Fighting Bleb Fibrosis After Glaucoma Surgery: Updated Focus on Key Players and Novel Targets for Therapy
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
4.1. Main Features of Extra-Ocular Fibrosis
4.1.1. Skin Fibrosis
Pathogenesis: Early Phase
Pathogenesis: Late Phase
Anti-Skin Fibrosis Treatments
Key Features and Therapy Highlights
4.1.2. Idiopathic Pulmonary Fibrosis
Pathogenesis
Anti-IPF Treatments
Key Features and Therapy Highlights
4.2. Main Features of Post-GFS Fibrosis
4.2.1. Existing Model of Filtration Bleb Fibrosis
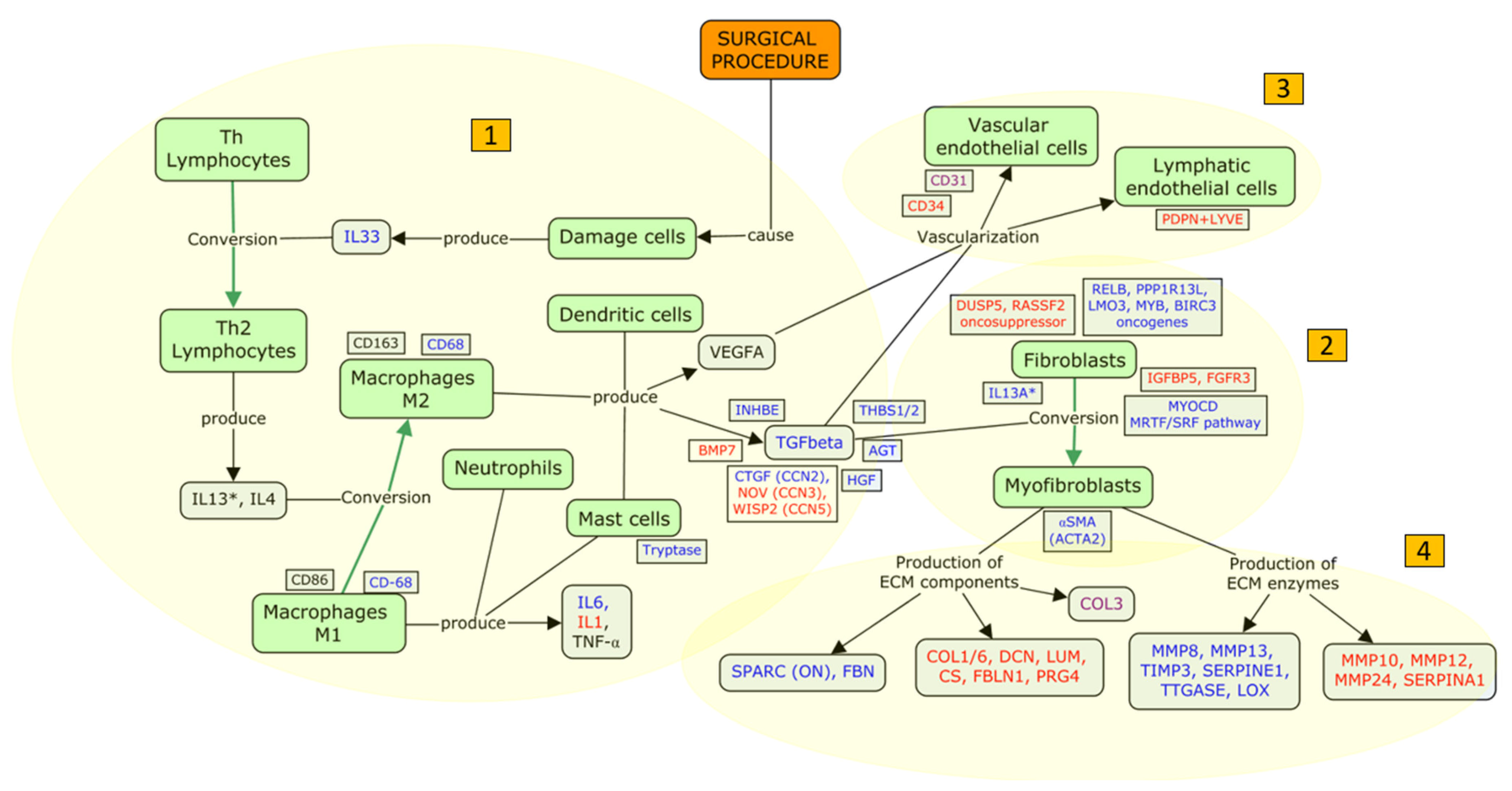
4.2.2. An Updated Model of FB Fibrosis
- (i)
- Inflammation: humoral and cellular response
- (ii)
- Proliferation of fibroblasts and myofibroblast conversion
- (iii)
- Filtration bleb vascularization
- (iv)
- Tissue remodeling
4.3. Focus Update and Proposed Targets
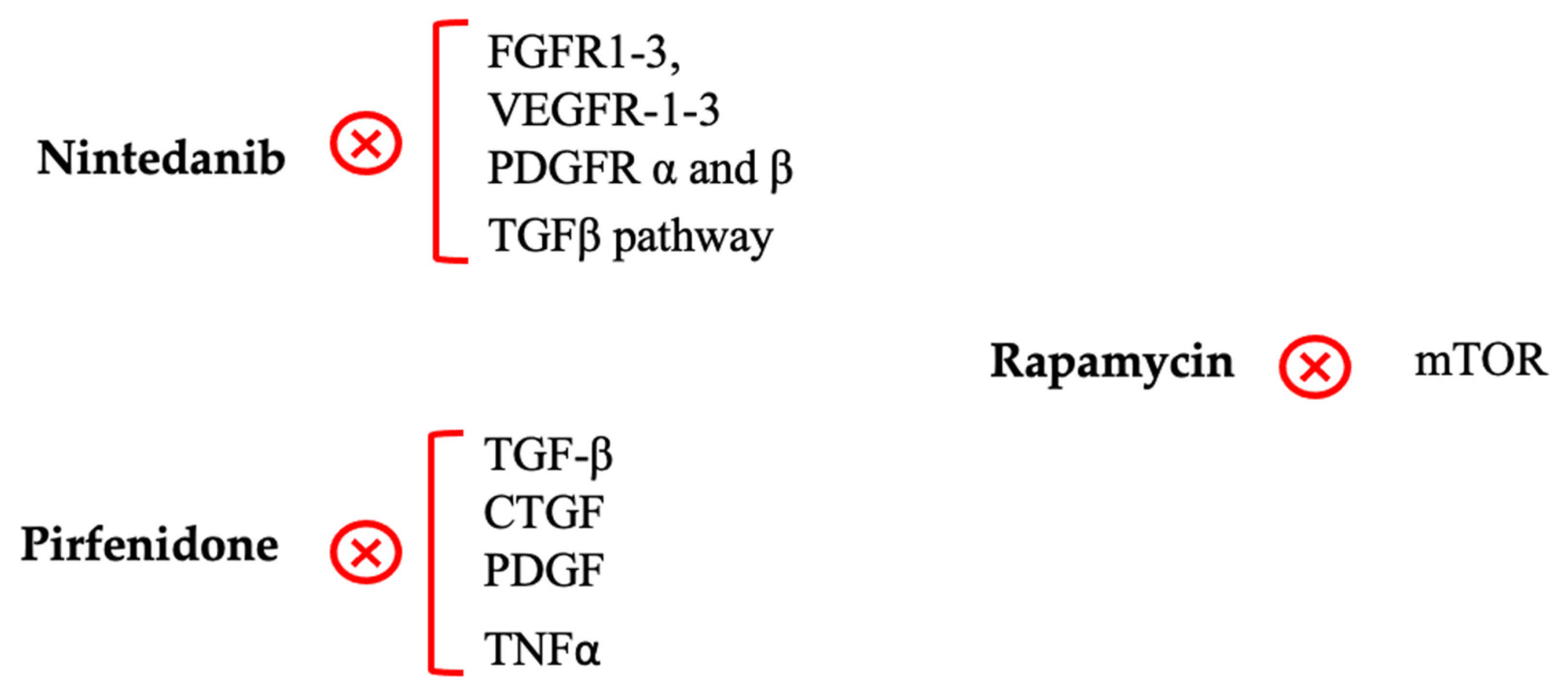
4.4. New Investigated Treatments to Fight Post-GFS Fibrosis
4.4.1. Nintedanib
4.4.2. Pirfenidone
4.4.3. Rapamycin
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, Y.; Chen, A.; Zou, M.; Zhang, Y.; Jin, L.; Li, Y.; Zheng, D.; Jin, G.; Congdon, N. Time Trends, Associations and Prevalence of Blindness and Vision Loss Due to Glaucoma: An Analysis of Observational Data from the Global Burden of Disease Study 2017. BMJ Open 2022, 12, e053805. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, A.; Haukka, J.; Loukovaara, S.; Harju, M. Incidence of Glaucoma Filtration Surgery from Disease Onset of Open-Angle Glaucoma. Acta Ophthalmol. 2024, 102, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, M.; Agnifili, L.; Brescia, L.; Oddone, F.; Villani, E.; Nucci, P.; Mastropasqua, L. Structural Imaging of Conjunctival Filtering Blebs in XEN Gel Implantation and Trabeculectomy: A Confocal and Anterior Segment Optical Coherence Tomography Study. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Fontana, H.; Nouri-Mahdavi, K.; Lumba, J.; Ralli, M.; Caprioli, J. Trabeculectomy with Mitomycin C: Outcomes and Risk Factors for Failure in Phakic Open-Angle Glaucoma. Ophthalmology 2006, 113, 930–936. [Google Scholar] [CrossRef]
- Mastropasqua, R.; Fasanella, V.; Brescia, L.; Oddone, F.; Mariotti, C.; Di Staso, S.; Agnifili, L. In Vivo Confocal Imaging of the Conjunctiva as a Predictive Tool for the Glaucoma Filtration Surgery Outcome. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO114–BIO120. [Google Scholar] [CrossRef]
- Atabai, K.; Yang, C.D.; Podolsky, M.J. You Say You Want a Resolution (of Fibrosis). Am. J. Respir. Cell Mol. Biol. 2020, 63, 424–435. [Google Scholar] [CrossRef]
- Jun, J.-I.; Lau, L.F. Resolution of Organ Fibrosis. J. Clin. Investig. 2018, 128, 97–107. [Google Scholar] [CrossRef]
- Hinz, B.; Lagares, D. Evasion of Apoptosis by Myofibroblasts: A Hallmark of Fibrotic Diseases. Nat. Rev. Rheumatol. 2020, 16, 11–31. [Google Scholar] [CrossRef]
- Wynn, T.A. Common and Unique Mechanisms Regulate Fibrosis in Various Fibroproliferative Diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and Molecular Mechanisms of Fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Khalil, H.; Kanisicak, O.; Prasad, V.; Correll, R.N.; Fu, X.; Schips, T.; Vagnozzi, R.J.; Liu, R.; Huynh, T.; Lee, S.-J.; et al. Fibroblast-Specific TGFβ-Smad2/3 Signaling Underlies Cardiac Fibrosis. J. Clin. Investig. 2017, 127, 3770–3783. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGFβ and the TGFβ Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef] [PubMed]
- Agnifili, L.; Sacchi, M.; Figus, M.; Posarelli, C.; Lizzio, R.A.U.; Nucci, P.; Mastropasqua, L. Preparing the Ocular Surface for Glaucoma Filtration Surgery: An Unmet Clinical Need. Acta Ophthalmol. 2022, 100, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Agnifili, L.; Figus, M.; Sacchi, M.; Oddone, F.; Villani, E.; Ferrari, G.; Posarelli, C.; Carnevale, C.; Nucci, P.; Nubile, M.; et al. Managing the Ocular Surface after Glaucoma Filtration Surgery: An Orphan Topic. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 262, 2039–2056. [Google Scholar] [CrossRef]
- Schlunck, G.; Meyer-ter-Vehn, T.; Klink, T.; Grehn, F. Conjunctival Fibrosis Following Filtering Glaucoma Surgery. Exp. Eye Res. 2016, 142, 76–82. [Google Scholar] [CrossRef]
- Amoozgar, B.; Lin, S.C.; Han, Y.; Kuo, J. A Role for Antimetabolites in Glaucoma Tube Surgery: Current Evidence and Future Directions. Curr. Opin. Ophthalmol. 2016, 27, 164–169. [Google Scholar] [CrossRef]
- Khaw, P.T.; Doyle, J.W.; Sherwood, M.B.; Grierson, I.; Schultz, G.; McGorray, S. Prolonged Localized Tissue Effects from 5-min Exposures to Fluorouracil and Mitomycin C. Arch. Ophthalmol. 1993, 111, 263–267. [Google Scholar] [CrossRef]
- Bass, P.D.; Gubler, D.A.; Judd, T.C.; Williamsa, R.M. The Mitomycinoid Alkaloids: Mechanism of Action, Biosynthesis, Total Syntheses and Synthetic Approaches. Chem. Rev. 2013, 113, 6816–6863. [Google Scholar] [CrossRef]
- Collotta, D.; Colletta, S.; Carlucci, V.; Fruttero, C.; Fea, A.M.; Collino, M. Pharmacological Approaches to Modulate the Scarring Process after Glaucoma Surgery. Pharmaceuticals 2023, 16, 898. [Google Scholar] [CrossRef]
- Al Habash, A.; Aljasim, L.A.; Owaidhah, O.; Edward, D.P. A Review of the Efficacy of Mitomycin C in Glaucoma Filtration Surgery. Clin. Ophthalmol. 2015, 9, 1945–1951. [Google Scholar] [CrossRef]
- Ang, B.C.H.; Lim, S.Y.; Betzler, B.K.; Wong, H.J.; Stewart, M.W.; Dorairaj, S. Recent Advancements in Glaucoma Surgery-A Review. Bioengineering 2023, 10, 1096. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.F.; Reichel, M.B.; Gay, J.A.; D’Esposita, F.; Alexander, R.A.; Khaw, P.T. Transforming Growth Factor-Beta1, -Beta2, and -Beta3 in Vivo: Effects on Normal and Mitomycin C-Modulated Conjunctival Scarring. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1975–1982. [Google Scholar]
- Jampel, H.D.; Roche, N.; Stark, W.J.; Roberts, A.B. Transforming Growth Factor-Beta in Human Aqueous Humor. Curr. Eye Res. 1990, 9, 963–969. [Google Scholar] [CrossRef] [PubMed]
- CAT-152 0102 Trabeculectomy Study Group; Khaw, P.; Grehn, F.; Holló, G.; Overton, B.; Wilson, R.; Vogel, R.; Smith, Z. A Phase III Study of Subconjunctival Human Anti-Transforming Growth Factor Beta(2) Monoclonal Antibody (CAT-152) to Prevent Scarring after First-Time Trabeculectomy. Ophthalmology 2007, 114, 1822–1830. [Google Scholar] [CrossRef]
- Figueiredo, R.; Barbosa-Breda, J. The Efficacy of Adjunctive Mitomycin C and/or Anti-VEGF Agents on Glaucoma Tube Shunt Drainage Device Surgeries: A Systematic Review. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 3273–3286. [Google Scholar] [CrossRef]
- Ramji, S.; Nagi, G.; Ansari, A.S.; Kailani, O. A Systematic Review and Meta-Analysis of Randomised Controlled Trials in the Management of Neovascular Glaucoma: Absence of Consensus and Variability in Practice. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 477–501. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Mietz, H.; Arnold, G.; Kirchhof, B.; Diestelhorst, M.; Krieglstein, G.K. Histopathology of Episcleral Fibrosis after Trabeculectomy with and without Mitomycin C. Graefes Arch. Clin. Exp. Ophthalmol. 1996, 234, 364–368. [Google Scholar] [CrossRef]
- Priglinger, S.G.; Alge, C.S.; Kook, D.; Thiel, M.; Schumann, R.; Eibl, K.; Yu, A.; Neubauer, A.S.; Kampik, A.; Welge-Lussen, U. Potential Role of Tissue Transglutaminase in Glaucoma Filtering Surgery. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3835–3845. [Google Scholar] [CrossRef]
- McCluskey, P.; Molteno, A.; Wakefield, D.; Di Girolamo, N. Otago Glaucoma Surgery Outcome Study: The Pattern of Expression of MMPs and TIMPs in Bleb Capsules Surrounding Molteno Implants. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2161–2164. [Google Scholar] [CrossRef]
- Chang, L.; Wong, T.; Ohbayashi, M.; Bunce, C.; Barton, K.; Ono, S.J.; Khaw, P.T. Increased Mast Cell Numbers in the Conjunctiva of Glaucoma Patients: A Possible Indicator of Preoperative Glaucoma Surgery Inflammation. Eye 2009, 23, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Fuchshofer, R.; Kottler, U.B.; Ohlmann, A.V.; Schlötzer-Schrehardt, U.; Jünemann, A.; Kruse, F.E.; Ohlmann, A. SPARC Is Expressed in Scars of the Tenon’s Capsule and Mediates Scarring Properties of Human Tenon’s Fibroblasts in Vitro. Mol. Vis. 2011, 17, 177–185. [Google Scholar] [PubMed]
- Mahale, A.; Fikri, F.; Al Hati, K.; Al Shahwan, S.; Al Jadaan, I.; Al Katan, H.; Khandekar, R.; Maktabi, A.; Edward, D.P. Histopathologic and Immunohistochemical Features of Capsular Tissue around Failed Ahmed Glaucoma Valves. PLoS ONE 2017, 12, e0187506. [Google Scholar] [CrossRef] [PubMed]
- Välimäki, J.; Uusitalo, H. Matrix Metalloproteinases (MMP-1, MMP-2, MMP-3 and MMP-9, and TIMP-1, TIMP-2 and TIMP-3) and Markers for Vascularization in Functioning and Non-Functioning Bleb Capsules of Glaucoma Drainage Implants. Acta Ophthalmol. 2015, 93, 450–456. [Google Scholar] [CrossRef]
- Yu-Wai-Man, C.; Tagalakis, A.D.; Meng, J.; Bouremel, Y.; Lee, R.M.H.; Virasami, A.; Hart, S.L.; Khaw, P.T. Genotype-Phenotype Associations of IL6 and PRG4 With Conjunctival Fibrosis After Glaucoma Surgery. JAMA Ophthalmol. 2017, 135, 1147–1155. [Google Scholar] [CrossRef]
- Siggel, R.; Schroedl, F.; Dietlein, T.; Koch, K.R.; Platzl, C.; Kaser-Eichberger, A.; Cursiefen, C.; Heindl, L.M. Absence of Lymphatic Vessels in Non-Functioning Bleb Capsules of Glaucoma Drainage Devices. Histol. Histopathol. 2020, 35, 1521–1531. [Google Scholar] [CrossRef]
- Yu-Wai-Man, C.; Owen, N.; Lees, J.; Tagalakis, A.D.; Hart, S.L.; Webster, A.R.; Orengo, C.A.; Khaw, P.T. Genome-Wide RNA-Sequencing Analysis Identifies a Distinct Fibrosis Gene Signature in the Conjunctiva after Glaucoma Surgery. Sci. Rep. 2017, 7, 5644. [Google Scholar] [CrossRef]
- Mahale, A.; Othman, M.W.; Al Shahwan, S.; Al Jadaan, I.; Owaydha, O.; Khan, Z.; Edward, D.P. Altered Expression of Fibrosis Genes in Capsules of Failed Ahmed Glaucoma Valve Implants. PLoS ONE 2015, 10, e0122409. [Google Scholar] [CrossRef]
- Wilgus, T.A. Inflammation as an Orchestrator of Cutaneous Scar Formation: A Review of the Literature. Plast. Aesthet. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Faour, S.; Farahat, M.; Aijaz, A.; Jeschke, M.G. Fibrosis in Burns: An Overview of Mechanisms and Therapies. Am. J. Physiol. Cell Physiol. 2023, 325, C1545–C1557. [Google Scholar] [CrossRef]
- Moss, B.J.; Ryter, S.W.; Rosas, I.O. Pathogenic Mechanisms Underlying Idiopathic Pulmonary Fibrosis. Annu. Rev. Pathol. 2022, 17, 515–546. [Google Scholar] [CrossRef] [PubMed]
- Podolanczuk, A.J.; Thomson, C.C.; Remy-Jardin, M.; Richeldi, L.; Martinez, F.J.; Kolb, M.; Raghu, G. Idiopathic Pulmonary Fibrosis: State of the Art for 2023. Eur. Respir. J. 2023, 61, 2200957. [Google Scholar] [CrossRef] [PubMed]
- Valentine, L.; Norris, M.R.; Bielory, L. Comparison of Structural Components and Functional Mechanisms within the Skin vs. the Conjunctival Surface. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 472–479. [Google Scholar] [CrossRef]
- Chen, J.; Bielory, L. Comparison of Cytokine Mediators in Type 2 Inflammatory Conditions on the Skin and Ocular Surface. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Oskeritzian, C.A. Mast Cells and Wound Healing. Adv. Wound Care 2012, 1, 23–28. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Ud-Din, S.; Bayat, A. A Review of the Evidence for and against a Role for Mast Cells in Cutaneous Scarring and Fibrosis. Int. J. Mol. Sci. 2020, 21, 9673. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, C.; Ma, S.; Wen, H. Mast Cell Chymase in Keloid Induces Profibrotic Response via Transforming Growth Factor-Β1/Smad Activation in Keloid Fibroblasts. Int. J. Clin. Exp. Pathol. 2014, 7, 3596–3607. [Google Scholar]
- Jordan, P.M.; Andreas, N.; Groth, M.; Wegner, P.; Weber, F.; Jäger, U.; Küchler, C.; Werz, O.; Serfling, E.; Kamradt, T.; et al. ATP/IL-33-Triggered Hyperactivation of Mast Cells Results in an Amplified Production of pro-Inflammatory Cytokines and Eicosanoids. Immunology 2021, 164, 541–554. [Google Scholar] [CrossRef]
- Tredget, E.E.; Yang, L.; Delehanty, M.; Shankowsky, H.; Scott, P.G. Polarized Th2 Cytokine Production in Patients with Hypertrophic Scar Following Thermal Injury. J. Interferon Cytokine Res. 2006, 26, 179–189. [Google Scholar] [CrossRef]
- Penatzer, J.A.; Srinivas, S.; Thakkar, R.K. The Role of Macrophages in Thermal Injury. Int. J. Burns Trauma 2022, 12, 1–12. [Google Scholar]
- Chen, C.; Liu, T.; Tang, Y.; Luo, G.; Liang, G.; He, W. Epigenetic Regulation of Macrophage Polarization in Wound Healing. Burns Trauma 2023, 11, tkac057. [Google Scholar] [CrossRef] [PubMed]
- Ca2+ Influx Is an Essential Component of the Positive-Feedback Loop That Maintains Leading-Edge Structure and Activity in Macrophages|PNAS. Available online: https://www.pnas.org/doi/10.1073/pnas.0707719104 (accessed on 24 February 2025).
- Wei, C.; Wang, X.; Chen, M.; Ouyang, K.; Song, L.-S.; Cheng, H. Calcium Flickers Steer Cell Migration. Nature 2009, 457, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Abdelnaby, A.E.; Trebak, M. Store-Operated Ca2+ Entry in Fibrosis and Tissue Remodeling. Contact 2024, 7, 25152564241291374. [Google Scholar] [CrossRef] [PubMed]
- Roeb, E. Interleukin-13 (IL-13)-A Pleiotropic Cytokine Involved in Wound Healing and Fibrosis. Int. J. Mol. Sci. 2023, 24, 12884. [Google Scholar] [CrossRef]
- Ong, C.T.; Khoo, Y.T.; Mukhopadhyay, A.; Do, D.V.; Lim, I.J.; Aalami, O.; Phan, T.T. mTOR as a Potential Therapeutic Target for Treatment of Keloids and Excessive Scars. Exp. Dermatol. 2007, 16, 394–404. [Google Scholar] [CrossRef]
- Shah, V.V.; Aldahan, A.S.; Mlacker, S.; Alsaidan, M.; Samarkandy, S.; Nouri, K. 5-Fluorouracil in the Treatment of Keloids and Hypertrophic Scars: A Comprehensive Review of the Literature. Dermatol. Ther. 2016, 6, 169–183. [Google Scholar] [CrossRef]
- Bi, M.; Sun, P.; Li, D.; Dong, Z.; Chen, Z. Intralesional Injection of Botulinum Toxin Type A Compared with Intralesional Injection of Corticosteroid for the Treatment of Hypertrophic Scar and Keloid: A Systematic Review and Meta-Analysis. Med. Sci. Monit. 2019, 25, 2950–2958. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, X.; Ding, K.; Liang, Y.; Jiang, D.; Dai, H. Rapamycin Inhibits Transforming Growth Factor Β1-Induced Fibrogenesis in Primary Human Lung Fibroblasts. Yonsei Med. J. 2013, 54, 437–444. [Google Scholar] [CrossRef]
- Osman, B.; Doller, A.; Akool, E.-S.; Holdener, M.; Hintermann, E.; Pfeilschifter, J.; Eberhardt, W. Rapamycin Induces the TGFbeta1/Smad Signaling Cascade in Renal Mesangial Cells Upstream of mTOR. Cell. Signal. 2009, 21, 1806–1817. [Google Scholar] [CrossRef]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. mTOR: From Growth Signal Integration to Cancer, Diabetes and Ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef]
- Hardt, M.; Chantaravisoot, N.; Tamanoi, F. Activating Mutations of TOR (Target of Rapamycin). Genes Cells 2011, 16, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wu, J.; Frizell, E.; Liu, S.L.; Bashey, R.; Rubin, R.; Norton, P.; Zern, M.A. Rapamycin Inhibits Hepatic Stellate Cell Proliferation in Vitro and Limits Fibrogenesis in an in Vivo Model of Liver Fibrosis. Gastroenterology 1999, 117, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; You, F.; Januszyk, M.; Gurtner, G.C.; Kuang, A.A. Transcriptional Profiling of Rapamycin-Treated Fibroblasts from Hypertrophic and Keloid Scars. Ann. Plast. Surg. 2014, 72, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.; Maderal, A.; Raphael, B. Keloids and Hypertrophic Scars: Pathophysiology, Classification, and Treatment. Dermatol. Surg. 2017, 43 (Suppl. S1), S3–S18. [Google Scholar] [CrossRef]
- Leszczynski, R.; da Silva, C.A.; Pinto, A.C.P.N.; Kuczynski, U.; da Silva, E.M. Laser Therapy for Treating Hypertrophic and Keloid Scars. Cochrane Database Syst. Rev. 2022, 9, CD011642. [Google Scholar] [CrossRef]
- Simonacci, F.; Bertozzi, N.; Grieco, M.P.; Grignaffini, E.; Raposio, E. Procedure, Applications, and Outcomes of Autologous Fat Grafting. Ann. Med. Surg. 2017, 20, 49–60. [Google Scholar] [CrossRef]
- Ekstein, S.F.; Wyles, S.P.; Moran, S.L.; Meves, A. Keloids: A Review of Therapeutic Management. Int. J. Dermatol. 2021, 60, 661–671. [Google Scholar] [CrossRef]
- Barratt, S.L.; Creamer, A.; Hayton, C.; Chaudhuri, N. Idiopathic Pulmonary Fibrosis (IPF): An Overview. J. Clin. Med. 2018, 7, 201. [Google Scholar] [CrossRef]
- Zhu, W.; Tan, C.; Zhang, J. Alveolar Epithelial Type 2 Cell Dysfunction in Idiopathic Pulmonary Fibrosis. Lung 2022, 200, 539–547. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular Senescence Mediates Fibrotic Pulmonary Disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
- Moiseeva, V.; Cisneros, A.; Cobos, A.C.; Tarrega, A.B.; Oñate, C.S.; Perdiguero, E.; Serrano, A.L.; Muñoz-Cánoves, P. Context-Dependent Roles of Cellular Senescence in Normal, Aged, and Disease States. FEBS J. 2023, 290, 1161–1185. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Baker, J.; Donnelly, L.E. Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases. Am. J. Respir. Crit. Care Med. 2019, 200, 556–564. [Google Scholar] [CrossRef]
- Carraro, G.; Mulay, A.; Yao, C.; Mizuno, T.; Konda, B.; Petrov, M.; Lafkas, D.; Arron, J.R.; Hogaboam, C.M.; Chen, P.; et al. Single-Cell Reconstruction of Human Basal Cell Diversity in Normal and Idiopathic Pulmonary Fibrosis Lungs. Am. J. Respir. Crit. Care Med. 2020, 202, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, Z. Fibroblast Senescence in Idiopathic Pulmonary Fibrosis. Front. Cell Dev. Biol. 2020, 8, 593283. [Google Scholar] [CrossRef] [PubMed]
- Ca2+ Signalling in Fibroblasts and the Therapeutic Potential of KCa3.1 Channel Blockers in Fibrotic Diseases—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31758702/ (accessed on 24 February 2025).
- Platé, M.; Guillotin, D.; Chambers, R.C. The Promise of mTOR as a Therapeutic Target Pathway in Idiopathic Pulmonary Fibrosis. Eur. Respir. Rev. 2020, 29, 200269. [Google Scholar] [CrossRef]
- Albert, V.; Hall, M.N. mTOR Signaling in Cellular and Organismal Energetics. Curr. Opin. Cell Biol. 2015, 33, 55–66. [Google Scholar] [CrossRef]
- Romero, Y.; Bueno, M.; Ramirez, R.; Álvarez, D.; Sembrat, J.C.; Goncharova, E.A.; Rojas, M.; Selman, M.; Mora, A.L.; Pardo, A. mTORC1 Activation Decreases Autophagy in Aging and Idiopathic Pulmonary Fibrosis and Contributes to Apoptosis Resistance in IPF Fibroblasts. Aging Cell 2016, 15, 1103–1112. [Google Scholar] [CrossRef]
- Glass, D.S.; Grossfeld, D.; Renna, H.A.; Agarwala, P.; Spiegler, P.; DeLeon, J.; Reiss, A.B. Idiopathic Pulmonary Fibrosis: Current and Future Treatment. Clin. Respir. J. 2022, 16, 84–96. [Google Scholar] [CrossRef]
- Malouf, M.A.; Hopkins, P.; Snell, G.; Glanville, A.R. Everolimus in IPF Study Investigators An Investigator-Driven Study of Everolimus in Surgical Lung Biopsy Confirmed Idiopathic Pulmonary Fibrosis. Respirology 2011, 16, 776–783. [Google Scholar] [CrossRef]
- Kolb, M.; Bonella, F.; Wollin, L. Therapeutic Targets in Idiopathic Pulmonary Fibrosis. Respir. Med. 2017, 131, 49–57. [Google Scholar] [CrossRef]
- Lachapelle, P.; Li, M.; Douglass, J.; Stewart, A. Safer Approaches to Therapeutic Modulation of TGFβ Signaling for Respiratory Disease. Pharmacol. Ther. 2018, 187, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Overed-Sayer, C.; Miranda, E.; Dunmore, R.; Liarte Marin, E.; Beloki, L.; Rassl, D.; Parfrey, H.; Carruthers, A.; Chahboub, A.; Koch, S.; et al. Inhibition of Mast Cells: A Novel Mechanism by Which Nintedanib May Elicit Anti-Fibrotic Effects. Thorax 2020, 75, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Khaw, P.T.; Bouremel, Y.; Brocchini, S.; Henein, C. The Control of Conjunctival Fibrosis as a Paradigm for the Prevention of Ocular Fibrosis-Related Blindness. “Fibrosis Has Many Friends”. Eye 2020, 34, 2163–2174. [Google Scholar] [CrossRef]
- Shu, D.Y.; Lovicu, F.J. Myofibroblast Transdifferentiation: The Dark Force in Ocular Wound Healing and Fibrosis. Prog. Retin. Eye Res. 2017, 60, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Gajda-Deryło, B.; Stahnke, T.; Struckmann, S.; Warsow, G.; Birke, K.; Birke, M.T.; Hohberger, B.; Rejdak, R.; Fuellen, G.; Jünemann, A.G. Comparison of Cytokine/Chemokine Levels in Aqueous Humor of Primary Open-Angle Glaucoma Patients with Positive or Negative Outcome Following Trabeculectomy. Biosci. Rep. 2019, 39, BSR20181894. [Google Scholar] [CrossRef]
- Zhu, J.-D.; Xie, L.-L.; Li, Z.-Y.; Lu, X.-H. The Prognosis of Trabeculectomy in Primary Angle-Closure Glaucoma Patients. Int. J. Ophthalmol. 2019, 12, 66–72. [Google Scholar] [CrossRef]
- Aeschlimann, D.; Paulsson, M. Cross-Linking of Laminin-Nidogen Complexes by Tissue Transglutaminase. A Novel Mechanism for Basement Membrane Stabilization. J. Biol. Chem. 1991, 266, 15308–15317. [Google Scholar] [CrossRef]
- Schiemann, B.J.; Neil, J.R.; Schiemann, W.P. SPARC Inhibits Epithelial Cell Proliferation in Part through Stimulation of the Transforming Growth Factor-{beta}-Signaling System. Mol. Biol. Cell 2003, 14, 3977–3988. [Google Scholar] [CrossRef]
- Esnault, C.; Stewart, A.; Gualdrini, F.; East, P.; Horswell, S.; Matthews, N.; Treisman, R. Rho-Actin Signaling to the MRTF Coactivators Dominates the Immediate Transcriptional Response to Serum in Fibroblasts. Genes Dev. 2014, 28, 943–958. [Google Scholar] [CrossRef]
- Alquraini, A.; Garguilo, S.; D’Souza, G.; Zhang, L.X.; Schmidt, T.A.; Jay, G.D.; Elsaid, K.A. The Interaction of Lubricin/Proteoglycan 4 (PRG4) with Toll-like Receptors 2 and 4: An Anti-Inflammatory Role of PRG4 in Synovial Fluid. Arthritis Res. Ther. 2015, 17, 353. [Google Scholar] [CrossRef]
- Novince, C.M.; Koh, A.J.; Michalski, M.N.; Marchesan, J.T.; Wang, J.; Jung, Y.; Berry, J.E.; Eber, M.R.; Rosol, T.J.; Taichman, R.S.; et al. Proteoglycan 4, a Novel Immunomodulatory Factor, Regulates Parathyroid Hormone Actions on Hematopoietic Cells. Am. J. Pathol. 2011, 179, 2431–2442. [Google Scholar] [CrossRef] [PubMed]
- Law, R.H.; Zhang, Q.; McGowan, S.; Buckle, A.M.; Silverman, G.A.; Wong, W.; Rosado, C.J.; Langendorf, C.G.; Pike, R.N.; Bird, P.I.; et al. An Overview of the Serpin Superfamily. Genome Biol. 2006, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H. Metalloproteases. Curr. Protoc. Protein Sci. 2001, 24, 21.4.1–21.4.13. [Google Scholar] [CrossRef] [PubMed]
- Pichery, M.; Mirey, E.; Mercier, P.; Lefrancais, E.; Dujardin, A.; Ortega, N.; Girard, J.-P. Endogenous IL-33 Is Highly Expressed in Mouse Epithelial Barrier Tissues, Lymphoid Organs, Brain, Embryos, and Inflamed Tissues: In Situ Analysis Using a Novel Il-33-LacZ Gene Trap Reporter Strain. J. Immunol. 2012, 188, 3488–3495. [Google Scholar] [CrossRef]
- Irnaten, M.; Zhdanov, A.; Brennan, D.; Crotty, T.; Clark, A.; Papkovsky, D.; O’Brien, C. Activation of the NFAT-Calcium Signaling Pathway in Human Lamina Cribrosa Cells in Glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 831–842. [Google Scholar] [CrossRef]
- Meng, X.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGFβ: The Master Regulator of Fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Dammacco, R.; Merlini, G.; Lisch, W.; Kivelä, T.T.; Giancipoli, E.; Vacca, A.; Dammacco, F. Amyloidosis and Ocular Involvement: An Overview. Semin. Ophthalmol. 2020, 35, 7–26. [Google Scholar] [CrossRef]
- Kitahara, J.; Kakihara, S.; Mukawa, S.; Hirano, T.; Imai, A.; Miyahara, T.; Murata, T. Long-Term Surgical Results of Trabeculectomy for Secondary Glaucoma in Val30Met Hereditary Transthyretin Amyloidosis. Sci. Rep. 2023, 13, 12755. [Google Scholar] [CrossRef]
- Barbosa Ribeiro, B.; Vieira, R.; Ferreira, A.; Marta, A.; Figueiredo, A.; Reis, R.; Sampaio, I.; Melo Beirão, J.; Menéres, M.J. Modified Ex-PRESS Technique versus Ahmed Glaucoma Valve as Primary Glaucoma Surgery for Hereditary Transthyretin Amyloidosis Glaucoma. Amyloid 2024, 31, 302–308. [Google Scholar] [CrossRef]
- Wang, R.; Chen, B.; Wei, H.; Yan, W.; Wu, Y.; Wang, C.; Zhang, B.; Liu, F.; Tian, H.; Chen, X.; et al. Collecting and Deactivating TGFΒ1 Hydrogel for Anti-Scarring Therapy in Post-Glaucoma Filtration Surgery. Mater. Today Bio 2022, 14, 100260. [Google Scholar] [CrossRef]
- Yoshida, M.; Kokubun, T.; Sato, K.; Tsuda, S.; Yokoyama, Y.; Himori, N.; Nakazawa, T. DPP-4 Inhibitors Attenuate Fibrosis After Glaucoma Filtering Surgery by Suppressing the TGFβ/Smad Signaling Pathway. Investig. Ophthalmol. Vis. Sci. 2023, 64, 2. [Google Scholar] [CrossRef] [PubMed]
- Heukels, P.; Moor, C.C.; von der Thüsen, J.H.; Wijsenbeek, M.S.; Kool, M. Inflammation and Immunity in IPF Pathogenesis and Treatment. Respir. Med. 2019, 147, 79–91. [Google Scholar] [CrossRef]
- Yüksel, R.; Yüksel, N.; Yazır, Y.; Öztürk, A.; Furat Rençber, S.; Demirci Küçük, K. The Anti-Scar Effect of Tyrosine-Kinase Inhibitor Nintedanib in Experimental Glaucoma Filtration Surgery in Rabbits. Exp. Eye Res. 2023, 229, 109431. [Google Scholar] [CrossRef]
- Podolanczuk, A.J.; Cottin, V. A Narrative Review of Real-World Data on the Safety of Nintedanib in Patients with Idiopathic Pulmonary Fibrosis. Adv. Ther. 2023, 40, 2038–2050. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zeng, B.; Wu, C.; Chen, Z.; Yu, M.; Yang, Y. Inhibition of TGFΒ2-Induced Trabecular Meshwork Fibrosis by Pirfenidone. Transl. Vis. Sci. Technol. 2023, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Sun, G.; Lin, X.; Wu, K.; Yu, M. Evaluation of Pirfenidone as a New Postoperative Antiscarring Agent in Experimental Glaucoma Surgery. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3136–3142. [Google Scholar] [CrossRef]
- Kasar, K.; Demir, T.; Akin, M.M.; Gungor Kobat, S. The Effect of Halofugınone and Pirfenidone on Wound Healing in Experimental Glaucoma Filtration Surgery. J. Fr. Ophtalmol. 2021, 44, 340–349. [Google Scholar] [CrossRef]
- Jung, K.I.; Park, C.K. Pirfenidone Inhibits Fibrosis in Foreign Body Reaction after Glaucoma Drainage Device Implantation. Drug Des. Dev. Ther. 2016, 10, 1477–1488. [Google Scholar] [CrossRef]
- Na, J.H.; Sung, K.R.; Shin, J.A.; Moon, J.I. Antifibrotic Effects of Pirfenidone on Tenon’s Fibroblasts in Glaucomatous Eyes: Comparison with Mitomycin C and 5-Fluorouracil. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1537–1545. [Google Scholar] [CrossRef]
- Ma, Y.-J.; Zhang, Q.; Wang, C.-X.; Wu, W. The Efficacy and Safety of Pirfenidone in the Treatment of HPS-Related Pulmonary Fibrosis and Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8411–8424. [Google Scholar] [CrossRef]
- Sun, G.; Lin, X.; Zhong, H.; Yang, Y.; Qiu, X.; Ye, C.; Wu, K.; Yu, M. Pharmacokinetics of Pirfenidone after Topical Administration in Rabbit Eye. Mol. Vis. 2011, 17, 2191–2196. [Google Scholar] [PubMed]
- Stahnke, T.; Siewert, S.; Reske, T.; Schmidt, W.; Schmitz, K.-P.; Grabow, N.; Guthoff, R.F.; Wree, A. Development of a Biodegradable Antifibrotic Local Drug Delivery System for Glaucoma Microstents. Biosci. Rep. 2018, 38, BSR20180628. [Google Scholar] [CrossRef] [PubMed]
- Stahnke, T.; Kowtharapu, B.S.; Stachs, O.; Schmitz, K.-P.; Wurm, J.; Wree, A.; Guthoff, R.F.; Hovakimyan, M. Suppression of TGFβ Pathway by Pirfenidone Decreases Extracellular Matrix Deposition in Ocular Fibroblasts in Vitro. PLoS ONE 2017, 12, e0172592. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, P.P.; Maddala, R.; Rao, P.V. Regulation of Plasticity and Fibrogenic Activity of Trabecular Meshwork Cells by Rho GTPase Signaling. J. Cell. Physiol. 2014, 229, 927–942. [Google Scholar] [CrossRef]
- Westermeyer, H.D.; Salmon, B.; Baynes, R.; Yeatts, J.; Khattab, A.; Oh, A.; Mowat, F. Safety and Efficacy of Topically Applied 0.5% and 1% Pirfenidone in a Canine Model of Subconjunctival Fibrosis. Vet. Ophthalmol. 2019, 22, 502–509. [Google Scholar] [CrossRef]
- Targeting Ageing with Rapamycin and Its Derivatives in Humans: A Systematic Review—The Lancet Healthy Longevity. Available online: https://www.thelancet.com/journals/lanhl/article/PIIS2666-7568(23)00258-1/fulltext (accessed on 24 February 2025).
- Salas-Prato, M.; Assalian, A.; Mehdi, A.Z.; Duperré, J.; Thompson, P.; Brazeau, P. Inhibition by Rapamycin of PDGF- and bFGF-Induced Human Tenon Fibroblast Proliferation in Vitro. J. Glaucoma 1996, 5, 54–59. [Google Scholar] [CrossRef]
- Igarashi, N.; Honjo, M.; Aihara, M. Effects of Mammalian Target of Rapamycin Inhibitors on Fibrosis after Trabeculectomy. Exp. Eye Res. 2021, 203, 108421. [Google Scholar] [CrossRef]
- Eren, K.; Turgut, B.; Akin, M.M.; Demir, T. The Suppression of Wound Healing Response with Sirolimus and Sunitinib Following Experimental Trabeculectomy in a Rabbit Model. Curr. Eye Res. 2016, 41, 367–376. [Google Scholar] [CrossRef]
- Yan, Z.; Bai, Y.; Tian, Z.; Hu, H.; You, X.; Lin, J.; Liu, S.; Zhuo, Y.; Luo, R. Anti-Proliferation Effects of Sirolimus Sustained Delivery Film in Rabbit Glaucoma Filtration Surgery. Mol. Vis. 2011, 17, 2495–2506. [Google Scholar]
- Kang, X.; Shen, Y.; Zhao, H.; Wang, Z.; Guan, W.; Ge, R.; Wang, R.; Tai, X. [Anti-scarring effect of rapamycin in rabbits following glaucoma filtering surgery]. Nan Fang Yi Ke Da Xue Xue Bao 2018, 38, 1389–1394. [Google Scholar] [CrossRef]
- Tai, X.; Shen, Y.; Zhao, H.; Wang, Z.; Guan, W.; Kang, X.; Guo, W. [Anti-scarring effect of rapamycin following filtering surgery in rabbit eyes]. Nan Fang Yi Ke Da Xue Xue Bao 2020, 40, 1346–1352. [Google Scholar] [CrossRef]

| Year | Author | Main Group Features | Control Groups Features | Molecular Targets | Analysis | Results |
|---|---|---|---|---|---|---|
| 1996 | Mietz et al. [28] | GROUP 1: (n = 7) Bleb tissue (Tenon’s capsule) in eyes with failed trabe + MMC | GROUP 2: (n = 5) Bleb tissue (Tenon’s capsule) in eyes with failed trabe (no MMC/5-FU); GROUP 3: (n = 23) Conjunctiva in non-glaucomatous eyes | αSMA | IHC on slices | +αSMA GROUP 1 vs. GROUP 2, GROUP 1 vs. GROUP 3 |
| 2006 | Priglinger et al. [29] | GROUP 1: (n = 8) Bleb tissue (Tenon’s capsule) in eyes with failed trabe | GROUP 2: (n = 6) Bleb tissue (Tenon’s capsule) of in vitro functioning trabe models before (GROUP 2a) and after in vitro TGFβ2 treatment (GROUP 2b) | tTgase, FBN, epsilon-gamma-glutamyl-lysine | IHC on slices; RNA/protein study on HTFs with RT-PCR and blot analysis | +tTgase, FBN, epsilon-gamma-glutamyl-lysine GROUP 1 vs. GROUP 2 AND GROUP 2b vs. GROUP 2a |
| 2009 | McCluskey et al. [30] | GROUP 1: (n = 10) Bleb tissue (Tenon’s capsule) in eyes with functioning Molteno implant | Not reported | MMP1, MMP2, MMP3, TIMP1, TIMP2, TIMP3 | IHC on slices | +MMP1, MMP2, MMP3, TIMP2 |
| 2009 | Chang et al. [31] | GROUP 1: (n = 8) Peri-bleb tissue (conjunctiva) in eyes with failed trabe | (n = 6) Conjunctiva in medically treated glaucoma (GROUP 2); (n = 7) uveitic glaucoma (GROUP 3); (n = 8) non glaucomatous eyes (GROUP 4) | Mast cells | IHC on slices | +Mast cells GROUP 1 vs. GROUP 4 |
| 2011 | Fuchshofer et al. [32] | GROUP 1: (n = 3) Bleb tissue (Tenon’s capsule) in eyes with failed trabe | GROUP 2: (n = 3) Tenon’s capsule in non-glaucomatous eyes before (GROUP 2a) and after in vitro TGFβ1 (GROUP 2b), TGFβ2 (GROUP 2c), SPARC (GROUP 2d), SPARC + TGFβ1 (GROUP 2e) treatment | SPARC (secreted acidic cysteine-rich glycoprotein) | IHC on slices; RNA/protein study on HTFs with RT-PCR and blot analysis | +SPARC GROUP 1 vs. GROUP 2a AND GROUP 2b vs. GROUP 2c, +contractility & cells proliferation GROUP 2e vs. GROUP 2d, GROUP 2e vs. GROUP 2b |
| 2015 | Mahale et al. [38] | GROUP 1: (n = 7 PCR Array/n = 20 TaqMan) Bleb tissue (Tenon’s capsule) in eyes with failed Ahmed implant | GROUP 2: (n = 2 PCR Array/n = 8 TaqMan) Tenon’s capsule in medically treated glaucomatous eyes | 84 selected genes | RNA study on HTFs with RT-PCR; TaqMan assay for 9 selected genes that are >2 fold at PCR | >2 fold: 39 genes. +in ≥5/7: CTGF*, THBS1/2*, INBHE*, IL13RA2*, HGF, AGT, CCL11, MMP1, MMP3*, MMP8, MMP13*, LOX, SERPINE1, ITGA2, ITGB5, αSMA, COL3A1*. -in ≥5/7: IL1A*, BMP7, SERPINA1. Taqman validated: *. |
| 2015 | Välimäki et al. [34] | GROUP 1: (n = 3) Bleb tissue (Tenon’s capsule) in eyes with failed implant (Molteno, Baerveldt, Ahmed) | GROUP 2: (n = 3) Tenon’s capsule in eyes with functioning implant (Molteno, Ahmed) | MMP1, MMP2, MMP3, MMP9, TIMP1, TIMP2, TIMP3, CD31 | IHC on slices | +TIMP3 GROUP 1, correlate with CD31- avascular profile, +MMP9 GROUP 2, correlate with CD31+ vascular profile |
| 2017 | Yu-Wai-Man, Owen, et al. [37] | GROUP 1: (n = 10) Bleb tissue (conjunctiva) in eyes with failed glaucoma surgery | GROUP 2: (n = 7) Conjunctiva in medically treated glaucoma eyes | Genome wide | RNA study on HTFs with RNA-Seq; RT-PCR for 11 selected genes that are >2 fold at NGS | >2 fold up/downregulation: 246 genes. PCR validated: +MYOCD, LMO3, IL6, RELB; -PRG4, CD34, IL33, COL6A6, MMP10, WISP2, IGFBP5. Significant others: +PPP1R13L, MYB, BIRC3; -DUSP5, FGFR3, NOV, FBLN1, MMP12, MMP24, RASSF2 |
| 2017 | Yu-Wai-Man, Tagalakis, et al. [35] | GROUP 1: (n = 24) Bleb tissue (conjunctiva) in eyes with failed glaucoma surgery (trabe, implants) | GROUP 2: (n = 14) Conjunctiva in medically treated glaucomatous eyes | IL6, PRG4 | IHC on slices; RNA study on HCFs with RT-PCR and TaqMan assay | +IL6, -PRG4 GROUP 1 vs. GROUP 2 |
| 2017 | Mahale et al. [33] | GROUP 1: (n = 14) Bleb tissue (Tenon’s capsule) in eyes with failed Ahmed implant | GROUP 2: (n = 8) Tenon’s capsule in eyes in non-treated glaucomatous eyes | COL1, COL3, DCN, LUM, CS, ACAN, KS, αSMA and TGFβ | IHC on slices | +αSMA, TGFβ, -COL3, DCN, LUM, CS in the inner layers GROUP 1 vs. GROUP 2 |
| 2020 | Siggel et al. [36] | GROUP 1: (n = 15) Bleb tissue (Tenon’s capsule) in eyes with failed Baerveldt implant | Not reported | CD31, CD68, PDPN (D2-40, Podoplanin), LYVE-1 | IHC on slices | +++CD31 and +CD68 in the outer layers, +PDPN in the inner/outer layers, -PDPN AND LYVE1 |
| Year | Author | Age | Ethnicity | Glaucoma Type | Surgical Technique | Perioperative Antimetabolites | “Failure” Definition (GROUP 1) | Time from Surgery to Biopsy |
|---|---|---|---|---|---|---|---|---|
| 1996 | Mietz et al. [28] | Adults, children | Not reported | POAG, PG, PXG, ICE, aniridia, Peter’s anomaly, trauma | GROUP 1: trabe; GROUP 2: trabe | GROUP 1: MMC; GROUP 2: no | Clinically failed secondary to fibrosis at the level of the episclera or related to the formation of Tenon’s capsule cysts | GROUP 1: 1 weeks–10 months; GROUP 2: 1–11 months |
| 2006 | Priglinger et al. [29] | Adults | Not reported | POAG, PXG, CG | GROUP 1: trabe; GROUP 2: trabe | GROUP 1: 3/8 yes; GROUP 2: not reported | Formation of Tenon cysts | 7 weeks–2 years |
| 2009 | McCluskey et al. [30] | Adults | Not reported | POAG, PXG, NVG, trauma, ghost cells | Molteno implant | Not reported | Not reported | 2 months–22.9 years |
| 2009 | Chang et al. [31] | Adults | Asian, Afro-Caribbean, Caucasian, Turkish | Specified only for GROUP 3: UG | Trabe | Not reported | Not reported | Not reported |
| 2011 | Fuchshofer et al. [32] | Not reported | Not reported | Not reported | Trabe | Not reported | Not reported | Not reported |
| 2015 | Mahale et al. [38] | Adults, children | Not reported | Not reported | Ahmed implant | Not reported | Uncontrolled IOP with maximal tolerated medically therapy | GROUP 1 for PCR Array: 12–144 months; GROUP 1 for TaqMan: 6–156 months |
| 2015 | Välimäki et al. [34] | Adults | Not reported | POAG, PXG, NVG, UG | Molteno, Baerveldt, Ahmed implants | Not reported | IOP > 21 mmHg or <20% reduction in IOP from baseline with maximal tolerated medication | GROUP 1: 6–108 months; GROUP 2: 31–67 months |
| 2017 | Yu-Wai-Man, Owen, et al. [37] | Adults | Asian, Afro-Caribbean, Caucasian | POAG, SOAG, CG | Not reported | Not reported | Not reported | Not reported |
| 2017 | Yu-Wai-Man, Tagalakis, et al. [35] | Adults | Asian, Afro-Caribbean, White | Not reported | Trabe, implants | Not reported | Not reported | Not reported |
| 2017 | Mahale et al. [33] | Adults, children | Not reported | POAG, SOAG (no NVG), CG | Ahmed implant | Not reported | Intraocular pressure above target levels on maximum medical therapy determined by the treating physician | 3–156 months |
| 2020 | Siggel et al. [36] | Adults, children | Not reported | POAG, CG, silicon oil, steroid induced, aphakic, ICE | Baerveldt implant | Not reported | Poor function | 1.1 months–6.3 years |
| Processes | Evidences from IHC | Evidences from Genetic/Genomic |
|---|---|---|
| Inflammation: humoral and cellular response | Mast cells (tryptase), macrophages (CD68), humoral response (IL6, TGFβ) | Interleukines (IL1, IL6, IL13RA2, IL33), chemokines (CCL11), integrins (ITGA2, ITGB5), growth factors (CTGF/CCN2, HGF, AGT, IGFBP5, FGFR3), TGFβ pathways (CTGF/CCN2, NOV/CCN3, WISP2/CCN5, THBS1, THBS2, INBHE, BMP7) |
| Proliferation of fibroblasts and myofibroblast conversion | Activity (αSMA/ACTA2) | Activity (αSMA/ACTA2), differentiation (MYOCD), proliferation/apoptosis (DUSP5, RELB, PPP1R13L, LMO3, MYB, BIRC3, RASSF2) |
| Filtration bleb vascularization | Arterial vessels (CD31, CD31), lymphatic vessels (PDPN + LYVE) | Arterial vessels (CD34) |
| Tissue remodelling | ECM composition (PRG4, SPARC/ON, FBN, COL1, COL3, DCN, LUM, CS), ECM turnover (MMP1*, MMP2*, MMP3*, MMP9*, TIMP2*, TIMP3) | ECM composition (PRG4, SPARC/ON, FBN, FBLN1, COL3A1, COL6A6), ECM turnover (MMP1, MMP3, MMP8, MMP10, MMP12, MMP13, MMP24, TIMP3, SERPINE1, SERPINA1, LOX) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacchi, M.; Tomaselli, D.; Ruggeri, M.L.; Aiello, F.B.; Sabella, P.; Dore, S.; Pinna, A.; Mastropasqua, R.; Nubile, M.; Agnifili, L. Fighting Bleb Fibrosis After Glaucoma Surgery: Updated Focus on Key Players and Novel Targets for Therapy. Int. J. Mol. Sci. 2025, 26, 2327. https://doi.org/10.3390/ijms26052327
Sacchi M, Tomaselli D, Ruggeri ML, Aiello FB, Sabella P, Dore S, Pinna A, Mastropasqua R, Nubile M, Agnifili L. Fighting Bleb Fibrosis After Glaucoma Surgery: Updated Focus on Key Players and Novel Targets for Therapy. International Journal of Molecular Sciences. 2025; 26(5):2327. https://doi.org/10.3390/ijms26052327
Chicago/Turabian StyleSacchi, Matteo, Davide Tomaselli, Maria Ludovica Ruggeri, Francesca Bianca Aiello, Pierfilippo Sabella, Stefano Dore, Antonio Pinna, Rodolfo Mastropasqua, Mario Nubile, and Luca Agnifili. 2025. "Fighting Bleb Fibrosis After Glaucoma Surgery: Updated Focus on Key Players and Novel Targets for Therapy" International Journal of Molecular Sciences 26, no. 5: 2327. https://doi.org/10.3390/ijms26052327
APA StyleSacchi, M., Tomaselli, D., Ruggeri, M. L., Aiello, F. B., Sabella, P., Dore, S., Pinna, A., Mastropasqua, R., Nubile, M., & Agnifili, L. (2025). Fighting Bleb Fibrosis After Glaucoma Surgery: Updated Focus on Key Players and Novel Targets for Therapy. International Journal of Molecular Sciences, 26(5), 2327. https://doi.org/10.3390/ijms26052327







