Metabolic Syndrome and Insulin Resistance in Romania
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Anamnestic, Lifestyle and Socio Demographic Data
4.3. Clinical and Biochemical Data
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamali, Z.; Ayoobi, F.; Jalali, Z.; Bidaki, R.; Lotfi, M.A.; Esmaeili-Nadimi, A.; Khalili, P. Metabolic syndrome: A population-based study of prevalence and risk factors. Sci. Rep. 2024, 14, 3987. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.; Elsayes, K.M.; Lubner, M.G.; Shehata, M.A.; Fowler, K.; Kaoud, A.; Pickhardt, P.J. Metabolic syndrome: Imaging features and clinical outcomes. Br. J. Radiol. 2024, 97, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- Vesa, C.M.; Zaha, D.C.; Bungău, S.G. Molecular Mechanisms of Metabolic Syndrome. Int. J. Mol. Sci. 2024, 25, 5452. [Google Scholar] [CrossRef]
- Scurt, F.G.; Ganz, M.J.; Herzog, C.; Bose, K.; Mertens, P.R.; Chatzikyrkou, C. Association of metabolic syndrome and chronic kidney disease. Obes. Rev. 2024, 25, e13649. [Google Scholar] [CrossRef]
- Tirandi, A.; Carbone, F.; Montecucco, F.; Liberale, L. The role of metabolic syndrome in sudden cardiac death risk: Recent evidence and future directions. Eur. J. Clin. Investig. 2022, 52, e13693. [Google Scholar] [CrossRef]
- Protasiewicz-Timofticiuc, D.C.; Bădescu, D.; Moța, M.; Ștefan, A.G.; Mitrea, A.; Clenciu, D.; Efrem, I.C.; Roșu, M.M.; Vladu, B.E.; Gheonea, T.C.; et al. Back to Roots: Dysbiosis, Obesity, Metabolic Syndrome, Type 2 Diabetes Mellitus, and Obstructive Sleep Apnea-Is There an Objective Connection? A Narrative Review. Nutrients 2024, 16, 4057. [Google Scholar] [CrossRef]
- Kurnool, S.; McCowen, K.C.; Bernstein, N.A.; Malhotra, A. Sleep Apnea, Obesity, and Diabetes—An Intertwined Trio. Curr. Diab. Rep. 2023, 23, 165–171. [Google Scholar] [CrossRef]
- Nolan, P.B.; Carrick-Ranson, G.; Stinear, J.W.; Reading, S.A.; Dalleck, L.C. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: A pooled analysis. Prev. Med. Rep. 2017, 7, 211–215. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, N.M. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch. Intern. Med. 1989, 149, 1514–1520. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, C.; Li, M.; Yan, G.; Tang, C. Sex Differences in the Associations among Insulin Resistance Indexes with Metabolic Syndrome: A Large Cross-Sectional Study. Int. J. Endocrinol. 2024, 2024, 3352531. [Google Scholar] [CrossRef]
- Paniagua, J.A. Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J. Diabetes 2016, 7, 483–514. [Google Scholar] [CrossRef]
- Dichi, I.; Simão, A.N.; Vannucchi, H.; Curi, R.; Calder, P.C. Metabolic syndrome: Epidemiology, pathophysiology, and nutrition intervention. J. Nutr. Metab. 2012, 2012, 584541. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: A systematic review and modelling analysis. Lancet Child Adolesc. Health 2022, 6, 158–170. [Google Scholar] [CrossRef]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011-2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef]
- Popa, S.; Moţa, M.; Popa, A.; Moţa, E.; Serafinceanu, C.; Guja, C.; Catrinoiu, D.; Hâncu, N.; Lichiardopol, R.; Bala, C.; et al. Prevalence of overweight/obesity, abdominal obesity and metabolic syndrome and atypical cardiometabolic phenotypes in the adult Romanian population: PREDATORR study. J. Endocrinol. Investig. 2016, 39, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Vasques, A.C.; Novaes, F.S.; de Oliveira Mda, S.; Souza, J.R.; Yamanaka, A.; Pareja, J.C.; Tambascia, M.A.; Saad, M.J.; Geloneze, B. TyG index performs better than HOMA in a Brazilian population: A hyperglycemic clamp validated study. Diabetes Res. Clin. Pract. 2011, 93, e98–e100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.; Tang, J.; Shen, L.; He, J.; Chen, Y. Association of triglyceride glucose-related parameters with all-cause mortality and cardiovascular disease in NAFLD patients: NHANES 1999-2018. Cardiovasc. Diabetol. 2024, 23, 262. [Google Scholar] [CrossRef]
- Mirr, M.; Skrypnik, D.; Bogdański, P.; Owecki, M. Newly proposed insulin resistance indexes called TyG-NC and TyG-NHtR show efficacy in diagnosing the metabolic syndrome. J. Endocrinol. Investig. 2021, 44, 2831–2843. [Google Scholar] [CrossRef]
- Bala, C.; Gheorghe-Fronea, O.; Pop, D.; Pop, C.; Caloian, B.; Comsa, H.; Bozan, C.; Matei, C.; Dorobantu, M. The Association Between Six Surrogate Insulin Resistance Indexes and Hypertension: A Population-Based Study. Metab. Syndr. Relat. Disord. 2019, 17, 328–333. [Google Scholar] [CrossRef]
- Efrem, I.C.; Moța, M.; Vladu, I.M.; Mitrea, A.; Clenciu, D.; Timofticiuc, D.C.P.; Diaconu, I.-D.; Turcu, A.; Crișan, A.E.; Geormăneanu, C.; et al. A Study of Biomarkers Associated with Metabolic Dysfunction-Associated Fatty Liver Disease in Patients with Type 2 Diabetes. Diagnostics 2022, 12, 2426. [Google Scholar] [CrossRef]
- Amzolini, A.M.; Forţofoiu, M.C.; Barău Abu-Alhija, A.; Vladu, I.M.; Clenciu, D.; Mitrea, A.; Forţofoiu, M.; Matei, D.; Enăchescu, V.; Predescu, O.I.; et al. Triglyceride and glucose index: A useful tool for non-alcoholic liver disease assessed by liver biopsy in patients with metabolic syndrome? Rom. J. Morphol. Embryol. 2021, 62, 475–480. [Google Scholar] [CrossRef]
- Amzolini, A.M.; Forțofoiu, M.-C.; Alhija, A.B.; Vladu, I.M.; Clenciu, D.; Mitrea, A.; Forțofoiu, M.; Matei, D.; Diaconu, M.; Tudor, M.S.; et al. Triglyceride and Glucose Index as a Screening Tool for Nonalcoholic Liver Disease in Patients with Metabolic Syndrome. J. Clin. Med. 2022, 11, 3043. [Google Scholar] [CrossRef]
- Raimi, T.H.; Dele-Ojo, B.F.; Dada, S.A.; Fadare, J.O.; Ajayi, D.D.; Ajayi, E.A.; Ajayi, O.A. Triglyceride-Glucose Index and Related Parameters Predicted Metabolic Syndrome in Nigerians. Metab. Syndr. Relat. Disord. 2021, 19, 76–82. [Google Scholar] [CrossRef]
- Khan, S.H.; Sobia, F.; Niazi, N.K.; Manzoor, S.M.; Fazal, N.; Ahmad, F. Metabolic clustering of risk factors: Evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol. Metab. Syndr. 2018, 10, 74. [Google Scholar] [CrossRef]
- Lim, J.; Kim, J.; Koo, S.H.; Kwon, G.C. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007-2010 Korean National Health and Nutrition Examination Survey. PLoS ONE 2019, 14, e0212963. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, L.; Zhang, W.; Ming, J.; Jia, A.; Xu, S.; Li, Q.; Ji, Q. Fasting triglycerides and glucose index is more suitable for the identification of metabolically unhealthy individuals in the Chinese adult population: A nationwide study. J. Diabetes Investig. 2019, 10, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Reaven, G.M. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: Triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism 2011, 60, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Fan, J.; Pan, S.J. METS-IR, a novel simple insulin resistance indexes, is associated with hypertension in normal-weight Chinese adults. J. Clin. Hypertens. 2019, 21, 1075–1081. [Google Scholar] [CrossRef]
- Nabipoorashrafi, S.A.; Seyedi, S.A.; Rabizadeh, S.; Ebrahimi, M.; Ranjbar, S.A.; Reyhan, S.K.; Meysamie, A.; Nakhjavani, M.; Esteghamati, A. The accuracy of triglyceride-glucose (TyG) index for the screening of metabolic syndrome in adults: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2677–2688. [Google Scholar] [CrossRef]
- Wan, H.; Cao, H.; Ning, P. Superiority of the triglyceride glucose index over the homeostasis model in predicting metabolic syndrome based on NHANES data analysis. Sci. Rep. 2024, 14, 15499. [Google Scholar] [CrossRef]
- Son, D.H.; Lee, H.S.; Lee, Y.J.; Lee, J.H.; Han, J.H. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 596–604. [Google Scholar] [CrossRef]
- Mansoori, A.; Nosrati, M.; Dorchin, M.; Mohammadyari, F.; Derakhshan-Nezhad, E.; Ferns, G.; Esmaily, H.; Ghayour-Mobarhan, M. A novel index for diagnosis of type 2 diabetes mellitus: Cholesterol, High density lipoprotein, and Glucose (CHG) index. J. Diabetes Investig. 2025, 16, 309–314. [Google Scholar] [CrossRef]
- Laohabut, I.; Udol, K.; Phisalprapa, P.; Srivanichakorn, W.; Chaisathaphol, T.; Washirasaksiri, C.; Sitasuwan, T.; Chouriyagune, C.; Auesomwang, C. Neck circumference as a predictor of metabolic syndrome: A cross-sectional study. Prim. Care Diabetes 2020, 14, 265–273. [Google Scholar] [CrossRef]
- Yang, G.R.; Dye, T.D.; Zand, M.S.; Fogg, T.T.; Yuan, S.Y.; Yang, J.K.; Li, D. Association Between Neck Circumference and Coronary Heart Disease: A Meta-analysis. Asian Pac. Isl. Nurs. J. 2019, 4, 34–46. [Google Scholar] [CrossRef]
- Bello-Chavolla, O.Y.; Almeda-Valdes, P.; Gomez-Velasco, D.; Viveros-Ruiz, T.; Cruz-Bautista, I.; Romo-Romo, A.; Sánchez-Lázaro, D.; Meza-Oviedo, D.; Vargas-Vázquez, A.; Campos, O.A.; et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur. J. Endocrinol. 2018, 178, 533–544. [Google Scholar] [CrossRef]
- Widjaja, N.A.; Irawan, R.; Hanindita, M.H.; Ugrasena, I.; Handajani, R. METS-IR vs. HOMA-AD and Metabolic Syndrome in Obese Adolescents. J. Med. Investig. 2023, 70, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, Y.; Fujihara, K.; Yamada-Harada, M.; Mitsuma, Y.; Sato, T.; Yaguchi, Y.; Osawa, T.; Yamamoto, M.; Kitazawa, M.; Yamada, T.; et al. Impact of metabolic syndrome and metabolic dysfunction-associated fatty liver disease on cardiovascular risk by the presence or absence of type 2 diabetes and according to sex. Cardiovasc. Diabetol. 2022, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Leoni, S.; Tovoli, F.; Napoli, L.; Serio, I.; Ferri, S.; Bolondi, L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J. Gastroenterol. 2018, 24, 3361–3373. [Google Scholar] [CrossRef]
- Kouvari, M.; Chrysohoou, C.; Skoumas, J.; Pitsavos, C.; Panagiotakos, D.B.; Mantzoros, C.S.; ATTICA study Investigators. The presence of NAFLD influences the transition of metabolically healthy to metabolically unhealthy obesity and the ten-year cardiovascular disease risk: A population-based cohort study. Metabolism 2022, 128, 154893. [Google Scholar] [CrossRef]
- Mota, M.; Popa, S.G.; Mota, E.; Mitrea, A.; Catrinoiu, D.; Cheta, D.M.; Guja, C.; Hancu, N.; Ionescu-Tirgoviste, C.; Lichiardopol, R.; et al. Prevalence of diabetes mellitus and prediabetes in the adult Romanian population: PREDATORR study. J. Diabetes 2016, 8, 336–344. [Google Scholar] [CrossRef]
- World Health Organization. Waist circumference and waist–hip ratio. In Proceedings of the Report of a WHO Expert Consultation, Geneva, Switzerland, 8–11 December 2008; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Weststrate, J.A.; Deurenberg, P. Body composition in children: Proposal for a method for calculating body fat percentage from total body density or skinfold-thickness measurements. Am. J. Clin. Nutr. 1989, 50, 1104–1115, Erratum in Am. J. Clin. Nutr. 1991, 54, 428. Erratum in Am. J. Clin. Nutr. 1991, 54, 590. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
| Characteristics | Female Group | Male Group | ||||
|---|---|---|---|---|---|---|
| MetS (+) | MetS (−) | p-Value | MetS (+) | MetS (−) | p-Value | |
| Participants, no. | 595 | 782 | 590 | 627 | ||
| Age (years) | 63 (13) | 53 (22) | <0.001 | 61 (15) | 57 (24) | <0.001 |
| BMI (kg/m2) | 30.8 (6.9) | 26 (7.6) | <0.001 | 29.4 (5.5) | 26.3 (5.4) | <0.001 |
| WC (cm) | 101.5 (16) | 88 (20) | <0.001 | 106 (13) | 97.2 (16) | <0.001 |
| WHtR | 0.63 (0.10) | 0.54 (0.13) | <0.001 | 0.61 (0.08) | 0.56 (0.09) | <0.001 |
| NC (cm) | 37 (4) | 34 (5) | <0.001 | 42 (4) | 40 (5) | <0.001 |
| NhtR | 0.23 (0.03) | 0.21 (0.03) | <0.001 | 0.23 (0.03) | 0.22 (0.03) | <0.001 |
| SBP (mmHg) | 140 (25) | 127 (28) | <0.001 | 145 (28) | 133 (29) | <0.001 |
| DBP (mmHg) | 81 (16) | 76 (15) | <0.001 | 84 (15) | 79 (17) | <0.001 |
| FPG (mg/dL) | 96.17 (31) | 80 (14) | <0.001 | 99 (32) | 81.99 (16) | <0.001 |
| HbA1c (%) | 5.80 (0.9) | 5.38 (0.5) | <0.001 | 5.70 (1.0) | 5.40 (0.4) | <0.001 |
| TC (mg/dL) | 212 (75) | 204.5 (59) | 0.006 | 204 (64) | 200 (57) | 0.014 |
| HDL-c (mg/dL) | 48 (14) | 62 (17) | <0.001 | 42 (14) | 53.36 (17) | <0.001 |
| LDL-c (mg/dL) | 133 (64) | 124 (50) | <0.001 | 123 (54) | 121.34 (50) | 0.638 |
| TG (mg/dL) | 153.57 (79) | 88 (49) | <0.001 | 172 (110) | 98.94 (53) | <0.001 |
| %BF | 45.68 (8.28) | 38.66 (11.66) | <0.001 | 32.94 (7.37) | 28.54 (8.39) | <0.001 |
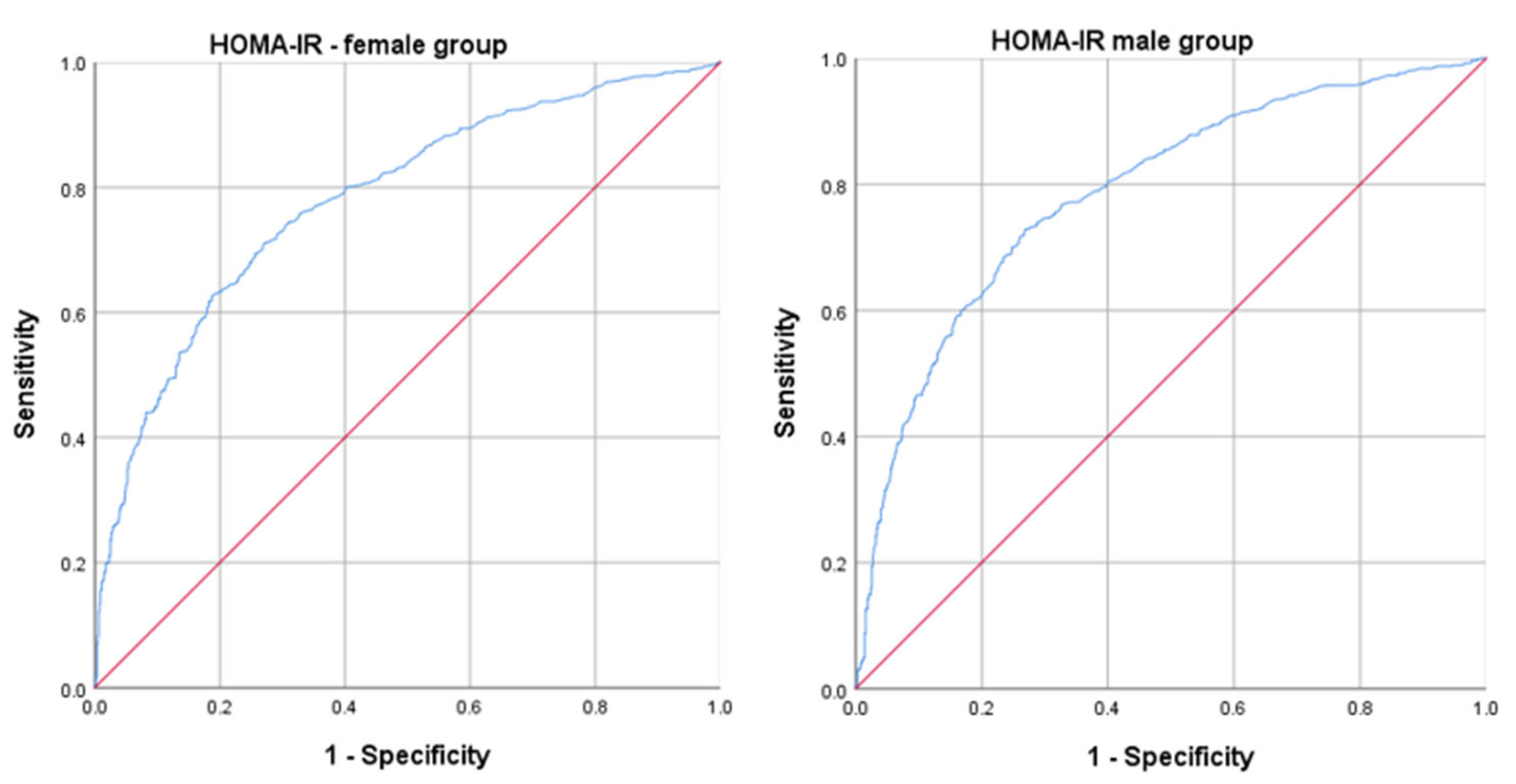
| HOMA-IR | 4.01 (2.72) | 1.53 (1.17) | <0.001 | 4.07 (3.04) | 1.85 (1.21) | <0.001 |
| Marital status (%) | ||||||
| Married | 62.5 | 69.1 | <0.001 | 86.7 | 78.9 | <0.001 |
| Single | 4.1 | 9.0 | 3.2 | 11.8 | ||
| Divorced | 6.1 | 7.7 | 5.1 | 4.3 | ||
| Widowed | 27.3 | 14.2 | 5.0 | 4.9 | ||
| High educational level (%) | 55.7 | 67.0 | <0.001 | 59.1 | 59.9 | 0.772 |
| Sedentarism (%) | 18.1 | 19.8 | 0.425 | 19.9 | 15.6 | 0.047 |
| Reduced sleep duration (%) | 43.2 | 32.8 | <0.001 | 28.5 | 28.8 | 0.922 |
| Alcohol consumption (%) | 34.6 | 36.2 | 0.550 | 76.0 | 79.8 | 0.107 |
| Smoking (%) | ||||||
| Daily smokers | 12.9 | 15.1 | 0.021 | 19.5 | 22.1 | 0.125 |
| Occasional smokers | 1.3 | 3.7 | 2.6 | 4.2 | ||
| Former smokers | 16.9 | 17.1 | 43.7 | 38.3 | ||
| Non-smokers | 68.9 | 64.1 | 34.2 | 35.4 | ||
| IR Indicators | Female Group | Male Group | ||||
|---|---|---|---|---|---|---|
| MetS (+) | MetS (−) | p-Value | MetS (+) | MetS (−) | p-Value | |
| TyG | 8.90 [0.64] | 8.18 (0.60) | <0.001 | 9.05 (0.72) | 8.30 (0.57) | <0.001 |
| TyG–BMI | 276.35 (64.57) | 213.82 (67.02) | <0.001 | 268.50 (59.93) | 217.41 (50.68) | <0.001 |
| TyG–WC | 912.20 ± 128.78 | 724.17 ± 131.97 | <0.001 | 982.33 ± 135.99 | 810.24 ± 122.20 | <0.001 |
| TyG–WHtR | 5.70 ± 0.81 | 4.49 ± 0.84 | <0.001 | 5.65 ± 0.80 | 4.66 ± 0.69 | <0.001 |
| TyG–NC | 329.12 (52.59) | 281.60 (49.68) | <0.001 | 378.90 (59.30) | 328.60 (46.33) | <0.001 |
| TyG–NHtR | 2.04 (0.35) | 1.75 (0.32) | <0.001 | 2.18 (0.34) | 1.89 (0.28) | <0.001 |
| TG/HDL-c | 3.08 (2.04) | 1.40 (0.94) | <0.001 | 4.17 (3.62) | 1.83 (1.37) | <0.001 |
| MetS-IR | 47.30 (12.44) | 34.87 (11.00) | <0.001 | 47.36 (11.95) | 37.23 (9.24) | <0.001 |
| IR Indicators | TyG | TyG–BMI | TyG–WC | TyG–WHtR | TyG–NC | TyG–NHtR | TG/HDL-c | MetS-IR |
|---|---|---|---|---|---|---|---|---|
| TyG | 1 | |||||||
| TyG–BMI | 0.611 * | 1 | ||||||
| TyG–WC | 0.682 * | 0.874 * | 1 | |||||
| TyG–WHtR | 0.680 * | 0.883 * | 0.975 * | 1 | ||||
| TyG–NC | 0.713 * | 0.727 * | 0.779 * | 0.746 * | 1 | |||
| TyG–NHtR | 0.714 * | 0.746 * | 0.757 * | 0.787 * | 0.953 * | 1 | ||
| TG/HDL-c | 0.873 * | 0.543 * | 0.605 * | 0.594 * | 0.623 * | 0.612 * | 1 | |
| MetS-IR | 0.606 * | 0.971 * | 0.865 * | 0.868 * | 0.715 * | 0.728 * | 0.615 * | 1 |
| IR Indicators | TyG | TyG–BMI | TyG–WC | TyG–WHtR | TyG–NC | TyG–NHtR | TG/HDL-c | MetS-IR |
|---|---|---|---|---|---|---|---|---|
| TyG | 1 | |||||||
| TyG–BMI | 0.619 * | 1 | ||||||
| TyG–WC | 0.678 * | 0.818 * | 1 | |||||
| TyG–WHtR | 0.674 * | 0.824 * | 0.969 * | 1 | ||||
| TyG–NC | 0.588 * | 0.593 * | 0.621 * | 0.600 * | 1 | |||
| TyG–NHtR | 0.578 * | 0.595 * | 0.588 * | 0.626 * | 0.971 * | 1 | ||
| TG/HDL-c | 0.757 * | 0.416 * | 0.453 * | 0.443 * | 0.418 * | 0.404 * | 1 | |
| MetS-IR | 0.629 * | 0.954 * | 0.795 * | 0.791 * | 0.582 * | 0.576 * | 0.540 * | 1 |
| Variables | Female Group | Male Group | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age (years) | 1.057 | 1.047–1.066 | <0.001 | 1.022 | 1.013–1.030 | <0.001 |
| BMI (kg/m2) | 1.135 | 1.111–1.159 | <0.001 | 1.202 | 1.165–1.239 | <0.001 |
| WC (cm) | 1.076 | 1.066–1.086 | <0.001 | 1.070 | 1.058–1.082 | <0.001 |
| WHtR (≧0.5852) | 5.868 | 4.634–7.430 | <0.001 | 4.116 | 3.253–5.209 | <0.001 |
| NC (cm) | 1.162 | 1.126–1.198 | <0.001 | 1.101 | 1.069–1.134 | <0.001 |
| NhtR (≧0.2293) | 3.099 | 2.471–3.886 | <0.001 | 2.888 | 2.285–3.650 | <0.001 |
| SBP (mmHg) | 1.030 | 1.024–1.035 | <0.001 | 1.024 | 1.018–1.030 | <0.001 |
| DBP (mmHg) | 1.034 | 1.024–1.044 | <0.001 | 1.030 | 1.020–1.039 | <0.001 |
| FPG (mg/dL) | 1.069 | 1.059–1.079 | <0.001 | 1.054 | 1.045–1.062 | <0.001 |
| HbA1c (%) | 6.834 | 5.189–9.000 | <0.001 | 4.247 | 3.302–5.463 | <0.001 |
| TC (mg/dL) | 1.003 | 1.001–1.005 | 0.007 | 1.003 | 1.001–1.006 | 0.008 |
| HDL-c (mg/dL) | 0.916 | 0.905–0.926 | <0.001 | 0.926 | 0.916–0.937 | <0.001 |
| LDL-c (mg/dL) | 1.006 | 1.003–1.008 | <0.001 | 1.001 | 0.998–1.003 | 0.685 |
| TG (mg/dL) | 1.028 | 1.025–1.032 | <0.001 | 1.019 | 1.017–1.022 | <0.001 |
| % BF | 1.124 | 1.106–1.143 | <0.001 | 1.141 | 1.117–1.166 | <0.001 |
| HOMA-IR | 1.821 | 1.667–1.988 | <0.001 | 1.704 | 1.564–1.855 | <0.001 |
| TyG | 52.985 | 34.600–81.139 | <0.001 | 29.123 | 19.900–42.622 | <0.001 |
| TyG–BMI | 1.021 | 1.019–1.024 | <0.001 | 1.032 | 1.028–1.036 | <0.001 |
| TyG–WC | 1.011 | 1.010–1.012 | <0.001 | 1.012 | 1.011–1.013 | <0.001 |
| TyG–WHtR | 5.820 | 4.811–7.040 | <0.001 | 7.363 | 5.808–9.335 | <0.001 |
| TyG–NC | 1.031 | 1.027–1.035 | <0.001 | 1.027 | 1.023–1.030 | <0.001 |
| TyG–NHtR | 145.452 | 80.931–261.410 | <0.001 | 79.122 | 44.325–141.234 | <0.001 |
| TG/HDL-c | 4.484 | 3.792–5.302 | <0.001 | 2.135 | 1.933–2.358 | <0.001 |
| MetS-IR | 1.149 | 1.131–1.168 | <0.001 | 1.204 | 1.178–1.231 | <0.001 |
| Variables | Female Group | Male Group | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| TyG | 86.426 | 40.023–186.631 | <0.001 | 40.242 | 21.488–75.365 | <0.001 |
| TyG–BMI | 0.898 | 0.880–0.915 | <0.001 | 0.944 | 0.930–0.959 | <0.001 |
| TyG–WC | 1.002 | 0.961–1.044 | 0.933 | 1.006 | 0.973–1.040 | 0.728 |
| TyG–WHtR | 2.377 | 0.003–1836.364 | 0.799 | 0.662 | 0.002–202.588 | 0.888 |
| TyG–NC | 0.999 | 0.893–1.119 | 0.990 | 1.002 | 0.921–1.089 | 0.970 |
| TyG–NHtR | 0.618 | 0.000–46,825,902.32 | 0.959 | 0.896 | 0.000–1,981,435.421 | 0.988 |
| TG/HDL-c | 0.677 | 0.561–0.818 | <0.001 | 0.793 | 0.720–0.874 | <0.001 |
| MetS-IR | 1.881 | 1.684–2.101 | <0.001 | 1.448 | 1.336–1.569 | <0.001 |
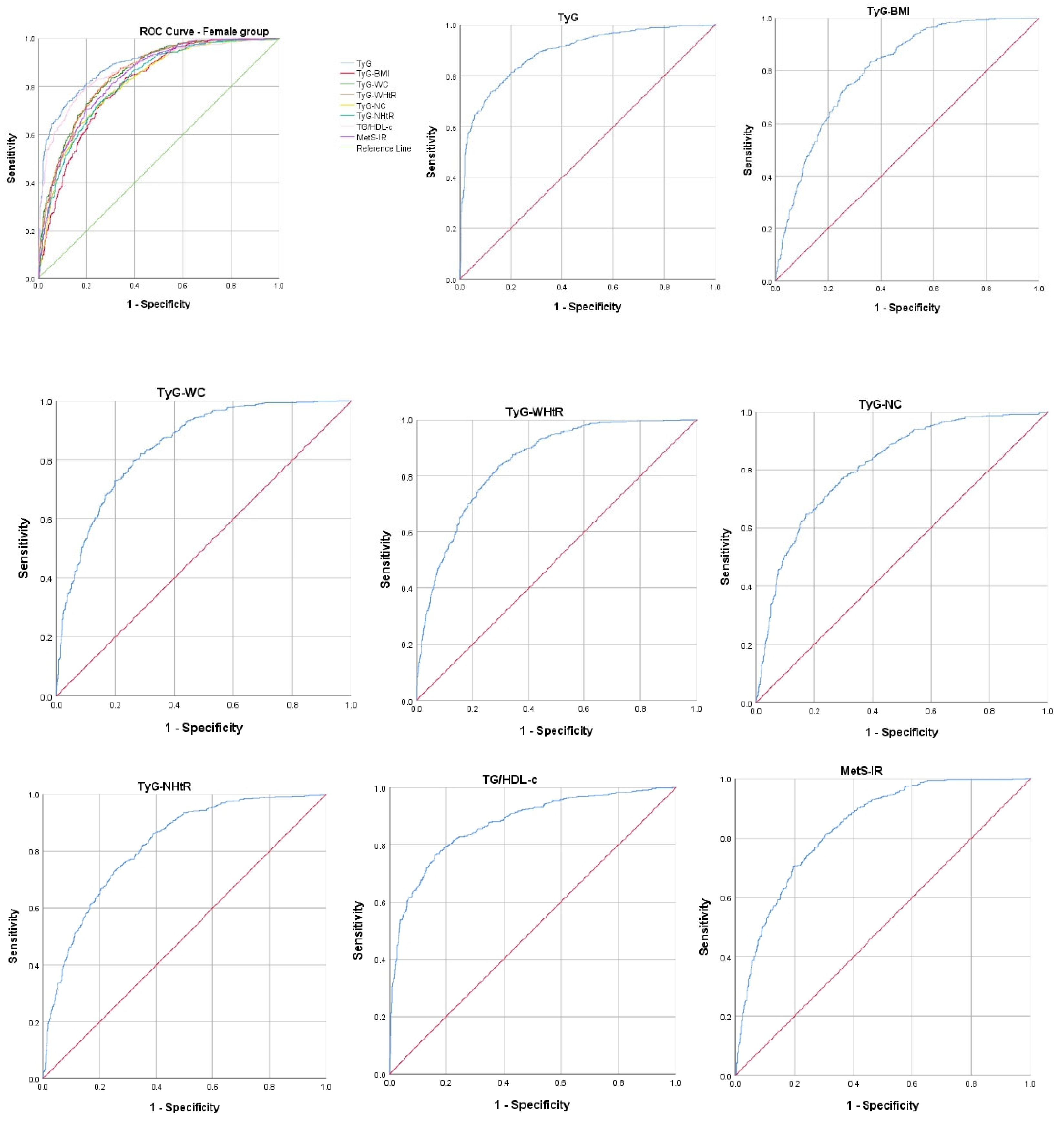
| IR Indicators | AUROC Curve | Standard Error | 95% CI | p-Value | Cut-Off Value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|
| TyG | 0.890 | 0.009 | 0.873–0.907 | <0.001 | 8.51 | 81.4% | 80.0% |
| TyG–BMI | 0.806 | 0.011 | 0.784–0.828 | <0.001 | 244.52 | 74.4% | 86.0% |
| TyG–WC | 0.848 | 0.010 | 0.829–0.868 | <0.001 | 805.72 | 79.5% | 74.0% |
| TyG–WHtR | 0.849 | 0.010 | 0.829–0.868 | <0.001 | 5.06 | 79.4% | 74.2% |
| TyG–NC | 0.814 | 0.011 | 0.791–0.836 | <0.001 | 306.46 | 72.4% | 74.9% |
| TyG–NHtR | 0.817 | 0.011 | 0.795–0.839 | <0.001 | 1.89 | 74.3% | 73.2% |
| TG/HDL-c | 0.872 | 0.010 | 0.853–0.891 | <0.001 | 2.05 | 80.4% | 79.0% |
| MetS-IR | 0.836 | 0.010 | 0.815–0.857 | <0.001 | 41.02 | 74.3% | 75.8% |
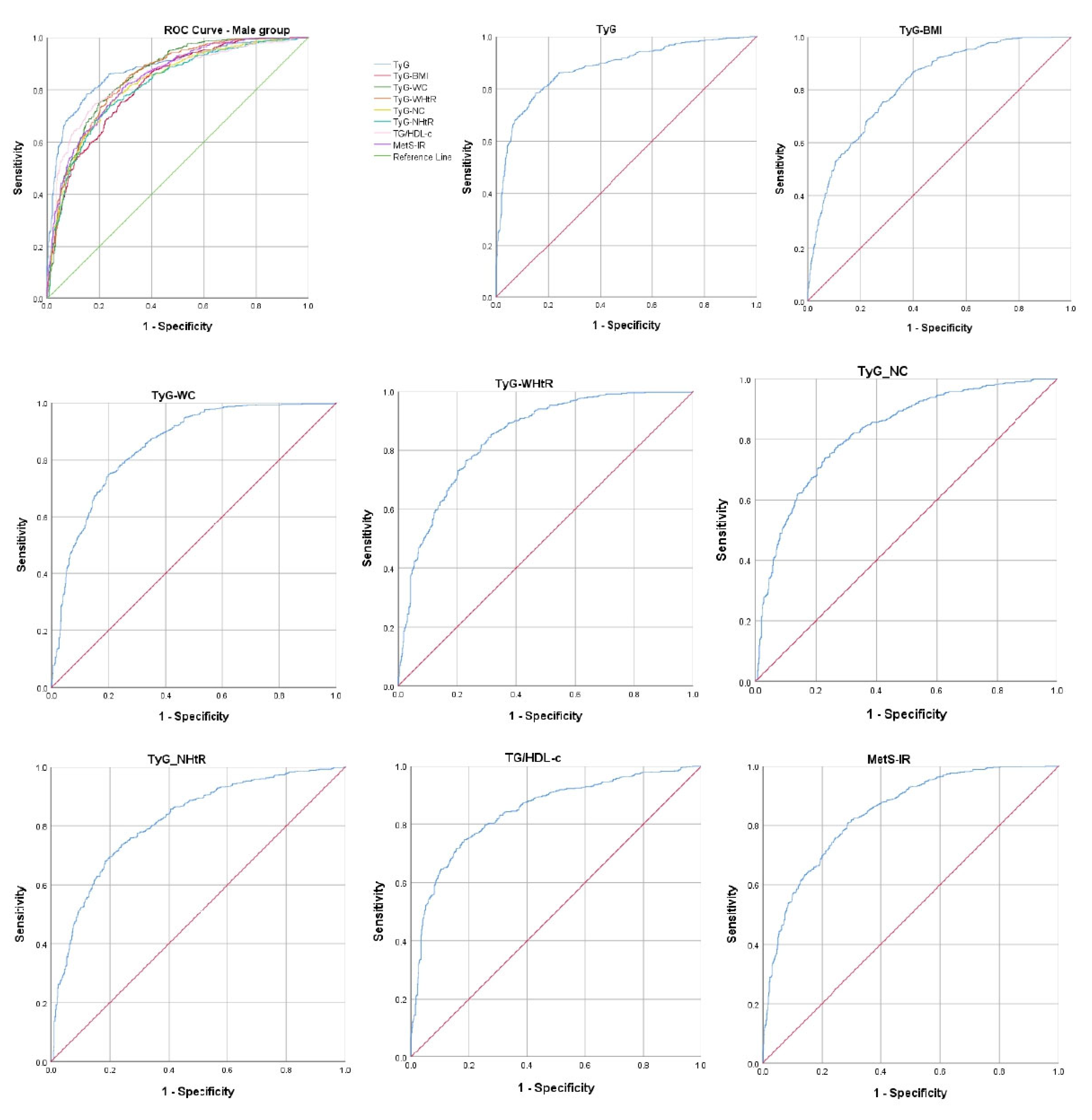
| IR Indicators | AUROC Curve | Standard Error | 95% CI | p-Value | Cut-Off Value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|
| TyG | 0.880 | 0.010 | 0.861–0.899 | <0.001 | 8.69 | 78.5% | 84.6% |
| TyG–BMI | 0.818 | 0.012 | 0.795–0.840 | <0.001 | 241.54 | 75.3% | 71.7% |
| TyG–WC | 0.851 | 0.011 | 0.831–0.872 | <0.001 | 895.51 | 74.8% | 80.3% |
| TyG–WHtR | 0.845 | 0.011 | 0.824–0.866 | <0.001 | 5.07 | 76.9% | 76.6% |
| TyG–NC | 0.823 | 0.012 | 0.800–0.846 | <0.001 | 352.06 | 75.3% | 75.5% |
| TyG–NHtR | 0.815 | 0.012 | 0.792–0.838 | <0.001 | 2.03 | 73.8% | 76.0% |
| TG/HDL-c | 0.845 | 0.011 | 0.824–0.867 | <0.001 | 2.87 | 74.6% | 81.5% |
| MetS-IR | 0.840 | 0.011 | 0.819–0.862 | <0.001 | 41.07 | 80.5% | 71.2% |
| Measure | Categorical Cut Points |
|---|---|
| Elevated waist circumference * | Population- and country-specific definitions (Europid: Men ≥ 94 cm, Women ≥ 80 cm) |
| Elevated triglycerides (or drug treatment for elevated triglycerides †) | ≥150 mg/dL |
| Reduced HDL-c (or drug treatment for reduced HDL-c †) | <40 mg/dL in males; <50 mg/dL in females |
| Elevated blood pressure (or antihypertensive drug treatment in a patient with a history of hypertension) | Systolic ≥ 130 and/or diastolic ≥ 85 mmHg |
| Elevated fasting glucose ‡ (or drug treatment of elevated glucose) | ≥100 mg/dL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ştefan, A.G.; Clenciu, D.; Mitrea, A.; Vladu, I.M.; Protasiewicz-Timofticiuc, D.C.; Roşu, M.M.; Maria, D.T.; Dinu, I.R.; Gheonea, T.C.; Vladu, B.E.; et al. Metabolic Syndrome and Insulin Resistance in Romania. Int. J. Mol. Sci. 2025, 26, 2389. https://doi.org/10.3390/ijms26062389
Ştefan AG, Clenciu D, Mitrea A, Vladu IM, Protasiewicz-Timofticiuc DC, Roşu MM, Maria DT, Dinu IR, Gheonea TC, Vladu BE, et al. Metabolic Syndrome and Insulin Resistance in Romania. International Journal of Molecular Sciences. 2025; 26(6):2389. https://doi.org/10.3390/ijms26062389
Chicago/Turabian StyleŞtefan, Adela Gabriela, Diana Clenciu, Adina Mitrea, Ionela Mihaela Vladu, Diana Cristina Protasiewicz-Timofticiuc, Maria Magdalena Roşu, Daniela Teodora Maria, Ilie Robert Dinu, Theodora Claudia Gheonea, Beatrice Elena Vladu, and et al. 2025. "Metabolic Syndrome and Insulin Resistance in Romania" International Journal of Molecular Sciences 26, no. 6: 2389. https://doi.org/10.3390/ijms26062389
APA StyleŞtefan, A. G., Clenciu, D., Mitrea, A., Vladu, I. M., Protasiewicz-Timofticiuc, D. C., Roşu, M. M., Maria, D. T., Dinu, I. R., Gheonea, T. C., Vladu, B. E., Efrem, I. C., Moţa, E., & Moţa, M. (2025). Metabolic Syndrome and Insulin Resistance in Romania. International Journal of Molecular Sciences, 26(6), 2389. https://doi.org/10.3390/ijms26062389






