Molecular Biomarkers in Neurological Diseases: Advances in Diagnosis and Prognosis
Abstract
1. Introduction
2. Results
2.1. Genomic and Transcriptomic Biomarkers
2.2. Epigenomic and Proteomic Insights
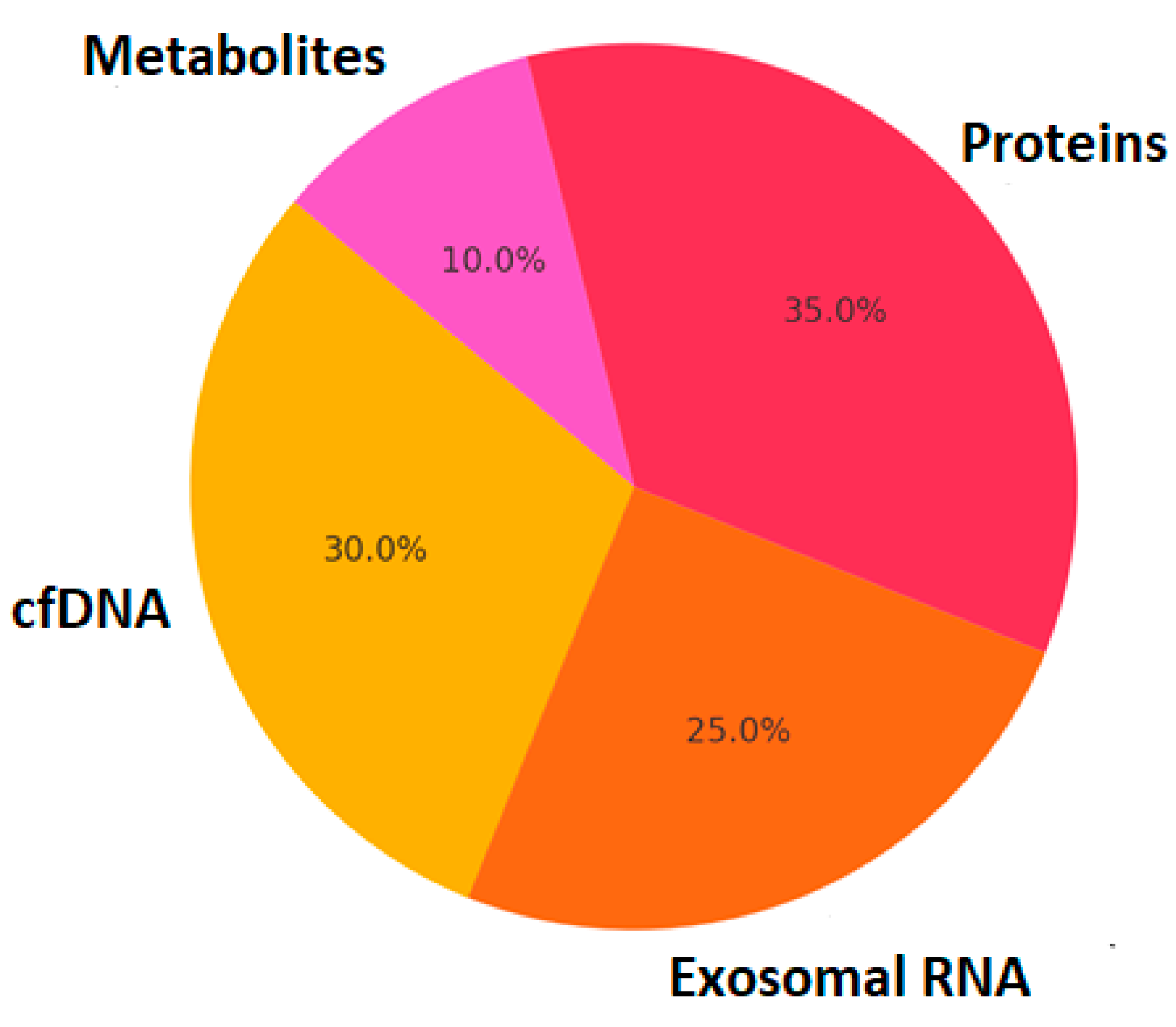
2.3. Liquid Biopsy and Multi-Omics Integration
- -
- Large-scale multi-omics integration with well-characterized patient cohorts.
- -
- Standardization of biomarker assessment protocols across different populations.
- -
- AI-driven approaches to analyze high-dimensional biomarker data, improving diagnostic accuracy.
- -
- Translation of biomarker panels into clinical decision-making tools through regulatory harmonization [5].
3. Discussion
- Standardization and Validation: biomarkers must undergo rigorous validation across independent cohorts before being implemented in clinical practice [9].
- Inter-individual Variability: differences in genetics, lifestyle, and comorbidities influence biomarker expression, making it necessary to develop personalized reference ranges [9].
- Data Integration and Interpretation: multi-omics datasets generate large volumes of information that require sophisticated computational tools and robust statistical frameworks to extract clinically relevant insights [43].
4. Materials and Methods
- “molecular biomarkers”;
- “neurological diseases”;
- “genomics”;
- “proteomics”;
- “liquid biopsy”;
- “precision medicine”.
- Genomic biomarkers: studies investigating single nucleotide polymorphisms (SNPs), gene mutations, and genome-wide association studies (GWASs) in neurological diseases.
- Transcriptomic biomarkers: RNA sequencing (RNA-seq) and differential gene expression studies.
- Epigenomic biomarkers: DNA methylation, histone modifications, and chromatin remodeling studies.
- Proteomic biomarkers: studies identifying cerebrospinal fluid (CSF) and blood-based protein biomarkers.
- Liquid biopsy: analysis of exosomal RNA, circulating cfDNA, and extracellular vesicles as diagnostic tools.
- Multi-omics approaches: integration of genomics, transcriptomics, proteomics, and metabolomics in biomarker discovery.
- AI and machine learning in biomarker analysis: studies utilizing AI-driven approaches for biomarker identification and clinical applications.
5. Conclusions
- Standardization and Validation: large-scale multi-center studies are needed to validate molecular biomarkers across diverse populations before clinical implementation.
- Inter-Individual Variability: biomarker expression varies based on genetics, environment, and comorbidities, highlighting the need for personalized reference ranges.
- Data Integration and Interpretation: the complexity of multi-omics datasets requires advanced bioinformatics tools and robust statistical models to extract meaningful insights.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| MS | multiple sclerosis |
| ALS | amyotrophic lateral sclerosis |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
References
- GBD 2021 Nervous System Disorders Collaborators. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381, Erratum in Lancet Neurol. 2024, 23, e9; Erratum in Lancet Neurol. 2024, 23, e11.. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hampel, H.; O’Bryant, S.E.; Molinuevo, J.L.; Zetterberg, H.; Masters, C.L.; Lista, S.; Kiddle, S.J.; Batrla, R.; Blennow, K. Blood-based biomarkers for Alzheimer disease: Mapping the road to the clinic. Nat. Rev. Neurol. 2018, 14, 639–652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol. 2017, 18, 83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, S.W.; Xu, J.Y.; Zhang, T. DGMP: Identifying cancer driver genes by jointing DGCN and MLP from multi-omics genomic data. Genom. Proteom. Bioinform. 2022, 20, 928–938. [Google Scholar] [CrossRef] [PubMed]
- De Jager, P.L.; Srivastava, G.; Lunnon, K.; Burgess, J.; Schalkwyk, L.C.; Yu, L.; Eaton, M.L.; Keenan, B.T.; Ernst, J.; McCabe, C. Alzheimer’s disease: Early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat. Neurosci. 2014, 17, 1156–1163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olsson, B.; Lautner, R.; Andreasson, U.; Öhrfelt, A.; Portelius, E.; Bjerke, M.; Hölttä, M.; Rosén, C.; Olsson, C.; Strobel, G. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol. 2016, 15, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Gulisano, W.; Maugeri, D.; Baltrons, M.A.; Fà, M.; Amato, A.; Palmeri, A.; D’Adamio, L.; Grassi, C.; Devanand, D.P.; Honig, L.S. Role of Amyloid-β and Tau Proteins in Alzheimer’s Disease: Confuting the Amyloid Cascade. J. Alzheimer’s Dis. 2018, 64 (Suppl. S1), S611–S631, Erratum in J. Alzheimer’s Dis. 2019, 68, 415.. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teunissen, C.E.; Verberk, I.M.W.; Thijssen, E.H.; Vermunt, L.; Hansson, O.; Zetterberg, H.; van der Flier, W.M.; Mielke, M.M.; Del Campo, M. Blood-based biomarkers for Alzheimer’s disease: Towards clinical implementation. Lancet Neurol. 2022, 21, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Kodam, P.; Sai Swaroop, R.; Pradhan, S.S.; Sivaramakrishnan, V.; Vadrevu, R. Integrated multi-omics analysis of Alzheimer’s disease shows molecular signatures associated with disease progression and potential therapeutic targets. Sci. Rep. 2023, 13, 3695. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hampel, H.; Vergallo, A.; Afshar, M.; Akman-Anderson, L.; Arenas, J.; Benda, N.; Batrla, R.; Broich, K.; Caraci, F.; Cuello, A.C. Blood-based systems biology biomarkers for next-generation clinical trials in Alzheimer’s diseases. Dialogues Clin. Neurosci. 2019, 21, 177–191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hardy, J. The genetic causes of neurodegenerative diseases. J. Alzheimer’s Dis. 2001, 3, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Van der Brug, M.P.; Singleton, A.; Gasser, T. Parkinson’s disease: From human genetics to clinical trials. Sci. Transl. Med. 2015, 7, 305ps20. [Google Scholar] [CrossRef] [PubMed]
- Jansen, I.E.; Savage, J.E.; Watanabe, K.; Bryois, J.; Williams, D.M.; Steinberg, S.; Sealock, J.; Karlsson, I.K.; Hägg, S.; Athanasiu, L. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 2019, 51, 404–413, Erratum in Nat. Genet. 2020, 52, 354.. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahley, R.W. Apolipoprotein E: From cardiovascular disease to neurodegenerative disorders. J. Mol. Med. 2016, 94, 739–746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ulrich, J.D.; Ulland, T.K.; Colonna, M.; Holtzman, D.M. Elucidating the Role of TREM2 in Alzheimer’s Disease. Neuron 2017, 94, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Brás, J.; Guerreiro, R.; Hardy, J. SnapShot: Genetics of Parkinson’s disease. Cell 2015, 160, 570.e1. [Google Scholar] [CrossRef] [PubMed]
- International Multiple Sclerosis Genetics Consortium. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 2011, 476, 214–219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, B.; Gaiteri, C.; Bodea, L.G.; Wang, Z.; McElwee, J.; Podtelezhnikov, A.A.; Zhang, C.; Xie, T.; Tran, L.; Dobrin, R. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 2013, 153, 707–720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prinz, M.; Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 2017, 20, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Jeong, H.H.; Hsieh, Y.C.; Klein, H.U.; Bennett, D.A.; De Jager, P.L.; Liu, Z.; Shulman, J.M. Tau Activates Transposable Elements in Alzheimer’s Disease. Cell Rep. 2018, 23, 2874–2880. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faghihi, M.A.; Modarresi, F.; Khalil, A.M.; Wood, D.E.; Sahagan, B.G.; Morgan, T.E.; Finch, C.E.; St. Laurent, G., 3rd; Kenny, P.J.; Wahlestedt, C. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat. Med. 2008, 14, 723–730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baranzini, S.E.; Oksenberg, J.R. The Genetics of Multiple Sclerosis: From 0 to 200 in 50 Years. Trends Genet. 2017, 33, 960–970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Habib, N.; McCabe, C.; Medina, S.; Varshavsky, M.; Kitsberg, D.; Dvir-Szternfeld, R.; Green, G.; Dionne, D.; Nguyen, L.; Marshall, J.L. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 2020, 23, 701–706. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lardenoije, R.; Iatrou, A.; Kenis, G.; Kompotis, K.; Steinbusch, H.W.; Mastroeni, D.; Coleman, P.; Lemere, C.A.; Hof, P.R.; van den Hove, D.L. The epigenetics of aging and neurodegeneration. Prog. Neurobiol. 2015, 131, 21–64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watson, C.T.; Roussos, P.; Garg, P.; Ho, D.J.; Azam, N.; Katsel, P.L.; Haroutunian, V.; Sharp, A.J. Genome-wide DNA methylation profiling in the superior temporal gyrus reveals epigenetic signatures associated with Alzheimer’s disease. Genome Med. 2016, 8, 5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masliah, E.; Dumaop, W.; Galasko, D.; Desplats, P. Distinctive patterns of DNA methylation associated with Parkinson disease: Identification of concordant epigenetic changes in brain and peripheral blood leukocytes. Epigenetics 2013, 8, 1030–1038. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fischer, A. Epigenetic memory: The Lamarckian brain. EMBO J. 2014, 33, 945–967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Govindarajan, N.; Rao, P.; Burkhardt, S.; Sananbenesi, F.; Schlüter, O.M.; Bradke, F.; Lu, J.; Fischer, A. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer’s disease. EMBO Mol. Med. 2013, 5, 52–63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huynh, J.L.; Casaccia, P. Epigenetic mechanisms in multiple sclerosis: Implications for pathogenesis and treatment. Lancet Neurol. 2013, 12, 195–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blennow, K.; Zetterberg, H. Biomarkers for Alzheimer’s disease: Current status and prospects for the future. J. Intern. Med. 2018, 284, 643–663. [Google Scholar] [CrossRef] [PubMed]
- Mollenhauer, B.; Locascio, J.J.; Schulz-Schaeffer, W.; Sixel-Döring, F.; Trenkwalder, C.; Schlossmacher, M.G. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: A cohort study. Lancet Neurol. 2011, 10, 230–240, Erratum in Lancet Neurol. 2011, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, N.; Andreasson, U.; Zetterberg, H.; Blennow, K. Alzheimer’s Disease Neuroimaging Initiative. Association of Plasma Neurofilament Light with Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol. 2017, 74, 557–566. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; He, Y.; Han, J.; Wei, W.; Chen, F. Blood-brain barrier dysfunction and Alzheimer’s disease: Associations, pathogenic mechanisms, and therapeutic potential. Front. Aging Neurosci. 2023, 15, 1258640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Podlesniy, P.; Llorens, F.; Puigròs, M.; Serra, N.; Sepúlveda-Falla, D.; Schmidt, C.; Hermann, P.; Zerr, I.; Trullas, R. Cerebrospinal Fluid Mitochondrial DNA in Rapid and Slow Progressive Forms of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, M.; Liu, C.; Cook, T.J.; Bullock, K.M.; Zhao, Y.; Ginghina, C.; Li, Y.; Aro, P.; Dator, R.; He, C. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014, 128, 639–650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Selmaj, I.; Cichalewska, M.; Namiecinska, M.; Galazka, G.; Horzelski, W.; Selmaj, K.W.; Mycko, M.P. Global exosome transcriptome profiling reveals biomarkers for multiple sclerosis. Ann. Neurol. 2017, 81, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Le, W. Recent advances and perspectives of metabolomics-based investigations in Parkinson’s disease. Mol. Neurodegener. 2019, 14, 3. [Google Scholar] [CrossRef]
- Baranzini, S.E.; Mudge, J.; van Velkinburgh, J.C.; Khankhanian, P.; Khrebtukova, I.; Miller, N.A.; Zhang, L.; Farmer, A.D.; Bell, C.J.; Kim, R.W. Genome, epigenome and RNA sequences of monozygotic twins discordant for multiple sclerosis. Nature 2010, 464, 1351–1356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barthélemy, N.R.; Salvadó, G.; Schindler, S.E.; He, Y.; Janelidze, S.; Collij, L.E.; Saef, B.; Henson, R.L.; Chen, C.D.; Gordon, B.A. Highly accurate blood test for Alzheimer’s disease is similar or superior to clinical cerebrospinal fluid tests. Nat. Med. 2024, 30, 1085–1095. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suppa, A.; Costantini, G.; Asci, F.; Di Leo, P.; Al-Wardat, M.S.; Di Lazzaro, G.; Scalise, S.; Pisani, A.; Saggio, G. Voice in Parkinson’s Disease: A Machine Learning Study. Front. Neurol 2022, 13, 831428. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.C.; Uricchio, L.H.; Torgerson, D.G.; Hernandez, R.D. Population genetics of rare variants and complex diseases. Hum. Hered. 2012, 74, 118–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robinson, W.H.; Fontoura, P.; Lee, B.J.; de Vegvar, H.E.; Tom, J.; Pedotti, R.; DiGennaro, C.D.; Mitchell, D.J.; Fong, D.; Ho, P.P. Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat. Biotechnol. 2003, 21, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
| Disease | Biomarkers | Type | Clinical Utility |
|---|---|---|---|
| Alzheimer’s Disease (AD) | p-Tau, Aβ42/Aβ40, NfL | Protein-based, Genetic | Early diagnosis, Monitoring |
| Parkinson’s Disease (PD) | α-synuclein, DJ-1, NfL | Protein-based, Extracellular vesicles | Differential diagnosis, Disease progression |
| Multiple Sclerosis (MS) | NfL, CXCL13, miR-21 | Protein-based, RNA-based | Immune profiling |
| Amyotrophic Lateral Sclerosis (ALS) | TDP-43, NfL, SOD1 | Protein-based, Genetic | Neurodegeneration marker |
| Technology | Application | Advantages |
|---|---|---|
| RNA-seq | Transcriptomic analysis | High sensitivity |
| Mass Spectometry | Proteomic analysis | Accurate protein quantification |
| AI-driven analysis | Predictive modeling and diagnostics | Big data analysis |
| Single-cell sequencing | Single-cell molecular characterization | Individual cell characterization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myrou, A.; Barmpagiannos, K.; Ioakimidou, A.; Savopoulos, C. Molecular Biomarkers in Neurological Diseases: Advances in Diagnosis and Prognosis. Int. J. Mol. Sci. 2025, 26, 2231. https://doi.org/10.3390/ijms26052231
Myrou A, Barmpagiannos K, Ioakimidou A, Savopoulos C. Molecular Biomarkers in Neurological Diseases: Advances in Diagnosis and Prognosis. International Journal of Molecular Sciences. 2025; 26(5):2231. https://doi.org/10.3390/ijms26052231
Chicago/Turabian StyleMyrou, Athena, Konstantinos Barmpagiannos, Aliki Ioakimidou, and Christos Savopoulos. 2025. "Molecular Biomarkers in Neurological Diseases: Advances in Diagnosis and Prognosis" International Journal of Molecular Sciences 26, no. 5: 2231. https://doi.org/10.3390/ijms26052231
APA StyleMyrou, A., Barmpagiannos, K., Ioakimidou, A., & Savopoulos, C. (2025). Molecular Biomarkers in Neurological Diseases: Advances in Diagnosis and Prognosis. International Journal of Molecular Sciences, 26(5), 2231. https://doi.org/10.3390/ijms26052231






