Poria cocos-Derived Exosome-like Nanovesicles Alleviate Metabolic Dysfunction-Associated Fatty Liver Disease by Promoting Mitophagy and Inhibiting NLRP3 Inflammasome Activation
Abstract
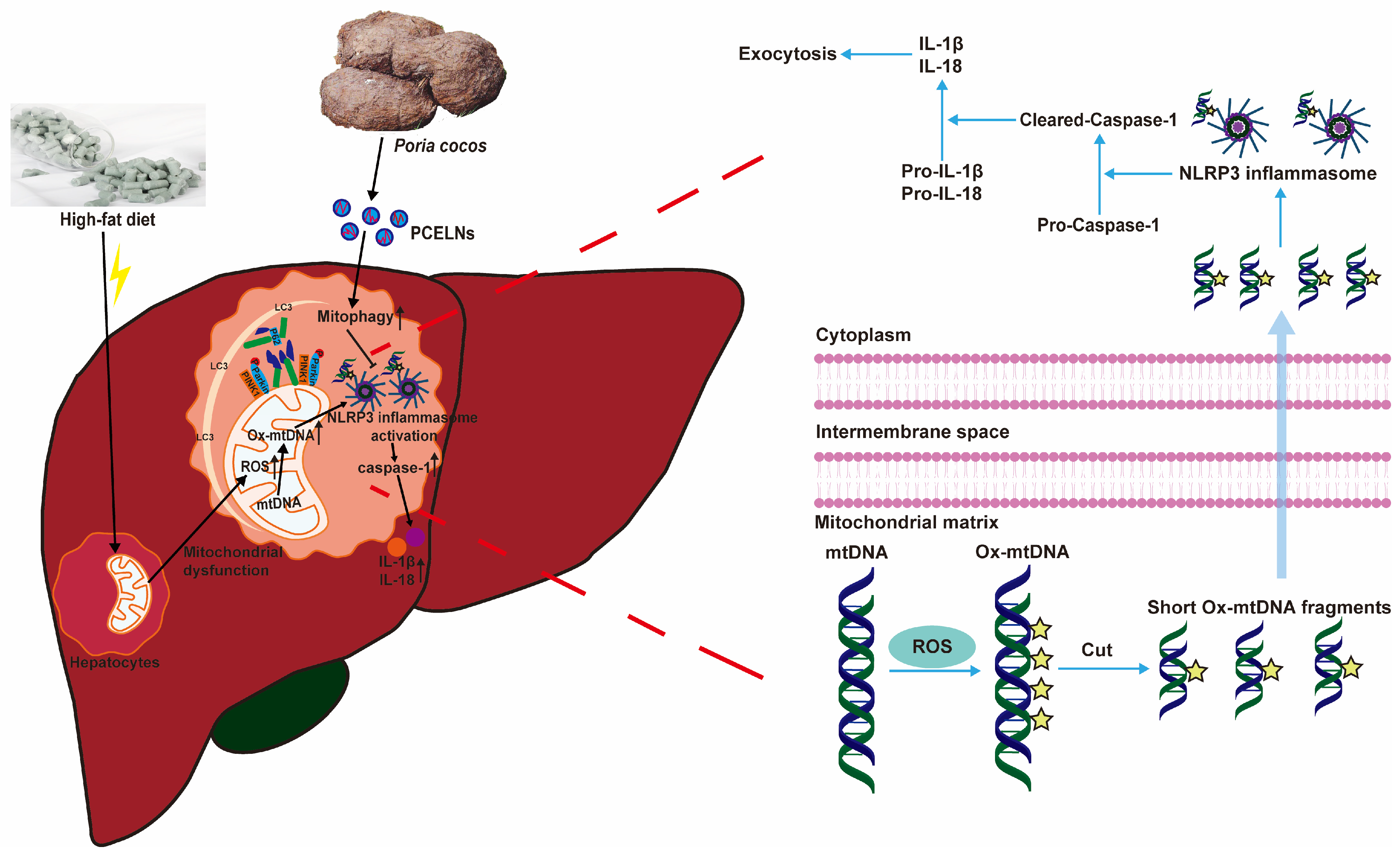
:1. Introduction
2. Results
2.1. Characteristics of PCELNs
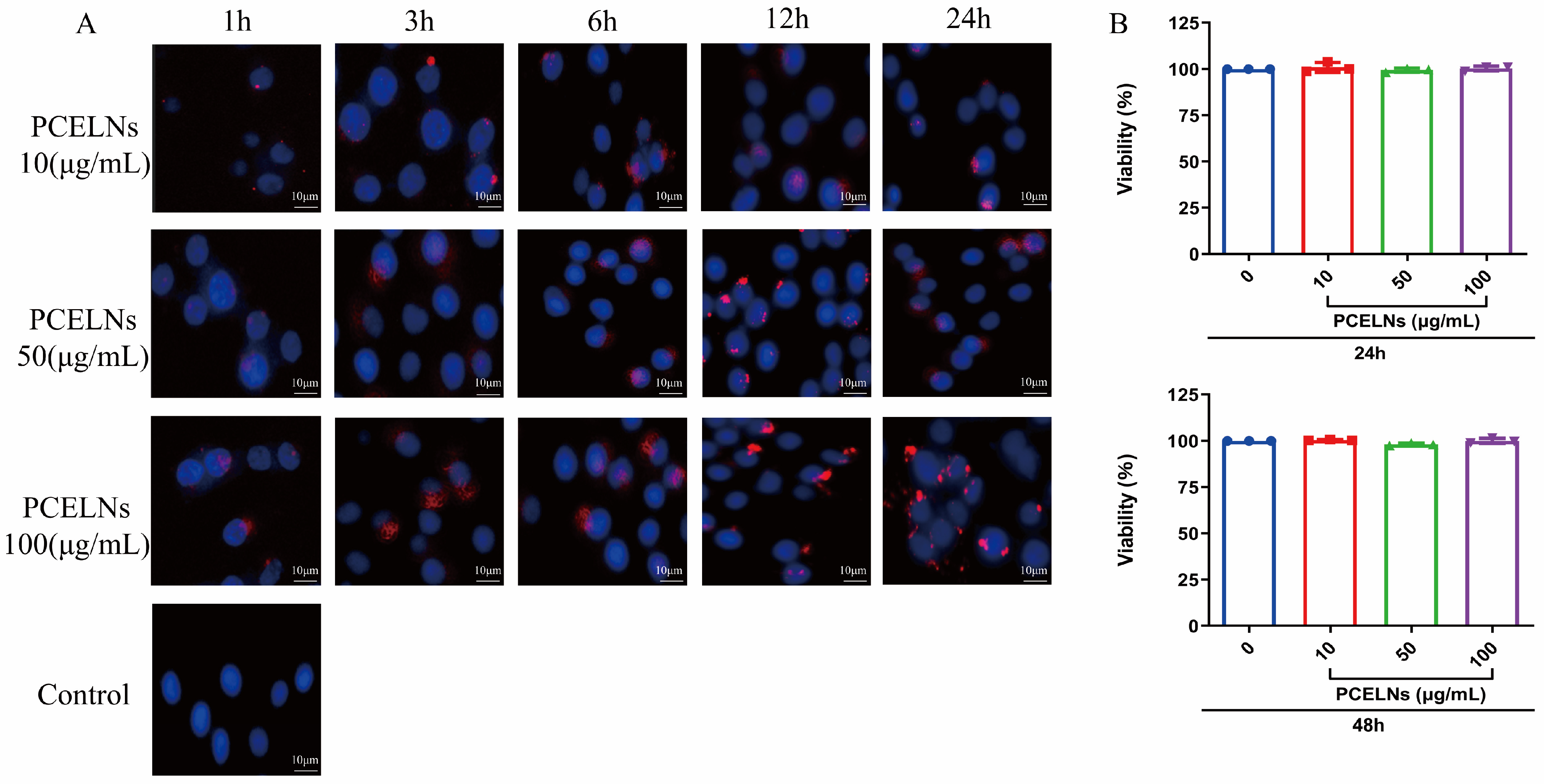
2.2. In Vitro Biocompatibility and Cytotoxicity of PCELNs
2.3. In Vitro Anti-Steatotic Effects of PCELNs
2.4. Effect of PCELNs on PINK1/Parkin-Mediated Mitophagy in L02 Cells
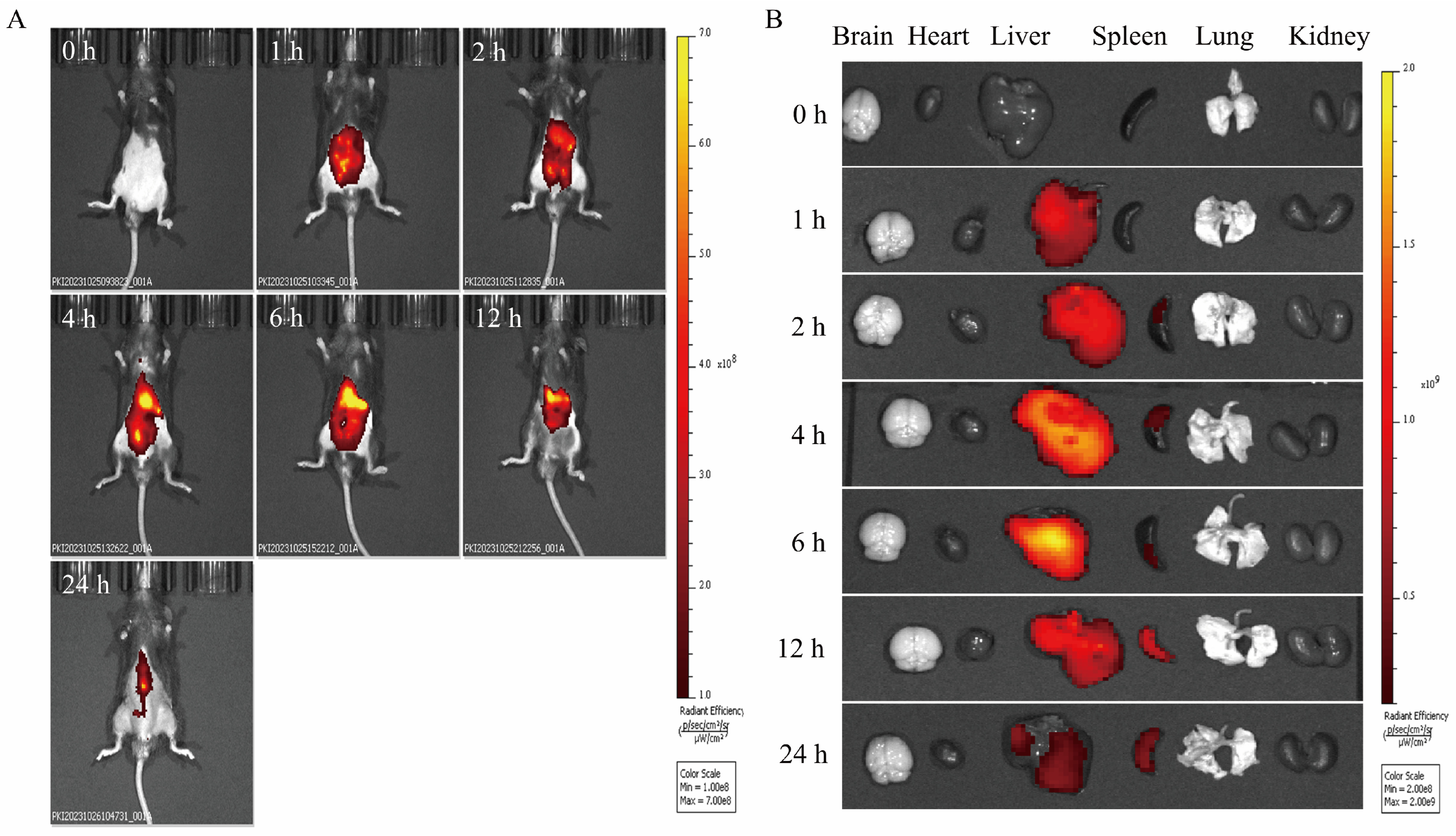
2.5. In Vivo Biodistribution of PCELNs
2.6. In Vivo Anti-MAFLD Effects of PCELNs
2.6.1. Effects of PCELNs on Body Weight, Food Intake, Organs, and Fat Index
2.6.2. Effects of PCELNs on Serum Lipid Parameters
2.6.3. Effects of PCELNs on Liver Lipid Metabolism
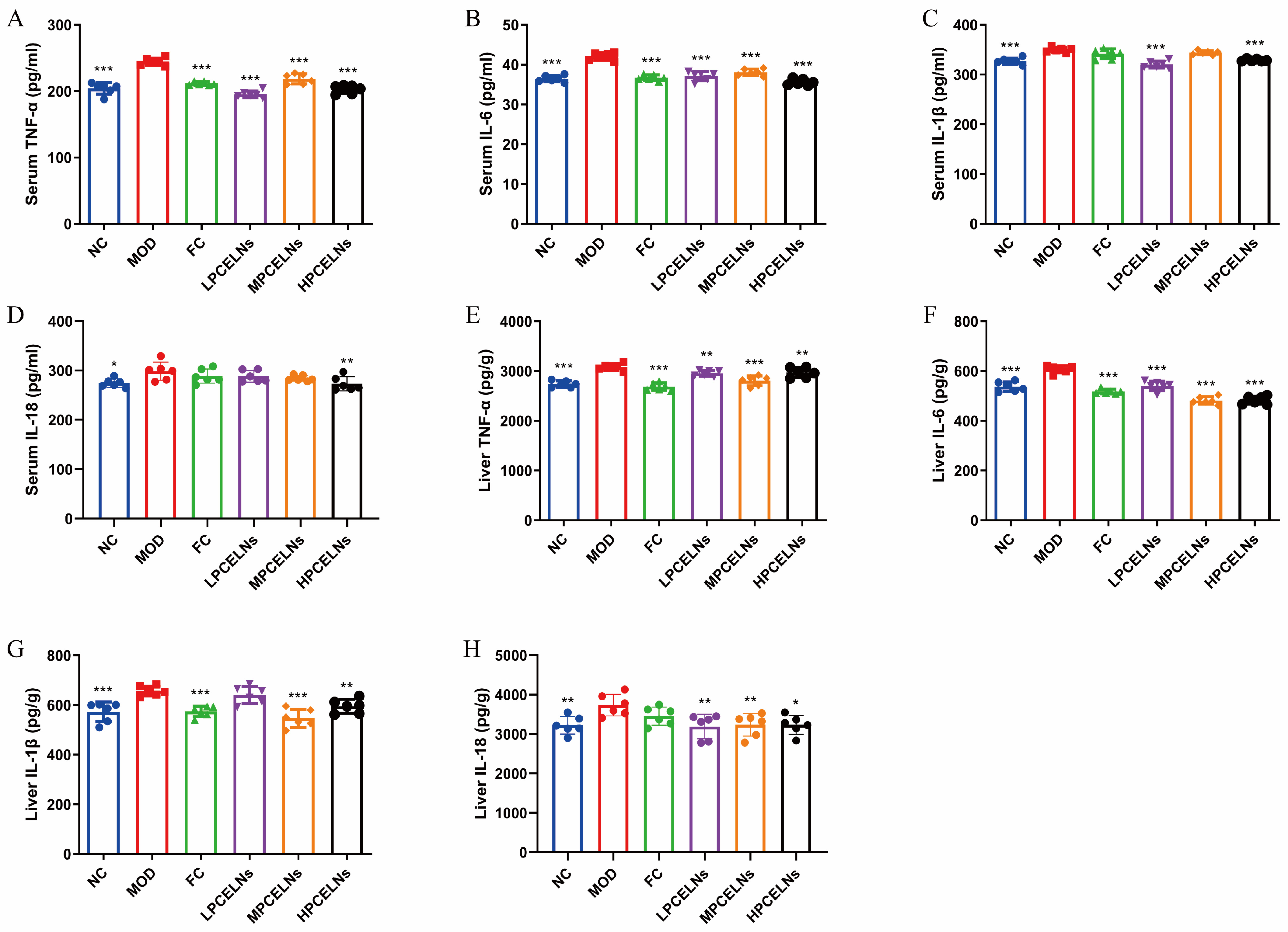
2.6.4. Effects of PCELNs on Serum and Liver Inflammatory Factors
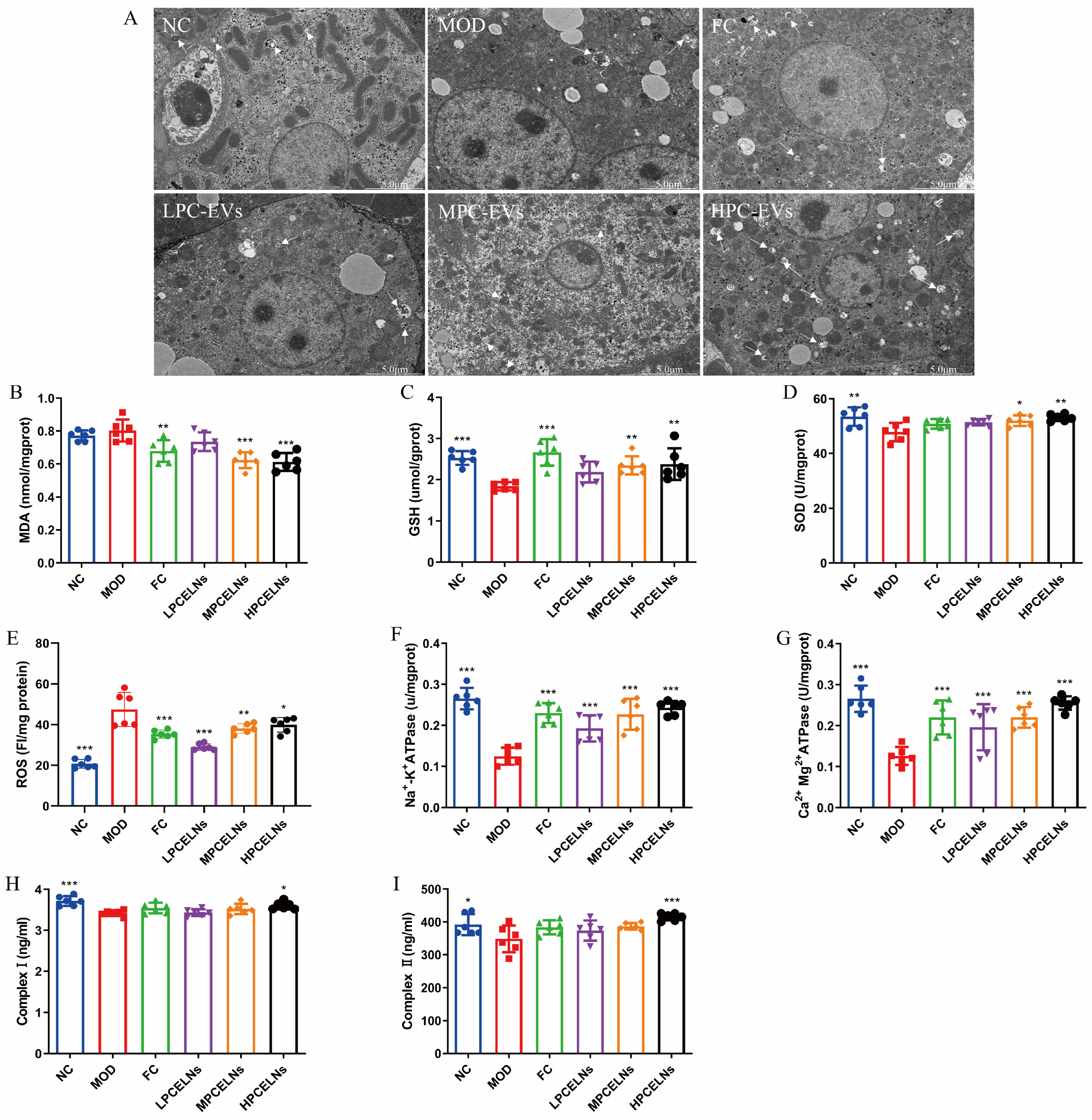
2.6.5. Effects of PCELNs on Mitochondrial Ultrastructure and Function in Liver
2.6.6. Mitophagy Was Involved in the Inhibition of NLRP3 Inflammasome by PCELNs
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Materials
4.2. Preparation and Characterization of PCELNs
4.3. Cell Experiments
4.3.1. Cell Culture
4.3.2. Labeling and Uptake of PCELNs
4.3.3. Cytotoxicity Assay
4.3.4. Therapeutic Effect of PCELNs on Steatosis of L02 Cells
4.4. Animal Experiments
4.4.1. Animals
4.4.2. Imaging In Vivo and Organ Distribution of PCELNs
4.4.3. Therapeutic Effect of PCELNs Against MAFLD
Design
Sample Collection
Histopathological Assessment
TEM of the Liver
Serum and Liver Biochemical Parameters
Oxidative Stress and Energy Metabolism in the Liver
Ox-mtDNA in Liver Mitochondria and Cytoplasm
Western Blot Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef]
- Mantovani, A.; Dalbeni, A. Treatments for NAFLD: State of Art. Int. J. Mol. Sci. 2021, 22, 2350. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zuo, Z.T.; Wang, Y.Z. The Traditional Usages, Chemical Components and Pharmacological Activities of Wolfiporia cocos: A Review. Am. J. Chin. Med. 2022, 50, 389–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Zhang, J.; Zhao, Y.L.; Li, T.; Shen, T.; Li, J.Q.; Li, W.Y.; Liu, H.G. Mycology, cultivation, traditional uses, phytochemistry and pharmacology of Wolfiporia cocos (Schwein.) Ryvarden et Gilb.: A review. J. Ethnopharmacol. 2013, 147, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Sim, H.A.; Jung, D.Y.; Lim, E.Y.; Kim, Y.T.; Kim, B.J.; Jung, M.H. Poria cocus Wolf extract ameliorates hepatic steatosis through regulation of lipid metabolism, inhibition of ER stress, and activation of autophagy via AMPK activation. Int. J. Mol. Sci. 2019, 20, 4801. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, Y.; Zhang, F.; Li, Y.; Li, X.; Pu, X.; He, X.; Zhang, M.; Yang, X.; Yu, Q.; et al. Effects of Poria cocos extract on metabolic dysfunction-associated fatty liver disease via the FXR/PPARα-SREBPs pathway. Front. Pharmacol. 2022, 13, 1007274. [Google Scholar] [CrossRef]
- Ye, H.; Ma, S.; Qiu, Z.; Huang, S.; Deng, G.; Li, Y.; Xu, S.; Yang, M.; Shi, H.; Wu, C.; et al. Poria cocos polysaccharides rescue pyroptosis-driven gut vascular barrier disruption in order to alleviates non-alcoholic steatohepatitis. J. Ethnopharmacol. 2022, 296, 115457. [Google Scholar] [CrossRef]
- Duan, Y.; Huang, J.; Sun, M.; Jiang, Y.; Wang, S.; Wang, L.; Yu, N.; Peng, D.; Wang, Y.; Chen, W.; et al. Poria cocos polysaccharide improves intestinal barrier function and maintains intestinal homeostasis in mice. Int. J. Biol. Macromol. 2023, 249, 125953. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.; Yang, H.; He, J.; Yang, Y.; Tao, Y.; Zhang, M.; Zhang, M.; Yu, J.; Yang, X. In vivo identification of bioactive components of Poria cocos for adjusting mitochondria against metabolic dysfunction-associated fatty liver disease. Heliyon 2024, 10, e35645. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, D.; Huang, F.; Zhao, A.; Kuang, J.; Ren, Z.; Chen, T.; Lei, J.; Lin, J.; Wang, X.; et al. Theabrownin and Poria cocos polysaccharide improve lipid metabolism via modulation of bile acid and fatty acid metabolism. Front. Pharmacol. 2022, 13, 875549. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Urzì, O.; Gasparro, R.; Ganji, N.R.; Alessandro, R.; Raimondo, S. Plant-RNA in Extracellular Vesicles: The Secret of Cross-Kingdom Communication. Membranes 2022, 12, 352. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Cui, M.; Fang, Z.; Liu, K. Advanced research on extracellular vesicles based oral drug delivery systems. J. Control Release 2022, 351, 560–572. [Google Scholar] [CrossRef]
- Alfieri, M.; Leone, A.; Ambrosone, A. Plant-Derived Nano and Microvesicles for Human Health and Therapeutic Potential in Nanomedicine. Pharmaceutics 2021, 13, 498. [Google Scholar] [CrossRef]
- Urzì, O.; Raimondo, S.; Alessandro, R. Extracellular Vesicles from Plants: Current Knowledge and Open Questions. Int. J. Mol. Sci. 2021, 22, 5366. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.W.; Zhu, A.Q.; Huang, L.Q.; Peng, L.H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X.; et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. Immunother. Cancer. 2019, 7, 326. [Google Scholar] [CrossRef]
- Zhang, L.; He, F.; Gao, L.; Cong, M.; Sun, J.; Xu, J.; Wang, Y.; Hu, Y.; Asghar, S.; Hu, L.; et al. Engineering Exosome-Like Nanovesicles Derived from Asparagus cochinchinensis Can Inhibit the Proliferation of Hepatocellular Carcinoma Cells with Better Safety Profile. Int. J. Nanomed. 2021, 16, 1575–1586. [Google Scholar] [CrossRef]
- Ou, X.; Wang, H.; Tie, H.; Liao, J.; Luo, Y.; Huang, W.; Yu, R.; Song, L.; Zhu, J. Novel plant-derived exosome-like nanovesicles from Catharanthus roseus: Preparation, characterization, and immunostimulatory effect via TNF-α/NF-κB/PU.1 axis. J. Nanobiotechnol. 2023, 21, 160. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Xu, H.M.; Liang, Y.J.; Xu, J.; Yue, N.N.; Zhang, Y.; Tian, C.M.; Yao, J.; Wang, L.S.; Nie, Y.Q.; et al. Edible exosome-like nanoparticles from portulaca oleracea L mitigate DSS-induced colitis via facilitating double-positive CD4+CD8+ T cells expansion. J. Nanobiotechnol. 2023, 21, 309. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ruan, Y.; Zhu, X.; Lin, X.; Xin, Y.; Li, X.; Mai, M.; Guo, H. Deoxycholic Acid Promotes Pyroptosis in Free Fatty Acid-Induced Steatotic Hepatocytes by Inhibiting PINK1-Mediated Mitophagy. Inflammation 2022, 45, 639–650. [Google Scholar] [CrossRef]
- Hallal, S.; Tűzesi, Á.; Grau, G.E.; Buckland, M.E.; Alexander, K.L. Understanding the extracellular vesicle surface for clinical molecular biology. J. Extracell. Vesicles 2022, 11, e12260. [Google Scholar] [CrossRef]
- Ghadami, S.; Dellinger, K. The lipid composition of extracellular vesicles: Applications in diagnostics and therapeutic delivery. Front. Mol. Biosci. 2023, 10, 1198044. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Cai, R.; Zeng, H.; Zhu, H.; Dou, Y.; Sun, S. Exosome may be the next generation of promising cell-free vaccines. Hum. Vaccin. Immunother. 2024, 20, 2345940. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Commun. Signal 2022, 20, 145. [Google Scholar] [CrossRef]
- Wang, H.; Mu, J.; Chen, Y.; Liu, Y.; Li, X.; Li, H.; Cao, P. Hybrid Ginseng-derived Extracellular Vesicles-Like Particles with Autologous Tumor Cell Membrane for Personalized Vaccination to Inhibit Tumor Recurrence and Metastasis. Adv. Sci. 2024, 11, e2308235. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, F.; Wang, W.; Zhang, X.J.; Ji, Y.X.; Zhang, P.; She, Z.G.; Zhu, L.; Cai, J.; Li, H. Epidemiological Features of NAFLD From 1999 to 2018 in China. Hepatology 2022, 71, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Kowdley, K.V.; Lacaille, F.; Loomba, R.; Mitchell-Thain, R.; Morgan, T.R.; Powell, E.E.; Roden, M.; Romero-Gómez, M.; Silva, M.; Singh, S.P.; et al. NAFLD Nomenclature consensus group, A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Benedé-Ubieto, R.; Cubero, F.J.; Nevzorova, Y.A. Breaking the barriers: The role of gut homeostasis in Metabolic-Associated Steatotic Liver Disease (MASLD). Gut Microbes 2024, 16, 2331460. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Pan, M.; Zheng, C.; Shen, H.; Pi, D.; Song, Q.; Liang, Z.; Zhen, J.; Pan, J.; Liu, L.; et al. Leonurine Inhibits Hepatic Lipid Synthesis to Ameliorate NAFLD via the ADRA1a/AMPK/SCD1 Axis. Int. J. Mol. Sci. 2024, 25, 10855. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, Y.; Zhan, B.; Wang, Y.; Cheng, J.; Yu, H.; Lv, M.; Zhang, Y.; Zhai, Y.; Guan, Y.; et al. Asiaticoside alleviated NAFLD by activating Nrf2 and inhibiting the NF-κB pathway. Phytomedicine 2025, 136, 156317. [Google Scholar] [CrossRef]
- Mei, Y.; Hu, H.; Deng, L.; Sun, X.; Tan, W. Therapeutic effects of isosteviol sodium on non-alcoholic fatty liver disease by regulating autophagy via Sirt1/AMPK pathway. Sci. Rep. 2022, 12, 12857. [Google Scholar] [CrossRef]
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef]
- Ramanathan, R.; Ali, A.H.; Ibdah, J.A. Mitochondrial Dysfunction Plays Central Role in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 7280. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wu, X.; Liao, R. Mechanism and regulation of mitophagy in nonalcoholic fatty liver disease (NAFLD): A mini-review. Life Sci. 2023, 312, 121162. [Google Scholar] [CrossRef] [PubMed]
- Xian, H.; Watari, K.; Sanchez-Lopez, E.; Offenberger, J.; Onyuru, J.; Sampath, H.; Ying, W.; Hoffman, H.M.; Shadel, G.S.; Karin, M. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 2022, 55, 1370–1385. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Faitg, J.; Auwerx, J.; Ferrucci, L.; D’Amico, D. Mitophagy in human health, ageing and disease. Nat. Metab. 2023, 5, 2047–2061. [Google Scholar] [CrossRef]
- Lueangarun, S.; Cho, B.S.; Tempark, T. Hair repigmentation of poliosis circumscripta in androgenetic alopecia patient treated with exosomes and fractional picosecond laser. J. Cosmet. Dermatol. 2024, 23, 2307–2311. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Ko, H.J.; Kim, K.; Sohn, Y.; Min, S.Y.; Kim, J.A.; Na, D.; Yeon, J.H. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J. Extracell. Vesicles 2019, 9, 1703480. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Gao, Y.; Chen, H.; Yin, Y.; Zhang, W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients 2019, 11, 2062. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Zhao, J.; Li, Q.-Y.; Yang, H.-Q.; Li, M.; Duan, R.; Zhang, M.; Qi, Y.; Yu, J.; Yang, X.-X. Poria cocos-Derived Exosome-like Nanovesicles Alleviate Metabolic Dysfunction-Associated Fatty Liver Disease by Promoting Mitophagy and Inhibiting NLRP3 Inflammasome Activation. Int. J. Mol. Sci. 2025, 26, 2253. https://doi.org/10.3390/ijms26052253
Wang T, Zhao J, Li Q-Y, Yang H-Q, Li M, Duan R, Zhang M, Qi Y, Yu J, Yang X-X. Poria cocos-Derived Exosome-like Nanovesicles Alleviate Metabolic Dysfunction-Associated Fatty Liver Disease by Promoting Mitophagy and Inhibiting NLRP3 Inflammasome Activation. International Journal of Molecular Sciences. 2025; 26(5):2253. https://doi.org/10.3390/ijms26052253
Chicago/Turabian StyleWang, Tao, Jun Zhao, Qiu-Yi Li, Hui-Qiong Yang, Min Li, Rong Duan, Mei Zhang, Yan Qi, Jie Yu, and Xing-Xin Yang. 2025. "Poria cocos-Derived Exosome-like Nanovesicles Alleviate Metabolic Dysfunction-Associated Fatty Liver Disease by Promoting Mitophagy and Inhibiting NLRP3 Inflammasome Activation" International Journal of Molecular Sciences 26, no. 5: 2253. https://doi.org/10.3390/ijms26052253
APA StyleWang, T., Zhao, J., Li, Q.-Y., Yang, H.-Q., Li, M., Duan, R., Zhang, M., Qi, Y., Yu, J., & Yang, X.-X. (2025). Poria cocos-Derived Exosome-like Nanovesicles Alleviate Metabolic Dysfunction-Associated Fatty Liver Disease by Promoting Mitophagy and Inhibiting NLRP3 Inflammasome Activation. International Journal of Molecular Sciences, 26(5), 2253. https://doi.org/10.3390/ijms26052253






