Blood Biomarkers Reflect Dementia Symptoms and Are Influenced by Cerebrovascular Lesions
Abstract
1. Introduction
2. Results
2.1. Background of All Patients
2.2. Correlation Between Blood Biomarker Level and Clinical Diagnosis
2.3. Association Between Plasma Aβ Level and Amyloid PET
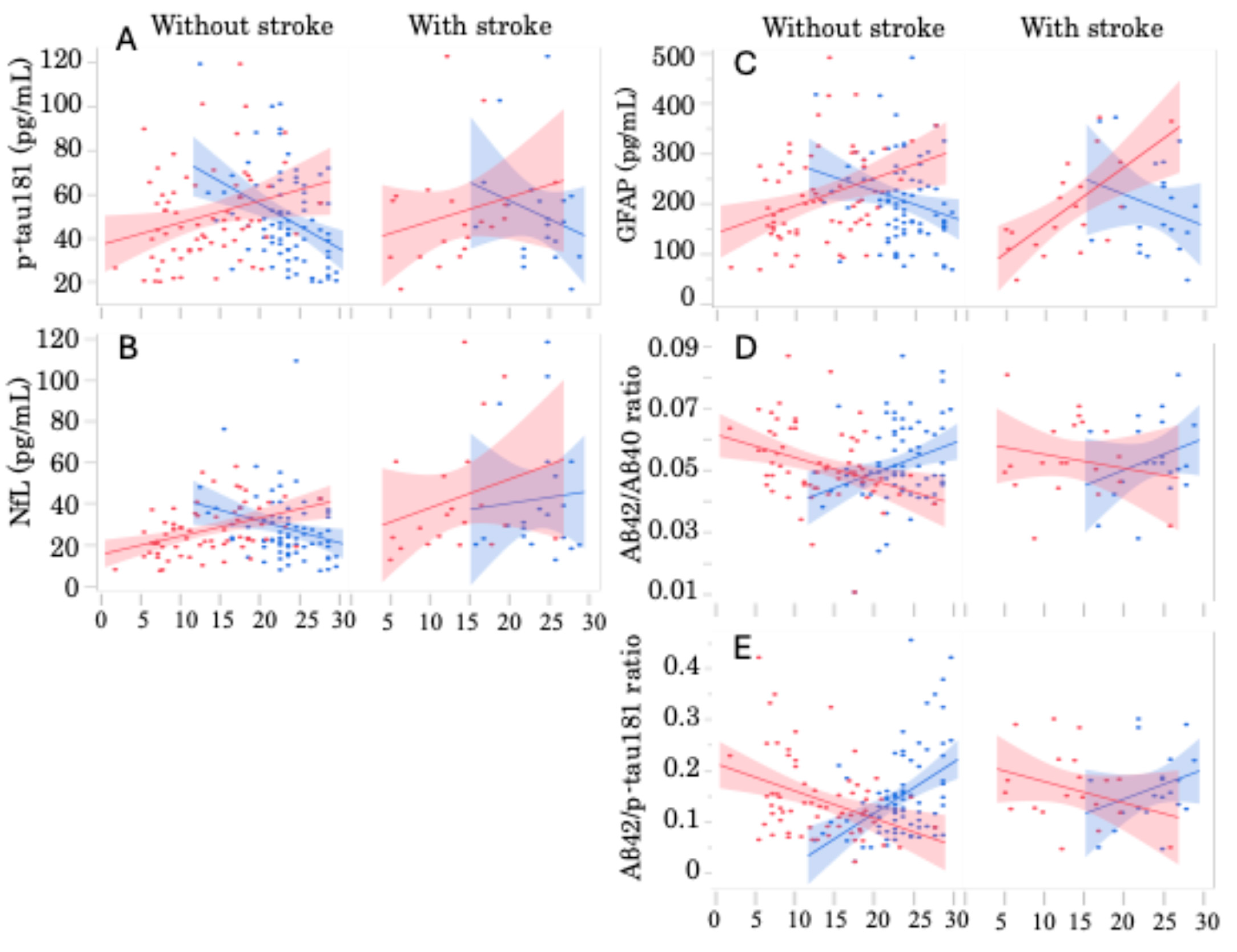
2.4. Association of Plasma Biomarker Level and Severity of Cognitive Impairment
2.5. Correlation Between Plasma Biomarkers and Cognitive Decline by Multivariate Analysis
2.6. Correlation Between Plasma Biomarkers and Neuropsychological Symptoms Using Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Biomarker Measurement
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-beta |
| AD | Alzheimer’s disease |
| ADD | AD type dementia |
| ADAS | Alzheimer’s dementia assessment scale |
| aMCI | Amnestic mild cognitive impairment |
| AUC | Area under curve |
| BMI | Body mass index |
| DM | Diabetes mellitus |
| GFAP | Glial fibrillary acidic protein |
| HL | Hyperlipidemia |
| HT | Hypertension |
| LDL | Low-density lipoprotein |
| MMSE | Mini-mental state examination |
| MRI | Magnetic resonance imaging |
| NfL | Neurofilament light chain |
| PET | Positron emission tomography |
| p-tau | Phosphorylated tau |
| ROC | Receiver operating characteristic |
| SCI | Subjective cognitive impairment |
| CSF | Cerebrospinal fluid |
References
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Koyama, A.; Okereke, O.I.; Yang, T.; Blacker, D.; Selkoe, D.J.; Grodstein, F. Plasma amyloid-beta as a predictor of dementia and cognitive decline: A systematic review and meta-analysis. Arch. Neurol. 2012, 69, 824–831. [Google Scholar] [CrossRef]
- de Wolf, F.; Ghanbari, M.; Licher, S.; McRae-McKee, K.; Gras, L.; Weverling, G.J.; Wermeling, P.; Sedaghat, S.; Ikram, M.K.; Waziry, R.; et al. Plasma tau, neurofilament light chain and amyloid-beta levels and risk of dementia; a population-based cohort study. Brain 2020, 143, 1220–1232. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.N.; Huang, Y.Y.; Chen, S.D.; Guo, Y.; Tan, L.; Dong, Q.; Yu, J.T.; Alzheimer’s Disease Neuroimaging Initiative. Plasma phosphorylated-tau181 as a predictive biomarker for Alzheimer’s amyloid, tau and FDG PET status. Transl. Psychiatry 2021, 11, 585. [Google Scholar] [CrossRef]
- Brickman, A.M.; Manly, J.J.; Honig, L.S.; Sanchez, D.; Reyes-Dumeyer, D.; Lantigua, R.A.; Lao, P.J.; Stern, Y.; Vonsattel, J.P.; Teich, A.F.; et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 2021, 17, 1353–1364. [Google Scholar] [CrossRef]
- Ebenau, J.L.; Timmers, T.; Wesselman, L.M.P.; Verberk, I.M.W.; Verfaillie, S.C.J.; Slot, R.E.R.; van Harten, A.C.; Teunissen, C.E.; Barkhof, F.; van den Bosch, K.A.; et al. ATN classification and clinical progression in subjective cognitive decline: The SCIENCe project. Neurology 2020, 95, e46–e58. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H.; et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020, 26, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M.; Goldman, S.A. Glymphatic failure as a final common pathway to dementia. Science 2020, 370, 50–56. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef]
- Mok, V.C.T.; Cai, Y.; Markus, H.S. Vascular cognitive impairment and dementia: Mechanisms, treatment, and future directions. Int. J. Stroke 2024, 19, 838–856. [Google Scholar] [CrossRef]
- Ferri, C.; Croce, G.; Cofini, V.; De Berardinis, G.; Grassi, D.; Casale, R.; Properzi, G.; Desideri, G. C-reactive protein: Interaction with the vascular endothelium and possible role in human atherosclerosis. Curr. Pharm. Des. 2007, 13, 1631–1645. [Google Scholar] [CrossRef] [PubMed]
- Dettori, P.; Paliogiannis, P.; Pascale, R.M.; Zinellu, A.; Mangoni, A.A.; Pintus, G. Blood Cell Count Indexes of Systemic Inflammation in Carotid Artery Disease: Current Evidence and Future Perspectives. Curr. Pharm. Des. 2021, 27, 2170–2179. [Google Scholar] [CrossRef] [PubMed]
- Tatebe, H.; Kasai, T.; Ohmichi, T.; Kishi, Y.; Kakeya, T.; Waragai, M.; Kondo, M.; Allsop, D.; Tokuda, T. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: Pilot case-control studies including patients with Alzheimer’s disease and down syndrome. Mol. Neurodegener. 2017, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Pike, J.R.; Chen, J.; Walker, K.A.; Sullivan, K.J.; Thyagarajan, B.; Mielke, M.M.; Lutsey, P.L.; Knopman, D.; Gottesman, R.F.; et al. Changes in Alzheimer disease blood biomarkers and associations with incident all-cause dementia. JAMA 2024, 332, 1258–1269. [Google Scholar] [CrossRef]
- Ohara, T.; Tatebe, H.; Hata, J.; Honda, T.; Shibata, M.; Matsuura, S.; Mikami, T.; Maeda, T.; Ono, K.; Mimura, M.; et al. Plasma biomarkers for predicting the development of dementia in a community-dwelling older Japanese population. Psychiat Clin. Neurosci. 2024, 78, 362–371. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease is a synaptic failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef]
- Hamelin, L.; Lagarde, J.; Dorothée, G.; Potier, M.C.; Corlier, F.; Kuhnast, B.; Caillé, F.; Dubois, B.; Fillon, L.; Chupin, M.; et al. Distinct dynamic profiles of microglial activation are associated with progression of Alzheimer’s disease. Brain 2018, 141, 1855–1870. [Google Scholar] [CrossRef]
- Rabe, C.; Bittner, T.; Jethwa, A.; Suridjan, I.; Manuilove, E.; Friesenhahn, M.; Stomurud, E.; Zetterberg, H.; Blennow, K.; Hansson, O.; et al. Clinical performance and robustness evaluation of plasma amyloid-β42/40 prescreening. Alzheimers Dement. 2023, 19, 1393–1402. [Google Scholar] [CrossRef]
- Filley, C.M.; Fields, R.D. White matter and cognition: Making the connection. J. Neurophysiol. 2016, 116, 2093–2104. [Google Scholar] [CrossRef]
- Shizadi, Z.; Schultz, S.A.; Yau, W.Y.W.; Mathurin, N.J.; Fitzpatrick, C.D.; Levin, R.; Kantarci, K.; Preboske, G.M.; Jack, C.R.; Farlow, M.R.; et al. Etiology of white matter hyperintensities in autosomal dominant and sporadic Alzheimer disease. JAMA Neurol. 2023, 80, 1353–1363. [Google Scholar] [CrossRef]
- Bilello, M.; Doshi, J.; Nabavizadeh, A.; Toledo, J.B.; Erus, G.; Xie, S.X.; Trojanowski, J.Q.; Han, X.; Davatzikos, C. Correlating cognitive decline with white matter lesion and brain atrophy MRI measurements in Alzheimer’s disease. J. Alzheimers Dis. 2015, 48, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Osborn, K.E.; Liu, D.; Samuels, L.R.; Moore, E.E.; Cambronero, F.E.; Acosta, L.M.Y.; Bell, S.P.; Babicz, M.A.; Gordon, E.A.; Pechman, K.R.; et al. Cerebrospinal fluid beta-amyloid42 and neurofilament light relate to white matter hyperintensities. Neurobiol. Aging 2018, 68, 18–25. [Google Scholar] [CrossRef]
- Takeishi, J.; Tatewaki, Y.; Nakase, T.; Takano, Y.; Tomita, N.; Yamamoto, S.; Mutoh, T.; Taki, Y. Alzheimer’s disease and type 2 diabetes mellitus: The se of MCT oil and a ketogenic diet. Int. J. Mol. Sci. 2021, 22, 12310. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomarker Insights 2016, 11, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Nakase, T.; Thyreau, B.; Tatewaki, Y.; Tomita, N.; Takano, Y.; Muranaka, M.; Taki, Y. Association between gray and white matter lesions and its involvement in clinical symptoms of Alzheimer’s-type dementia. J. Clin. Med. 2023, 12, 7642. [Google Scholar] [CrossRef]
- Lamar, M.; Boots, E.A.; Arfanakis, K.; Barnes, L.L.; Schneider, J.A. Common brain structural alterations associated with cardiovascular disease risk factors and Alzheimer’s dementia: Future directions and implications. Neuropsychol. Rev. 2020, 30, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, T.; Zhang, Y.; Zhou, J.; Qi, B.; Wang, X.; Chen, Z.; Li, P. Aging neurovascular unit and potential role of DNA damage and repair in combating vascular and neurodegenerative disorders. Front. Neurosci. 2019, 13, 778. [Google Scholar] [CrossRef]
- Pluta, R.; Miziak, B.; Czuczwar, S.J. Post-ischemic permeability of the blood-brain barrier to amyloid and platelets as a factor in the maturation of Alzheimer’s disease-type brain neurodegeneration. Int. J. Mol. Sci. 2023, 24, 10739. [Google Scholar] [CrossRef]
- Wilson, D.H.; Rissin, D.M.; Kan, C.W.; Fournier, D.R.; Piech, T.; Campbell, T.G.; Meyer, R.E.; Fishburn, M.W.; Cabrera, C.; Patel, P.P.; et al. The Simoa HD-1 Analyzer: A Novel Fully Automated Digital Immunoassay Analyzer with Single-Molecule Sensitivity and Multiplexing. J. Lab. Autom. 2016, 21, 533–547. [Google Scholar] [CrossRef]


| Total | ADD | aMCI | SCI | Depression | p | |
|---|---|---|---|---|---|---|
| n | 111 | 45 | 56 | 4 | 8 | |
| Female (%) | 62.2% | 71.1% | 53.7% | 50.0% | 75.0% | 0.2629 |
| Age (ave. ± SD years-old) | 74.5 ± 9.1 | 74.8 ± 10.3 | 75.6 ± 7.0 | 68.5 ± 1.7 | 67.9 ± 13.1 | 0.0758 |
| MMSE | 23.8 ± 4.4 | 21.6 ± 4.6 | 24.6 ± 3.3 | 29.5 ± 0.6 | 28.6 ± 2.2 | <0.0001 |
| ADAS | 13.6 ± 5.8 | 16.7 ± 5.7 | 12.0 ± 5.0 | 6.8 ± 1.1 | 7.6 ± 3.3 | <0.0001 |
| HT (%) | 55.9% | 53.3% | 57.4% | 0.0% | 87.5% | 0.0371 |
| HL (%) | 45.0% | 42.2% | 44.4% | 50.0% | 62.5% | 0.7585 |
| LDL (ave. ± SD mg/L) | 109.6 ± 37.2 | 103.3 ± 35.9 | 117.1 ± 5.3 | 95.0 ± 23.8 | 101.1 ± 26.1 | 0.2600 |
| DM (%) | 20.7% | 20.0% | 22.2% | 0.0% | 25.0% | 0.7475 |
| HbA1c (ave. ± SD %) | 6.0 ± 0.8 | 6.1 ± 1.0 | 6.0 ± 0.8 | 5.9 ± 0.3 | 5.7 ± 0.2 | 0.6056 |
| Smoking (%) | 20.7% | 22.2% | 20.4% | 0.0% | 25.0% | 0.7529 |
| BMI | 22.4 ± 3.4 | 22.0 ± 3.7 | 22.3 ± 2.9 | 22.2 ± 2.0 | 24.6 ± 5.2 | 0.3056 |
| Stroke (n, %) | 20, 18.0% | 7, 13.3% | 12, 25.9% | 1, 25.0% | 0, 0.0% | 0.1332 |
| Subtype: embolic (n) | 1 | 0 | 1 | 0 | 0 | na |
| atherosclerotic (n) | 4 | 2 | 2 (1) | 0 | 0 | na |
| lacunar (n) | 11 | 4 (1) | 7 (3) | 0 | 0 | na |
| Hemorrhage (n) | 4 | 1 | 2 | 1 | 0 | na |
| Duration (ave. ± SD years) | 6.5 ± 3.2 | 7.3 ± 3.5 | 6.0 ± 3.1 | 5.0 | 0 | 0.4271 |
| ave. ± SD | ADD | aMCI | SCI | Depression | p |
|---|---|---|---|---|---|
| Aβ40 (pg/mL) | 123.4 ± 23.9 | 122.6 ± 24.1 | 110.8 ± 14.3 | 102.2 ± 20.6 | 0.0942 |
| Aβ42 (pg/mL) | 6.12 ± 2.12 | 6.45 ± 1.72 | 7.91 ± 1.19 | 6.60 ± 2.14 | 0.3166 |
| p-tau181 (pg/mL) | 55.6 ± 19.2 | 45.6 ± 21.7 | 31.3 ± 16.6 | 32.5 ± 13.6 | 0.0032 |
| NfL (pg/mL) | 37.5 ± 25.0 | 30.7 ± 16.7 | 16.5 ± 44.9 | 17.7 ± 9.5 | 0.0216 |
| GFAP (pg/mL) | 227.6 ± 78.9 | 195.5 ± 89.2 | 125.0 ± 55.8 | 94.0 ± 32.8 | 0.0002 |
| Aβ42/Aβ40 | 0.049 ± 0.013 | 0.053 ± 0.013 | 0.071 ± 0.005 | 0.064 ± 0.017 | 0.0007 |
| Aβ42/p-tau181 | 0.121 ± 0.056 | 0.170 ± 0.088 | 0.299 ± 0.130 | 0.239 ± 0.111 | <0.0001 |
| MMSE | ADAS | |||
|---|---|---|---|---|
| r | p | r | p | |
| p-tau181 | −0.3485 | 0.0004 | 0.2561 | 0.0167 |
| NfL | −0.1416 | 0.1600 | 0.2738 | 0.0103 |
| GFAP | −0.2403 | 0.0160 | 0.3936 | 0.0002 |
| Aβ42/Aβ40 | 0.2907 | 0.0033 | −0.3015 | 0.0045 |
| Aβ42/p-tau181 | 0.4159 | <0.0001 | −0.3335 | 0.0016 |
| Without Stroke | With Stroke | |||||||
|---|---|---|---|---|---|---|---|---|
| MMSE | ADAS | MMSE | ADAS | |||||
| r | p | r | p | r | p | r | p | |
| p-tau181 | −0.3801 | 0.0005 | 0.2288 | 0.1139 | −0.2460 | 0.2957 | 0.2401 | 0.3080 |
| NfL | −0.2625 | 0.0186 | 0.4988 | 0.0003 | 0.0721 | 0.7626 | 0.2540 | 0.2799 |
| GFAP | −0.2382 | 0.0334 | 0.2646 | 0.0662 | −0.2475 | 0.2928 | 0.6749 | 0.0011 |
| Aβ42/Aβ40 | 0.2906 | 0.0089 | −0.2609 | 0.0702 | 0.2894 | 0.2159 | −0.1898 | 0.4227 |
| Aβ42/p-tau181 | 0.4399 | <0.0001 | −0.2289 | 0.1136 | 0.2932 | 0.2096 | −0.3117 | 0.1809 |
| Depression | Irritability | Hallucinations | Delusions | Apathy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | 28 | 18 | 7 | 12 | 27 | |||||
| r | p | r | p | r | p | r | p | r | p | |
| p-tau181 | −0.0045 | 0.9644 | 0.0547 | 0.5891 | 0.0902 | 0.3722 | 0.0323 | 0.7493 | 0.0726 | 0.4726 |
| NfL | −0.0084 | 0.9343 | 0.1513 | 0.1328 | 0.2204 | 0.0275 | 0.3159 | 0.0014 | 0.0627 | 0.5352 |
| GFAP | −0.0182 | 0.8575 | −0.0617 | 0.5421 | 0.0060 | 0.9526 | 0.0057 | 0.9551 | 0.0381 | 0.7066 |
| Aβ42/Aβ40 | 0.2049 | 0.0409 | −0.0647 | 0.5225 | 0.1443 | 0.1521 | 0.1386 | 0.1689 | −0.0813 | 0.4215 |
| Aβ42/p-tau181 | 0.0096 | 0.9242 | −0.1118 | 0.2679 | 0.0149 | 0.8830 | −0.0085 | 0.9333 | 0.0053 | 0.9581 |
| Without Stroke Lesions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Depression | Irritability | Hallucinations | Delusions | Apathy | ||||||
| n | 23 | 13 | 4 | 9 | 22 | |||||
| r | p | r | p | r | p | r | p | r | p | |
| p-tau181 | 0.0393 | 0.7290 | 0.0668 | 0.5561 | 0.1349 | 0.2328 | 0.0661 | 0.5601 | 0.1692 | 0.1336 |
| NfL | −0.0103 | 0.9278 | 0.1384 | 0.2209 | 0.2079 | 0.0642 | 0.2067 | 0.0659 | 0.0530 | 0.6406 |
| GFAP | 0.0076 | 0.9470 | −0.0160 | 0.8881 | −0.0473 | 0.6767 | −0.0160 | 0.8880 | 0.0395 | 0.7277 |
| Aβ42/Aβ40 | 0.2294 | 0.0407 | 0.0082 | 0.9421 | 0.1633 | 0.1477 | 0.0931 | 0.4114 | −0.0445 | 0.6950 |
| Aβ42/p-tau181 | −0.0181 | 0.8733 | −0.0904 | 0.4252 | −0.0084 | 0.9407 | −0.0400 | 0.7248 | −0.0680 | 0.5489 |
| With stroke lesions | ||||||||||
| n | 5 | 5 | 3 | 3 | 5 | |||||
| p-tau181 | −0.1510 | 0.5251 | 0.0074 | 0.9753 | −0.0131 | 0.9563 | −0.0750 | 0.7534 | −0.2399 | 0.3082 |
| NfL | −0.0289 | 0.9039 | 0.1149 | 0.6296 | 0.1579 | 0.5061 | 0.5492 | 0.0121 | 0.0644 | 0.7875 |
| GFAP | −0.1072 | 0.6528 | −0.1832 | 0.4393 | 0.1629 | 0.4925 | 0.0912 | 0.7022 | 0.0429 | 0.8574 |
| Aβ42/Aβ40 | 0.1051 | 0.6591 | −0.3341 | 0.1499 | 0.0901 | 0.7056 | 0.2941 | 0.2081 | −0.2313 | 0.3264 |
| Aβ42/p-tau181 | 0.1318 | 0.5795 | −0.2542 | 0.2794 | 0.0378 | 0.8744 | 0.1095 | 0.6459 | 0.3323 | 0.1523 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakase, T.; Tatewaki, Y.; Takano, Y.; Nomura, S.; Baek, H.W.; Taki, Y. Blood Biomarkers Reflect Dementia Symptoms and Are Influenced by Cerebrovascular Lesions. Int. J. Mol. Sci. 2025, 26, 2325. https://doi.org/10.3390/ijms26052325
Nakase T, Tatewaki Y, Takano Y, Nomura S, Baek HW, Taki Y. Blood Biomarkers Reflect Dementia Symptoms and Are Influenced by Cerebrovascular Lesions. International Journal of Molecular Sciences. 2025; 26(5):2325. https://doi.org/10.3390/ijms26052325
Chicago/Turabian StyleNakase, Taizen, Yasuko Tatewaki, Yumi Takano, Shuko Nomura, Hae Woon Baek, and Yasuyuki Taki. 2025. "Blood Biomarkers Reflect Dementia Symptoms and Are Influenced by Cerebrovascular Lesions" International Journal of Molecular Sciences 26, no. 5: 2325. https://doi.org/10.3390/ijms26052325
APA StyleNakase, T., Tatewaki, Y., Takano, Y., Nomura, S., Baek, H. W., & Taki, Y. (2025). Blood Biomarkers Reflect Dementia Symptoms and Are Influenced by Cerebrovascular Lesions. International Journal of Molecular Sciences, 26(5), 2325. https://doi.org/10.3390/ijms26052325







