Mineral Carbonation for Carbon Sequestration: A Case for MCP and MICP
Abstract
1. Introduction
2. Biochemical Precipitation
2.1. Carbonate Precipitates
2.2. Biogenic Carbonate Precipitates
| Mechanism | Precipitate Location | Conditions | Organisms | Level of Organism Control | Precipitated Minerals |
|---|---|---|---|---|---|
| BCM | Intracellular, intercellular, extracellular | Controlled by cellular activities | Eukaryotes | High | Magnetite, greigite, amorphous silica, calcite |
| BIM | Extracellular | Reactive surfaces & metabolism | Prokaryotes | Moderate | Iron hydroxides, magnetite, manganese oxides, clays, amorphous silica, carbonates, phosphates, sulfates, sulfide minerals |
| BMM | EPS matrix | Alkalinity engine & organic matter | Not required | Low | Carbonate minerals |
| Carbonate Precipitation | Mechanism | Microbial Involvement | Application | Research Topics | Advantages | Drawbacks |
|---|---|---|---|---|---|---|
| MICP | BIM | Active | In situ 1 & ex situ 2 | Restoration of calcareous stones & construction materials, soil strengthening, selective plugging for oil recovery, bio-clogging, soil thermal conductivity, dust suppression, erosion control, liquefaction mitigation, wastewater treatment, bioremediation, CO2 sequestration [45] | Wide range of applicable microorganisms, applicable to a wide range of environments, low costs, high CaCO3 conversion, short timeframes [45] | Potential for harmful byproducts, bio-clogging at injection site, requires specific conditions |
| MCP | BMM | Passive | In situ 1 & ex situ 2 | Wastewater treatment, oil recovery, biofilm barriers, bioremediation [46] | Wide range of environments, adaptable to versatile environmental conditions | Variable efficacy for carbonate precipitation, slower rates of precipitation |
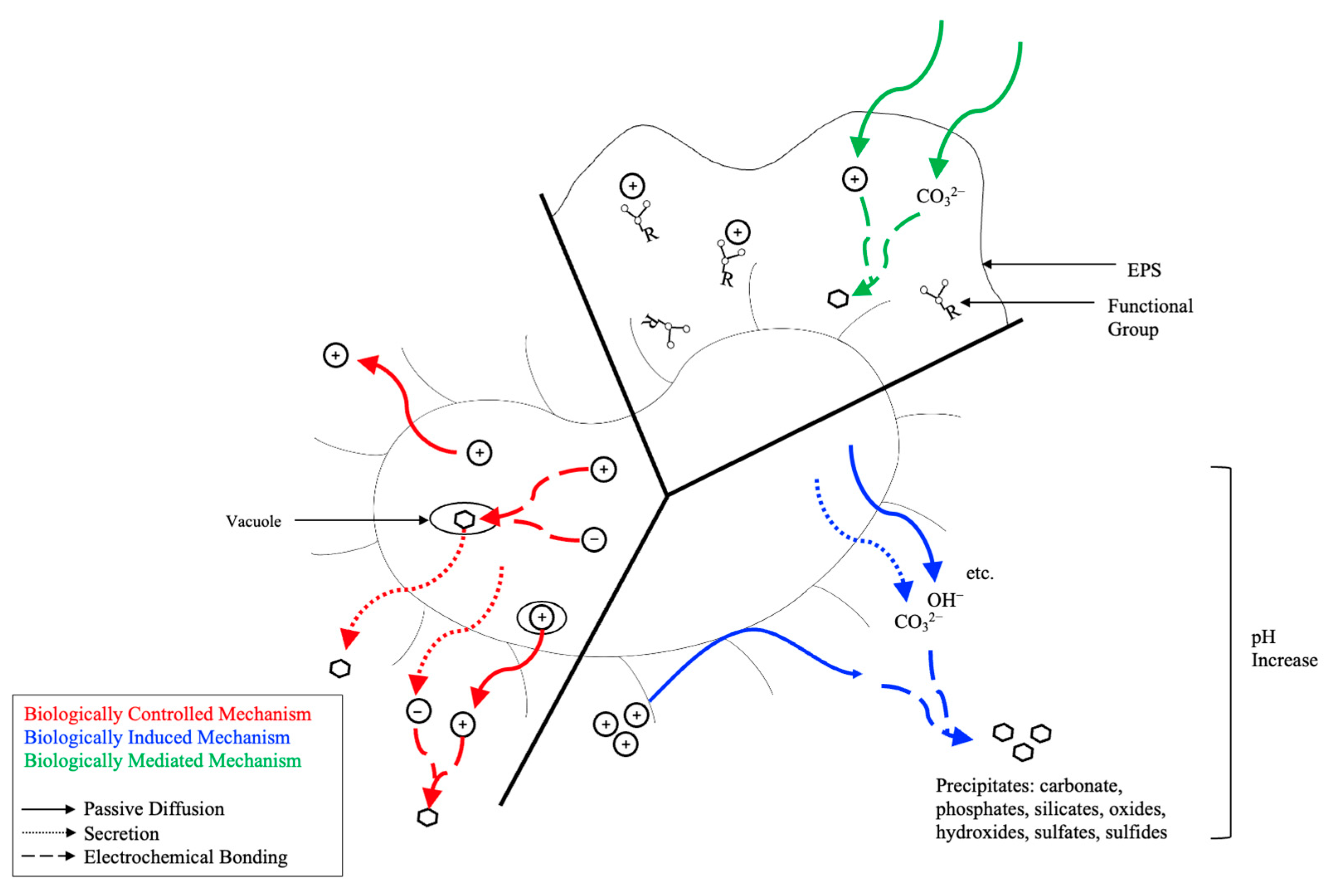
2.3. Microbial Carbonate Precipitation (MCP)
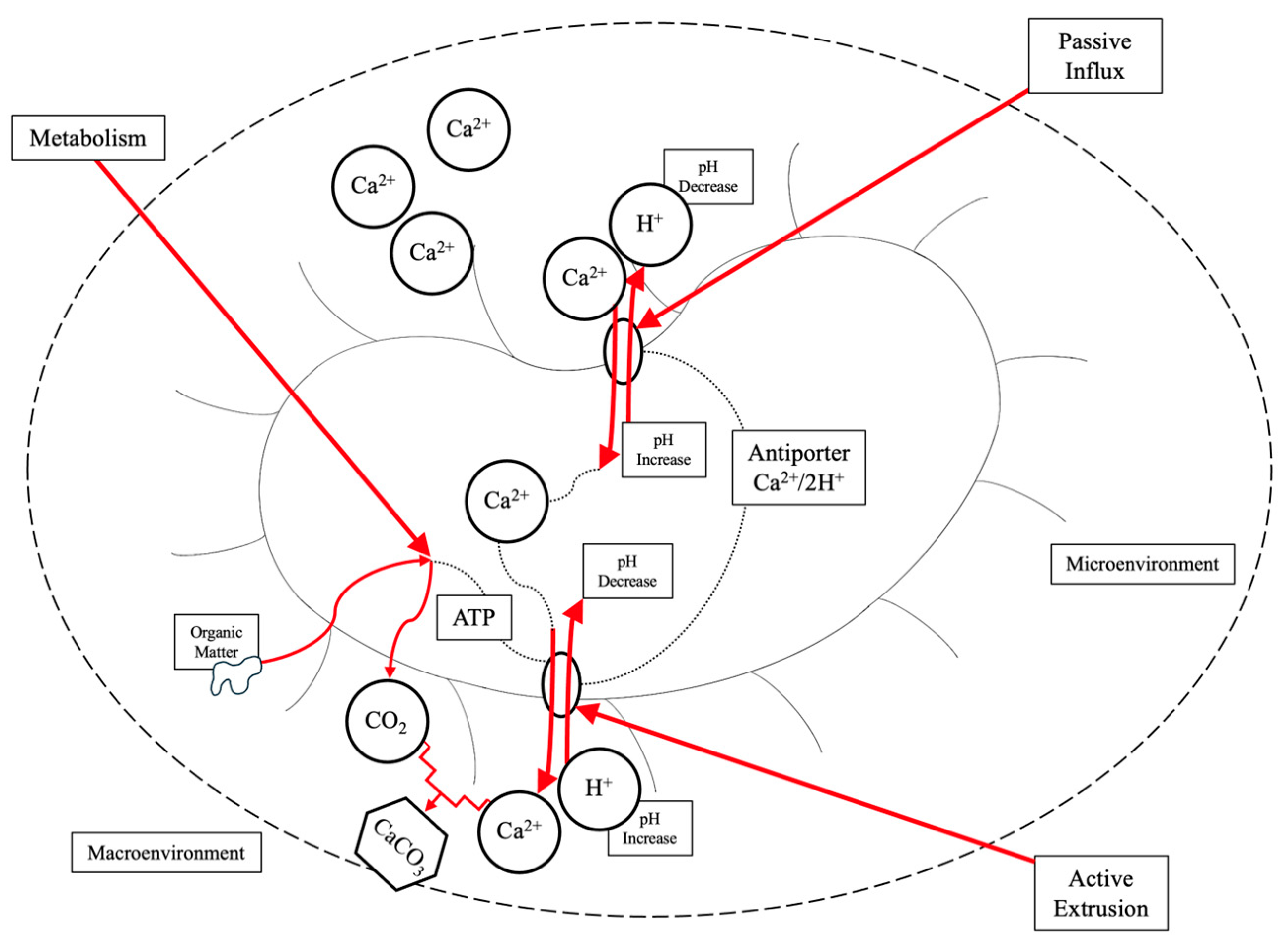
2.4. Microbial-Induced Carbonate Precipitation (MICP)
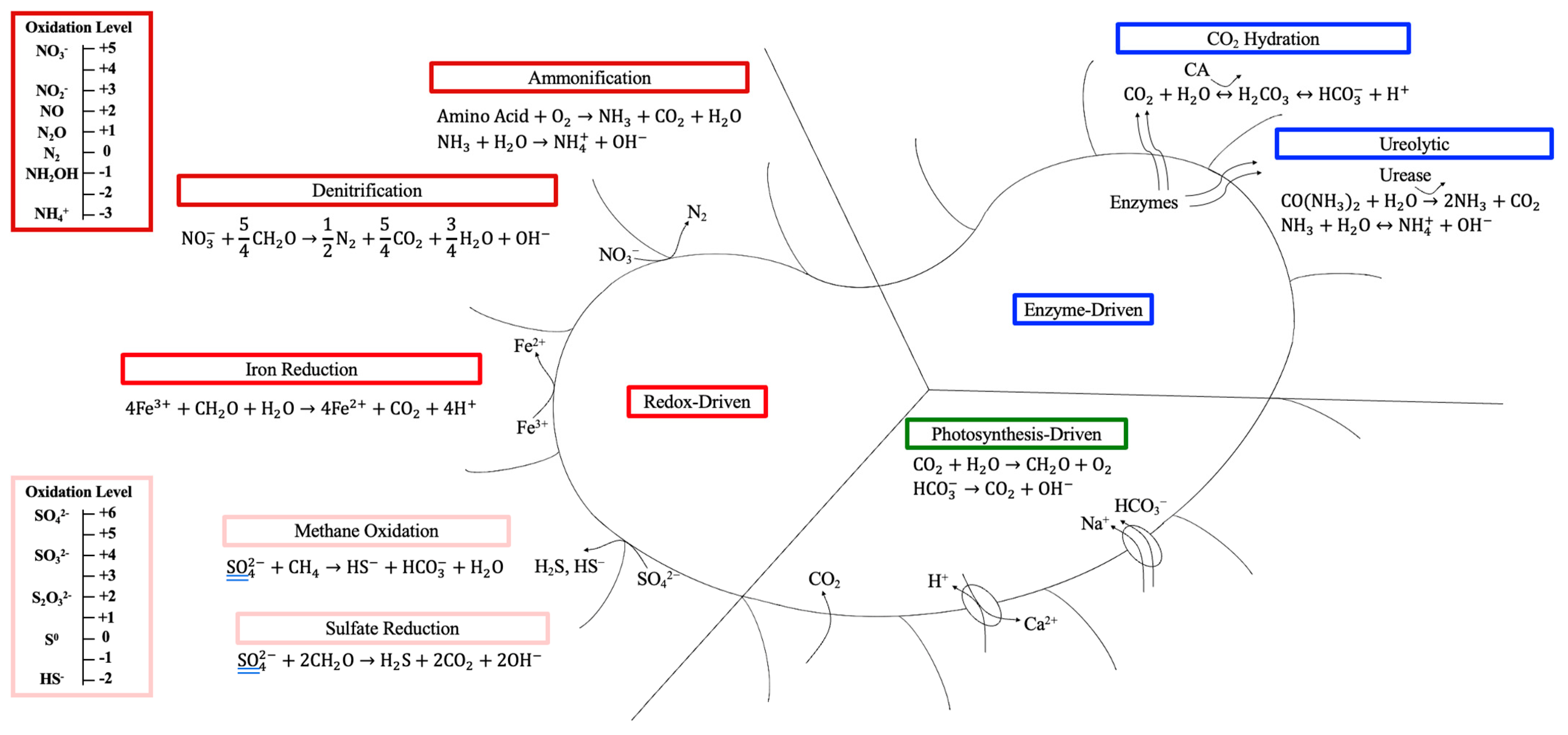
2.4.1. Nitrogen Cycle
2.4.2. Sulfur Cycle
2.4.3. Photosynthesis
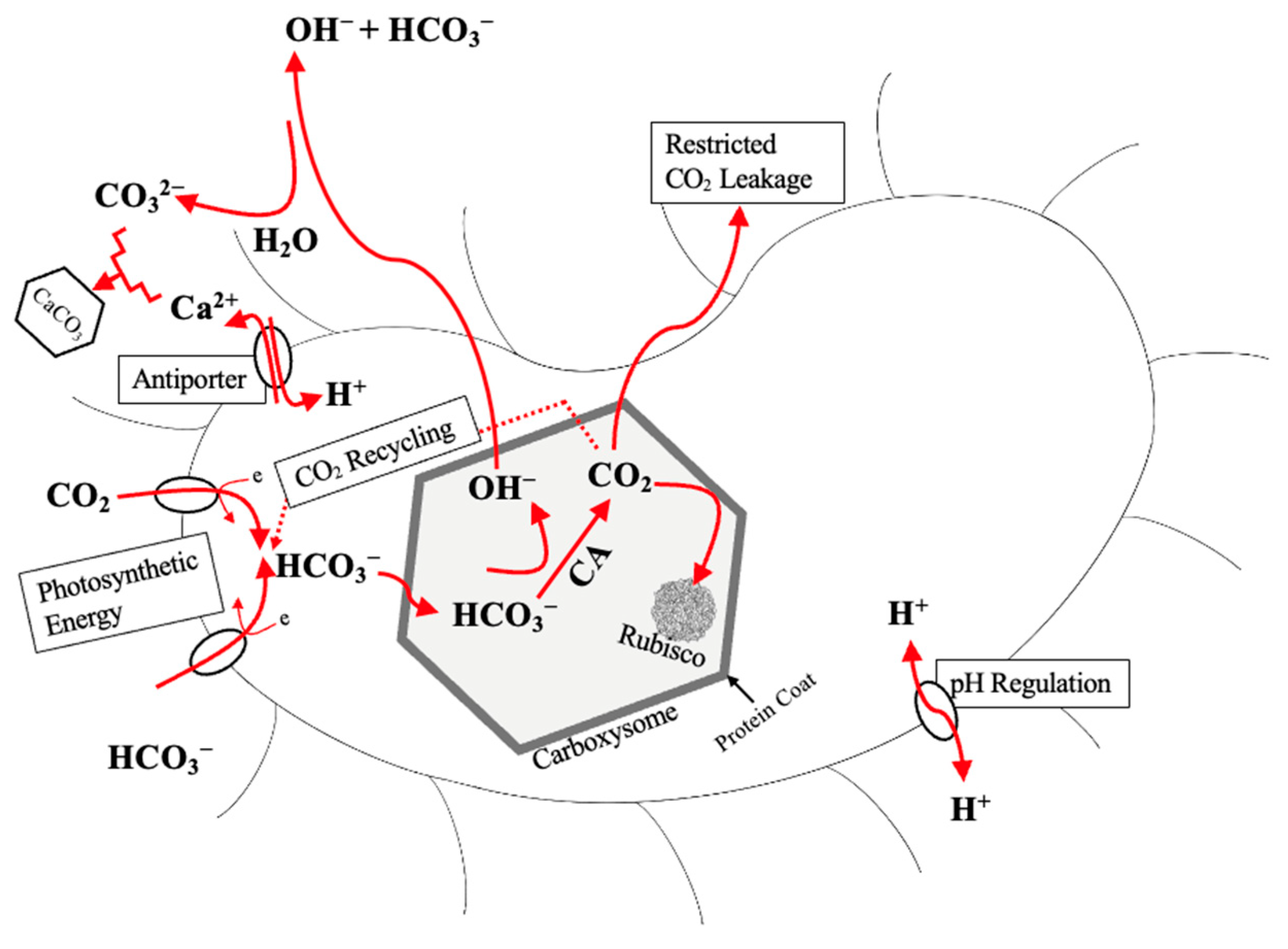
3. Carbon Sequestration
3.1. Mineral Carbonation and Carbon Sequestration
3.2. Advancements to Mineral Carbonation for Carbon Sequestration
4. Future Research
- Comparisons of MICP utilizing alternative bacteria species to induce different metabolic pathways for the assessment of optimal carbon sequestration.
- Suitability of specific bacterial species for use with different material types to establish conducive environmental conditions for their metabolic pathways and activity. To date, most MICP research evaluates its usage with soil. However, additional research is required regarding alternative materials that are less hospitable environments for microorganisms to determine the practicality of biochemical carbon sequestration near GHG point-source emissions.
- Analysis of biochemical alterations for enhancement and optimal use of enzyme-driven metabolic pathways. In addition to optimal growth conditions for bacteria, which is regularly incorporated into biochemical analyses, an evaluation of chemical additives and their impact on the efficacy of metalloenzymes (i.e., Ni and Zn) with the objective of carbon sequestration.
- Evaluation of MICP and CO2 injection to better understand preferable CO2 phases (liquid, gas, supercritical state) for biocalcification and pressures microorganisms can withstand to maximize the rate-limiting CO2 supply for carbonate precipitation, while minimizing damage to bacterial cells, biomass concentration, and the organic matrix.
- Comprehensive assessment of bacterial carbonation and its impact on precipitate composition, morphology, and stability for long-term storage of inorganic carbon. Impacts at the micro-particle scale and the large-scale feasibility of carbonate precipitation, considering MICP application and its impact on carbonate stability.
- Life-cycle assessments of the MICP process comparing different MICP application methods (i.e., in situ biostimulation, ex situ biostimulation, bioaugmentation, amended bioaugmentation) with traditional carbon sequestration techniques to determine quantitively the carbon emissions vs. carbon sequestered from “cradle” to “grave”.
- Evaluation of the long-term feasibility of MICP with the changing environment due to climate change. The geophysical and biochemical environmental changes (temperature, groundwater conditions, etc.) attributed to climate change and their impacts on specific bacterial species and community diversity, their metabolic activity, and their ability to precipitate carbonates.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.W.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; Blanco, G.; et al. IPCC, 2023: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; Intergovernmental Panel on Climate Change (IPCC), 2023; IPCC: Geneva, Switzerland, 2023.
- Gayathri, R.; Mahboob, S.; Govindarajan, M.; Al-Ghanim, K.A.; Ahmed, Z.; Al-Mulhm, N.; Vodovnik, M.; Vijayalakshmi, S. A Review on Biological Carbon Sequestration: A Sustainable Solution for a Cleaner Air Environment, Less Pollution and Lower Health Risks. J. King Saud Univ. Sci. 2021, 33, 101282. [Google Scholar] [CrossRef]
- Oves, M.; Hussain, F.M.; Ismail, I.M.I.; Felemban, N.M.; Qari, H.A. Microbiological Carbon Sequestration: A Novel Solution for Atmospheric Carbon–Carbon Sequestration through Biological Approach. In Handbook of Research on Inventive Bioremediation Techniques; IGI Global: Hershey, PA, USA, 2017; pp. 108–133. ISBN 978-1-5225-2325-3. [Google Scholar]
- Karpiński, P.H.; Bałdyga, J. Chapter 8—Precipitation Processes. In Handbook of Industrial Crystallization; Myerson, A.S., Erdemir, D., Lee, A.Y., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 216–265. ISBN 978-1-139-02694-9. [Google Scholar]
- Lewis, A. Chapter 4—Precipitation of Heavy Metals. In Sustainable Heavy Metal Remediation: Volume 1: Principles and Processes; Rene, E.R., Sahinkaya, E., Lewis, A., Lens, P.N.L., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2017; pp. 101–120. ISBN 978-3-319-58622-9. [Google Scholar]
- The Editors of Encyclopaedia Britannica Chemical Precipitation|Britannica. Available online: https://www.britannica.com/science/chemical-precipitation (accessed on 29 March 2023).
- Wang, L.K.; Vaccari, D.A.; Li, Y.; Shammas, N.K. Chapter 5—Chemical Precipitation. In Physicochemical Treatment Processes; Wang, L.K., Hung, Y.-T., Shammas, N.K., Eds.; Handbook of Environmental Engineering; Humana Press: Totowa, NJ, USA, 2005; pp. 141–197. ISBN 978-1-59259-820-5. [Google Scholar]
- Yong, R.N.; Mulligan, C.N.; Fukue, M. Sustainable Practices in Geoenvironmental Engineering, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-0-429-16839-0. [Google Scholar]
- Chakhmouradian, A.R.; Zaitsev, A.N. Rare Earth Mineralization in Igneous Rocks: Sources and Processes. Elements 2012, 8, 347–353. [Google Scholar] [CrossRef]
- Morse, J.W.; Arvidson, R.S. The Dissolution Kinetics of Major Sedimentary Carbonate Minerals. Earth Sci. Rev. 2002, 58, 51–84. [Google Scholar] [CrossRef]
- Wada, H.; Tomita, T.; Matsuura, K.; Tuchi, K.; Ito, M.; Morikiyo, T. Graphitization of Carbonaceous Matter during Metamorphism with References to Carbonate and Pelitic Rocks of Contact and Regional Metamorphisms, Japan. Contr. Mineral. Petrol. 1994, 118, 217–228. [Google Scholar] [CrossRef]
- James, N.P.; Jones, B. Origin of Carbonate Sedimentary Rocks; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 978-1-118-65270-1. [Google Scholar]
- Gregg, J.M.; Bish, D.L.; Kaczmarek, S.E.; Machel, H.G. Mineralogy, Nucleation and Growth of Dolomite in the Laboratory and Sedimentary Environment: A Review. Sedimentology 2015, 62, 1749–1769. [Google Scholar] [CrossRef]
- Zhang, G.; Morales, J.; Manuel García-Ruiz, J. Growth Behaviour of Silica/Carbonate Nanocrystalline Composites of Calcite and Aragonite. J. Mater. Chem. B 2017, 5, 1658–1663. [Google Scholar] [CrossRef]
- Gonzalez, L.A.; Carpenter, S.J.; Lohmann, K.C. Inorganic Calcite Morphology; Roles of Fluid Chemistry and Fluid Flow. J. Sediment. Res. 1992, 62, 382–399. [Google Scholar] [CrossRef]
- Folk, R.L. The Natural History of Crystalline Calcium Carbonate; Effect of Magnesium Content and Salinity. J. Sediment. Res. 1974, 44, 40–53. [Google Scholar] [CrossRef]
- Lahann, R.W. A Chemical Model for Calcite Crystal Growth and Morphology Control. J. Sediment. Res. 1978, 48, 337–347. [Google Scholar] [CrossRef]
- Zhang, Y.; Dawe, R.A. Influence of Mg2+ on the Kinetics of Calcite Precipitation and Calcite Crystal Morphology. Chem. Geol. 2000, 163, 129–138. [Google Scholar] [CrossRef]
- Weyl, P.K. The Change in Solubility of Calcium Carbonate with Temperature and Carbon Dioxide Content. Geochim. Cosmochim. Acta 1959, 17, 214–225. [Google Scholar] [CrossRef]
- Brečević, L.; Nielsen, A.E. Solubility of Amorphous Calcium Carbonate. J. Cryst. Growth 1989, 98, 504–510. [Google Scholar] [CrossRef]
- Harvey, O.R.; Qafoku, N.P.; Cantrell, K.J.; Lee, G.; Amonette, J.E.; Brown, C.F. Geochemical Implications of Gas Leakage Associated with Geologic CO2 Storage—A Qualitative Review. Environ. Sci. Technol. 2013, 47, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Taft, W. Modern Carbonate Sediments. In Carbonate Rocks: Origin, Occurence and Classification; Chilingar, G.V., Bissell, H.J., Fairbridge, R.W., Eds.; Developments in Sedimentology; Elsevier Pub. Co.: Amsterdam, The Netherlands, 1967. [Google Scholar]
- Wu, S. Classification of Biogenic Carbonate Rocks. Biopetrology 2021, 1, 19–29. [Google Scholar]
- Chilingar, G.V.; Bissell, H.J.; Wolf, K.H. Chapter 5 Diagenesis of Carbonate Rocks. In Developments in Sedimentology; Larsen, G., Chilingar, G.V., Eds.; Diagenesis in Sediments; Elsevier: Amsterdam, The Netherlands, 1967; Volume 8, pp. 179–322. [Google Scholar]
- Fairbridge, R.W.; Chilingar, G.V.; Bissell, H.J. Introduction. In Carbonate Rocks: Origin, Occurence and Classification; Chilingarian, G.V., Bissell, H.J., Fairbridge, R.W., Eds.; Developments in Sedimentology; Elsevier Pub. Co.: Amsterdam, The Netherlands, 1967. [Google Scholar]
- Castro-Alonso, M.J.; Montañez-Hernandez, L.E.; Sanchez-Muñoz, M.A.; Macias Franco, M.R.; Narayanasamy, R.; Balagurusamy, N. Microbially Induced Calcium Carbonate Precipitation (MICP) and Its Potential in Bioconcrete: Microbiological and Molecular Concepts. Front. Mater. 2019, 6, 126. [Google Scholar] [CrossRef]
- Joshi, S.; Goyal, S.; Sudhakara Reddy, M. Influence of Biogenic Treatment in Improving the Durability Properties of Waste Amended Concrete: A Review. Constr. Build. Mater. 2020, 263, 120170. [Google Scholar] [CrossRef]
- Kumar, R.; Nongkhlaw, M.; Acharya, C.; Joshi, S.R. Bacterial Community Structure from the Perspective of the Uranium Ore Deposits of Domiasiat in India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2013, 83, 485–497. [Google Scholar] [CrossRef]
- Weiner, S.; Dove, P.M. An Overview of Biomineralization Processes and the Problem of the Vital Effect. Rev. Mineral. Geochem. 2003, 54, 1–29. [Google Scholar] [CrossRef]
- Zhuang, D.; Yan, H.; Tucker, M.E.; Zhao, H.; Han, Z.; Zhao, Y.; Sun, B.; Li, D.; Pan, J.; Zhao, Y.; et al. Calcite Precipitation Induced by Bacillus Cereus MRR2 Cultured at Different Ca2+ Concentrations: Further Insights into Biotic and Abiotic Calcite. Chem. Geol. 2018, 500, 64–87. [Google Scholar] [CrossRef]
- Kelts, K.; Talbot, M. Lacustrine Carbonates as Geochemical Archives of Environmental Change and Biotic/Abiotic Interactions. In Large Lakes: Ecological Structure and Function; Tilzer, M.M., Serruya, C., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 288–315. ISBN 978-3-642-84077-7. [Google Scholar]
- Rainey, D.K.; Jones, B. Abiotic versus Biotic Controls on the Development of the Fairmont Hot Springs Carbonate Deposit, British Columbia, Canada. Sedimentology 2009, 56, 1832–1857. [Google Scholar] [CrossRef]
- Okyay, T.O.; Rodrigues, D.F. Biotic and Abiotic Effects on CO2 Sequestration during Microbially-Induced Calcium Carbonate Precipitation. FEMS Microbiol. Ecol. 2015, 91, fiv017. [Google Scholar] [CrossRef] [PubMed]
- Anbu, P.; Kang, C.-H.; Shin, Y.-J.; So, J.-S. Formations of Calcium Carbonate Minerals by Bacteria and Its Multiple Applications. Springerplus 2016, 5, 250. [Google Scholar] [CrossRef] [PubMed]
- Mujah, D.; Shahin, M.; Cheng, L. State-of-the-Art Review of Biocementation by Microbially Induced Calcite Precipitation (MICP) for Soil Stabilization. Geomicrobiology 2016, 34, 524–537. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Biomineralization of Calcium Carbonates and Their Engineered Applications: A Review. Front. Microbiol. 2013, 4, 314. [Google Scholar] [CrossRef]
- Benzerara, K.; Miot, J.; Morin, G.; Ona-Nguema, G.; Skouri-Panet, F.; Férard, C. Significance, Mechanisms and Environmental Implications of Microbial Biomineralization. Comptes Rendus Geosci. 2011, 343, 160–167. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of Carbonate Precipitation in Modern Microbial Mats. Earth Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Cornelissen, J.H.C.; Bardgett, R.D. Plant Functional Traits and Soil Carbon Sequestration in Contrasting Biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef]
- Lal, R. Carbon Sequestration. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 815–830. [Google Scholar] [CrossRef]
- Farrelly, D.J.; Everard, C.D.; Fagan, C.C.; McDonnell, K.P. Carbon Sequestration and the Role of Biological Carbon Mitigation: A Review. Renew. Sustain. Energy Rev. 2013, 21, 712–727. [Google Scholar] [CrossRef]
- Liu, D.-X.; Mai, Z.-M.; Sun, C.-C.; Zhou, Y.-W.; Liao, H.-H.; Wang, Y.-S.; Cheng, H. Dynamics of Extracellular Polymeric Substances and Soil Organic Carbon with Mangrove Zonation along a Continuous Tidal Gradient. Front. Mar. Sci. 2022, 9, 967767. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon Cycling and Storage in Mangrove Forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Konhauser, K. Introduction to Geomicrobiology; Blackwell Publishing: Oxford, UK, 2007; ISBN 978-0-632-05454-1. [Google Scholar]
- Wilcox, S.M.; Mulligan, C.N.; Neculita, C.M. Microbially Induced Calcium Carbonate Precipitation as a Bioremediation Technique for Mining Waste. Toxics 2024, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.C.; Phillips, A.J.; Hiebert, R.; Gerlach, R.; Spangler, L.H.; Cunningham, A.B. Biofilm Enhanced Geologic Sequestration of Supercritical CO2. Int. J. Greenh. Gas Control 2009, 3, 90–99. [Google Scholar] [CrossRef]
- Jiang, N.-J.; Wang, Y.-J.; Chu, J.; Kawasaki, S.; Tang, C.-S.; Cheng, L.; Du, Y.-J.; Shashank, B.S.; Singh, D.N.; Han, X.-L.; et al. Bio-Mediated Soil Improvement: An Introspection into Processes, Materials, Characterization and Applications. Soil Use Manag. 2022, 38, 68–93. [Google Scholar] [CrossRef]
- Madigan, M.T.; Bender, K.S.; Buckley, D.H.; Sattley, W.M.; Stahl, D.A. Brock Biology of Microorganisms, 15th ed.; Global Edition; Pearson: New York, NY, USA, 2019; ISBN 978-1-292-23510-3. [Google Scholar]
- Westbroek, P.; Brown, C.W.; van Bleijswijk, J.; Brownlee, C.; Brummer, G.J.; Conte, M.; Egge, J.; Fernández, E.; Jordan, R.; Knappertsbusch, M.; et al. A Model System Approach to Biological Climate Forcing. The Example of Emiliania Huxleyi. Glob. Planet. Change 1993, 8, 27–46. [Google Scholar] [CrossRef]
- Riebesell, U.; Zondervan, I.; Rost, B.; Tortell, P.D.; Zeebe, R.E.; Morel, F.M.M. Reduced Calcification of Marine Plankton in Response to Increased Atmospheric CO2. Nature 2000, 407, 364–367. [Google Scholar] [CrossRef]
- Berg, I.A.; Kockelkorn, D.; Ramos-Vera, W.H.; Say, R.F.; Zarzycki, J.; Hügler, M.; Alber, B.E.; Fuchs, G. Autotrophic Carbon Fixation in Archaea. Nat. Rev. Microbiol 2010, 8, 447–460. [Google Scholar] [CrossRef]
- Hammes, F.; Verstraete, W. Key Roles of pH and Calcium Metabolism in Microbial Carbonate Precipitation. Rev. Environ. Sci. Biotechnol. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- Phillips, A.J.; Gerlach, R.; Lauchnor, E.; Mitchell, A.C.; Cunningham, A.B.; Spangler, L. Engineered Applications of Ureolytic Biomineralization: A Review. Biofouling 2013, 29, 715–733. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X. Characterization of Urease and Carbonic Anhydrase Producing Bacteria and Their Role in Calcite Precipitation. Curr. Microbiol. 2011, 62, 894–902. [Google Scholar] [CrossRef]
- Douglas, S.; Beveridge, T.J. Mineral Formation by Bacteria in Natural Microbial Communities. FEMS Microbiol. Ecol. 1998, 26, 79–88. [Google Scholar] [CrossRef]
- Kapahi, M.; Sachdeva, S. Bioremediation Options for Heavy Metal Pollution. J. Health Pollut. 2019, 9, 191203. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.; Fein, J.B.; Daughney, C.J. Experimental Study of the pH, Ionic Strength, and Reversibility Behavior of Bacteria–Mineral Adsorption. Geochim. Cosmochim. Acta 2000, 64, 609–617. [Google Scholar] [CrossRef]
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef]
- Xiangliang, P. Micrologically Induced Carbonate Precipitation as a Promising Way to in Situ Immobilize Heavy Metals in Groundwater and Sediment. Res. J. Chem. Environ. 2009, 13, 3–4. [Google Scholar]
- Achal, V.; Mukherjee, A.; Basu, P.C.; Reddy, M.S. Strain Improvement of Sporosarcina pasteurii for Enhanced Urease and Calcite Production. J. Ind. Microbiol. Biotechnol. 2009, 36, 981–988. [Google Scholar] [CrossRef]
- Ferris, F.G.; Fyfe, W.S.; Beveridge, T.J. Bacteria as Nucleation Sites for Authigenic Minerals in a Metal-Contaminated Lake Sediment. Chem. Geol. 1987, 63, 225–232. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Dittrich, M.; Sibler, S. Calcium Carbonate Precipitation by Cyanobacterial Polysaccharides. Geol. Soc. Lond. Spec. Publ. 2010, 336, 51–63. [Google Scholar] [CrossRef]
- Tourney, J.; Ngwenya, B.T.; Mosselmans, J.W.F.; Tetley, L.; Cowie, G.L. The Effect of Extracellular Polymers (EPS) on the Proton Adsorption Characteristics of the Thermophile Bacillus Licheniformis S-86. Chem. Geol. 2008, 247, 1–15. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Decho, A.W. A Laboratory Investigation of Cyanobacterial Extracellular Polymeric Secretions (EPS) in Influencing CaCO3 Polymorphism. J. Cryst. Growth 2002, 240, 230–235. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-Y. An Optimum Condition of MICP Indigenous Bacteria with Contaminated Wastes of Heavy Metal. J. Mater. Cycles Waste Manag. 2019, 21, 239–247. [Google Scholar] [CrossRef]
- Tourney, J.; Ngwenya, B.T. Bacterial Extracellular Polymeric Substances (EPS) Mediate CaCO3 Morphology and Polymorphism. Chem. Geol. 2009, 262, 138–146. [Google Scholar] [CrossRef]
- Dejong, J.t.; Soga, K.; Kavazanjian, E.; Burns, S.; Van Paassen, L.a.; Al Qabany, A.; Aydilek, A.; Bang, S.s.; Burbank, M.; Caslake, L.f.; et al. Biogeochemical Processes and Geotechnical Applications: Progress, Opportunities and Challenges. Géotechnique 2013, 63, 287–301. [Google Scholar] [CrossRef]
- Achal, V.; Li, M.; Zhang, Q. Biocement, Recent Research in Construction Engineering: Status of China against Rest of World. Adv. Cem. Res. 2014, 26, 281–291. [Google Scholar] [CrossRef]
- Fenchel, T.; King, G.M.; Blackburn, T.H. Bacterial Biogeochemistry: The Ecophysiology of Mineral Cycling; Academic Press: Cambridge, MA, USA, 2012; ISBN 978-0-12-415836-8. [Google Scholar]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The Priming Effect of Organic Matter: A Question of Microbial Competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Pepper, I.L.; Gerba, C.P.; Gentry, T.J. Environmental Microbiology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-0-12-394626-3. [Google Scholar]
- Govarthanan, M.; Lee, K.-J.; Cho, M.; Kim, J.S.; Kamala-Kannan, S.; Oh, B.-T. Significance of Autochthonous bacillus sp. KK1 on Biomineralization of Lead in Mine Tailings. Chemosphere 2013, 90, 2267–2272. [Google Scholar] [CrossRef]
- Bang, S. Microbiologically-Enhanced Crack Remediation (MECR). Proc. Microbiol. Soc. Korea Conf. 2001, 11, 26–36. [Google Scholar]
- Chuo, S.C.; Mohamed, S.F.; Mohd Setapar, S.H.; Ahmad, A.; Jawaid, M.; Wani, W.A.; Yaqoob, A.A.; Mohamad Ibrahim, M.N. Insights into the Current Trends in the Utilization of Bacteria for Microbially Induced Calcium Carbonate Precipitation. Materials 2020, 13, 4993. [Google Scholar] [CrossRef]
- Graddy, C.M.R.; Gomez, M.G.; DeJong, J.T.; Nelson, D.C. Native Bacterial Community Convergence in Augmented and Stimulated Ureolytic MICP Biocementation. Environ. Sci. Technol. 2021, 55, 10784–10793. [Google Scholar] [CrossRef]
- McConnaughey, T.A.; Whelan, J.F. Calcification Generates Protons for Nutrient and Bicarbonate Uptake. Earth Sci. Rev. 1997, 42, 95–117. [Google Scholar] [CrossRef]
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.-P. Ca-Carbonates Precipitation and Limestone Genesis—The Microbiogeologist Point of View. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Norris, V.; Grant, S.; Freestone, P.; Canvin, J.; Sheikh, F.N.; Toth, I.; Trinei, M.; Modha, K.; Norman, R.I. Calcium Signalling in Bacteria. J. Bacteriol. 1996, 178, 3677–3682. [Google Scholar] [CrossRef]
- Kumar, A.; Song, H.-W.; Mishra, S.; Zhang, W.; Zhang, Y.-L.; Zhang, Q.-R.; Yu, Z.-G. Application of Microbial-Induced Carbonate Precipitation (MICP) Techniques to Remove Heavy Metal in the Natural Environment: A Critical Review. Chemosphere 2023, 318, 137894. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hora, R.N.; Ahenkorah, I.; Beecham, S.; Karim, M.R.; Iqbal, A. State-of-the-Art Review of Microbial-Induced Calcite Precipitation and Its Sustainability in Engineering Applications. Sustainability 2020, 12, 6281. [Google Scholar] [CrossRef]
- Olaya-Abril, A.; Hidalgo-Carrillo, J.; Luque-Almagro, V.M.; Fuentes-Almagro, C.; Urbano, F.J.; Moreno-Vivián, C.; Richardson, D.J.; Roldán, M.D. Exploring the Denitrification Proteome of Paracoccus Denitrificans PD1222. Front. Microbiol. 2018, 9, 1137. [Google Scholar] [CrossRef]
- van Paassen, L.A.; Daza, C.M.; Staal, M.; Sorokin, D.Y.; van der Zon, W.; van Loosdrecht, M.C.M. Potential Soil Reinforcement by Biological Denitrification. Ecol. Eng. 2010, 36, 168–175. [Google Scholar] [CrossRef]
- Hamdan, N.; Kavazanjian, E.; Rittmann, B.E.; Karatas, I. Carbonate Mineral Precipitation for Soil Improvement through Microbial Denitrification. Geomicrobiol. J. 2017, 34, 139–146. [Google Scholar] [CrossRef]
- Liang, Y.; Fu, R.; Sailike, A.; Hao, H.; Yu, Z.; Wang, R.; Peng, N.; Li, S.; Zhang, W.; Liu, Y. Soil Labile Organic Carbon and Nitrate Nitrogen Are the Main Factors Driving Carbon-Fixing Pathways during Vegetation Restoration in the Loess Plateau, China. Agric. Ecosyst. Environ. 2025, 378, 109283. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, Z.; Wang, X.; Zhou, Z.; Chen, D.; Zeng, H.; Zhao, S.; Chen, L.; Hu, Y.; Zhang, C.; et al. Diversity and Contributions to Nitrogen Cycling and Carbon Fixation of Soil Salinity Shaped Microbial Communities in Tarim Basin. Front. Microbiol. 2018, 9, 431. [Google Scholar] [CrossRef]
- Chekroun, K.B.; Rodríguez-Navarro, C.; González-Muñoz, M.T.; Arias, J.M.; Cultrone, G.; Rodríguez-Gallego, M. Precipitation and Growth Morphology of Calcium Carbonate Induced by Myxococcus xanthus: Implications for Recognition of Bacterial Carbonates. J. Sediment. Res. 2004, 74, 868–876. [Google Scholar] [CrossRef]
- Jiménez-López, C.; Jroundi, F.; Rodríguez-Gallego, M.; Arias, J.; Gonzalez, M.T. Biomineralization Induced by Myxobacteria. In Communicating Current Research and Educational Topics and Trends in Applied Microbiology; Méndez-Vilas, A., Ed.; Formatex Research Center: Oviedo, Spain, 2007. [Google Scholar]
- Marín-Ortega, S.; Torras, M.À.C.i.; Iglesias-Campos, M.Á. Microbially Induced Calcium Carbonate Precipitation in Fossil Consolidation Treatments: Preliminary Results Inducing Exogenous Myxococcus xanthus Bacteria in a Miocene Cheirogaster Richardi Specimen. Heliyon 2023, 9, e17597. [Google Scholar] [CrossRef] [PubMed]
- Keena, M.; Meehan, M.; Scherer, T. Nitrogen Behaviour in the Environment; North Dakota State University: Fargo, ND, USA, 2022. [Google Scholar]
- Lee, M.; Gomez, M.G.; San Pablo, A.C.M.; Kolbus, C.M.; Graddy, C.M.R.; DeJong, J.T.; Nelson, D.C. Investigating Ammonium by-Product Removal for Ureolytic Bio-Cementation Using Meter-Scale Experiments. Sci. Rep. 2019, 9, 18313. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Goyal, S.; Mukherjee, A.; Reddy, M.S. Microbial Healing of Cracks in Concrete: A Review. J. Ind. Microbiol. Biotechnol. 2017, 44, 1511–1525. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Synergistic Role of Bacterial Urease and Carbonic Anhydrase in Carbonate Mineralization. Appl. Biochem. Biotechnol. 2014, 172, 2552–2561. [Google Scholar] [CrossRef]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological Precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Hasan, H.A.H. Ureolytic Microorganisms and Soil Fertility: A Review. Commun. Soil Sci. Plant Anal. 2000, 31, 2565–2589. [Google Scholar] [CrossRef]
- Zerner, B. Recent Advances in the Chemistry of an Old Enzyme, Urease. Bioorganic Chem. 1991, 19, 116–131. [Google Scholar] [CrossRef]
- Callahan, B.P.; Yuan, Y.; Wolfenden, R. The Burden Borne by Urease. J. Am. Chem. Soc. 2005, 127, 10828–10829. [Google Scholar] [CrossRef]
- Ciurli, S. Urease: Recent Insights on the Role of Nickel. In Nickel and Its Surprising Impact in Nature; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 241–277. ISBN 978-0-470-02813-1. [Google Scholar]
- Su, F.; Yang, Y.Y. Microbially Induced Carbonate Precipitation via Methanogenesis Pathway by a Microbial Consortium Enriched from Activated Anaerobic Sludge. J. Appl. Microbiol. 2021, 131, 236–256. [Google Scholar] [CrossRef]
- DeJong, J.; Proto, C.; Kuo, M.; Gomez, M. Bacteria, Biofilms, and Invertebrates: The next Generation of Geotechnical Engineers? In Proceedings of the Geo-Congress 2014, Atlanta, GA, USA, 23–26 February 2014; pp. 3959–3968. [Google Scholar] [CrossRef]
- Buikema, N.D.; Zwissler, B.E.; Seagren, E.A.; Oommen, T.; Vitton, S. Stabilisation of Iron Mine Tailings through Biocalcification. Environ. Geotech. 2018, 5, 94–106. [Google Scholar] [CrossRef]
- Stabnikov, V.; Ivanov, V. Biotechnological Production of Biogrout from Iron Ore and Cellulose. J. Chem. Technol. Biotechnol. 2017, 92, 180–187. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, L.; Hemamali Peduruhewa, J.; Van Zwieten, L.; Gong, L.; Tan, B.; Zhang, G. The Coupling between Iron and Carbon and Iron Reducing Bacteria Control Carbon Sequestration in Paddy Soils. CATENA 2023, 223, 106937. [Google Scholar] [CrossRef]
- Chen, C.; Dynes, J.J.; Wang, J.; Sparks, D.L. Properties of Fe-Organic Matter Associations via Coprecipitation versus Adsorption. Environ. Sci. Technol. 2014, 48, 13751–13759. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gélinas, Y. Preservation of Organic Matter in Sediments Promoted by Iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef]
- Bergdale, T.E.; Pinkelman, R.J.; Hughes, S.R.; Zambelli, B.; Ciurli, S.; Bang, S.S. Engineered Biosealant Strains Producing Inorganic and Organic Biopolymers. J. Biotechnol. 2012, 161, 181–189. [Google Scholar] [CrossRef]
- Zambelli, B.; Musiani, F.; Benini, S.; Ciurli, S. Chemistry of Ni2+ in Urease: Sensing, Trafficking, and Catalysis. Acc. Chem. Res. 2011, 44, 520–530. [Google Scholar] [CrossRef]
- Svane, S.; Sigurdarson, J.J.; Finkenwirth, F.; Eitinger, T.; Karring, H. Inhibition of Urease Activity by Different Compounds Provides Insight into the Modulation and Association of Bacterial Nickel Import and Ureolysis. Sci. Rep. 2020, 10, 8503. [Google Scholar] [CrossRef]
- Lv, J.; Jiang, Y.; Yu, Q.; Lu, S. Structural and Functional Role of Nickel Ions in Urease by Molecular Dynamics Simulation. J. Biol. Inorg. Chem. 2011, 16, 125–135. [Google Scholar] [CrossRef]
- Estiu, G.; Merz, K.M. Catalyzed Decomposition of Urea. Molecular Dynamics Simulations of the Binding of Urea to Urease. Biochemistry 2006, 45, 4429–4443. [Google Scholar] [CrossRef][Green Version]
- Bachmeier, K.L.; Williams, A.E.; Warmington, J.R.; Bang, S.S. Urease Activity in Microbiologically-Induced Calcite Precipitation. J. Biotechnol. 2002, 93, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Okyay, T.O.; Rodrigues, D.F. Optimized Carbonate Micro-Particle Production by Sporosarcina pasteurii Using Response Surface Methodology. Ecol. Eng. 2014, 62, 168–174. [Google Scholar] [CrossRef]
- Okyay, T.O.; Nguyen, H.N.; Castro, S.L.; Rodrigues, D.F. CO2 Sequestration by Ureolytic Microbial Consortia through Microbially-Induced Calcite Precipitation. Sci. Total Environ. 2016, 572, 671–680. [Google Scholar] [CrossRef]
- Kaur, G.; Dhami, N.K.; Goyal, S.; Mukherjee, A.; Reddy, M.S. Utilization of Carbon Dioxide as an Alternative to Urea in Biocementation. Constr. Build. Mater. 2016, 123, 527–533. [Google Scholar] [CrossRef]
- Sánchez-Andrea, I.; Sanz, J.L.; Bijmans, M.F.M.; Stams, A.J.M. Sulfate Reduction at Low pH to Remediate Acid Mine Drainage. J. Hazard. Mater. 2014, 269, 98–109. [Google Scholar] [CrossRef]
- Sánchez-Andrea, I.; Stams, A.J.M.; Weijma, J.; Gonzalez Contreras, P.; Dijkman, H.; Rozendal, R.A.; Johnson, D.B. A Case in Support of Implementing Innovative Bio-Processes in the Metal Mining Industry. FEMS Microbiol. Lett. 2016, 363, 1–4. [Google Scholar] [CrossRef][Green Version]
- Paul, V.G.; Wronkiewicz, D.J.; Mormile, M.R. Impact of Elevated CO2 Concentrations on Carbonate Mineral Precipitation Ability of Sulfate-Reducing Bacteria and Implications for CO2 Sequestration. Appl. Geochem. 2017, 78, 250–271. [Google Scholar] [CrossRef]
- Pester, M.; Knorr, K.-H.; Friedrich, M.W.; Wagner, M.; Loy, A. Sulfate-Reducing Microorganisms in Wetlands—Fameless Actors in Carbon Cycling and Climate Change. Front. Microbiol. 2012, 3, 19769. [Google Scholar] [CrossRef]
- Visscher, P.T.; Reid, R.P.; Bebout, B.M.; Hoeft, S.E.; Macintyre, I.G.; Thompson, J.A. Formation of Lithified Micritic Laminae in Modern Marine Stromatolites (Bahamas); The Role of Sulfur Cycling. Am. Mineral. 1998, 83, 1482–1493. [Google Scholar] [CrossRef]
- Görgen, S.; Benzerara, K.; Skouri-Panet, F.; Gugger, M.; Chauvat, F.; Cassier-Chauvat, C. The Diversity of Molecular Mechanisms of Carbonate Biomineralization by Bacteria. Discov. Mater. 2020, 1, 2. [Google Scholar] [CrossRef]
- Akam, S.A.; Swanner, E.D.; Yao, H.; Hong, W.-L.; Peckmann, J. Methane-Derived Authigenic Carbonates—A Case for a Globally Relevant Marine Carbonate Factory. Earth Sci. Rev. 2023, 243, 104487. [Google Scholar] [CrossRef]
- Caesar, K.H.; Kyle, J.R.; Lyons, T.W.; Tripati, A.; Loyd, S.J. Carbonate Formation in Salt Dome Cap Rocks by Microbial Anaerobic Oxidation of Methane. Nat. Commun. 2019, 10, 808. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Fang, C.; Achal, V. A Critical Review on Microbial Carbonate Precipitation via Denitrification Process in Building Materials. Bioengineered 2021, 12, 7529–7551. [Google Scholar] [CrossRef] [PubMed]
- Rasigraf, O.; Kool, D.M.; Jetten, M.S.M.; Sinninghe Damsté, J.S.; Ettwig, K.F. Autotrophic Carbon Dioxide Fixation via the Calvin-Benson-Bassham Cycle by the Denitrifying Methanotroph “Candidatus Methylomirabilis Oxyfera”. Appl. Environ. Microbiol. 2014, 80, 2451–2460. [Google Scholar] [CrossRef]
- Smith, K.S.; Ferry, J.G. Prokaryotic Carbonic Anhydrases. FEMS Microbiol. Rev. 2000, 24, 335–366. [Google Scholar] [CrossRef]
- Altermann, W.; Kazmierczak, J.; Oren, A.; Wright, D.T. Cyanobacterial Calcification and Its Rock-Building Potential during 3.5 Billion Years of Earth History. Geobiology 2006, 4, 147–166. [Google Scholar] [CrossRef]
- Verrecchia, E.P.; Freytet, P.; Verrecchia, K.E.; Dumont, J.-L. Spherulites in Calcrete Laminar Crusts; Biogenic CaCO 3 Precipitation as a Major Contributor to Crust Formation. J. Sediment. Res. 1995, 65, 690–700. [Google Scholar] [CrossRef]
- Pentecost, A. The Formation of Travertine Shrubs: Mammoth Hot Springs, Wyoming. Geol. Mag. 1990, 127, 159–168. [Google Scholar] [CrossRef]
- Jones, T.R.; Poitras, J.; Levett, A.; Langendam, A.; Vietti, A.; Southam, G. Accelerated Carbonate Biomineralisation of Venetia Diamond Mine Coarse Residue Deposit (CRD) Material—A Field Trial Study. Sci. Total Environ. 2023, 893, 164853. [Google Scholar] [CrossRef]
- Jones, T.R.; Poitras, J.; Paterson, D.; Southam, G. Historical Diamond Mine Waste Reveals Carbon Sequestration Resource in Kimberlite Residue. Chem. Geol. 2023, 617, 121270. [Google Scholar] [CrossRef]
- Sundaram, S.; Thakur, I.S. Induction of Calcite Precipitation through Heightened Production of Extracellular Carbonic Anhydrase by CO2 Sequestering Bacteria. Bioresour. Technol. 2018, 253, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.; Ilies, M.A. Evaluation of the Impact of Esterases and Lipases from the Circulatory System against Substrates of Different Lipophilicity. Int. J. Mol. Sci. 2022, 23, 1262. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Reinhold, L. CO2 Concentrating Mechanisms in Photosynthetic Microorganisms. Annu. Rev. Plant Biol. 1999, 50, 539–570. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Capasso, C. An Overview of the Bacterial Carbonic Anhydrases. Metabolites 2017, 7, 56. [Google Scholar] [CrossRef]
- Badger, M.R.; Hanson, D.; Price, G.D. Evolution and Diversity of CO2 Concentrating Mechanisms in Cyanobacteria. Funct. Plant Biol. 2002, 29, 161–173. [Google Scholar] [CrossRef]
- Klanchui, A.; Cheevadhanarak, S.; Prommeenate, P.; Meechai, A. Exploring Components of the CO2-Concentrating Mechanism in Alkaliphilic Cyanobacteria Through Genome-Based Analysis. Comput. Struct. Biotechnol. J. 2017, 15, 340–350. [Google Scholar] [CrossRef]
- Price, G.D.; Badger, M.R.; Woodger, F.J.; Long, B.M. Advances in Understanding the Cyanobacterial CO2-Concentrating-Mechanism (CCM): Functional Components, Ci Transporters, Diversity, Genetic Regulation and Prospects for Engineering into Plants. J. Exp. Bot. 2008, 59, 1441–1461. [Google Scholar] [CrossRef]
- Badger, M.R.; Price, G.D.; Long, B.M.; Woodger, F.J. The Environmental Plasticity and Ecological Genomics of the Cyanobacterial CO2 Concentrating Mechanism. J. Exp. Bot. 2006, 57, 249–265. [Google Scholar] [CrossRef]
- Capasso, C.; Supuran, C.T. An Overview of the Alpha-, Beta- and Gamma-Carbonic Anhydrases from Bacteria: Can Bacterial Carbonic Anhydrases Shed New Light on Evolution of Bacteria? J. Enzym. Inhib. Med. Chem. 2015, 30, 325–332. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattacharya, A.; Singh, S. Purification and Characterization of an Extracellular Carbonic Anhydrase from Pseudomonas Fragi. Process Biochem. 2009, 44, 1293–1297. [Google Scholar] [CrossRef]
- Kupriyanova, E.; Villarejo, A.; Markelova, A.; Gerasimenko, L.; Zavarzin, G.; Samuelsson, G.; Los, D.A.; Pronina, N. Extracellular Carbonic Anhydrases of the Stromatolite-Forming Cyanobacterium Microcoleus Chthonoplastes. Microbiology 2007, 153, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lian, B.; Chen, M.; Li, X.; Li, Y. Bacillus Mucilaginosus Can Capture Atmospheric CO2 by Carbonic Anhydrase. Afr. J. Microbiol. Res. 2011, 5, 106–112. [Google Scholar]
- Li, W.; Yu, L.; He, Q.; Wu, Y.; Yuan, D.; Cao, J. Effects of Microbes and Their Carbonic Anhydrase on Ca2+ and Mg2+ Migration in Column-Built Leached Soil-Limestone Karst Systems. Appl. Soil Ecol. 2005, 29, 274–281. [Google Scholar] [CrossRef]
- Nathan, V.K.; Ammini, P. Carbon Dioxide Sequestering Ability of Bacterial Carbonic Anhydrase in a Mangrove Soil Microcosm and Its Bio-Mineralization Properties. Water Air Soil Pollut. 2019, 230, 192. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, J.; Pang, C.; Li, Q.; Yang, Z.; Li, G. Isolation, Identification, and Carbonate Mineralization Characteristics of a Newly Carbonic Anhydrase-Producing Strain. Appl. Biochem. Biotechnol. 2024, 196, 8009–8025. [Google Scholar] [CrossRef]
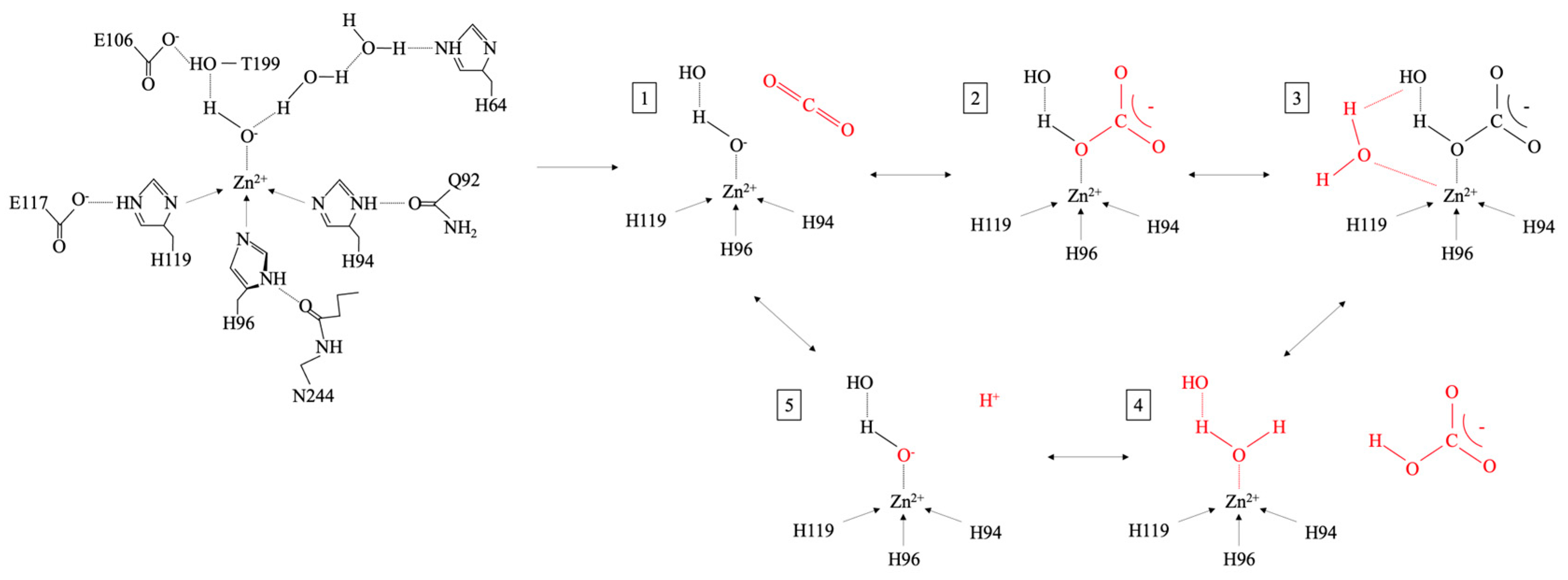
- Christianson, D.W.; Fierke, C.A. Carbonic Anhydrase: Evolution of the Zinc Binding Site by Nature and by Design. Acc. Chem. Res. 1996, 29, 331–339. [Google Scholar] [CrossRef]
- Kiefer, L.L.; Paterno, S.A.; Fierke, C.A. Hydrogen Bond Network in the Metal Binding Site of Carbonic Anhydrase Enhances Zinc Affinity and Catalytic Efficiency. J. Am. Chem. Soc. 1995, 117, 6831–6837. [Google Scholar] [CrossRef]
- Supuran, C.T.; Scozzafava, A.; Casini, A. Carbonic Anhydrase Inhibitors. Med. Res. Rev. 2003, 23, 146–189. [Google Scholar] [CrossRef]
- Tupper, R.; Watts, R.W.E.; Wormall, A. Some Observations on the Zinc in Carbonic Anhydrase. Biochem. J. 1952, 50, 429–432. [Google Scholar] [CrossRef]
- Håkansson, K.; Carlsson, M.; Svensson, L.A.; Liljas, A. Structure of Native and Apo Carbonic Anhydrase II and Structure of Some of Its Anion-Ligand Complexes. J. Mol. Biol. 1992, 227, 1192–1204. [Google Scholar] [CrossRef]
- McCall, K.A.; Huang, C.; Fierke, C.A. Function and Mechanism of Zinc Metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.; Reddy, M.; Mukherjee, A. Biomineralization of Calcium Carbonate Polymorphs by the Bacterial Strains Isolated from Calcareous Sites. J. Microbiol. Biotechnol. 2013, 23, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, W.; Zhang, F.; Lu, C.; Du, J.; Zhu, R.; Sun, L. Evaluation of CO2 Solubility-Trapping and Mineral-Trapping in Microbial-Mediated CO2–Brine–Sandstone Interaction. Mar. Pollut. Bull. 2014, 85, 78–85. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Lee, D.-J.; Kumari, D.; Zhang, D. Remediation of Cr(VI) from Chromium Slag by Biocementation. Chemosphere 2013, 93, 1352–1358. [Google Scholar] [CrossRef]
- Park, I.-S.; Hausinger, R.P. Requirement of Carbon Dioxide for in Vitro Assembly of the Urease Nickel Metallocenter. Science 1995, 267, 1156–1158. [Google Scholar] [CrossRef]
- Lal, R. Forest Soils and Carbon Sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Dhanwantri, K.; Sharma, P.; Mehta, S.; Prakash, P. Carbon Sequestration, Its Methods and Significance. In Environmental Sustainability: Concepts, Principles, Evidences and Innovations; Mishra, G.C., Ed.; Excellent Publishing House: New Delhi, India, 2014; pp. 94–98. ISBN 978-93-83083-75-6. [Google Scholar]
- Jiao, N.; Robinson, C.; Azam, F.; Thomas, H.; Baltar, F.; Dang, H.; Hardman-Mountford, N.J.; Johnson, M.; Kirchman, D.L.; Koch, B.P.; et al. Mechanisms of Microbial Carbon Sequestration in the Ocean—Future Research Directions. Biogeosciences 2014, 11, 5285–5306. [Google Scholar] [CrossRef]
- Ricour, F.; Guidi, L.; Gehlen, M.; DeVries, T.; Legendre, L. Century-Scale Carbon Sequestration Flux throughout the Ocean by the Biological Pump. Nat. Geosci. 2023, 16, 1105–1113. [Google Scholar] [CrossRef]
- Jiao, N.; Azam, F. Microbial Carbon Pump and Its Significance for Carbon Sequestration in the Ocean. In Microbial Carbon Pump in the Ocean; Jiao, N., Azam, F., Sanders, S., Eds.; The American Association for the Advancement of Science: Washington, DC, USA, 2011. [Google Scholar]
- Dhamu, V.; Qureshi, M.F.; Barckholtz, T.A.; Mhadeshwar, A.B.; Linga, P. Evaluating Liquid CO2 Hydrate Formation Kinetics, Morphology, and Stability in Oceanic Sediments on a Lab Scale Using Top Injection. Chem. Eng. J. 2023, 478, 147200. [Google Scholar] [CrossRef]
- Cunningham, A.B.; Gerlach, R.; Spangler, L.; Mitchell, A.C. Microbially Enhanced Geologic Containment of Sequestered Supercritical CO2. Energy Procedia 2009, 1, 3245–3252. [Google Scholar] [CrossRef]
- Benson, S.M.; Cole, D.R. CO2 Sequestration in Deep Sedimentary Formations. Elements 2008, 4, 325–331. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Chang, E.E.; Chiang, P.-C. CO2 Capture by Accelerated Carbonation of Alkaline Wastes: A Review on Its Principles and Applications. Aerosol Air Qual. Res. 2012, 12, 770–791. [Google Scholar] [CrossRef]
- Bodor, M.; Santos, R.; Gerven, T.; Vlad, M. Recent Developments and Perspectives on the Treatment of Industrial Wastes by Mineral Carbonation—A Review. Open Eng. 2013, 3, 566–584. [Google Scholar] [CrossRef]
- Gomes, H.I.; Mayes, W.M.; Rogerson, M.; Stewart, D.I.; Burke, I.T. Alkaline Residues and the Environment: A Review of Impacts, Management Practices and Opportunities. J. Clean. Prod. 2016, 112, 3571–3582. [Google Scholar] [CrossRef]
- Eloneva, S.; Puheloinen, E.-M.; Kanweva, J.; Ekroos, A.; Zevenhoven, R.; Fogelholm, C.-J. Co-Utilisation of CO2 and Steelmaking Slags for Production of Pure CaCO3—Legislative Issues. J. Clean. Prod. 2010, 18, 1833–1839. [Google Scholar] [CrossRef]
- Rashid, M.I.; Yaqoob, Z.; Mujtaba, M.A.; Fayaz, H.; Saleel, C.A. Developments in Mineral Carbonation for Carbon Sequestration. Heliyon 2023, 9, e21796. [Google Scholar] [CrossRef]
- Chang, R.; Kim, S.; Lee, S.; Choi, S.; Kim, M.; Park, Y. Calcium Carbonate Precipitation for CO2 Storage and Utilization: A Review of the Carbonate Crystallization and Polymorphism. Front. Energy Res. 2017, 5, 17. [Google Scholar] [CrossRef]
- Mayes, W.M.; Younger, P.L. Buffering of Alkaline Steel Slag Leachate across a Natural Wetland. Environ. Sci. Technol. 2006, 40, 1237–1243. [Google Scholar] [CrossRef]
- Lim, M.; Han, G.-C.; Ahn, J.-W.; You, K.-S. Environmental Remediation and Conversion of Carbon Dioxide (CO2) into Useful Green Products by Accelerated Carbonation Technology. Int. J. Environ. Res. Public Health 2010, 7, 203–228. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon Capture and Storage Using Alkaline Industrial Wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Eloneva, S.; Teir, S.; Salminen, J.; Fogelholm, C.-J.; Zevenhoven, R. Fixation of CO2 by Carbonating Calcium Derived from Blast Furnace Slag. Energy 2008, 33, 1461–1467. [Google Scholar] [CrossRef]
- Olajire, A.A. A Review of Mineral Carbonation Technology in Sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A Review of Mineral Carbonation Technologies to Sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed]
- Khudhur, F.W.K.; MacDonald, J.M.; Macente, A.; Daly, L. The Utilization of Alkaline Wastes in Passive Carbon Capture and Sequestration: Promises, Challenges and Environmental Aspects. Sci. Total Environ. 2022, 823, 153553. [Google Scholar] [CrossRef]
- Wilson, S.A.; Dipple, G.M.; Power, I.M.; Thom, J.M.; Anderson, R.G.; Raudsepp, M.; Gabites, J.E.; Southam, G. Carbon Dioxide Fixation within Mine Wastes of Ultramafic-Hosted Ore Deposits: Examples from the Clinton Creek and Cassiar Chrysotile Deposits, Canada. Econ. Geol. 2009, 104, 95–112. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M. Accelerating Mineral Carbonation Using Carbonic Anhydrase. Environ. Sci. Technol. 2016, 50, 2610–2618. [Google Scholar] [CrossRef]
- Li, X.; Bertos, M.F.; Hills, C.D.; Carey, P.J.; Simon, S. Accelerated Carbonation of Municipal Solid Waste Incineration Fly Ashes. Waste Manag. 2007, 27, 1200–1206. [Google Scholar] [CrossRef]
- Rendek, E.; Ducom, G.; Germain, P. Carbon Dioxide Sequestration in Municipal Solid Waste Incinerator (MSWI) Bottom Ash. J. Hazard. Mater. 2006, 128, 73–79. [Google Scholar] [CrossRef]
- Liu, W.; Su, S.; Xu, K.; Chen, Q.; Xu, J.; Sun, Z.; Wang, Y.; Hu, S.; Wang, X.; Xue, Y.; et al. CO2 Sequestration by Direct Gas–Solid Carbonation of Fly Ash with Steam Addition. J. Clean. Prod. 2018, 178, 98–107. [Google Scholar] [CrossRef]
- Veetil, S.P.; Pasquier, L.-C.; Blais, J.-F.; Cecchi, E.; Kentish, S.; Mercier, G. Direct Gas–Solid Carbonation of Serpentinite Residues in the Absence and Presence of Water Vapor: A Feasibility Study for Carbon Dioxide Sequestration. Environ. Sci. Pollut. Res. 2015, 22, 13486–13495. [Google Scholar] [CrossRef]
- Fernández Bertos, M.; Li, X.; Simons, S.J.R.; Hills, C.D.; Carey, P.J. Investigation of Accelerated Carbonation for the Stabilisation of MSW Incinerator Ashes and the Sequestration of CO2. Green Chem. 2004, 6, 428–436. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Hamidi, H.; Junin, R.; Karaei, M.A. A Review on Carbon Dioxide Mineral Carbonation through pH-Swing Process. Chem. Eng. J. 2015, 279, 615–630. [Google Scholar] [CrossRef]
- Fagerlund, J.; Nduagu, E.; Romão, I.; Zevenhoven, R. A Stepwise Process for Carbon Dioxide Sequestration Using Magnesium Silicates. Front. Chem. Eng. China 2010, 4, 133–141. [Google Scholar] [CrossRef]
- Ben Ghacham, A.; Cecchi, E.; Pasquier, L.-C.; Blais, J.-F.; Mercier, G. CO2 Sequestration Using Waste Concrete and Anorthosite Tailings by Direct Mineral Carbonation in Gas–Solid–Liquid and Gas–Solid Routes. J. Environ. Manag. 2015, 163, 70–77. [Google Scholar] [CrossRef]
- El-Naas, M.H.; El Gamal, M.; Hameedi, S.; Mohamed, A.-M.O. CO2 Sequestration Using Accelerated Gas-Solid Carbonation of Pre-Treated EAF Steel-Making Bag House Dust. J. Environ. Manag. 2015, 156, 218–224. [Google Scholar] [CrossRef]
- Baciocchi, R.; Polettini, A.; Pomi, R.; Prigiobbe, V.; Von Zedwitz, V.N.; Steinfeld, A. CO2 Sequestration by Direct Gas−Solid Carbonation of Air Pollution Control (APC) Residues. Energy Fuels 2006, 20, 1933–1940. [Google Scholar] [CrossRef]
- Ho, H.-J.; Iizuka, A.; Shibata, E.; Tomita, H.; Takano, K.; Endo, T. CO2 Utilization via Direct Aqueous Carbonation of Synthesized Concrete Fines under Atmospheric Pressure. ACS Omega 2020, 5, 15877–15890. [Google Scholar] [CrossRef]
- Li, J.; Jacobs, A.D.; Hitch, M. Direct Aqueous Carbonation on Olivine at a CO2 Partial Pressure of 6.5 MPa. Energy 2019, 173, 902–910. [Google Scholar] [CrossRef]
- Song, K.; Jang, Y.-N.; Kim, W.; Lee, M.G.; Shin, D.; Bang, J.-H.; Jeon, C.W.; Chae, S.C. Factors Affecting the Precipitation of Pure Calcium Carbonate during the Direct Aqueous Carbonation of Flue Gas Desulfurization Gypsum. Energy 2014, 65, 527–532. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Witkamp, G.-J.; Comans, R.N.J. Mineral CO2 Sequestration by Steel Slag Carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef]
- Yadav, V.S.; Prasad, M.; Khan, J.; Amritphale, S.S.; Singh, M.; Raju, C.B. Sequestration of Carbon Dioxide (CO2) Using Red Mud. J. Hazard. Mater. 2010, 176, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Uibu, M.; Velts, O.; Kuusik, R. Developments in CO2 Mineral Carbonation of Oil Shale Ash. J. Hazard. Mater. 2010, 174, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Montes-Hernandez, G.; Pérez-López, R.; Renard, F.; Nieto, J.M.; Charlet, L. Mineral Sequestration of CO2 by Aqueous Carbonation of Coal Combustion Fly-Ash. J. Hazard. Mater. 2009, 161, 1347–1354. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Lv, Z.; Tong, Y.; Ran, J.; Qin, C. Evaluation on Direct Aqueous Carbonation of Industrial/Mining Solid Wastes for CO2 Mineralization. J. Ind. Eng. Chem. 2023, 122, 359–365. [Google Scholar] [CrossRef]
- Ho, H.-J.; Iizuka, A.; Shibata, E.; Tomita, H.; Takano, K.; Endo, T. Utilization of CO2 in Direct Aqueous Carbonation of Concrete Fines Generated from Aggregate Recycling: Influences of the Solid–Liquid Ratio and CO2 Concentration. J. Clean. Prod. 2021, 312, 127832. [Google Scholar] [CrossRef]
- Ho, H.-J.; Iizuka, A.; Shibata, E. Utilization of Low-Calcium Fly Ash via Direct Aqueous Carbonation with a Low-Energy Input: Determination of Carbonation Reaction and Evaluation of the Potential for CO2 Sequestration and Utilization. J. Environ. Manag. 2021, 288, 112411. [Google Scholar] [CrossRef]
- O’Connor, W.K.; Dahlin, D.C.; Nilsen, D.N.; Rush, G.E.; Walters, R.P.; Turner, P.C. CO2 Storage in Solid Form: A Study of Direct Mineral Carbonation; CSIRO: Collinwood, Australia, 2000.
- Mun, M.; Cho, H.; Kwon, J. Study on Characteristics of Various Extractants for Mineral Carbonation of Industrial Wastes. J. Environ. Chem. Eng. 2017, 5, 3803–3821. [Google Scholar] [CrossRef]
- Park, A.-H.A.; Fan, L.-S. CO2 Mineral Sequestration: Physically Activated Dissolution of Serpentine and pH Swing Process. Chem. Eng. Sci. 2004, 59, 5241–5247. [Google Scholar] [CrossRef]
- Wang, X.; Maroto-Valer, M.M. Dissolution of Serpentine Using Recyclable Ammonium Salts for CO2 Mineral Carbonation. Fuel 2011, 90, 1229–1237. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching: Metal Solubilization by Microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Schwartzman, D.W.; Volk, T. Biotic Enhancement of Weathering and the Habitability of Earth. Nature 1989, 340, 457–460. [Google Scholar] [CrossRef]
- Chiang, Y.W.; Santos, R.M.; Monballiu, A.; Ghyselbrecht, K.; Martens, J.A.; Mattos, M.L.T.; Gerven, T.V.; Meesschaert, B. Effects of Bioleaching on the Chemical, Mineralogical and Morphological Properties of Natural and Waste-Derived Alkaline Materials. Miner. Eng. 2013, 48, 116–125. [Google Scholar] [CrossRef]
- Power, I.M.; McCutcheon, J.; Harrison, A.L.; Wilson, S.; Dipple, G.M.; Kelly, S.; Southam, C.; Southam, G. Strategizing Carbon-Neutral Mines: A Case for Pilot Projects. Minerals 2014, 4, 399–436. [Google Scholar] [CrossRef]
- Power, I.M.; Dipple, G.M.; Southam, G. Bioleaching of Ultramafic Tailings by Acidithiobacillus Spp. for CO2 Sequestration. Environ. Sci. Technol. 2010, 44, 456–462. [Google Scholar] [CrossRef]
- Fang, C.; Achal, V. Enhancing Carbon Neutrality: A Perspective on the Role of Microbially Induced Carbonate Precipitation (MICP). Biogeotechnics 2024, 2, 100083. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Fu, Q.; Zhang, D. Biomineralization Based Remediation of As(III) Contaminated Soil by Sporosarcina Ginsengisoli. J. Hazard. Mater. 2012, 201–202, 178–184. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Zhang, D. Bioremediation of Strontium (Sr) Contaminated Aquifer Quartz Sand Based on Carbonate Precipitation Induced by Sr Resistant Halomonas sp. Chemosphere 2012, 89, 764–768. [Google Scholar] [CrossRef]
- Stabnikov, V.; Jian, C.; Ivanov, V.; Li, Y. Halotolerant, Alkaliphilic Urease-Producing Bacteria from Different Climate Zones and Their Application for Biocementation of Sand. World J. Microbiol. Biotechnol. 2013, 29, 1453–1460. [Google Scholar] [CrossRef]
- Duan, Y.; Niu, L.; Li, B.; He, Y.; Xu, X.; Yu, C.; Wang, Z.; Xiao, C.; Zheng, C. Montmorillonite-Coupled Microbially Induced Carbonate Precipitation (MICP) Enhanced Contaminant Removal and Carbon Capture in Cyanide Tailings. J. Environ. Chem. Eng. 2024, 12, 113498. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A. A Review of Microbial Precipitation for Sustainable Construction. Constr. Build. Mater. 2015, 93, 1224–1235. [Google Scholar] [CrossRef]
- Kang, B.; Zha, F.; Deng, W.; Wang, R.; Sun, X.; Lu, Z. Biocementation of Pyrite Tailings Using Microbially Induced Calcite Carbonate Precipitation. Molecules 2022, 27, 3608. [Google Scholar] [CrossRef] [PubMed]
- Maureira, A.; Zapata, M.; Olave, J.; Jeison, D.; Wong, L.-S.; Panico, A.; Hernández, P.; Cisternas, L.A.; Rivas, M. MICP Mediated by Indigenous Bacteria Isolated from Tailings for Biocementation for Reduction of Wind Erosion. Front. Bioeng. Biotechnol. 2024, 12, 1393334. [Google Scholar] [CrossRef] [PubMed]
- Mwandira, W.; Nakashima, K.; Kawasaki, S.; Ito, M.; Sato, T.; Igarashi, T.; Banda, K.; Chirwa, M.; Nyambe, I.; Nakayama, S.; et al. Efficacy of Biocementation of Lead Mine Waste from the Kabwe Mine Site Evaluated Using Pararhodobacter sp. Environ. Sci. Pollut. Res. Int. 2019, 26, 15653–15664. [Google Scholar] [CrossRef] [PubMed]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial Carbonate Precipitation in Construction Materials: A Review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Saha, A.; Ghosh, D.; Dam, B.; Samanta, A.K.; Dutta, S. Microbial Repairing of Concrete & Its Role in CO2 Sequestration: A Critical Review. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 7. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, D.; Koner, S.; Hseu, Z.-Y.; Hsu, B.-M. Microbial Induce Carbonate Precipitation Derive Bio-Concrete Formation: A Sustainable Solution for Carbon Sequestration and Eco-Friendly Construction. Environ. Res 2025, 270, 121006. [Google Scholar] [CrossRef]
- Wong, P.Y.; Mal, J.; Sandak, A.; Luo, L.; Jian, J.; Pradhan, N. Advances in Microbial Self-Healing Concrete: A Critical Review of Mechanisms, Developments, and Future Directions. Sci. Total Environ. 2024, 947, 174553. [Google Scholar] [CrossRef]
- Fu, T.; Saracho, A.C.; Haigh, S.K. Microbially Induced Carbonate Precipitation (MICP) for Soil Strengthening: A Comprehensive Review. Biogeotechnics 2023, 1, 100002. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Mujah, D. Influence of Key Environmental Conditions on Microbially Induced Cementation for Soil Stabilization. J. Geotech. Geoenviron. Eng. 2017, 143, 04016083. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-Mediated Soil Improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Ramanan, R.; Kannan, K.; Sivanesan, S.D.; Mudliar, S.; Kaur, S.; Tripathi, A.K.; Chakrabarti, T. Bio-Sequestration of Carbon Dioxide Using Carbonic Anhydrase Enzyme Purified from Citrobacter Freundii. World J. Microbiol. Biotechnol. 2009, 25, 981–987. [Google Scholar] [CrossRef]
- Gilmour, K.A.; Ghimire, P.S.; Wright, J.; Haystead, J.; Dade-Robertson, M.; Zhang, M.; James, P. Microbially Induced Calcium Carbonate Precipitation through CO2 Sequestration via an Engineered Bacillus Subtilis. Microb. Cell Factories 2024, 23, 168. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, W.-S.; Zhou, P.-P.; Yu, L.-J. Influence of Enzyme Concentration on Bio-Sequestration of CO2 in Carbonate Form Using Bacterial Carbonic Anhydrase. Chem. Eng. J. 2013, 232, 149–156. [Google Scholar] [CrossRef]
- Silva-Castro, G.A.; Uad, I.; Gonzalez-Martinez, A.; Rivadeneyra, A.; Gonzalez-Lopez, J.; Rivadeneyra, M.A. Bioprecipitation of Calcium Carbonate Crystals by Bacteria Isolated from Saline Environments Grown in Culture Media Amended with Seawater and Real Brine. BioMed Res. Int. 2015, 2015, 816102. [Google Scholar] [CrossRef]
- Zheng, T.; Qian, C. Influencing Factors and Formation Mechanism of CaCO3 Precipitation Induced by Microbial Carbonic Anhydrase. Process Biochem. 2020, 91, 271–281. [Google Scholar] [CrossRef]
- Xiao, L.; Lian, B. Heterologously Expressed Carbonic Anhydrase from Bacillus mucilaginosus Promoting CaCO3 Formation by Capturing Atmospheric CO2. Carbonates Evaporites 2016, 31, 39–45. [Google Scholar] [CrossRef]
- Abdelsamad, R.; Disi, Z.A.; Abu-Dieyeh, M.; Al-Ghouti, M.A.; Zouari, N. Evidencing the Role of Carbonic Anhydrase in the Formation of Carbonate Minerals by Bacterial Strains Isolated from Extreme Environments in Qatar. Heliyon 2022, 8, e11151. [Google Scholar] [CrossRef]
- Huang, L.; Li, F.; Ji, C.; Wang, Y.; Yang, G. Carbon Isotope Fractionation and Its Tracer Significance to Carbon Source during Precipitation of Calcium Carbonate in the Presence of Bacillus Cereus LV-1. Chem. Geol. 2022, 609, 121029. [Google Scholar] [CrossRef]
- Yang, G.; Li, L.; Li, F.; Zhang, C.; Lyu, J. Mechanism of Carbonate Mineralization Induced by Microbes: Taking Curvibacter Lanceolatus Strain HJ-1 as an Example. Micron 2021, 140, 102980. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Dideriksen, K.; Spangler, L.H.; Cunningham, A.B.; Gerlach, R. Microbially Enhanced Carbon Capture and Storage by Mineral-Trapping and Solubility-Trapping. Environ. Sci. Technol. 2010, 44, 5270–5276. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, X.; Wang, Y.; Jiang, N. A Critical Review of Biomineralization in Environmental Geotechnics: Applications, Trends, and Perspectives. Biogeotechnics 2023, 1, 100003. [Google Scholar] [CrossRef]
- Government of Canada; Innovation, Science and Economic Development Canada; Cement Association of Canada. Roadmap to Net-Zero Carbon Concrete by 2050; Innovation, Science and Economic Development Canada: Ottawa, ON, Canada, 2024.
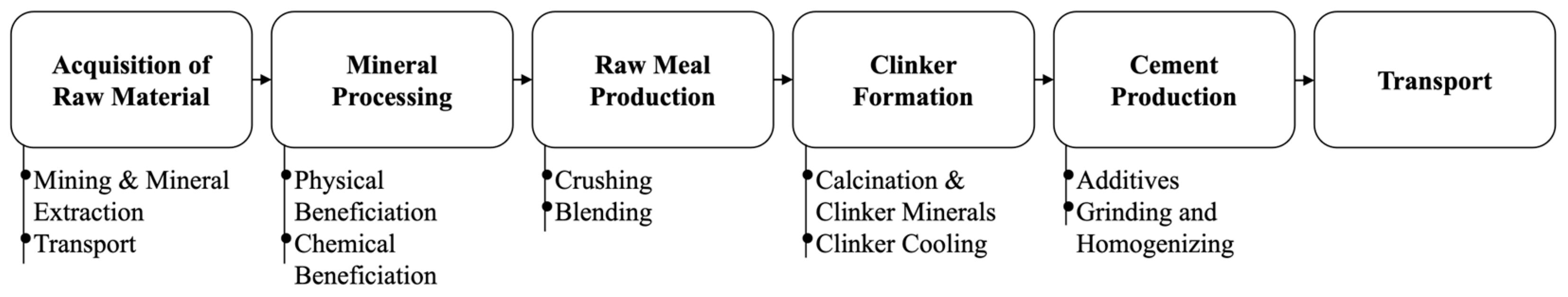
- del Strother, P. 2—Manufacture of Portland Cement. In Lea’s Chemistry of Cement and Concrete, 15th ed.; Hewlett, P.C., Liska, M., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 31–56. ISBN 978-0-08-100773-0. [Google Scholar]
- Hendriks, C.A.; Worrell, E.; Price, L.; Martin, N.; Ozawa Meida, L.; De Jager, D.; Riemer, P. Emission Reduction of Greenhouse Gases from the Cement Industry. In Greenhouse Gas Control Technologies 4; Elsevier: Amsterdam, The Netherlands, 1999; pp. 939–944. ISBN 978-0-08-043018-8. [Google Scholar]
- Sharma, M.; Satyam, N.; Reddy, K.R. State of the Art Review of Emerging and Biogeotechnical Methods for Liquefaction Mitigation in Sands. J. Hazard. Toxic Radioact. Waste 2021, 25, 03120002. [Google Scholar] [CrossRef]
- Burbank, M.B.; Weaver, T.J.; Green, T.L.; Williams, B.C.; Crawford, R.L. Precipitation of Calcite by Indigenous Microorganisms to Strengthen Liquefiable Soils. Geomicrobiol. J. 2011, 28, 301–312. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, C.-S.; Jiang, N.-J.; Pan, X.-H.; Liu, B.; Wang, Y.-J.; Shi, B. Microbial-induced Carbonate Precipitation (MICP) Technology: A Review on the Fundamentals and Engineering Applications. Environ. Earth Sci. 2023, 82, 229. [Google Scholar] [CrossRef]
- Liu, J.; Zhen, B.; Qiu, H.; Zhou, X.; Zhang, H. Impact of Waterlogging and Heat Stress on Rice Rhizosphere Microbiome Assembly and Potential Function in Carbon and Nitrogen Transformation. Arch. Agron. Soil Sci. 2023, 69, 1920–1932. [Google Scholar] [CrossRef]
- Liu, B.; Tang, C.-S.; Pan, X.-H.; Zhu, C.; Cheng, Y.-J.; Xu, J.-J.; Shi, B. Potential Drought Mitigation through Microbial Induced Calcite Precipitation—MICP. Water Resour. Res. 2021, 57, e2020WR029434. [Google Scholar] [CrossRef]

| Method | Material | CO2 Application | CO2 Input 1 | Results 1 | Findings | Reference |
|---|---|---|---|---|---|---|
| Direct Gas–Solid Carbonation | Municipal Solid Waste Incinerator | CO2 flow | 100% CO2, 3 bars, 2.5 h | 3.19% CaCO3 gain bottom ash 7.31% CaCO3 gain fly ash | More suitable to small particle size. | [183] |
| 100% CO2, 3 bars, 3 h | 11% CaCO3 gain fly ash | Optimal CO2 capture at water/solid ratio 0.3. | [179] | |||
| 17 bars, 3 h | 3% CaCO3 gain bottom ash | Optimal CO2 capture 20% w/w moisture and 4 mm sieving. | [180] | |||
| 1 bar, 1 h | 60 g CO2/kg fly ash | Temperature (600 °C) and H2O(g) (20%) are more significant than CO2 content. | [181] | |||
| Waste Concrete & Anorthosite Tailings | 18.2 vol% CO2, 4 & 5 bar, 30 min | 66% CO2 removal waste concrete 34% CO2 removal anorthosite | Aqueous phase carbonation resulted in 34.6% removal in 15 min. | [186] | ||
| Pre-treated EAF steel-making bag house dust | 3 bar inlet, 1 bar (outlet), 12 L/min | 0.657 kg CO2/kg dust | Carbonation was based on the total calcium content. | [187] | ||
| Air Pollution Control Residues from a Medical Solid Waste Incinerator | 100% CO2, 6 h | 0.12 kg CO2/kg dry solid waste | Maximum carbonation at 400 °C. | [188] | ||
| Serpentinite Mining Residue | CO2 concentration | 18 vol% CO2 | 0.07 g CO2/g residue | Water vapor (10 vol%) required for carbonation. | [182] | |
| Direct Aqueous Carbonation | Concrete Fines | CO2 flow | 14% CO2, 90 min | 0.19 g CO2/g concrete fines | Almost all absorbed CO2 was converted to CaCO3, and increased CO2 concentration requires higher solid–liquid ratio. | [189] |
| Olivine with NaHCO3 & NaOH Buffers | pCO2 6.5 MPa, 6 h | <80% carbonation | Agitation is necessary to prevent solids settlement. Low pCO2 requires high NaHCO3 concentration. | [190] | ||
| Flue Gas Desulfurization Gypsum | 1 L/min, 15 min | 90% CaCO3 efficiency | CaCO3 precipitation increased linearly with ammonia content. | [191] | ||
| Steel Slag | 19 bar CO2, 30 min | 0.25 kg CO2/kg steel slag | Primary factors: particle size <2mm to <38 μm and temperature 25–225 °C. | [192] | ||
| Red-Mud | 3.5 bar, 3.5 h | 5.3 g CO2/100 g red mud | At liquid–solid ratio of 0.35. | [193] | ||
| Oil Shale Ash | Continuous flow (0.7 m/10 L), 15% CO2 | 17–20% bound CO2 | Size and structure of CaCO3 depended on end-point pH. | [194] | ||
| Coal Fly Ash | 10 bars, 18 h | 26 kg CO2/ton fly-ash | Pressure was independent of carbonation efficiency and not affected by temperature of fly ash weight. | [195] | ||
| Industrial/Mining Wastes | CO2 concentration | 15% CO2 | 544.6 g CO2/kg carbide slag | Ca content in material produces increased carbonation. Max carbon sequestration occurred at < 75 μm particle size, 60 °C, 100 g/L liquid–solid ratio. | [196] | |
| Aggregate Recycling Concrete Fines | 5% CO2 | 0.13 g CO2/g concrete fines | 0.10 CO2/g concrete fines captured as CaCO3, and 0.02 CO2/g concrete fines dissolved in aqueous. | [197] | ||
| Low-Calcium Fly Ash | 30% CO2 | 0.016 g CO2/g fly ash | Good carbonation potential despite low energy input and low calcium content. | [198] |
| Metabolic Pathway | Microbial Strain | Material | Findings | Reference |
|---|---|---|---|---|
| CA | Citrobacter freundii | Wastewater | CaCO3 precipitated with CO2 catalyzed by CA. Can sequester CO2 at high concentrations, but HCO3− inhibits CA enzyme activity due to pH decrease. | [224] |
| Bacillus subtilis | Agar & Liquid Medium | CA converted CO2 to CaCO3 minerals. | [225] | |
| Bacillus cereus | Karst Soil | CA enzyme activity influenced CaCO3 crystal morphology. | [226] | |
| Bacillus megaterium | Mortar Specimens | CO2 influx precipitated comparable CaCO3 to ureolysis-precipitated CaCO3 | [114] | |
| Bacillus pumilus, Bacillus marisflavi | Seawater | H2O and CaCO3, showing the potential for carbon sequestration. | [227] | |
| Bacillus altitudinis | Mangrove Soil | Impact of CO2 sequestration with bacteria showed 75% removal and 97% removal with bacteria and CA. | [144] | |
| Bacillus mucilaginosus | Liquid Medium | Optimal CA at 30 °C and alkaline environment to enhance CO2 hydration. | [228] | |
| Bacillus mucilaginosus | Liquid Medium | CO2 is more easily captured by CA, which alters the size and morphology of CaCO3 crystals. | [229] | |
| Psychrobacter sp., Vibrio alginolyticus | Marine Sediments | Strong potential for carbonate precipitation with high CA, meaning capture of CO2. | [230] | |
| EPS & CA | Bacillus cereus | Liquid Medium | Calcite induced by bacteria can fix CO2 from air since CO2 released from organic matter is less than in air. | [231] |
| Curvibacter lanceolatus | Liquid Medium | CA precipitated only calcite, whereas CA and EPA precipitated calcite and aragonite to enhance CO2 fixation. | [232] | |
| Phototrophic | Phragmoplastophyta | Diamond Mine | Secondary carbonate precipitation capable of offsetting CO2e by 20%. | [129] |
| Oscillatoria sp., Porphyrobacter sp., Blastomas sp., Rhodobacter sp. | Diamond Mine | Kimberlite weathering and secondary carbonate precipitation can sequester carbon through photosynthetic bacteria acting as a catalyst to convert CO2 to CaCO3/MgCO3. | [130] | |
| Ureolysis | Sporosarcina, Sphingobacterium, Stenotrophomonas, Acinetobacter, Elizabethkingia | Cave & Tavern Water | CO2 sequestration depended on pH and the consortia of bacteria. | [113] |
| Sporosarcina, Brevudimonas, Sphingobacterium, Stenotrophomonas, Acinetobacter | Cave & Tavern Water | Abiotic CO2 sequestration depended on pH and medium, whereas biotic CO2 sequestration depended on the bacterial species or strains. | [33] | |
| Sporosarcina pasteurii | Tailings | MICP increased CO2 capture from tailings by 27.15–34.55% | [212] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilcox, S.M.; Mulligan, C.N.; Neculita, C.M. Mineral Carbonation for Carbon Sequestration: A Case for MCP and MICP. Int. J. Mol. Sci. 2025, 26, 2230. https://doi.org/10.3390/ijms26052230
Wilcox SM, Mulligan CN, Neculita CM. Mineral Carbonation for Carbon Sequestration: A Case for MCP and MICP. International Journal of Molecular Sciences. 2025; 26(5):2230. https://doi.org/10.3390/ijms26052230
Chicago/Turabian StyleWilcox, Samantha M., Catherine N. Mulligan, and Carmen Mihaela Neculita. 2025. "Mineral Carbonation for Carbon Sequestration: A Case for MCP and MICP" International Journal of Molecular Sciences 26, no. 5: 2230. https://doi.org/10.3390/ijms26052230
APA StyleWilcox, S. M., Mulligan, C. N., & Neculita, C. M. (2025). Mineral Carbonation for Carbon Sequestration: A Case for MCP and MICP. International Journal of Molecular Sciences, 26(5), 2230. https://doi.org/10.3390/ijms26052230









