates are generally intermediate, with changes manifesting over months or years due to longer lifespans and more complex life cycles. However, some species, such as Drosophila melanogaster (fruit flies), exhibit relatively rapid adaptive changes, with phenotypic shifts observable within weeks under controlled laboratory conditions. Despite these exceptions, most animals fall into the intermediate range of adaptive rates.
In plants, adaptive changes typically occur at a slower pace compared with microorganisms and animals, primarily due to their sessile nature, longer generation times, and reliance on epigenetic mechanisms for stress responses. The extended life cycles of many plant species pose significant challenges for studying adaptive changes, as experiments often require years or even decades to observe measurable evolutionary shifts. However, understanding plants’ adaptation is crucial for unraveling the mechanisms of evolutionary processes in sessile organisms. To overcome these limitations, researchers could leverage rapid propagation techniques, such as tissue culture or clonal propagation, to accelerate the generation of plant materials under controlled conditions. These approaches would enable the study of adaptive changes over shorter timeframes, providing insights into the genetic and epigenetic mechanisms driving plant evolution. By adopting methodologies inspired by microbial and animal studies, plant research can bridge the gap in understanding adaptive rates across taxa and contribute to a more comprehensive framework for evolutionary biology.
5. Conclusions
Adaptive changes under controlled conditions arise from the complex interplay of environmental factors, genetic and epigenetic mechanisms, and species-specific biological traits. This review systematically examines the processes and genetic foundations of adaptive changes in microorganisms, animals, and plants, revealing that while research on animals and microorganisms is relatively comprehensive, studies on plant adaptation remain limited. The well-established mechanisms and technologies in animal and microbial systems provide a valuable framework for advancing plant adaptation research. Future studies should focus on elucidating the molecular basis of adaptive changes, particularly in understudied taxa such as plants, by leveraging advanced genomic and epigenomic tools to explore the roles of genetic variation, chromosomal instability, and non-coding RNA regulation. Such investigations will not only deepen our understanding of fundamental evolutionary processes but also offer practical applications for optimizing agricultural, biotechnological, and conservation systems. For instance, harnessing adaptive mechanisms in crops could enhance stress tolerance and yield stability, addressing global challenges such as climate change and food security.
Author Contributions
Y.-W.G.: Ideas, data curation, formal analysis, investigation, writing—original draft, writing—review and editing. Y.L.: Data curation, formal analysis, writing—original draft. P.-C.H.: Data curation, formal analysis. M.R.: Formal analysis, visualization. W.W.: Visualization. Y.-H.X.: Ideas, formulation or evolution of overarching research goals and aims, writing—review and editing. J.-H.W.: Ideas, formulation or evolution of overarching research goals and aims, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Key Research and Development Program of China (2022YFC3501500), the National Administration of Traditional Chinese Medicine (GZY-KJS-2023-008), and the CAMS Innovation Fund for Medical Sciences (CIFMS)—Construction of the Germplasm Resource Bank of Medicinal Plants (2021-I2M-1-032).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets generated for this study are included in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Parsons, P.A. The metabolic cost of multiple environmental stresses: Implications for climatic change and conservation. Trends Ecol. Evol. 1990, 5, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Seaborn, T.; Griffith, D.; Kliskey, A.; Caudill, C.C. Building a bridge between adaptive capacity and adaptive potential to understand responses to environmental change. Glob. Chang. Biol. 2021, 27, 2656–2668. [Google Scholar] [CrossRef] [PubMed]
- Kingsolver, J.G.; Buckley, L.B. Evolution of plasticity and adaptive responses to climate change along climate gradients. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170386. [Google Scholar] [CrossRef]
- Grant, P.R. Evolution, climate change, and extreme events. Science 2017, 357, 451–452. [Google Scholar] [CrossRef]
- Zheng, J.; Payne, J.L.; Wagner, A. Cryptic genetic variation accelerates evolution by opening access to diverse adaptive peaks. Science 2019, 365, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, T.; Morrissey, M.B.; de Villemereuil, P.; Alberts, S.C.; Arcese, P.; Bailey, L.D.; Boutin, S.; Brekke, P.; Brent, L.J.N.; Camenisch, G. Genetic variance in fitness indicates rapid contemporary adaptive evolution in wild animals. Science 2022, 376, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Ghalambor, C.K.; Hoke, K.L.; Ruell, E.W.; Fischer, E.K.; Reznick, D.N.; Hughes, K.A. Erratum: Non-adaptive plasticity potentiates rapid adaptive evolution of gene expression in nature. Nature 2018, 555, 688. [Google Scholar] [CrossRef]
- Lustenhouwer, N.; Wilschut, R.A.; Williams, J.L.; van der Putten, W.H.; Levine, J.M. Rapid evolution of phenology during range expansion with recent climate change. Glob. Chang. Biol. 2018, 24, e534–e544. [Google Scholar] [CrossRef] [PubMed]
- Ashe, A.; Sapetschnig, A.; Weick, E.-M.; Mitchell, J.; Bagijn, M.P.; Cording, A.C.; Doebley, A.-L.; Goldstein, L.D.; Lehrbach, N.J.; Le Pen, J. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell 2012, 150, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.M.; Hellmann, J.K. An integrative framework for understanding the mechanisms and multigenerational consequences of transgenerational plasticity. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 97–118. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Cui, J.; Wang, L.; Teng, N.; Zhang, S.; Lam, H.-M.; Zhu, Y.; Xiao, S.; Ke, W.; Lin, J. Genome-wide DNA mutations in Arabidopsis plants after multigenerational exposure to high temperatures. Genome Biol. 2021, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.Q.; Cho, M.; Zhu, H.; Puppala, H.L.; Escalera, G.; Zhong, W.; Colvin, V.L. Toxicity of quantum dots and cadmium salt to Caenorhabditis elegans after multigenerational exposure. Environ. Sci. Technol. 2013, 47, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kwak, J.I.; An, Y.-J. Multigenerational study of gold nanoparticles in Caenorhabditis elegans: Transgenerational effect of maternal exposure. Environ. Sci. Technol. 2013, 47, 5393–5399. [Google Scholar] [CrossRef]
- Byrne, M.; Foo, S.A.; Ross, P.M.; Putnam, H.M. Limitations of cross-and multigenerational plasticity for marine invertebrates faced with global climate change. Glob. Chang. Biol. 2020, 26, 80–102. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ye, Q.; Dai, W.; Zheng, J.; Li, Y.; Wang, C.; Luo, Z.; Yang, J.; Zhuo, W.; Wan, Q.-L. Cadmium exposure induces multigenerational inheritance of germ cell apoptosis and fertility suppression in Caenorhabditis elegans. Environ. Int. 2024, 191, 108952. [Google Scholar] [CrossRef]
- Ramanan, D.; Sefik, E.; Galván-Peña, S.; Wu, M.; Yang, L.; Yang, Z.; Kostic, A.; Golovkina, T.V.; Kasper, D.L.; Mathis, D. An immunologic mode of multigenerational transmission governs a gut Treg setpoint. Cell 2020, 181, 1276–1290.e13. [Google Scholar] [CrossRef]
- Kwak, J.I.; Kim, L.; An, Y.J. Microplastics promote the accumulation of negative fungal groups and cause multigenerational effects in springtails. J. Hazard. Mater. 2024, 466, 133574. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Jander, G. Molecular ecology of plant volatiles in interactions with insect herbivores. J. Exp. Bot. 2022, 73, 449–462. [Google Scholar] [CrossRef]
- Sakauchi, K.; Otaki, J.M. Soil microbes and plant-associated microbes in response to radioactive pollution may indirectly affect plants and insect herbivores: Evidence for indirect field effects from chernobyl and fukushima. Microorganisms 2024, 12, 364. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Bao, Y.; Zhang, M.; Liu, S.; Yu, C.; Dai, J.; Wu, C.; Tang, D.; Fang, W. An inactivating mutation in the vacuolar arginine exporter gene Vae results in culture degeneration in the fungus Metarhizium robertsii. Environ. Microbiol. 2022, 24, 2924–2937. [Google Scholar] [CrossRef]
- Pichersky, E.; Lewinsohn, E. Convergent evolution in plant specialized metabolism. Annu. Rev. Plant Biol. 2011, 62, 549–566. [Google Scholar] [CrossRef]
- Ono, E.; Murata, J. Exploring the evolvability of plant specialized metabolism: Uniqueness out of uniformity and uniqueness behind uniformity. Plant Cell Physiol. 2023, 64, 1449–1465. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Liu, X.; Cui, B.; Miao, W.; Cheng, W.; Zhao, F. Characteristics analysis reveals the progress of Volvariella volvacea mycelium subculture degeneration. Front. Microbiol. 2019, 10, 2045. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, Q.; An, X.; Tan, Q.; Yun, J.; Zhang, Y. Oxidative damage from repeated tissue isolation for subculturing causes degeneration in Volvariella volvacea. Front. Microbiol. 2023, 14, 1210496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Liu, X.; Chen, C.; Cheng, Z.; Wang, W.; Yun, J. Successive mycelial subculturing decreased lignocellulase activity and increased ROS accumulation in Volvariella volvacea. Front. Microbiol. 2022, 13, 997485. [Google Scholar] [CrossRef]
- Chen, A.; Wang, Y.; Shao, Y.; Huang, B. A novel technique for rejuvenation of degenerated caterpillar medicinal mushroom, Cordyceps militaris (Ascomycetes), a valued traditional Chinese medicine. Int. J. Med. Mushrooms 2017, 19, 87–91. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.S.A.; Hassan, W.H.B.; Sweilam, S.H.; Alqarni, M.H.S.; El Sayed, Z.I.; Abdel-Aal, M.M.; Abdelsalam, E.; Abdelaziz, S. Production, bioprocessing and anti-proliferative activity of camptothecin from Penicillium chrysogenum, “an endozoic of marine sponge, Cliona sp.”, as a metabolically stable camptothecin producing isolate. Molecules 2022, 27, 3033. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.S.A.; Mohamed, N.Z.; Safan, S.; Yassin, M.A.; Shaban, L.; Shindia, A.A.; Shad Ali, G.; Sitohy, M.Z. Restoring the Taxol biosynthetic machinery of Aspergillus terreus by Podocarpus gracilior Pilger microbiome, with retrieving the ribosome biogenesis proteins of WD40 superfamily. Sci. Rep. 2019, 9, 11534. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, B.; Song, J.; Pang, S.; Song, T.; Gao, S.; Zhang, Y.; Huang, H.; Qi, T. A molecular framework for signaling crosstalk between jasmonate and ethylene in anthocyanin biosynthesis, trichome development, and defenses against insect herbivores in Arabidopsis. J. Integr. Plant Biol. 2022, 64, 1770–1788. [Google Scholar] [CrossRef] [PubMed]
- Wellham, P.A.D.; Hafeez, A.; Gregori, A.; Brock, M.; Kim, D.-H.; Chandler, D.; de Moor, C.H. Culture degeneration reduces sex-related gene expression, alters metabolite production and reduces insect pathogenic response in Cordyceps militaris. Microorganisms 2021, 9, 1559. [Google Scholar] [CrossRef] [PubMed]
- Sgrò, C.M.; Terblanche, J.S.; Hoffmann, A.A. What can plasticity contribute to insect responses to climate change? Annu. Rev. Entomol. 2016, 61, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Rudman, S.M.; Greenblum, S.I.; Rajpurohit, S.; Betancourt, N.J.; Hanna, J.; Tilk, S.; Yokoyama, T.; Petrov, D.A.; Schmidt, P. Direct observation of adaptive tracking on ecological time scales in Drosophila. Science 2022, 375, eabj7484. [Google Scholar] [CrossRef] [PubMed]
- Levell, S.T.; Bedgood, S.A.; Travis, J. Plastic maternal effects of social density on reproduction and fitness in the least killifish, Heterandria formosa. Ecol. Evol. 2023, 13, e10074. [Google Scholar] [CrossRef] [PubMed]
- Castano-Sanz, V.; Gomez-Mestre, I.; Garcia-Gonzalez, F. Evolutionary consequences of pesticide exposure include transgenerational plasticity and potential terminal investment transgenerational effects. Evolution 2022, 76, 2649–2668. [Google Scholar] [CrossRef] [PubMed]
- Gillis, M.K.; Walsh, M.R. Individual variation in plasticity dulls transgenerational responses to stress. Funct. Ecol. 2019, 33, 1993–2002. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, F.; Sun, S. Transgenerational effects and temperature variation alter life history traits of the moon jellyfish. Front. Mar. Sci. 2022, 9, 913654. [Google Scholar] [CrossRef]
- Webster, A.K.; Jordan, J.M.; Hibshman, J.D.; Chitrakar, R.; Baugh, L.R. Transgenerational effects of extended dauer diapause on starvation survival and gene expression plasticity in Caenorhabditis elegans. Genetics 2018, 210, 263–274. [Google Scholar] [CrossRef]
- Castano-Sanz, V.; Gomez-Mestre, I.; Rodriguez-Exposito, E.; Garcia-Gonzalez, F. Pesticide exposure triggers sex-specific inter-and transgenerational effects conditioned by past sexual selection. Proc. R. Soc. B Biol. Sci. 2024, 291, 20241037. [Google Scholar] [CrossRef] [PubMed]
- Beaty, L.E.; Wormington, J.D.; Kensinger, B.J.; Bayley, K.N.; Goeppner, S.R.; Gustafson, K.D.; Luttbeg, B. Shaped by the past, acting in the present: Transgenerational plasticity of anti-predatory traits. Oikos 2016, 125, 1570–1576. [Google Scholar] [CrossRef]
- Graeve, A.; Janßen, M.; Villalba de la Pena, M.; Tollrian, R.; Weiss, L.C. Higher, faster, better: Maternal effects shorten time lags and increase morphological defenses in Daphnia lumholtzi offspring generations. Front. Ecol. Evol. 2021, 9, 637421. [Google Scholar] [CrossRef]
- Sha, Y.; Hansson, L.-A. Ancestral environment determines the current reaction to ultraviolet radiation in Daphnia magna. Evolution 2022, 76, 1821–1835. [Google Scholar] [CrossRef]
- Sentis, A.; Bertram, R.; Dardenne, N.; Ramon-Portugal, F.; Espinasse, G.; Louit, I.; Negri, L.; Haeler, E.; Ashkar, T.; Pannetier, T. Evolution without standing genetic variation: Change in transgenerational plastic response under persistent predation pressure. Heredity 2018, 121, 266–281. [Google Scholar] [CrossRef]
- Smithson, M.; Thorson, J.L.M.; Sadler-Riggleman, I.; Beck, D.; Skinner, M.K.; Dybdahl, M. Between-generation phenotypic and epigenetic stability in a clonal snail. Genome Biol. Evol. 2020, 12, 1604–1615. [Google Scholar] [CrossRef]
- Nelson, A.C.; Cauceglia, J.W.; Merkley, S.D.; Youngson, N.A.; Oler, A.J.; Nelson, R.J.; Cairns, B.R.; Whitelaw, E.; Potts, W.K. Reintroducing domesticated wild mice to sociality induces adaptive transgenerational effects on MUP expression. Proc. Natl. Acad. Sci. USA 2013, 110, 19848–19853. [Google Scholar] [CrossRef]
- Gervasi, D.D.L.; Schiestl, F.P. Real-time divergent evolution in plants driven by pollinators. Nat. Commun. 2017, 8, 14691. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.E.; Schiestl, F.P. Rapid plant evolution driven by the interaction of pollination and herbivory. Science 2019, 364, 193–196. [Google Scholar] [CrossRef]
- Yu, X.; Lu, M.; Zhou, M.; Wang, H.; Feng, J.; Wen, Y. Reduction of pectin may decrease the embryogenicity of grapevine (Vitis vinifera) pro-embryonic masses after 10 years of in vitro culture. Sci. Hortic. 2023, 309, 111690. [Google Scholar] [CrossRef]
- Lin, X.; Yin, J.; Wang, Y.; Yao, J.; Li, Q.Q.; Latzel, V.; Bossdorf, O.; Zhang, Y.-Y. Environment-induced heritable variations are common in Arabidopsis thaliana. Nat. Commun. 2024, 15, 4615. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.; Becker, C.; Marconi, G.; Durr, J.; Price, J.; Hagmann, J.; Papareddy, R.; Putra, H.; Kageyama, J.; Becker, J. Correction: Hyperosmotic stress memory in Arabidopsis is mediated by distinct epigenetically labile sites in the genome and is restricted in the male germline by DNA glycosylase activity. eLife 2018, 7, e44302. [Google Scholar] [CrossRef]
- Smit, S.J.; Lichman, B.R. Plant biosynthetic gene clusters in the context of metabolic evolution. Nat. Prod. Rep. 2022, 39, 1465–1482. [Google Scholar] [CrossRef]
- Zhan, C.; Shen, S.; Yang, C.; Liu, Z.; Fernie, A.R.; Graham, I.A.; Luo, J. Plant metabolic gene clusters in the multi-omics era. Trends Plant Sci. 2022, 27, 981–1001. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Z. Unlocking plant metabolic diversity: A (pan)-genomic view. Plant Commun. 2022, 3, 100300. [Google Scholar] [CrossRef] [PubMed]
- Kominek, J.; Doering, D.T.; Opulente, D.A.; Shen, X.; Zhou, X.; DeVirgilio, J.; Hulfachor, A.B.; Groenewald, M.; McGee, M.A.; Karlen, S.D. Eukaryotic acquisition of a bacterial operon. Cell 2019, 176, 1356–1366.e10. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Guo, Z.; Yang, Z.; Han, H.; Wang, S.; Xu, H.; Yang, X.; Yang, F.; Wu, Q.; Xie, W. Whitefly hijacks a plant detoxification gene that neutralizes plant toxins. Cell 2021, 184, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- Nanney, D.L. Epigenetic control systems. Proc. Natl. Acad. Sci. USA 1958, 44, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Portela, A.; Esteller, M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010, 28, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Biswas, I. Genetic tools for manipulating Acinetobacter baumannii genome: An overview. J. Med. Microbiol. 2015, 64, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Forche, A.; Cromie, G.; Gerstein, A.C.; Solis, N.V.; Pisithkul, T.; Srifa, W.; Jeffery, E.; Abbey, D.; Filler, S.G.; Dudley, A.M. Rapid phenotypic and genotypic diversification after exposure to the oral host niche in Candida albicans. Genetics 2018, 209, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Danner, C.; Mach, R.L.; Mach-Aigner, A.R. The phenomenon of strain degeneration in biotechnologically relevant fungi. Microbiol. Biotechnol. 2023, 107, 4745–4758. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Lu, X.; Xing, L.; Ho, S.W.A.; Kwan, H.S. Genomic and transcriptomic comparison of Aspergillus oryzae strains: A case study in soy sauce koji fermentation. J. Ind. Microbiol. Biotechnol. 2018, 45, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.H.; Henriksen, C.M.; Nielsen, J.; Villadsen, J.; Egel-Mitani, M. Continuous cultivation of Penicillium chrysogenum. Growth on glucose and penicillin production. J. Biotechnol. 1995, 42, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Barredo, J.L.; Díez, B.; Alvarez, E.; Martín, J.F. Large amplification of a 35-kb DNA fragment carrying two penicillin biosynthetic genes in high penicillin producing strains of Penicillium chrysogenum. Curr. Genet. 1989, 16, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Fierro, F.; Barredo, J.L.; Díez, B.; Gutierrez, S.; Fernández, F.J.; Martin, J.F. The penicillin gene cluster is amplified in tandem repeats linked by conserved hexanucleotide sequences. Proc. Natl. Acad. Sci. USA 1995, 92, 6200–6204. [Google Scholar] [CrossRef]
- Newbert, R.W.; Barton, B.; Greaves, P.; Harper, J.; Turner, G. Analysis of a commercially improved Penicillium chrysogenum strain series: Involvement of recombinogenic regions in amplification and deletion of the penicillin biosynthesis gene cluster. J. Ind. Microbiol. Biotechnol. 1997, 19, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Douma, R.D.; Batista, J.M.; Touw, K.M.; Kiel, J.A.K.W.; Krikken, A.M.; Zhao, Z.; Veiga, T.; Klaassen, P.; Bovenberg, R.A.L.; Daran, J.-M. Degeneration of penicillin production in ethanol-limited chemostat cultivations of Penicillium chrysogenum: A systems biology approach. BMC Syst. Biol. 2011, 5, 132. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Lin, J.; Guo, L.; Wang, X.; Tian, S.; Liu, C.; Zhao, Y.; Zhao, R. Advances in research on Cordyceps militaris degeneration. Microbiol. Biotechnol. 2019, 103, 7835–7841. [Google Scholar] [CrossRef]
- Xie, H.; Ma, Q.; Wei, D.; Wang, F. Transcriptomic analysis of Aspergillus niger strains reveals the mechanism underlying high citric acid productivity. Bioresour. Bioprocess. 2018, 5, 21. [Google Scholar] [CrossRef]
- Li, X.; Huang, L.; Pan, L.; Wang, B.; Pan, L. CRISPR/dCas9-mediated epigenetic modification reveals differential regulation of histone acetylation on Aspergillus niger secondary metabolite. Microbiol. Res. 2021, 245, 126694. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, M.; Cai, X.; Han, Z.; Si, J.; Chen, D. Jasmonate signaling pathway modulates plant defense, growth, and their trade-offs. Int. J. Mol. Sci. 2022, 23, 3945. [Google Scholar] [CrossRef]
- Pierron, F.; Daffe, G.; Daramy, F.; Heroin, D.; Barré, A.; Bouchez, O.; Clérendeau, C.; Romero-Ramirez, A.; Nikolski, M. Transgenerational endocrine disruptor effects of cadmium in zebrafish and contribution of standing epigenetic variation to adaptation. J. Hazard. Mater. 2023, 455, 131579. [Google Scholar] [CrossRef] [PubMed]
- Maamar, M.B.; Nilsson, E.; Sadler-Riggleman, I.; Beck, D.; McCarrey, J.R.; Skinner, M.K. Developmental origins of transgenerational sperm DNA methylation epimutations following ancestral DDT exposure. Dev. Biol. 2019, 445, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Sgrò, C.M.; Weeks, A.R. Chromosomal inversion polymorphisms and adaptation. Trends Ecol. Evol. 2004, 19, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Rando, O.J.; Verstrepen, K.J. Timescales of genetic and epigenetic inheritance. Cell 2007, 128, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Tenaillon, O.; Rodríguez-Verdugo, A.; Gaut, R.L.; McDonald, P.; Bennett, A.F.; Long, A.D.; Gaut, B.S. The molecular diversity of adaptive convergence. Science 2012, 335, 457–461. [Google Scholar] [CrossRef]
- Shor, E.; Fox, C.A.; Broach, J.R. The yeast environmental stress response regulates mutagenesis induced by proteotoxic stress. PLoS Genet. 2013, 9, e1003680. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Mithani, A.; Belfield, E.J.; Mott, R.; Hurst, L.D.; Harberd, N.P. Environmentally responsive genome-wide accumulation of de novo Arabidopsis thaliana mutations and epimutations. Genome Res. 2014, 24, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Gorter, F.A.; Derks, M.F.L.; van den Heuvel, J.; Aarts, M.G.M.; Zwaan, B.J.; de Ridder, D.; de Visser, J.A.G.M. Genomics of adaptation depends on the rate of environmental change in experimental yeast populations. Mol. Biol. Evol. 2017, 34, 2613–2626. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Lu, M.Y.; Chang, Y.W.; Li, W.H. Experimental evolution of yeast for high-temperature tolerance. Mol. Biol. Evol. 2018, 35, 1823–1839. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.E.; Tittes, S.; Franks, S.J. Rapid, nonparallel genomic evolution of Brassica rapa (field mustard) under experimental drought. J. Evol. Biol. 2023, 36, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Zhu, X.; Xie, S.; Lang, Z.; Zhu, J.-K. Transgenerational increases in DNA methylation in Arabidopsis plants defective in active DNA demethylation. Proc. Natl. Acad. Sci. USA 2024, 121, e2320468121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tian, C.; Zhu, C.; Lai, Z.; Lin, Y.; Guo, Y. Hidden players in the regulation of secondary metabolism in tea plant: Focus on non-coding RNAs. Beverage Plant Res. 2022, 2, 19. [Google Scholar] [CrossRef]
- Yang, J.; Gu, D.; Wu, S.; Zhou, X.; Chen, J.; Liao, Y.; Zeng, L.; Yang, Z. Feasible strategies for studying the involvement of DNA methylation and histone acetylation in the stress-induced formation of quality-related metabolites in tea (Camellia sinensis). Hortic. Res. 2021, 8, 253. [Google Scholar] [CrossRef]
- Jia, H.; Jia, H.; Lu, S.; Zhang, Z.; Su, Z.; Sadeghnezhad, E.; Li, T.; Xiao, X.; Wang, M.; Pervaiz, T. DNA and histone methylation regulates different types of fruit ripening by transcriptome and proteome analyses. J. Agric. Food Chem. 2022, 70, 3541–3556. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Duncan, S.; Li, Y.; Huang, S.; Luo, M. Decoding plant specialized metabolism: New mechanistic insights. Trends Plant Sci. 2024, 29, 535–545. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).