Reduced Respiratory Sinus Arrhythmia in Infants with the FMR1 Premutation
Abstract
1. Introduction
- Do baseline RSA and IBI differ in 12-month-old infants with the FXpm compared to NT controls?
- Is there a relationship between CGG repeat length and baseline RSA or IBI in 12-month-old infants with the FXpm?
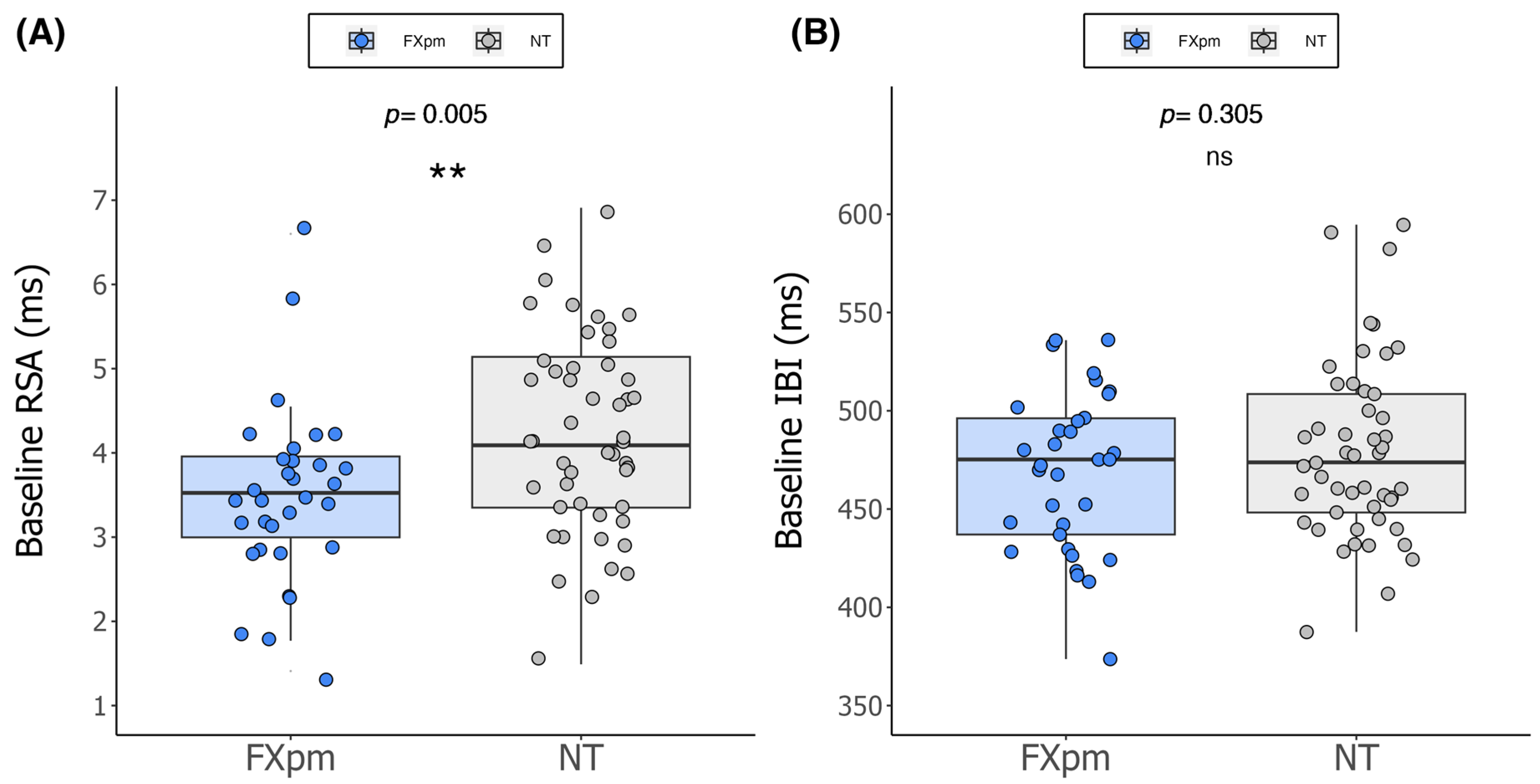
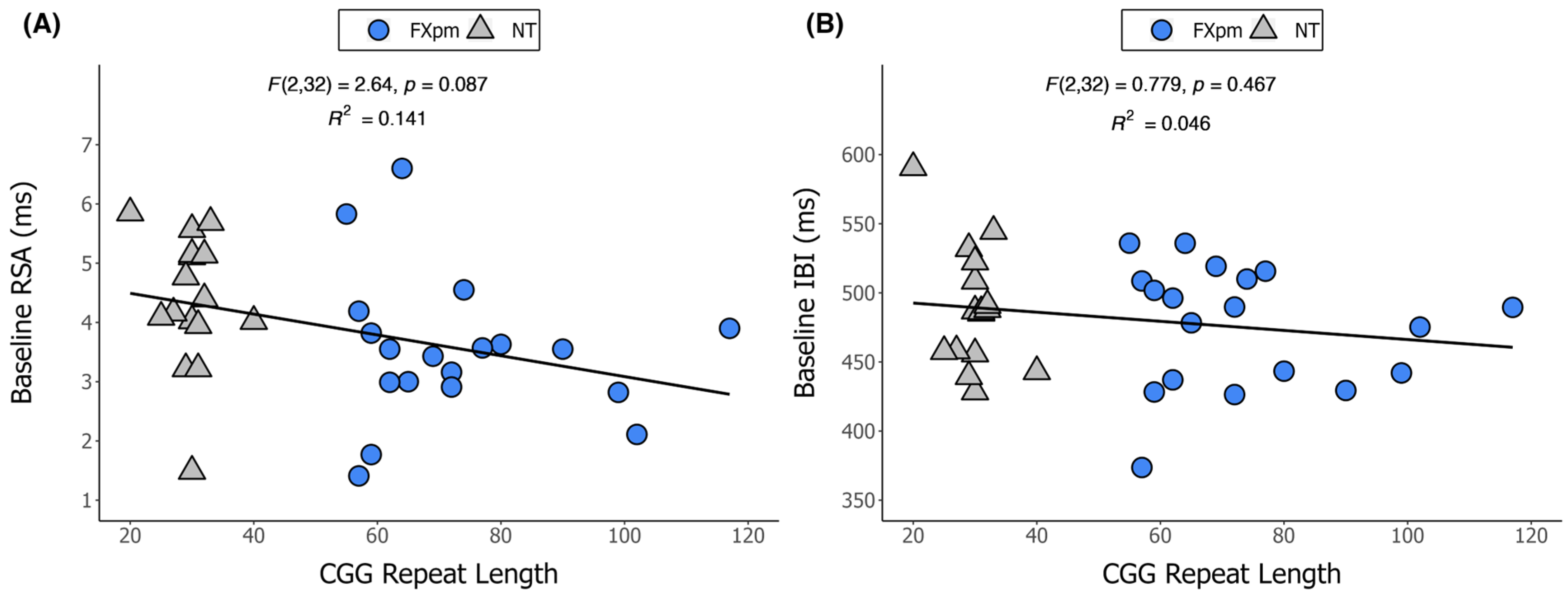
2. Results
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. ANS Function
4.3. CGG Repeat Length
4.4. Procedure
4.5. Statistical Plan
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANS | Autonomic nervous system |
| ASD | Autism spectrum disorder |
| BSID | Bayley Scales of Infant and Toddler Development |
| CGG | Cytosine–guanine–guanine |
| FMR1 | Fragile X messenger ribonucleoprotein 1 |
| FMRP | FMR1 protein |
| FXAND | Fragile X-associated neuropsychiatric disorders |
| FXPAC | Fragile X premutation associated conditions |
| FXpm | Fragile X premutation |
| FXS | Fragile X syndrome |
| FXTAS | Fragile X-associated tremor/ataxia syndrome |
| IBI | Interbeat interval |
| IBR SCL | Institute for Basic Research Specialty Clinical Laboratories |
| MSEL | Mullen Scales of Early Learning |
| NT | Neurotypical |
| PNS | Parasympathetic nervous system |
| RSA | Respiratory sinus arrhythmia |
References
- Cornish, K.; Turk, J.; Hagerman, R. The fragile X continuum: New advances and perspectives. J. Intellect. Disabil. Res. 2008, 52, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.J.; Hagerman, P.J. Fragile X Syndrome: Diagnosis, Treatment, and Research, 3rd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2002; pp. 3–68. [Google Scholar]
- Raspa, M.; Wylie, A.; Wheeler, A.C.; Kolacz, J.; Edwards, A.; Heilman, K.; Porges, S.W. Sensory Difficulties in Children With an FMR1 Premutation. Front. Genet. 2018, 9, 351. [Google Scholar] [CrossRef]
- Hessl, D.; Rivera, S.; Koldewyn, K.; Cordeiro, L.; Adams, J.; Tassone, F.; Hagerman, P.J.; Hagerman, R.J. Amygdala dysfunction in men with the fragile X premutation. Brain 2007, 130, 404–416. [Google Scholar] [CrossRef]
- Klusek, J.; LaFauci, G.; Adayev, T.; Brown, W.T.; Tassone, F.; Roberts, J.E. Reduced vagal tone in women with the FMR1 premutation is associated with FMR1 mRNA but not depression or anxiety. J. Neurodev. Disord. 2017, 9, 16. [Google Scholar] [CrossRef]
- Cornish, K.; Kogan, C.; Turk, J.; Manly, T.; James, N.; Mills, A.; Dalton, A. The emerging fragile X premutation phenotype: Evidence from the domain of social cognition. Brain Cogn. 2005, 57, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.; Abucayan, F.; Hagerman, R.; Tassone, F.; Hessl, D. Anxiety disorders in fragile X premutation carriers: Preliminary characterization of probands and non-probands. Intractable Rare Dis. Res. 2015, 4, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Klusek, J.; Ruber, A.; Roberts, J.E. Impaired eye contact in the FMR1 premutation is not associated with social anxiety or the broad autism phenotype. Clin. Neuropsychol. 2018, 32, 1337–1352. [Google Scholar] [CrossRef] [PubMed]
- Hantash, F.M.; Goos, D.M.; Crossley, B.; Anderson, B.; Zhang, K.; Sun, W.; Strom, C.M. FMR1 premutation carrier frequency in patients undergoing routine population-based carrier screening: Insights into the prevalence of fragile X syndrome, fragile X-associated tremor/ataxia syndrome, and fragile X-associated primary ovarian insufficiency in the United States. Genet. Med. 2011, 13, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.; Rivero-Arias, O.; Angelov, A.; Kim, E.; Fotheringham, I.; Leal, J. Epidemiology of fragile X syndrome: A systematic review and meta-analysis. Am. J. Med. Genet. A 2014, 164A, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, M.M.; Baker, M.W.; Hong, J.; Maenner, M.; Greenberg, J.; Mandel, D. Prevalence of CGG expansions of the FMR1 gene in a US population-based sample. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012, 159B, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Zucker, A.; Hinton, V.J. Autistic Traits Associated with the Fragile X Premutation Allele: The Neurodevelopmental Profile. Dev. Neuropsychol. 2024, 49, 153–166. [Google Scholar] [CrossRef]
- Klusek, J.; Will, E.; Christensen, T.; Caravella, K.; Hogan, A.; Sun, J.; Smith, J.; Fairchild, A.J.; Roberts, J.E. Social Communication Delay in an Unbiased Sample of Preschoolers with the FMR1 Premutation. J. Speech Lang. Hear. Res. 2024, 67, 2316–2332. [Google Scholar] [CrossRef]
- Wheeler, A.C.; Sideris, J.; Hagerman, R.; Berry-Kravis, E.; Tassone, F.; Bailey, D.B., Jr. Developmental profiles of infants with an FMR1 premutation. J. Neurodev. Disord. 2016, 8, 40. [Google Scholar] [CrossRef]
- Sagaser, K.G.; Malinowski, J.; Westerfield, L.; Proffitt, J.; Hicks, M.A.; Toler, T.L.; Blakemore, K.J.; Stevens, B.K.; Oakes, L.M. Expanded carrier screening for reproductive risk assessment: An evidence-based practice guideline from the National Society of Genetic Counselors. J. Genet. Couns. 2023, 32, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Fuller, E.A.; Kaiser, A.P. The Effects of Early Intervention on Social Communication Outcomes for Children with Autism Spectrum Disorder: A Meta-analysis. J. Autism Dev. Disord. 2020, 50, 1683–1700. [Google Scholar] [CrossRef] [PubMed]
- Rodas, N.V.; Eisenhower, A.; Blacher, J. Structural and Pragmatic Language in Children with ASD: Longitudinal Impact on Anxiety and Externalizing Behaviors. J. Autism Dev. Disord. 2017, 47, 3479–3488. [Google Scholar] [CrossRef]
- Baughman, N.; Prescott, S.L.; Rooney, R. The Prevention of Anxiety and Depression in Early Childhood. Front. Psychol. 2020, 11, 517896. [Google Scholar] [CrossRef] [PubMed]
- Rapee, R.M.; Kennedy, S.; Ingram, M.; Edwards, S.; Sweeney, L. Prevention and early intervention of anxiety disorders in inhibited preschool children. J. Consult. Clin. Psychol. 2005, 73, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, K.A.; Schultz, J.R.; Newsom, C. A comparison of two group-delivered social skills programs for young children with autism. J. Autism Dev. Disord. 2007, 37, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Johnston, C.; Tassone, F.; Sansone, S.; Hagerman, R.J.; Ferrer, E.; Rivera, S.M.; Hessl, D. Broad autism spectrum and obsessive-compulsive symptoms in adults with the fragile X premutation. Clin. Neuropsychol. 2016, 30, 929–943. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Herring, J.; Richstein, J. Fragile X Premutation Associated Conditions (FXPAC). Front. Pediatr. 2020, 8, 266. [Google Scholar] [CrossRef]
- Jacquemont, S.; Hagerman, R.J.; Leehey, M.A.; Hall, D.A.; Levine, R.A.; Brunberg, J.A.; Zhang, L.; Jardini, T.; Gane, L.W.; Harris, S.W.; et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA 2004, 291, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Revenga, L.; Madrigal, I.; Pagonabarraga, J.; Xuncla, M.; Badenas, C.; Kulisevsky, J.; Gomez, B.; Mila, M. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur. J. Hum. Genet. 2009, 17, 1359–1362. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.D.; Welt, C.; Sherman, S. FMR1 and the continuum of primary ovarian insufficiency. Semin. Reprod. Med. 2011, 29, 299–307. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Protic, D.; Rajaratnam, A.; Salcedo-Arellano, M.J.; Aydin, E.Y.; Schneider, A. Fragile X-Associated Neuropsychiatric Disorders (FXAND). Front. Psychiatry 2018, 9, 564. [Google Scholar] [CrossRef] [PubMed]
- Klusek, J.; Fairchild, A.J.; Roberts, J.E. Vagal Tone as a Putative Mechanism for Pragmatic Competence: An Investigation of Carriers of the FMR1 Premutation. J. Autism Dev. Disord. 2019, 49, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Maltman, N.; DaWalt, L.S.; Hong, J.; Baker, M.W.; Berry-Kravis, E.M.; Brilliant, M.H.; Mailick, M. FMR1 CGG Repeats and Stress Influence Self-Reported Cognitive Functioning in Mothers. Am. J. Intellect. Dev. Disabil. 2023, 128, 1–20. [Google Scholar] [CrossRef]
- Hong, J.; Dembo, R.S.; DaWalt, L.S.; Brilliant, M.; Berry-Kravis, E.M.; Mailick, M. The effect of college degree attainment on neurodegenerative symptoms in genetically at-risk women. SSM Popul. Health 2022, 19, 101262. [Google Scholar] [CrossRef]
- Klusek, J. Genetic risk for FXTAS neuromotor signs in women with the FMR1 premutation buffered by education. In Proceedings of the NFXF International Fragile X Conference, Orlando, FL, USA, 25–28 July 2024. [Google Scholar]
- Dias, C.M.; Issac, B.; Sun, L.; Lukowicz, A.; Talukdar, M.; Akula, S.K.; Miller, M.B.; Walsh, K.; Rockowitz, S.; Walsh, C.A. Glial dysregulation in the human brain in fragile X-associated tremor/ataxia syndrome. Proc. Natl. Acad. Sci. USA 2023, 120, e2300052120. [Google Scholar] [CrossRef] [PubMed]
- Elizur, S.E.; Friedman Gohas, M.; Dratviman-Storobinsky, O.; Cohen, Y. Pathophysiology Mechanisms in Fragile-X Primary Ovarian Insufficiency. Methods Mol. Biol. 2019, 1942, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Gohel, D.; Sripada, L.; Prajapati, P.; Currim, F.; Roy, M.; Singh, K.; Shinde, A.; Mane, M.; Kotadia, D.; Tassone, F.; et al. Expression of expanded FMR1-CGG repeats alters mitochondrial miRNAs and modulates mitochondrial functions and cell death in cellular model of FXTAS. Free Radic. Biol. Med. 2021, 165, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Kraan, C.M.; Godler, D.E.; Amor, D.J. Epigenetics of fragile X syndrome and fragile X-related disorders. Dev. Med. Child. Neurol. 2019, 61, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Loomis, E.W.; Sanz, L.A.; Chedin, F.; Hagerman, P.J. Transcription-associated R-loop formation across the human FMR1 CGG-repeat region. PLoS Genet. 2014, 10, e1004294. [Google Scholar] [CrossRef]
- Todd, P.K.; Oh, S.Y.; Krans, A.; He, F.; Sellier, C.; Frazer, M.; Renoux, A.J.; Chen, K.C.; Scaglione, K.M.; Basrur, V.; et al. CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron 2013, 78, 440–455. [Google Scholar] [CrossRef]
- Usdin, K.; Kumari, D. Repeat-mediated epigenetic dysregulation of the FMR1 gene in the fragile X-related disorders. Front. Genet. 2015, 6, 192. [Google Scholar] [CrossRef]
- Tassone, F.; Beilina, A.; Carosi, C.; Albertosi, S.; Bagni, C.; Li, L.; Glover, K.; Bentley, D.; Hagerman, P.J. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA 2007, 13, 555–562. [Google Scholar] [CrossRef]
- Berry-Kravis, E.; Abrams, L.; Coffey, S.M.; Hall, D.A.; Greco, C.; Gane, L.W.; Grigsby, J.; Bourgeois, J.A.; Finucane, B.; Jacquemont, S.; et al. Fragile X-associated tremor/ataxia syndrome: Clinical features, genetics, and testing guidelines. Mov. Disord. 2007, 22, 2018–2030. [Google Scholar] [CrossRef] [PubMed]
- Klusek, J.; Hong, J.; Sterling, A.; Berry-Kravis, E.; Mailick, M.R. Inhibition deficits are modulated by age and CGG repeat length in carriers of the FMR1 premutation allele who are mothers of children with fragile X syndrome. Brain Cogn. 2020, 139, 105511. [Google Scholar] [CrossRef]
- Klusek, J.; Porter, A.; Abbeduto, L.; Adayev, T.; Tassone, F.; Mailick, M.R.; Glicksman, A.; Tonnsen, B.L.; Roberts, J.E. Curvilinear Association Between Language Disfluency and FMR1 CGG Repeat Size Across the Normal, Intermediate, and Premutation Range. Front. Genet. 2018, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Mailick, M.R.; Hong, J.; Greenberg, J.; Smith, L.; Sherman, S. Curvilinear association of CGG repeats and age at menopause in women with FMR1 premutation expansions. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2014, 165B, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.G.; Sullivan, A.K.; Marcus, M.; Small, C.; Dominguez, C.; Epstein, M.P.; Charen, K.; He, W.; Taylor, K.C.; Sherman, S.L. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum. Reprod. 2007, 22, 2142–2152. [Google Scholar] [CrossRef] [PubMed]
- Hocking, D.R.; Loesch, D.Z.; Stimpson, P.; Tassone, F.; Atkinson, A.; Storey, E. Relationships of Motor Changes with Cognitive and Neuropsychiatric Features in FMR1 Male Carriers Affected with Fragile X-Associated Tremor/Ataxia Syndrome. Brain Sci. 2022, 12, 1549. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Hashimoto, R.; Tassone, F.; Simon, T.J.; Rivera, S.M. Altered neural activity of magnitude estimation processing in adults with the fragile X premutation. J. Psychiatr. Res. 2013, 47, 1909–1916. [Google Scholar] [CrossRef][Green Version]
- Benevides, T.W.; Lane, S.J. A review of cardiac autonomic measures: Considerations for examination of physiological response in children with autism spectrum disorder. J. Autism Dev. Disord. 2015, 45, 560–575. [Google Scholar] [CrossRef]
- Porges, S.W. Cardiac vagal tone: A physiological index of stress. Neurosci. Biobehav. Rev. 1995, 19, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W.; Doussard-Roosevelt, J.A.; Portales, A.L.; Greenspan, S.I. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Dev. Psychobiol. 1996, 29, 697–712. [Google Scholar] [CrossRef]
- Beauchaine, T.P. Respiratory Sinus Arrhythmia: A Transdiagnostic Biomarker of Emotion Dysregulation and Psychopathology. Curr. Opin. Psychol. 2015, 3, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.L. A theoretical analysis of the role of hyperarousal in the learning and behavior of fragile X males. Ment. Retard. Dev. Disabil. Res. Rev. 1995, 1, 286–291. [Google Scholar] [CrossRef]
- Belser, R.C.; Sudhalter, V. Arousal difficulties in males with fragile X syndrome: A preliminary report. Dev. Brain Dysfunct. 1995, 8, 270–279. [Google Scholar]
- Hogan, A.; Hunt, E.; Smith, K.; Black, C.; Bangert, K.; Klusek, J.; Roberts, J. Trajectories of Heart Activity Across Infancy to Early Childhood Differentially Predict Autism and Anxiety Symptoms in Fragile X Syndrome. Front. Psychiatry 2021, 12, 727559. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E.; Bradshaw, J.; Will, E.; Hogan, A.L.; McQuillin, S.; Hills, K. Emergence and rate of autism in fragile X syndrome across the first years of life. Dev. Psychopathol. 2020, 32, 1335–1352. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E.; Tonnsen, B.; Robinson, A.; Shinkareva, S.V. Heart activity and autistic behavior in infants and toddlers with fragile X syndrome. Am. J. Intellect. Dev. Disabil. 2012, 117, 90–102. [Google Scholar] [CrossRef][Green Version]
- Heilman, K.J.; Harden, E.R.; Zageris, D.M.; Berry-Kravis, E.; Porges, S.W. Autonomic regulation in fragile X syndrome. Dev. Psychobiol. 2011, 53, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Klusek, J.; Martin, G.E.; Losh, M. Physiological arousal in autism and fragile X syndrome: Group comparisons and links with pragmatic language. Am. J. Intellect. Dev. Disabil. 2013, 118, 475–495. [Google Scholar] [CrossRef]
- Hall, S.S.; Lightbody, A.A.; Huffman, L.C.; Lazzeroni, L.C.; Reiss, A.L. Physiological correlates of social avoidance behavior in children and adolescents with fragile x syndrome. J. Am. Acad. Child. Adolesc. Psychiatry 2009, 48, 320–329. [Google Scholar] [CrossRef]
- Boccia, M.L.; Roberts, J.E. Behavior and autonomic nervous system function assessed via heart period measures: The case of hyperarousal in boys with fragile X syndrome. Behav. Res. Methods Instrum. Comput. 2000, 32, 5–10. [Google Scholar] [CrossRef]
- Patriquin, M.A.; Lorenzi, J.; Scarpa, A.; Bell, M.A. Developmental trajectories of respiratory sinus arrhythmia: Associations with social responsiveness. Dev. Psychobiol. 2014, 56, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Patriquin, M.A.; Lorenzi, J.; Scarpa, A.; Calkins, S.D.; Bell, M.A. Broad implications for respiratory sinus arrhythmia development: Associations with childhood symptoms of psychopathology in a community sample. Dev. Psychobiol. 2015, 57, 120–130. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.E.B.; Sheinkopf, S.J.; Levine, T.P.; LaGasse, L.L.; Tronick, E.; Lester, B.L. Diminished respiratory sinus arrhythmia response in infants later diagnosed with autism spectrum disorder. Autism Res. 2018, 11, 726–731. [Google Scholar] [CrossRef]
- Tassone, F.; Protic, D.; Allen, E.G.; Archibald, A.D.; Baud, A.; Brown, T.W.; Budimirovic, D.B.; Cohen, J.; Dufour, B.; Eiges, R.; et al. Insight and Recommendations for Fragile X-Premutation-Associated Conditions from the Fifth International Conference on FMR1 Premutation. Cells 2023, 12, 2330. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Flavell, J.; Franklin, C.; Nestor, P.J. A Systematic Review of Fragile X-Associated Neuropsychiatric Disorders. J. Neuropsychiatry Clin. Neurosci. 2023, 35, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, J.A.; Seritan, A.L.; Casillas, E.M.; Hessl, D.; Schneider, A.; Yang, Y.; Kaur, I.; Cogswell, J.B.; Nguyen, D.V.; Hagerman, R.J. Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J. Clin. Psychiatry 2011, 72, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Tassanakijpanich, N.; Hagerman, R.J.; Worachotekamjorn, J. Fragile X premutation and associated health conditions: A review. Clin. Genet. 2021, 99, 751–760. [Google Scholar] [CrossRef]
- Grigsby, J.; Cornish, K.; Hocking, D.; Kraan, C.; Olichney, J.M.; Rivera, S.M.; Schneider, A.; Sherman, S.; Wang, J.Y.; Yang, J.C. The cognitive neuropsychological phenotype of carriers of the FMR1 premutation. J. Neurodev. Disord. 2014, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Aishworiya, R.; Protic, D.; Tang, S.J.; Schneider, A.; Tassone, F.; Hagerman, R. Fragile X-Associated Neuropsychiatric Disorders (FXAND) in Young Fragile X Premutation Carriers. Genes 2022, 13, 2399. [Google Scholar] [CrossRef] [PubMed]
- Patriquin, M.A.; Scarpa, A.; Friedman, B.H.; Porges, S.W. Respiratory sinus arrhythmia: A marker for positive social functioning and receptive language skills in children with autism spectrum disorders. Dev. Psychobiol. 2013, 55, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Guy, L.; Souders, M.; Bradstreet, L.; DeLussey, C.; Herrington, J.D. Brief report: Emotion regulation and respiratory sinus arrhythmia in autism spectrum disorder. J. Autism Dev. Disord. 2014, 44, 2614–2620. [Google Scholar] [CrossRef]
- Sheinkopf, S.J.; Levine, T.P.; McCormick, C.E.B.; Puggioni, G.; Conradt, E.; Lagasse, L.L.; Lester, B.M. Developmental trajectories of autonomic functioning in autism from birth to early childhood. Biol. Psychol. 2019, 142, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E.; Tonnsen, B.L.; McCary, L.M.; Ford, A.L.; Golden, R.N.; Bailey, D.B., Jr. Trajectory and Predictors of Depression and Anxiety Disorders in Mothers With the FMR1 Premutation. Biol. Psychiatry 2016, 79, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Bayley, N.; Aylward, G.P. Bayley 4: Scales of Infant and Toddler Development: Technical Manual, 4th ed.; NCS Pearson, Inc.: Bloomington, MN, USA, 2019. [Google Scholar]
- Mullen, E.M. Mullen Scales of Early Learning, AGS ed.; Pearson Clinical Assessment: Bloomington, MN, USA, 1995. [Google Scholar]
- Actiheart 5, version 5.1.14; CamNtech Ltd./Inc.: Cambridge, UK, 2019.
- Actiwave Cardio, version 1.6; CamNtech Ltd./Inc.: Cambridge, UK, 2009.
- CardioEdit Plus Software, version 7; Brain-Body Center for Psychophysiology and Bioengineering: University of North Carolina: Chapel Hill, NC, USA, 2022.
- CardioBatch Plus Software, version 7; Brain-Body Center for Psychophysiology and Bioengineering: University of North Carolina: Chapel Hill, NC, USA, 2016.
- AmplideX, version 3.0.0.107; Asuragen: Austin, TX, USA, 2023.
- Chen, L.; Hadd, A.; Sah, S.; Filipovic-Sadic, S.; Krosting, J.; Sekinger, E.; Pan, R.; Hagerman, P.J.; Stenzel, T.T.; Tassone, F.; et al. An information-rich CGG repeat primed PCR that detects the full range of fragile X expanded alleles and minimizes the need for southern blot analysis. J. Mol. Diagn. 2010, 12, 589–600. [Google Scholar] [CrossRef]
- Filipovic-Sadic, S.; Sah, S.; Chen, L.; Krosting, J.; Sekinger, E.; Zhang, W.; Hagerman, P.J.; Stenzel, T.T.; Hadd, A.G.; Latham, G.J.; et al. A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin. Chem. 2010, 56, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.; Zhou, L.; Jackson, J.; Tassone, F. Diagnostic profile of the AmplideX Fragile X Dx and Carrier Screen Kit for diagnosis and screening of fragile X syndrome and other FMR1-related disorders. Expert. Rev. Mol. Diagn. 2021, 21, 255–267. [Google Scholar] [CrossRef] [PubMed]
- IBM SPSS Statistics, version 27.0; IBM Corp: Armonk, NY, USA, 2020.
- Cook, R.D. Detection of Influential Observation in Linear-Regression. Technometrics 1977, 19, 15–18. [Google Scholar] [CrossRef]
| FXpm (n = 33) | NT (n = 49) | Total (n = 82) | |
|---|---|---|---|
| Age (in months) | |||
| Mean (SD) | 12.1 (1.5) | 11.8 (1.4) | 11.9 (1.4) |
| Range | 8.7–14.5 | 8.8–13.9 | 8.7–14.5 |
| Sex | |||
| Male | 19 (57.6%) | 33 (67.4%) | 52 (63.4%) |
| Female | 14 (42.4%) | 16 (32.7%) | 30 (36.6%) |
| Race | |||
| White | 28 (84.8%) | 38 (77.6%) | 66 (80.5%) |
| Black/African Am. | 0 (0%) | 6 (12.2%) | 6 (7.3%) |
| Asian | 1 (3.0%) | 0 (0%) | 1 (1.2%) |
| More than one race | 4 (12.2%) | 5 (10.2%) | 9 (11.0%) |
| Ethnicity | |||
| Hispanic | 2 (6.1%) | 5 (10.2%) | 7 (8.5%) |
| Non-Hispanic | 31 (93.9%) | 44 (89.8%) | 75 (91.5%) |
| MSEL ELC a | |||
| N | 9 | 28 | 37 |
| Mean (SD) | 93.9 (9.5) | 98.7 (14.8) | 97.5 (13.7) |
| Range | 82–111 | 71–134 | 71–134 |
| BSID Cognitive SS b | |||
| N | 24 | 21 | 45 |
| Mean (SD) | 93.1 (13.6) | 97.6 (8.5) | 95.2 (11.6) |
| Range | 70–115 | 80–110 | 70–115 |
| FXpm (n = 33) | NT (n = 49) | |||
|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | |
| CGG Repeat Length a | 73.3 (17.4) | 55–117 | 29.9 (4.1) | 20–40 |
| RSA | 3.5 (1.0) | 1.4–6.6 | 4.2 (1.2) | 1.5–6.9 |
| IBI | 469.3 (40.5) | 373.7–535.9 | 479.4 (45.3) | 387.5–594.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chase, A.; Hamrick, L.; Arnold, H.; Smith, J.; Hantman, R.; Cortez, K.; Adayev, T.; Tortora, N.D.; Dahlman, A.; Roberts, J. Reduced Respiratory Sinus Arrhythmia in Infants with the FMR1 Premutation. Int. J. Mol. Sci. 2025, 26, 2186. https://doi.org/10.3390/ijms26052186
Chase A, Hamrick L, Arnold H, Smith J, Hantman R, Cortez K, Adayev T, Tortora ND, Dahlman A, Roberts J. Reduced Respiratory Sinus Arrhythmia in Infants with the FMR1 Premutation. International Journal of Molecular Sciences. 2025; 26(5):2186. https://doi.org/10.3390/ijms26052186
Chicago/Turabian StyleChase, Abigail, Lisa Hamrick, Holley Arnold, Jenna Smith, Rachel Hantman, Kaitlyn Cortez, Tatyana Adayev, Nicole D. Tortora, Alison Dahlman, and Jane Roberts. 2025. "Reduced Respiratory Sinus Arrhythmia in Infants with the FMR1 Premutation" International Journal of Molecular Sciences 26, no. 5: 2186. https://doi.org/10.3390/ijms26052186
APA StyleChase, A., Hamrick, L., Arnold, H., Smith, J., Hantman, R., Cortez, K., Adayev, T., Tortora, N. D., Dahlman, A., & Roberts, J. (2025). Reduced Respiratory Sinus Arrhythmia in Infants with the FMR1 Premutation. International Journal of Molecular Sciences, 26(5), 2186. https://doi.org/10.3390/ijms26052186







